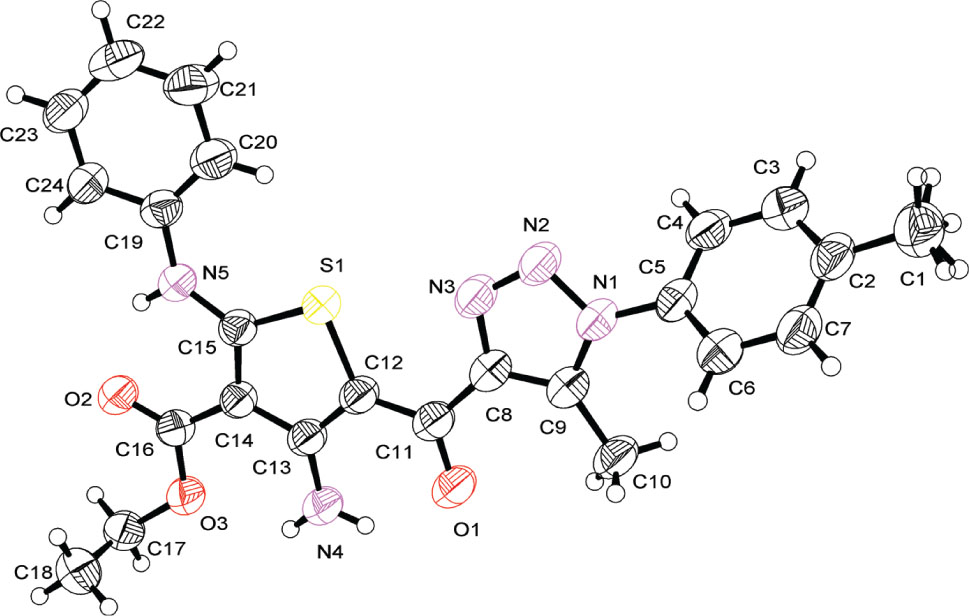
Crystal structure of ethyl 4-amino-5-(5-methyl-1-(4-tolyl)-1H-1,2,3-triazole-4-carbonyl)-2-(phenylamino)thiophene-3-carboxylate, C24H23N5O3S
-
Mohammed H. Geesi
Abstract
C24H23N5O3S, triclinic, P1̅ (no. 2), a = 9.1704(9) Å, b = 10.1253(11) Å, c = 12.2182(14) Å, α = 83.686(10)°, β = 89.542(9)°, γ = 76.982(9)°, V = 1098.5(2) Å3, Z = 2, Rgt(F) = 0.0551, wRref(F2) = 0.1510, T = 296(2) K.
Data collection and handling.
| Crystal: | Needle, yellow |
| Size: | 0.37 × 0.21 × 0.13 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 1.85 mm−1 |
| Diffractometer, scan mode: | SuperNova φ and ω-scans |
| θmax, completeness: | 29.99°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 9769, 5171, 0.032 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 3341 |
| N(param)refined: | 300 |
| Programs: | CrysAlisPro [1], SHELX [2, 3] , WinGX and Ortep [11] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| C1 | 0.1586(3) | −0.1973(3) | 0.5590(3) | 0.0885(9) |
| H1A | 0.117670 | −0.240273 | 0.504099 | 0.133 |
| H1B | 0.241182 | −0.261384 | 0.596358 | 0.133 |
| H1C | 0.082841 | −0.167440 | 0.611197 | 0.133 |
| H1D | 0.176792 | −0.205792 | 0.637004 | 0.133 |
| H1E | 0.053280 | −0.184680 | 0.544744 | 0.133 |
| H1F | 0.211621 | −0.278625 | 0.529906 | 0.133 |
| C2 | 0.2120(3) | −0.0766(3) | 0.5048(2) | 0.0643(7) |
| C3 | 0.2024(3) | −0.0452(3) | 0.3915(2) | 0.0692(7) |
| H3 | 0.159406 | −0.098227 | 0.349584 | 0.083 |
| C4 | 0.2544(3) | 0.0619(3) | 0.3393(2) | 0.0616(6) |
| H4 | 0.245519 | 0.081672 | 0.263131 | 0.074 |
| C5 | 0.3197(2) | 0.1395(3) | 0.40043(18) | 0.0538(6) |
| C6 | 0.3314(3) | 0.1108(3) | 0.51376(19) | 0.0627(6) |
| H6 | 0.376190 | 0.162912 | 0.555366 | 0.075 |
| C7 | 0.2761(3) | 0.0043(3) | 0.5643(2) | 0.0668(7) |
| H7 | 0.282234 | −0.013530 | 0.640638 | 0.080 |
| C8 | 0.4566(2) | 0.4286(3) | 0.28995(18) | 0.0542(6) |
| C9 | 0.3712(2) | 0.3756(3) | 0.36957(17) | 0.0538(6) |
| C10 | 0.2778(3) | 0.4372(3) | 0.4594(2) | 0.0682(7) |
| H10A | 0.196194 | 0.392898 | 0.473156 | 0.102 |
| H10B | 0.238963 | 0.532683 | 0.437652 | 0.102 |
| H10C | 0.337917 | 0.425725 | 0.525161 | 0.102 |
| C11 | 0.4792(2) | 0.5688(3) | 0.27327(18) | 0.0557(6) |
| C12 | 0.5619(2) | 0.6132(3) | 0.18264(18) | 0.0523(6) |
| C13 | 0.5799(2) | 0.7468(3) | 0.15920(18) | 0.0517(6) |
| C14 | 0.6680(2) | 0.7656(2) | 0.06442(17) | 0.0490(5) |
| C15 | 0.7184(2) | 0.6425(2) | 0.01817(17) | 0.0491(5) |
| C16 | 0.7118(2) | 0.8890(3) | 0.02047(19) | 0.0533(6) |
| C17 | 0.7086(3) | 1.1197(3) | 0.0473(2) | 0.0644(7) |
| H17A | 0.632652 | 1.198032 | 0.064235 | 0.077 |
| H17B | 0.726857 | 1.130091 | −0.031099 | 0.077 |
| C18 | 0.8490(3) | 1.1136(3) | 0.1097(2) | 0.0778(8) |
| H18A | 0.831615 | 1.099620 | 0.187221 | 0.117 |
| H18B | 0.880319 | 1.197818 | 0.092837 | 0.117 |
| H18C | 0.925835 | 1.039572 | 0.089198 | 0.117 |
| C19 | 0.8743(2) | 0.5255(3) | −0.12913(18) | 0.0526(6) |
| C20 | 0.8436(3) | 0.3976(3) | −0.1163(2) | 0.0673(7) |
| H20 | 0.772925 | 0.378557 | −0.065559 | 0.081 |
| C21 | 0.9173(3) | 0.2974(3) | −0.1785(2) | 0.0792(8) |
| H21 | 0.897219 | 0.210909 | −0.168209 | 0.095 |
| C22 | 1.0195(3) | 0.3244(3) | −0.2550(2) | 0.0783(8) |
| H22 | 1.069031 | 0.256887 | −0.296611 | 0.094 |
| C23 | 1.0481(3) | 0.4525(4) | −0.2696(2) | 0.0756(8) |
| H23 | 1.116341 | 0.472029 | −0.322156 | 0.091 |
| C24 | 0.9774(3) | 0.5513(3) | −0.2078(2) | 0.0638(7) |
| H24 | 0.998551 | 0.637401 | −0.218484 | 0.077 |
| N1 | 0.38313(19) | 0.2453(2) | 0.34652(14) | 0.0545(5) |
| N2 | 0.4711(2) | 0.2189(2) | 0.25693(16) | 0.0622(5) |
| N3 | 0.5141(2) | 0.3305(2) | 0.22421(16) | 0.0607(5) |
| N4 | 0.5175(2) | 0.8463(2) | 0.22077(16) | 0.0643(6) |
| H4A | 0.464554 | 0.828679 | 0.276588 | 0.077 |
| H4B | 0.530521 | 0.927610 | 0.204329 | 0.077 |
| N5 | 0.80860(19) | 0.6336(2) | −0.06973(14) | 0.0546(5) |
| H5 | 0.830101 | 0.709702 | −0.094230 | 0.066 |
| O1 | 0.4214(2) | 0.64891(19) | 0.34080(14) | 0.0713(5) |
| O2 | 0.79200(18) | 0.89881(18) | −0.05869(13) | 0.0640(5) |
| O3 | 0.65593(18) | 0.99586(18) | 0.07665(14) | 0.0653(5) |
| S1 | 0.65561(6) | 0.50883(6) | 0.08608(5) | 0.05279(19) |
Source of material
The title compound was synthesized from reaction of an equimolar mixture of ethyl 2-cyano-2-(4-hydroxy-4-(5-methyl-1-(4-tolyl)-1H-1,2,3-triazol-4-yl)-3-phenylthiazolidin-2-ylidene)acetate, hydroxylamine hydrochloride and anhydrous potassium carbonate in anhydrous ethanol under reflux for 4 h. The mixture was allowed to cool to room temperature and poured into ice-water. The solid obtained was collected by filtration, dried and recrystallized from DMF to give pale-yellow crystals (Mp. 211−212 °C; lit. Mp. 210 °C [4]) of the title compound (68%).
Experimental details
All hydrogen atoms were placed in calculated positions and refined using a riding model. Difference Fourier calculation indicated disorder in the tolyl methyl group and the group was refined with two positions rotated by 60° from each other (AFIX 123 instruction in SHELXL [3]) The other methyl groups (AFIX 137) were allowed to rotate about the C-C bond and Uiso values for all methyl groups were set to 1.5Ueq(C). The Uiso values for the amine (AFIX 93), methylene (AFIX 23), aromatic hydrogens (AFIX 43) were set to 1.2Ueq(C, N).
Comment
Thiophene derivatives have been used as building blocks in drugs since they show various pharmacological activities such as antibacterial, anti-inflammatory, anticancer, and antiviral [5], [6], [7] properties. Compounds containing the 1,2,3-triazole moiety have also shown a variety of biological activities [8], [9], [10].
The asymmetric unit consists of one molecule. The methyltriazole-carbonyl-aminothiophene-carboxylate group (A) is planar with a maximum deviation of 0.124(2) Å from the least squares plane. With a twist of just 8.3(1)°, the phenylamine group is also almost co-planar with A, whereas the angle is 48.0(1)° for the tolyl group. The ethoxy group also deviates from the plane with a C18-C17-O3-C16 torsion angle of 86.4(3)°. In the crystal, the molecules pack in layers parallel to (101). For adjacent layers, the planes through A and the phenylamine groups are separated by about 3.65 Å.
Acknowledgements
The project was supported by King Saud University, Deanship of Scientific Research, Research Chairs.
References
CrysAlisPRO. Agilent Technologies, Yarnton, England (2014).Search in Google Scholar
Sheldrick, G. M.: A short history of SHELX. Acta Crystallogr. A64 (2008) 112–122.10.1107/S0108767307043930Search in Google Scholar PubMed
Sheldrick, G. M.: Crystal structure refinement with SHELXL. Acta Cryst. C71 (2015) 3–8.10.1107/S2053229614024218Search in Google Scholar PubMed PubMed Central
Mohamed, H. A.; Abdel-Wahab, B. F.; El-Hiti, G. A.: synthesis of some novel thiophene and thiazole derivatives and their antimicrobial evaluation. Heterocycles 94 (2017) 716–726.10.3987/COM-17-13668Search in Google Scholar
Gramec, D.; Mašič, L. P.; Dolenc, M. S.: Bioactivation potential of thiophene-containing drugs. Chem. Res. Toxicol. 27 (2014) 1344–1358.10.1021/tx500134gSearch in Google Scholar PubMed
Pillai, A. D.; Rathod, P. D.; Xavier, F. P.; Padh, H.; Sudarsanam, V.; Vasu, K. K.: Tetra substituted thiophenes as anti-inflammatory agents: exploitation of analogue-based drug design. Bioorg. Med. Chem. 13 (2005) 6685–6692.10.1016/j.bmc.2005.07.044Search in Google Scholar PubMed
Ghorab, M. M.; Bashandy, M. S.; Alsaid, M. S.: Novel thiophene derivatives with sulfonamide, isoxazole, benzothiazole, quinoline and anthracene moieties as potential anticancer agents. Acta Pharm. 64 (2014) 419–431.10.2478/acph-2014-0035Search in Google Scholar PubMed
Shaikh, M. H.; Subhedar, D. D.; Nawale, L.; Sarkar, D.; Khan, F. A. K.; Sangshetti, J. N.; Shingate, B. B.: 1,2,3-Triazole derivatives as antitubercular agents: synthesis, biological evaluation and molecular docking study. MedChemComm 6 (2015) 1104–1116.10.1039/C5MD00057BSearch in Google Scholar
Pokhodylo, N.; Shyyka, O.; Matiychuk, V.: Synthesis of 1,2,3-triazole derivatives and evaluation of their anticancer activity. Sci. Pharm. 81 (2013) 663–676.10.3797/scipharm.1302-04Search in Google Scholar PubMed PubMed Central
Slámová, K.; Marhol, P.; Bezouska, K.; Lindkvist, L.; Hansen, S. G.; Kren, V.; Jensen, H. H.: Synthesis and biological activity of glycosyl-1H-1,2,3-triazoles. Bioorg. Med. Chem. Lett. 20 (2010) 4263–4265.10.1016/j.bmcl.2010.04.151Search in Google Scholar PubMed
Farrugia, L. J.: WinGX and ORTEP for Windows: an update. J. Appl. Crystallogr. 45 (2012) 849–854.10.1107/S0021889812029111Search in Google Scholar
©2018 Mohammed H. Geesi et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 License.
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of (E)-1-(4-(((E)-2-hydroxy-5-methylbenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C17H18N2O2
- Crystal structure of 1,3,5,7-tetraazaadamantane-1,3-diium 2,5-dicarboxyterephthalate, C16H18N4O8
- Crystal structure of guanidinium tetrabutyl-ammonium 5-hydroxyisophthalate dihydrate, C25H50N4O7
- Crystal structure of poly[aqua-(μ2-5-methoxyisophthalate-κ3O,O′:O′′)-(μ2-1,4-bis((1H-1,2,4-triazol-1-yl)methyl)benzene-κ2N:N′)nickel(II), NiC21H20N6O6
- Crystal structure of aqua-bis(3,4-dimethoxybenzoato-κ1O)-(2,2′-bipyridine-κ2N,N′)copper(II), C28H26CuN2O9
- Crystal structure of catena-poly[aqua-(μ2-(3,5-di(1H-imidazol-1-yl)-pyridine-κ2N:N′)-(μ2-2-(carboxylatomethyl)benzoato-κ2O:O′)] cadmium(II), C20H17CdN5O5
- The crystal structure of catena-poly[chlorido-(μ2-5-methyl-1,3,4-thiadiazole-2-thiolato-κ2S:N)mercury(II)], C3H3ClHgN2S2
- Crystal structure of (E)-2,4-dichloro-6-(((4-methyl-2-nitrophenyl)imino)methyl)phenol, C14H10Cl2N2O3
- Crystal structure of a new polymorph of bis[μ-1,3-bis(diphenylphosphino)propane-κ2P:P′-disilver(I)] bis(tetrafluoroborate), [Ag(dppp)]2(BF4)2, C54H52Ag2B2F8P4
- The crystal structure of 2-phenyl-4,6-bis(R-tert-butylsulfonamido)-1,3,5-triazine – ethyl acetate (2/1), C38H58N10O6S4
- Crystal structure of 6-amino-8-(2-methoxy-phenyl)-2-methyl-2,3,8,8a-tetrahydro-1H-iso-quinoline-5,7,7-tricarbonitrile monohydrate, C20H21N5O2
- Crystal structure of methyl (1-phenylethyl)carbamate, C10H13NO2
- Crystal structure of dimethanol-(μ2-squarato-κ2O:O′)-tetrakis(tri-p-tolylphosphane-κP)disilver(I) – methanol (1/2), C92H98Ag2O8P4
- Crystal structure of catena-poly[bis(μ2-1,4-bis(triazol-1-ylmethyl)benzene-κ2N:N′)-bis(5-tert-butyl-isophthalate-κO)copper(II)]tetrahydrate, C36H46CuN6O12
- Crystal structure of 4-aminopyridinium 4-acetyl-(pyridin-4-yl)-1H-1,2,3-triazol-5-olate monohydrate, C14H16N6O3
- Crystal structure of 2-(8-bromo-2-phenylimidazo[1,2-α]pyridin-3-yl)-6,7-dimethyl-3-phenylquinoxaline, C29H21BrN4
- Crystal structure of aqua(1-(2-pyridyl)ethanone oxime-κ2N,N′)(1-(2-pyridyl)ethanone oximato-κ2N,N′) nitrate monohydrate, C14H19N5O7Cu
- Crystal structure of poly[tetraaqua-(μ4-oxalato-κ4O,O′:O′′,O′′′)-(μ8-benzene-1,2,4,5-tetracarboxylato-κ8O1:O2:O3:O4:O5:O6:O7:O8)yttrium(III)], C6H5O8Y
- Crystal structure of bis{catena-poly[(μ2-1,2-bis(4-pyridyl)ethane-κ2N:N′)silver(I)]} diaqua-bis(5-(4-carboxyphenyl)pyridine-2-carboxylato-κ2N,O)-(μ2-1,2-bis(4-pyridyl)ethane-κ2N:N′)disilver(I) octahydrate, C31H35Ag2N4O9
- Crystal structure of (E)-N-(2-(benzylamino)-2-oxo-1-(4-oxo-4H-chromen-3-yl)ethyl)-N-(4-bromophenyl)-3-chloroacrylamide hydrate, C27H22BrClN2O5
- Crystal structure of catena-poly[octaaqua-bis(μ2-4,6-dicarboxyisophthalate-κ2O:O′)cadmium(II)disodium(I)] dihydrate, C20H28CdNa2O26
- Crystal structure of acetonitrile{bis(2-benzimidazolylmethyl)amine-κ3N,N′,N′′}-{maleato-κO}zinc(II) perchlorate - acetonitrile (1/1), C24H24ClN7O8Zn
- Crystal structure of 2-amino-4-(3,5-dibromo-4-hydroxyphenyl)-7-methyl-5-oxo-2H,5H-pyrano[4,3-b]pyran-3-carbonitrile, C16H10Br2N2O4
- Crystal structure of catena-poly[diaqua-(μ2-3,5-bis(pyridin-4-ylmethoxy)benzoate-κ2N:O) manganese(II)] tetrahydrate [(3,5-bis-(pyridin-4-ylmethoxy)-benzoic-κ1Oκ1N) manganese(II)] trihydrate, C38H42MnN4O14
- The crystal structure of 2-carboxybenzaldehyde-2-phenylacetohydrazone, C16H14N2O3
- The crystal structure of poly[μ2-aqua-(μ2-2-naphthylamine-1-sulfonato-κ3O,O′:O′′)sodium(I)], C10H10N1O4S1Na
- The crystal structure of phthalazin-1(2H)-one, C8H6N2O1
- Crystal structure of 3,5-bis(trifluoromethyl)benzyl(Z)-N-(adamantan-1-yl)morpholine-4-carbothioimidate, C24H28F6N2OS
- Crystal structure of diazido-bis(μ2-pyridin-2-ylmethanolato-κ2N:O)-bis(pyridin-2-ylmethanolato-κ2N,O)dicobalt(III) – methanol (1/3), C27H35Co2N10O7
- Crystal structure of N-[[(4,6-dimethoxy-2-pyrimidinyl)amino]carbonyl]-3-(2,2,2-trifluoroethoxy)-2-pyridinesulfonamide, C14H14F3N5O6S
- Crystal structure of 1-phenyl-N′-(1-phenyl-5-(thiophen-2-yl)-1H-pyrazole-3-carbonyl)-5-(thiophen-2-yl)-1H-pyrazole-3-carbohydrazide, C28H20N6O2S2
- The crystal structure of poly[bis(4-hydroxybenzoato-κO)-(μ2-4,4′-bipyridine-κ2N:N′)copper(II)] hydrate, C24H20N2O7Cu
- Crystal structure of poly[μ3-5-(4-(2,6-di(pyridine-2-yl)pyridine-4-yl)phenoxy)isophthalato-κ5O:O′,O′′:N,N′,N′′cobalt(II)], C29H17CoN3O5
- Crystal structure of poly[μ3-5-(4-(2,6-di(pyridine-2-yl)pyridine-4-yl)phenoxy)isophthalato-κ6O:O′,O′′:N,N′,N′′)cobalt(II)] C29H17CoN3O5
- Crystal structure of diaqua-(acetato-κ3O,O′:O′′)-(μ3-4,6-di(1H-imidazol-1-yl)isophthalato-κ4O:O′:O′′,O′′′)lanthanum(III), C16H15LaN4O8
- Synthesis and crystal structure of 6-carboxy-1-(3,5-dicarboxyphenyl)-1H-benzo[d]imidazol-3-ium-5-carboxylate dihydrate, C18H12N2O8
- Crystal structure of (E)-2-hydroxybenzaldehyde O-(2-(((E)-(4-(dimethylamino)benzylidene)amino)oxy)ethyl)oxime, C18H21N3O3
- Crystal structure of bis{2-((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}zinc(II), C32H30N4O4Zn
- Crystal structure of bis(9-aminoacridin-10-ium) tetrachloridocuprate(II) monohydrate, C26H24Cl4CuN4O
- The crystal structure of 4-tert-butyl-N′-[(E)-(4-fluoro-3-methoxyphenyl)methylidene]benzohydrazide, C19H21F1N2O2
- Crystal structure of (E)-3-(3-(5-methyl-1-4-tolyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazol-4-yl)-1-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)prop-2-en-1-one, C31H26N8O
- Crystal structure of (E)-N′-(4-methoxybenzylidene)-5-methyl-1-(4-tolyl)-1H-1,2,3-triazole-4-carbohydrazide, C19H19N5O2
- Crystal structure and molecular packing of O-ethyl (2-chlorophenyl)carbamothioate, C9H10ClNOS
- Crystal structure of pyrene-2-carbaldehyde, C17H10O
- Crystal structure of (E)-2,4-diiodo-6-(4-methyl-2-nitrostyryl)phenol, C14H10I2N2O3
- Crystal structure of (E)-2,4-dichloro-6-(((4-methoxy-2-nitrophenyl)imino)methyl)phenol, C14H10Cl2N2O4
- Crystal structure of (E)-2-bromo-4-chloro-6-(4-methoxy-2-nitrostyryl)phenol, C14H10BrClN2O4
- Crystal structure of (E)-4,6-diiodo-2-(((4-methoxy-2-nitrophenyl)imino)methyl)-3-methylphenol, C14H10I2N2O4
- The crystal structure of 7-bromo-1-cyclopropyl-8-methyl-4-oxo-1,4-dihydroquinoline-3-carboxylic acid an intermediate of the ozenoxacin synthesis, C14H12BrNO3
- Crystal structure of bis(N-(1-(pyrazin-2-yl)ethylidene)nicotinohydrazonato-κ3N,N′,O)copper(II) C24H20N10O2Cu
- Crystal structure of diaqua-dinitrato-k2O,O′((Z)-N-((E)-1-(pyrazin-2-yl)ethylidene)nicotinohydrazonato-k3N,N′,O)europium(II), C12H14N7O9Eu
- Crystal structure of ethyl 4-amino-5-(5-methyl-1-(4-tolyl)-1H-1,2,3-triazole-4-carbonyl)-2-(phenylamino)thiophene-3-carboxylate, C24H23N5O3S
- The crystal structure of acridin-10-ium2-carboxybenzoate, C21H15NO4
- The crystal structure of 3-((phenylamino)methylene)-1,5-dioxaspiro[5.5]undecane-2,4-dione, C16H17N1O4
- Crystal structure of 12-chloro-5,6,7,12-tetrahydrodibenzo[c,f][1,5]oxastibocine, C14H12ClOSb
- Crystal structure of 4-((1,3-dioxoisoindolin-2-yl)methyl)phenethyl 4-methylbenzenesulfonate, C24H21NO5S
- Crystal structure of 3-methyl-2,3-dihydro-2-thioxoquinazolin-4(1H)-one, C9H8N2OS
- Crystal structure of tert-butyl (2-(4-oxo-2-thioxo-1,4-dihydroquinazolin-3(2H)-yl)ethyl)carbamate, C15H19N3O3S
- Crystal structure of ethyl 5-formyl-3,4-dimethylpyrrole-2-carboxylate–1-(propan-2-ylidene)thiosemicarbazide (1/1), C14H22N4O3S
- Crystal structure of bis-(N′-(5-ethoxycarbonyl-3,4-dimethyl-pyrrol-2-yl-methylidene)-3-hydroxybenzohydrazide-κ2O,N)copper(II) – dimethylformamide (1/2), C40H50N8O10Cu
- Crystal structure of bis(acetato-κO)bis{2-((1H-tetrazol-1-yl)methyl)-1H-benzo[d]imidazole-κN}zinc(II), C22H22N12O4Zn
- Crystal structure of 4-phenyl-3-((4-phenyl-1H-1,2,3-triazol-1-yl)methyl)-1H-1,2,4-triazole-5(4H)-thione, C17H14N6S
- Crystal structure of (Z)-N-(4-nitrophenyl)-3-phenyl-3-(phenylamino)acrylamide, C21H17N3O3
- Crystal structure of 1,1′-(pentane-1,5-diyl)bis(3-methyl-1H-imidazol-3-ium)bis(hexafluorophosphate), C13H22F12N4P2
- Synthesis and crystal structure of bis(furan-2-ylmethanaminium)-catena-[bis(μ2-phthalato-κ2O:O′)cobalt(II)], C26H24CoN2O10
- Crystal structure of methyl (R)-4-(o-chlorobenzoyl)-1-thia-4-azaspiro[4.5]decane-3-carboxylate, C17H20ClNO3S
- Crystal structure of 2-[[4-[2-[4-(4-methoxyphenyl)-1-piperazinyl]ethyl]phenyl] methyl]-1H-isoindole-1,3(2H)-dione, C28H29N3O3
- The crystal structure of benzenaminium 5,7-dihydroxy-4-oxo-2-phenyl-4H-chromene-8-sulfonate hydrate, C21H19NO8S
- Crystal structure of semiconducting potassium poly[(μ2-tetraselenido-κ2Se1:Se4)(μ2-pentaselenido-κ1Se1:Se1)argentate(I)], K3AgSe9
- Crystal structure of 2-isopropyl-8-methyl-phenanthrene-3,4-dione, C18H16O2
- Crystal structure of 2-isopropyl-8,8-dimethyl-5,6,7,8-tetrahydrophenanthrene-3,4-dione, C19H22O2
- Crystal structure of (E)-2-(1-((2-aminophenyl)imino)ethyl)-4-bromophenol, C14H13BrN2O
- Crystal structure of 1,1-di(4-cyanophenyl)-2,2-diphenylethene, C28H18N2
- Crystal structure of bis(hydroxylamido-κ2O,N)-oxido(1H-pyrazole-3-carboxylato-κ2O,N)vanadium(V), C4H7N4O5V
- The crystal structure of In1.2B3O5.6(OH)1.4
- The crystal structure of chlorido(2-(1H-pyrazol-3-yl)phenolato-κ2N,O)(2-(1H-pyrazol-3-yl)phenol-κN)copper(II), C18H15ClCuN4O2
- Crystal structure of 1-heptylpyridazin-1-ium iodide, C11H19N2I
- The crystal structure of N-butylpyridinium bis(μ2-dichlorido)-tetrachloridodicopper(II), C18H28N2Cu2Cl6
- Crystal structure of 6-hydroxy-5-((2-hydroxy-6-oxocyclohex-1-en-1-yl)(4-methoxyphenyl)methyl)-1,3-dimethylpyrimidine-2,4(1H,3H)-dione, C20H22N2O6
- Crystal structure of bis(acetonitrile)-diaqua-dichloridoiron(II), C4H10Cl2N2O2Fe
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of (E)-1-(4-(((E)-2-hydroxy-5-methylbenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C17H18N2O2
- Crystal structure of 1,3,5,7-tetraazaadamantane-1,3-diium 2,5-dicarboxyterephthalate, C16H18N4O8
- Crystal structure of guanidinium tetrabutyl-ammonium 5-hydroxyisophthalate dihydrate, C25H50N4O7
- Crystal structure of poly[aqua-(μ2-5-methoxyisophthalate-κ3O,O′:O′′)-(μ2-1,4-bis((1H-1,2,4-triazol-1-yl)methyl)benzene-κ2N:N′)nickel(II), NiC21H20N6O6
- Crystal structure of aqua-bis(3,4-dimethoxybenzoato-κ1O)-(2,2′-bipyridine-κ2N,N′)copper(II), C28H26CuN2O9
- Crystal structure of catena-poly[aqua-(μ2-(3,5-di(1H-imidazol-1-yl)-pyridine-κ2N:N′)-(μ2-2-(carboxylatomethyl)benzoato-κ2O:O′)] cadmium(II), C20H17CdN5O5
- The crystal structure of catena-poly[chlorido-(μ2-5-methyl-1,3,4-thiadiazole-2-thiolato-κ2S:N)mercury(II)], C3H3ClHgN2S2
- Crystal structure of (E)-2,4-dichloro-6-(((4-methyl-2-nitrophenyl)imino)methyl)phenol, C14H10Cl2N2O3
- Crystal structure of a new polymorph of bis[μ-1,3-bis(diphenylphosphino)propane-κ2P:P′-disilver(I)] bis(tetrafluoroborate), [Ag(dppp)]2(BF4)2, C54H52Ag2B2F8P4
- The crystal structure of 2-phenyl-4,6-bis(R-tert-butylsulfonamido)-1,3,5-triazine – ethyl acetate (2/1), C38H58N10O6S4
- Crystal structure of 6-amino-8-(2-methoxy-phenyl)-2-methyl-2,3,8,8a-tetrahydro-1H-iso-quinoline-5,7,7-tricarbonitrile monohydrate, C20H21N5O2
- Crystal structure of methyl (1-phenylethyl)carbamate, C10H13NO2
- Crystal structure of dimethanol-(μ2-squarato-κ2O:O′)-tetrakis(tri-p-tolylphosphane-κP)disilver(I) – methanol (1/2), C92H98Ag2O8P4
- Crystal structure of catena-poly[bis(μ2-1,4-bis(triazol-1-ylmethyl)benzene-κ2N:N′)-bis(5-tert-butyl-isophthalate-κO)copper(II)]tetrahydrate, C36H46CuN6O12
- Crystal structure of 4-aminopyridinium 4-acetyl-(pyridin-4-yl)-1H-1,2,3-triazol-5-olate monohydrate, C14H16N6O3
- Crystal structure of 2-(8-bromo-2-phenylimidazo[1,2-α]pyridin-3-yl)-6,7-dimethyl-3-phenylquinoxaline, C29H21BrN4
- Crystal structure of aqua(1-(2-pyridyl)ethanone oxime-κ2N,N′)(1-(2-pyridyl)ethanone oximato-κ2N,N′) nitrate monohydrate, C14H19N5O7Cu
- Crystal structure of poly[tetraaqua-(μ4-oxalato-κ4O,O′:O′′,O′′′)-(μ8-benzene-1,2,4,5-tetracarboxylato-κ8O1:O2:O3:O4:O5:O6:O7:O8)yttrium(III)], C6H5O8Y
- Crystal structure of bis{catena-poly[(μ2-1,2-bis(4-pyridyl)ethane-κ2N:N′)silver(I)]} diaqua-bis(5-(4-carboxyphenyl)pyridine-2-carboxylato-κ2N,O)-(μ2-1,2-bis(4-pyridyl)ethane-κ2N:N′)disilver(I) octahydrate, C31H35Ag2N4O9
- Crystal structure of (E)-N-(2-(benzylamino)-2-oxo-1-(4-oxo-4H-chromen-3-yl)ethyl)-N-(4-bromophenyl)-3-chloroacrylamide hydrate, C27H22BrClN2O5
- Crystal structure of catena-poly[octaaqua-bis(μ2-4,6-dicarboxyisophthalate-κ2O:O′)cadmium(II)disodium(I)] dihydrate, C20H28CdNa2O26
- Crystal structure of acetonitrile{bis(2-benzimidazolylmethyl)amine-κ3N,N′,N′′}-{maleato-κO}zinc(II) perchlorate - acetonitrile (1/1), C24H24ClN7O8Zn
- Crystal structure of 2-amino-4-(3,5-dibromo-4-hydroxyphenyl)-7-methyl-5-oxo-2H,5H-pyrano[4,3-b]pyran-3-carbonitrile, C16H10Br2N2O4
- Crystal structure of catena-poly[diaqua-(μ2-3,5-bis(pyridin-4-ylmethoxy)benzoate-κ2N:O) manganese(II)] tetrahydrate [(3,5-bis-(pyridin-4-ylmethoxy)-benzoic-κ1Oκ1N) manganese(II)] trihydrate, C38H42MnN4O14
- The crystal structure of 2-carboxybenzaldehyde-2-phenylacetohydrazone, C16H14N2O3
- The crystal structure of poly[μ2-aqua-(μ2-2-naphthylamine-1-sulfonato-κ3O,O′:O′′)sodium(I)], C10H10N1O4S1Na
- The crystal structure of phthalazin-1(2H)-one, C8H6N2O1
- Crystal structure of 3,5-bis(trifluoromethyl)benzyl(Z)-N-(adamantan-1-yl)morpholine-4-carbothioimidate, C24H28F6N2OS
- Crystal structure of diazido-bis(μ2-pyridin-2-ylmethanolato-κ2N:O)-bis(pyridin-2-ylmethanolato-κ2N,O)dicobalt(III) – methanol (1/3), C27H35Co2N10O7
- Crystal structure of N-[[(4,6-dimethoxy-2-pyrimidinyl)amino]carbonyl]-3-(2,2,2-trifluoroethoxy)-2-pyridinesulfonamide, C14H14F3N5O6S
- Crystal structure of 1-phenyl-N′-(1-phenyl-5-(thiophen-2-yl)-1H-pyrazole-3-carbonyl)-5-(thiophen-2-yl)-1H-pyrazole-3-carbohydrazide, C28H20N6O2S2
- The crystal structure of poly[bis(4-hydroxybenzoato-κO)-(μ2-4,4′-bipyridine-κ2N:N′)copper(II)] hydrate, C24H20N2O7Cu
- Crystal structure of poly[μ3-5-(4-(2,6-di(pyridine-2-yl)pyridine-4-yl)phenoxy)isophthalato-κ5O:O′,O′′:N,N′,N′′cobalt(II)], C29H17CoN3O5
- Crystal structure of poly[μ3-5-(4-(2,6-di(pyridine-2-yl)pyridine-4-yl)phenoxy)isophthalato-κ6O:O′,O′′:N,N′,N′′)cobalt(II)] C29H17CoN3O5
- Crystal structure of diaqua-(acetato-κ3O,O′:O′′)-(μ3-4,6-di(1H-imidazol-1-yl)isophthalato-κ4O:O′:O′′,O′′′)lanthanum(III), C16H15LaN4O8
- Synthesis and crystal structure of 6-carboxy-1-(3,5-dicarboxyphenyl)-1H-benzo[d]imidazol-3-ium-5-carboxylate dihydrate, C18H12N2O8
- Crystal structure of (E)-2-hydroxybenzaldehyde O-(2-(((E)-(4-(dimethylamino)benzylidene)amino)oxy)ethyl)oxime, C18H21N3O3
- Crystal structure of bis{2-((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}zinc(II), C32H30N4O4Zn
- Crystal structure of bis(9-aminoacridin-10-ium) tetrachloridocuprate(II) monohydrate, C26H24Cl4CuN4O
- The crystal structure of 4-tert-butyl-N′-[(E)-(4-fluoro-3-methoxyphenyl)methylidene]benzohydrazide, C19H21F1N2O2
- Crystal structure of (E)-3-(3-(5-methyl-1-4-tolyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazol-4-yl)-1-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)prop-2-en-1-one, C31H26N8O
- Crystal structure of (E)-N′-(4-methoxybenzylidene)-5-methyl-1-(4-tolyl)-1H-1,2,3-triazole-4-carbohydrazide, C19H19N5O2
- Crystal structure and molecular packing of O-ethyl (2-chlorophenyl)carbamothioate, C9H10ClNOS
- Crystal structure of pyrene-2-carbaldehyde, C17H10O
- Crystal structure of (E)-2,4-diiodo-6-(4-methyl-2-nitrostyryl)phenol, C14H10I2N2O3
- Crystal structure of (E)-2,4-dichloro-6-(((4-methoxy-2-nitrophenyl)imino)methyl)phenol, C14H10Cl2N2O4
- Crystal structure of (E)-2-bromo-4-chloro-6-(4-methoxy-2-nitrostyryl)phenol, C14H10BrClN2O4
- Crystal structure of (E)-4,6-diiodo-2-(((4-methoxy-2-nitrophenyl)imino)methyl)-3-methylphenol, C14H10I2N2O4
- The crystal structure of 7-bromo-1-cyclopropyl-8-methyl-4-oxo-1,4-dihydroquinoline-3-carboxylic acid an intermediate of the ozenoxacin synthesis, C14H12BrNO3
- Crystal structure of bis(N-(1-(pyrazin-2-yl)ethylidene)nicotinohydrazonato-κ3N,N′,O)copper(II) C24H20N10O2Cu
- Crystal structure of diaqua-dinitrato-k2O,O′((Z)-N-((E)-1-(pyrazin-2-yl)ethylidene)nicotinohydrazonato-k3N,N′,O)europium(II), C12H14N7O9Eu
- Crystal structure of ethyl 4-amino-5-(5-methyl-1-(4-tolyl)-1H-1,2,3-triazole-4-carbonyl)-2-(phenylamino)thiophene-3-carboxylate, C24H23N5O3S
- The crystal structure of acridin-10-ium2-carboxybenzoate, C21H15NO4
- The crystal structure of 3-((phenylamino)methylene)-1,5-dioxaspiro[5.5]undecane-2,4-dione, C16H17N1O4
- Crystal structure of 12-chloro-5,6,7,12-tetrahydrodibenzo[c,f][1,5]oxastibocine, C14H12ClOSb
- Crystal structure of 4-((1,3-dioxoisoindolin-2-yl)methyl)phenethyl 4-methylbenzenesulfonate, C24H21NO5S
- Crystal structure of 3-methyl-2,3-dihydro-2-thioxoquinazolin-4(1H)-one, C9H8N2OS
- Crystal structure of tert-butyl (2-(4-oxo-2-thioxo-1,4-dihydroquinazolin-3(2H)-yl)ethyl)carbamate, C15H19N3O3S
- Crystal structure of ethyl 5-formyl-3,4-dimethylpyrrole-2-carboxylate–1-(propan-2-ylidene)thiosemicarbazide (1/1), C14H22N4O3S
- Crystal structure of bis-(N′-(5-ethoxycarbonyl-3,4-dimethyl-pyrrol-2-yl-methylidene)-3-hydroxybenzohydrazide-κ2O,N)copper(II) – dimethylformamide (1/2), C40H50N8O10Cu
- Crystal structure of bis(acetato-κO)bis{2-((1H-tetrazol-1-yl)methyl)-1H-benzo[d]imidazole-κN}zinc(II), C22H22N12O4Zn
- Crystal structure of 4-phenyl-3-((4-phenyl-1H-1,2,3-triazol-1-yl)methyl)-1H-1,2,4-triazole-5(4H)-thione, C17H14N6S
- Crystal structure of (Z)-N-(4-nitrophenyl)-3-phenyl-3-(phenylamino)acrylamide, C21H17N3O3
- Crystal structure of 1,1′-(pentane-1,5-diyl)bis(3-methyl-1H-imidazol-3-ium)bis(hexafluorophosphate), C13H22F12N4P2
- Synthesis and crystal structure of bis(furan-2-ylmethanaminium)-catena-[bis(μ2-phthalato-κ2O:O′)cobalt(II)], C26H24CoN2O10
- Crystal structure of methyl (R)-4-(o-chlorobenzoyl)-1-thia-4-azaspiro[4.5]decane-3-carboxylate, C17H20ClNO3S
- Crystal structure of 2-[[4-[2-[4-(4-methoxyphenyl)-1-piperazinyl]ethyl]phenyl] methyl]-1H-isoindole-1,3(2H)-dione, C28H29N3O3
- The crystal structure of benzenaminium 5,7-dihydroxy-4-oxo-2-phenyl-4H-chromene-8-sulfonate hydrate, C21H19NO8S
- Crystal structure of semiconducting potassium poly[(μ2-tetraselenido-κ2Se1:Se4)(μ2-pentaselenido-κ1Se1:Se1)argentate(I)], K3AgSe9
- Crystal structure of 2-isopropyl-8-methyl-phenanthrene-3,4-dione, C18H16O2
- Crystal structure of 2-isopropyl-8,8-dimethyl-5,6,7,8-tetrahydrophenanthrene-3,4-dione, C19H22O2
- Crystal structure of (E)-2-(1-((2-aminophenyl)imino)ethyl)-4-bromophenol, C14H13BrN2O
- Crystal structure of 1,1-di(4-cyanophenyl)-2,2-diphenylethene, C28H18N2
- Crystal structure of bis(hydroxylamido-κ2O,N)-oxido(1H-pyrazole-3-carboxylato-κ2O,N)vanadium(V), C4H7N4O5V
- The crystal structure of In1.2B3O5.6(OH)1.4
- The crystal structure of chlorido(2-(1H-pyrazol-3-yl)phenolato-κ2N,O)(2-(1H-pyrazol-3-yl)phenol-κN)copper(II), C18H15ClCuN4O2
- Crystal structure of 1-heptylpyridazin-1-ium iodide, C11H19N2I
- The crystal structure of N-butylpyridinium bis(μ2-dichlorido)-tetrachloridodicopper(II), C18H28N2Cu2Cl6
- Crystal structure of 6-hydroxy-5-((2-hydroxy-6-oxocyclohex-1-en-1-yl)(4-methoxyphenyl)methyl)-1,3-dimethylpyrimidine-2,4(1H,3H)-dione, C20H22N2O6
- Crystal structure of bis(acetonitrile)-diaqua-dichloridoiron(II), C4H10Cl2N2O2Fe

