Abstract
This contribution investigates chlorine (Cl) interaction with the Fe(100) surface, with a focus on governing adsorption energies and geometrical features at the nanoscale using the density functional theory (DFT) approach. The Cl/Fe(100) system can be considered as a building block to create nanosystems with specific and desired electronic, material, mechanical, or environmental properties. We report adsorption energies, surface relaxations. and buckling distances for Cl adsorbed as a function of Cl coverage. The computational DFT framework employs a vdW-DF functional with coverages varying from 0.25 to 1 ML. Adsorption at a bridge site with coverage of 0.5 ML appears to be the most preferred site, with an adsorption energy of −4.44 eV. For all coverages, Cl adsorption at the bridge and hollow sites incurs slightly higher adsorption energies than adsorption at the top (T) site. The potential energy surface (PES) for the dissociation of a Cl molecule over the Fe(100) surface was calculated. Dissociative adsorption of the Cl molecule on Fe(100) ensues via a modest activation barrier of only 0.58 eV in a noticeably exothermic reaction of 2.94 eV. In agreement with experimental observations, the work function decreases upon Cl addition in reference to the clean iron surface. The electronic interaction between Cl and the Fe(100) surface was examined by calculating the differential charge density distribution of the most stable structure (B-0.5 ML). The vdW-DF interactions increase the adsorption energies and reduce the equilibrium distances when compared with the corresponding results from plain DFT.
1 Introduction
The profound chemical reactivity of iron surfaces with chlorine species is of prime importance for several environmental and industrial concerns, including chlorination of aromatic pollutants (most notably notorious dioxins) and corrosion of equipment [1,2,3]. For instance, the system of halogens–iron plays a central role in deriving the halogenation cycle of aromatic compounds in scenarios pertinent to municipal waste incineration and recycling of electronic wastes [4]. From a safety perspective, the interaction of iron and chlorine has been extensively studied to comprehend chemical phenomena that prevail under corrosive HCl environments [5] and chlorine-containing salts (i.e., seawater) [6]. Chlorine chemisorption on the low-index (100) and (110) iron surfaces have been investigated on the basis of many aspects [7,8,9]. Dowbin and Jones [1] investigated the adsorption of Cl2 on the Fe(100) surface using Auger electron spectroscopy (AES) and low energy electron diffraction (LEED) approaches at room temperature. They illustrated that the change in the work function is proportional to the chlorine coverage, with a maximum value of 1.43 eV at complete saturation. Chlorine adsorption over a four-fold site (hollow site) is more stable than that at other sites. Upon heating, chlorine either desorbs into the vacuum or undergoes bulk diffusion. Along the same line of enquiry, Hino and Lambert [2] studied the interaction of chlorine with the Fe(100) surface at low pressure using Auger spectroscopy, thermal desorption, X-ray and ultraviolet photoemission spectroscopy (XPS and UPS) techniques. They demonstrated that the first adsorbate layer comprises FeCl2 rather than FeCl or nondissociated chlorine molecules. After the complete formation of a chemisorbed overlayer and at 300 K, the growth of the iron chloride layer is sustained.
Computationally, several density functional theory (DFT) studies have provided atomic-based insights into the Cl/Fe configurations with a focus on governing electronic/structural properties and thermodynamic stability at different chlorine coverages [3,7,8,9]. The binding, structures, and reactions of Cl, Cl2, HCl, and HCl + O with the Fe(100) structure were investigated based on atom superposition and electron delocalization (ASED)molecular orbital theory [3]. Cl2 was found to readily dissociate over one-fold and two-fold sites [3]. Previous theoretical calculations provided mechanistic insight into the growth of the FeCl2 phase via the continuous adsorption of Cl atoms over the Fe(100) surface using generalized gradient approximation-projector augmented wave (GGA-PAW) [9] but without providing an intrinsic reaction barrier. At a 0.75 ML coverage, the four-fold (hollow) and two-fold (bridge) sites were found to be slightly more preferred than the top sites, with adsorption energies of −3.50, −3.39 and −3.29 eV, respectively. Nonetheless, the marginal difference in energies among the former values most likely resides within the accuracy benchmark of the adapted DFT calculations. Our comprehensive DFT study [9] on the interaction of atomic chlorine over the Fe(100) surface presented binding chemisorption energies for on-surface and substitutional adsorption of chlorine using a plain DFT functional. A thermodynamic stability diagram was constructed over a wide range of chlorine chemical potentials to simulate real operational conditions encountered in iron corrosion by chlorine. Nonetheless, our previous study deployed a standard DFT functional without accounting for the long-term interactions that may result from van der Waals (vdW) forces.
To this end, this contribution reports values for adsorption energies, equilibrium geometries, and differential charge density distributions pertinent to the Cl/Fe(100) system. Furthermore, activation energies and work functions are calculated. Computed binding energies take into account the contribution from long-term interactions [10] using the van der Waals correction functional (vdE–DF) [11,12], which has been accounted for via the inclusion of a dispersion correction function (DFT-D). An important goal of the current study is to provide underlying kinetic parameters for the uptake of chlorine molecules by the Fe(100) surface. Such information is vital to attain a detailed understanding of processes that dictates the chlorination of iron into its chloride forms. Likewise, the acquired thermodynamic values are expected to enable the construction of stability phase diagrams of Fe–Cl phases at practical conditions germane to varying values of chlorine chemical potential.
2 Methodology
We carry out all computations using spin-polarized density functional theory (DFT) calculations. The PAW-GGA method of PW91 is used in all spin-polarized calculations [13,14,15] as implemented in the Vienna ab initio simulation (VASP) package [16,17]. The van der Waals correction functional (vdE–DF) by Dion et al. [11] refines adsorption energies taking into account the effect of long-range interactions. The calculations consider the ferromagnetism character of Fe atoms in which the magnetic moment of partially filled Fe sites rests at ±5.0 μB [18].
The Fe(100) surface was modeled using a supercell with six layers. To identify the most favorable sites, the bottom two layers were kept fixed at their bulk positions with the first four layers, and the adsorbate atoms were allowed to relax during the optimization. A sampling of the irreducible part of the Brillouin zone was carried out based on automatic generation of κ-points based on a Monkhost-Pack mesh of (3 × 3 × 1) [19]. In the z direction, a vacuum spacing of 15 Å separates images along the z-direction in which a dipole correction is imposed. The kinetic energy cutoff was set at 400 eV. Optimizing one structure with eight atomic layers (H-0.25) at 500 eV cutoff energy and 4 × 4 × 1 κ-points sampling has changed its associated adsorption energy marginally within 2.6%.
Relaxations
The adsorption energy (E ads.) and the chemisorption energy (E C) are computed based on
where E
Cl+surface represents the total energy of the optimized surface with atomic Cl adsorbed, n stands for the number of adsorbed Cl atoms, E
Cl is the single Cl atom’s ground state energy, E
surface is the total energy of the clean surface without adsorption of the Cl atoms, and
Charge transfer was examined by calculating the differential charge density:
where
3 Results and discussion
3.1 Fe(100) clean surface
The calculated lattice constant for the bulk Fe is 2.828 Å. This value was obtained by minimizing energy with respect to the volume of the unit cell. This value appears in very good agreement with previous experimental measurements of 2.845 Å [22] and other theoretically calculated values of 2.828 Å [9], 2.830 Å [23], and 2.834 Å [24]. The derived value of the bulk interlayer spacing (d
o) is 1.1 Å. The calculated relaxations between the first and second layers as well as second and third layers for the Fe(100) surface with analogous literature values are listed in Table 1. For the Fe(100) surface, the spacing between the first and second layers is lower than the bulk interlayer spacing (d
12 = −4.15%). This effect is compensated by an expansion between the second and third layers (d
23 = +2.65%). Bearing in mind the accuracy margin of the experimental measurements (i.e.,
Comparison between values of relaxation computed herein and corresponding literature values
| Relaxation | Refs. [9,25,26,27] | Current calculations | |
|---|---|---|---|
| Experimental values | Other theoretical values | ||
|
|
−1.40 ± 3 | −3, −3.50 | −4.15 |
|
|
5.0 ± 2 | 1.7, 2.30 | 2.65 |
As shown in Figure 1(a), the Fe(100) surface displays three distinct adsorption sites on top of one of the Fe atoms (one-fold, T), a bridge between two surface Fe atoms (two-fold, B), and at a hollow between four surface Fe atoms (four-fold, H).
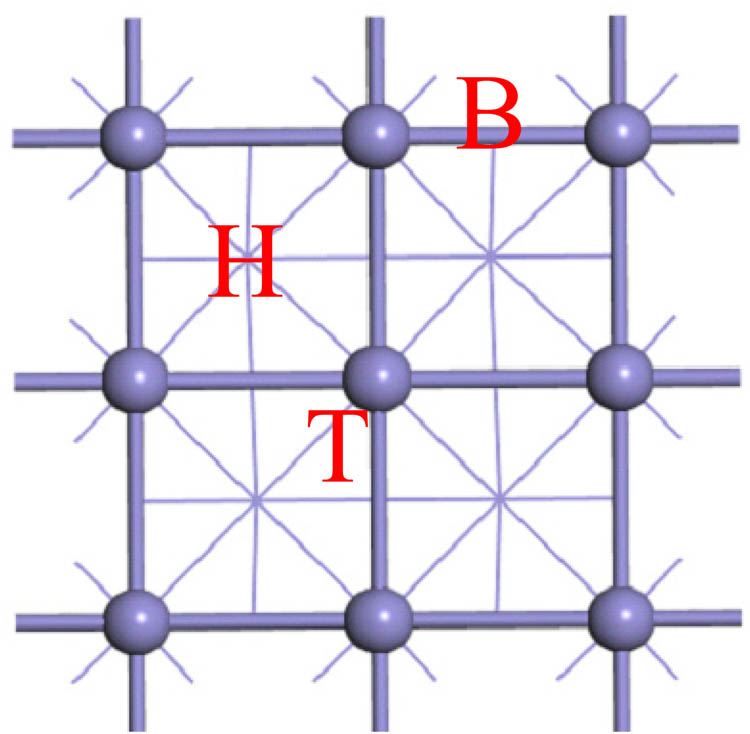
Potential adsorption sites for atomic Cl on the Fe(100) surface. For clarity, atoms in the second layer are denoted by lines.
3.2 Cl adsorbed on the Fe(100) surface
Different Cl/Fe(100) configurations at coverages varying from 0.25 to 1 ML are studied. Adsorption and chemisorption energies and some important geometrical features for all configurations are presented in Table 2. For all coverages, adsorption at two-fold (B) and four-fold (H) adsorption sites is more favored than adsorption at the one-fold (T) sites. At 0.5 ML coverage, B and H sites are found to be more preferred than T sites with adsorption energies (chemisorption energy) of −4.44 (−3.00), −4.39 (−2.97), and −4.06 (−2.62) eV, respectively. For all sites, 0.5 ML coverage is shown to be the most stable configuration among the same site based on the values of adsorption and chemisorption energies. Overall, B site with coverage of 0.5 ML appears to be the most preferred site with chemisorption energy of −3.0 eV. At this site, the average Cl–Fe bond length is 2.31 Å, and the metal–adsorbate distance is 1.84 Å.
Adsorption energies and structural parameters for Cl/Fe(100)
| Site | Coverage Θ (ML) | E ads. (eV) | E C (eV) | (Cl–Fe) (Å) | R (Å) | H (Å) | (d 12)% | (d 23)% |
|---|---|---|---|---|---|---|---|---|
| T | 0.25 | −2.75 | −1.31 | 2.15 | 2.08 | 0.11 | −1.01 | −0.77 |
| 0.5 | −4.06 | −2.62 | 2.14 | 2.16 | 0.03 | 5.87 | −2.94 | |
| 0.75 | −3.64 | −2.20 | 2.15 | 2.30 | 0.36 | −4.93 | 0.09 | |
| 1 | −3.08 | −1.64 | 2.10 | 2.11 | 0.00 | −5.81 | −0.78 | |
| H | 0.25 | −3.93 | −2.49 | 2.58 | 1.45 | 0.02 | 7.62 | −2.60 |
| 0.5 | −4.39 | −2.97 | 2.60 | 1.65 | 0.01 | 9.76 | −4.21 | |
| 0.75 | −3.86 | −2.42 | 2.59 | 1.48 | 0.06 | 5.75 | −4.33 | |
| B | 0.25 | −4.38 | −2.94 | 2.34 | 1.99 | 0.17 | 5.65 | −2.01 |
| 0.5 | −4.44 | −3.00 | 2.31 | 1.84 | 0.04 | 1.27 | −4.18 | |
| 0.75 | −3.65 | −2.21 | 2.30 | 1.81 | 0.08 | 7.27 | −2.06 | |
| 1 | −3.44 | −2.00 | 2.27 | 1.78 | 0.01 | 9.60 | −4.57 |
E ads.: adsorption energies, E C: chemisorption energies, (Cl–Fe): average distance between nearest Fe atoms and Cl atom(s), R: average heights of Cl atoms above the first layers, H: the height between the lowest and highest Fe layers, (d 12)% relaxation of first and second Fe layers with respect to the clean surface (calculated based on equation (1)), and (d 23)% relaxation of second and third Fe layers with respect to the clean surface (calculated based on equation (1)).
At the 0.75 ML coverage, the four-fold site (H) is found to be more preferred than the bridge (B) and top (T) sites, with adsorption energies of −3.86, −3.65 and −3.64 eV and chemisorption energies of −2.42, −2.21 and −2.20 eV, respectively. The latter results are in very good agreement with the result of Dowbin and Jones [1] and previous theoretical calculations [9]. The relaxations of the first three Fe layers of the considered surface after adding the adsorbate with respect to the interlayer spacing of the clean surface are presented in Table 2. In all coverages for H and B sites, the presence of chlorine causes an expansion of the first and second interlayer distance and a compression of the second and third interlayer distance. This behavior shows that the first layer of iron relaxes up after interaction with chlorine, while the second layer relaxes down with the attraction of the lower iron atoms. This is in contrast with the case at site T, where each coverage obtained a different behavior. The average Cl–Fe nearest distance (Cl–Fe) increases in the order of T < B < H. The vertical buckling based on the H values in Table 2 in all configurations is very minimal (0.01–0.36) Å, with nearly no buckling observed at full coverages for all sites. The lateral repulsive interactions are expected to play a noticeable role in reducing the binding energy at high coverages [28].
In a corrosive chlorine-containing environment, interactions of chlorine with iron surfaces produce iron chlorides and oxychlorides. Thus, it is of interest to compare distances in Cl–Fe configurations with their corresponding values in bulk iron chlorides. The important geometrical parameters of the most stable configuration B (0.5 ML) are contrasted with analogous distances of bulk FeCl2 and FeCl3 [29], as presented in Table 3. The Cl–Cl distances in the latter structure overshoot their corresponding distances in bulk FeCl2 and FeCl3 by 0.45 and 0.79 Å, respectively. The shortest Fe–Cl spacing in B (0.5 ML) site deviates marginally by 6.5 and 4.9% relative to the analogous values in bulk FeCl2 and FeCl3, respectively. Despite certain notable differences in some interatomic distances, comparing geometries of B (0.5 ML) with bulk FeCl2 reveals that B (0.5 ML) site retains analogous geometries of bulk FeCl2 to some extent.
Nearest atomic distances (in Å) for the optimized bulk of FeCl2 and FeCl3 and B (0.5 ML) site
| Bond length (Å) | FeCl2 [29] | FeCl3 [29] | B (0.5 ML) |
|---|---|---|---|
| Cl–Cl | 3.54 | 3.20 | 3.99 |
| Cl–Fe | 2.47 | 2.26 | 2.31 |
Next, we turn our attention to computing activation energies for the dissociation of a chlorine molecule above the surface. Iron readily corrodes in a chlorine-containing environment [30]. This indicates that iron chlorination ensues modest reaction barriers in real scenarios. Figure 2 shows a potential energy surface (PES) for the dissociation of a gas-phase chlorine molecule over the Fe(100) surface. We found the chlorine molecule to be very weakly adsorbed (−0.15 eV, DFT–vdW) while adapting a horizontal-like configuration. This value slightly decreases to −0.10 eV when a plain DFT method is deployed. Rupture of the Cl–Cl bond at two hollow sites demands a modest barrier of only 0.58 eV in a noticeably exothermic reaction of 2.94 eV. The profound surface-mediated effect of the iron surface becomes evident when contrasting the sizable Cl–Cl bond dissociation energy in the gas-phase chlorine molecule at 2.52 eV. The nature of the located transition state as a saddle point was confirmed by evaluating the vibrational frequencies of the structure using the linear response method [31]. The configuration retains one major frequency of (258i)/cm along with a few other minimal values in the range (34i–87i)/cm. The latter values most likely correspond to the movements of surface iron atoms.
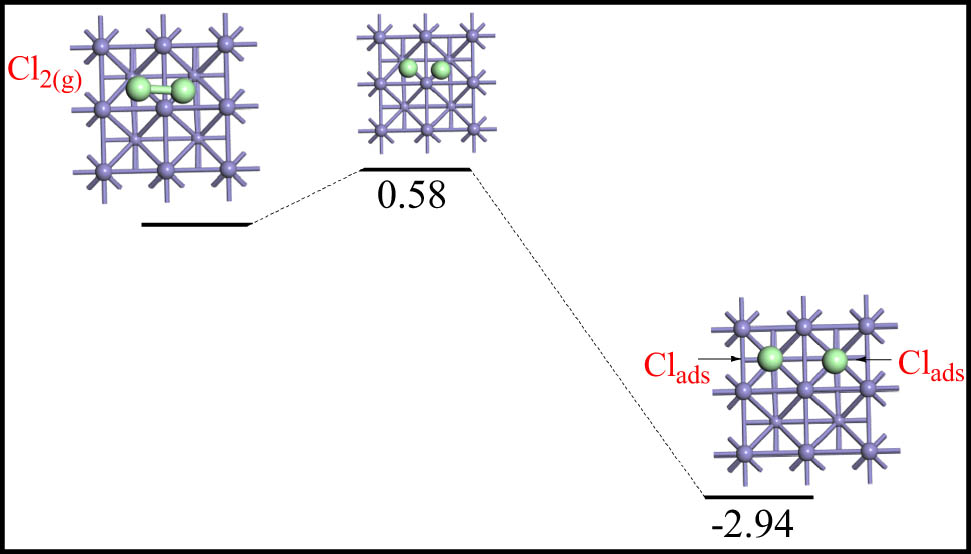
PES for the Fe(100)-assisted fission of a chlorine molecule. Values are in eV in reference to the adsorbed chlorine molecule.
By considering the surface area of iron surfaces (0.3 m2/g) and the density of active sites (
where s, M, E a, and T signify the sticking coefficient (assumed to be 1.0), the molecular weight of Cl2, activation energy, and temperature, respectively. The calculations of the reaction rate considered a gas-phase concentration at 500 ppm Cl2 that mimicked mild corrosion conditions. To the best of our knowledge, the literature presents no analogous experimental measurements to compare with computed values herein.
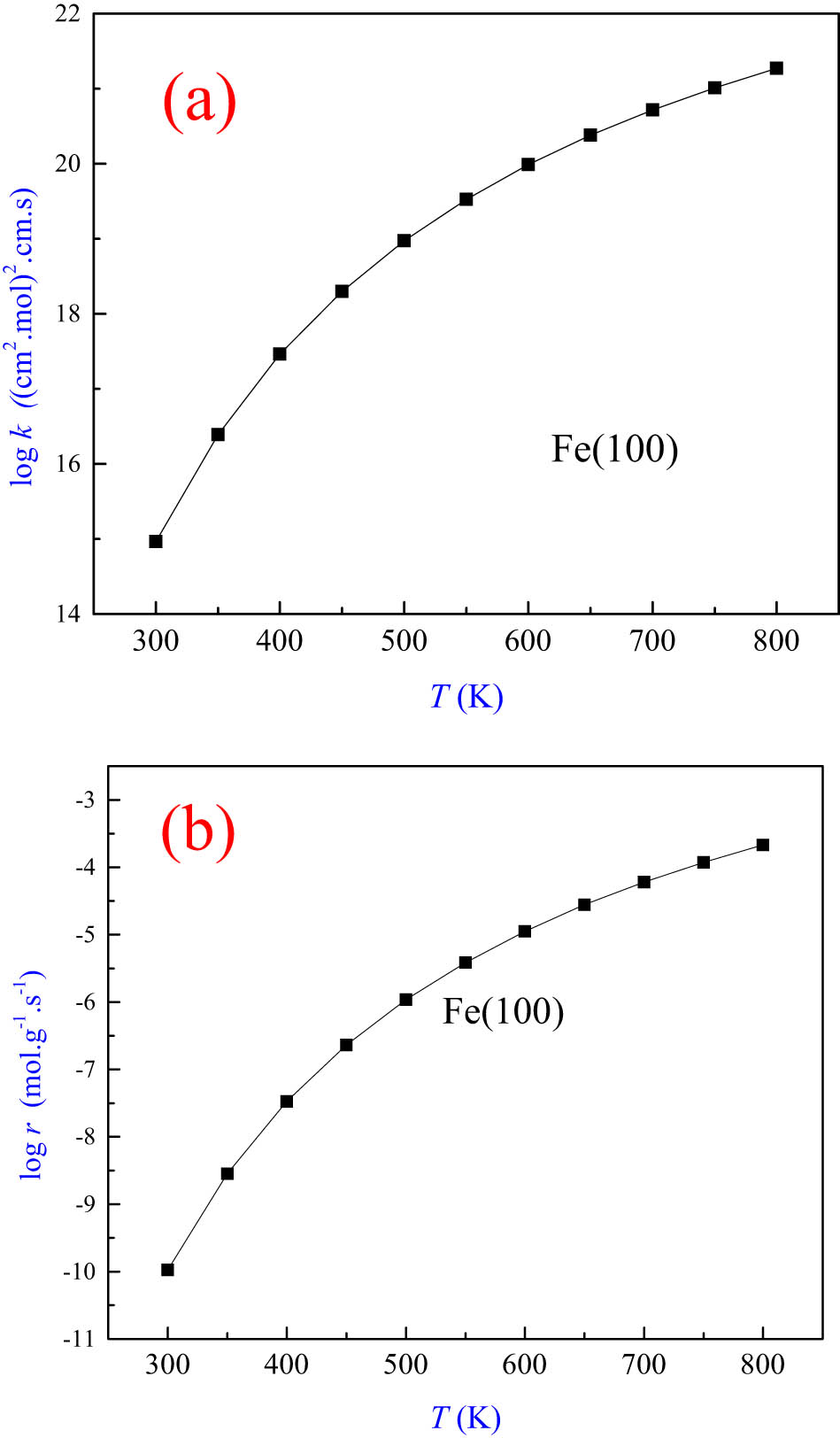
The reaction rate constant (a) and the reaction rate for the dissociative adsorption (b) of a chlorine molecule over the Fe(100) surface.
3.3 Comparison between GGA and van der Waals corrections
The most stable adsorption sites identified above were examined using the GGA-PAW functional. The computed adsorption energies and selected distances are shown in Table 4. The stability ordering of the configurations using GGA-PAW for Cl/Fe(100) configurations is the same as that presented in our previous study [9]. The recalculated GGA-PAW adsorption energies herein depart minimally from our previously computed values [9] in the narrow range of 0.01–0.11 eV. This is due to the number of surface layers of the Fe(100) surface for each study; the current study used six layers, while the previous study used five layers.
Values of E ads. and distance (R) using vdW-DF and GGA-PAW functional for chlorine atom adsorbates on the Fe(100) surface
| Site | Surface | vdW-DF | GGA-PAW | α | ||
|---|---|---|---|---|---|---|
| E ads.(eV) | (R) (Å) | E ads. (eV) | (R) (Å) | |||
| B (0.5 ML) | Cl/Fe(100) | −4.44 | 1.84 | −3.99 | 1.88 | 1.11 |
| H (0.5 ML) | −4.39 | 1.65 | −3.96 | 1.66 | 1.11 | |
| T (1 ML) | −3.08 | 2.11 | −2.73 | 2.11 | 1.13 | |
| B (1 ML) | −3.44 | 1.78 | −2.98 | 1.79 | 1.15 | |
For Fe(100) at 0.5 ML, the adsorption energy of the B and H configurations was found to increase by ∼0.45 and 0.43 eV, respectively, after the inclusion of van der Waals corrections. For T and B at full coverage, the corresponding adsorption energies increased by ∼0.35 and 0.46 eV, respectively. The inclusion of vdW interactions into standard DFT within the GGA brings a large increase in binding for closed-shell species, i.e., singlet-state molecules with surfaces. The enhancement factor, the calculated ratio of the adsorption energy with the inclusion of vdW to the adsorption energy without the inclusion of vdW
Considering the B (0.5 ML) configuration at the Cl/Fe(100) system as an example, the adsorption energy calculated by the vdW-DF functional overshoot calculated by the plain DFT method was a factor of 1.11. This rather small increase in the adsorption energy translates into a negligible difference in the measured height among the two methods at only 0.02%. This finding can now be attributed to the ionic nature of the formed Cl–Fe bonds. Nonlocal interaction increases the adsorption energy on both Fe surfaces and shrinks the equilibrium distance (Table 4). The overlap between the wavefunctions of the iron slab and the Cl atoms accounts for the movement of the electron to higher energy states, and the Cl atoms reach a shorter distance where the magnitude of the attractive force is larger, and the binding energy increases. An inconsistent influence of the inclusion of the vdW function (on geometries and binding energies) with the degree of chlorination most likely originates from the repulsion between electronegatively charged chlorine atoms at high coverages.
3.4 Electronic analysis
The electronic interaction between chlorine and the Fe(100) surface was examined by calculating the differential charge density distribution of the most thermodynamically stable structure (B-0.5 ML). As chlorine adsorbed on the Fe(100) surface, in B (0.5 ML) site, a large degree of charge transfer takes place between Cl atoms and the surface Fe atoms using both GGA-PAW and van der Waals correction functions (Figure 4). Charge transfer from the surface to the adsorbate has significantly decreased using the van der Waals correction function in comparison to that obtained using the GGA function. The weak electrostatic interaction and dispersion forces contribute to the adsorption of Cl on the Fe(100) surface. According to the comparison between the above two functions, the charge that accumulates because of the electrostatic effect is larger and extends over a wider domain. Atomic partial charges for selected atoms in the B (0.5 ML) structure are presented in Figure 5. Across most of the investigated configurations, surface iron atoms are reduced by ∼0.2 e, while adsorbed chlorine atoms entail a net negative charge of 0.3 e.
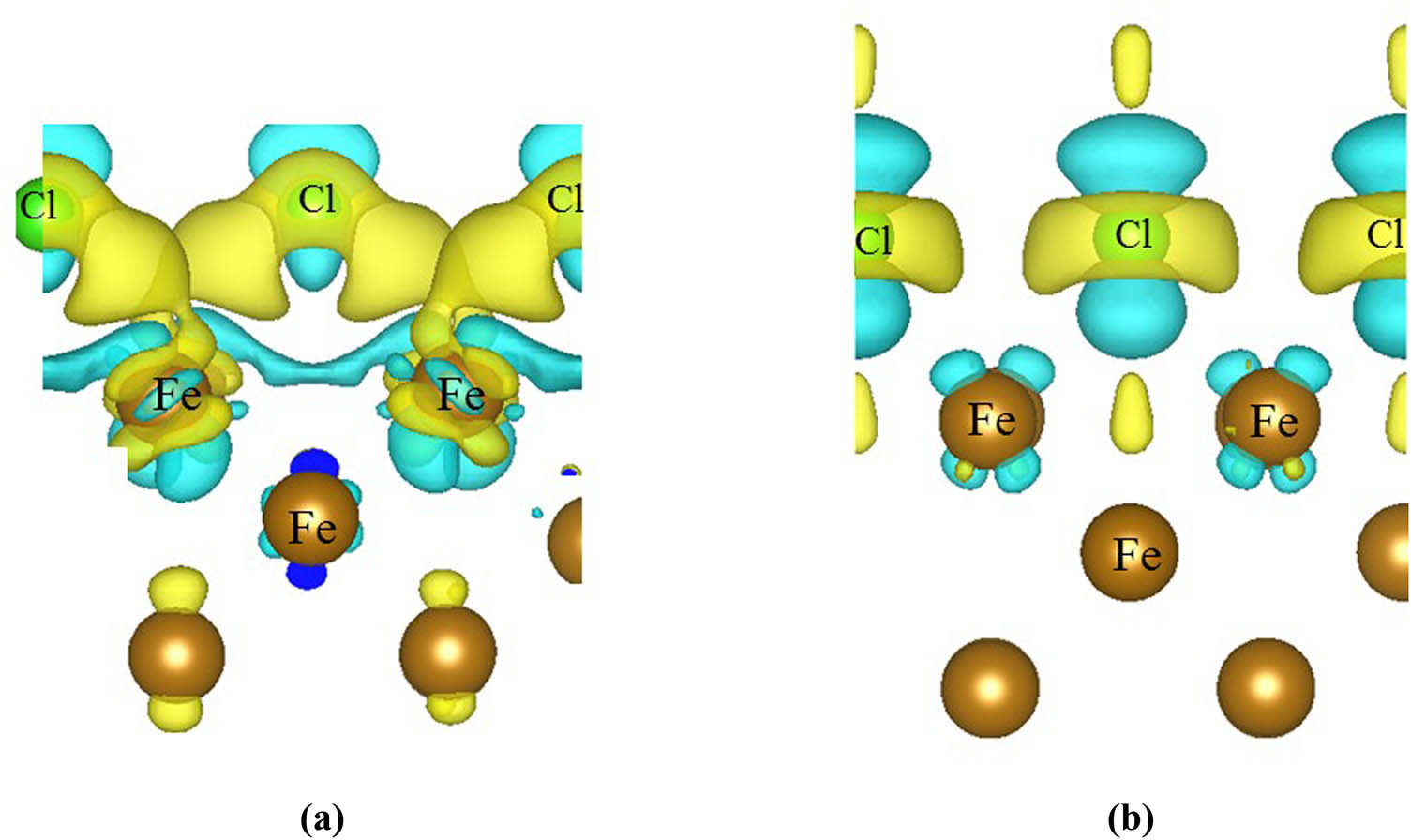
Charge density difference of Cl adsorbed on the Fe(100) surface in the B (0.5 ML) site. The isosurface was set as 0.005 e/Å3. The yellow and blue areas represent a gain and a loss in charge, respectively. The brown and light green spheres represent Fe and Cl atoms, respectively. (a) GGA and (b) vdW-DF.
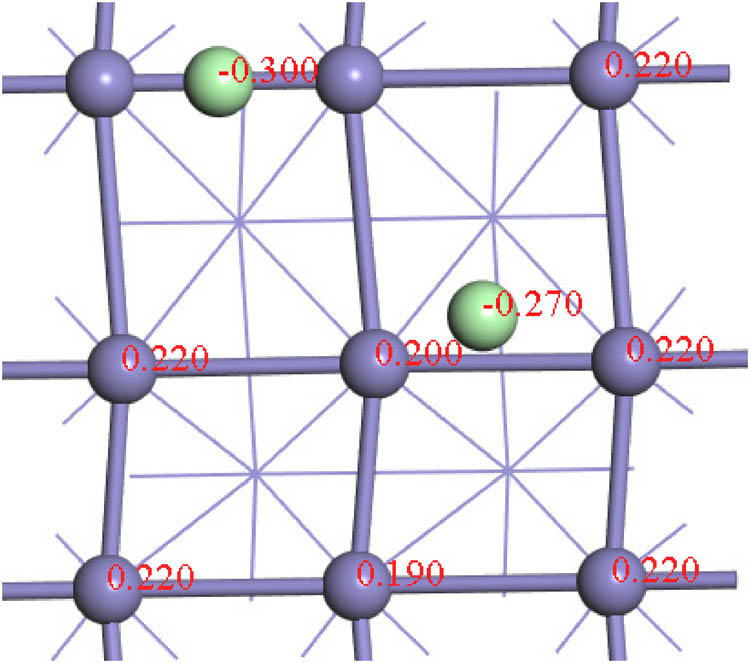
Atomic charges (in e) at selected atoms in the B (0.5 ML) configuration.
The work function (Φ) represents an important electronic fingerprint halogen/iron system. Through combined AES-LEED experiments, Yang et al. [33] observed a noticeable change in the work function of Fe(100) and Fe(110) surfaces upon bromine adsorption. The addition of a bromine atom was shown to decrease the work function of the Fe(110) surface while increasing it for Fe(100). The variation in Φ was primarily attributed to a surface charge redistribution that creates an inward pointing dipole. The latter prevails more in the open Fe(100) surface and is sustained by a larger probability of subsurface bromine penetration in reference to the more compact Fe(110) surface. Along the same note of enquiry, the inconsistent change in the work function among halogen (Br/I) –iron configurations prompted Wu and Klepeis [34] to propose a multidipole model in which the change in the work function is governed by the competition between dipoles in different directions. Chen et al. [35] illustrated a positive correlation between the changes in vertical dipole moments (with respect to the degree of sulfur coverage above the Fe(100) surface) and that of the work function. Our calculated Φ values for unsubstituted Fe(100) and Fe(110) surfaces amount to 3.92 and 4.09 eV, respectively. These values are in accord with analogous experimental and theoretical values [36,37,38]. To the best of our knowledge, the literature presents no account of the values of work functions for Cl–iron systems. Figure 6 contrasts Φ values between pure iron surfaces and selected Cl–Fe structures. Dissociation of a chlorine molecule over the Fe(100) surface increases its Φ value by 0.67 eV, while chlorine addition decreases the work function in reference to the pure Fe(110) surface. This trend prevails in bromine addition to the iron surfaces, as in previous studies [34].
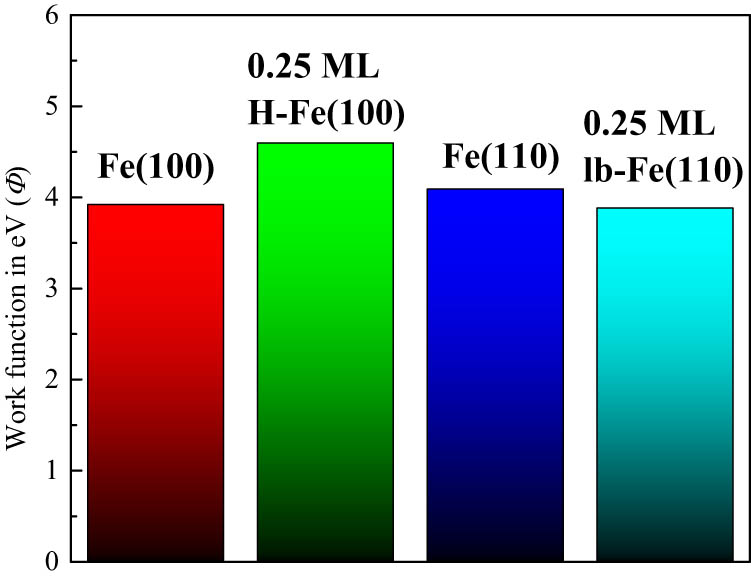
Values of the work function for the Fe(100)/Fe(110) surface and selected Cl–iron structures.
4 Conclusions
In this study, DFT–vdW calculations were performed to determine the geometrical features and thermodynamic and kinetic parameters for the adsorption of chlorine on the Fe(100) surface. The obtained activation energy for the dissociative adsorption of the chlorine molecule, along with the density of active sites on the Fe(100) surface, was used to develop a reaction constant and reaction rate for the initial uptake of gaseous chlorine molecules by the Fe(100) surface. As a result of the ionic character of the interaction between chlorine and iron atoms, the enhancement factor characterizing the influence of the vdW functional resides around unity. Chlorine adsorption reduces the work function of the Fe(100) surface. Future research directions may include mapping out the mechanism for the growth of iron chlorides from the subsurface adsorption of chlorine atoms into the iron bulk.
Acknowledgements
The authors acknowledge access to High Performance Supercomputing facility at the United Arab Emirates University (UAEU).
-
Funding information: M.A. acknowledges a UPAR grant from the UAEU (grant ID: 21N225).
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
References
[1] Dowben P, Jones R. Halogen adsorption on Fe(100): I. The adsorption of Cl2 studied by AES, LEED, work function change and thermal desorption. Surf Sci. 1979;84(2):449–61.10.1016/0039-6028(79)90148-1Search in Google Scholar
[2] Hino S, Lambert R. Chlorine chemisorption and surface chloride formation on iron: adsorption/desorption and photoelectron spectroscopy. Langmuir. 1986;2(2):147–50.10.1021/la00068a005Search in Google Scholar
[3] Li X, Binnemans K. Oxidative dissolution of metals in organic solvents. Chem Rev. 2021;121(8):4506–30.10.1021/acs.chemrev.0c00917Search in Google Scholar PubMed PubMed Central
[4] Altarawneh M, Saeed A, Al-Harahsheh M, Dlugogorski MBZ. Thermal decomposition of brominated flame retardants (BFRs): products and mechanisms. Prog Energ Combust. 2019;70:212–59.10.1016/j.pecs.2018.10.004Search in Google Scholar
[5] Khaing Aye K, Nguyen TD, Zhang J, Young DJ. Effect of silicon on corrosion of Fe-20Cr and Fe-20Cr-20Ni alloys in wet CO2 with and without HCl at 650°C. Corros Sci. 2021;179:109096.10.1016/j.corsci.2020.109096Search in Google Scholar
[6] Sui J, Lehmusto J, Bergelin MI, Hupa L. Initial oxidation mechanisms of stainless steel Sanicro 28 (35Fe27Cr31Ni) exposed to KCl, NaCl, and K2CO3 under dry and humid conditions at 535°C. Corros Sci. 2021;155:29–45.10.1016/j.corsci.2019.04.010Search in Google Scholar
[7] Pick Š. Comparison of chlorine and oxygen adsorption on the ferromagnetic Fe(001) surface: density-functional theory study. Surf Sci. 2008;602(24):3733–6.10.1016/j.susc.2008.10.017Search in Google Scholar
[8] Zhao W, Wang J, Liu F, Chen D. Equilibrium geometric structure and electronic properties of Cl and H2O co-adsorption on Fe(100) surface. Sci Bull. 2009;54(8):1295–301.10.1007/s11434-009-0199-ySearch in Google Scholar
[9] Altarawneh M, Saraireh SA. Theoretical insight into chlorine adsorption on the Fe(100) surface. Phys Chem Chem Phys. 2014;16(18):8575.10.1039/C4CP00220BSearch in Google Scholar PubMed
[10] Kannemann FO, Becke AD. van der Waals interactions in density-functional theory: intermolecular complexes. J Chem Theory Comput. 2010;6(4):1081–8.10.1021/ct900699rSearch in Google Scholar
[11] Dion M, Rydberg H, Schröder E, Langreth DC, Lundqvist BI. Van der Waals density functional for general geometries. Phys Rev Lett. 2004;92(24):246401.10.1103/PhysRevLett.92.246401Search in Google Scholar
[12] Berland K, Cooper VR, Lee K, Schröder E, Thonhauser T, Hyldgaard P, et al. van der Waals forces in density functional theory: a review of the vdW-DF method. Rep Prog Phys. 2015;78(6):066501.10.1088/0034-4885/78/6/066501Search in Google Scholar
[13] Blöchl PE. Projector augmented-wave method. Phys Rev B. 1994;50(24):17953.10.1103/PhysRevB.50.17953Search in Google Scholar
[14] Kresse G, Joubert D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys Rev. 1999;59(3):1758.10.1103/PhysRevB.59.1758Search in Google Scholar
[15] Perdew JP, Chevary JA, Vosko SH, Jackson KA, Pederson MR, Singh DJ, et al. Atoms, molecules, solids, and surfaces: applications of the generalized gradient approximation for exchange and correlation. Phys Rev B. 1993;48:4978.10.1103/PhysRevB.46.6671Search in Google Scholar
[16] Kresse G, Hafner J. Ab initio molecular dynamics for liquid metals. Phys Rev B. 1993;47(1):558.10.1016/0022-3093(95)00355-XSearch in Google Scholar
[17] Kresse G, Furthmüller J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput Mater Sci. 1996;6(1):15–50.10.1016/0927-0256(96)00008-0Search in Google Scholar
[18] Lin SY, Tran NTT, Lin MF. Diversified phenomena in metal- and transition-metal-adsorbed graphene nanoribbons. Nanomaterials. 2021;11(3):630.10.3390/nano11030630Search in Google Scholar PubMed PubMed Central
[19] Monkhorst HJ, Pack JD. Special points for brillouin-zone integrations. Phys Rev B. 1976;13(12):5188.10.1103/PhysRevB.13.5188Search in Google Scholar
[20] Tang W, Sanville E, Henkelman GA. grid-based bader analysis algorithm without lattice bias. J Condens Matter Phys. 2009;21(8):084204.10.1088/0953-8984/21/8/084204Search in Google Scholar PubMed
[21] Sheppard D, Terrell R, Henkelman G. Optimization methods for finding minimum energy paths. J Chem Phys. 2008;128(13):134106.10.1063/1.2841941Search in Google Scholar PubMed
[22] Kittel C, McEuen P. Introduction to solid state physics. New York: Wiley; 2004.Search in Google Scholar
[23] Nguyen TQ, Sato K, Shibutani Y. First-principles study of BCC/FCC phase transition promoted by interstitial carbon in iron. Mater Trans. 2018;59(6):870–5.10.2320/matertrans.M2018014Search in Google Scholar
[24] Zhong W, Overney G, Toma D. Structural properties of Fe crystals. Phys Rev B. 1993;47(1):95.10.1103/PhysRevB.47.95Search in Google Scholar PubMed
[25] Jona F, Legg K, Shih H, Jepsen D, Marcus P. Random occupation of adsorption sites in the c(2 × 2) structure of CO on Fe{001}. Phys Rev Lett. 1978;40(22):1466.10.1103/PhysRevLett.40.1466Search in Google Scholar
[26] Eder M, Terakura, K, Hafner J. Initial stages of oxidation of (100) and (110) surfaces of iron caused by water. Phys Rev B. 2001;64(11):115426.10.1103/PhysRevB.64.115426Search in Google Scholar
[27] Alde M, Mirbt S, Skriver HL, Rosengaard N, Johansson BJ. Surface magnetism in iron, cobalt, and nickel. Phys Rev B. 1992;46(10):6303.10.1103/PhysRevB.46.6303Search in Google Scholar PubMed
[28] Poberžnik M, Kokalj A. Surprising lateral interactions between negatively charged adatoms on metal surfaces. J Phys Chem Lett. 2020;11(17):7122–6.10.1021/acs.jpclett.0c01373Search in Google Scholar PubMed PubMed Central
[29] Saraireh SA, Altarawneh M. Thermodynamic stability and structures of iron chloride surfaces: a first-principles investigation. J Chem Phys. 2014;141(5):054709.10.1063/1.4891577Search in Google Scholar PubMed
[30] Cao W. Adsorption of surface active elements on the iron (100) surface, a study based on ab initio calculations. (PhD thesis). Sweden: Royal Institute of Technology; 2009.Search in Google Scholar
[31] Baroni S, Gironcoli Sde, Corso AD, Giannozzi P. Phonons and related crystal properties from density-functional perturbation theory. Rev Mod Phys. 2001;73:515.10.1103/RevModPhys.73.515Search in Google Scholar
[32] Stöhr M, Voorhis TV, Tkatchenko A. Theory and practice of modeling van der Waals interactions in electronic-structure calculation. Chem Soc Rev. 2019;48:4118–54.10.1039/C9CS00060GSearch in Google Scholar
[33] Yang Y, Sroubek Z, Yarmoff J. Internal electronic structure of adatoms on Fe(110) and Fe(100) surfaces: a low-energy Li + scattering study. Phys Rev B. 2004;69(4):045420.10.1103/PhysRevB.69.045420Search in Google Scholar
[34] Wu CJ, Klepeis JE. Halogen adsorption on transition-metal surfaces: a case study of Cl on Ta(110). Phys Rev B. 1997;55(16):10848.10.1103/PhysRevB.55.10848Search in Google Scholar
[35] Chen H, Li B, Wen B, Ye Q, Zhang N. Corrosion resistance of iron-chromium-aluminium steel in eutectic molten salts under thermal cycling conditions. Corros Sci. 2020;173:108798.10.1016/j.corsci.2020.108798Search in Google Scholar
[36] Pirug G, Brodén G, Bonzel H. Coadsorption of potassium and oxygen on Fe(110). Surf Sci. 1980;94(2–3):323–38.10.1016/0039-6028(80)90010-2Search in Google Scholar
[37] Nelson S, Spencer M, Snook I, Yarovsky I. Effect of S contamination on properties of Fe(100) surfaces. Surf Sci. 2005;590(1):63–75.10.1016/j.susc.2005.06.014Search in Google Scholar
[38] Ueda K, Shimizu R. LEED-work function studies on Fe (100). Jpn J Appl Phys. 1972;11(6):916.10.1143/JJAP.11.916Search in Google Scholar
© 2021 Sherin A. Saraireh et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Improved impedance matching by multi-componential metal-hybridized rGO toward high performance of microwave absorption
- Pure-silk fibroin hydrogel with stable aligned micropattern toward peripheral nerve regeneration
- Effective ion pathways and 3D conductive carbon networks in bentonite host enable stable and high-rate lithium–sulfur batteries
- Fabrication and characterization of 3D-printed gellan gum/starch composite scaffold for Schwann cells growth
- Synergistic strengthening mechanism of copper matrix composite reinforced with nano-Al2O3 particles and micro-SiC whiskers
- Deformation mechanisms and plasticity of ultrafine-grained Al under complex stress state revealed by digital image correlation technique
- On the deformation-induced grain rotations in gradient nano-grained copper based on molecular dynamics simulations
- Removal of sulfate from aqueous solution using Mg–Al nano-layered double hydroxides synthesized under different dual solvent systems
- Microwave-assisted sol–gel synthesis of TiO2-mixed metal oxide nanocatalyst for degradation of organic pollutant
- Electrophoretic deposition of graphene on basalt fiber for composite applications
- Polyphenylene sulfide-coated wrench composites by nanopinning effect
- Thermal conductivity and thermoelectric properties in 3D macroscopic pure carbon nanotube materials
- An effective thermal conductivity and thermomechanical homogenization scheme for a multiscale Nb3Sn filaments
- Friction stir spot welding of AA5052 with additional carbon fiber-reinforced polymer composite interlayer
- Improvement of long-term cycling performance of high-nickel cathode materials by ZnO coating
- Quantum effects of gas flow in nanochannels
- An approach to effectively improve the interfacial bonding of nano-perfused composites by in situ growth of CNTs
- Effects of nano-modified polymer cement-based materials on the bending behavior of repaired concrete beams
- Effects of the combined usage of nanomaterials and steel fibres on the workability, compressive strength, and microstructure of ultra-high performance concrete
- One-pot solvothermal synthesis and characterization of highly stable nickel nanoparticles
- Comparative study on mechanisms for improving mechanical properties and microstructure of cement paste modified by different types of nanomaterials
- Effect of in situ graphene-doped nano-CeO2 on microstructure and electrical contact properties of Cu30Cr10W contacts
- The experimental study of CFRP interlayer of dissimilar joint AA7075-T651/Ti-6Al-4V alloys by friction stir spot welding on mechanical and microstructural properties
- Vibration analysis of a sandwich cylindrical shell in hygrothermal environment
- Water barrier and mechanical properties of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch (TPS)/poly(lactic acid) (PLA) blend bionanocomposites
- Strong quadratic acousto-optic coupling in 1D multilayer phoxonic crystal cavity
- Three-dimensional shape analysis of peripapillary retinal pigment epithelium-basement membrane layer based on OCT radial images
- Solvent regulation synthesis of single-component white emission carbon quantum dots for white light-emitting diodes
- Xanthate-modified nanoTiO2 as a novel vulcanization accelerator enhancing mechanical and antibacterial properties of natural rubber
- Effect of steel fiber on impact resistance and durability of concrete containing nano-SiO2
- Ultrasound-enhanced biosynthesis of uniform ZnO nanorice using Swietenia macrophylla seed extract and its in vitro anticancer activity
- Temperature dependence of hardness prediction for high-temperature structural ceramics and their composites
- Study on the frequency of acoustic emission signal during crystal growth of salicylic acid
- Controllable modification of helical carbon nanotubes for high-performance microwave absorption
- Role of dry ozonization of basalt fibers on interfacial properties and fracture toughness of epoxy matrix composites
- Nanosystem’s density functional theory study of the chlorine adsorption on the Fe(100) surface
- A rapid nanobiosensing platform based on herceptin-conjugated graphene for ultrasensitive detection of circulating tumor cells in early breast cancer
- Improving flexural strength of UHPC with sustainably synthesized graphene oxide
- The role of graphene/graphene oxide in cement hydration
- Structural characterization of microcrystalline and nanocrystalline cellulose from Ananas comosus L. leaves: Cytocompatibility and molecular docking studies
- Evaluation of the nanostructure of calcium silicate hydrate based on atomic force microscopy-infrared spectroscopy experiments
- Combined effects of nano-silica and silica fume on the mechanical behavior of recycled aggregate concrete
- Safety study of malapposition of the bio-corrodible nitrided iron stent in vivo
- Triethanolamine interface modification of crystallized ZnO nanospheres enabling fast photocatalytic hazard-free treatment of Cr(vi) ions
- Novel electrodes for precise and accurate droplet dispensing and splitting in digital microfluidics
- Construction of Chi(Zn/BMP2)/HA composite coating on AZ31B magnesium alloy surface to improve the corrosion resistance and biocompatibility
- Experimental and multiscale numerical investigations on low-velocity impact responses of syntactic foam composites reinforced with modified MWCNTs
- Comprehensive performance analysis and optimal design of smart light pole for cooperative vehicle infrastructure system
- Room temperature growth of ZnO with highly active exposed facets for photocatalytic application
- Influences of poling temperature and elongation ratio on PVDF-HFP piezoelectric films
- Large strain hardening of magnesium containing in situ nanoparticles
- Super stable water-based magnetic fluid as a dual-mode contrast agent
- Photocatalytic activity of biogenic zinc oxide nanoparticles: In vitro antimicrobial, biocompatibility, and molecular docking studies
- Hygrothermal environment effect on the critical buckling load of FGP microbeams with initial curvature integrated by CNT-reinforced skins considering the influence of thickness stretching
- Thermal aging behavior characteristics of asphalt binder modified by nano-stabilizer based on DSR and AFM
- Building effective core/shell polymer nanoparticles for epoxy composite toughening based on Hansen solubility parameters
- Structural characterization and nanoscale strain field analysis of α/β interface layer of a near α titanium alloy
- Optimization of thermal and hydrophobic properties of GO-doped epoxy nanocomposite coatings
- The properties of nano-CaCO3/nano-ZnO/SBR composite-modified asphalt
- Three-dimensional metallic carbon allotropes with superhardness
- Physical stability and rheological behavior of Pickering emulsions stabilized by protein–polysaccharide hybrid nanoconjugates
- Optimization of volume fraction and microstructure evolution during thermal deformation of nano-SiCp/Al–7Si composites
- Phase analysis and corrosion behavior of brazing Cu/Al dissimilar metal joint with BAl88Si filler metal
- High-efficiency nano polishing of steel materials
- On the rheological properties of multi-walled carbon nano-polyvinylpyrrolidone/silicon-based shear thickening fluid
- Fabrication of Ag/ZnO hollow nanospheres and cubic TiO2/ZnO heterojunction photocatalysts for RhB degradation
- Fabrication and properties of PLA/nano-HA composite scaffolds with balanced mechanical properties and biological functions for bone tissue engineering application
- Investigation of the early-age performance and microstructure of nano-C–S–H blended cement-based materials
- Reduced graphene oxide coating on basalt fabric using electrophoretic deposition and its role in the mechanical and tribological performance of epoxy/basalt fiber composites
- Effect of nano-silica as cementitious materials-reducing admixtures on the workability, mechanical properties and durability of concrete
- Machine-learning-assisted microstructure–property linkages of carbon nanotube-reinforced aluminum matrix nanocomposites produced by laser powder bed fusion
- Physical, thermal, and mechanical properties of highly porous polylactic acid/cellulose nanofibre scaffolds prepared by salt leaching technique
- A comparative study on characterizations and synthesis of pure lead sulfide (PbS) and Ag-doped PbS for photovoltaic applications
- Clean preparation of washable antibacterial polyester fibers by high temperature and high pressure hydrothermal self-assembly
- Al 5251-based hybrid nanocomposite by FSP reinforced with graphene nanoplates and boron nitride nanoparticles: Microstructure, wear, and mechanical characterization
- Interlaminar fracture toughness properties of hybrid glass fiber-reinforced composite interlayered with carbon nanotube using electrospray deposition
- Microstructure and life prediction model of steel slag concrete under freezing-thawing environment
- Synthesis of biogenic silver nanoparticles from the seed coat waste of pistachio (Pistacia vera) and their effect on the growth of eggplant
- Study on adaptability of rheological index of nano-PUA-modified asphalt based on geometric parameters of parallel plate
- Preparation and adsorption properties of nano-graphene oxide/tourmaline composites
- A study on interfacial behaviors of epoxy/graphene oxide derived from pitch-based graphite fibers
- Multiresponsive carboxylated graphene oxide-grafted aptamer as a multifunctional nanocarrier for targeted delivery of chemotherapeutics and bioactive compounds in cancer therapy
- Piezoresistive/piezoelectric intrinsic sensing properties of carbon nanotube cement-based smart composite and its electromechanical sensing mechanisms: A review
- Smart stimuli-responsive biofunctionalized niosomal nanocarriers for programmed release of bioactive compounds into cancer cells in vitro and in vivo
- Photoremediation of methylene blue by biosynthesized ZnO/Fe3O4 nanocomposites using Callistemon viminalis leaves aqueous extract: A comparative study
- Study of gold nanoparticles’ preparation through ultrasonic spray pyrolysis and lyophilisation for possible use as markers in LFIA tests
- Review Articles
- Advance on the dispersion treatment of graphene oxide and the graphene oxide modified cement-based materials
- Development of ionic liquid-based electroactive polymer composites using nanotechnology
- Nanostructured multifunctional electrocatalysts for efficient energy conversion systems: Recent perspectives
- Recent advances on the fabrication methods of nanocomposite yarn-based strain sensor
- Review on nanocomposites based on aerospace applications
- Overview of nanocellulose as additives in paper processing and paper products
- The frontiers of functionalized graphene-based nanocomposites as chemical sensors
- Material advancement in tissue-engineered nerve conduit
- Carbon nanostructure-based superhydrophobic surfaces and coatings
- Functionalized graphene-based nanocomposites for smart optoelectronic applications
- Interfacial technology for enhancement in steel fiber reinforced cementitious composite from nano to macroscale
- Metal nanoparticles and biomaterials: The multipronged approach for potential diabetic wound therapy
- Review on resistive switching mechanisms of bio-organic thin film for non-volatile memory application
- Nanotechnology-enabled biomedical engineering: Current trends, future scopes, and perspectives
- Research progress on key problems of nanomaterials-modified geopolymer concrete
- Smart stimuli-responsive nanocarriers for the cancer therapy – nanomedicine
- An overview of methods for production and detection of silver nanoparticles, with emphasis on their fate and toxicological effects on human, soil, and aquatic environment
- Effects of chemical modification and nanotechnology on wood properties
- Mechanisms, influencing factors, and applications of electrohydrodynamic jet printing
- Application of antiviral materials in textiles: A review
- Phase transformation and strengthening mechanisms of nanostructured high-entropy alloys
- Research progress on individual effect of graphene oxide in cement-based materials and its synergistic effect with other nanomaterials
- Catalytic defense against fungal pathogens using nanozymes
- A mini-review of three-dimensional network topological structure nanocomposites: Preparation and mechanical properties
- Mechanical properties and structural health monitoring performance of carbon nanotube-modified FRP composites: A review
- Nano-scale delivery: A comprehensive review of nano-structured devices, preparative techniques, site-specificity designs, biomedical applications, commercial products, and references to safety, cellular uptake, and organ toxicity
- Effects of alloying, heat treatment and nanoreinforcement on mechanical properties and damping performances of Cu–Al-based alloys: A review
- Recent progress in the synthesis and applications of vertically aligned carbon nanotube materials
- Thermal conductivity and dynamic viscosity of mono and hybrid organic- and synthetic-based nanofluids: A critical review
- Recent advances in waste-recycled nanomaterials for biomedical applications: Waste-to-wealth
- Layup sequence and interfacial bonding of additively manufactured polymeric composite: A brief review
- Quantum dots synthetization and future prospect applications
- Approved and marketed nanoparticles for disease targeting and applications in COVID-19
- Strategies for improving rechargeable lithium-ion batteries: From active materials to CO2 emissions
Articles in the same Issue
- Research Articles
- Improved impedance matching by multi-componential metal-hybridized rGO toward high performance of microwave absorption
- Pure-silk fibroin hydrogel with stable aligned micropattern toward peripheral nerve regeneration
- Effective ion pathways and 3D conductive carbon networks in bentonite host enable stable and high-rate lithium–sulfur batteries
- Fabrication and characterization of 3D-printed gellan gum/starch composite scaffold for Schwann cells growth
- Synergistic strengthening mechanism of copper matrix composite reinforced with nano-Al2O3 particles and micro-SiC whiskers
- Deformation mechanisms and plasticity of ultrafine-grained Al under complex stress state revealed by digital image correlation technique
- On the deformation-induced grain rotations in gradient nano-grained copper based on molecular dynamics simulations
- Removal of sulfate from aqueous solution using Mg–Al nano-layered double hydroxides synthesized under different dual solvent systems
- Microwave-assisted sol–gel synthesis of TiO2-mixed metal oxide nanocatalyst for degradation of organic pollutant
- Electrophoretic deposition of graphene on basalt fiber for composite applications
- Polyphenylene sulfide-coated wrench composites by nanopinning effect
- Thermal conductivity and thermoelectric properties in 3D macroscopic pure carbon nanotube materials
- An effective thermal conductivity and thermomechanical homogenization scheme for a multiscale Nb3Sn filaments
- Friction stir spot welding of AA5052 with additional carbon fiber-reinforced polymer composite interlayer
- Improvement of long-term cycling performance of high-nickel cathode materials by ZnO coating
- Quantum effects of gas flow in nanochannels
- An approach to effectively improve the interfacial bonding of nano-perfused composites by in situ growth of CNTs
- Effects of nano-modified polymer cement-based materials on the bending behavior of repaired concrete beams
- Effects of the combined usage of nanomaterials and steel fibres on the workability, compressive strength, and microstructure of ultra-high performance concrete
- One-pot solvothermal synthesis and characterization of highly stable nickel nanoparticles
- Comparative study on mechanisms for improving mechanical properties and microstructure of cement paste modified by different types of nanomaterials
- Effect of in situ graphene-doped nano-CeO2 on microstructure and electrical contact properties of Cu30Cr10W contacts
- The experimental study of CFRP interlayer of dissimilar joint AA7075-T651/Ti-6Al-4V alloys by friction stir spot welding on mechanical and microstructural properties
- Vibration analysis of a sandwich cylindrical shell in hygrothermal environment
- Water barrier and mechanical properties of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch (TPS)/poly(lactic acid) (PLA) blend bionanocomposites
- Strong quadratic acousto-optic coupling in 1D multilayer phoxonic crystal cavity
- Three-dimensional shape analysis of peripapillary retinal pigment epithelium-basement membrane layer based on OCT radial images
- Solvent regulation synthesis of single-component white emission carbon quantum dots for white light-emitting diodes
- Xanthate-modified nanoTiO2 as a novel vulcanization accelerator enhancing mechanical and antibacterial properties of natural rubber
- Effect of steel fiber on impact resistance and durability of concrete containing nano-SiO2
- Ultrasound-enhanced biosynthesis of uniform ZnO nanorice using Swietenia macrophylla seed extract and its in vitro anticancer activity
- Temperature dependence of hardness prediction for high-temperature structural ceramics and their composites
- Study on the frequency of acoustic emission signal during crystal growth of salicylic acid
- Controllable modification of helical carbon nanotubes for high-performance microwave absorption
- Role of dry ozonization of basalt fibers on interfacial properties and fracture toughness of epoxy matrix composites
- Nanosystem’s density functional theory study of the chlorine adsorption on the Fe(100) surface
- A rapid nanobiosensing platform based on herceptin-conjugated graphene for ultrasensitive detection of circulating tumor cells in early breast cancer
- Improving flexural strength of UHPC with sustainably synthesized graphene oxide
- The role of graphene/graphene oxide in cement hydration
- Structural characterization of microcrystalline and nanocrystalline cellulose from Ananas comosus L. leaves: Cytocompatibility and molecular docking studies
- Evaluation of the nanostructure of calcium silicate hydrate based on atomic force microscopy-infrared spectroscopy experiments
- Combined effects of nano-silica and silica fume on the mechanical behavior of recycled aggregate concrete
- Safety study of malapposition of the bio-corrodible nitrided iron stent in vivo
- Triethanolamine interface modification of crystallized ZnO nanospheres enabling fast photocatalytic hazard-free treatment of Cr(vi) ions
- Novel electrodes for precise and accurate droplet dispensing and splitting in digital microfluidics
- Construction of Chi(Zn/BMP2)/HA composite coating on AZ31B magnesium alloy surface to improve the corrosion resistance and biocompatibility
- Experimental and multiscale numerical investigations on low-velocity impact responses of syntactic foam composites reinforced with modified MWCNTs
- Comprehensive performance analysis and optimal design of smart light pole for cooperative vehicle infrastructure system
- Room temperature growth of ZnO with highly active exposed facets for photocatalytic application
- Influences of poling temperature and elongation ratio on PVDF-HFP piezoelectric films
- Large strain hardening of magnesium containing in situ nanoparticles
- Super stable water-based magnetic fluid as a dual-mode contrast agent
- Photocatalytic activity of biogenic zinc oxide nanoparticles: In vitro antimicrobial, biocompatibility, and molecular docking studies
- Hygrothermal environment effect on the critical buckling load of FGP microbeams with initial curvature integrated by CNT-reinforced skins considering the influence of thickness stretching
- Thermal aging behavior characteristics of asphalt binder modified by nano-stabilizer based on DSR and AFM
- Building effective core/shell polymer nanoparticles for epoxy composite toughening based on Hansen solubility parameters
- Structural characterization and nanoscale strain field analysis of α/β interface layer of a near α titanium alloy
- Optimization of thermal and hydrophobic properties of GO-doped epoxy nanocomposite coatings
- The properties of nano-CaCO3/nano-ZnO/SBR composite-modified asphalt
- Three-dimensional metallic carbon allotropes with superhardness
- Physical stability and rheological behavior of Pickering emulsions stabilized by protein–polysaccharide hybrid nanoconjugates
- Optimization of volume fraction and microstructure evolution during thermal deformation of nano-SiCp/Al–7Si composites
- Phase analysis and corrosion behavior of brazing Cu/Al dissimilar metal joint with BAl88Si filler metal
- High-efficiency nano polishing of steel materials
- On the rheological properties of multi-walled carbon nano-polyvinylpyrrolidone/silicon-based shear thickening fluid
- Fabrication of Ag/ZnO hollow nanospheres and cubic TiO2/ZnO heterojunction photocatalysts for RhB degradation
- Fabrication and properties of PLA/nano-HA composite scaffolds with balanced mechanical properties and biological functions for bone tissue engineering application
- Investigation of the early-age performance and microstructure of nano-C–S–H blended cement-based materials
- Reduced graphene oxide coating on basalt fabric using electrophoretic deposition and its role in the mechanical and tribological performance of epoxy/basalt fiber composites
- Effect of nano-silica as cementitious materials-reducing admixtures on the workability, mechanical properties and durability of concrete
- Machine-learning-assisted microstructure–property linkages of carbon nanotube-reinforced aluminum matrix nanocomposites produced by laser powder bed fusion
- Physical, thermal, and mechanical properties of highly porous polylactic acid/cellulose nanofibre scaffolds prepared by salt leaching technique
- A comparative study on characterizations and synthesis of pure lead sulfide (PbS) and Ag-doped PbS for photovoltaic applications
- Clean preparation of washable antibacterial polyester fibers by high temperature and high pressure hydrothermal self-assembly
- Al 5251-based hybrid nanocomposite by FSP reinforced with graphene nanoplates and boron nitride nanoparticles: Microstructure, wear, and mechanical characterization
- Interlaminar fracture toughness properties of hybrid glass fiber-reinforced composite interlayered with carbon nanotube using electrospray deposition
- Microstructure and life prediction model of steel slag concrete under freezing-thawing environment
- Synthesis of biogenic silver nanoparticles from the seed coat waste of pistachio (Pistacia vera) and their effect on the growth of eggplant
- Study on adaptability of rheological index of nano-PUA-modified asphalt based on geometric parameters of parallel plate
- Preparation and adsorption properties of nano-graphene oxide/tourmaline composites
- A study on interfacial behaviors of epoxy/graphene oxide derived from pitch-based graphite fibers
- Multiresponsive carboxylated graphene oxide-grafted aptamer as a multifunctional nanocarrier for targeted delivery of chemotherapeutics and bioactive compounds in cancer therapy
- Piezoresistive/piezoelectric intrinsic sensing properties of carbon nanotube cement-based smart composite and its electromechanical sensing mechanisms: A review
- Smart stimuli-responsive biofunctionalized niosomal nanocarriers for programmed release of bioactive compounds into cancer cells in vitro and in vivo
- Photoremediation of methylene blue by biosynthesized ZnO/Fe3O4 nanocomposites using Callistemon viminalis leaves aqueous extract: A comparative study
- Study of gold nanoparticles’ preparation through ultrasonic spray pyrolysis and lyophilisation for possible use as markers in LFIA tests
- Review Articles
- Advance on the dispersion treatment of graphene oxide and the graphene oxide modified cement-based materials
- Development of ionic liquid-based electroactive polymer composites using nanotechnology
- Nanostructured multifunctional electrocatalysts for efficient energy conversion systems: Recent perspectives
- Recent advances on the fabrication methods of nanocomposite yarn-based strain sensor
- Review on nanocomposites based on aerospace applications
- Overview of nanocellulose as additives in paper processing and paper products
- The frontiers of functionalized graphene-based nanocomposites as chemical sensors
- Material advancement in tissue-engineered nerve conduit
- Carbon nanostructure-based superhydrophobic surfaces and coatings
- Functionalized graphene-based nanocomposites for smart optoelectronic applications
- Interfacial technology for enhancement in steel fiber reinforced cementitious composite from nano to macroscale
- Metal nanoparticles and biomaterials: The multipronged approach for potential diabetic wound therapy
- Review on resistive switching mechanisms of bio-organic thin film for non-volatile memory application
- Nanotechnology-enabled biomedical engineering: Current trends, future scopes, and perspectives
- Research progress on key problems of nanomaterials-modified geopolymer concrete
- Smart stimuli-responsive nanocarriers for the cancer therapy – nanomedicine
- An overview of methods for production and detection of silver nanoparticles, with emphasis on their fate and toxicological effects on human, soil, and aquatic environment
- Effects of chemical modification and nanotechnology on wood properties
- Mechanisms, influencing factors, and applications of electrohydrodynamic jet printing
- Application of antiviral materials in textiles: A review
- Phase transformation and strengthening mechanisms of nanostructured high-entropy alloys
- Research progress on individual effect of graphene oxide in cement-based materials and its synergistic effect with other nanomaterials
- Catalytic defense against fungal pathogens using nanozymes
- A mini-review of three-dimensional network topological structure nanocomposites: Preparation and mechanical properties
- Mechanical properties and structural health monitoring performance of carbon nanotube-modified FRP composites: A review
- Nano-scale delivery: A comprehensive review of nano-structured devices, preparative techniques, site-specificity designs, biomedical applications, commercial products, and references to safety, cellular uptake, and organ toxicity
- Effects of alloying, heat treatment and nanoreinforcement on mechanical properties and damping performances of Cu–Al-based alloys: A review
- Recent progress in the synthesis and applications of vertically aligned carbon nanotube materials
- Thermal conductivity and dynamic viscosity of mono and hybrid organic- and synthetic-based nanofluids: A critical review
- Recent advances in waste-recycled nanomaterials for biomedical applications: Waste-to-wealth
- Layup sequence and interfacial bonding of additively manufactured polymeric composite: A brief review
- Quantum dots synthetization and future prospect applications
- Approved and marketed nanoparticles for disease targeting and applications in COVID-19
- Strategies for improving rechargeable lithium-ion batteries: From active materials to CO2 emissions

