Abstract
C13H22F12N4P2, monoclinic, P21/c, a = 12.9171(13) Å, b = 13.3953(13) Å, c = 12.5808(12) Å, β = 97.766(1)°, V = 2156.9(4) Å3, Z = 4,Rgt(F) = 0.0583, wRref(F2) = 0.1557, T = 296(2) K.
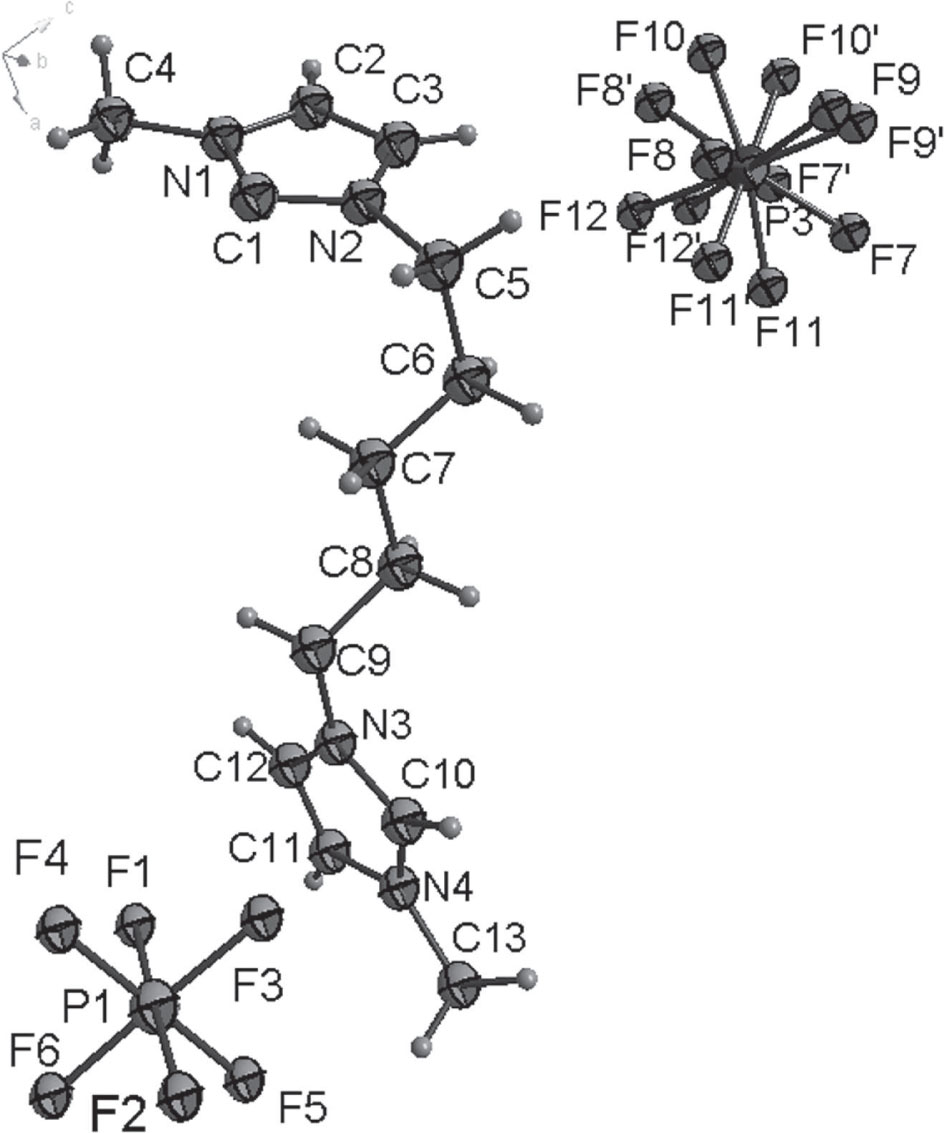
The crystal structure is shown in the figure. Tables 1 and 2 contain details on crystal structure and measurement conditions and a list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colorless block |
| Size: | 0.21 × 0.20 × 0.19 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.31 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω-scans |
| θmax, completeness: | 25.5°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 16239, 4010, 0.033 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 2890 |
| N(param)refined: | 338 |
| Programs: | Bruker programs [1], SHELX [2] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| N1 | −0.1664(2) | 0.2727(2) | 0.4032(2) | 0.0650(7) |
| N2 | −0.02379(19) | 0.28810(19) | 0.5099(2) | 0.0575(7) |
| N3 | 0.3493(2) | 0.4298(2) | 0.2098(2) | 0.0652(7) |
| N4 | 0.4863(2) | 0.5092(2) | 0.1792(2) | 0.0686(8) |
| P1 | 0.52608(7) | 0.20209(6) | 0.07292(7) | 0.0603(3) |
| P3 | 0.07664(8) | 0.51739(8) | 0.81503(8) | 0.0724(3) |
| F1 | 0.4346(2) | 0.2733(2) | 0.0285(3) | 0.1443(12) |
| F2 | 0.61817(18) | 0.13149(18) | 0.1215(2) | 0.0992(8) |
| F3 | 0.5080(2) | 0.2244(2) | 0.1927(2) | 0.1141(9) |
| F4 | 0.44853(19) | 0.11101(19) | 0.0688(2) | 0.1060(8) |
| F5 | 0.6052(2) | 0.2914(2) | 0.0786(2) | 0.1276(11) |
| F6 | 0.5471(2) | 0.1799(3) | −0.04392(19) | 0.1306(11) |
| F7a | 0.1675(11) | 0.5845(8) | 0.8689(11) | 0.190(5) |
| F8a | 0.0986(13) | 0.4094(5) | 0.8066(12) | 0.189(5) |
| F9 a | 0.1122(9) | 0.4474(10) | 0.9202(10) | 0.152(4) |
| F10a | −0.0404(5) | 0.4992(14) | 0.8311(12) | 0.173(6) |
| F11a | 0.1830(7) | 0.5365(10) | 0.7795(16) | 0.179(6) |
| F12a | 0.0365(11) | 0.5282(6) | 0.6908(5) | 0.134(4) |
| F7’b | 0.0548(10) | 0.6326(6) | 0.8133(8) | 0.132(4) |
| F8’b | 0.0027(13) | 0.4322(12) | 0.7749(6) | 0.165(6) |
| F9’b | 0.1056(12) | 0.5216(16) | 0.9296(7) | 0.192(6) |
| F10’b | −0.0001(12) | 0.5584(13) | 0.8789(12) | 0.200(6) |
| F11’b | 0.1527(12) | 0.476(2) | 0.7517(12) | 0.272(11) |
| F12’b | 0.0396(11) | 0.590(3) | 0.733(3) | 0.337(14) |
| C1 | −0.0707(3) | 0.2384(2) | 0.4278(3) | 0.0645(8) |
| H1 | −0.041181 | 0.186915 | 0.392340 | 0.077* |
| C2 | −0.1801(3) | 0.3470(3) | 0.4727(3) | 0.0782(10) |
| H2 | −0.240510 | 0.384554 | 0.473694 | 0.094* |
| C3 | −0.0921(3) | 0.3568(3) | 0.5389(3) | 0.0747(10) |
| H3 | −0.079358 | 0.402278 | 0.595040 | 0.090* |
| C4 | −0.2423(3) | 0.2406(4) | 0.3117(3) | 0.0999(14) |
| H4A | −0.248121 | 0.291045 | 0.257087 | 0.150* |
| H4B | −0.309208 | 0.230400 | 0.335190 | 0.150* |
| H4C | −0.219075 | 0.179265 | 0.283233 | 0.150* |
| C5 | 0.0838(3) | 0.2752(3) | 0.5611(3) | 0.0737(10) |
| H5A | 0.105875 | 0.207134 | 0.550145 | 0.088* |
| H5B | 0.086822 | 0.285836 | 0.637739 | 0.088* |
| C6 | 0.1583(3) | 0.3466(3) | 0.5171(3) | 0.0739(10) |
| H6A | 0.129306 | 0.413406 | 0.516620 | 0.089* |
| H6B | 0.223874 | 0.347000 | 0.564912 | 0.089* |
| C7 | 0.1801(3) | 0.3216(3) | 0.4062(3) | 0.0704(9) |
| H7A | 0.114245 | 0.317584 | 0.359115 | 0.085* |
| H7B | 0.212818 | 0.256411 | 0.407454 | 0.085* |
| C8 | 0.2501(3) | 0.3968(3) | 0.3602(3) | 0.0650(8) |
| H8A | 0.213024 | 0.459562 | 0.347692 | 0.078* |
| H8B | 0.311672 | 0.408674 | 0.411869 | 0.078* |
| C9 | 0.2822(4) | 0.3611(3) | 0.2589(4) | 0.0971(14) |
| H9A | 0.318817 | 0.298191 | 0.272298 | 0.117* |
| H9B | 0.220072 | 0.348424 | 0.208327 | 0.117* |
| C10 | 0.4486(3) | 0.4454(2) | 0.2431(3) | 0.0641(8) |
| H10 | 0.486480 | 0.415782 | 0.302964 | 0.077* |
| C11 | 0.4081(4) | 0.5357(3) | 0.1021(3) | 0.0852(12) |
| H11 | 0.412862 | 0.580438 | 0.046474 | 0.102* |
| C12 | 0.3230(4) | 0.4863(3) | 0.1200(3) | 0.0849(12) |
| H12 | 0.257583 | 0.489575 | 0.078931 | 0.102* |
| C13 | 0.5941(4) | 0.5428(4) | 0.1882(4) | 0.1133(17) |
| H13A | 0.632832 | 0.499230 | 0.147563 | 0.170* |
| H13B | 0.624474 | 0.541794 | 0.262166 | 0.170* |
| H13C | 0.596154 | 0.609555 | 0.160811 | 0.170* |
aOccupancies: a = 526(7), b = 0.474(7).
Source of materials
1-Methylimidazole (8.21 g, 0.1 mol) was dissolved in methylbenzene (20 mL), 1,5-dibromopentane (11.35 g, 0.05 mol) was quickly added under stirring. The mixture first reacted at 90 °C for 10 min, and then heated to 110 °C for 7–10 hours. After the reaction has completed (monitored by TLC), a white solid was produced. The resulting suspension was filtered, crushed and washed with hexane, ethylacetate and diethyl ether three times respectively. Then the intermediate (C5M—Br)(0.5 g, 0.00127 mol), potassium hexafluorophosphate (0.6 g, 0.0029 mol) was dissolved in deionized water (20 mL). The mixture stirred well for 12 h at 85 °C and then cooled slowly. Colorless crystals were produced and filtered. They were washed with deionized water many times until silver bromide precipitation occurs anymore. The product (0.1 g) was dissolved in ethanol: water (2:1) solution and (7 mL), then the mixture reacted in the microwave synthesizer at 100 °C for 2 min. Crystals suitable for X-ray analysis were obtained after cooled slowly.
Experimental details
All H atoms were included in calculated positions and refined as riding atoms, with C–H = 0.93–0.97 Å with Uiso(H) = 1.5 Ueq(C) for methyl H atoms and 1.2 Ueq(C) for all other H atoms. There is a disorder of one of the two hexafluorophosphate anions (cf. the figure).
Discussion
Ionic liquid, as a new type of environmentally friendly solvent and liquid acid base catalyst, owing to the advantages of adjustable structure, high catalytic efficiency, mild conditions, and can be recycled, etc, has been widely used in catalytic science, electrochemistry, environmental science, extraction and separation, biomass energy, resource conversion and other fields [3], [4], [5]. Because of the unique physical and chemical properties of ionic liquids, ionic liquids have the unique potential advantages of biodiesel preparation. In recent years, various functional ionic liquids have been synthesised, and have been used to prepare biodiesel highly efficiently and environmental friendly [6], [7]. It was found that dinuclear alkaline ionic liquid bis-(3-methyl-1-imidazolium-)-ethlyene dihydroxide([MC2]OH) shows excellent catalytic efficiency, the highest conversion rate of cotton seed oil was up to 98.5%, and the stability of and separation effect of the catalyst was very ideal [8].
Recently, our group still focused on the preparation of biodiesel catalyzed by ionic liquid [9], [10] and reported two crystal structures of 1,1′-butanebis(3-methyl-1H-imidazol-3-ium),bis(hexafluorophosphate) and 1,1′-(hexane-1,6-diyl)bis(3-methyl-1H-imidazol-3-ium), bis(hexafluorophosphate) [11], [12]. In order to find the ionic liquid catalyst with better catalytic efficiency, we were engaged in synthesising the novel ionic liquid catalyst with imidazole. Herein, we report the synthesis and structure of the bisimidazoles ionic liquid. Bond lengths and angles within the imidazole ring are very similar to those given in the literature for diimidazole ionic liquid [13]. The title structure consists of one C5M2+ cation(1,1′-(pentane-1,5-diyl)bis(3-methyl-1H-imidazol-3-ium)), and two PF−6 anions (cf. the figure). Two cationic 1-ethylimidazolium rings were bound to the both sides of pentyl group. The two imidazole rings are almost crystallographically dependent planar and the dihedral angle of two imidazole rings is 89.207°.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 31760193, 31760068) and the Research Foundation of Educational Department of Jiangxi Province[GJJ160382, 160408]. X-ray data were collected at Instrumental Analysis Center Nanchang Hangkong University, Nanchang, 330063, People’s Republic of China.
References
Bruker. APEX2, SAINT and SADABS. Brucker AXS Inc., Madison, WI, USA (2009).Search in Google Scholar
Sheldrick, G. M.: A short history of SHELX. Acta Crystallogr. A64 (2008) 112–122.10.1107/S0108767307043930Search in Google Scholar
Zhen, B.; Jiao, Q. Z.; Wu, Q.; Li, H. S.: Catalytic performance of acidic ionic liquid-functionalized silica in biodiesel production. J. Energy Chem. 23 (2014) 97–104.10.1016/S2095-4956(14)60122-4Search in Google Scholar
Rafiee, E.; Eavani, S.: A new organic–inorganic hybrid ionic liquid polyoxometalate for biodiesel production. J. Mol. Liq. 199 (2014) 96–101.10.1016/j.molliq.2014.08.034Search in Google Scholar
Fan, M. M.; Zhou, J. J.; Han, Q. J.; Zhang. P. B.: Effect of various functional groups on biodiesel synthesis from soybean oils by acidic ionic liquids. Chin. Chem. Lett. 23 (2012) 1107–1110.10.1016/j.cclet.2012.07.009Search in Google Scholar
Han, X. X.; He, Y. F.; Hung, C. T.; Liu, L. L.; Huang, S. J.; Liu, S. B.: Efficient and reusable polyoxometalate-based sulfonated ionic liquid catalysts for palmitic acid esterification to biodiesel. Chem. Eng. Sci. 104 (2013) 64–72.10.1016/j.ces.2013.08.059Search in Google Scholar
Li, Y.; Hu, S. G.; Cheng, J. H.; Lou, W. Y.: Acidic ionic liquid catalyzed esterification of oleic acid for biodiesel synthesis. Chin. J. Catal. 35 (2014) 396–406.10.1016/S1872-2067(14)60005-XSearch in Google Scholar
Liang, J. H.; Ren, X. Q.; Wang, J. T.: Preparation of biodiesel by transesterification from cottonseed oil using thebasic dication ionic liquids as catalysts. Wood. Chem. Techn. J. 38 (2010) 275–280.10.1016/S1872-5813(10)60033-3Search in Google Scholar
Xiong, W. M.; Zhu, M. Z.; Deng, L.; Fu, Y.; Guo, Q. X.: Esterification of organic acid in bio-oil using acidic ionic liquid catalysts. Energy. Fuel. 23 (2009) 2278–2283.10.1021/ef801021jSearch in Google Scholar
Kong, J. H.; Lan, Y. D.; Chen, J.; Huang, C. G.; Xiong, W. M.: Preparation and component analysis of biodiesel catalyzed by functionalized dication ionic liquid. Acta Agric. Univ. Jiangxiensis. 38 (2016) 386–390.Search in Google Scholar
Xiong, W. M.; Chen, J.; Peng, D. Y.; Nie, X. L.; Huang, C. G.: Crystal structure of 1,1′-butanebis(3-methyl-1H-imidazol-3-ium)bis(hexafluorophosphate),C12H20F12N4P2. Kristallogr. NCS. 232 (2017) 1007–1008.10.1515/ncrs-2017-0135Search in Google Scholar
Nie, X. L.; Kong, J. H.; Chen, J.; Chen, J. Z.; Xiong, W. M.: Crystal structure of 1,1′-(hexane-1,6-diyl)bis(3-methyl-1H-imidazol-3-ium)bis(hexafluorophosphate),C9H14F12N4P2. Kristallogr. NCS. 232 (2017) 73–74.10.1515/ncrs-2016-0153Search in Google Scholar
Noh, T. H.; Ahn, J. M.; Na, Y. M.; Jung, O. S.: Anion effects on structure and properties of bis(3-methylimidazolium-1-yl) salts. Bull. Korean. Chem. Soc. 32 (2011) 2795–2798.10.5012/bkcs.2011.32.8.2795Search in Google Scholar
©2018 Xiong Wan-Ming et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 License.
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of (E)-1-(4-(((E)-2-hydroxy-5-methylbenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C17H18N2O2
- Crystal structure of 1,3,5,7-tetraazaadamantane-1,3-diium 2,5-dicarboxyterephthalate, C16H18N4O8
- Crystal structure of guanidinium tetrabutyl-ammonium 5-hydroxyisophthalate dihydrate, C25H50N4O7
- Crystal structure of poly[aqua-(μ2-5-methoxyisophthalate-κ3O,O′:O′′)-(μ2-1,4-bis((1H-1,2,4-triazol-1-yl)methyl)benzene-κ2N:N′)nickel(II), NiC21H20N6O6
- Crystal structure of aqua-bis(3,4-dimethoxybenzoato-κ1O)-(2,2′-bipyridine-κ2N,N′)copper(II), C28H26CuN2O9
- Crystal structure of catena-poly[aqua-(μ2-(3,5-di(1H-imidazol-1-yl)-pyridine-κ2N:N′)-(μ2-2-(carboxylatomethyl)benzoato-κ2O:O′)] cadmium(II), C20H17CdN5O5
- The crystal structure of catena-poly[chlorido-(μ2-5-methyl-1,3,4-thiadiazole-2-thiolato-κ2S:N)mercury(II)], C3H3ClHgN2S2
- Crystal structure of (E)-2,4-dichloro-6-(((4-methyl-2-nitrophenyl)imino)methyl)phenol, C14H10Cl2N2O3
- Crystal structure of a new polymorph of bis[μ-1,3-bis(diphenylphosphino)propane-κ2P:P′-disilver(I)] bis(tetrafluoroborate), [Ag(dppp)]2(BF4)2, C54H52Ag2B2F8P4
- The crystal structure of 2-phenyl-4,6-bis(R-tert-butylsulfonamido)-1,3,5-triazine – ethyl acetate (2/1), C38H58N10O6S4
- Crystal structure of 6-amino-8-(2-methoxy-phenyl)-2-methyl-2,3,8,8a-tetrahydro-1H-iso-quinoline-5,7,7-tricarbonitrile monohydrate, C20H21N5O2
- Crystal structure of methyl (1-phenylethyl)carbamate, C10H13NO2
- Crystal structure of dimethanol-(μ2-squarato-κ2O:O′)-tetrakis(tri-p-tolylphosphane-κP)disilver(I) – methanol (1/2), C92H98Ag2O8P4
- Crystal structure of catena-poly[bis(μ2-1,4-bis(triazol-1-ylmethyl)benzene-κ2N:N′)-bis(5-tert-butyl-isophthalate-κO)copper(II)]tetrahydrate, C36H46CuN6O12
- Crystal structure of 4-aminopyridinium 4-acetyl-(pyridin-4-yl)-1H-1,2,3-triazol-5-olate monohydrate, C14H16N6O3
- Crystal structure of 2-(8-bromo-2-phenylimidazo[1,2-α]pyridin-3-yl)-6,7-dimethyl-3-phenylquinoxaline, C29H21BrN4
- Crystal structure of aqua(1-(2-pyridyl)ethanone oxime-κ2N,N′)(1-(2-pyridyl)ethanone oximato-κ2N,N′) nitrate monohydrate, C14H19N5O7Cu
- Crystal structure of poly[tetraaqua-(μ4-oxalato-κ4O,O′:O′′,O′′′)-(μ8-benzene-1,2,4,5-tetracarboxylato-κ8O1:O2:O3:O4:O5:O6:O7:O8)yttrium(III)], C6H5O8Y
- Crystal structure of bis{catena-poly[(μ2-1,2-bis(4-pyridyl)ethane-κ2N:N′)silver(I)]} diaqua-bis(5-(4-carboxyphenyl)pyridine-2-carboxylato-κ2N,O)-(μ2-1,2-bis(4-pyridyl)ethane-κ2N:N′)disilver(I) octahydrate, C31H35Ag2N4O9
- Crystal structure of (E)-N-(2-(benzylamino)-2-oxo-1-(4-oxo-4H-chromen-3-yl)ethyl)-N-(4-bromophenyl)-3-chloroacrylamide hydrate, C27H22BrClN2O5
- Crystal structure of catena-poly[octaaqua-bis(μ2-4,6-dicarboxyisophthalate-κ2O:O′)cadmium(II)disodium(I)] dihydrate, C20H28CdNa2O26
- Crystal structure of acetonitrile{bis(2-benzimidazolylmethyl)amine-κ3N,N′,N′′}-{maleato-κO}zinc(II) perchlorate - acetonitrile (1/1), C24H24ClN7O8Zn
- Crystal structure of 2-amino-4-(3,5-dibromo-4-hydroxyphenyl)-7-methyl-5-oxo-2H,5H-pyrano[4,3-b]pyran-3-carbonitrile, C16H10Br2N2O4
- Crystal structure of catena-poly[diaqua-(μ2-3,5-bis(pyridin-4-ylmethoxy)benzoate-κ2N:O) manganese(II)] tetrahydrate [(3,5-bis-(pyridin-4-ylmethoxy)-benzoic-κ1Oκ1N) manganese(II)] trihydrate, C38H42MnN4O14
- The crystal structure of 2-carboxybenzaldehyde-2-phenylacetohydrazone, C16H14N2O3
- The crystal structure of poly[μ2-aqua-(μ2-2-naphthylamine-1-sulfonato-κ3O,O′:O′′)sodium(I)], C10H10N1O4S1Na
- The crystal structure of phthalazin-1(2H)-one, C8H6N2O1
- Crystal structure of 3,5-bis(trifluoromethyl)benzyl(Z)-N-(adamantan-1-yl)morpholine-4-carbothioimidate, C24H28F6N2OS
- Crystal structure of diazido-bis(μ2-pyridin-2-ylmethanolato-κ2N:O)-bis(pyridin-2-ylmethanolato-κ2N,O)dicobalt(III) – methanol (1/3), C27H35Co2N10O7
- Crystal structure of N-[[(4,6-dimethoxy-2-pyrimidinyl)amino]carbonyl]-3-(2,2,2-trifluoroethoxy)-2-pyridinesulfonamide, C14H14F3N5O6S
- Crystal structure of 1-phenyl-N′-(1-phenyl-5-(thiophen-2-yl)-1H-pyrazole-3-carbonyl)-5-(thiophen-2-yl)-1H-pyrazole-3-carbohydrazide, C28H20N6O2S2
- The crystal structure of poly[bis(4-hydroxybenzoato-κO)-(μ2-4,4′-bipyridine-κ2N:N′)copper(II)] hydrate, C24H20N2O7Cu
- Crystal structure of poly[μ3-5-(4-(2,6-di(pyridine-2-yl)pyridine-4-yl)phenoxy)isophthalato-κ5O:O′,O′′:N,N′,N′′cobalt(II)], C29H17CoN3O5
- Crystal structure of poly[μ3-5-(4-(2,6-di(pyridine-2-yl)pyridine-4-yl)phenoxy)isophthalato-κ6O:O′,O′′:N,N′,N′′)cobalt(II)] C29H17CoN3O5
- Crystal structure of diaqua-(acetato-κ3O,O′:O′′)-(μ3-4,6-di(1H-imidazol-1-yl)isophthalato-κ4O:O′:O′′,O′′′)lanthanum(III), C16H15LaN4O8
- Synthesis and crystal structure of 6-carboxy-1-(3,5-dicarboxyphenyl)-1H-benzo[d]imidazol-3-ium-5-carboxylate dihydrate, C18H12N2O8
- Crystal structure of (E)-2-hydroxybenzaldehyde O-(2-(((E)-(4-(dimethylamino)benzylidene)amino)oxy)ethyl)oxime, C18H21N3O3
- Crystal structure of bis{2-((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}zinc(II), C32H30N4O4Zn
- Crystal structure of bis(9-aminoacridin-10-ium) tetrachloridocuprate(II) monohydrate, C26H24Cl4CuN4O
- The crystal structure of 4-tert-butyl-N′-[(E)-(4-fluoro-3-methoxyphenyl)methylidene]benzohydrazide, C19H21F1N2O2
- Crystal structure of (E)-3-(3-(5-methyl-1-4-tolyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazol-4-yl)-1-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)prop-2-en-1-one, C31H26N8O
- Crystal structure of (E)-N′-(4-methoxybenzylidene)-5-methyl-1-(4-tolyl)-1H-1,2,3-triazole-4-carbohydrazide, C19H19N5O2
- Crystal structure and molecular packing of O-ethyl (2-chlorophenyl)carbamothioate, C9H10ClNOS
- Crystal structure of pyrene-2-carbaldehyde, C17H10O
- Crystal structure of (E)-2,4-diiodo-6-(4-methyl-2-nitrostyryl)phenol, C14H10I2N2O3
- Crystal structure of (E)-2,4-dichloro-6-(((4-methoxy-2-nitrophenyl)imino)methyl)phenol, C14H10Cl2N2O4
- Crystal structure of (E)-2-bromo-4-chloro-6-(4-methoxy-2-nitrostyryl)phenol, C14H10BrClN2O4
- Crystal structure of (E)-4,6-diiodo-2-(((4-methoxy-2-nitrophenyl)imino)methyl)-3-methylphenol, C14H10I2N2O4
- The crystal structure of 7-bromo-1-cyclopropyl-8-methyl-4-oxo-1,4-dihydroquinoline-3-carboxylic acid an intermediate of the ozenoxacin synthesis, C14H12BrNO3
- Crystal structure of bis(N-(1-(pyrazin-2-yl)ethylidene)nicotinohydrazonato-κ3N,N′,O)copper(II) C24H20N10O2Cu
- Crystal structure of diaqua-dinitrato-k2O,O′((Z)-N-((E)-1-(pyrazin-2-yl)ethylidene)nicotinohydrazonato-k3N,N′,O)europium(II), C12H14N7O9Eu
- Crystal structure of ethyl 4-amino-5-(5-methyl-1-(4-tolyl)-1H-1,2,3-triazole-4-carbonyl)-2-(phenylamino)thiophene-3-carboxylate, C24H23N5O3S
- The crystal structure of acridin-10-ium2-carboxybenzoate, C21H15NO4
- The crystal structure of 3-((phenylamino)methylene)-1,5-dioxaspiro[5.5]undecane-2,4-dione, C16H17N1O4
- Crystal structure of 12-chloro-5,6,7,12-tetrahydrodibenzo[c,f][1,5]oxastibocine, C14H12ClOSb
- Crystal structure of 4-((1,3-dioxoisoindolin-2-yl)methyl)phenethyl 4-methylbenzenesulfonate, C24H21NO5S
- Crystal structure of 3-methyl-2,3-dihydro-2-thioxoquinazolin-4(1H)-one, C9H8N2OS
- Crystal structure of tert-butyl (2-(4-oxo-2-thioxo-1,4-dihydroquinazolin-3(2H)-yl)ethyl)carbamate, C15H19N3O3S
- Crystal structure of ethyl 5-formyl-3,4-dimethylpyrrole-2-carboxylate–1-(propan-2-ylidene)thiosemicarbazide (1/1), C14H22N4O3S
- Crystal structure of bis-(N′-(5-ethoxycarbonyl-3,4-dimethyl-pyrrol-2-yl-methylidene)-3-hydroxybenzohydrazide-κ2O,N)copper(II) – dimethylformamide (1/2), C40H50N8O10Cu
- Crystal structure of bis(acetato-κO)bis{2-((1H-tetrazol-1-yl)methyl)-1H-benzo[d]imidazole-κN}zinc(II), C22H22N12O4Zn
- Crystal structure of 4-phenyl-3-((4-phenyl-1H-1,2,3-triazol-1-yl)methyl)-1H-1,2,4-triazole-5(4H)-thione, C17H14N6S
- Crystal structure of (Z)-N-(4-nitrophenyl)-3-phenyl-3-(phenylamino)acrylamide, C21H17N3O3
- Crystal structure of 1,1′-(pentane-1,5-diyl)bis(3-methyl-1H-imidazol-3-ium)bis(hexafluorophosphate), C13H22F12N4P2
- Synthesis and crystal structure of bis(furan-2-ylmethanaminium)-catena-[bis(μ2-phthalato-κ2O:O′)cobalt(II)], C26H24CoN2O10
- Crystal structure of methyl (R)-4-(o-chlorobenzoyl)-1-thia-4-azaspiro[4.5]decane-3-carboxylate, C17H20ClNO3S
- Crystal structure of 2-[[4-[2-[4-(4-methoxyphenyl)-1-piperazinyl]ethyl]phenyl] methyl]-1H-isoindole-1,3(2H)-dione, C28H29N3O3
- The crystal structure of benzenaminium 5,7-dihydroxy-4-oxo-2-phenyl-4H-chromene-8-sulfonate hydrate, C21H19NO8S
- Crystal structure of semiconducting potassium poly[(μ2-tetraselenido-κ2Se1:Se4)(μ2-pentaselenido-κ1Se1:Se1)argentate(I)], K3AgSe9
- Crystal structure of 2-isopropyl-8-methyl-phenanthrene-3,4-dione, C18H16O2
- Crystal structure of 2-isopropyl-8,8-dimethyl-5,6,7,8-tetrahydrophenanthrene-3,4-dione, C19H22O2
- Crystal structure of (E)-2-(1-((2-aminophenyl)imino)ethyl)-4-bromophenol, C14H13BrN2O
- Crystal structure of 1,1-di(4-cyanophenyl)-2,2-diphenylethene, C28H18N2
- Crystal structure of bis(hydroxylamido-κ2O,N)-oxido(1H-pyrazole-3-carboxylato-κ2O,N)vanadium(V), C4H7N4O5V
- The crystal structure of In1.2B3O5.6(OH)1.4
- The crystal structure of chlorido(2-(1H-pyrazol-3-yl)phenolato-κ2N,O)(2-(1H-pyrazol-3-yl)phenol-κN)copper(II), C18H15ClCuN4O2
- Crystal structure of 1-heptylpyridazin-1-ium iodide, C11H19N2I
- The crystal structure of N-butylpyridinium bis(μ2-dichlorido)-tetrachloridodicopper(II), C18H28N2Cu2Cl6
- Crystal structure of 6-hydroxy-5-((2-hydroxy-6-oxocyclohex-1-en-1-yl)(4-methoxyphenyl)methyl)-1,3-dimethylpyrimidine-2,4(1H,3H)-dione, C20H22N2O6
- Crystal structure of bis(acetonitrile)-diaqua-dichloridoiron(II), C4H10Cl2N2O2Fe
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of (E)-1-(4-(((E)-2-hydroxy-5-methylbenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C17H18N2O2
- Crystal structure of 1,3,5,7-tetraazaadamantane-1,3-diium 2,5-dicarboxyterephthalate, C16H18N4O8
- Crystal structure of guanidinium tetrabutyl-ammonium 5-hydroxyisophthalate dihydrate, C25H50N4O7
- Crystal structure of poly[aqua-(μ2-5-methoxyisophthalate-κ3O,O′:O′′)-(μ2-1,4-bis((1H-1,2,4-triazol-1-yl)methyl)benzene-κ2N:N′)nickel(II), NiC21H20N6O6
- Crystal structure of aqua-bis(3,4-dimethoxybenzoato-κ1O)-(2,2′-bipyridine-κ2N,N′)copper(II), C28H26CuN2O9
- Crystal structure of catena-poly[aqua-(μ2-(3,5-di(1H-imidazol-1-yl)-pyridine-κ2N:N′)-(μ2-2-(carboxylatomethyl)benzoato-κ2O:O′)] cadmium(II), C20H17CdN5O5
- The crystal structure of catena-poly[chlorido-(μ2-5-methyl-1,3,4-thiadiazole-2-thiolato-κ2S:N)mercury(II)], C3H3ClHgN2S2
- Crystal structure of (E)-2,4-dichloro-6-(((4-methyl-2-nitrophenyl)imino)methyl)phenol, C14H10Cl2N2O3
- Crystal structure of a new polymorph of bis[μ-1,3-bis(diphenylphosphino)propane-κ2P:P′-disilver(I)] bis(tetrafluoroborate), [Ag(dppp)]2(BF4)2, C54H52Ag2B2F8P4
- The crystal structure of 2-phenyl-4,6-bis(R-tert-butylsulfonamido)-1,3,5-triazine – ethyl acetate (2/1), C38H58N10O6S4
- Crystal structure of 6-amino-8-(2-methoxy-phenyl)-2-methyl-2,3,8,8a-tetrahydro-1H-iso-quinoline-5,7,7-tricarbonitrile monohydrate, C20H21N5O2
- Crystal structure of methyl (1-phenylethyl)carbamate, C10H13NO2
- Crystal structure of dimethanol-(μ2-squarato-κ2O:O′)-tetrakis(tri-p-tolylphosphane-κP)disilver(I) – methanol (1/2), C92H98Ag2O8P4
- Crystal structure of catena-poly[bis(μ2-1,4-bis(triazol-1-ylmethyl)benzene-κ2N:N′)-bis(5-tert-butyl-isophthalate-κO)copper(II)]tetrahydrate, C36H46CuN6O12
- Crystal structure of 4-aminopyridinium 4-acetyl-(pyridin-4-yl)-1H-1,2,3-triazol-5-olate monohydrate, C14H16N6O3
- Crystal structure of 2-(8-bromo-2-phenylimidazo[1,2-α]pyridin-3-yl)-6,7-dimethyl-3-phenylquinoxaline, C29H21BrN4
- Crystal structure of aqua(1-(2-pyridyl)ethanone oxime-κ2N,N′)(1-(2-pyridyl)ethanone oximato-κ2N,N′) nitrate monohydrate, C14H19N5O7Cu
- Crystal structure of poly[tetraaqua-(μ4-oxalato-κ4O,O′:O′′,O′′′)-(μ8-benzene-1,2,4,5-tetracarboxylato-κ8O1:O2:O3:O4:O5:O6:O7:O8)yttrium(III)], C6H5O8Y
- Crystal structure of bis{catena-poly[(μ2-1,2-bis(4-pyridyl)ethane-κ2N:N′)silver(I)]} diaqua-bis(5-(4-carboxyphenyl)pyridine-2-carboxylato-κ2N,O)-(μ2-1,2-bis(4-pyridyl)ethane-κ2N:N′)disilver(I) octahydrate, C31H35Ag2N4O9
- Crystal structure of (E)-N-(2-(benzylamino)-2-oxo-1-(4-oxo-4H-chromen-3-yl)ethyl)-N-(4-bromophenyl)-3-chloroacrylamide hydrate, C27H22BrClN2O5
- Crystal structure of catena-poly[octaaqua-bis(μ2-4,6-dicarboxyisophthalate-κ2O:O′)cadmium(II)disodium(I)] dihydrate, C20H28CdNa2O26
- Crystal structure of acetonitrile{bis(2-benzimidazolylmethyl)amine-κ3N,N′,N′′}-{maleato-κO}zinc(II) perchlorate - acetonitrile (1/1), C24H24ClN7O8Zn
- Crystal structure of 2-amino-4-(3,5-dibromo-4-hydroxyphenyl)-7-methyl-5-oxo-2H,5H-pyrano[4,3-b]pyran-3-carbonitrile, C16H10Br2N2O4
- Crystal structure of catena-poly[diaqua-(μ2-3,5-bis(pyridin-4-ylmethoxy)benzoate-κ2N:O) manganese(II)] tetrahydrate [(3,5-bis-(pyridin-4-ylmethoxy)-benzoic-κ1Oκ1N) manganese(II)] trihydrate, C38H42MnN4O14
- The crystal structure of 2-carboxybenzaldehyde-2-phenylacetohydrazone, C16H14N2O3
- The crystal structure of poly[μ2-aqua-(μ2-2-naphthylamine-1-sulfonato-κ3O,O′:O′′)sodium(I)], C10H10N1O4S1Na
- The crystal structure of phthalazin-1(2H)-one, C8H6N2O1
- Crystal structure of 3,5-bis(trifluoromethyl)benzyl(Z)-N-(adamantan-1-yl)morpholine-4-carbothioimidate, C24H28F6N2OS
- Crystal structure of diazido-bis(μ2-pyridin-2-ylmethanolato-κ2N:O)-bis(pyridin-2-ylmethanolato-κ2N,O)dicobalt(III) – methanol (1/3), C27H35Co2N10O7
- Crystal structure of N-[[(4,6-dimethoxy-2-pyrimidinyl)amino]carbonyl]-3-(2,2,2-trifluoroethoxy)-2-pyridinesulfonamide, C14H14F3N5O6S
- Crystal structure of 1-phenyl-N′-(1-phenyl-5-(thiophen-2-yl)-1H-pyrazole-3-carbonyl)-5-(thiophen-2-yl)-1H-pyrazole-3-carbohydrazide, C28H20N6O2S2
- The crystal structure of poly[bis(4-hydroxybenzoato-κO)-(μ2-4,4′-bipyridine-κ2N:N′)copper(II)] hydrate, C24H20N2O7Cu
- Crystal structure of poly[μ3-5-(4-(2,6-di(pyridine-2-yl)pyridine-4-yl)phenoxy)isophthalato-κ5O:O′,O′′:N,N′,N′′cobalt(II)], C29H17CoN3O5
- Crystal structure of poly[μ3-5-(4-(2,6-di(pyridine-2-yl)pyridine-4-yl)phenoxy)isophthalato-κ6O:O′,O′′:N,N′,N′′)cobalt(II)] C29H17CoN3O5
- Crystal structure of diaqua-(acetato-κ3O,O′:O′′)-(μ3-4,6-di(1H-imidazol-1-yl)isophthalato-κ4O:O′:O′′,O′′′)lanthanum(III), C16H15LaN4O8
- Synthesis and crystal structure of 6-carboxy-1-(3,5-dicarboxyphenyl)-1H-benzo[d]imidazol-3-ium-5-carboxylate dihydrate, C18H12N2O8
- Crystal structure of (E)-2-hydroxybenzaldehyde O-(2-(((E)-(4-(dimethylamino)benzylidene)amino)oxy)ethyl)oxime, C18H21N3O3
- Crystal structure of bis{2-((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}zinc(II), C32H30N4O4Zn
- Crystal structure of bis(9-aminoacridin-10-ium) tetrachloridocuprate(II) monohydrate, C26H24Cl4CuN4O
- The crystal structure of 4-tert-butyl-N′-[(E)-(4-fluoro-3-methoxyphenyl)methylidene]benzohydrazide, C19H21F1N2O2
- Crystal structure of (E)-3-(3-(5-methyl-1-4-tolyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazol-4-yl)-1-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)prop-2-en-1-one, C31H26N8O
- Crystal structure of (E)-N′-(4-methoxybenzylidene)-5-methyl-1-(4-tolyl)-1H-1,2,3-triazole-4-carbohydrazide, C19H19N5O2
- Crystal structure and molecular packing of O-ethyl (2-chlorophenyl)carbamothioate, C9H10ClNOS
- Crystal structure of pyrene-2-carbaldehyde, C17H10O
- Crystal structure of (E)-2,4-diiodo-6-(4-methyl-2-nitrostyryl)phenol, C14H10I2N2O3
- Crystal structure of (E)-2,4-dichloro-6-(((4-methoxy-2-nitrophenyl)imino)methyl)phenol, C14H10Cl2N2O4
- Crystal structure of (E)-2-bromo-4-chloro-6-(4-methoxy-2-nitrostyryl)phenol, C14H10BrClN2O4
- Crystal structure of (E)-4,6-diiodo-2-(((4-methoxy-2-nitrophenyl)imino)methyl)-3-methylphenol, C14H10I2N2O4
- The crystal structure of 7-bromo-1-cyclopropyl-8-methyl-4-oxo-1,4-dihydroquinoline-3-carboxylic acid an intermediate of the ozenoxacin synthesis, C14H12BrNO3
- Crystal structure of bis(N-(1-(pyrazin-2-yl)ethylidene)nicotinohydrazonato-κ3N,N′,O)copper(II) C24H20N10O2Cu
- Crystal structure of diaqua-dinitrato-k2O,O′((Z)-N-((E)-1-(pyrazin-2-yl)ethylidene)nicotinohydrazonato-k3N,N′,O)europium(II), C12H14N7O9Eu
- Crystal structure of ethyl 4-amino-5-(5-methyl-1-(4-tolyl)-1H-1,2,3-triazole-4-carbonyl)-2-(phenylamino)thiophene-3-carboxylate, C24H23N5O3S
- The crystal structure of acridin-10-ium2-carboxybenzoate, C21H15NO4
- The crystal structure of 3-((phenylamino)methylene)-1,5-dioxaspiro[5.5]undecane-2,4-dione, C16H17N1O4
- Crystal structure of 12-chloro-5,6,7,12-tetrahydrodibenzo[c,f][1,5]oxastibocine, C14H12ClOSb
- Crystal structure of 4-((1,3-dioxoisoindolin-2-yl)methyl)phenethyl 4-methylbenzenesulfonate, C24H21NO5S
- Crystal structure of 3-methyl-2,3-dihydro-2-thioxoquinazolin-4(1H)-one, C9H8N2OS
- Crystal structure of tert-butyl (2-(4-oxo-2-thioxo-1,4-dihydroquinazolin-3(2H)-yl)ethyl)carbamate, C15H19N3O3S
- Crystal structure of ethyl 5-formyl-3,4-dimethylpyrrole-2-carboxylate–1-(propan-2-ylidene)thiosemicarbazide (1/1), C14H22N4O3S
- Crystal structure of bis-(N′-(5-ethoxycarbonyl-3,4-dimethyl-pyrrol-2-yl-methylidene)-3-hydroxybenzohydrazide-κ2O,N)copper(II) – dimethylformamide (1/2), C40H50N8O10Cu
- Crystal structure of bis(acetato-κO)bis{2-((1H-tetrazol-1-yl)methyl)-1H-benzo[d]imidazole-κN}zinc(II), C22H22N12O4Zn
- Crystal structure of 4-phenyl-3-((4-phenyl-1H-1,2,3-triazol-1-yl)methyl)-1H-1,2,4-triazole-5(4H)-thione, C17H14N6S
- Crystal structure of (Z)-N-(4-nitrophenyl)-3-phenyl-3-(phenylamino)acrylamide, C21H17N3O3
- Crystal structure of 1,1′-(pentane-1,5-diyl)bis(3-methyl-1H-imidazol-3-ium)bis(hexafluorophosphate), C13H22F12N4P2
- Synthesis and crystal structure of bis(furan-2-ylmethanaminium)-catena-[bis(μ2-phthalato-κ2O:O′)cobalt(II)], C26H24CoN2O10
- Crystal structure of methyl (R)-4-(o-chlorobenzoyl)-1-thia-4-azaspiro[4.5]decane-3-carboxylate, C17H20ClNO3S
- Crystal structure of 2-[[4-[2-[4-(4-methoxyphenyl)-1-piperazinyl]ethyl]phenyl] methyl]-1H-isoindole-1,3(2H)-dione, C28H29N3O3
- The crystal structure of benzenaminium 5,7-dihydroxy-4-oxo-2-phenyl-4H-chromene-8-sulfonate hydrate, C21H19NO8S
- Crystal structure of semiconducting potassium poly[(μ2-tetraselenido-κ2Se1:Se4)(μ2-pentaselenido-κ1Se1:Se1)argentate(I)], K3AgSe9
- Crystal structure of 2-isopropyl-8-methyl-phenanthrene-3,4-dione, C18H16O2
- Crystal structure of 2-isopropyl-8,8-dimethyl-5,6,7,8-tetrahydrophenanthrene-3,4-dione, C19H22O2
- Crystal structure of (E)-2-(1-((2-aminophenyl)imino)ethyl)-4-bromophenol, C14H13BrN2O
- Crystal structure of 1,1-di(4-cyanophenyl)-2,2-diphenylethene, C28H18N2
- Crystal structure of bis(hydroxylamido-κ2O,N)-oxido(1H-pyrazole-3-carboxylato-κ2O,N)vanadium(V), C4H7N4O5V
- The crystal structure of In1.2B3O5.6(OH)1.4
- The crystal structure of chlorido(2-(1H-pyrazol-3-yl)phenolato-κ2N,O)(2-(1H-pyrazol-3-yl)phenol-κN)copper(II), C18H15ClCuN4O2
- Crystal structure of 1-heptylpyridazin-1-ium iodide, C11H19N2I
- The crystal structure of N-butylpyridinium bis(μ2-dichlorido)-tetrachloridodicopper(II), C18H28N2Cu2Cl6
- Crystal structure of 6-hydroxy-5-((2-hydroxy-6-oxocyclohex-1-en-1-yl)(4-methoxyphenyl)methyl)-1,3-dimethylpyrimidine-2,4(1H,3H)-dione, C20H22N2O6
- Crystal structure of bis(acetonitrile)-diaqua-dichloridoiron(II), C4H10Cl2N2O2Fe

