Abstract
RhC30H25Cl2NO2P, orthorhombic P212121 (no. 19), a = 7.6656(5) Å, b = 16.6097(11) Å, c = 20.9423(14) Å, V = 2666.4(3) Å3, Z = 4, Rgt(F) = 0.0649, wRref(F2) = 0.1632, T = 100 K.
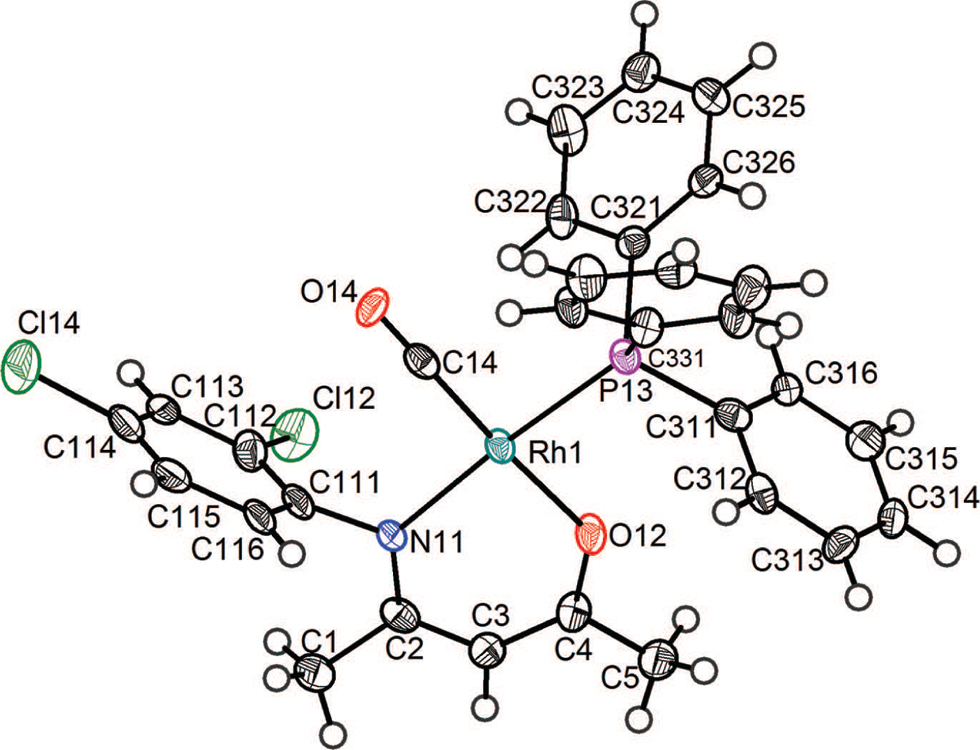
The crystal structure is shown in the figure. Tables 1 and 2 contain details on crystal structure and measurement conditions and a list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Vuboid, colourless |
| Size: | 0.22 × 0.15 × 0.08 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.93 mm−1 |
| Diffractometer, scan mode: | X8 ApexII Kappa CCD, φ and ω-scans |
| 2θmax, completeness: | 28.3°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 9906, 6127, 0.052 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 4921 |
| N(param)refined: | 330 |
| Programs: | Bruker programs [12, 13] , via SIR2014 [14], DIAMOND [15], WinGX, ORTEP [16], SHELX [17] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| Rh1 | 0.86210(7) | 0.18073(3) | 0.28939(2) | 0.01997(14) |
| N11 | 0.7378(8) | 0.2079(4) | 0.3749(3) | 0.0215(13) |
| O12 | 1.0336(7) | 0.2713(3) | 0.3076(2) | 0.0290(13) |
| O14 | 0.6362(8) | 0.0407(3) | 0.2629(2) | 0.0284(11) |
| P13 | 1.0094(2) | 0.15912(11) | 0.19709(8) | 0.0199(4) |
| Cl12 | 0.8431(3) | 0.07631(14) | 0.46271(12) | 0.0446(6) |
| Cl14 | 0.1983(4) | −0.03765(17) | 0.43525(14) | 0.0603(8) |
| C1 | 0.6752(11) | 0.2786(5) | 0.4747(4) | 0.0316(18) |
| H1A | 0.5511 | 0.2776 | 0.4635 | 0.047* |
| H1B | 0.7044 | 0.3308 | 0.4936 | 0.047* |
| H1C | 0.7 | 0.2356 | 0.5054 | 0.047* |
| C2 | 0.7824(10) | 0.2659(5) | 0.4157(3) | 0.0259(16) |
| C3 | 0.9233(9) | 0.3188(5) | 0.4052(3) | 0.0226(14) |
| H3 | 0.9411 | 0.3587 | 0.437 | 0.027* |
| C4 | 1.0375(9) | 0.3201(5) | 0.3552(3) | 0.0233(14) |
| C5 | 1.1822(11) | 0.3806(5) | 0.3536(4) | 0.0333(19) |
| H5A | 1.2948 | 0.3527 | 0.3554 | 0.05* |
| H5B | 1.1716 | 0.4169 | 0.3903 | 0.05* |
| H5C | 1.1751 | 0.4119 | 0.314 | 0.05* |
| C14 | 0.7209(10) | 0.0951(5) | 0.2721(3) | 0.0226(16) |
| C111 | 0.6021(10) | 0.1525(5) | 0.3937(4) | 0.0306(13) |
| C112 | 0.6404(11) | 0.0879(5) | 0.4315(3) | 0.0291(16) |
| C113 | 0.5154(13) | 0.0288(5) | 0.4454(4) | 0.038(2) |
| H113 | 0.5434 | −0.0166 | 0.471 | 0.046* |
| C114 | 0.3462(13) | 0.0393(6) | 0.4197(4) | 0.041(2) |
| C115 | 0.3043(11) | 0.1034(5) | 0.3835(4) | 0.036(2) |
| H115 | 0.1891 | 0.1088 | 0.3673 | 0.043* |
| C116 | 0.4319(10) | 0.1630(5) | 0.3693(4) | 0.0306(13) |
| H116 | 0.4032 | 0.2087 | 0.3441 | 0.037* |
| C311 | 1.1696(10) | 0.2372(4) | 0.1843(3) | 0.0237(16) |
| C312 | 1.1152(9) | 0.3145(5) | 0.1688(3) | 0.0249(15) |
| H312 | 0.9944 | 0.3241 | 0.1623 | 0.03* |
| C313 | 1.2307(10) | 0.3778(5) | 0.1623(4) | 0.0272(17) |
| H313 | 1.1894 | 0.4298 | 0.1509 | 0.033* |
| C314 | 1.4076(10) | 0.3651(5) | 0.1725(3) | 0.0253(17) |
| H314 | 1.4879 | 0.4083 | 0.1684 | 0.03* |
| C315 | 1.4661(10) | 0.2888(5) | 0.1888(4) | 0.0286(17) |
| H315 | 1.5867 | 0.2798 | 0.1964 | 0.034* |
| C316 | 1.3484(10) | 0.2252(4) | 0.1939(3) | 0.0259(16) |
| H316 | 1.3902 | 0.1729 | 0.2041 | 0.031* |
| C321 | 1.1351(10) | 0.0655(4) | 0.1931(3) | 0.0203(14) |
| C322 | 1.1317(10) | 0.0130(5) | 0.2454(4) | 0.0281(16) |
| H322 | 1.0644 | 0.0258 | 0.2821 | 0.034* |
| C323 | 1.2276(10) | −0.0583(5) | 0.2433(4) | 0.034(2) |
| H323 | 1.2237 | −0.0946 | 0.2783 | 0.041* |
| C324 | 1.3266(10) | −0.0760(5) | 0.1914(4) | 0.0296(18) |
| H324 | 1.3935 | −0.1241 | 0.1908 | 0.036* |
| C325 | 1.3307(11) | −0.0249(5) | 0.1398(4) | 0.0307(18) |
| H325 | 1.4017 | −0.0374 | 0.104 | 0.037* |
| C326 | 1.2314(10) | 0.0449(4) | 0.1399(4) | 0.0237(16) |
| H326 | 1.2298 | 0.0787 | 0.1033 | 0.028* |
| C331 | 0.8886(9) | 0.1600(4) | 0.1207(3) | 0.0212(15) |
| C332 | 0.7241(10) | 0.1266(5) | 0.1168(4) | 0.0262(17) |
| H332 | 0.6693 | 0.1069 | 0.1543 | 0.031* |
| C333 | 0.6376(11) | 0.1213(5) | 0.0588(4) | 0.0326(18) |
| H333 | 0.525 | 0.0976 | 0.0571 | 0.039* |
| C334 | 0.7131(10) | 0.1502(5) | 0.0038(4) | 0.0273(17) |
| H334 | 0.6518 | 0.1475 | −0.0356 | 0.033* |
| C335 | 0.8783(11) | 0.1831(5) | 0.0061(3) | 0.0351(18) |
| H335 | 0.9311 | 0.2034 | −0.0316 | 0.042* |
| C336 | 0.9688(11) | 0.1865(6) | 0.0650(3) | 0.0312(18) |
| H336 | 1.0846 | 0.2068 | 0.0664 | 0.037* |
Source of material
To a 5 mL acetone solution of [Rh(2,4-Cl2-Phony)(CO)2] (0.0207 g, 51.49 μmol) was added PPh3 (0.0137 g, 52.23 μmol) resulting in the immediate evolution of gas. The solution was left to crystallize. Crystals suitable for X-ray diffraction were obtained in quantitative yield (0.0328 g, 100%). IR (KBr): νCO 1963.4 (s) cm−1. UV/Vis: λmax = 328 nm, ϵ = 12678 M−1⋅cm−1. 1H NMR (600.28 MHz, CD2Cl2, 25 °C): 1.34 (s, 5H), 1.98 (s, 1H), 4.98 (s, 3H), 6.26 (d, 116H), 6.62 (dd, 115H), 7.02 (m, Arom), 7.71 (d, 113H). 13C NMR (150.96 MHz, CD2Cl2, 25 °C): 19.15 (s, 1C), 29.22 (s, 5C), 99.17 (s, 3C), 127.18 (s, 111C), 127.21 (s, 116C), 128.07 (s, Arom), 128.31 (s, Arom), 128.44 (s, 115C), 129.91 (s, 112C), 130.59 (s, 114C), 152.25 (s, 113C), 165.99 (s, 2C), 180.30 (s, 4C), 188.20(d, 14C). 31P NMR (242.99 MHz, CD2Cl2, H3PO4, 25 °C): 43.52 (d, JRh-P 161.35 Hz).
Experimental details
The methyl groups were generated to fit the difference electron density and the groups were then refined as rigid rotors, while the aromatic and methine H atoms were placed in geometrically idealized positions and constrained to ride on their parent atoms. C—H = 0.98 Å and 0.95 Å and Uiso(H) = 1.5Ueq(C) and 1.2Ueq(C), respectively. During the structure refinement it became apparent to constrain the Uij parameters of the atoms C111 and C116 using the EADP command of the SHELX system.
Discussion
Rhodium(I) dicarbonyl complexes of the type [Rh(L,L′)(CO)2] containing chelating mono-anionic bidentate (L,L′) ligands coordinated to rhodium via (O,O) donor atoms have been studied as catalyst precursors [1], [2], [3]. The title complex forms part of a study which investigates complexes containing bidentate β-enaminoketonato ligands, starting with 4-(phenylamino)pent-3-en-2-onato (PhonyH [4]) coordinated to rhodium via (N,O) donor atoms. The proposal that only one CO-group in a [Rh(N,O-bid)(CO)2]-type complex will be substituted by triphenyl phosphine, with the product being one of two possible isomers [5], has been confirmed [6], [7], [8]. In each case the CO-group trans to the N-atom will be substituted, attributed to the larger trans-influence of the N-donor atom over the O-atom. This is also evident in the title compound, where [Rh(2,4-diCl-Phony)(CO)(PPh3)] is formed by the substitution of the carbonyl ligand in the dicarbonyl rhodium(I) complex [Rh(2,4-diCl-Phony)(CO)2] [9] by PPh3. Rh1 is displaced from the plane formed by N11, O12, P13 and C14 by 0.01 Å. It may appear that a C—O⋯π interaction might be possible between the carbonyl oxygen, O14, and the delocalized electrons of the phenyl ring of the bidentate ligand, with the distance from O14 to the centroid of the phenyl moiety of the bidentate N,O-ligand being 3.393(5) Å. However, the angle between a line between the O14 atom to the centroid and a plane through the phenyl ring is 75.0°, and this angle, in conjunction with the C14—O14⋯centroid angle of 81.2° refutes this possibility. A similar observation may be made concerning a Cl⋯Cl interaction. At first glance a Type II [10] close intermolecular Cl⋯Cl interaction appears to be present, with a Cl⋯Cl distance of 3.367(4) Å; the C—Cl⋯Cl angles of 137.5(4)° and 98.5(4)° once more refute the possibility. Bond distances and angles involving rhodium in the complex do not differ significantly from those in the related complex [Rh(2,6-diCl-Phony)(CO)(PPh3)] [8], with the exception of the Rh—N and Rh—C distances. These distances of 2.076(6) Å and 1.825(8) Å, respectively, are in turn shorter and significantly longer than in [Rh(2,6-diCl-Phony)(CO)(PPh3)], illustrating the effect of the position of the second chloride atom on the phenyl ring on the electronic effect of the ligand. The effective cone angle, θE, [11] of 155.80(9)° is similar to the angles in the related compounds.
Acknowledgements
Financial assistance from the University of the Free State is gratefully acknowledged. This work is based on the research supported in part by the National Research Foundation of South Africa for the grant, Unique Grant no. 93957, as well as the SA-NRF/THRIP under grant number GUN 2068915. Opinions, findings, conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the SA-NRF.
References
Cornils, B.; Herrmann, W. A.: Applied homogeneous catalysis with organometallic compounds. A Comprehensive handbook, VCH, Weinheim (1996).10.1002/9783527619351Suche in Google Scholar
Trzeciak, A. M.; Ziolkowski, J. J.: 1,5-Hexadiene selective hydroformylation reaction catalyzed with Rh(acac){P(OPh)3}2/P(OPh)3 and Rh(acac)(CO)(PPh3)/PPh3 complexes. J. Organomet. Chem. 464 (1994) 107–111.10.1016/0022-328X(94)87017-9Suche in Google Scholar
Van Rooy, A.; Orji, E. N.; Kramer, P. G. J.; Van Leeuwen, P. W. M. N.: Hydroformylation with a rhodium/bulky phosphite modified catalyst. catalyst comparison for oct-1-ene, cyclohexene, and styrene. Organometallics 14 (1995) 34–43.10.1021/om00001a010Suche in Google Scholar
Shaheen, F.; Marchio, L.; Badshaha, A.; Khosa, M. K.: (Z)-4-anilinopent-3-en-2-one. Acta Crystallogr. E62 (2006) o873–o874.10.1107/S1600536806003205Suche in Google Scholar
Bonati, F.; Wilkinson, G.: Dicarbonyl-β-diketonato- and related complexes of rhodium(I). J. Chem. Soc. (1964) 3156–3160.10.1039/JR9640003156Suche in Google Scholar
Venter, G. J. S.; Steyl, G.; Roodt, A.: Carbonyl[4-(2,3-dimethylphenylamino)-pent-3-en-2-onato-κ2N,O](triphenylphosphine-κP)rhodium(I). Acta Crystallogr. E65 (2009) m1321–m1322.10.1107/S1600536809039816Suche in Google Scholar PubMed PubMed Central
Venter, G. J. S.; Steyl, G.; Roodt, A.: Carbonyl[4-(2,6-dimethylphenylamino)-pent-3-en-2-onato-κ2N,O](triphenylphosphine-kP)rhodium(I) acetone hemisolvate. Acta Crystallogr. E65 (2009) m1606–m1607.10.1107/S160053680904817XSuche in Google Scholar PubMed PubMed Central
Venter, G. J. S.; Steyl, G.; Roodt, A.: Crystal structure of carbonyl-(4-(2,6-dichlorophenylamino)pent-3-en-2-onato-κ2N,O)-(triphenylphosphine-κP)-rhodium(I) acetone solvate, C31.5H28Cl2NO2.5PRh. Z. Kristallogr. NCS 228 (2013) 410–412.10.1524/ncrs.2013.0172Suche in Google Scholar
Venter, G. J. S.; Steyl, G.; Roodt, A.: Solid state and theoretical study of structural properties induced by step-wise chloro functionalization in dicarbonyl-[2-(phenylamino)pent-3-en-4-onato]rhodium(I) complexes. J. Coord. Chem. 67 (2014) 176–193.10.1080/00958972.2013.878801Suche in Google Scholar
Awwadi, F. F.; Willett, R. D.; Peterson, K. A.; Twamley, B.: The nature of halogen⋯halogen synthons: crystallographic and theoretical studies. Chem. Eur. J. 12 (2006) 8952–8960.10.1002/chem.200600523Suche in Google Scholar PubMed
Tolman, C. A.: Steric effects of phosphorus ligands in organometallic chemistry and homogeneous catalysis. Chem. Rev. 77 (1977) 313–348.10.1021/cr60307a002Suche in Google Scholar
Bruker: APEX2 (Version 1.0-27). Bruker AXS Inc., Madison, WI, USA (2012).Suche in Google Scholar
Bruker: SAINT-Plus (Version 7.12) and SADABS (Version 2004/1). Bruker AXS Inc., Madison, WI, USA (2012).Suche in Google Scholar
Burla, M. C.; Caliandro, R.; Carrozzini, B.; Cascarano, G. L.; Cuocci, C.; Giacovazzo, C.; Mallamo, M.; Mazzone, A.; Polidori, G.: Crystal structure determination and refinement via SIR2014. J. Appl. Crystallogr. 48 (2015) 306–309.10.1107/S1600576715001132Suche in Google Scholar
Brandenburg, K.; Putz, H.: DIAMOND. Visual Crystal Structure Information System. Release 4.1.3. Crystal Impact GbR, Bonn, Germany, (2014).Suche in Google Scholar
Farrugia, L. J.: WinGX and ORTEP for Windows: an update. J. Appl. Cryst. 45 (2012) 849–854.10.1107/S0021889812029111Suche in Google Scholar
Sheldrick, G. M.: Crystal structure refinement with SHELXL. Acta Crystallogr. C71 (2015) 3–8.10.1107/S2053229614024218Suche in Google Scholar PubMed PubMed Central
©2017 Gertruida J.S. Venter, published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 License.
Artikel in diesem Heft
- Cover and Frontmatter
- Crystal structure of potassium 1-methyl-1H-1,2,3,4-tetrazole-5-thiolate, C2H3N4SK
- Crystal structure of bis(3-(3-ethylureido)-N,N-dimethylpropan-1-aminium) bis (μ3-2-(hydroxymethyl)-2-(oxidomethyl)propane-1,3-bis(olato))-(μ6-oxo)-hexakis(μ2-oxo)-hexaoxo-hexavanadium(V) – dichloromethane (1/1), C27H60Cl2N6O23V6
- Crystal structure of bis(μ3-methanolato-κ3O:O:O)-bis(μ2-methanolato-κ2O:O)-dimethanol-bis{6,6′-(1,3-dihydroxyl-2-acetylpropane-1,3-diyl)bis(2-chloro-4-bromophenolato)}tetramanganese(III) C40H40Br4Cl4Mn4O16
- Synthesis and crystal structure of tetrakis(μ2-methanolato)-dimethanol-bis(μ2-2-acetyl-1,3-bis(3,5-dibromo-2-oxidophenyl)propane-1,3-bis(olato)-κ5O1,O2,O3:O3,O4,O5)tetramanganese(III), C40H40Br8Mn4O16
- Synthesis and crystal structure tetrakis(μ2-methanolato)-dimethanol-bis(μ2-2-acetyl-1,3-bis(3,5-dichloro-2-oxidophenyl)propane-1,3-bis(olato)-κ5O1,O2,O3:O3,O4,O5), tetramanganese(III), C40H40Cl8Mn4O16
- Crystal structure of bis{5-methoxy-2-((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O)}copper(II), C34H34CuN4O6
- Crystal structure of (E)-1-(2-hydroxy-3-{[2-hydroxybenzylidene]amino}phenyl)ethan-1-one, C15H13NO3
- Crystal structure of bis(μ2-acetato-κ2O:O′)-bis{μ2-4-chloro-2-(8-(4-(diethylamino)-2-oxidophenyl)-3,6-dioxa-2,7-diazaocta-1,7-dien-1-yl)phenolato-κ6O:O,N,N′,O′′:O′′}tricobalt(II), C44H49Cl2Co3N6O12
- Crystal structure of bis{μ2-2,4-dichloro-6-(8-(4-(diethylamino)-2-oxidophenyl)-3,6-dioxa-2,7-diazaocta-1,7-dien-1-yl)phenolato-κ5O:O,N,N′,O′}dicobalt(II) acetone solvate, C43H48Br4Co2N6O9
- Synthesis and crystal structure of bis(μ2-acetato-κ2O:O′)-bis{μ2-4-chloro-2-(8-(4-(diethylamino)-2-oxidophenyl)-3,6-dioxa-2,7-diazaocta-1,7-dien-1-yl)phenolato-κ6O:O,N,N′,O′:O′}trizinc(II), C44H49Cl2N6O12Zn3
- Crystal structure of bis{5-(N,N′-diethylamine)-5′-methoxy -2,2′-[ethylenediyldioxybis(nitrilomethylidyne)]diphenolato}-bis(μ2-acetato-κ2O:O′)trizinc(II), C46H56Zn3N6O14
- Crystal structure of tris(cyano-(hydrogen tris(3,5-dimethylpyrazolyl)borate))-iron(III) 4-methoxypyridinium monohydrate, C24H32BN10O2Fe
- Synthesis and crystal structure of bis{1-(((4-(1-(hydroxyimino)ethyl)phenyl)imino)methyl)naphthalen-2-olato-κ2O,N}copper(II), C38H30CuN4O4
- Crystal structure of (E)-1-(4-{[(E)-2-Hydroxy-1-naphthalenylmethylene] amino}phenyl)ethanone oxime, C19H16N2O2
- The pseudosymmetric crystal structure of 2-(2-naphthalenyl)-3-nitro-2H-1-benzopyran, C38H26N2O6
- Crystal structure of 2-benzoyl-3-(4-methoxyphenyl)cyclopropane-1,1-dicarbonitrile, C19H14N2O2
- N′-(5-ethoxycarbonyl-3,4-dimethyl-pyrrol-2-yl-methylidene)-4-hydroxybenzohydrazide monohydrate, C17H21N3O5
- The crystal structure of carbonyl-[4-(2,4-dichlorophenylamino)pent-3-en-2-onato-κ2N,O]-(triphenylphosphine-κP)rhodium(I), RhC30H25Cl2NO2P
- Crystal structure of (E)-5-(diethylamino)-2-(((1,1,2-trihydroxyethyl)iminio)methyl)phenolate, C15H24N2O4
- Crystal structure of poly[(μ2-1,4-bis((2-ethyl-1H-benzo[d]imidazol-1-yl)methyl)benzene-κ2N:N′)(μ2-isophthalato-κ4O,O′:O′′,O′′′)cadmium(II)], C34H30N4O4Cd
- Crystal structure of 5,6-Dihydro-9,10-dimethoxybenzo[g]-1,3-benzodioxolo[5,6-a]chinolizinium 3-(4-hydroxyphenyl)-4-oxo-4H-chromen-7-olate - methanol - water (1/1/1), C36H33NO10
- A single crystal study on 2-(methylcarbamoyl)benzoic acid, C9H9NO3
- Crystal structure of the salt 1,1′-(ethane-1,2-diyl)bis(1,4-diazabicyclo[2.2.2]octan-1-ium) diperchlorate, C14H28N4(ClO4)2
- Crystal structure of 2,5-bis((E)-2-(trifluoromethyl)benzylidene)cyclopentan-1-one, C21H14F6O
- Crystal structure of catena-poly[(μ2-benzene-1,4-dicarboxylato-κ2O:O′)-(1-ethyl-6-fluoro-7-(4-methylpiperazin-1-ium-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylato-κ2O,O′)zinc(II)] 1.25 hydrate, C25H26.5N3O8.25FZn
- Crystal structure of 1-methyl-1,4-diazabicyclo[2.2.2]octan-1-ium poly[aqua-bis(μ2-perchlorato-κ3O,O′:O′′)sodium], C7H17Cl2N2NaO9
- Crystal structure of trimethyammonium 2,6-dicarboxyisonicotinate monohydrate, C11H16N2O7
- Crystal structure of dodecaguanidinium bis(tetrapropylammonium) heptacarbonate, C43H128N38O21
- Crystal structure of poly[tetraaqua-bis(μ6-benzene-1,2,4,5-tetracarboxylato)nickel(II)diyttrium(III)]dihydrate, C20H16NiO22Y2
- Halogen bonds and π–π interactions in the crystal structure of 1,3,5-trifluoro-2,4,6-triiodobenzene–N,N-dimethylformamide (1/1), C9H7F3I3NO
- Crystal structure of guanidinium tetraethylammonium carbonate dihydrate, C10H30N4O5
- The crystal structure of oxonium chlorido-ethylenediaminetetraactetotin(IV) hydrate, C10H17ClN2O10Sn
- Crystal structure of 8-((E)-((4-((E)-1-((benzyloxy)imino)ethyl)phenyl)imino)methyl)-7-hydroxy-4-methyl-2H-chromen-2-one, C26H22N2O4
- Crystal structure of bis{5-(diethylamino)-2-(((2-oxo-2H-chromen-6-yl)imino)methyl)phenolato-κ2O,N}copper(II), C40H38CuN4O6
- Crystal structure of fac-(acetylacetonato-κ2O,O′)tricarbonyl(tri(p-tolyl)phosphine-κP)rhenium(I), C29H28O5PRe
- Crystal structure of fac-(acetylacetonato-κ2O,O′)tricarbonyl(benzyldiphenylphosphine-κP)rhenium(I), C27H24O5PRe
- Crystal structure of triethylammonium bis{3-(((3-oxidonaphthalen-1-yl)methylene)amino)-2-oxo-2H-chromen-4-olato-κ3O,N,O′}manganese(III), C46H38MnN3O8
- Crystal structure of bis(N,N,N-ethyldimethylethanaminium) bis(heptaselenido-κ2Se1,Se7)palladate(II), C12H32N2PdSe14
- Crystal structure of bis(4-(1H-pyrazol-5-yl)pyridine-κ2N:N′)-bis(2-(2-((2,6-difluorophenyl)amino)phenyl)acetate-κO)cadmium(II), C44H34N8CdCl4O4
- Crystal structure of 4,4′-(1,4-phenylene)bis(pyridin-1-ium) catena-poly[diaqua-bis(μ2-3′,5′-dicarboxy-[1,1′-biphenyl]-2,5-dicarboxylato-κ2O:O′] dihydrate, C48H42O22N2Ca
- The crystal structure of (S)-2-benzylsuccinic acid, C11H12O4
- Crystal structure of poly[aqua-(μ3-4-(pyridin-4-yl)isophthalato-κ4O,O′:O′′:O′′′)-(μ2-5-carboxy-2-(pyridin-4-yl)benzoato-κ2O:O′)yttrium(III)], C26H17N2O9Y
- Crystal structure of ethyl 1-(3,4-dimethylphenyl)-5-methyl-1H-1,2,3-triazole-4-carboxylate, C14H17N3O2
- Crystal structure of tetrakis(methanol-κO)-bis{μ2-3-((4-methoxy-2-oxidobenzylidene)amino)-2-oxo-2H-chromen-4-olate-κ4O,N;O′:O′}dizinc(II), C38H38Zn2N2O14
- Crystal structure of bromido(4,4′-dimethoxy-2,2′-bipyridine-κ2N,N′)(isopropyl(diphenyl)phosphane-κP)copper(I), C27H29BrCuN2O2P
- Crystal structure of bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane)-9,9-dioctylfluorene, C41H64B2O4
- Crystal structure of 11-oxo-2,3,6,7-tetrahydro-1H,5H,11H-pyrano[2,3-f]pyrido[3,2,1-ij]quinoline-10-carbaldehyde - a julolidine derivative, C16H15NO3
- Crystal structure of 1,3-dimethyl-5,5-dibenzylbarbituric acid, C20H20N2O3
- Crystal structure of bis(N,N,N-trimethylethanaminium) poly[bis(μ2-heptaselenido-κ2Se1,Se7)palladate(II)], C10H28N2PdSe14
- Crystal structure of 1-{4-[(5-Chloro-2-hydroxy-benzylidene)amino]phenyl} ethanone O-ethyl-oxime, C17H17ClN2O2
- The crystal structure of methyl N-(4-bromophenyl)carbamate, C8H8BrNO2
- Synthesis and crystal structure of 1-{4-[(2-hydroxy-benzylidene)amino]phenyl}ethanone oxime, C15H14N2O2
- Crystal structure of 1,1′-butanebis(3-methyl-1H-imidazol-3-ium)bis(hexafluorophosphate), C12H20F12N4P2
- (E)-N-benzylidene-3-(benzylthio)-5-p-tolyl-4H-1,2,4-triazol-4-amine, C23H20N4S
- Crystal structure of ethyl 5-methyl-1-(pyridin-3-yl)-1H-1,2,3-triazole-4-carboxylate, C11H12N4O2
- Crystal structure of 5,5-difluoro-10-(4-fluorophenyl)-1,3,7,9-tetramethyl-5H-4l4,5l4-dipyrrolo[1,2-c:2′,1′-f][1,3,2]diazaborinine - a Z′ = 3 structure, C19H18B2F3N2
- The crystal structure of the Matrine derivative: 12-(1H-indol-1-yl)dodecahydro-1H,5H,10H-dipyrido[2,1-f:3′,2′,1′-ij][1,6]naphthyridin-10-one hydrate, C23H29N3O
- The crystal structure of tris(μ2-1,3-bis(4,4,4-trifluoro-3-oxido-1-(oxo)but-2-en-1-yl)phenyl-κ4O,O′:O′′,O′′′)-bis(1,2-dimethoxyethane-κ2O,O′)dilutetium(III), C50H38F18Lu2O16
- The crystal structure of (Z)-2-(3-(2-(4-chlorobenzoyl)hydrazono)-2-oxoindolin-1-yl) acetic acid, C17H12ClN3O4
- Synthesis and crystal structure of trans-tetraqua-bis(2-(((7-hydroxy-3-(4-hydroxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)ammonio)acetato-κO)cobalt(II) hexahydrate, C36H48CoN2O28S2
- The crystal structure of N,N-dimethyl-2,6-di-p-tolylpyrimidin-4-amine, C20H21N3
- The crystal structure (E)-4-methyl-N′-(2-nitrobenzylidene)benzenesulfonohydrazide, C14H13N3O4S
- Crystal structure of catena-poly[(μ2-1,3-bis(benzimidazol-1-yl)propane κ2N:N′)-(μ2-5-methoxyisophthalato-κ2O:O′)zinc(II)] hydrate, C26H24ZnN4O6
- The crystal structure of (E)-N′-(quinolin-2-ylmethylene)furan-2-carbohydrazide monohydrate, C15H13N3O3
- Crystal structure of 2,8-diphenyl-3,7,9-trioxa-1-azaspiro[4.5]dec-1-ene, C18H17N1O3
- Crystal structure of diethyl 2-(4-chlorophenyl)-1,3-dioxane-5,5-dicarboxylate, C16H19ClO6
- Crystal structure of 1-(carboxymethyl)-1H-benzo[d][1,2,3]triazole 3-oxide, C8H7N3O3
- Crystal structure of (acetylacetonato-κ2O,O′)-(2-amino-6-chlorobenzoato-κO)-oxido(1,10-phenanthroline-κ2N,N′)vanadium(IV) – trichloromethane (1/1)
- Crystal structure of (1E,4E)-1,5-bis(4-chlorophenyl)penta-1,4-dien-3-one, C17H12Cl2O
- The crystal structure of trans-dibromido-bis(pyridine-κN)platinum(II), C10H10Br2N2Pt
Artikel in diesem Heft
- Cover and Frontmatter
- Crystal structure of potassium 1-methyl-1H-1,2,3,4-tetrazole-5-thiolate, C2H3N4SK
- Crystal structure of bis(3-(3-ethylureido)-N,N-dimethylpropan-1-aminium) bis (μ3-2-(hydroxymethyl)-2-(oxidomethyl)propane-1,3-bis(olato))-(μ6-oxo)-hexakis(μ2-oxo)-hexaoxo-hexavanadium(V) – dichloromethane (1/1), C27H60Cl2N6O23V6
- Crystal structure of bis(μ3-methanolato-κ3O:O:O)-bis(μ2-methanolato-κ2O:O)-dimethanol-bis{6,6′-(1,3-dihydroxyl-2-acetylpropane-1,3-diyl)bis(2-chloro-4-bromophenolato)}tetramanganese(III) C40H40Br4Cl4Mn4O16
- Synthesis and crystal structure of tetrakis(μ2-methanolato)-dimethanol-bis(μ2-2-acetyl-1,3-bis(3,5-dibromo-2-oxidophenyl)propane-1,3-bis(olato)-κ5O1,O2,O3:O3,O4,O5)tetramanganese(III), C40H40Br8Mn4O16
- Synthesis and crystal structure tetrakis(μ2-methanolato)-dimethanol-bis(μ2-2-acetyl-1,3-bis(3,5-dichloro-2-oxidophenyl)propane-1,3-bis(olato)-κ5O1,O2,O3:O3,O4,O5), tetramanganese(III), C40H40Cl8Mn4O16
- Crystal structure of bis{5-methoxy-2-((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O)}copper(II), C34H34CuN4O6
- Crystal structure of (E)-1-(2-hydroxy-3-{[2-hydroxybenzylidene]amino}phenyl)ethan-1-one, C15H13NO3
- Crystal structure of bis(μ2-acetato-κ2O:O′)-bis{μ2-4-chloro-2-(8-(4-(diethylamino)-2-oxidophenyl)-3,6-dioxa-2,7-diazaocta-1,7-dien-1-yl)phenolato-κ6O:O,N,N′,O′′:O′′}tricobalt(II), C44H49Cl2Co3N6O12
- Crystal structure of bis{μ2-2,4-dichloro-6-(8-(4-(diethylamino)-2-oxidophenyl)-3,6-dioxa-2,7-diazaocta-1,7-dien-1-yl)phenolato-κ5O:O,N,N′,O′}dicobalt(II) acetone solvate, C43H48Br4Co2N6O9
- Synthesis and crystal structure of bis(μ2-acetato-κ2O:O′)-bis{μ2-4-chloro-2-(8-(4-(diethylamino)-2-oxidophenyl)-3,6-dioxa-2,7-diazaocta-1,7-dien-1-yl)phenolato-κ6O:O,N,N′,O′:O′}trizinc(II), C44H49Cl2N6O12Zn3
- Crystal structure of bis{5-(N,N′-diethylamine)-5′-methoxy -2,2′-[ethylenediyldioxybis(nitrilomethylidyne)]diphenolato}-bis(μ2-acetato-κ2O:O′)trizinc(II), C46H56Zn3N6O14
- Crystal structure of tris(cyano-(hydrogen tris(3,5-dimethylpyrazolyl)borate))-iron(III) 4-methoxypyridinium monohydrate, C24H32BN10O2Fe
- Synthesis and crystal structure of bis{1-(((4-(1-(hydroxyimino)ethyl)phenyl)imino)methyl)naphthalen-2-olato-κ2O,N}copper(II), C38H30CuN4O4
- Crystal structure of (E)-1-(4-{[(E)-2-Hydroxy-1-naphthalenylmethylene] amino}phenyl)ethanone oxime, C19H16N2O2
- The pseudosymmetric crystal structure of 2-(2-naphthalenyl)-3-nitro-2H-1-benzopyran, C38H26N2O6
- Crystal structure of 2-benzoyl-3-(4-methoxyphenyl)cyclopropane-1,1-dicarbonitrile, C19H14N2O2
- N′-(5-ethoxycarbonyl-3,4-dimethyl-pyrrol-2-yl-methylidene)-4-hydroxybenzohydrazide monohydrate, C17H21N3O5
- The crystal structure of carbonyl-[4-(2,4-dichlorophenylamino)pent-3-en-2-onato-κ2N,O]-(triphenylphosphine-κP)rhodium(I), RhC30H25Cl2NO2P
- Crystal structure of (E)-5-(diethylamino)-2-(((1,1,2-trihydroxyethyl)iminio)methyl)phenolate, C15H24N2O4
- Crystal structure of poly[(μ2-1,4-bis((2-ethyl-1H-benzo[d]imidazol-1-yl)methyl)benzene-κ2N:N′)(μ2-isophthalato-κ4O,O′:O′′,O′′′)cadmium(II)], C34H30N4O4Cd
- Crystal structure of 5,6-Dihydro-9,10-dimethoxybenzo[g]-1,3-benzodioxolo[5,6-a]chinolizinium 3-(4-hydroxyphenyl)-4-oxo-4H-chromen-7-olate - methanol - water (1/1/1), C36H33NO10
- A single crystal study on 2-(methylcarbamoyl)benzoic acid, C9H9NO3
- Crystal structure of the salt 1,1′-(ethane-1,2-diyl)bis(1,4-diazabicyclo[2.2.2]octan-1-ium) diperchlorate, C14H28N4(ClO4)2
- Crystal structure of 2,5-bis((E)-2-(trifluoromethyl)benzylidene)cyclopentan-1-one, C21H14F6O
- Crystal structure of catena-poly[(μ2-benzene-1,4-dicarboxylato-κ2O:O′)-(1-ethyl-6-fluoro-7-(4-methylpiperazin-1-ium-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylato-κ2O,O′)zinc(II)] 1.25 hydrate, C25H26.5N3O8.25FZn
- Crystal structure of 1-methyl-1,4-diazabicyclo[2.2.2]octan-1-ium poly[aqua-bis(μ2-perchlorato-κ3O,O′:O′′)sodium], C7H17Cl2N2NaO9
- Crystal structure of trimethyammonium 2,6-dicarboxyisonicotinate monohydrate, C11H16N2O7
- Crystal structure of dodecaguanidinium bis(tetrapropylammonium) heptacarbonate, C43H128N38O21
- Crystal structure of poly[tetraaqua-bis(μ6-benzene-1,2,4,5-tetracarboxylato)nickel(II)diyttrium(III)]dihydrate, C20H16NiO22Y2
- Halogen bonds and π–π interactions in the crystal structure of 1,3,5-trifluoro-2,4,6-triiodobenzene–N,N-dimethylformamide (1/1), C9H7F3I3NO
- Crystal structure of guanidinium tetraethylammonium carbonate dihydrate, C10H30N4O5
- The crystal structure of oxonium chlorido-ethylenediaminetetraactetotin(IV) hydrate, C10H17ClN2O10Sn
- Crystal structure of 8-((E)-((4-((E)-1-((benzyloxy)imino)ethyl)phenyl)imino)methyl)-7-hydroxy-4-methyl-2H-chromen-2-one, C26H22N2O4
- Crystal structure of bis{5-(diethylamino)-2-(((2-oxo-2H-chromen-6-yl)imino)methyl)phenolato-κ2O,N}copper(II), C40H38CuN4O6
- Crystal structure of fac-(acetylacetonato-κ2O,O′)tricarbonyl(tri(p-tolyl)phosphine-κP)rhenium(I), C29H28O5PRe
- Crystal structure of fac-(acetylacetonato-κ2O,O′)tricarbonyl(benzyldiphenylphosphine-κP)rhenium(I), C27H24O5PRe
- Crystal structure of triethylammonium bis{3-(((3-oxidonaphthalen-1-yl)methylene)amino)-2-oxo-2H-chromen-4-olato-κ3O,N,O′}manganese(III), C46H38MnN3O8
- Crystal structure of bis(N,N,N-ethyldimethylethanaminium) bis(heptaselenido-κ2Se1,Se7)palladate(II), C12H32N2PdSe14
- Crystal structure of bis(4-(1H-pyrazol-5-yl)pyridine-κ2N:N′)-bis(2-(2-((2,6-difluorophenyl)amino)phenyl)acetate-κO)cadmium(II), C44H34N8CdCl4O4
- Crystal structure of 4,4′-(1,4-phenylene)bis(pyridin-1-ium) catena-poly[diaqua-bis(μ2-3′,5′-dicarboxy-[1,1′-biphenyl]-2,5-dicarboxylato-κ2O:O′] dihydrate, C48H42O22N2Ca
- The crystal structure of (S)-2-benzylsuccinic acid, C11H12O4
- Crystal structure of poly[aqua-(μ3-4-(pyridin-4-yl)isophthalato-κ4O,O′:O′′:O′′′)-(μ2-5-carboxy-2-(pyridin-4-yl)benzoato-κ2O:O′)yttrium(III)], C26H17N2O9Y
- Crystal structure of ethyl 1-(3,4-dimethylphenyl)-5-methyl-1H-1,2,3-triazole-4-carboxylate, C14H17N3O2
- Crystal structure of tetrakis(methanol-κO)-bis{μ2-3-((4-methoxy-2-oxidobenzylidene)amino)-2-oxo-2H-chromen-4-olate-κ4O,N;O′:O′}dizinc(II), C38H38Zn2N2O14
- Crystal structure of bromido(4,4′-dimethoxy-2,2′-bipyridine-κ2N,N′)(isopropyl(diphenyl)phosphane-κP)copper(I), C27H29BrCuN2O2P
- Crystal structure of bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane)-9,9-dioctylfluorene, C41H64B2O4
- Crystal structure of 11-oxo-2,3,6,7-tetrahydro-1H,5H,11H-pyrano[2,3-f]pyrido[3,2,1-ij]quinoline-10-carbaldehyde - a julolidine derivative, C16H15NO3
- Crystal structure of 1,3-dimethyl-5,5-dibenzylbarbituric acid, C20H20N2O3
- Crystal structure of bis(N,N,N-trimethylethanaminium) poly[bis(μ2-heptaselenido-κ2Se1,Se7)palladate(II)], C10H28N2PdSe14
- Crystal structure of 1-{4-[(5-Chloro-2-hydroxy-benzylidene)amino]phenyl} ethanone O-ethyl-oxime, C17H17ClN2O2
- The crystal structure of methyl N-(4-bromophenyl)carbamate, C8H8BrNO2
- Synthesis and crystal structure of 1-{4-[(2-hydroxy-benzylidene)amino]phenyl}ethanone oxime, C15H14N2O2
- Crystal structure of 1,1′-butanebis(3-methyl-1H-imidazol-3-ium)bis(hexafluorophosphate), C12H20F12N4P2
- (E)-N-benzylidene-3-(benzylthio)-5-p-tolyl-4H-1,2,4-triazol-4-amine, C23H20N4S
- Crystal structure of ethyl 5-methyl-1-(pyridin-3-yl)-1H-1,2,3-triazole-4-carboxylate, C11H12N4O2
- Crystal structure of 5,5-difluoro-10-(4-fluorophenyl)-1,3,7,9-tetramethyl-5H-4l4,5l4-dipyrrolo[1,2-c:2′,1′-f][1,3,2]diazaborinine - a Z′ = 3 structure, C19H18B2F3N2
- The crystal structure of the Matrine derivative: 12-(1H-indol-1-yl)dodecahydro-1H,5H,10H-dipyrido[2,1-f:3′,2′,1′-ij][1,6]naphthyridin-10-one hydrate, C23H29N3O
- The crystal structure of tris(μ2-1,3-bis(4,4,4-trifluoro-3-oxido-1-(oxo)but-2-en-1-yl)phenyl-κ4O,O′:O′′,O′′′)-bis(1,2-dimethoxyethane-κ2O,O′)dilutetium(III), C50H38F18Lu2O16
- The crystal structure of (Z)-2-(3-(2-(4-chlorobenzoyl)hydrazono)-2-oxoindolin-1-yl) acetic acid, C17H12ClN3O4
- Synthesis and crystal structure of trans-tetraqua-bis(2-(((7-hydroxy-3-(4-hydroxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)ammonio)acetato-κO)cobalt(II) hexahydrate, C36H48CoN2O28S2
- The crystal structure of N,N-dimethyl-2,6-di-p-tolylpyrimidin-4-amine, C20H21N3
- The crystal structure (E)-4-methyl-N′-(2-nitrobenzylidene)benzenesulfonohydrazide, C14H13N3O4S
- Crystal structure of catena-poly[(μ2-1,3-bis(benzimidazol-1-yl)propane κ2N:N′)-(μ2-5-methoxyisophthalato-κ2O:O′)zinc(II)] hydrate, C26H24ZnN4O6
- The crystal structure of (E)-N′-(quinolin-2-ylmethylene)furan-2-carbohydrazide monohydrate, C15H13N3O3
- Crystal structure of 2,8-diphenyl-3,7,9-trioxa-1-azaspiro[4.5]dec-1-ene, C18H17N1O3
- Crystal structure of diethyl 2-(4-chlorophenyl)-1,3-dioxane-5,5-dicarboxylate, C16H19ClO6
- Crystal structure of 1-(carboxymethyl)-1H-benzo[d][1,2,3]triazole 3-oxide, C8H7N3O3
- Crystal structure of (acetylacetonato-κ2O,O′)-(2-amino-6-chlorobenzoato-κO)-oxido(1,10-phenanthroline-κ2N,N′)vanadium(IV) – trichloromethane (1/1)
- Crystal structure of (1E,4E)-1,5-bis(4-chlorophenyl)penta-1,4-dien-3-one, C17H12Cl2O
- The crystal structure of trans-dibromido-bis(pyridine-κN)platinum(II), C10H10Br2N2Pt

