Abstract
In this article, the flower-like, urchin-like, and rod-like ZnOs were synthesized by a convenient atmospheric hydrothermal method. The crystalline structures, morphologies, exposed crystal faces, and specific surface areas of the as-prepared ZnO samples were analyzed. Rhodamine B (RhB) was used as the simulated pollutant to evaluate the photocatalytic performance of the ZnO nanostructures. The flower-like ZnO prepared by controlled hydrothermal method at room temperature for 2 h displayed highest specific surface area and exposed more high active
1 Introduction
Semiconductor materials are widely studied by a number of researchers for the significant roles, which are used in sensors [1], photocatalysis [2], reduction of CO2 [3], air purification [4], and other fields. The different morphologies of semiconductor materials show special characteristics and are beneficial to explore novel functions. Common metal oxides such as TiO2 [5], ZnO [6], SnO2 [7], BiVO4 [8], and CuO [9] recently have become research hotspots in many fields. Among them, ZnO, owning various morphologies, good thermal stability, low cost, and biocompatible, has the potential to substitute TiO2 [10] to be used in photocatalysis and antibacterial field [11].
As a semiconductor material with a wide band gap (E
g = 3.37 eV) and large exciton binding energy (60 meV), ZnO has been extensively studied in solar cells [12], antibacterial materials [13], photocatalytic degradation of pollutants [14], and catalytic hydrogen production [15]. In recent years, researchers have commonly used many methods such as material recombination [16], morphology control, and doping [17] to enhance various properties of materials. Among them, ZnO with different morphologies prepared by morphology control exhibits a significant effect on their photocatalytic performance. The difference in morphologies not only leads to different specific surface areas and surface energy, but also results in difference in atom arrangement, which affects the activities and defect concentration. So far, the methods for synthesizing ZnO include hydrothermal [18], chemical vapor deposition [19], ion sputtering [20], sol–gel [21], precipitation method [22], etc. The exposure of different facets of ZnO can be controlled by using different solvents, adjusting raw material molar ratio, and changing the traction temperature. Through a large number of theoretical calculations, it can be known that the {0001} facets have the highest catalytic activity [23], followed by the
In this article, a mild condition for preparing the highly active ZnO by atmospheric hydrothermal method, without any surfactants, templates, and organic solvents, is reported. The structure of ZnO could be modified by adjusting the molar ratio of raw materials, reaction time, and temperature. The result shows that the flower-like ZnO prepared by hydrothermal method under room temperature exhibits obvious advantage for the photocatalytic performance over the other two kinds of ZnO under simulated sunlight. Due to the different exposed facets of ZnO, this study proposes that the exposed
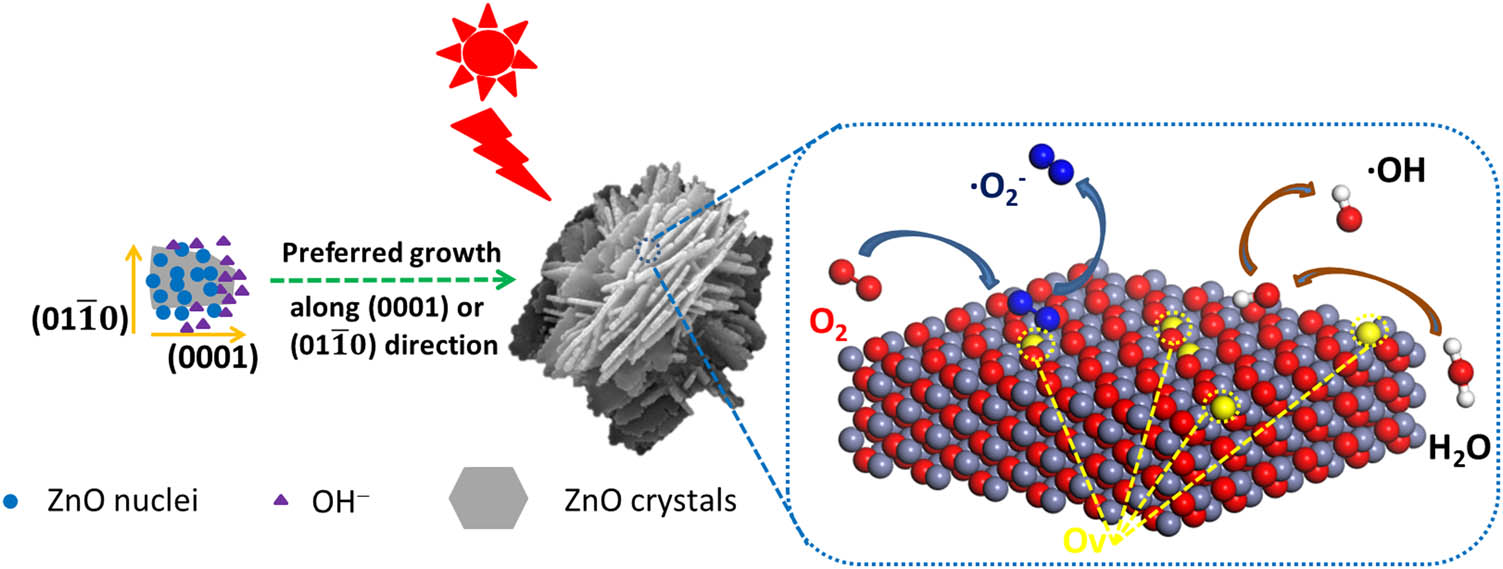
Schematic diagram of growth and photocatalysis of flower-like ZnO.
2 Experimental methods
2.1 Materials
Zinc nitrate hexahydrate (Zn(NO3)2·6H2O), nitro blue tetrazolium chloride (NBT), terephthalic acid (TA), 2-hydroxyterephthalic acid (2-OH-TA), and hexamethylenetetramine (HMTA, (CH2)6N4) were purchased from Aladdin Bio-Chem Technology Co., Ltd. (Shanghai, China). NBT, sodium hydroxide (NaOH), ammonia (NH3·H2O), and rhodamine B (RhB) were purchased from Chengdu Kelong Chemical Co., Ltd., P.R. China. All chemical reagents used in this study were used without any further purification.
2.2 Synthesis of ZnO
Flower-like ZnO were synthesized using the room temperature hydrothermal method. 2.23 g of Zn(NO3)2·6H2O and 3.00 g of NaOH were dissolved in 150 mL of deionized water followed by adding the NaOH solution into the zinc nitrate solution. The mixed solution was reacted for 2 h under magnetic stirring at room temperature. The corresponding product was washed and centrifuged for several times with deionized water and ethanol, and finally dried in air at 60°C for 12 h.
The preparation of urchin-like ZnO was prepared by the hydrothermal method. Typically, 8.92 g of Zn(NO3)2·6H2O and 10.50 g of NH3·H2O were added into 50 mL of deionized water and 200 mL of deionized water, respectively. Then, ammonia solution was added dropwise to the continuously stirred ZnO solution at a rate of 10 mL/min. The mixture was then reacted at 90°C for 20 h. After reaction, the precipitate was naturally cooled and washed with deionized water and ethanol. Then, it was centrifuged for several times and finally dried in air at 60°C for 12 h.
Rod-like ZnOs were also synthesized by hydrothermal route. Briefly, 8.94 g of Zn(NO3)2·6H2O were dissolved in 300 mL of deionized water. 4.20 g of HMTA were added to the above solution. Then, the mixture solution was stirred magnetically at room temperature for 24 h. After stirring, the above solution was transferred into a water bath reacting for 8 h at 90°C. Similarly, the white precipitate obtained after the reaction was washed with water and ethanol followed by centrifuging and then drying in an oven at 60°C for 12 h.
The preparation flow chart of three morphologies of ZnO is shown in Figure 2.
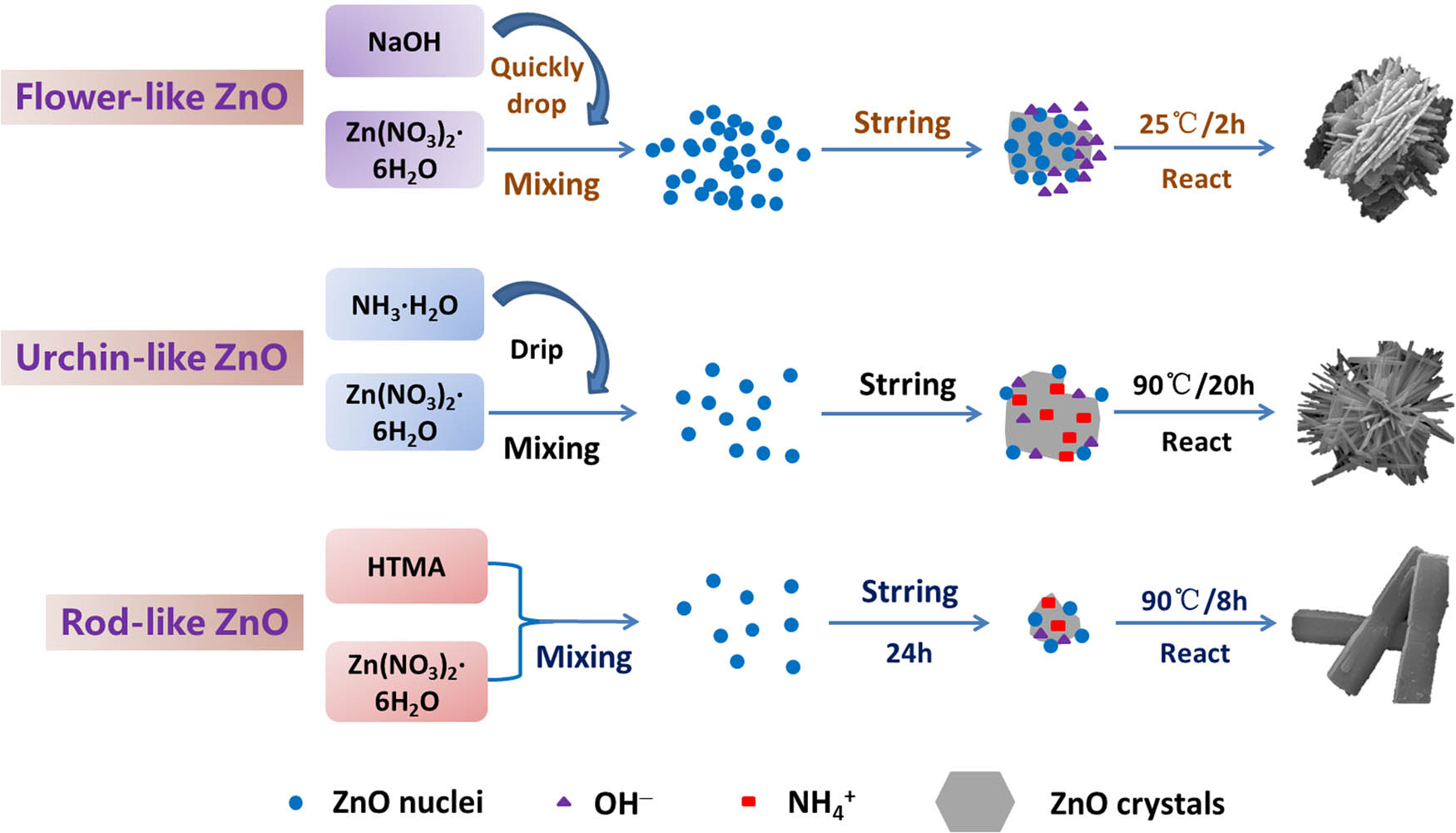
Preparation flow chart of ZnO with different morphologies.
2.3 Characterization
The crystal structure of the samples was tested by X-ray diffraction (XRD, Bruker D8 ADVANCE), with CuKα radiation in the 2θ range of 5–85°. The surface morphologies of three kinds of samples were studied by field emission scanning electron microscopy (FE-SEM; Inspect F, FEI, Netherlands), operated at 20.0 kV. More details about the structure of ZnO were studied by transmission electron microscopy (TEM; JSM2100F, JEOL, Japan) and selected-area electron diffraction (SAED) patterns were obtained at an accelerating voltage of 200.0 kV. X-ray photoelectron spectroscopy (XPS) analysis was performed on a Thermo Fisher ESCALAB Xi+ multifunctional imaging electron spectrometer, and the data were corrected by setting the adventitious C 1s peak at a fixed value of 284.8 eV. The Brunauer–Emmett–Teller (BET) special specific surface areas of the samples were detected through measuring the N2 adsorption–desorption isotherms at 77 K using a surface area analyzer (Autosorb iQ, Quantachrome, USA). All samples were studied by ultraviolet-visible (UV-Vis) spectrophotometer (UV-2600, Shimadzu, Japan) to analyze the light absorption ability and detect superoxide radical content. The fluorescence spectrophotometer (Hitachi F-7000) was used to detect the amount of hydroxyl radicals produced by the samples.
2.4 Photocatalytic activity tests
Photocatalytic performance of different samples under simulated sunlight were evaluated by degrading RhB at the same temperature using a 150W Xe lamp (XBO 150W/CR OFR, Osram) as the light source. Typically, 40 mg of photocatalysts were dispersed in 40 mL of 10 mg/L RhB solution under continuous stirring. The mixed solution was stirred for 30 min to achieve adsorption–desorption equilibrium at room temperature before irradiation. After photocatalytic reaction for every 20 min, 3 mL of the suspension was taken out followed by centrifuging to remove the catalysts. The UV-Vis spectrophotometer was used to record the concentration change in RhB during the reaction at a wavelength of 553 nm.
2.5 Analysis of the hydroxyl radicals
The content of hydroxyl radicals (˙OH) was mainly detected by using TA as the probe molecule. TA can react with hydroxyl radicals to generate 2-TA-OH which can emit a strong fluorescence signal at around 425 nm under an excitation wavelength of 315 nm. Its production in the solution was detected with the fluorescence spectrophotometer [28,29]. It is known that the PL peak intensity of 2-TA-OH is proportional to the output of hydroxyl radicals. Therefore, through analyzing the PL intensity of 2-OH-TA in the reaction solution, the amount of hydroxyl radicals can be further obtained. This process is similar to the photocatalytic reaction with the difference that the pollutants solution was replaced by TA (1 × 10−3 M) dissolved in NaOH solution (1 × 10−2 M). At different time intervals, the suspension was collected and centrifuged to determine the PL intensity using the fluorescence spectrophotometer with excitation at 315 nm.
2.6 Analysis of the superoxide radicals
The production of superoxide radicals (˙
3 Results and discussion
3.1 Characterization of ZnO
The crystal structure of the prepared samples was studied by XRD as shown in Figure 3. All diffraction peaks are well matched to the wurtzite ZnO (space group P63 mc; JCPDS 36-1451). No other peaks were observed, indicating the successful preparation of pure ZnO crystal. At the same time, the grain size and crystallinity of the samples were calculated by Scherrer’s formula, and the results are shown in Table 1. It can be seen from Table 1 that the crystal grain size of flower-like ZnO was 37.1 nm, which is smaller than the urchin-like and rod-like ZnOs. The corresponding crystallinity was only 93.88%, which is also the lowest compared with other samples.
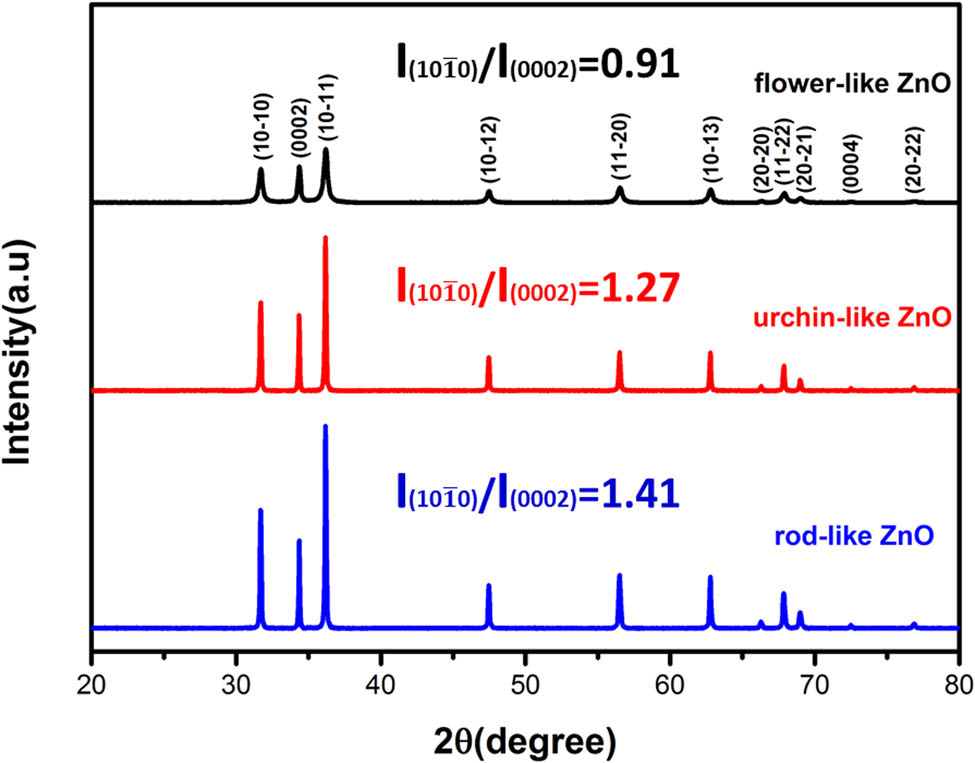
XRD patterns of the three samples.
The grain size and crystallinity of ZnO with different morphologies
| Sample | Grain size (nm) | Crystallinity (%) |
|---|---|---|
| Flower-like ZnO | 37.1 | 93.88 |
| Urchin-like ZnO | >100 | 98.41 |
| Rod-like ZnO | >100 | 97.71 |
According to Figure 3, the diffraction peaks of the flower-like ZnO were broad and the full width at half maxima were large. Unlike the flower-like ZnO, the diffraction peaks of the urchin-like ZnO and rod-like ZnO were both sharp. Combined with the ZnO crystal data as shown in Table 1, the crystallization and formation of flower-like ZnO was incomplete due to the short reaction time and moderate preparation temperature, leading to the lower crystallinity than the other two ZnOs. In addition, The relative intensity ratio
After analyzing the crystal structure of the prepared samples, their microscopic morphologies were further studied. As shown in Figure 4, the SEM micrographs of the three different morphologies of ZnO could be observed. It can be seen obviously from Figure 4(a) and (d) that the flower-like ZnOs consisted of multiple slice structures whose thicknesses were 30–60 nm.The size of the flower-like ZnOs were approximately 2–3 μm. Figure 4(b) and (e) illustrates that the urchin-like ZnOs were made up of needle-like structures whose diameters were about 5–6 μm. Figure 4(c) and (f) showed that the rod-like ZnOs are composed of hexagonal prism structures with lengths of 3–4 μm and diameters of 300–600 nm. In combination with Figure 3, it can be concluded that the difference in the growth direction of ZnO can lead to the difference in its morphology.
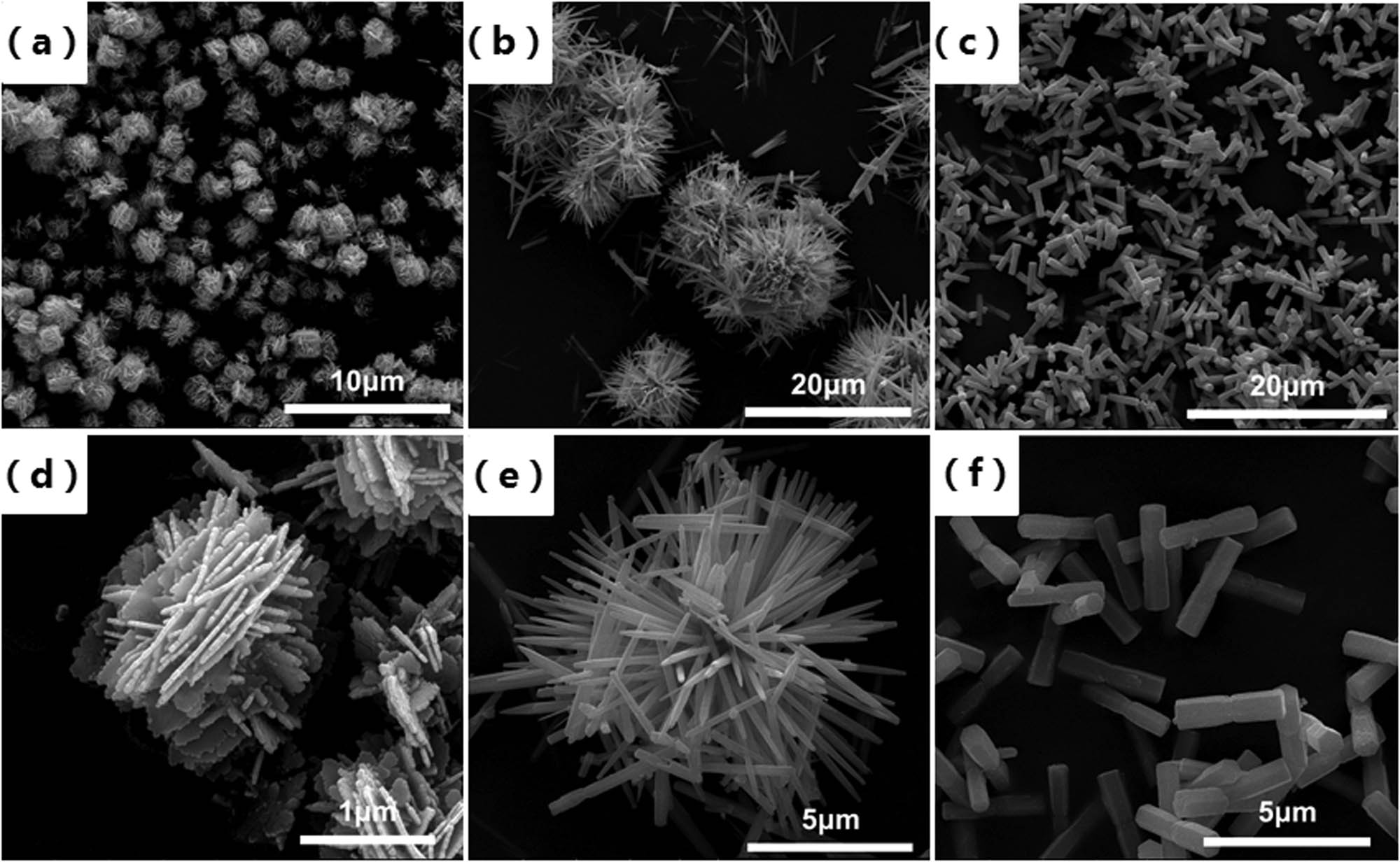
SEM images of (a and d) flower-like ZnO, (b and e) sea urchin ZnO, and (c and f) rod-like ZnO.
The exposed facets will change when ZnOs are formed under different conditions. So it is necessary to observe the exposed surface of ZnO by TEM. According to Figure 5, different facets were exposed as the surface of the samples when the morphologies of ZnO were changed. It can be seen from Figure 5(a) that the morphology of ZnO was flower-like shape and Figure 5(a1) is the corresponding HRTEM image of this sample. It revealed that the lattice spacing between neighboring lattices is 0.28 and 0.52 nm corresponding to the
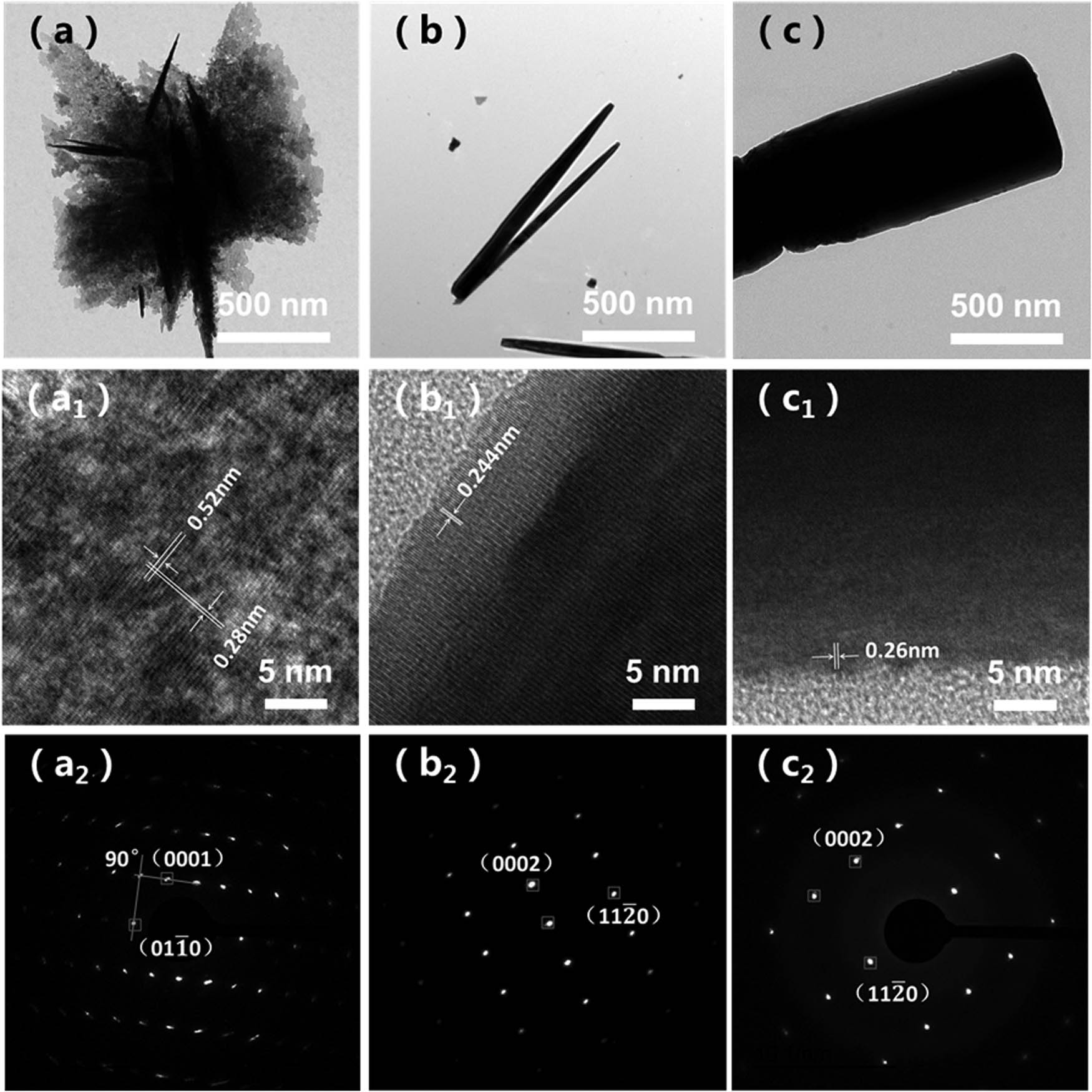
TEM and HRTEM images of samples, (a, a1, and a2) flower-like ZnO, (b, b1, and b2) urchin-like ZnO, and (c, c1, and c2) rod-like ZnO.
Figure 5(b)–(b2) are the TEM images that exhibit the morphologies and exposed facets of urchin-like ZnO. Figure 5(b1) indicated that the clear lattice fringe with lattice distance was 0.244 nm which corresponded to the
The TEM images of rod-like ZnO are shown in Figure 5(c)–(c2). Figure 5(c1) exhibits the growth direction of a single rod-like ZnO along the c-axis with the lattice fringe of 0.26 nm, corresponding to the distance between the {0001} facets. So that the
The nitrogen adsorption–desorption isotherms of flower-like ZnO, urchin-like ZnO, and rod-like ZnO are shown in Figure 6. All the samples displayed type-IV isotherms. According to Table 2, the specific surface areas of the samples can be calculated. All three kinds of ZnO had small specific surface areas value (27.0, 9.1 and 4.8 m2/g). The specific surface area of the flower-like ZnO was larger than the others. Therefore, the exposure of active surface of flower-like ZnO was larger than the others [32].
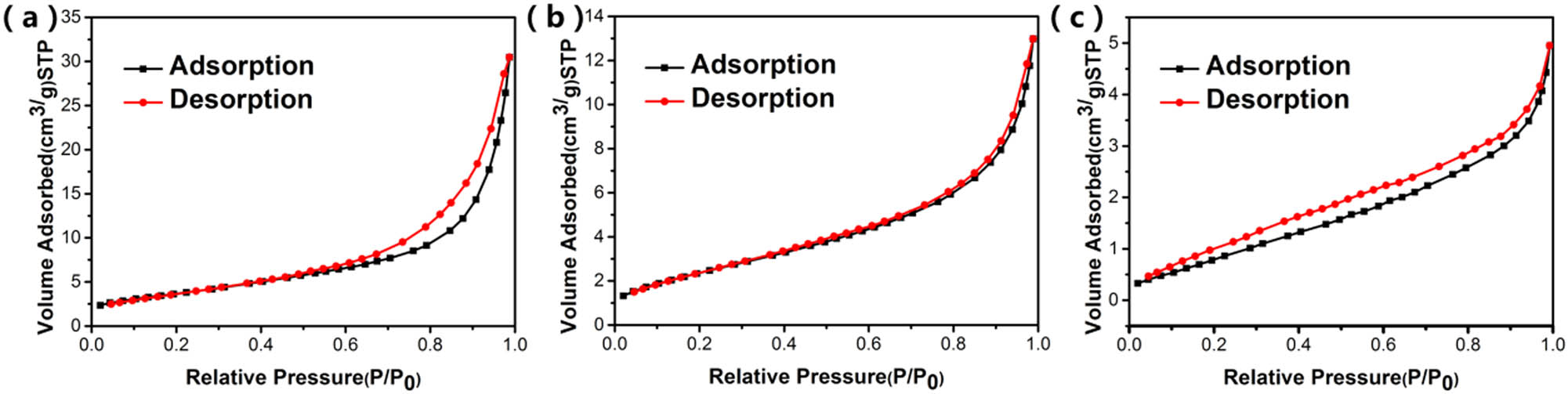
N2 adsorption–desorption isotherm of (a) flower-like ZnO, (b) urchin-like ZnO, and (c) rod-like ZnO.
Specific surface areas and oxygen vacancy concentration of ZnO with different morphologies
| Samples | BET (m2/g) | Ov (%) |
|---|---|---|
| Flower-like ZnO | 27.0 | 47.4 |
| Urchin-like ZnO | 9.1 | 42.1 |
| Rod-like ZnO | 4.8 | 44.2 |
3.2 Growth mechanism of ZnO with different morphologies
Based on the above analysis, formation mechanisms of ZnO with different morphologies were proposed. The formation of ZnO mainly go through two steps: nucleation and growth. The related reaction chemistry processes are shown as follows:
The basic principle was to mix zinc salt with alkali to form growth units, which acted as the nucleus for growth. The growth units finally dehydrated to form ZnO [33]. For flower-like ZnO, the molar ratio
When NH3·H2O was used as the structural regulator for preparing ZnO, there were both OH− and
For rod-like ZnO, HMTA can decompose into methanol and ammonia which lead to the formation of OH− and
Therefore, it can be seen that the different morphologies of ZnO comes from the ionization of different alkalis to produce different anions and cations, and the ionized concentrations of ions are also different, so that the interaction between different ions and ZnO is different, thus forming the three specific morphologies.
3.3 Photocatalytic performance of ZnO with different morphologies
After successful preparation of the three kinds of ZnO with different exposed facets, the systematic study about photocatalytic properties was carried out. RhB was used as a model pollutant to analyze the catalytic performance. The photocatalytic activities of the three kinds of samples were evaluated by the degradation efficiency of RhB under simulated sunlight irradiation. Figure 7 shows the detailed behavior of RhB photodegradation of flower-like ZnO, urchin-like ZnO, and rod-like ZnO.
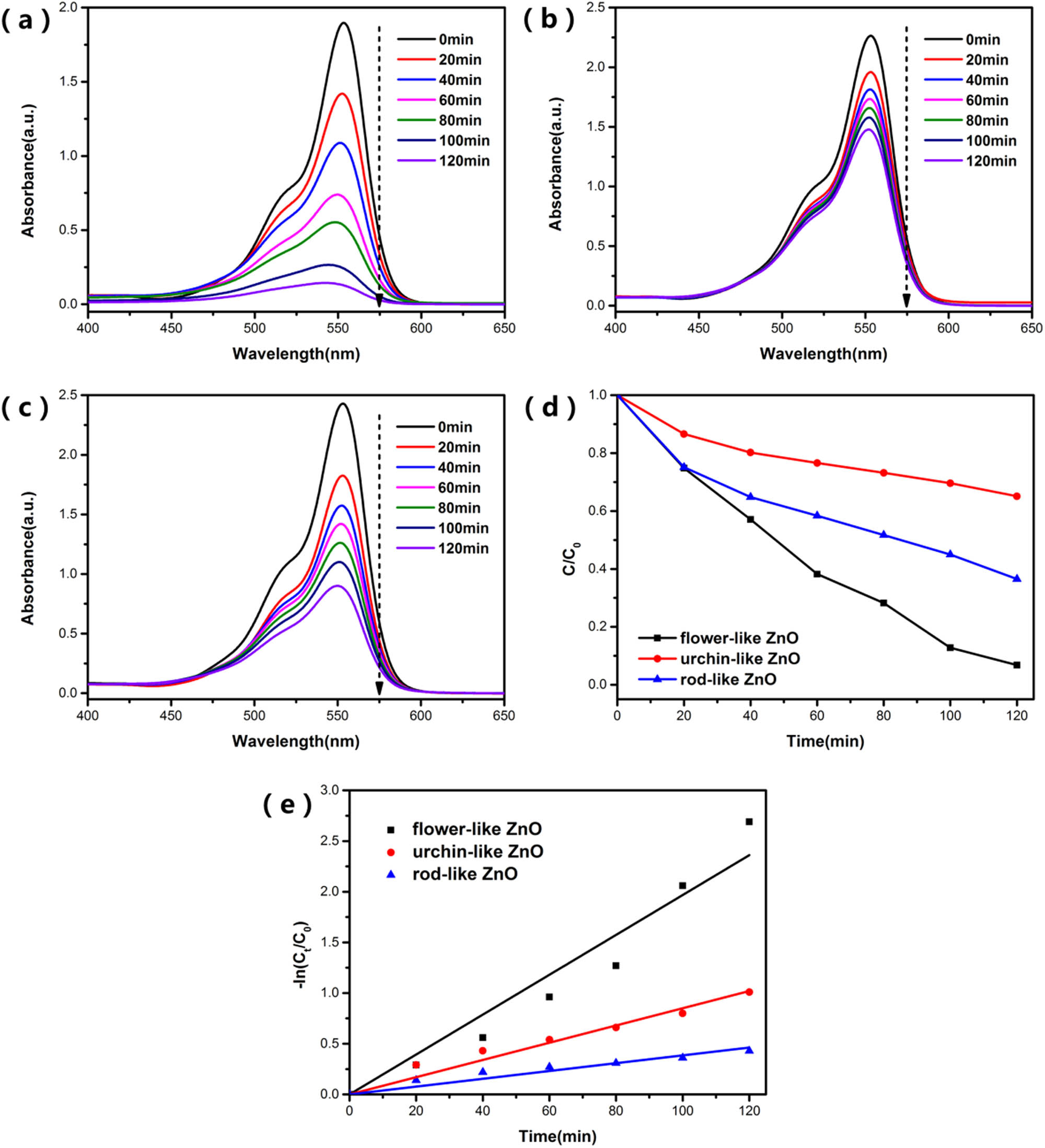
The UV-visible absorbance spectra of RhB during degradation by (a) flower-like ZnO, (b) urchin-like ZnO, and (c) rod-like ZnO. (d) Photocatalytic activities of the three samples evaluated by the degradation of RhB under simulated sunlight, and (e) fitted curves with pseudo-first-order kinetic model of samples under simulated sunlight.
Figure 7(a)–(c) shows the UV-visible absorption spectra of RhB during degradation and it can be seen that the characteristic absorption wavelength of RhB is 553 nm. This characteristic absorption peak of RhB had significant decrease. It proves that flower-like ZnO showed the best photocatalytic performance. After 2 h of simulated sunlight irradiation, the photodegradation efficiencies of RhB were about 99.3, 35.1, and 63.6% for flower-like ZnO, urchin-like ZnO, and rod-like ZnO, respectively. This result does not correspond to the previous BET test results of ZnO. Furthermore, Figure 7(e) represents that the degradation kinetic behaviors of RhB quite fit the pseudo-first-order kinetic model: In (C/C 0) = kt, where C and C 0 are the concentrations of the RhB at time t and at the original time (t = 0), respectively. The slope k of these curves represents the apparent reaction rate constant. The bigger degradation rate constant means higher photocatalytic degradation efficiency. The degradation rate constant of the flower-like ZnO is up to 0.0197, which is about 2.3 times that of the urchin-like ZnO and 5.1 times that of the rod-like ZnO. This result also indicates that the flower-like ZnO has a great degradation rate for RhB.
Based on the above results shown in Figure 7, the three types of ZnO have different photocatalytic properties. Besides, there is no correspondence between the difference in photocatalytic performance and the specific surface areas of ZnO, which indicates that the size of the specific surface area of ZnO has a limited effect on its photocatalytic performance.
3.4 Photocatalytic mechanism
Since ZnO has different exposed facets, it will result in a difference in the surface oxygen vacancies concentration [37]. Oxygen vacancies will affect the photocatalytic performance of ZnO. Therefore, it is vital to study the surface oxygen vacancies concentration of different samples. Figure 8 shows the high-resolution of Zn and O of the samples. The binding energy of Zn element in ZnO had two peaks at 1,022 and 1,044 eV due to spin splitting. The O 1s peak was mainly fitted to two band, as shown in Figure 8(b)–(d). The lattice oxygen (OL) was 530.2 eV which corresponds to the O–Zn bond. The other peak at 531.6 eV was attributed to the binding energy of the ZnO surface to adsorb oxygen from the environment. Because most of the oxygen adsorbed on the surface of ZnO was caused by highly active oxygen vacancies, to a certain extent, the concentration of oxygen vacancies on the surface of ZnO can be expressed by the concentration of adsorbed oxygen on the surface [38]. As displayed in Table 2, the concentration of oxygen vacancies in various ZnO can be calculated by the results of O 1s peak splitting. The results showed that the percentages of oxygen vacancies of these samples were 47.4, 42.1, and 44.2%, respectively. Flower-like ZnO has the most oxygen vacancies content and urchin-like ZnO has the least content.
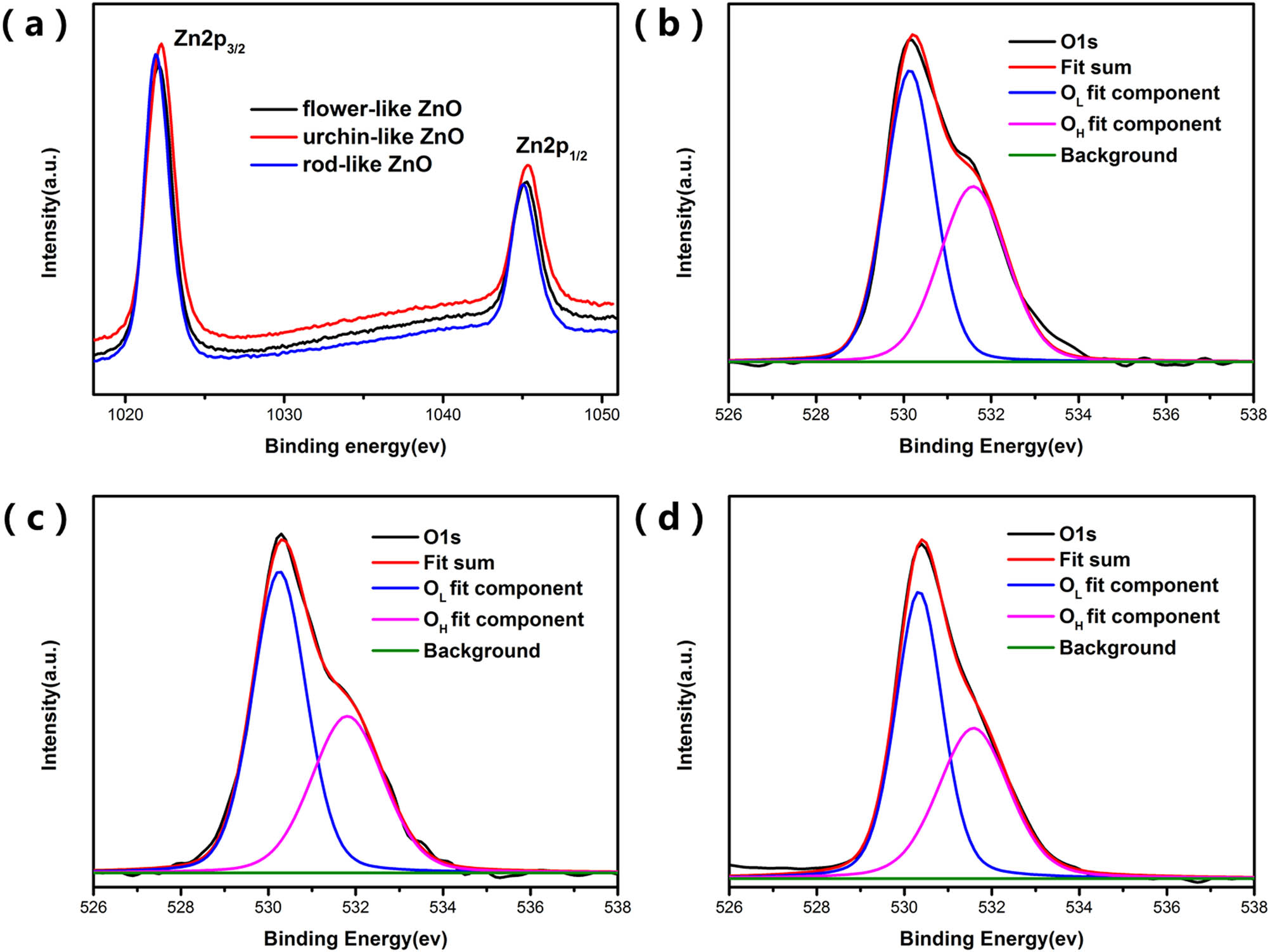
XPS analysis of samples showing (a) Zn 2p, (b–d) O 1s spectra of flower-like ZnO, urchin-like ZnO, and rod-like ZnO.
Since the photocatalytic performance of ZnO is related to the content of reactive oxygen species produced, the photocatalytic mechanism was further analyzed by quantitatively detecting the amount of ˙
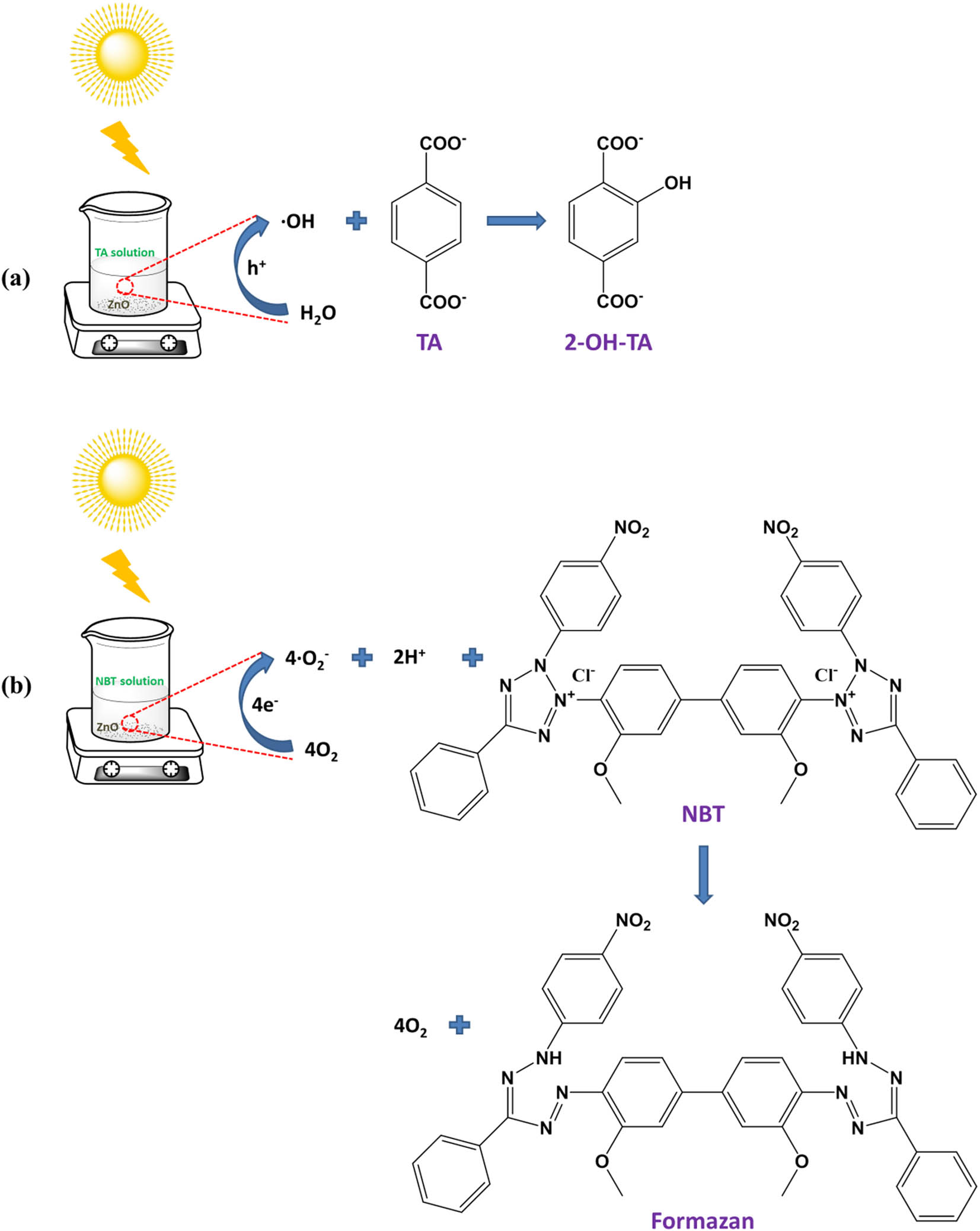
Schematic diagram of the principle of ZnO producing active oxygen under simulated sunlight: (a) hydroxyl radical and (b) superoxide anion.
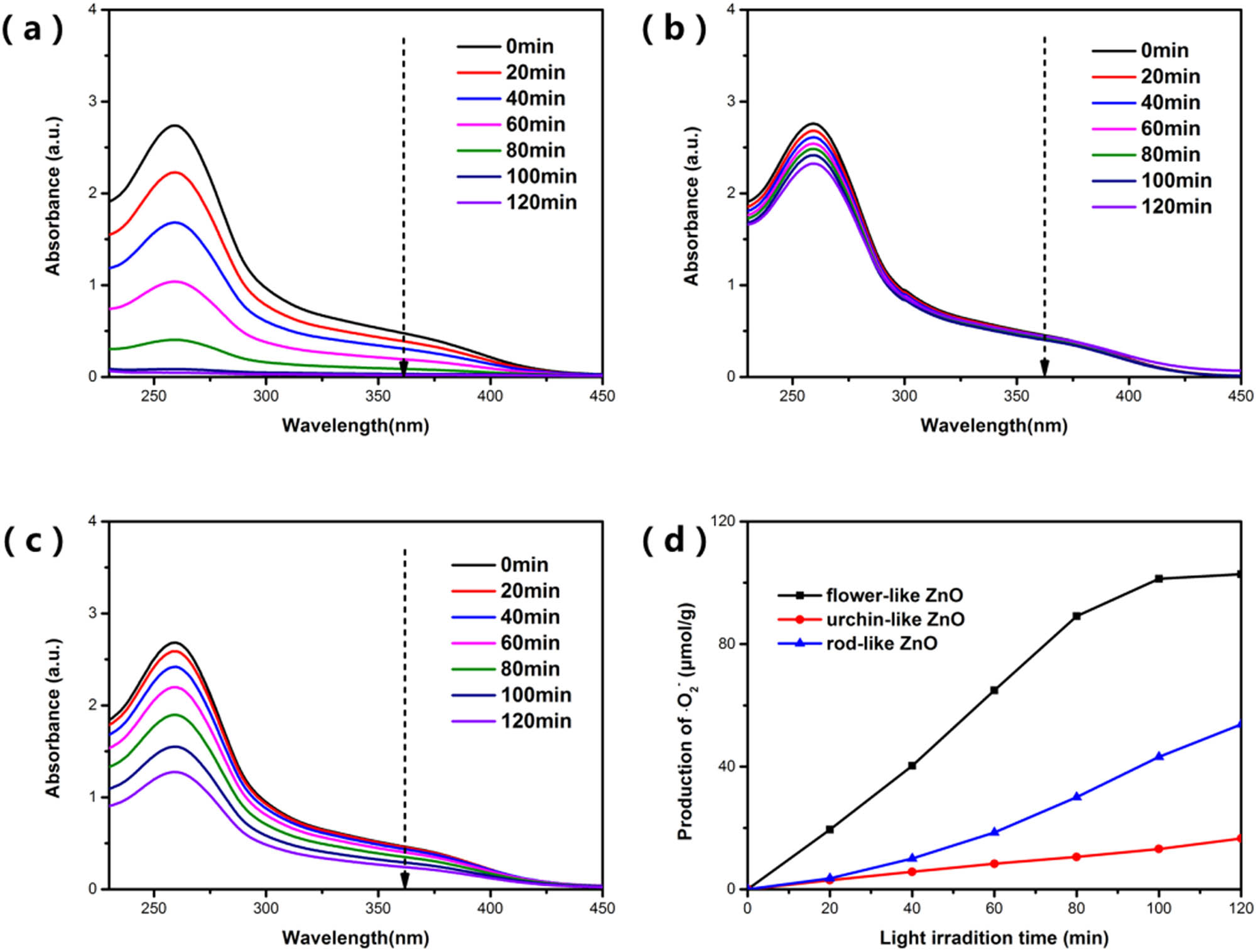
UV-Vis absorption spectra of NBT degraded by ZnO with three morphologies of (a–c) flower-like ZnO, urchin-like ZnO, and rod-like ZnO, and (d) the yield of ˙
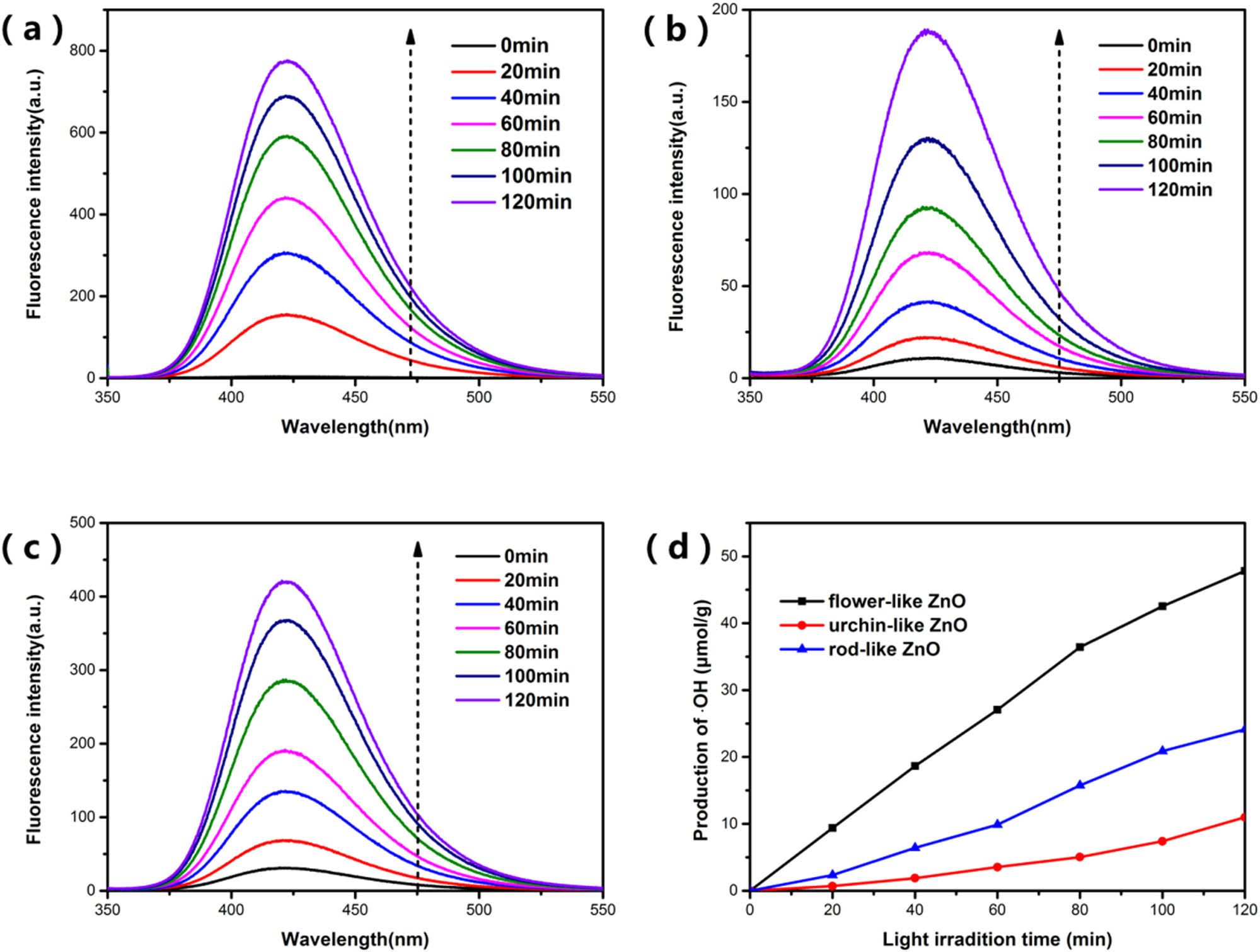
Fluorescence spectra of the amount of 2-OH-TA produced by the samples under simulated sunlight over time: (a–c) are the spectra of flower-like ZnO, urchin-like ZnO, and rod-like ZnO, respectively, (d) is the yield of ˙OH in ZnO suspension under simulated sunlight with time.
Based on the above analysis, we can know from Figures 10 and 11 that under simulated sunlight, flower-like ZnO with
In short, it can be known that the specific surface area are not the main reasons for the difference in photocatalytic performance. Because ZnOs with different morphologies have different exposed facets, their oxygen vacancy concentration is also different. According to the previous research work of our research group [34], the surface oxygen vacancies of flower-like ZnO with exposed
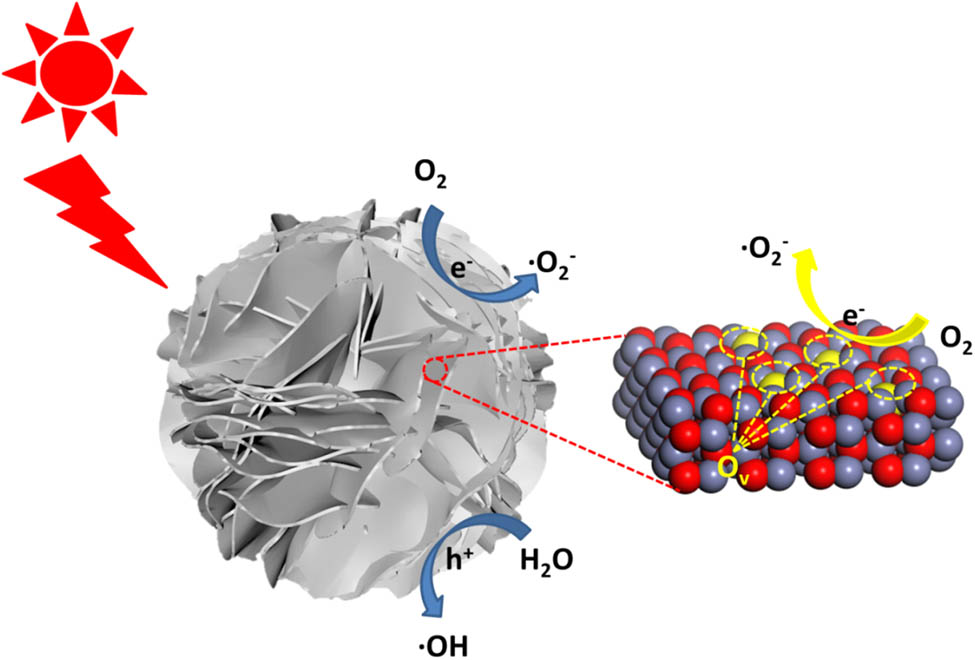
The photocatalytic mechanism of flower-like ZnO with exposed
4 Conclusion
In summary, ZnO with different morphologies including flower-like ZnO, urchin-like ZnO, and rod-like ZnO were synthesized by an atmospheric hydrothermal method. The electrostatic adsorption between different ions in the solution and the ZnO crystal nucleus had a significant effect on the growth of ZnO, which further affected the formation of various morphologies. Photocatalytic results showed that under the simulated sunlight, the flower-like ZnO exhibited higher photocatalytic activity and produced more active oxygen compared with the other two kinds of ZnO. The excellent photocatalytic performance of flower-like ZnO was attributed to the larger amount of oxygen vacancies on the exposed
-
Funding information: This research was financially supported by the National Natural Science Foundation of China (No. 51772251), the Science and Technology Planning Project of Sichuan Province (Nos. 2020ZDZX0008, 2020ZDZX0005, 2018GZ0431), the Space Station Engineering Aerospace Technology Test Field Project and the Fundamental Research Funds for the Central Universities (No. 2682020XG05).
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
References
[1] Zhang D, Wu X, Han N, Chen Y. Chemical vapor deposition preparation of nanostructured ZnO particles and their gas-sensing properties. J Nanopart Res. 2013;15(4):1–10.10.1007/s11051-013-1580-ySearch in Google Scholar
[2] Wang Y, Feng J, Jin L, Li C. Photocatalytic reduction of graphene oxide with cuprous oxide film under UV-vis irradiation. Rev Adv Mater Sci. 2020;59(1):207–14.10.1515/rams-2020-0022Search in Google Scholar
[3] Liu X, Ye L, Liu S, Li Y, Ji X. Photocatalytic reduction of CO2 by ZnO micro/nanomaterials with different morphologies and ratios of {0001} facets. Sci Rep. 2016;6(1):1–9.10.1038/srep38474Search in Google Scholar PubMed PubMed Central
[4] Pokhum C, Intasanta V, Yaipimai W, Subjalearndee N, Srisitthiratkul C, Pongsorrarith V, et al. A facile and cost-effective method for removal of indoor airborne psychrotrophic bacterial and fungal flora based on silver and zinc oxide nanoparticles decorated on fibrous air filter. Atmos Pollut Res. 2018;9(1):172–7.10.1016/j.apr.2017.08.005Search in Google Scholar
[5] Xu H, Reunchan P, Ouyang S, Tong H, Umezawa N, Kako T, et al. Anatase TiO2 single crystals exposed with high-reactive {111} facets toward efficient H2 evolution. Chem Mater. 2013;25(3):405–11.10.1021/cm303502bSearch in Google Scholar
[6] Jin Y, Long J, Ma X, Zhou T, Zhang Z, Lin H, et al. Synthesis of caged iodine-modified ZnO nanomaterials and study on their visible light photocatalytic antibacterial properties. Appl Catal B Environ. 2019;256:117873.10.1016/j.apcatb.2019.117873Search in Google Scholar
[7] Wang H, Kalytchuk S, Yang H, He L, Hu C, Teoh WY, et al. Hierarchical growth of SnO2 nanostructured films on FTO substrates: structural defects induced by Sn(ii) self-doping and their effects on optical and photoelectrochemical properties. Nanoscale. 2014;6(11):6084–91.10.1039/c4nr00672kSearch in Google Scholar PubMed
[8] Li R, Zhang F, Wang D, Yang J, Li M, Zhu J, et al. Spatial separation of photogenerated electrons and holes among {010} and {110} crystal facets of BiVO4. Nat Commun. 2013;4(1):1–7.10.1038/ncomms2401Search in Google Scholar PubMed
[9] Cam TS, Vishnievskaia TA, Popkov VI. Catalytic oxidation of CO over CuO/CeO2 nanocomposites synthesized via solution combustion method: effect of fuels. Rev Adv Mater Sci. 2020;59(1):131–43.10.1515/rams-2020-0002Search in Google Scholar
[10] Guo Q, Zhou C, Ma Z, Yang X. Fundamentals of TiO2 photocatalysis: concepts, mechanisms, and challenges. Adv Mater. 2019;31(50):1901997.10.1002/adma.201901997Search in Google Scholar PubMed
[11] Luo L, Zhou Y, Xu X, Shi W, Hu J, Li G, et al. Progress in construction of bio-inspired physico-antimicrobial surfaces. Nanotechnol Rev. 2020;9(1):1562–75.10.1515/ntrev-2020-0089Search in Google Scholar
[12] Saleh SM, Soliman AM, Sharaf MA, Kale V, Gadgil B. Influence of solvent in the synthesis of nano-structured ZnO by hydrothermal method and their application in solar-still. J Environ Chem Eng. 2017;5(1):1219–26.10.1016/j.jece.2017.02.004Search in Google Scholar
[13] Dejen KD, Zereffa EA, Murthy HCA, Merga A. Synthesis of ZnO and ZnO/PVA nanocomposite using aqueous Moringa oleifeira leaf extract template: antibacterial and electrochemical activities. Rev Adv Mater Sci. 2020;59(1):464–76.10.1515/rams-2020-0021Search in Google Scholar
[14] Yang T, Peng J, Zheng Y, He X, Hou Y, Wu L, et al. Enhanced photocatalytic ozonation degradation of organic pollutants by ZnO modified TiO2 nanocomposites. Appl Catal B Environ. 2018;221:223–34.10.1016/j.apcatb.2017.09.025Search in Google Scholar
[15] Núñez J, Fresno F, Platero-Prats AE, Jana P, Fierro JL, Coronado JM, et al. Ga-promoted photocatalytic H2 production over Pt/ZnO nanostructures. ACS Appl Mater Interfaces. 2016;8(36):23729–38.10.1021/acsami.6b07599Search in Google Scholar PubMed
[16] Zhang C, Fei W, Wang H, Li N, Chen D, Xu Q, et al. p-n Heterojunction of BiOI/ZnO nanorod arrays for piezo-photocatalytic degradation of bisphenol A in water. J Hazard Mater. 2020;399:123109.10.1016/j.jhazmat.2020.123109Search in Google Scholar PubMed
[17] Chen A, Zhu H, Wu Y, Chen M, Zhu Y, Gui X, et al. Beryllium-assisted p-type doping for ZnO homojunction light-emitting devices. Adv Funct Mater. 2016;26(21):3696–702.10.1002/adfm.201600163Search in Google Scholar
[18] Xu L, Hu Y-L, Pelligra C, Chen CH, Jin L, Huang H, et al. ZnO with different morphologies synthesized by solvothermal methods for enhanced photocatalytic activity. Chem Mater. 2009;21(13):2875–85.10.1021/cm900608dSearch in Google Scholar
[19] Liu Z, Liu S, Wu W, Liu CR. The mechanism of controlled integration of ZnO nanowires using pulsed-laser-induced chemical deposition. Nanoscale. 2019;11(6):2617–23.10.1039/C8NR06890ASearch in Google Scholar
[20] Nandi R, Major S. The mechanism of growth of ZnO nanorods by reactive sputtering. Appl Surf Sci. 2017;399:305–12.10.1016/j.apsusc.2016.12.097Search in Google Scholar
[21] Mahendraprabhu K, Sharma AS, Elumalai PCO. Sensing performances of YSZ-based sensor attached with sol–gel derived ZnO nanospheres. Sens Actuators B Chem. 2019;283:842–7.10.1016/j.snb.2018.11.164Search in Google Scholar
[22] Li S-M, Zhang L-X, Zhu M-Y, Ji GJ, Zhao LX, Yin J, et al. Acetone sensing of ZnO nanosheets synthesized using room-temperature precipitation. Sens Actuators B Chem. 2017;249:611–23.10.1016/j.snb.2017.04.007Search in Google Scholar
[23] Sun Y, Chen L, Bao Y, Zhang Y, Wang J, Fu M, et al. The applications of morphology controlled ZnO in catalysis. Catalysts. 2016;6(12):188.10.3390/catal6120188Search in Google Scholar
[24] Zhao Y, Cui T, Wu T, Jin C, Qiao R, Qian Y, et al. Polymorphous ZnO nanostructures: zn polar surface-guided size and shape evolution mechanism and enhanced photocatalytic activity. Chem Cat Chem. 2017;9(16):3180–90.10.1002/cctc.201700135Search in Google Scholar
[25] Xu X, Pang H, Zhou Z, Fan X, Hu S, Wang Y. Preparation of multi-interfacial ZnO particles and their growth mechanism. Adv Powder Technol. 2011;22(5):634–8.10.1016/j.apt.2010.09.017Search in Google Scholar
[26] Chen X, Song X, Qiao W, Zhang X, Sun Y, Xu X, et al. Solvent-directed and anion-modulated self-assemblies of nanoparticles: a case of ZnO. Cryst Eng Comm. 2016;18(47):9139–51.10.1039/C6CE02056ASearch in Google Scholar
[27] Rezapour M, Talebian N. Comparison of structural, optical properties and photocatalytic activity of ZnO with different morphologies: effect of synthesis methods and reaction media. Mater Chem Phys. 2011;129(1–2):249–55.10.1016/j.matchemphys.2011.04.012Search in Google Scholar
[28] Wang Q, Guan S, Li B. 2D graphitic-C3N4 hybridized with 1D flux-grown Na-modified K2Ti6O13 nanobelts for enhanced simulated sunlight and visible-light photocatalytic performance. Catal Sci Technol. 2017;7(18):4064–78.10.1039/C7CY01134BSearch in Google Scholar
[29] Wang J-C, Yao H-C, Fan Z-Y, Zhang L, Wang JS, Zang SQ, et al. Indirect Z-scheme BiOI/g-C3N4 photocatalysts with enhanced photoreduction CO2 activity under visible light irradiation. ACS Appl Mater Interfaces. 2016;8(6):3765–75.10.1021/acsami.5b09901Search in Google Scholar PubMed
[30] Bajpai V, Baek K-H, Kang S. Antioxidant and free radical scavenging activities of taxoquinone, a diterpenoid isolated from Metasequoia glyptostroboides. South Afr J Botany. 2017;111:93–8.10.1016/j.sajb.2017.03.004Search in Google Scholar
[31] Huang M, Weng S, Wang B, Hu J, Fu X, Liu P. Various facet tunable ZnO crystals by a scalable solvothermal synthesis and their facet-dependent photocatalytic activities. J Phys Chem C. 2014;118(44):25434–40.10.1021/jp5072567Search in Google Scholar
[32] Flores NM, Pal U, Galeazzi R, Sandoval A. Effects of morphology, surface area, and defect content on the photocatalytic dye degradation performance of ZnO nanostructures. RSC Adv. 2014;4(77):41099–110.10.1039/C4RA04522JSearch in Google Scholar
[33] Li P, Wei Y, Liu H, Wang X. A simple low-temperature growth of ZnO nanowhiskers directly from aqueous solution containing Zn(OH)42− ions. Chem Commun. 2004;24:2856–7.10.1039/b409425eSearch in Google Scholar PubMed
[34] Qi K, Cheng B, Yu J, Ho W. Review on the improvement of the photocatalytic and antibacterial activities of ZnO. J Alloy Compd. 2017;727:792–820.10.1016/j.jallcom.2017.08.142Search in Google Scholar
[35] Zhou Y, Guo Y, Li J, Wei W, Li D, Luo L, et al. Excellent antibacterial activities in the dark of ZnO nanoflakes with oxygen vacancies on exposed {2110} facets. J Mater Chem A. 2020;8(23):11511–4.10.1039/C9TA14044ASearch in Google Scholar
[36] Baruah S, Dutta J. Hydrothermal growth of ZnO nanostructures. Sci Technol Adv Mater. 2009;10(1):013001.10.1088/1468-6996/10/1/013001Search in Google Scholar PubMed PubMed Central
[37] Wang J, Wang Z, Huang B, Ma Y, Liu Y, Qin X, et al. Oxygen vacancy induced band-gap narrowing and enhanced visible light photocatalytic activity of ZnO. ACS Appl Mater Interfaces. 2012;4(8):4024–30.10.1021/am300835pSearch in Google Scholar PubMed
[38] Tang Y, Zhou H, Zhang K, Ding J, Fan T, Zhang D. Visible-light-active ZnO via oxygen vacancy manipulation for efficient formaldehyde photodegradation. Chem Eng J. 2015;262:260–7.10.1016/j.cej.2014.09.095Search in Google Scholar
© 2021 Jiahao Hu et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Improved impedance matching by multi-componential metal-hybridized rGO toward high performance of microwave absorption
- Pure-silk fibroin hydrogel with stable aligned micropattern toward peripheral nerve regeneration
- Effective ion pathways and 3D conductive carbon networks in bentonite host enable stable and high-rate lithium–sulfur batteries
- Fabrication and characterization of 3D-printed gellan gum/starch composite scaffold for Schwann cells growth
- Synergistic strengthening mechanism of copper matrix composite reinforced with nano-Al2O3 particles and micro-SiC whiskers
- Deformation mechanisms and plasticity of ultrafine-grained Al under complex stress state revealed by digital image correlation technique
- On the deformation-induced grain rotations in gradient nano-grained copper based on molecular dynamics simulations
- Removal of sulfate from aqueous solution using Mg–Al nano-layered double hydroxides synthesized under different dual solvent systems
- Microwave-assisted sol–gel synthesis of TiO2-mixed metal oxide nanocatalyst for degradation of organic pollutant
- Electrophoretic deposition of graphene on basalt fiber for composite applications
- Polyphenylene sulfide-coated wrench composites by nanopinning effect
- Thermal conductivity and thermoelectric properties in 3D macroscopic pure carbon nanotube materials
- An effective thermal conductivity and thermomechanical homogenization scheme for a multiscale Nb3Sn filaments
- Friction stir spot welding of AA5052 with additional carbon fiber-reinforced polymer composite interlayer
- Improvement of long-term cycling performance of high-nickel cathode materials by ZnO coating
- Quantum effects of gas flow in nanochannels
- An approach to effectively improve the interfacial bonding of nano-perfused composites by in situ growth of CNTs
- Effects of nano-modified polymer cement-based materials on the bending behavior of repaired concrete beams
- Effects of the combined usage of nanomaterials and steel fibres on the workability, compressive strength, and microstructure of ultra-high performance concrete
- One-pot solvothermal synthesis and characterization of highly stable nickel nanoparticles
- Comparative study on mechanisms for improving mechanical properties and microstructure of cement paste modified by different types of nanomaterials
- Effect of in situ graphene-doped nano-CeO2 on microstructure and electrical contact properties of Cu30Cr10W contacts
- The experimental study of CFRP interlayer of dissimilar joint AA7075-T651/Ti-6Al-4V alloys by friction stir spot welding on mechanical and microstructural properties
- Vibration analysis of a sandwich cylindrical shell in hygrothermal environment
- Water barrier and mechanical properties of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch (TPS)/poly(lactic acid) (PLA) blend bionanocomposites
- Strong quadratic acousto-optic coupling in 1D multilayer phoxonic crystal cavity
- Three-dimensional shape analysis of peripapillary retinal pigment epithelium-basement membrane layer based on OCT radial images
- Solvent regulation synthesis of single-component white emission carbon quantum dots for white light-emitting diodes
- Xanthate-modified nanoTiO2 as a novel vulcanization accelerator enhancing mechanical and antibacterial properties of natural rubber
- Effect of steel fiber on impact resistance and durability of concrete containing nano-SiO2
- Ultrasound-enhanced biosynthesis of uniform ZnO nanorice using Swietenia macrophylla seed extract and its in vitro anticancer activity
- Temperature dependence of hardness prediction for high-temperature structural ceramics and their composites
- Study on the frequency of acoustic emission signal during crystal growth of salicylic acid
- Controllable modification of helical carbon nanotubes for high-performance microwave absorption
- Role of dry ozonization of basalt fibers on interfacial properties and fracture toughness of epoxy matrix composites
- Nanosystem’s density functional theory study of the chlorine adsorption on the Fe(100) surface
- A rapid nanobiosensing platform based on herceptin-conjugated graphene for ultrasensitive detection of circulating tumor cells in early breast cancer
- Improving flexural strength of UHPC with sustainably synthesized graphene oxide
- The role of graphene/graphene oxide in cement hydration
- Structural characterization of microcrystalline and nanocrystalline cellulose from Ananas comosus L. leaves: Cytocompatibility and molecular docking studies
- Evaluation of the nanostructure of calcium silicate hydrate based on atomic force microscopy-infrared spectroscopy experiments
- Combined effects of nano-silica and silica fume on the mechanical behavior of recycled aggregate concrete
- Safety study of malapposition of the bio-corrodible nitrided iron stent in vivo
- Triethanolamine interface modification of crystallized ZnO nanospheres enabling fast photocatalytic hazard-free treatment of Cr(vi) ions
- Novel electrodes for precise and accurate droplet dispensing and splitting in digital microfluidics
- Construction of Chi(Zn/BMP2)/HA composite coating on AZ31B magnesium alloy surface to improve the corrosion resistance and biocompatibility
- Experimental and multiscale numerical investigations on low-velocity impact responses of syntactic foam composites reinforced with modified MWCNTs
- Comprehensive performance analysis and optimal design of smart light pole for cooperative vehicle infrastructure system
- Room temperature growth of ZnO with highly active exposed facets for photocatalytic application
- Influences of poling temperature and elongation ratio on PVDF-HFP piezoelectric films
- Large strain hardening of magnesium containing in situ nanoparticles
- Super stable water-based magnetic fluid as a dual-mode contrast agent
- Photocatalytic activity of biogenic zinc oxide nanoparticles: In vitro antimicrobial, biocompatibility, and molecular docking studies
- Hygrothermal environment effect on the critical buckling load of FGP microbeams with initial curvature integrated by CNT-reinforced skins considering the influence of thickness stretching
- Thermal aging behavior characteristics of asphalt binder modified by nano-stabilizer based on DSR and AFM
- Building effective core/shell polymer nanoparticles for epoxy composite toughening based on Hansen solubility parameters
- Structural characterization and nanoscale strain field analysis of α/β interface layer of a near α titanium alloy
- Optimization of thermal and hydrophobic properties of GO-doped epoxy nanocomposite coatings
- The properties of nano-CaCO3/nano-ZnO/SBR composite-modified asphalt
- Three-dimensional metallic carbon allotropes with superhardness
- Physical stability and rheological behavior of Pickering emulsions stabilized by protein–polysaccharide hybrid nanoconjugates
- Optimization of volume fraction and microstructure evolution during thermal deformation of nano-SiCp/Al–7Si composites
- Phase analysis and corrosion behavior of brazing Cu/Al dissimilar metal joint with BAl88Si filler metal
- High-efficiency nano polishing of steel materials
- On the rheological properties of multi-walled carbon nano-polyvinylpyrrolidone/silicon-based shear thickening fluid
- Fabrication of Ag/ZnO hollow nanospheres and cubic TiO2/ZnO heterojunction photocatalysts for RhB degradation
- Fabrication and properties of PLA/nano-HA composite scaffolds with balanced mechanical properties and biological functions for bone tissue engineering application
- Investigation of the early-age performance and microstructure of nano-C–S–H blended cement-based materials
- Reduced graphene oxide coating on basalt fabric using electrophoretic deposition and its role in the mechanical and tribological performance of epoxy/basalt fiber composites
- Effect of nano-silica as cementitious materials-reducing admixtures on the workability, mechanical properties and durability of concrete
- Machine-learning-assisted microstructure–property linkages of carbon nanotube-reinforced aluminum matrix nanocomposites produced by laser powder bed fusion
- Physical, thermal, and mechanical properties of highly porous polylactic acid/cellulose nanofibre scaffolds prepared by salt leaching technique
- A comparative study on characterizations and synthesis of pure lead sulfide (PbS) and Ag-doped PbS for photovoltaic applications
- Clean preparation of washable antibacterial polyester fibers by high temperature and high pressure hydrothermal self-assembly
- Al 5251-based hybrid nanocomposite by FSP reinforced with graphene nanoplates and boron nitride nanoparticles: Microstructure, wear, and mechanical characterization
- Interlaminar fracture toughness properties of hybrid glass fiber-reinforced composite interlayered with carbon nanotube using electrospray deposition
- Microstructure and life prediction model of steel slag concrete under freezing-thawing environment
- Synthesis of biogenic silver nanoparticles from the seed coat waste of pistachio (Pistacia vera) and their effect on the growth of eggplant
- Study on adaptability of rheological index of nano-PUA-modified asphalt based on geometric parameters of parallel plate
- Preparation and adsorption properties of nano-graphene oxide/tourmaline composites
- A study on interfacial behaviors of epoxy/graphene oxide derived from pitch-based graphite fibers
- Multiresponsive carboxylated graphene oxide-grafted aptamer as a multifunctional nanocarrier for targeted delivery of chemotherapeutics and bioactive compounds in cancer therapy
- Piezoresistive/piezoelectric intrinsic sensing properties of carbon nanotube cement-based smart composite and its electromechanical sensing mechanisms: A review
- Smart stimuli-responsive biofunctionalized niosomal nanocarriers for programmed release of bioactive compounds into cancer cells in vitro and in vivo
- Photoremediation of methylene blue by biosynthesized ZnO/Fe3O4 nanocomposites using Callistemon viminalis leaves aqueous extract: A comparative study
- Study of gold nanoparticles’ preparation through ultrasonic spray pyrolysis and lyophilisation for possible use as markers in LFIA tests
- Review Articles
- Advance on the dispersion treatment of graphene oxide and the graphene oxide modified cement-based materials
- Development of ionic liquid-based electroactive polymer composites using nanotechnology
- Nanostructured multifunctional electrocatalysts for efficient energy conversion systems: Recent perspectives
- Recent advances on the fabrication methods of nanocomposite yarn-based strain sensor
- Review on nanocomposites based on aerospace applications
- Overview of nanocellulose as additives in paper processing and paper products
- The frontiers of functionalized graphene-based nanocomposites as chemical sensors
- Material advancement in tissue-engineered nerve conduit
- Carbon nanostructure-based superhydrophobic surfaces and coatings
- Functionalized graphene-based nanocomposites for smart optoelectronic applications
- Interfacial technology for enhancement in steel fiber reinforced cementitious composite from nano to macroscale
- Metal nanoparticles and biomaterials: The multipronged approach for potential diabetic wound therapy
- Review on resistive switching mechanisms of bio-organic thin film for non-volatile memory application
- Nanotechnology-enabled biomedical engineering: Current trends, future scopes, and perspectives
- Research progress on key problems of nanomaterials-modified geopolymer concrete
- Smart stimuli-responsive nanocarriers for the cancer therapy – nanomedicine
- An overview of methods for production and detection of silver nanoparticles, with emphasis on their fate and toxicological effects on human, soil, and aquatic environment
- Effects of chemical modification and nanotechnology on wood properties
- Mechanisms, influencing factors, and applications of electrohydrodynamic jet printing
- Application of antiviral materials in textiles: A review
- Phase transformation and strengthening mechanisms of nanostructured high-entropy alloys
- Research progress on individual effect of graphene oxide in cement-based materials and its synergistic effect with other nanomaterials
- Catalytic defense against fungal pathogens using nanozymes
- A mini-review of three-dimensional network topological structure nanocomposites: Preparation and mechanical properties
- Mechanical properties and structural health monitoring performance of carbon nanotube-modified FRP composites: A review
- Nano-scale delivery: A comprehensive review of nano-structured devices, preparative techniques, site-specificity designs, biomedical applications, commercial products, and references to safety, cellular uptake, and organ toxicity
- Effects of alloying, heat treatment and nanoreinforcement on mechanical properties and damping performances of Cu–Al-based alloys: A review
- Recent progress in the synthesis and applications of vertically aligned carbon nanotube materials
- Thermal conductivity and dynamic viscosity of mono and hybrid organic- and synthetic-based nanofluids: A critical review
- Recent advances in waste-recycled nanomaterials for biomedical applications: Waste-to-wealth
- Layup sequence and interfacial bonding of additively manufactured polymeric composite: A brief review
- Quantum dots synthetization and future prospect applications
- Approved and marketed nanoparticles for disease targeting and applications in COVID-19
- Strategies for improving rechargeable lithium-ion batteries: From active materials to CO2 emissions
Articles in the same Issue
- Research Articles
- Improved impedance matching by multi-componential metal-hybridized rGO toward high performance of microwave absorption
- Pure-silk fibroin hydrogel with stable aligned micropattern toward peripheral nerve regeneration
- Effective ion pathways and 3D conductive carbon networks in bentonite host enable stable and high-rate lithium–sulfur batteries
- Fabrication and characterization of 3D-printed gellan gum/starch composite scaffold for Schwann cells growth
- Synergistic strengthening mechanism of copper matrix composite reinforced with nano-Al2O3 particles and micro-SiC whiskers
- Deformation mechanisms and plasticity of ultrafine-grained Al under complex stress state revealed by digital image correlation technique
- On the deformation-induced grain rotations in gradient nano-grained copper based on molecular dynamics simulations
- Removal of sulfate from aqueous solution using Mg–Al nano-layered double hydroxides synthesized under different dual solvent systems
- Microwave-assisted sol–gel synthesis of TiO2-mixed metal oxide nanocatalyst for degradation of organic pollutant
- Electrophoretic deposition of graphene on basalt fiber for composite applications
- Polyphenylene sulfide-coated wrench composites by nanopinning effect
- Thermal conductivity and thermoelectric properties in 3D macroscopic pure carbon nanotube materials
- An effective thermal conductivity and thermomechanical homogenization scheme for a multiscale Nb3Sn filaments
- Friction stir spot welding of AA5052 with additional carbon fiber-reinforced polymer composite interlayer
- Improvement of long-term cycling performance of high-nickel cathode materials by ZnO coating
- Quantum effects of gas flow in nanochannels
- An approach to effectively improve the interfacial bonding of nano-perfused composites by in situ growth of CNTs
- Effects of nano-modified polymer cement-based materials on the bending behavior of repaired concrete beams
- Effects of the combined usage of nanomaterials and steel fibres on the workability, compressive strength, and microstructure of ultra-high performance concrete
- One-pot solvothermal synthesis and characterization of highly stable nickel nanoparticles
- Comparative study on mechanisms for improving mechanical properties and microstructure of cement paste modified by different types of nanomaterials
- Effect of in situ graphene-doped nano-CeO2 on microstructure and electrical contact properties of Cu30Cr10W contacts
- The experimental study of CFRP interlayer of dissimilar joint AA7075-T651/Ti-6Al-4V alloys by friction stir spot welding on mechanical and microstructural properties
- Vibration analysis of a sandwich cylindrical shell in hygrothermal environment
- Water barrier and mechanical properties of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch (TPS)/poly(lactic acid) (PLA) blend bionanocomposites
- Strong quadratic acousto-optic coupling in 1D multilayer phoxonic crystal cavity
- Three-dimensional shape analysis of peripapillary retinal pigment epithelium-basement membrane layer based on OCT radial images
- Solvent regulation synthesis of single-component white emission carbon quantum dots for white light-emitting diodes
- Xanthate-modified nanoTiO2 as a novel vulcanization accelerator enhancing mechanical and antibacterial properties of natural rubber
- Effect of steel fiber on impact resistance and durability of concrete containing nano-SiO2
- Ultrasound-enhanced biosynthesis of uniform ZnO nanorice using Swietenia macrophylla seed extract and its in vitro anticancer activity
- Temperature dependence of hardness prediction for high-temperature structural ceramics and their composites
- Study on the frequency of acoustic emission signal during crystal growth of salicylic acid
- Controllable modification of helical carbon nanotubes for high-performance microwave absorption
- Role of dry ozonization of basalt fibers on interfacial properties and fracture toughness of epoxy matrix composites
- Nanosystem’s density functional theory study of the chlorine adsorption on the Fe(100) surface
- A rapid nanobiosensing platform based on herceptin-conjugated graphene for ultrasensitive detection of circulating tumor cells in early breast cancer
- Improving flexural strength of UHPC with sustainably synthesized graphene oxide
- The role of graphene/graphene oxide in cement hydration
- Structural characterization of microcrystalline and nanocrystalline cellulose from Ananas comosus L. leaves: Cytocompatibility and molecular docking studies
- Evaluation of the nanostructure of calcium silicate hydrate based on atomic force microscopy-infrared spectroscopy experiments
- Combined effects of nano-silica and silica fume on the mechanical behavior of recycled aggregate concrete
- Safety study of malapposition of the bio-corrodible nitrided iron stent in vivo
- Triethanolamine interface modification of crystallized ZnO nanospheres enabling fast photocatalytic hazard-free treatment of Cr(vi) ions
- Novel electrodes for precise and accurate droplet dispensing and splitting in digital microfluidics
- Construction of Chi(Zn/BMP2)/HA composite coating on AZ31B magnesium alloy surface to improve the corrosion resistance and biocompatibility
- Experimental and multiscale numerical investigations on low-velocity impact responses of syntactic foam composites reinforced with modified MWCNTs
- Comprehensive performance analysis and optimal design of smart light pole for cooperative vehicle infrastructure system
- Room temperature growth of ZnO with highly active exposed facets for photocatalytic application
- Influences of poling temperature and elongation ratio on PVDF-HFP piezoelectric films
- Large strain hardening of magnesium containing in situ nanoparticles
- Super stable water-based magnetic fluid as a dual-mode contrast agent
- Photocatalytic activity of biogenic zinc oxide nanoparticles: In vitro antimicrobial, biocompatibility, and molecular docking studies
- Hygrothermal environment effect on the critical buckling load of FGP microbeams with initial curvature integrated by CNT-reinforced skins considering the influence of thickness stretching
- Thermal aging behavior characteristics of asphalt binder modified by nano-stabilizer based on DSR and AFM
- Building effective core/shell polymer nanoparticles for epoxy composite toughening based on Hansen solubility parameters
- Structural characterization and nanoscale strain field analysis of α/β interface layer of a near α titanium alloy
- Optimization of thermal and hydrophobic properties of GO-doped epoxy nanocomposite coatings
- The properties of nano-CaCO3/nano-ZnO/SBR composite-modified asphalt
- Three-dimensional metallic carbon allotropes with superhardness
- Physical stability and rheological behavior of Pickering emulsions stabilized by protein–polysaccharide hybrid nanoconjugates
- Optimization of volume fraction and microstructure evolution during thermal deformation of nano-SiCp/Al–7Si composites
- Phase analysis and corrosion behavior of brazing Cu/Al dissimilar metal joint with BAl88Si filler metal
- High-efficiency nano polishing of steel materials
- On the rheological properties of multi-walled carbon nano-polyvinylpyrrolidone/silicon-based shear thickening fluid
- Fabrication of Ag/ZnO hollow nanospheres and cubic TiO2/ZnO heterojunction photocatalysts for RhB degradation
- Fabrication and properties of PLA/nano-HA composite scaffolds with balanced mechanical properties and biological functions for bone tissue engineering application
- Investigation of the early-age performance and microstructure of nano-C–S–H blended cement-based materials
- Reduced graphene oxide coating on basalt fabric using electrophoretic deposition and its role in the mechanical and tribological performance of epoxy/basalt fiber composites
- Effect of nano-silica as cementitious materials-reducing admixtures on the workability, mechanical properties and durability of concrete
- Machine-learning-assisted microstructure–property linkages of carbon nanotube-reinforced aluminum matrix nanocomposites produced by laser powder bed fusion
- Physical, thermal, and mechanical properties of highly porous polylactic acid/cellulose nanofibre scaffolds prepared by salt leaching technique
- A comparative study on characterizations and synthesis of pure lead sulfide (PbS) and Ag-doped PbS for photovoltaic applications
- Clean preparation of washable antibacterial polyester fibers by high temperature and high pressure hydrothermal self-assembly
- Al 5251-based hybrid nanocomposite by FSP reinforced with graphene nanoplates and boron nitride nanoparticles: Microstructure, wear, and mechanical characterization
- Interlaminar fracture toughness properties of hybrid glass fiber-reinforced composite interlayered with carbon nanotube using electrospray deposition
- Microstructure and life prediction model of steel slag concrete under freezing-thawing environment
- Synthesis of biogenic silver nanoparticles from the seed coat waste of pistachio (Pistacia vera) and their effect on the growth of eggplant
- Study on adaptability of rheological index of nano-PUA-modified asphalt based on geometric parameters of parallel plate
- Preparation and adsorption properties of nano-graphene oxide/tourmaline composites
- A study on interfacial behaviors of epoxy/graphene oxide derived from pitch-based graphite fibers
- Multiresponsive carboxylated graphene oxide-grafted aptamer as a multifunctional nanocarrier for targeted delivery of chemotherapeutics and bioactive compounds in cancer therapy
- Piezoresistive/piezoelectric intrinsic sensing properties of carbon nanotube cement-based smart composite and its electromechanical sensing mechanisms: A review
- Smart stimuli-responsive biofunctionalized niosomal nanocarriers for programmed release of bioactive compounds into cancer cells in vitro and in vivo
- Photoremediation of methylene blue by biosynthesized ZnO/Fe3O4 nanocomposites using Callistemon viminalis leaves aqueous extract: A comparative study
- Study of gold nanoparticles’ preparation through ultrasonic spray pyrolysis and lyophilisation for possible use as markers in LFIA tests
- Review Articles
- Advance on the dispersion treatment of graphene oxide and the graphene oxide modified cement-based materials
- Development of ionic liquid-based electroactive polymer composites using nanotechnology
- Nanostructured multifunctional electrocatalysts for efficient energy conversion systems: Recent perspectives
- Recent advances on the fabrication methods of nanocomposite yarn-based strain sensor
- Review on nanocomposites based on aerospace applications
- Overview of nanocellulose as additives in paper processing and paper products
- The frontiers of functionalized graphene-based nanocomposites as chemical sensors
- Material advancement in tissue-engineered nerve conduit
- Carbon nanostructure-based superhydrophobic surfaces and coatings
- Functionalized graphene-based nanocomposites for smart optoelectronic applications
- Interfacial technology for enhancement in steel fiber reinforced cementitious composite from nano to macroscale
- Metal nanoparticles and biomaterials: The multipronged approach for potential diabetic wound therapy
- Review on resistive switching mechanisms of bio-organic thin film for non-volatile memory application
- Nanotechnology-enabled biomedical engineering: Current trends, future scopes, and perspectives
- Research progress on key problems of nanomaterials-modified geopolymer concrete
- Smart stimuli-responsive nanocarriers for the cancer therapy – nanomedicine
- An overview of methods for production and detection of silver nanoparticles, with emphasis on their fate and toxicological effects on human, soil, and aquatic environment
- Effects of chemical modification and nanotechnology on wood properties
- Mechanisms, influencing factors, and applications of electrohydrodynamic jet printing
- Application of antiviral materials in textiles: A review
- Phase transformation and strengthening mechanisms of nanostructured high-entropy alloys
- Research progress on individual effect of graphene oxide in cement-based materials and its synergistic effect with other nanomaterials
- Catalytic defense against fungal pathogens using nanozymes
- A mini-review of three-dimensional network topological structure nanocomposites: Preparation and mechanical properties
- Mechanical properties and structural health monitoring performance of carbon nanotube-modified FRP composites: A review
- Nano-scale delivery: A comprehensive review of nano-structured devices, preparative techniques, site-specificity designs, biomedical applications, commercial products, and references to safety, cellular uptake, and organ toxicity
- Effects of alloying, heat treatment and nanoreinforcement on mechanical properties and damping performances of Cu–Al-based alloys: A review
- Recent progress in the synthesis and applications of vertically aligned carbon nanotube materials
- Thermal conductivity and dynamic viscosity of mono and hybrid organic- and synthetic-based nanofluids: A critical review
- Recent advances in waste-recycled nanomaterials for biomedical applications: Waste-to-wealth
- Layup sequence and interfacial bonding of additively manufactured polymeric composite: A brief review
- Quantum dots synthetization and future prospect applications
- Approved and marketed nanoparticles for disease targeting and applications in COVID-19
- Strategies for improving rechargeable lithium-ion batteries: From active materials to CO2 emissions

