Abstract
C17H10O, monoclinic, P21/c (no. 14), a = 8.1312(16) Å, b = 7.8252(16) Å, c = 17.231(3) Å, β = 92.47(3)°, V = 1095.3(4) Å3, Z = 4, Rgt(F) = 0.0517, wRref(F2) = 0.1281, T = 293(2) K.
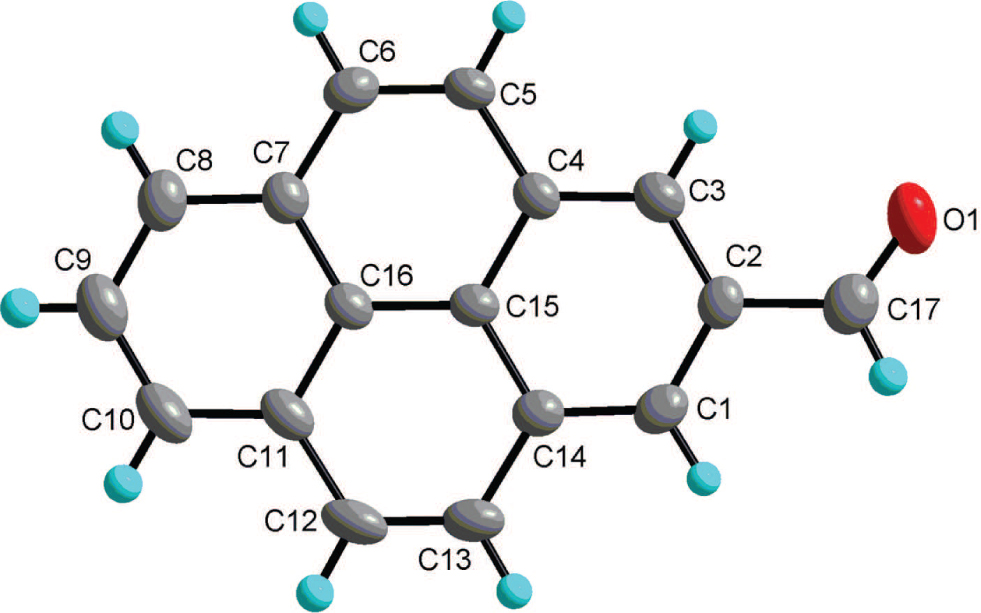
The crystal structure is shown in the figure. Tables 1 and 2 contain details on crystal structure and measurement conditions and a list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Yellow block |
| Size: | 0.15 × 0.14 × 0.12 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.09 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, ω-scans |
| θmax, completeness: | 26°, >98% |
| N(hkl)measured, N(hkl)unique, Rint: | 7793, 2121, 0.056 |
| Criterion for Iobs, N(hkl)gt: | Iobs >2 σ (Iobs), 1133 |
| N(param)refined: | 162 |
| Programs: | Bruker programs [1], SHELX [2] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| O1 | 0.9439(2) | 0.2050(2) | −0.10678(9) | 0.0507(5) |
| C1 | 0.5362(3) | 0.2907(2) | −0.04617(12) | 0.0326(6) |
| H1 | 0.5026 | 0.3529 | −0.0900 | 0.039* |
| C2 | 0.6942(3) | 0.2235(2) | −0.04090(12) | 0.0292(5) |
| C3 | 0.7474(3) | 0.1309(2) | 0.02406(12) | 0.0315(6) |
| H3 | 0.8537 | 0.0869 | 0.0269 | 0.038* |
| C4 | 0.6443(3) | 0.1027(2) | 0.08490(11) | 0.0268(5) |
| C5 | 0.6931(3) | 0.0038(2) | 0.15243(12) | 0.0288(5) |
| H5 | 0.7988 | −0.0418 | 0.1566 | 0.035* |
| C6 | 0.5892(3) | −0.0239(3) | 0.20932(12) | 0.0326(6) |
| H6 | 0.6252 | −0.0880 | 0.2522 | 0.039* |
| C7 | 0.4241(3) | 0.0420(2) | 0.20639(11) | 0.0300(5) |
| C8 | 0.3144(3) | 0.0129(3) | 0.26496(13) | 0.0384(6) |
| H8 | 0.3470 | −0.0537 | 0.3076 | 0.046* |
| C9 | 0.1562(3) | 0.0824(3) | 0.26035(14) | 0.0438(6) |
| H9 | 0.0848 | 0.0638 | 0.3002 | 0.053* |
| C10 | 0.1054(3) | 0.1797(3) | 0.19605(13) | 0.0406(6) |
| H10 | −0.0002 | 0.2257 | 0.1936 | 0.049* |
| C11 | 0.2095(3) | 0.2094(2) | 0.13542(13) | 0.0325(6) |
| C12 | 0.1593(3) | 0.3056(3) | 0.06735(14) | 0.0388(6) |
| H12 | 0.0533 | 0.3503 | 0.0631 | 0.047* |
| C13 | 0.2631(3) | 0.3320(3) | 0.00954(13) | 0.0345(6) |
| H13 | 0.2268 | 0.3947 | −0.0338 | 0.041* |
| C14 | 0.4269(3) | 0.2667(2) | 0.01291(12) | 0.0286(5) |
| C15 | 0.4810(3) | 0.1715(2) | 0.07997(12) | 0.0257(5) |
| C16 | 0.3715(3) | 0.1419(2) | 0.14055(11) | 0.0263(5) |
| C17 | 0.8042(3) | 0.2533(3) | −0.10544(14) | 0.0403(6) |
| H17 | 0.7620 | 0.3140 | −0.1482 | 0.048* |
Source of material
All chemicals were purchased from commercial sources and used as received without further purification. The title complex was prepared by two steps. The intermediate 2-formyl-4,5,9,10-tetrahydropyrene was prepared by the following procedure: To a stirred solution of 4,5,9,10-tetrahydropyrene (2.060 g, 10 mmol) and dichloromethyl methyl ether (1.495 g, 13 mmol) in CH2Cl2 (80 mL) was added at 0 °C a solution of titanium tetrachloride (10.95 mL, 100 mmol) in CH2Cl2 (20 mL). The mixture was stirred for 3 h at room temperature. The mixture was poured into ice-water and extracted with CH2Cl2 two times. The organic layer was washed with water, dried over MgSO4 and concentrated in vacuo. The residue was purified by silica gel chromatography using hexane/ CH2Cl2 as an eluent to afford 2.0358 g 2-formyl-4,5,9,10-tetrahydropyrene in 87% yield. 1H NMR (400 MHz, CDCl3) δ 9.98 (s, 1H), 7.62 (s, 2H), 7.23 (d, J = 6.8 Hz, 1H), 7.14 (d, J = 7.6 Hz, 2H), 2.97 (dd, J = 9.4, 3.6 Hz, 8H). GC/MS MS: (C17H14O) m/z 234(M+, 78), 216(18), 205(100), 189(38), 101(20).
The title compound was synthesized by dehydrogenation of the intermediate 2-formyl-4,5,9,10-tetrahydropyrene a solution of 2-formyl-4,5,9,10-tetrahydropyrene (1.170 g, 5 mmol) and 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (2.951 g, 13 mmol) in 100 mL of freshly-dried benzene was refluxed for two days. After removing the solvent by rotary evaporation, the residue was purified by silica gel chromatography using hexane/CH2Cl2 as an eluent to afford 1.081 g 2-pyrenyl aldehyde in 94% yield. 1H NMR (400 MHz, CDCl3) δ 10.47 (s, 1H), 8.68 (s, 2H), 8.27 (d, J = 7.6 Hz, 2H), 8.23–8.16 (m, 4H), 8.15–8.11 (m, 1H). GC—MS MS: (C17H10O) m/z 230(M+, 100), 201(95), 100(35). The yellow block crystals of the title compound were obtained by slow evaporation of hexane/CH2Cl2 solution (v:v = 1/1) and the selected crystal was structurally characterized by X-ray diffraction analysis.
Experimental details
All H atoms bond to C atoms were introduced using the HFIX commond in the SHELXL program [2], with the value of 0.93 Å or 0.96 Å for C—H bonds distances. All H atoms were allowed for as riding atoms with Uiso(H) = 1.2Ueq(C) for hydrogen atoms. The structure was checked using PLATON [3].
Discussion
In the last several decades, the research on organic fluorescent materials has gained important momentum due to their wide range of applications in organic light-emitting diodes (OLED), organic field effect transistor (OFET), organic lasers, fluorescent sensors and solar cells, etc. [4], [5], [6], [7], [8]. As a well known fluorophore, pyrene and its derivatives have been paid much attentions due to their pure blue fluorescence with high quantum yield, exceptionally long fluorescence lifetime, excellent thermal stability, and high charge carrier mobility [9], [10], [11]. Generally, the derivatization of pyrene is the key step for interesting pyrene-based functional materials. However, the active position of direct electrophilic mono substitution reaction on pyrene is almost exclusively at the electron-rich 1-position of pyrene [12], [13], [14], [15]. Compared to the derivatives with the substitutents at the 1-position of pyrene, the compounds with the substituents at 2-position are relatively limited, and only a few 2-substituted pyrenes (2-bromopyrene, 2-amionpyrrene, 2-acetylpyrene, and so on) have been obtained up to date [16], [17], [18], [19]. Recently, we synthesized one important pyrene-based derivative 2-pyrenyl aldehyde through the formylation and aromatization using 4,5,9,10-tetrahydropyrene as the starting material.
The single X-ray diffraction analysis agrees well with expected structure of the title compound. The functional group of aldehyde locates at the 2-position of pyrene. The C–O bond length is 1.198(3) Å, which is the typical double bond distance of aldehyde group. The C2–C17 bond length is 1.476(3) Å, indicating the π–π conjugation effect between the pyrene π-ring system and the aldehyde functional group. All carbon and oxygen atoms are nearly in a strict plane with the largest deviation to be 0.031(3) Å from the mean plane based on all the atoms. There is relatively strong intermolecular π–π interaction between adjacent molecules with the shortest interatomic distance is 3.366(3) Å, forming dimeric supramolecular structures. In addition, there exist weak intermolecular C–H⋯π and C–H⋯O interactions, which link the units of the title compounds into three-dimensional structure.
Acknowledgements
This work was supported by the Fundamental Research Funds for the Central Universities (2017BSCXA05).
References
Bruker. APEX2, SAINT and SADABS. Brucker AXS Inc., Madison, WI, USA (2012).Search in Google Scholar
Sheldrick, G. M.: Crystal structure refinement with SHELXL. Acta Crystallogr. C71 (2015) 3–8.10.1107/S2053229614024218Search in Google Scholar PubMed PubMed Central
Spek, A. L.: Single-crystal structure validation with the program PLATON. J. Appl. Crystallogr. 36 (2003) 7–13.10.1107/S0021889802022112Search in Google Scholar
Zhao, Z. J.; Chen, S. M.; Lam, J. W. Y.; Lu, P.; Zhong, Y. C.; Wong, K. S.; Kwok, H. S.; Tang, B. Z.: Creation of highly effcient solid emitter by decorating pyrene core with AIE-active tetraphenylethene peripheries. Chem. Commun. 46 (2010) 2221–2223.10.1039/b921451hSearch in Google Scholar PubMed
Mishra, A.; Uhrich, C.; Reinole, E.; Pfeiffer, M.; Bauerle, P.: Synthesis and characterization of acceptor-substituted oligothiophenes for solar cell applications. Adv. Enery Mater. 2 (2011) 265–273.10.1002/aenm.201100026Search in Google Scholar
Sasabe, H.; Kido, J.: Multifunctional materials in high-performance OLEDs: challenges for solid-state lighting. Chem. Mater. 23 (2010) 621–630.10.1021/cm1024052Search in Google Scholar
Nie, J.; Li, N.; Ni, Z. H.; Zhao, Y.; Zhang, L. F.: A sensitive teraphenylethene-based fluorescent probe for Zn2+ ion involving ESIPT and CHEF processes. Tetrahedron Lett. 58 (2017) 1980–1984.10.1016/j.tetlet.2017.04.027Search in Google Scholar
Indumathi, C.; Girisun, T. C. S.; Anitha, K.; Raj, S. A. C.: Structural, thermal, optical and optical properties of ethylenediaminium picrate single crystals. J. Phys. Chem. Solids. 106 (2017) 37–43.10.1016/j.jpcs.2017.03.003Search in Google Scholar
Zhang, R.; Zhao, Y.; Zhang, L. F.; Xu, L.; Ni, Z. H.: A series of short axially symmetrically 1,3,6,8-tetrasubstituted pyrene-based green and blue emitters with 4-tert-butylphenyl and aryamine attachments. Dyes Pigm. 130 (2016) 106–115.10.1016/j.dyepig.2016.03.020Search in Google Scholar
Figueira-Duarte, T. M.; Mullen, K.: Pyrene-based materials for organic electronics. Chem. Rev. 111 (2011) 7260–7314.10.1021/cr100428aSearch in Google Scholar PubMed
Zhang, R.; Zhang, T. F.; Xu, L.; Han, F. F.; Zhao, Y.; Ni, Z. H.: A new series of short axially symmetrically and asymmetrically 1,3,6,8-tetrasubtituted pyrenes with two types of substituents: syntheses, structures, photophysical properties and electroluminescence. J. Mol. Struct. 1127 (2017) 237–246.10.1016/j.molstruc.2016.07.105Search in Google Scholar
Xu, L. H.; Ni, Z. H.: Crystal structure of 1,3,6,8-tetrakis(p-tolylthio)pyrene, C44H34S4. Z. Kristallogr. NCS 229 (2016) 255–257.Search in Google Scholar
Zhang, X. M.; Wang, H. F.; Wang, S.; Shen, Y. T.; Yang, Y. L.; Deng, K.; Zhao, K. Q.; Zeng, Q. D.; Wang, C.: Triphenylene substituted pyrene derivative: synthesis and single molecule investigation. J. Phys. Chem. C 117 (2013) 307–312.10.1021/jp3095616Search in Google Scholar
Banerjee, M.; Vyas, V. S.; Lindeman, S. V.; Rathore, R.: Isolation and X-ray structural characterization of tetraisopropylpyrene cation radical. Chem. Commun. 16 (2008) 1889–1891.10.1039/b800168eSearch in Google Scholar PubMed
He, M. Q.; Pan, A. X.; Xie, J. M.; Li, H. M.; Yuan, X. H.; Cheng, X. N.; Chen, M.: Synthesis of 1-benzoylpyrene using silica-supported phosphotungstic heteropoly acid as an efficient and reusable catalyst. Korean J. Chem. Eng. 29 (2012) 1388–1392.10.1007/s11814-012-0039-7Search in Google Scholar
Zhang, R.; Han, F. F.; Zhang, L. F.: Crystal structure of 2-(4-methylbenzoyl)pyrene, C24H16O. Z. Kristallogr. NCS 231 (2016) 855–857.10.1515/ncrs-2015-0292Search in Google Scholar
Harvey, R. G.; Schmolka, S.; Cortez, C.; Lee, H.: Syntheses of 2-bromopyrene and 2-hydroxypyrene. Synth. Commun. 18 (1988) 2207–2209.10.1080/00397918808082362Search in Google Scholar
Krreyenschmidt, M.; Baumgarrten, M.; Tyutyulko, N.; Mullen, K.: 2,2′-Bipyrenyl and para-terpyrenyl-a new type of electronically decoupled oligoarylene. Angew. Chem. Int. Ed. Engl. 33 (1994) 1957–1959.10.1002/anie.199419571Search in Google Scholar
Suzuki, S.; Takeda, T.; Kuratsu, M.; Kozaki, M.; Sato, K.; Shiomi, D.; Takui, T.; Okada, K.: Pyrene-dihydrophenazine bis(radical cation) in a singlet ground state Org. Lett. 11 (2009) 2816–2818.Search in Google Scholar
©2018 Miao Bao-Xi et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 License.
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of (E)-1-(4-(((E)-2-hydroxy-5-methylbenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C17H18N2O2
- Crystal structure of 1,3,5,7-tetraazaadamantane-1,3-diium 2,5-dicarboxyterephthalate, C16H18N4O8
- Crystal structure of guanidinium tetrabutyl-ammonium 5-hydroxyisophthalate dihydrate, C25H50N4O7
- Crystal structure of poly[aqua-(μ2-5-methoxyisophthalate-κ3O,O′:O′′)-(μ2-1,4-bis((1H-1,2,4-triazol-1-yl)methyl)benzene-κ2N:N′)nickel(II), NiC21H20N6O6
- Crystal structure of aqua-bis(3,4-dimethoxybenzoato-κ1O)-(2,2′-bipyridine-κ2N,N′)copper(II), C28H26CuN2O9
- Crystal structure of catena-poly[aqua-(μ2-(3,5-di(1H-imidazol-1-yl)-pyridine-κ2N:N′)-(μ2-2-(carboxylatomethyl)benzoato-κ2O:O′)] cadmium(II), C20H17CdN5O5
- The crystal structure of catena-poly[chlorido-(μ2-5-methyl-1,3,4-thiadiazole-2-thiolato-κ2S:N)mercury(II)], C3H3ClHgN2S2
- Crystal structure of (E)-2,4-dichloro-6-(((4-methyl-2-nitrophenyl)imino)methyl)phenol, C14H10Cl2N2O3
- Crystal structure of a new polymorph of bis[μ-1,3-bis(diphenylphosphino)propane-κ2P:P′-disilver(I)] bis(tetrafluoroborate), [Ag(dppp)]2(BF4)2, C54H52Ag2B2F8P4
- The crystal structure of 2-phenyl-4,6-bis(R-tert-butylsulfonamido)-1,3,5-triazine – ethyl acetate (2/1), C38H58N10O6S4
- Crystal structure of 6-amino-8-(2-methoxy-phenyl)-2-methyl-2,3,8,8a-tetrahydro-1H-iso-quinoline-5,7,7-tricarbonitrile monohydrate, C20H21N5O2
- Crystal structure of methyl (1-phenylethyl)carbamate, C10H13NO2
- Crystal structure of dimethanol-(μ2-squarato-κ2O:O′)-tetrakis(tri-p-tolylphosphane-κP)disilver(I) – methanol (1/2), C92H98Ag2O8P4
- Crystal structure of catena-poly[bis(μ2-1,4-bis(triazol-1-ylmethyl)benzene-κ2N:N′)-bis(5-tert-butyl-isophthalate-κO)copper(II)]tetrahydrate, C36H46CuN6O12
- Crystal structure of 4-aminopyridinium 4-acetyl-(pyridin-4-yl)-1H-1,2,3-triazol-5-olate monohydrate, C14H16N6O3
- Crystal structure of 2-(8-bromo-2-phenylimidazo[1,2-α]pyridin-3-yl)-6,7-dimethyl-3-phenylquinoxaline, C29H21BrN4
- Crystal structure of aqua(1-(2-pyridyl)ethanone oxime-κ2N,N′)(1-(2-pyridyl)ethanone oximato-κ2N,N′) nitrate monohydrate, C14H19N5O7Cu
- Crystal structure of poly[tetraaqua-(μ4-oxalato-κ4O,O′:O′′,O′′′)-(μ8-benzene-1,2,4,5-tetracarboxylato-κ8O1:O2:O3:O4:O5:O6:O7:O8)yttrium(III)], C6H5O8Y
- Crystal structure of bis{catena-poly[(μ2-1,2-bis(4-pyridyl)ethane-κ2N:N′)silver(I)]} diaqua-bis(5-(4-carboxyphenyl)pyridine-2-carboxylato-κ2N,O)-(μ2-1,2-bis(4-pyridyl)ethane-κ2N:N′)disilver(I) octahydrate, C31H35Ag2N4O9
- Crystal structure of (E)-N-(2-(benzylamino)-2-oxo-1-(4-oxo-4H-chromen-3-yl)ethyl)-N-(4-bromophenyl)-3-chloroacrylamide hydrate, C27H22BrClN2O5
- Crystal structure of catena-poly[octaaqua-bis(μ2-4,6-dicarboxyisophthalate-κ2O:O′)cadmium(II)disodium(I)] dihydrate, C20H28CdNa2O26
- Crystal structure of acetonitrile{bis(2-benzimidazolylmethyl)amine-κ3N,N′,N′′}-{maleato-κO}zinc(II) perchlorate - acetonitrile (1/1), C24H24ClN7O8Zn
- Crystal structure of 2-amino-4-(3,5-dibromo-4-hydroxyphenyl)-7-methyl-5-oxo-2H,5H-pyrano[4,3-b]pyran-3-carbonitrile, C16H10Br2N2O4
- Crystal structure of catena-poly[diaqua-(μ2-3,5-bis(pyridin-4-ylmethoxy)benzoate-κ2N:O) manganese(II)] tetrahydrate [(3,5-bis-(pyridin-4-ylmethoxy)-benzoic-κ1Oκ1N) manganese(II)] trihydrate, C38H42MnN4O14
- The crystal structure of 2-carboxybenzaldehyde-2-phenylacetohydrazone, C16H14N2O3
- The crystal structure of poly[μ2-aqua-(μ2-2-naphthylamine-1-sulfonato-κ3O,O′:O′′)sodium(I)], C10H10N1O4S1Na
- The crystal structure of phthalazin-1(2H)-one, C8H6N2O1
- Crystal structure of 3,5-bis(trifluoromethyl)benzyl(Z)-N-(adamantan-1-yl)morpholine-4-carbothioimidate, C24H28F6N2OS
- Crystal structure of diazido-bis(μ2-pyridin-2-ylmethanolato-κ2N:O)-bis(pyridin-2-ylmethanolato-κ2N,O)dicobalt(III) – methanol (1/3), C27H35Co2N10O7
- Crystal structure of N-[[(4,6-dimethoxy-2-pyrimidinyl)amino]carbonyl]-3-(2,2,2-trifluoroethoxy)-2-pyridinesulfonamide, C14H14F3N5O6S
- Crystal structure of 1-phenyl-N′-(1-phenyl-5-(thiophen-2-yl)-1H-pyrazole-3-carbonyl)-5-(thiophen-2-yl)-1H-pyrazole-3-carbohydrazide, C28H20N6O2S2
- The crystal structure of poly[bis(4-hydroxybenzoato-κO)-(μ2-4,4′-bipyridine-κ2N:N′)copper(II)] hydrate, C24H20N2O7Cu
- Crystal structure of poly[μ3-5-(4-(2,6-di(pyridine-2-yl)pyridine-4-yl)phenoxy)isophthalato-κ5O:O′,O′′:N,N′,N′′cobalt(II)], C29H17CoN3O5
- Crystal structure of poly[μ3-5-(4-(2,6-di(pyridine-2-yl)pyridine-4-yl)phenoxy)isophthalato-κ6O:O′,O′′:N,N′,N′′)cobalt(II)] C29H17CoN3O5
- Crystal structure of diaqua-(acetato-κ3O,O′:O′′)-(μ3-4,6-di(1H-imidazol-1-yl)isophthalato-κ4O:O′:O′′,O′′′)lanthanum(III), C16H15LaN4O8
- Synthesis and crystal structure of 6-carboxy-1-(3,5-dicarboxyphenyl)-1H-benzo[d]imidazol-3-ium-5-carboxylate dihydrate, C18H12N2O8
- Crystal structure of (E)-2-hydroxybenzaldehyde O-(2-(((E)-(4-(dimethylamino)benzylidene)amino)oxy)ethyl)oxime, C18H21N3O3
- Crystal structure of bis{2-((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}zinc(II), C32H30N4O4Zn
- Crystal structure of bis(9-aminoacridin-10-ium) tetrachloridocuprate(II) monohydrate, C26H24Cl4CuN4O
- The crystal structure of 4-tert-butyl-N′-[(E)-(4-fluoro-3-methoxyphenyl)methylidene]benzohydrazide, C19H21F1N2O2
- Crystal structure of (E)-3-(3-(5-methyl-1-4-tolyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazol-4-yl)-1-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)prop-2-en-1-one, C31H26N8O
- Crystal structure of (E)-N′-(4-methoxybenzylidene)-5-methyl-1-(4-tolyl)-1H-1,2,3-triazole-4-carbohydrazide, C19H19N5O2
- Crystal structure and molecular packing of O-ethyl (2-chlorophenyl)carbamothioate, C9H10ClNOS
- Crystal structure of pyrene-2-carbaldehyde, C17H10O
- Crystal structure of (E)-2,4-diiodo-6-(4-methyl-2-nitrostyryl)phenol, C14H10I2N2O3
- Crystal structure of (E)-2,4-dichloro-6-(((4-methoxy-2-nitrophenyl)imino)methyl)phenol, C14H10Cl2N2O4
- Crystal structure of (E)-2-bromo-4-chloro-6-(4-methoxy-2-nitrostyryl)phenol, C14H10BrClN2O4
- Crystal structure of (E)-4,6-diiodo-2-(((4-methoxy-2-nitrophenyl)imino)methyl)-3-methylphenol, C14H10I2N2O4
- The crystal structure of 7-bromo-1-cyclopropyl-8-methyl-4-oxo-1,4-dihydroquinoline-3-carboxylic acid an intermediate of the ozenoxacin synthesis, C14H12BrNO3
- Crystal structure of bis(N-(1-(pyrazin-2-yl)ethylidene)nicotinohydrazonato-κ3N,N′,O)copper(II) C24H20N10O2Cu
- Crystal structure of diaqua-dinitrato-k2O,O′((Z)-N-((E)-1-(pyrazin-2-yl)ethylidene)nicotinohydrazonato-k3N,N′,O)europium(II), C12H14N7O9Eu
- Crystal structure of ethyl 4-amino-5-(5-methyl-1-(4-tolyl)-1H-1,2,3-triazole-4-carbonyl)-2-(phenylamino)thiophene-3-carboxylate, C24H23N5O3S
- The crystal structure of acridin-10-ium2-carboxybenzoate, C21H15NO4
- The crystal structure of 3-((phenylamino)methylene)-1,5-dioxaspiro[5.5]undecane-2,4-dione, C16H17N1O4
- Crystal structure of 12-chloro-5,6,7,12-tetrahydrodibenzo[c,f][1,5]oxastibocine, C14H12ClOSb
- Crystal structure of 4-((1,3-dioxoisoindolin-2-yl)methyl)phenethyl 4-methylbenzenesulfonate, C24H21NO5S
- Crystal structure of 3-methyl-2,3-dihydro-2-thioxoquinazolin-4(1H)-one, C9H8N2OS
- Crystal structure of tert-butyl (2-(4-oxo-2-thioxo-1,4-dihydroquinazolin-3(2H)-yl)ethyl)carbamate, C15H19N3O3S
- Crystal structure of ethyl 5-formyl-3,4-dimethylpyrrole-2-carboxylate–1-(propan-2-ylidene)thiosemicarbazide (1/1), C14H22N4O3S
- Crystal structure of bis-(N′-(5-ethoxycarbonyl-3,4-dimethyl-pyrrol-2-yl-methylidene)-3-hydroxybenzohydrazide-κ2O,N)copper(II) – dimethylformamide (1/2), C40H50N8O10Cu
- Crystal structure of bis(acetato-κO)bis{2-((1H-tetrazol-1-yl)methyl)-1H-benzo[d]imidazole-κN}zinc(II), C22H22N12O4Zn
- Crystal structure of 4-phenyl-3-((4-phenyl-1H-1,2,3-triazol-1-yl)methyl)-1H-1,2,4-triazole-5(4H)-thione, C17H14N6S
- Crystal structure of (Z)-N-(4-nitrophenyl)-3-phenyl-3-(phenylamino)acrylamide, C21H17N3O3
- Crystal structure of 1,1′-(pentane-1,5-diyl)bis(3-methyl-1H-imidazol-3-ium)bis(hexafluorophosphate), C13H22F12N4P2
- Synthesis and crystal structure of bis(furan-2-ylmethanaminium)-catena-[bis(μ2-phthalato-κ2O:O′)cobalt(II)], C26H24CoN2O10
- Crystal structure of methyl (R)-4-(o-chlorobenzoyl)-1-thia-4-azaspiro[4.5]decane-3-carboxylate, C17H20ClNO3S
- Crystal structure of 2-[[4-[2-[4-(4-methoxyphenyl)-1-piperazinyl]ethyl]phenyl] methyl]-1H-isoindole-1,3(2H)-dione, C28H29N3O3
- The crystal structure of benzenaminium 5,7-dihydroxy-4-oxo-2-phenyl-4H-chromene-8-sulfonate hydrate, C21H19NO8S
- Crystal structure of semiconducting potassium poly[(μ2-tetraselenido-κ2Se1:Se4)(μ2-pentaselenido-κ1Se1:Se1)argentate(I)], K3AgSe9
- Crystal structure of 2-isopropyl-8-methyl-phenanthrene-3,4-dione, C18H16O2
- Crystal structure of 2-isopropyl-8,8-dimethyl-5,6,7,8-tetrahydrophenanthrene-3,4-dione, C19H22O2
- Crystal structure of (E)-2-(1-((2-aminophenyl)imino)ethyl)-4-bromophenol, C14H13BrN2O
- Crystal structure of 1,1-di(4-cyanophenyl)-2,2-diphenylethene, C28H18N2
- Crystal structure of bis(hydroxylamido-κ2O,N)-oxido(1H-pyrazole-3-carboxylato-κ2O,N)vanadium(V), C4H7N4O5V
- The crystal structure of In1.2B3O5.6(OH)1.4
- The crystal structure of chlorido(2-(1H-pyrazol-3-yl)phenolato-κ2N,O)(2-(1H-pyrazol-3-yl)phenol-κN)copper(II), C18H15ClCuN4O2
- Crystal structure of 1-heptylpyridazin-1-ium iodide, C11H19N2I
- The crystal structure of N-butylpyridinium bis(μ2-dichlorido)-tetrachloridodicopper(II), C18H28N2Cu2Cl6
- Crystal structure of 6-hydroxy-5-((2-hydroxy-6-oxocyclohex-1-en-1-yl)(4-methoxyphenyl)methyl)-1,3-dimethylpyrimidine-2,4(1H,3H)-dione, C20H22N2O6
- Crystal structure of bis(acetonitrile)-diaqua-dichloridoiron(II), C4H10Cl2N2O2Fe
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of (E)-1-(4-(((E)-2-hydroxy-5-methylbenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C17H18N2O2
- Crystal structure of 1,3,5,7-tetraazaadamantane-1,3-diium 2,5-dicarboxyterephthalate, C16H18N4O8
- Crystal structure of guanidinium tetrabutyl-ammonium 5-hydroxyisophthalate dihydrate, C25H50N4O7
- Crystal structure of poly[aqua-(μ2-5-methoxyisophthalate-κ3O,O′:O′′)-(μ2-1,4-bis((1H-1,2,4-triazol-1-yl)methyl)benzene-κ2N:N′)nickel(II), NiC21H20N6O6
- Crystal structure of aqua-bis(3,4-dimethoxybenzoato-κ1O)-(2,2′-bipyridine-κ2N,N′)copper(II), C28H26CuN2O9
- Crystal structure of catena-poly[aqua-(μ2-(3,5-di(1H-imidazol-1-yl)-pyridine-κ2N:N′)-(μ2-2-(carboxylatomethyl)benzoato-κ2O:O′)] cadmium(II), C20H17CdN5O5
- The crystal structure of catena-poly[chlorido-(μ2-5-methyl-1,3,4-thiadiazole-2-thiolato-κ2S:N)mercury(II)], C3H3ClHgN2S2
- Crystal structure of (E)-2,4-dichloro-6-(((4-methyl-2-nitrophenyl)imino)methyl)phenol, C14H10Cl2N2O3
- Crystal structure of a new polymorph of bis[μ-1,3-bis(diphenylphosphino)propane-κ2P:P′-disilver(I)] bis(tetrafluoroborate), [Ag(dppp)]2(BF4)2, C54H52Ag2B2F8P4
- The crystal structure of 2-phenyl-4,6-bis(R-tert-butylsulfonamido)-1,3,5-triazine – ethyl acetate (2/1), C38H58N10O6S4
- Crystal structure of 6-amino-8-(2-methoxy-phenyl)-2-methyl-2,3,8,8a-tetrahydro-1H-iso-quinoline-5,7,7-tricarbonitrile monohydrate, C20H21N5O2
- Crystal structure of methyl (1-phenylethyl)carbamate, C10H13NO2
- Crystal structure of dimethanol-(μ2-squarato-κ2O:O′)-tetrakis(tri-p-tolylphosphane-κP)disilver(I) – methanol (1/2), C92H98Ag2O8P4
- Crystal structure of catena-poly[bis(μ2-1,4-bis(triazol-1-ylmethyl)benzene-κ2N:N′)-bis(5-tert-butyl-isophthalate-κO)copper(II)]tetrahydrate, C36H46CuN6O12
- Crystal structure of 4-aminopyridinium 4-acetyl-(pyridin-4-yl)-1H-1,2,3-triazol-5-olate monohydrate, C14H16N6O3
- Crystal structure of 2-(8-bromo-2-phenylimidazo[1,2-α]pyridin-3-yl)-6,7-dimethyl-3-phenylquinoxaline, C29H21BrN4
- Crystal structure of aqua(1-(2-pyridyl)ethanone oxime-κ2N,N′)(1-(2-pyridyl)ethanone oximato-κ2N,N′) nitrate monohydrate, C14H19N5O7Cu
- Crystal structure of poly[tetraaqua-(μ4-oxalato-κ4O,O′:O′′,O′′′)-(μ8-benzene-1,2,4,5-tetracarboxylato-κ8O1:O2:O3:O4:O5:O6:O7:O8)yttrium(III)], C6H5O8Y
- Crystal structure of bis{catena-poly[(μ2-1,2-bis(4-pyridyl)ethane-κ2N:N′)silver(I)]} diaqua-bis(5-(4-carboxyphenyl)pyridine-2-carboxylato-κ2N,O)-(μ2-1,2-bis(4-pyridyl)ethane-κ2N:N′)disilver(I) octahydrate, C31H35Ag2N4O9
- Crystal structure of (E)-N-(2-(benzylamino)-2-oxo-1-(4-oxo-4H-chromen-3-yl)ethyl)-N-(4-bromophenyl)-3-chloroacrylamide hydrate, C27H22BrClN2O5
- Crystal structure of catena-poly[octaaqua-bis(μ2-4,6-dicarboxyisophthalate-κ2O:O′)cadmium(II)disodium(I)] dihydrate, C20H28CdNa2O26
- Crystal structure of acetonitrile{bis(2-benzimidazolylmethyl)amine-κ3N,N′,N′′}-{maleato-κO}zinc(II) perchlorate - acetonitrile (1/1), C24H24ClN7O8Zn
- Crystal structure of 2-amino-4-(3,5-dibromo-4-hydroxyphenyl)-7-methyl-5-oxo-2H,5H-pyrano[4,3-b]pyran-3-carbonitrile, C16H10Br2N2O4
- Crystal structure of catena-poly[diaqua-(μ2-3,5-bis(pyridin-4-ylmethoxy)benzoate-κ2N:O) manganese(II)] tetrahydrate [(3,5-bis-(pyridin-4-ylmethoxy)-benzoic-κ1Oκ1N) manganese(II)] trihydrate, C38H42MnN4O14
- The crystal structure of 2-carboxybenzaldehyde-2-phenylacetohydrazone, C16H14N2O3
- The crystal structure of poly[μ2-aqua-(μ2-2-naphthylamine-1-sulfonato-κ3O,O′:O′′)sodium(I)], C10H10N1O4S1Na
- The crystal structure of phthalazin-1(2H)-one, C8H6N2O1
- Crystal structure of 3,5-bis(trifluoromethyl)benzyl(Z)-N-(adamantan-1-yl)morpholine-4-carbothioimidate, C24H28F6N2OS
- Crystal structure of diazido-bis(μ2-pyridin-2-ylmethanolato-κ2N:O)-bis(pyridin-2-ylmethanolato-κ2N,O)dicobalt(III) – methanol (1/3), C27H35Co2N10O7
- Crystal structure of N-[[(4,6-dimethoxy-2-pyrimidinyl)amino]carbonyl]-3-(2,2,2-trifluoroethoxy)-2-pyridinesulfonamide, C14H14F3N5O6S
- Crystal structure of 1-phenyl-N′-(1-phenyl-5-(thiophen-2-yl)-1H-pyrazole-3-carbonyl)-5-(thiophen-2-yl)-1H-pyrazole-3-carbohydrazide, C28H20N6O2S2
- The crystal structure of poly[bis(4-hydroxybenzoato-κO)-(μ2-4,4′-bipyridine-κ2N:N′)copper(II)] hydrate, C24H20N2O7Cu
- Crystal structure of poly[μ3-5-(4-(2,6-di(pyridine-2-yl)pyridine-4-yl)phenoxy)isophthalato-κ5O:O′,O′′:N,N′,N′′cobalt(II)], C29H17CoN3O5
- Crystal structure of poly[μ3-5-(4-(2,6-di(pyridine-2-yl)pyridine-4-yl)phenoxy)isophthalato-κ6O:O′,O′′:N,N′,N′′)cobalt(II)] C29H17CoN3O5
- Crystal structure of diaqua-(acetato-κ3O,O′:O′′)-(μ3-4,6-di(1H-imidazol-1-yl)isophthalato-κ4O:O′:O′′,O′′′)lanthanum(III), C16H15LaN4O8
- Synthesis and crystal structure of 6-carboxy-1-(3,5-dicarboxyphenyl)-1H-benzo[d]imidazol-3-ium-5-carboxylate dihydrate, C18H12N2O8
- Crystal structure of (E)-2-hydroxybenzaldehyde O-(2-(((E)-(4-(dimethylamino)benzylidene)amino)oxy)ethyl)oxime, C18H21N3O3
- Crystal structure of bis{2-((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}zinc(II), C32H30N4O4Zn
- Crystal structure of bis(9-aminoacridin-10-ium) tetrachloridocuprate(II) monohydrate, C26H24Cl4CuN4O
- The crystal structure of 4-tert-butyl-N′-[(E)-(4-fluoro-3-methoxyphenyl)methylidene]benzohydrazide, C19H21F1N2O2
- Crystal structure of (E)-3-(3-(5-methyl-1-4-tolyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazol-4-yl)-1-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)prop-2-en-1-one, C31H26N8O
- Crystal structure of (E)-N′-(4-methoxybenzylidene)-5-methyl-1-(4-tolyl)-1H-1,2,3-triazole-4-carbohydrazide, C19H19N5O2
- Crystal structure and molecular packing of O-ethyl (2-chlorophenyl)carbamothioate, C9H10ClNOS
- Crystal structure of pyrene-2-carbaldehyde, C17H10O
- Crystal structure of (E)-2,4-diiodo-6-(4-methyl-2-nitrostyryl)phenol, C14H10I2N2O3
- Crystal structure of (E)-2,4-dichloro-6-(((4-methoxy-2-nitrophenyl)imino)methyl)phenol, C14H10Cl2N2O4
- Crystal structure of (E)-2-bromo-4-chloro-6-(4-methoxy-2-nitrostyryl)phenol, C14H10BrClN2O4
- Crystal structure of (E)-4,6-diiodo-2-(((4-methoxy-2-nitrophenyl)imino)methyl)-3-methylphenol, C14H10I2N2O4
- The crystal structure of 7-bromo-1-cyclopropyl-8-methyl-4-oxo-1,4-dihydroquinoline-3-carboxylic acid an intermediate of the ozenoxacin synthesis, C14H12BrNO3
- Crystal structure of bis(N-(1-(pyrazin-2-yl)ethylidene)nicotinohydrazonato-κ3N,N′,O)copper(II) C24H20N10O2Cu
- Crystal structure of diaqua-dinitrato-k2O,O′((Z)-N-((E)-1-(pyrazin-2-yl)ethylidene)nicotinohydrazonato-k3N,N′,O)europium(II), C12H14N7O9Eu
- Crystal structure of ethyl 4-amino-5-(5-methyl-1-(4-tolyl)-1H-1,2,3-triazole-4-carbonyl)-2-(phenylamino)thiophene-3-carboxylate, C24H23N5O3S
- The crystal structure of acridin-10-ium2-carboxybenzoate, C21H15NO4
- The crystal structure of 3-((phenylamino)methylene)-1,5-dioxaspiro[5.5]undecane-2,4-dione, C16H17N1O4
- Crystal structure of 12-chloro-5,6,7,12-tetrahydrodibenzo[c,f][1,5]oxastibocine, C14H12ClOSb
- Crystal structure of 4-((1,3-dioxoisoindolin-2-yl)methyl)phenethyl 4-methylbenzenesulfonate, C24H21NO5S
- Crystal structure of 3-methyl-2,3-dihydro-2-thioxoquinazolin-4(1H)-one, C9H8N2OS
- Crystal structure of tert-butyl (2-(4-oxo-2-thioxo-1,4-dihydroquinazolin-3(2H)-yl)ethyl)carbamate, C15H19N3O3S
- Crystal structure of ethyl 5-formyl-3,4-dimethylpyrrole-2-carboxylate–1-(propan-2-ylidene)thiosemicarbazide (1/1), C14H22N4O3S
- Crystal structure of bis-(N′-(5-ethoxycarbonyl-3,4-dimethyl-pyrrol-2-yl-methylidene)-3-hydroxybenzohydrazide-κ2O,N)copper(II) – dimethylformamide (1/2), C40H50N8O10Cu
- Crystal structure of bis(acetato-κO)bis{2-((1H-tetrazol-1-yl)methyl)-1H-benzo[d]imidazole-κN}zinc(II), C22H22N12O4Zn
- Crystal structure of 4-phenyl-3-((4-phenyl-1H-1,2,3-triazol-1-yl)methyl)-1H-1,2,4-triazole-5(4H)-thione, C17H14N6S
- Crystal structure of (Z)-N-(4-nitrophenyl)-3-phenyl-3-(phenylamino)acrylamide, C21H17N3O3
- Crystal structure of 1,1′-(pentane-1,5-diyl)bis(3-methyl-1H-imidazol-3-ium)bis(hexafluorophosphate), C13H22F12N4P2
- Synthesis and crystal structure of bis(furan-2-ylmethanaminium)-catena-[bis(μ2-phthalato-κ2O:O′)cobalt(II)], C26H24CoN2O10
- Crystal structure of methyl (R)-4-(o-chlorobenzoyl)-1-thia-4-azaspiro[4.5]decane-3-carboxylate, C17H20ClNO3S
- Crystal structure of 2-[[4-[2-[4-(4-methoxyphenyl)-1-piperazinyl]ethyl]phenyl] methyl]-1H-isoindole-1,3(2H)-dione, C28H29N3O3
- The crystal structure of benzenaminium 5,7-dihydroxy-4-oxo-2-phenyl-4H-chromene-8-sulfonate hydrate, C21H19NO8S
- Crystal structure of semiconducting potassium poly[(μ2-tetraselenido-κ2Se1:Se4)(μ2-pentaselenido-κ1Se1:Se1)argentate(I)], K3AgSe9
- Crystal structure of 2-isopropyl-8-methyl-phenanthrene-3,4-dione, C18H16O2
- Crystal structure of 2-isopropyl-8,8-dimethyl-5,6,7,8-tetrahydrophenanthrene-3,4-dione, C19H22O2
- Crystal structure of (E)-2-(1-((2-aminophenyl)imino)ethyl)-4-bromophenol, C14H13BrN2O
- Crystal structure of 1,1-di(4-cyanophenyl)-2,2-diphenylethene, C28H18N2
- Crystal structure of bis(hydroxylamido-κ2O,N)-oxido(1H-pyrazole-3-carboxylato-κ2O,N)vanadium(V), C4H7N4O5V
- The crystal structure of In1.2B3O5.6(OH)1.4
- The crystal structure of chlorido(2-(1H-pyrazol-3-yl)phenolato-κ2N,O)(2-(1H-pyrazol-3-yl)phenol-κN)copper(II), C18H15ClCuN4O2
- Crystal structure of 1-heptylpyridazin-1-ium iodide, C11H19N2I
- The crystal structure of N-butylpyridinium bis(μ2-dichlorido)-tetrachloridodicopper(II), C18H28N2Cu2Cl6
- Crystal structure of 6-hydroxy-5-((2-hydroxy-6-oxocyclohex-1-en-1-yl)(4-methoxyphenyl)methyl)-1,3-dimethylpyrimidine-2,4(1H,3H)-dione, C20H22N2O6
- Crystal structure of bis(acetonitrile)-diaqua-dichloridoiron(II), C4H10Cl2N2O2Fe


