Abstract
Background and aim
Diclofenac is widely prescribed for the treatment of pain. Several network meta-analyses (NMA), largely of published trials have evaluated the efficacy, tolerability, and safety of nonsteroidal anti-inflammatory drugs (NSAIDs). The present NMA extends these analyses to unpublished older (legacy) diclofenac trials.
Methods
We identified randomised controlled trials (RCTs) of diclofenac with planned study duration of at least 4 weeks for the treatment of osteoarthritis (OA) from ‘legacy’ studies conducted by Novartis but not published in a peer reviewed journal or included in any previous pooled analyses. All studies reporting efficacy and/or safety of treatment with diclofenac or other active therapies or placebo were included. We used a Bayesian NMA model, and estimated relative treatment effects between pairwise treatments. Main outcomes included pain relief measured using visual analogue scale at 2, 4 and 12 weeks and patient global assessment (PGA) at 4 and 12 weeks for efficacy, all-cause withdrawals, and adverse events.
Results
A total of 19 RCTs (5030 patients) were included; 18 of which were double-blind and one singleblind. All studies were conducted before cyclooxygenase 2 inhibitors (COXIBs) became commercially available. Data permitted robust efficacy comparison between diclofenac and ibuprofen, but the amount of data for other comparators was limited. Diclofenac 150 mg/day was more efficacious than ibuprofen 1200 mg/day and had likely favourable outcomes for pain relief compared to ibuprofen 2400 mg/day. Diclofenac 100 mg/day had likely favourable outcomes compared to ibuprofen 1200 mg/day in alleviating pain. Based on PGA, diclofenac 150 mg/day was more efficacious and likely to be favourable than ibuprofen 1200 mg/day and 2400 mg/day, respectively. Risk of withdrawal due to all causes with diclofenac and ibuprofen were comparable. Diclofenac 150 mg/day was likely to have favourable efficacy and comparable tolerability with diclofenac 100 mg/day. Results comparing diclofenac and ibuprofen were similar to those from NMAs of published trials.
Conclusions
Results from these unpublished ‘legacy’ studies were similar to those from NMAs of published trials. The favourable efficacy results of diclofenac compared to ibuprofen expand the amount of available evidence comparing these two NSAIDs. The overall benefit-risk profile of diclofenac was comparable to that of ibuprofen in OA.
Implications
The present NMA results reassures that the older unpublished blinded trials have similar results compared to more recently published trials and also contributes to increase the transparency of clinical trials performed with diclofenac further back in the past.
1 Background and aims
Osteoarthritis (OA) is a common and progressive joint disorder, mostly affecting the adults and characterised by joint degeneration resulting in extreme pain, disability, and reduced quality of life. The most commonly affected joints include those in the hands, neck, and lower back and weight-bearing joints such as the knees and hip. OA affects over 250 million people worldwide, imposing a substantial burden on society [1]. Currently, no effective disease-modifying treatment options are available to cure OA; the existing symptomatic treatments can only relieve pain and improve joint function [2]. According to reports from a prospective, longitudinal cohort study conducted at 53 centres (1187 patients) in six European countries (United Kingdom [UK], France, Germany, Portugal, The Netherlands, and Italy), 54% of OA patients receiving treatment from general physicians or specialists reported inadequate pain relief [3]. Non-steroidal anti-inflammatory drugs (NSAIDs), both traditional NSAIDs (tNSAlDs) and cyclooxygenase 2 (COX-2) inhibitors (COXIBs), are the most frequently prescribed medicines and considered as cornerstones in the treatment of OA [2] as they intend to provide the desired relief from both pain and inflammation in OA patients.
Nevertheless, both benefits and risks associated with various treatments should be analysed to inform clinical decision making.Numerous clinical studies that included this treatment were performed in an era when publication of clinical studies was not as systematic as it is today. Today, there are more formalised good publication practice guidelines that are supported by researchers [4] and many research companies (including Novartis) have publically committed to publish sponsored clinical research [5]. The present review and NMA was conducted to gain insights on the data available from unpublished legacy studies with diclofenac conducted by Novartis in patients with OA. Its value lies in the fact that is presenting to the scientific community a wealth of data from 29 previously unpublished studies in osteoarthritis. The NMA is used as the appropriate quantitative method to synthesise these unpublished data and the authors consider this effort as complementary to a number of (network) meta-analyses and literature reviews that have been published over the last years. Since the legacy studies included in this meta-analysis were conducted before COXIBs became commercially available, comparators are limited to other tNSAlDs. Data from these legacy studies were systematically reviewed, and outcomes were synthesised by means of a Bayesian NMA. Based on these findings, the comparative efficacy and safety of diclofenac (100 and 150 mg/day) versus other NSAIDs in the management of OA were evaluated.
![Fig. 1
Interpretation of efficacy results of the NMA (adapted from Cope et al. [13]). CFB, change from baseline; diff, difference; RR, rate ratio; NMA, network meta-analysis.](/document/doi/10.1016/j.sjpain.2017.03.006/asset/graphic/j_j.sjpain.2017.03.006_fig_010.jpg)
Interpretation of efficacy results of the NMA (adapted from Cope et al. [13]). CFB, change from baseline; diff, difference; RR, rate ratio; NMA, network meta-analysis.
2 Methods
2.1 Study identification and data collection
A list of all legacy clinical trials conducted by Novartis was reviewed to identify randomised controlled trials (RCTs) of diclofenac with planned treatment duration of at least 4 weeks for the treatment of OA, so that their results have some relevance to the clinical treatment of a long-term condition. Blinded RCTs with diclofenac in OA, which were conducted by Novartis or its subsidiaries or predecessors and identified as not being included in a previous systematic review of published studies, were retrieved from the Novartis archives. Only 3 of the 19 studies had previously been published. The relevance of each identified clinical study report (CSR) was assessed according to pre-defined selection criteria (see Appendix 1A) by two independent reviewers in parallel (Anneloes van Walsem and Patricia Guyot), and any disagreement was resolved by consensus. All RCTs in OA that compared diclofenac versus placebo or other analgesic comparators with data on efficacy and/or safety were included. The most common comparators were ibuprofen (1200/2400 mg/day) and naproxen (500/750/1000 mg/day). Other less common comparators, such as piroxicam (20 mg/day), indomethacin (75 mg/day) and paracetamol (1950 mg/day) in combination with dextropropoxyphene (195 mg/day) were also included in a few RCTs.
Visual analogue scale (VAS) and Likert pain scale scores, VAS and Likert scale patients’ global assessments (PGA), and VAS and Likert scale investigators’ global assessments (IGA) were considered for analysing efficacy outcomes. Efficacy endpoints were assessed at 2, 4, and 12 weeks for VAS pain, at 4 and 12 weeks for PGA VAS, and at 4 weeks for IGA VAS. In addition safety (any adverse events [AEs] and serious adverse events [SAEs]) and tolerability (withdrawals due to all causes, lack of efficacy, and AEs) parameters were included in the analysis.
Study and patient characteristics, as well as efficacy, safety, and tolerability outcomes from the selected studies were recorded on a pre-designed data extraction form. Details on study characteristics such as study design, inclusion and exclusion criteria, comparator interventions, study duration, number of intention-to-treat (ITT) patients, and rescue medication use were extracted. In addition, baseline patient characteristics including age, gender, disease duration, and type of OA were extracted.
For each continuous outcome of interest, an estimate of the change from baseline (CFB) and the standard error of the estimate were extracted (see Appendix 2). For dichotomous outcomes, the number of patients experiencing an event was estimated based on reported percentages and size of the ITT population. Subsequently, the total person-years at-risk follow-up periods were estimated using the dropout rate. Data presented in graphs were extracted using the DigitizeIT software (version 1.5; DigitizeIT, Braunschweig, Germany).
The methodological and reporting quality of the included studies were assessed by using the Oxford quality scoring system for RCTs [6]. The risk of bias was assessed based on the following aspects: randomisation according to an appropriate method, allocation concealment of patients and investigators, and complete and non-selective reporting of study withdrawals and dropouts.
The SLR was conducted and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (see Appendix 3).
2.2 Data synthesis
Efficacy outcomes, tolerability and safety parameters were evaluated using a Bayesian NMA model [7,8,9]. The Bayesian framework analyses involve data, a likelihood distribution, and a model with parameters along with their prior distributions. A linear model with a normal likelihood distribution was used for continuous outcomes, whereas a Poisson likelihood with a log link was used for count outcomes [10,11]. The Poisson model for count data includes an offset term for study duration; a constant event rate is assumed. Flat (non-informative) prior distributions were assumed. In addition, random effects models, which assign random effects on the treatment effects, were evaluated to allow for heterogeneity between studies. The fixed- and random-effects models evaluated for each outcome were compared using the Deviance Information Criterion (DIC), and the model with a better fit (lower DIC) was selected [11]. Markov chain Monte Carlo simulations were applied to estimate the posterior densities for parameters. Convergence was assessed by visual inspection of trace plots. Accuracy of the posterior estimates was evaluated using the Monte Carlo error for each parameter. For each outcome, where a closed loop was present in the network, all available direct estimates were in line with those obtained from the consistency model (i.e. the NMA model), suggesting no significant inconsistencies between the direct and indirect treatment estimates. A fixed-effects model was selected for analyses of all outcomes except for CFB with respect to pain at 4 weeks. All models were implemented using WinBUGS (version 1.4.3; MRC Biostatistics Unit, Cambridge, UK) [10]. The detailed methodology used for data synthesis is described elsewhere [12].
Results of this NMA are presented as the median of the posterior distribution for relative treatment effects along with 95% credible intervals (CrIs). The efficacy results are presented as differences in CFB (ΔCFB). A negative ΔCFB indicates symptomatic improvements with diclofenac relative to the comparator. Tolerability and safety results are presented as rate ratios (RR); RR of <1 indicates that treatment with diclofenac has a lower risk relative to the comparator.
As depicted in Fig. 1 (adapted from Cope et al., 2013), treatments were categorised as follows: (1) ‘more efficacious’ if the ‘posterior probability (P) that the treatment is better than the comparator’ is ≥ 97.5%, (2) ‘likely to be favourable’, if P is ≥ 85%, (3) ‘comparable’, if P is between 15% and 85%, (4) ‘likely to be unfavourable’ if P is ≤ 15%, and (5) ‘less efficacious’ if P is ≤ 2.5%. Note that if P is ≥ 97.5% or P is ≤ 2.5%, then the 95% CrI does not include 0 (for continuous outcomes) or 1 (for dichotomous outcomes), whereas it does if 2.5% ≤ P ≤ 97.5% [13].
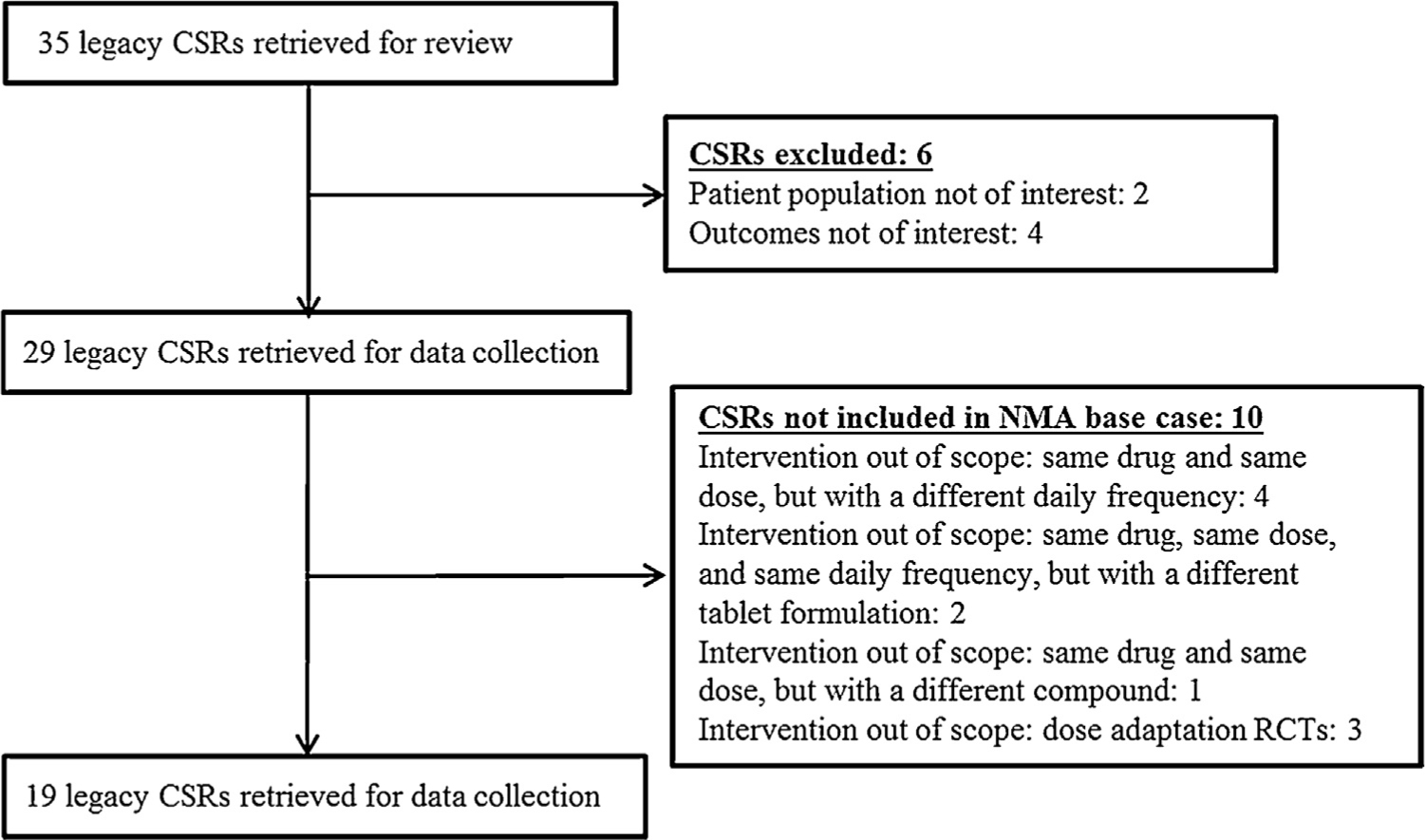
Study selection flow chart. CSR, clinical study report; NMA, network meta-analysis; RCT, randomised controlled trial.
3 Results
3.1 Evidence base
A total of 35 clinical trials were retrieved for review. Of these, six trials were excluded based on pre-defined study selection criteria (two because they included RA patient populations and four because of use of different outcomes); accordingly, 29 clinical trials were retrieved for complete data extraction. The trial selection process is outlined in Fig. 2.
After this phase, heterogeneity of the study evidence with respect to interventions and study design of interest was discussed. Based on these discussions, certain exclusion criteria were added (including comparison between diclofenac tablet formulations or daily frequency; comparison between diclofenac salts; dose escalation, see Appendix 1B), and eventually, 19 trials were selected for the NMA; hereafter, these 19 trials are referred to by letters from A to S. Details of the 10 trials not included in the NMA base case is provided in Appendix 4. Within the included studies (most including patients with osteoarthritis of knee and/or hip), certain treatment arms such as diclofenac (37.5 mg/day), lumiracoxib, and clomipramine were excluded, because these drugs are no longer used in clinical practice. Moreover, these arms were of no interest because they did not bridge to relevant drugs and doses in the network. Studies with diclofenac 200 mg/day were included although this is not a registered dose for OA.
Study designs of the RCTs included in the NMA, together with their methodological and reporting quality assessment, are provided in Appendix 5. The time interval between the beginning of the oldest and most recent studies was 17 years (1982–1999; trial E began in 1982, whereas trial N began in 1999). All 19 RCTs, except one (trial M was a single-blind study), were double-blind trials. In addition, 15 of the 19 RCTs were multicentre trials, whereas four were single-centre trials (trials I, L, Q, and S). With regard to study regions, these 15 trials included one international study (trial N), 10 US studies, three UK studies, two Italian studies, and one each, Brazilian, Mexican and German study. Inclusion and exclusion criteria used among these studies were similar: patients had to have a diagnosis of OA and be receiving aspirin or other NSAIDs on a continuing basis prior to enrolment in the study; patients receiving physiotherapy were usually eligible provided their programme was not altered during the study; patients with arthritis of any aetiology other than OA as well as those who were candidates for joint replacement surgery were excluded. Five studies had a duration of 28 days (4 weeks; trials K, N, O, P, and Q), six had a duration between 42 and 72 days (6–12 weeks; trials A, F, G, I, M, and S), and eight lasted for at least 84 days (≥12 weeks, trials B, C, D, E, H, J, L, and R). All studies included at least one diclofenac arm; however, the diclofenac salts used were not always the same. Diclofenac sodium was used in most studies (15 trials; trials D, F, B, H, R, I, Q, A, N, E, G, L, C, P, and S), while diclofenac potassium was used in five studies (trials M, J, O, K, and D) and diclofenac resinate was used in two studies (trials B and H). Based on differences in the diclofenac salts used, a scenario analysis was planned to investigate if the diclofenac salt used affected the NMA results.
Patient characteristics from all studies included in the analysis are presented in Appendix 6. The average number of ITT patients per arm was 107. Two studies (trials K and M) randomised over 300 patients per treatment arm. Five studies (trials A, I, P, Q, and S) randomised ≤30 patients per treatment arm. The mean weighted average proportion of men was 32% (range: 0–49%). Most studies included patients of either sex, except trial P, which included only female patients. The average age of patients across all studies ranged between 47 and 67 years (mean: 61 years). Trial A comprised all patients with OA-affected joints only in the hip, whereas trials C, D, F, and G comprised all patients with OA-affected joints only in the knee. The mean disease duration ranged from 0.3 to 12.6 years, with a weighted mean average of 7.4 years. Trial I included newly diagnosed patients with a shorter disease duration (0.3–0.5 years) compared with the rest of the studies (2.3–12.6 years).
The clinical outcome data for diclofenac (efficacy, tolerability and safety) compared with ibuprofen, the only comparator with enough data for robust comparisons, are presented in detail below. In addition, diclofenac (75 and 200 mg/day), naproxen, and other NSAIDs (piroxicam, indomethacin, paracetamol and dextropropoxyphene) were included in a few retrieved studies, but the number of patients was too small for reliable comparisons with diclofenac (100 and 150 mg/day). The clinical outcome results for these NSAIDs are described in Appendix 12.
3.2 Clinical outcomes
3.2.1 Efficacy outcomes
Comparative efficacy outcomes of diclofenac (150/100 mg/day) and ibuprofen (2400/1200 mg/day) are presented in this section.
The global evidence network for the 19 studies included in the analysis is presented in Fig. 3.
The networks according to outcome are provided in Appendix 7. The input data are in Appendix 2. The pain (VAS) data primarily included pain on motion; however, if pain on motion was not reported, then the overall pain and that at rest or at night were considered. The current identified evidence was appropriate to draw feasible networks for the following outcomes: pain (VAS) at 2, 4, and 12 weeks; PGA (VAS) at 4 and 12 weeks; IGA (VAS) at 4 weeks; withdrawals due to all causes, lack of efficacy and AEs; any SAEs. The evidence for PGA and IGA at 2 weeks and IGA at 12 weeks was too limited to provide pairwise comparison results versus the main comparator ibuprofen. Because none of these studies were primarily conducted to assess safety outcomes, evidence regarding other safety events was also somewhat limited.
Two scenario analyses were conducted: scenario 1 with exclusion of trial C, which had notably different results from those in the other studies at all time points, and scenario 2, based on separation of the diclofenac salts, to investigate if the diclofenac salts affected the NMA results.
In scenario 1 analysis, trial C was excluded to determine the extent to which it may affect the NMA results. The diclofenac 100 mg/day pain CFB values (mm) in trial C were ‘1.0 and 2.0’ versus ‘−25.6 and−27.4’ in the two other studies reporting results at 2 weeks: ‘−2.0 and 0.0’ versus ‘between−21.4 and−31.5’ at 4 weeks and ‘−2.0 and −1.0’ versus ‘between−29.2 and−34.9’ at 12 weeks. The diclofenac 100 mg/day PGA (VAS) CFB values (mm) in trial C were both ‘−1.0’ versus between ‘−18.4 and−28.9’ in the other studies at 4 weeks and both ‘−1’ versus ‘between−22.1 and−27.8’ at 12 weeks. In scenario 2 analysis, diclofenac treatments were separated according to the salts used (sodium, potassium and resinate).
3.2.1.1 Pain
Efficacy endpoints (VAS) were considered at 2, 4, and 12 weeks for pain CFB. Treatment with placebo had inferior outcomes at all time points compared to treatments with diclofenac and ibuprofen (data for ibuprofen vs placebo not shown).
Diclofenac 150 mg/day was more efficacious (P ≥ 97.5%) than ibuprofen 1200 mg/day at 4 and 12 weeks and was likely to be favourable (P ≥ 85%) compared to ibuprofen 2400 mg/day at 12 weeks in alleviating pain. The efficacy of diclofenac 150 mg/day was comparable (P: > 15% to < 85%) to that of ibuprofen 2400 mg/day at 2 and 4 weeks. In comparison with diclofenac 100 mg/day, diclofenac 150 mg/day was likely to be favourable (P ≥ 85%) at 2 and 4 weeks, but showed comparable efficacy (P: > 15% to < 85%) at 12 weeks (Fig. 4A).
Diclofenac 100 mg/day also demonstrated better results (i.e. was likely to be favourable; P ≥ 85%) than ibuprofen 1200 mg/day at 4 and 12 weeks for pain relief (data at 2 weeks not available). The results were not consistent when it was compared with ibuprofen 2400 mg/day: it was likely to be unfavourable (P ≤ 15%) at 2 weeks, but showed comparable efficacy (P: > 15% to < 85%) at 4 and 12 weeks (Fig. 4B).
3.2.1.2 Patient global assessment
Efficacy endpoints (VAS) on PGA CFB were assessed at 4 and 12 weeks. The results with placebo at 4 weeks were inferior to those with diclofenac and ibuprofen. At 12 weeks, diclofenac was likely to be favourable (P ≥ 85%), whereas ibuprofen was comparable (P = > 5% to < 85%) to placebo (data for ibuprofen vs placebo not shown).
Diclofenac 150 mg/day was more efficacious (higher PGA CFB; P ≥97.5%) than ibuprofen 1200 mg/day at 4 weeks and was likely to be favourable (P ≥ 85%) at 12 weeks. In comparison with ibuprofen 2400 mg/day, diclofenac 150 mg/day was likely to be favourable (P ≥ 85%) at both 4 and 12 weeks (Fig. 5A). Compared with diclofenac 100 mg/kg/day, diclofenac 150 mg/day was likely to be favourable (P ≥ 85%) at 4 weeks but was comparable (P = > 15% to < 85%) at 12 weeks (Fig. 5A). Diclofenac 100 mg/day was more efficacious (P ≥ 97.5%) than ibuprofen 1200 mg/day at 4 weeks and was likely to be favourable (P ≥ 85%) at 12 weeks. The efficacy of diclofenac 100 mg/day was comparable (P = > 15% to < 85%) to that of ibuprofen 2400 mg/day at 4 and 12 weeks (Fig. 5B).
3.2.1.2 Investigator global assessment
The efficacy endpoint on IGA (VAS) CFB was available only at 4 weeks. Based on IGA, diclofenac 150 and 100 mg/day, and ibuprofen 2400 mg/day was more efficacious (P ≥ 97.5%) compared with placebo (data for ibuprofen vs placebo not shown). In addition, the efficacy of diclofenac 150 and 100 mg/day were comparable (P: > 15% to < 85%) to that of ibuprofen 2400 mg/day (Fig. 6A and B).
3.2.2 Tolerability
3.2.2.1 Withdrawals due to all causes
Withdrawals due to all causes with diclofenac 150 and 100 mg/day and ibuprofen 1200 and 2400 mg/day were found to be lower than those with placebo (data for ibuprofen vs placebo not shown).
Withdrawals due to all causes with diclofenac 150 mg/day were comparable to those with ibuprofen 1200 and 2400 mg/day as well as diclofenac 100 mg/day (Fig. 7A). Withdrawals due to all causes with diclofenac 100 mg/day were comparable to those with ibuprofen 1200 and 2400 mg/day (Fig. 7B).
3.2.2.2 Withdrawals due to lack of efficacy
Compared with placebo, withdrawals due to lack of efficacy were lower with diclofenac 150 and 100 mg/day and likely to be lower with ibuprofen 1200 and 2400 mg/day as compared with placebo (data for ibuprofen vs placebo not shown).
Withdrawals due to lack of efficacy with diclofenac 150 mg/day were comparable with ibuprofen 1200 mg/day and lower than those with ibuprofen 2400 mg/day (Fig. 8A). Diclofenac 150 mg/day was likely to result in more withdrawals due to lack of efficacy versus those with diclofenac 100 mg/day.
Withdrawals due to lack of efficacy with diclofenac 100 mg/day were likely to be lower than those with ibuprofen 1200 mg/day and lower than with ibuprofen 2400 mg/day as well (Fig. 8B).
3.2.2.3 Withdrawals due to adverse events
Withdrawals due to AEs with diclofenac 150 mg/day were higher than with placebo and those with diclofenac 100 mg/day and ibuprofen 1200 and 2400 mg/day were likely to be higher than with placebo (data for ibuprofen vs placebo not shown).
Withdrawals due to AEs with diclofenac 150 mg/day were likely to be higher than with ibuprofen 2400 mg/day and comparable to those with ibuprofen 1200 mg/day and diclofenac 100 mg/day (Fig. 9A). Withdrawals due to AEs with diclofenac 100 mg/day were comparable to those with ibuprofen 2400 and 1200 mg/day (Fig. 9B).
The expected absolute effects and 95% CrI for each outcome (efficacy and safety) are presented in Appendix 8.
3.3 Scenario analyses
These results were slightly different in scenario 1 (Appendix 9). Diclofenac 150 mg/day was found likely to be favourable (P ≥ 85%) in comparison with ibuprofen 1200 mg/day and comparable (P: > 15% to < 85%) to ibuprofen 2400 mg/day in relieving pain at 12 weeks. Diclofenac 100 mg/day was found to be more efficacious (P ≥ 97.5%) than placebo for relieving pain at 4 weeks and comparable (P: > 15% to < 85%) with placebo and ibuprofen 2400 mg/day for relieving pain at 2 weeks. Based on PGA, the efficacy of diclofenac 150 and 100 mg/day was comparable (P: > 15% to < 85%) to that of placebo at 12 weeks. Based on IGA, the efficacy of diclofenac 100 mg/day was comparable (P: > 15% to < 85%) to that of placebo for IGA at 4 weeks. Withdrawals due to lack of efficacy with diclofenac 150 mg/day were likely to be lower than those with ibuprofen 1200 mg/day. Withdrawals due to lack of efficacy of diclofenac 100 mg/day were likely to be lower than those with placebo, while the withdrawals due to AEs of diclofenac 100 mg/day were comparable to those with placebo. On the other hand, a few changes were observed in scenario 2 (separation of diclofenac salts); these results are presented in Appendix 9.
3.4 Safety
3.4.1 Adverse events
The AEs, even if there are low number of events, were categorised by system organ class (SOC; terms pertaining to same organ system are grouped together). Appendix 10 presents a raw summary of the AEs across all the studies. The most frequent AEs were gastrointestinal disorders (e.g. peptic ulcer disease, gastritis, regional enteritis, or ulcerative colitis), nervous system disorders, respiratory, thoracic and mediastinal disorders, general disorders and administration site conditions, renal and urinary disorders, musculoskeletal and connective tissue disorders, infections and infestations, and cardiac disorders. No relevant differences were detected between diclofenac and ibuprofen.
3.4.2 Serious adverse events
A NMA of SAEs by SOC level was not possible owing to the very low number of reported events in these analysed studies. A summary of these data is presented in Appendix 11; this table has several ‘0’ values for exposure time (person-years), which indicates that none of the studies included treatment-provided information on the corresponding SAEs. Overall, SAEs appeared to be relatively rare in OA patients treated with tNSAIDs.
The summary results of the key benefits and risks of diclofenac 150 and 100 mg/day versus ibuprofen are summarised in Tables 1 and 2, respectively.
Relative benefits and risks of diclofenac 150 mg compared to placebo, and ibuprofen 1200 mg and 2400 mg.
| Outcome | Assessment time point | Placebo | Ibuprofen 1200 mg | Ibuprofen 2400 mg | |
|---|---|---|---|---|---|
| 2 weeks | −16.7 | NA | −1.3 | ||
| Pain (VAS) | (−23.3; −10.2) | (−4.3; 1.7) | |||
| Benefits | 4 weeks | −12.4 | −9.6 | −1.6 | |
| ΔCFB (mm) | (−19.7; −5.0) | (−16.9; −2.4) | (−6.5; 3.6) | ||
| 12 weeks | −8.8 | −6.0 | −3.1 | ||
| (−18.3; 0.6) | (−10.8; −1.2) | (−8.1; 1.8) | |||
| 4 weeks | −12.3 | −8.5 | −1.6 | ||
| PGA (VAS) | (−18.1; −6.6) | (−12.7; −4.4) | (−4.8; 1.4) | ||
| 12 weeks | −4.9 | −4.0 | −2.7 | ||
| (−13.9; 4.0) | (−8.6; 0.6) | (−7.5; 2.1) | |||
| IGA VAS | 4 weeks | −10.7 | NA | −1.2 | |
| (−17.4; −4.0) | (−3.9; 1.6) | ||||
| Serious adverse events | Duration of study | 0.45 | 0.65 | 1.37 | |
| Risks | (0.01; 6.08) | (0.18; 1.97) | (0.69; 2.92) | ||
| Rate ratio | Withdrawal due to all causes | Duration of study | 0.63 | 0.99 | 1.04 |
| (0.47; 0.84) | (0.72; 1.34) | (0.83; 1.31) | |||
| Withdrawal due to lack of efficacy | Duration of study | 0.37 | 0.74 | 0.63 | |
| (0.24; 0.56) | (0.39; 1.35) | (0.43; 0.93) | |||
| Withdrawal due to adverse events | Duration of study | 2.29 | 1.13 | 1.45 | |
| (1.27; 4.32) | (0.69; 1.81) | (0.96; 2.25) |
-
Mean and 95% credible intervals are presented; negative ΔCFBs favour diclofenac, rate ratios < 1 favour diclofenac.
ΔCFB, difference in change from baseline; IGA, investigator global assessment; NA, not available; PGA, patient global assessment; VAS, visual analogue scale.
Relative benefits and risks of diclofenac 100 mg compared to placebo, and ibuprofen 1200 mg and 2400 mg.
| Outcome | Assessment time point | Placebo | Ibuprofen 1200 mg | Ibuprofen 2400 mg | |
|---|---|---|---|---|---|
| 2 weeks | −10.5 | NA | 5.0 | ||
| Pain (VAS) | (−16.2; −4.9) | (−4.4; 14.2) | |||
| Benefits | 4 weeks | −7.2 | −4.5 | 3.5 | |
| ΔCFB (mm) | (−14.0; −0.4) | (−12.9; 4.0) | (−5.4; 12.8) | ||
| 12 weeks | −7.5 | −4.7 | −1.9 | ||
| (−14.3; −0.8) | (−10.8; 1.4) | (−10.2; 6.5) | |||
| 4 weeks | −9.4 | −5.6 | 1.2 | ||
| PGA (VAS) | (−14.4; −4.5) | (−10.8; −0.5) | (−4.8; 7.3) | ||
| 12 weeks | −5.0 | −4.1 | −2.7 | ||
| (−11.3; 1.4) | (−9.8; 1.7) | (−10.6; 5.2) | |||
| IGA VAS | 4 weeks | −6.5 | NA | 3.1 | |
| (−11.5; −1.4) | (−5.7; 11.9) | ||||
| Serious adverse events | Duration of study | 0.77 | 1.13 | 2.41 | |
| Risks | (0.21; 3.03) | (0.05; 69.69) | (0.12; 133.67) | ||
| Rate ratio | Withdrawal due to all causes | Duration of study | 0.54 | 0.85 | 0.90 |
| (0.39; 0.75) | (0.57; 1.26) | (0.58; 1.36) | |||
| Withdrawal due to lack of efficacy | Duration of study | 0.24 | 0.48 | 0.41 | |
| (0.14; 0.40) | (0.23; 1.00) | (0.20; 0.83) | |||
| Withdrawal due to adverse events | Duration of study | 1.96 | 0.96 | 1.24 | |
| (1.00; 4.10) | (0.47; 2.01) | (0.55; 2.89) |
-
Mean and 95% credible intervals are presented; negative ΔCFBs favour diclofenac, rate ratios < 1 favour diclofenac.
ΔCFB, difference in change from baseline; IGA, investigator global assessment; NA, not available; PGA, patient global assessment; VAS, visual analogue scale.
In addition to the most common comparators like diclofenac (150/100 mg/day) and ibuprofen (2400/1200 mg/day), some infrequent comparators like diclofenac (75 mg/day), naproxen (500/750/1000 mg/day), piroxicam (20 mg/day), indomethacin (75 mg/day) and paracetamol (1950 mg/day) in combination with dextropropoxyphene (195 mg/day) were also included in the efficacy and safety/tolerability analysis. Additional information regarding these infrequent comparators can be obtained from Appendix 12.

Global evidence network. Di, diclofenac; dex, dextropropoxyphene; Ibu, ibuprofen; Indo, indomethacin; Napro, naproxen; Para, paracetamol; Pbo, placebo; Piro, piroxicam.
4 Discussion
This study evaluated evidences of treatment with oral diclofenac formulations from various clinical trials to analyse potentially available but unpublished data. Data from clinical trials in OA with a study duration of at least 4 weeks (range 4–12 weeks) were reviewed, pooled, and analysed. Efficacy (pain relief, PGA and IGA), tolerability (withdrawals due to all causes, due to lack of efficacy and due to AEs), and safety (AEs and SAEs) outcomes were collated and various benefit and risk comparisons were undertaken in a rather homogenous population of OA patients (although with a variety of affected joints) using a similar methodology as used by van Walsem et al. [12].
To the best of our knowledge, there is no overlap between previous Coxib and tNSAIDTrialists’ (CNT) analysis [14], which included several unpublished studies, and our present NMA. By comparing the present data with that used in the NMA by van Walsem et al. [12], there is an overlap of three studies.
The overall efficacy outcomes of the present NMA indicate that diclofenac 150 mg/day was more efficacious than ibuprofen 1200 mg/day and likely to be more favourable than ibuprofen 2400 mg/day in relieving pain. Similarly, while comparing lower doses, diclofenac 100 mg/day was more efficacious than ibuprofen 1200 mg/day. This low dose of diclofenac was comparable to ibuprofen 2400 mg/day based on PGA and pain relief at 4 and 12 weeks, but it was likely to be unfavourable for pain relief at 2 weeks. The overall efficacy results are consistent with the results of a recently published systematic literature review NMA which included 176 published RCTs with a total of 146,524 patients with arthritis (van Walsem et al.), where diclofenac 150 mg/day was likely to be favourable than ibuprofen 2400 and 1200 mg/day, and diclofenac 100 mg/day was comparable with ibuprofen 2400 and 1200 mg/day [12]. The results of the present NMA are further supported by a very recently published NMA conducted by da Costa et al., where diclofenac at its maximum dose (150 mg/day) was reported to be the most effective option for the treatment of pain and physical disability in OA and superior to the maximum dose of ibuprofen (2400 mg/day) [15].
The present study results suggest that diclofenac was comparable to ibuprofen in terms of safety and tolerability. Withdrawal rates due to all causes with diclofenac at both doses (100 and 150 mg/day) were comparable to those with ibuprofen (at 1200 and 2400 mg/day). Despite the limitations in the comparison of these safety results with those from van Walsem et al., who pooled the different doses of each drug for safety analysis, there were no major contradictions in the results of these two NMAs.
As expected, diclofenac and ibuprofen have a better benefitrisk assessment compared to placebo. Both treatments were more efficacious and had lower withdrawal rates due to all causes than placebo. The analysis of SAEs did not reveal higher rates with these active drugs, although. Because of the wide Crls, no firm conclusions can be drawn when comparing the different treatments.
The main limitations of the present study were related to missing data for some of the planned comparisons, and the limited amount of data from these largely unpublished legacy studies. A maximum of 13 treatments used for pain relief in OA patients were included in the networks to estimate their comparisons. There was enough data for robust efficacy comparisons between diclofenac and ibuprofen, but the amount of data on naproxen, piroxicam, indomethacin, and paracetamol in combination with dextropropoxyphene were limited, and the number of patients in those treatment arms was too small for reliable comparisons with diclofenac. Therefore, the detailed presentation of the results had to be limited to comparison between diclofenac and ibuprofen. In addition, a few differences have been detected in the two scenario analyses. In scenario 1, with respect to pain CFB at 12 weeks, although the point estimate did not change, the exclusion of trial C added uncertainty in the estimates, thereby downgrading the difference between diclofenac 150 mg/day and ibuprofen 1200 mg/day from ‘more efficacious’ to ‘likely to be favourable’. In scenario 2, diclofenac potassium 150 mg/day was found to be better than diclofenac resinate 150 mg/day with respect to pain CFB at 2 weeks and better than diclofenac sodium 150 mg/day with respect to pain CFB at 4 weeks. The separation of diclofenac salts decreased the number of studies per treatment arm and added an extra link between the treatments. Additional data are needed to draw firm conclusions regarding the possible effects of diclofenac salts on pain relief. Nevertheless, the few differences detected in the two scenario analyses did not change the overall interpretation of the results.
In various networks (pain at 2 weeks, pain at 12 weeks, PGA at 12 weeks, IGA at 4 weeks, and SAEs), there were no closed loops because these time points were not consistent across all studies. For networks with closed loops, direct and indirect evidence was consistent (i.e. the consistency assumption was tested and found valid).
Moreover, the results are also important in another respect; they make otherwise unpublished data available. It is reassuring to see that these previously unpublished results are similar to those reported in published studies, and to the recent large NMA of published studies [12], as well as to a somewhat different analysis of similar published data which was conducted by da Costa et al. [15]. The fact that these unpublished clinical trial data present similar efficacy estimates as published ones is not new and has been shown before by Moore and Barden [16]. Thus, the present NMA results further extend that observation and also contributes to increase the transparency of clinical trials performed with diclofenac further back in the past.
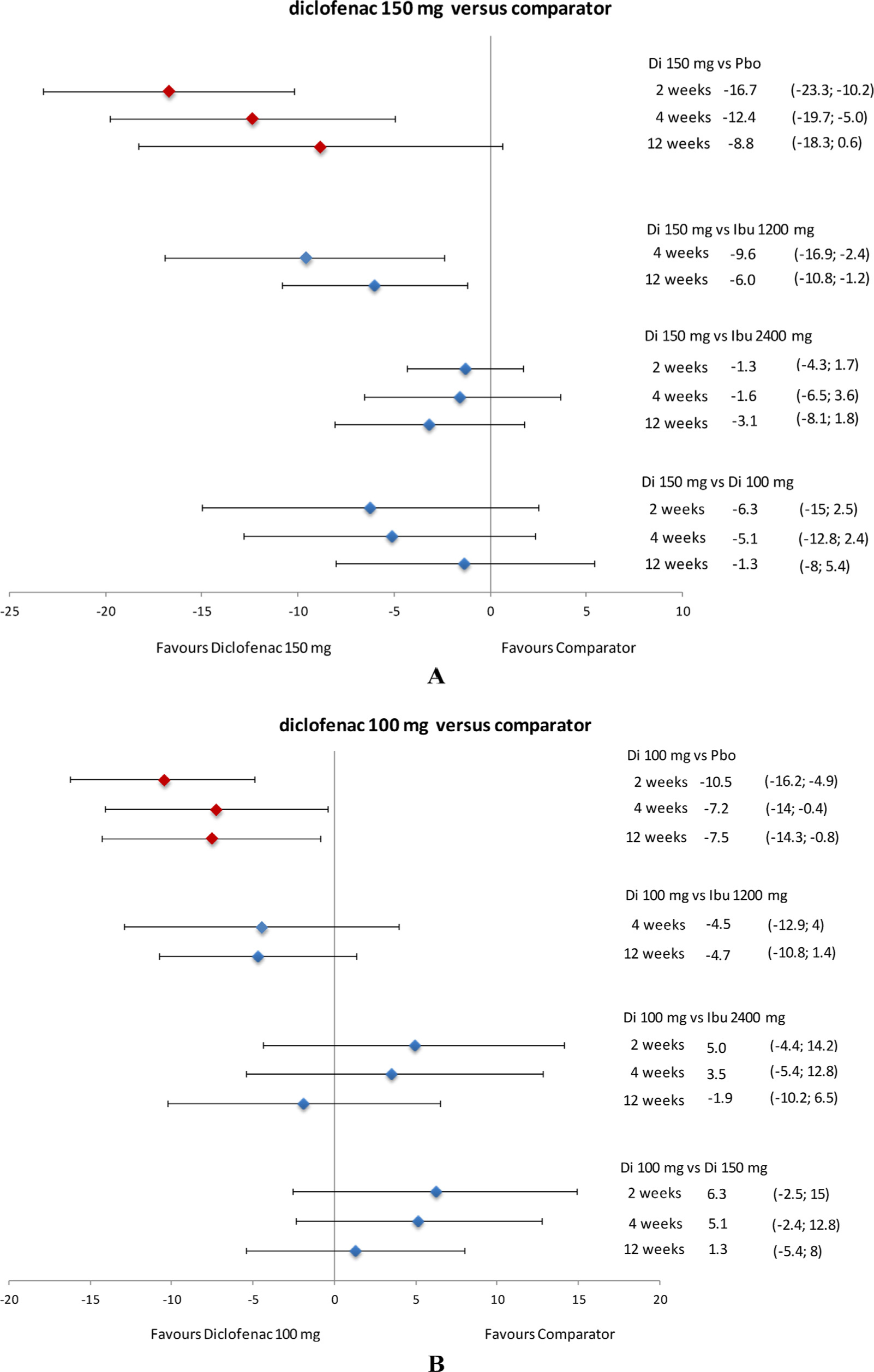
Relative efficacy of diclofenac in terms of pain (VAS) difference in CFB at 2, 4, and 12 weeks. Data presented as mean and 95% credible interval. CFB, change from baseline; Di, diclofenac; Ibu, ibuprofen; Pbo, placebo; VAS, visual analogue scale.
5 Conclusions
In the present NMA of unpublished legacy clinical trials, diclofenac 150 mg/day was more efficacious than ibuprofen 1200 mg/day and had likely favourable outcomes compared to ibuprofen 2400 mg/day for pain relief in OA. Diclofenac 100 mg/day had likely favourable outcomes compared to ibuprofen 1200 mg/day in alleviating pain. Based on PGA, diclofenac 150 mg/day was also more efficacious and likely to be more efficacious than ibuprofen 1200 mg/day and 2400 mg/day, respectively. The favourable efficacy results of diclofenac versus ibuprofen expand the amount of evidence comparing these two NSAIDs and may help physicians in making treatment decisions for patients with OA. The safety and tolerability results as well as the overall benefit-risk profile of these two drugs in OA were comparable. The results of this NMA were in line with the published study results of diclofenac in OA patients and give similar answers as those in published materials. Estimates of efficacy or risks were demonstrated to be similar in both unpublished and published trials.
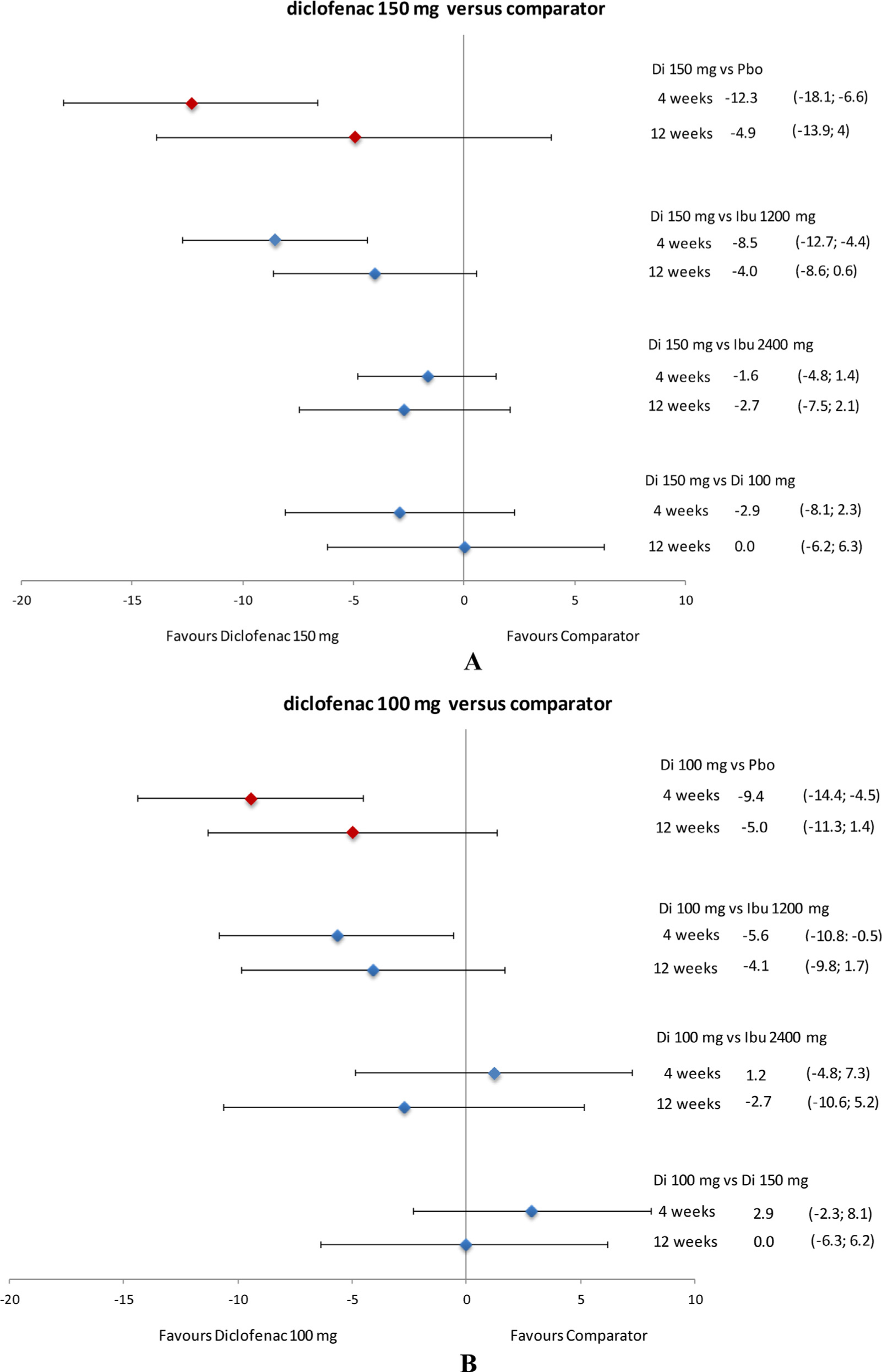
Relative efficacy of diclofenac in terms of PGA (VAS) difference in CFB at 4 and 12 weeks. Data presented as mean and 95% credible interval. CFB, change from baseline; Di, diclofenac; Ibu, ibuprofen; Pbo, placebo; PGA, patient global assessment; VAS, visual analogue scale.
6 Implications
The present NMA results reassures that the older unpublished blinded trials have similar results compared to more recently published trials and also contributes to increase the transparency of clinical trials performed with diclofenac further back in the past.

Relative efficacy of diclofenac in terms of IGA (VAS) difference in CFB at 4 weeks. Data presented as mean and 95% credible interval. IGA data on ibuprofen 1200 mg/day were not available. CFB, change from baseline; Di, diclofenac; Ibu, ibuprofen; IGA, investigator global assessment; Pbo, placebo; VAS, visual analogue scale.
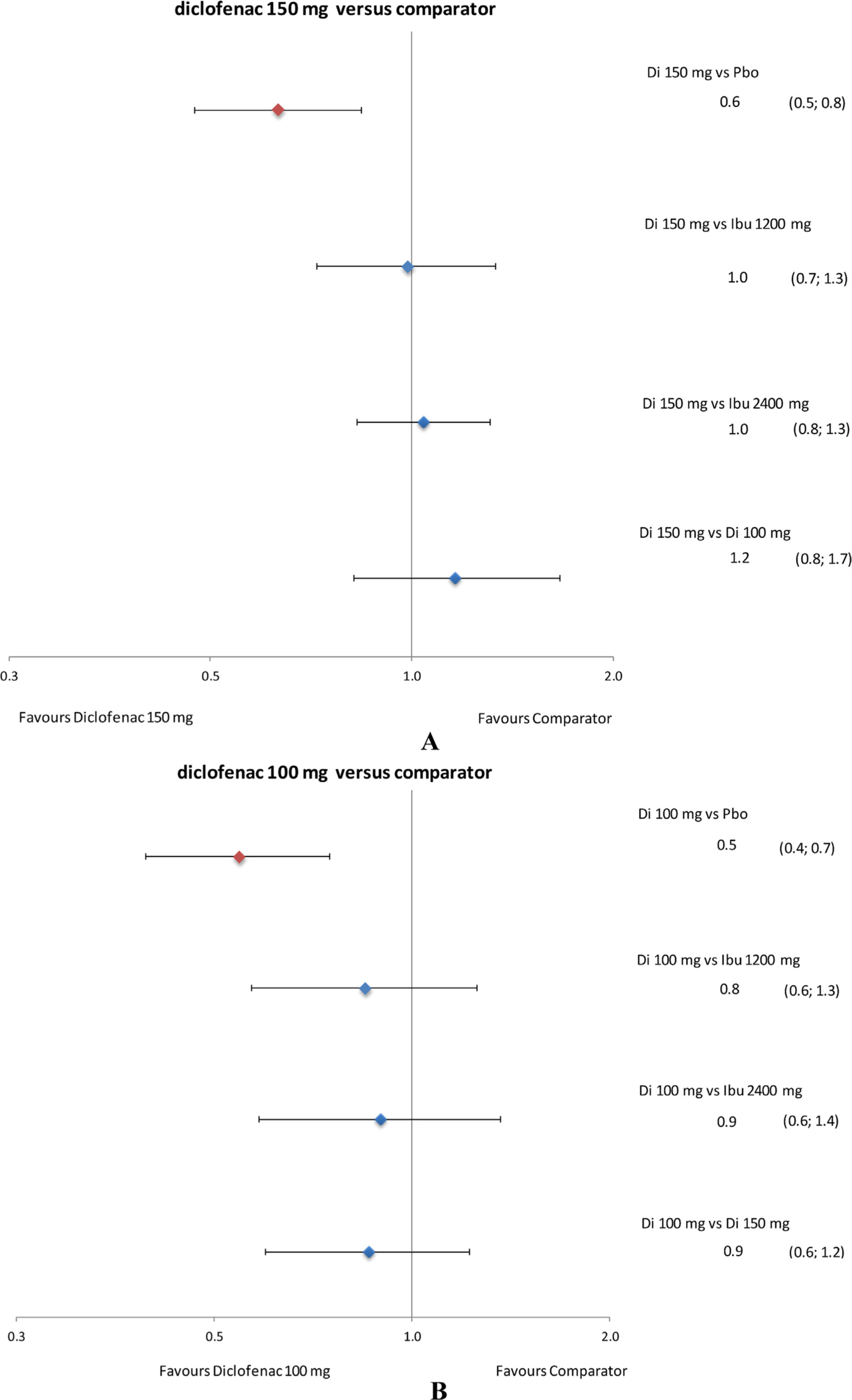
Forest plots of relative tolerability: withdrawal due to all causes. Data presented as rate ratio and 95% credible interval. Di, diclofenac; Ibu, ibuprofen; Pbo, placebo.

Forest plots of relative tolerability: withdrawal due to lack of efficacy. Data presented as rate ratio and 95% credible interval. Di: diclofenac, Ibu: ibuprofen; Pbo: placebo.
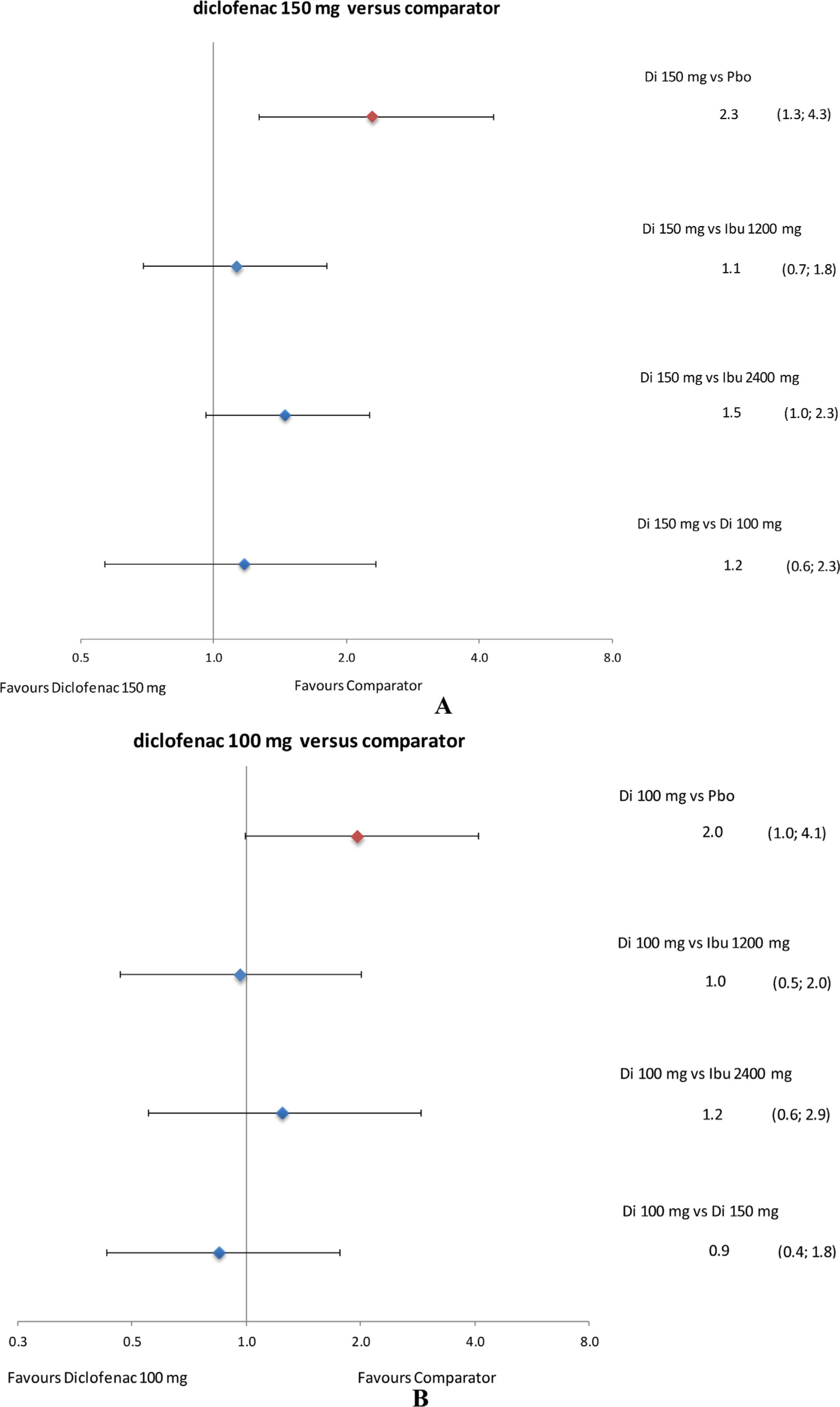
Forest plots of relative tolerability: withdrawal due to AEs. Data presented as rate ratio and 95% credible interval. AEs, adverse events; Di, diclofenac; Ibu, ibuprofen; Pbo, placebo.
Ethical issues
Ethics approval and consent to participate
All the studies were approved by the respective ethical committee of the countries and patient consent were obtained for the same. These studies form a ‘legacy’ of unpublished studies from an era when automatic registration and publication of every clinical trial just did not happen.
Consent to publish
Not applicable.
Availability of data and materials
The input data per study used for our analysis are provided in the manuscript and the supporting files.
Highlights
Legacy unpublished randomised controlled trials of diclofenac in osteoarthritis.
Bayesian NMA model estimated relative treatment effects between pairwise treatments.
Diclofenac 150 mg/day was more efficacious for pain relief than ibuprofen 1200 mg/day.
Diclofenac 150 mg/day had likely favourable outcomes for pain relief compared to ibuprofen 2400 mg/day.
Benefit-risk profile of diclofenac was comparable to that of ibuprofen in osteoarthritis.
DOI of refers to article: http://dx.doi.org/10.1016/j.sjpain.2017.05.009.
-
Authors’ contributions: RMN conceptualised and designed the study.
The study was conducted and the data were analysed by RMN and PG.
PG was involved in data collection.
Interpretation of data was done by RMN, PG, RAM, SP and RLC. All the authors drafted the manuscript and revised the contents. They also approved the final version and were responsible for the integrity of data analysis.
-
Conflict of interest: This study was conducted by Mapi on behalf of Novartis Pharma AG (Basel, Switzerland) who funded the study. PG is an employee of Mapi and served as paid consultant to Novartis during the conduct of this study and the preparation of this manuscript. SP, RN, AI, and RLC are employees of Novartis and are thus eligible for Novartis stock and stock options. RAM has no competing interests to declare in this work.
-
Funding: The study was funded by Novartis Pharma AG.
Acknowledgements
Authors thank Andreas Karabis (Mapi Group, The Netherlands) for his help in study conduct and preparation of the manuscript. The authors also thank Jitendriya Mishra (Novartis Healthcare Pvt. Ltd., Hyderabad, India) for providing medical writing assistance on this manuscript. Funding for writing assistance was provided by Novartis Pharma AG.
This manuscript is dedicated to the memory of Richard M. Nixon.
References
[1] Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, Abraham J, Ackerman I, Aggarwal R, Ahn SY, Ali MK, AlMazroa MA, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Bahalim AN, Barker-Collo S, Barrero LH, Bartels DH, Basáñez M-G, Baxter A, Bell ML, Benjamin EJ, Bennett D, Bernabé E, Bhalla K, Bhandari B, Bikbov B, Abdulhak AB, Birbeck G, Black JA, Blencowe H, Blore JD, Blyth F, Bolliger I, Bonaventure A, Boufous S, Bourne R, Boussinesq M, Braith-waite T, Brayne C, Bridgett L, Brooker S, Brooks P, Brugha TS, Bryan-Hancock C, Bucello C, Buchbinder R, Buckle G, Budke CM, Burch M, Burney P, Burstein R, Calabria B, Campbell B, Canter CE, Carabin H, Carapetis J, Carmona L, Cella C, Charlson F, Chen H, Cheng AT-A, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahiya M, Dahodwala N, Damsere-Derry J, Danaei G, Davis A, De Leo D, Degenhardt L, Dellavalle R, Delossantos A, Denenberg J, Derrett S, Des Jarlais DC, Dharmaratne SD, Dherani M, Diaz-Torne C, Dolk H, Dorsey ER, Driscoll T, Duber H, Ebel B, Edmond K, Elbaz A, Ali SE, Erskine H, Erwin PJ, Espindola P, Ewoigbokhan SE, Farzadfar F, Feigin V, Felson DT, Ferrari A, Ferri CP, Fèvre EM, Finucane MM, Flaxman S, Flood L, Foreman K, Forouzanfar MH, Fowkes FGR, Franklin R, Fransen M, Freeman MK, Gabbe BJ, Gabriel SE, Gakidou E, Ganatra HA, Garcia B, Gaspari F, Gillum RF, Gmel G, Gosselin R, Grainger R, Groeger J, Guillemin F, Gunnell D, Gupta R, Haagsma J, Hagan H, Halasa YA, Hall W, Haring D, Haro JM, Harrison JE, Havmoeller R, Hay RJ, Higashi H, Hill C, Hoen B, Hoffman H, Hotez PJ, Hoy D, Huang JJ, Ibeanusi SE, Jacobsen KH, James SL, Jarvis D, Jasrasaria R, Jayaraman S, Johns N, Jonas JB, Karthikeyan G, Kassebaum N, Kawakami N, Keren A, Khoo J-P, King CH, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lalloo R, Laslett LL, Lathlean T, Leasher JL, Lee YY, Leigh J, Lim SS, Limb E, Lin JK, Lipnick M, Lipshultz SE, Liu W, Loane M, Ohno SL, Lyons R, Ma J, Mabweijano J, MacIntyre MF, Malekzadeh R, Mallinger L, Manivannan S, Marcenes W, March L, Margolis DJ, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGill N, McGrath J, Medina-Mora ME, Meltzer M, Memish ZA, Mensah GA, Merriman TR, Meyer A-C, Miglioli V, Miller M, Miller TR, Mitchell PB, Mocumbi AO, Moffitt TE, Mokdad AA, Monasta L, Montico M, Moradi-Lakeh M, Moran A, Morawska L, Mori R, Murdoch ME, Mwaniki MK, Naidoo K, Nair MN, Naldi L, Narayan KMV, Nelson PK, Nelson RG, Nevitt MC, Newton CR, Nolte S, Norman P, Norman R, O’Donnell M, O’Hanlon S, Olives C, Omer SB, Ortblad K, Osborne R, Ozgediz D, Page A, Pahari B, Pandian JD, Rivero AP, Patten SB, Pearce N, Padilla RP, Perez-Ruiz F, Perico N, Pesudovs K, Phillips D, Phillips MR, Pierce K, Pion S, Polanczyk GV, Polinder S, Pope Iii CA, Popova S, Porrini E, Pourmalek F, Prince M, Pullan RL, Ramaiah KD, Ranganathan D, Razavi H, Regan M, Rehm JT, Rein DB, Remuzzi G, Richardson K, Rivara FP, Roberts T, Robinson C, De Leòn FR, Ronfani L, Room R, Rosenfeld LC, Rushton L, Sacco RL, Saha S, Sampson U, Sanchez-Riera L, Sanman E, Schwebel DC, Scott JG, Segui-Gomez M, Shahraz S, Shepard DS, Shin H, Shivakoti R, Silberberg D, Singh D, Singh GM, Singh JA, Singleton J, Sleet DA, Sliwa K, Smith E, Smith JL, Stapelberg NJC, Steer A, Steiner T, Stolk WA, Stovner LJ, Sudfeld C, Syed S, Tamburlini G, Tavakkoli M, Taylor HR, Taylor JA, Taylor WJ, Thomas B, Thomson WM, Thurston GD, Tleyjeh IM, Tonelli M, Towbin JA, Truelsen T, Tsilimbaris MK, Ubeda C, Undurraga EA, van der Werf MJ, van Os J, Vavilala MS, Venketasubramanian N, Wang M, Wang W, Watt K, Weatherall DJ, Weinstock MA, Weintraub R, Weisskopf MG, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams SRM, Witt E, Wolfe F, Woolf AD, Wulf S, Yeh P-H, Zaidi AKM, Zheng Z-J, Zonies D, Lopez AD, Murray CJL. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2163–96.Search in Google Scholar
[2] Bannuru RR, Schmid CH, Kent DM, Vaysbrot EE, Wong JB, McAlindon TE. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med 2015;162:46–54.Search in Google Scholar
[3] Conaghan PG, Peloso PM, Everett SV, Rajagopalan S, Black CM, Mavros P, Arden NK, Phillips CJ, Rannou F, van de Laar MA, Moore RA, Taylor SD. Inadequate pain relief and large functional loss among patients with knee osteoarthritis: evidence from a prospective multinational longitudinal study of osteoarthritis real-world therapies. Rheumatology (Oxford, England) 2015;54:270–7.Search in Google Scholar
[4] Battisti WP, Wager E, Baltzer L, Bridges D, Cairns A, Carswell CI, Citrome L, Gurr JA, Mooney LA, Moore BJ, Peña T, Sanes-Miller CH, Veitch K, Woolley KL, Yarker YE. Good Publication Practice for communicating company-sponsored medical research: GPP3Good Publication Practice for Company-Sponsored Research (GPP3). Ann Intern Med 2015;163:461–4.Search in Google Scholar
[5] https://www.novartis.com/sites/www.novartis.com/files/novartispublicationguidelinesposting.pdf.Search in Google Scholar
[6] Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials 1996;17:1–12.Search in Google Scholar
[7] Caldwell DM, Ades AE, Higgins JPT. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. Br Med J 2005;331:897–900.Search in Google Scholar
[8] Jansen JP, Fleurence R, Devine B, Itzler R, Barrett A, Hawkins N, Lee K, Boersma C, Annemans L, Cappelleri JC. Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 1. Value in Health 2011;14:417–28.Search in Google Scholar
[9] Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med 2004;23:3105–24.Search in Google Scholar
[10] Dias S, Sutton AJ, Ades AE, Welton NJ. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med Decis Making 2013;33:607–17.Search in Google Scholar
[11] Spiegelhalter DJ, Best NG, Carlin BP, Van Der Linde A. Bayesian measures of model complexity and fit. J R Stat Soc Series B Stat Methodol 2002;64:583–639.Search in Google Scholar
[12] van Walsem A, Pandhi S, Nixon RM, Guyot P, Karabis A, Moore RA. Relative benefit-risk comparing diclofenac to other traditional non-steroidal anti-inflammatory drugs and cyclooxygenase-2 inhibitors in patients with osteoarthritis or rheumatoid arthritis: a network meta-analysis. Arthritis Res Ther 2015;17:66.Search in Google Scholar
[13] Cope S, Donohue JF, Jansen JP, Kraemer M, Capkun-Niggli G, Baldwin M, Buckley F, Ellis A, Jones P. Comparative efficacy of long-acting bronchodilators for COPD – a network meta-analysis. Respir Res 2013;14:100.Search in Google Scholar
[14] Bhala N, Emberson J, Merhi A, Abramson S, Arber N, Baron JA, Bombardier C, Cannon C, Farkouh ME, FitzGerald GA, Goss P, Halls H, Hawk E, Hawkey C, Hennekens C, Hochberg M, Holland LE, Kearney PM, Laine L, Lanas A, Lance P, Laupacis A, Oates J, Patrono C, Schnitzer TJ, Solomon S, Tugwell P, Wilson K, Wittes J, Baigent C. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet 2013;382:769–79.Search in Google Scholar
[15] da Costa BR, Reichenbach S, Keller N, Nartey L, Wandel S, Juni P, Trelle S. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet 2016, http://dx.doi.org/10.1016/s0140-6736(16)30002-2.Search in Google Scholar
[16] Moore RA, Barden J. Systematic review of dexketoprofen in acute and chronic pain. BMC Clin Pharmacol 2008;8:11.Search in Google Scholar
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at http://dx.doi.org/10.1016/j.sjpain.2017.03.006.
- Abbreviations
- AE
-
adverse event
- CFB
-
change from baseline
- CNT
-
Coxib and tNSAID Trialists’
- CrI
-
credible interval
- CSR
-
clinical study reports
- DIC
-
deviance information criterion
- IGA
-
investigator global assessment
- ITT
-
intention-to-treat
- NMA
-
network meta-analysis
- OA
-
osteoarthritis
- PGA
-
patient global assessment
- RCT
-
randomised controlled trials
- SAE
-
serious adverse event
- tNSAID
-
traditional non-steroidal anti-inflammatory drug
- VAS
-
visual analogue scale
© 2017 Scandinavian Association for the Study of Pain
- Supplementary Material Details
- Supplementary Material Details
- Supplementary Material Details
- Supplementary Material Details
- Supplementary Material Details
- Supplementary Material Details
- Supplementary Material Details
- Supplementary Material Details
- Supplementary Material Details
- Supplementary Material Details
- Supplementary Material Details
Articles in the same Issue
- Scandinavian Journal of Pain
- Editorial comment
- Glucocorticoids – Efficient analgesics against postherpetic neuralgia?
- Original experimental
- Effect of intrathecal glucocorticoids on the central glucocorticoid receptor in a rat nerve ligation model
- Editorial comment
- Important new insight in pain and pain treatment induced changes in functional connectivity between the Pain Matrix and the Salience, Central Executive, and Sensorimotor networks
- Original experimental
- Salience, central executive, and sensorimotor network functional connectivity alterations in failed back surgery syndrome
- Editorial comment
- Education and support strategies improve assessment and management of pain by nurses
- Clinical pain research
- Using education and support strategies to improve the way nurses assess regular and transient pain – A quality improvement study of three hospitals
- Editorial comment
- The interference of pain with task performance: Increasing ecological validity in research
- Original experimental
- The disruptive effects of pain on multitasking in a virtual errands task
- Editorial comment
- Analyzing transition from acute back pain to chronic pain with linear mixed models reveals a continuous chronification of acute back pain
- Observational study
- From acute to chronic back pain: Using linear mixed models to explore changes in pain intensity, disability, and depression
- Editorial comment
- NSAIDs relieve osteoarthritis (OA) pain, but cardiovascular safety in question even for diclofenac, ibuprofen, naproxen, and celecoxib: what are the alternatives?
- Clinical pain research
- Efficacy and safety of diclofenac in osteoarthritis: Results of a network meta-analysis of unpublished legacy studies
- Editorial comment
- Editorial comment on Nina Kreddig’s and Monika Hasenbring’s study on pain anxiety and fear of (re) injury in patients with chronic back pain: Sex as a moderator
- Clinical pain research
- Pain anxiety and fear of (re) injury in patients with chronic back pain: Sex as a moderator
- Editorial comment
- Intraoral QST – Mission impossible or not?
- Clinical pain research
- Multifactorial assessment of measurement errors affecting intraoral quantitative sensory testing reliability
- Editorial comment
- Objective measurement of subjective pain-experience: Real nociceptive stimuli versus pain expectation
- Clinical pain research
- Cerebral oxygenation for pain monitoring in adults is ineffective: A sequence-randomized, sham controlled study in volunteers
- Editorial comment
- Association between adolescent and parental use of analgesics
- Observational study
- The association between adolescent and parental use of non-prescription analgesics for headache and other somatic pain – A cross-sectional study
- Editorial comment
- Cancer-pain intractable to high-doses systemic opioids can be relieved by intraspinal local anaesthetic plus an opioid and an alfa2-adrenoceptor agonist
- Clinical pain research
- Spinal analgesia for severe cancer pain: A retrospective analysis of 60 patients
- Editorial comment
- Specific symptoms and signs of unstable back segments and curative surgery?
- Clinical pain research
- Symptoms and signs possibly indicating segmental, discogenic pain. A fusion study with 18 years of follow-up
- Editorial comment
- Local anaesthesia methods for analgesia after total hip replacement: Problems of anatomy, methodology and interpretation?
- Clinical pain research
- Local infiltration analgesia or femoral nerve block for postoperative pain management in patients undergoing total hip arthroplasty. A randomized, double-blind study
- Editorial
- Scientific presentations at the 2017 annual meeting of the Scandinavian Association for the Study of Pain (SASP)
- Abstracts
- Correlation between quality of pain and depression: A post-operative assessment of pain after caesarian section among women in Ghana
- Abstracts
- Dynamic and static mechanical pain sensitivity is associated in women with migraine
- Abstracts
- The number of active trigger points is associated with sensory and emotional aspects of health-related quality of life in tension type headache
- Abstracts
- Chronic neuropathic pain following oxaliplatin and docetaxel: A 5-year follow-up questionnaire study
- Abstracts
- Expression of α1 adrenergic receptor subtypes by afferent fibers that innervate rat masseter muscle
- Abstracts
- Buprenorphine alleviation of pain does not compromise the rat monoarthritic pain model
- Abstracts
- Association between pain, disability, widespread pressure pain hypersensitivity and trigger points in subjects with neck pain
- Abstracts
- Association between widespread pressure pain hypersensitivity, health history, and trigger points in subjects with neck pain
- Abstracts
- Neuromas in patients with peripheral nerve injury and amputation - An ongoing study
- Abstracts
- The link between chronic musculoskeletal pain and sperm quality in overweight orthopedic patients
- Abstracts
- Several days of muscle hyperalgesia facilitates cortical somatosensory excitability
- Abstracts
- Social stress, epigenetic changes and pain
- Abstracts
- Characterization of released exosomes from satellite glial cells under normal and inflammatory conditions
- Abstracts
- Cell-based platform for studying trigeminal satellite glial cells under normal and inflammatory conditions
- Abstracts
- Tramadol in postoperative pain – 1 mg/ml IV gave no pain reduction but more side effects in third molar surgery
- Abstracts
- Tempo-spatial discrimination to non-noxious stimuli is better than for noxious stimuli
- Abstracts
- The encoding of the thermal grill illusion in the human spinal cord
- Abstracts
- Effect of cocoa on endorphin levels and craniofacial muscle sensitivity in healthy individuals
- Abstracts
- The impact of naloxegol treatment on gastrointestinal transit and colonic volume
- Abstracts
- Preoperative downregulation of long-noncoding RNA Meg3 in serum of patients with chronic postoperative pain after total knee replacement
- Abstracts
- Painful diabetic polyneuropathy and quality of life in Danish type 2 diabetic patients
- Abstracts
- “What about me?”: A qualitative explorative study on perspectives of spouses living with complex chronic pain patients
- Abstracts
- Increased postural stiffness in patients with knee osteoarthritis who are highly sensitized
- Abstracts
- Efficacy of dry needling on latent myofascial trigger points in male subjects with neck/shoulders musculoskeletal pain. A case series
- Abstracts
- Identification of pre-operative of risk factors associated with persistent post-operative pain by self-reporting tools in lower limb amputee patients – A feasibility study
- Abstracts
- Renal function estimations and dose recommendations for Gabapentin, Ibuprofen and Morphine in acute hip fracture patients
- Abstracts
- Evaluating the ability of non-rectangular electrical pulse forms to preferentially activate nociceptive fibers by comparing perception thresholds
- Abstracts
- Detection of systemic inflammation in severely impaired chronic pain patients, and effects of a CBT-ACT-based multi-modal pain rehabilitation program
- Abstracts
- Fixed or adapted conditioning intensity for repeated conditioned pain modulation
- Abstracts
- Combined treatment (Norspan, Gabapentin and Oxynorm) was found superior in pain management after total knee arthroplasty
- Abstracts
- Effects of conditioned pain modulation on the withdrawal pattern to nociceptive stimulation in humans – Preliminary results
- Abstracts
- Application of miR-223 onto the dorsal nerve roots in rats induces hypoexcitability in the pain pathways
- Abstracts
- Acute muscle pain alters corticomotor output of the affected muscle stronger than a synergistic, ipsilateral muscle
- Abstracts
- The subjective sensation induced by various thermal pulse stimulation in healthy volunteers
- Abstracts
- Assessing Offset Analgesia through electrical stimulations in healthy volunteers
- Abstracts
- Metastatic lung cancer in patient with non-malignant neck pain: A case report
- Abstracts
- The size of pain referral patterns from a tonic painful mechanical stimulus is increased in women
- Abstracts
- Oxycodone and macrogol 3350 treatment reduces anal sphincter relaxation compared to combined oxycodone and naloxone tablets
- Abstracts
- The effect of UVB-induced skin inflammation on histaminergic and non-histaminergic evoked itch and pain
- Abstracts
- Topical allyl-isothiocyanate (mustard oil) as a TRPA1-dependent human surrogate model of pain, hyperalgesia, and neurogenic inflammation – A dose response study
- Abstracts
- Dissatisfaction and persistent post-operative pain following total knee replacement – A 5 year follow-up of all patients from a whole region
- Abstracts
- Paradoxical differences in pain ratings of the same stimulus intensity
- Abstracts
- Pain assessment and post-operative pain management in orthopedic patients
- Abstracts
- Combined electric and pressure cuff pain stimuli for assessing conditioning pain modulation (CPM)
- Abstracts
- The effect of facilitated temporal summation of pain, widespread pressure hyperalgesia and pain intensity in patients with knee osteoarthritis on the responds to Non-Steroidal Anti-Inflammatory Drugs – A preliminary analysis
- Abstracts
- How to obtain the biopsychosocial record in multidisciplinary pain clinic? An action research study
- Abstracts
- Experimental neck muscle pain increase pressure pain threshold over cervical facet joints
- Abstracts
- Are we using Placebo effects in specialized Palliative Care?
- Abstracts
- Prevalence and pattern of helmet-induced headache among Danish military personnel
- Abstracts
- Aquaporin 4 expression on trigeminal satellite glial cells under normal and inflammatory conditions
- Abstracts
- Preoperative synovitis in knee osteoarthritis is predictive for pain 1 year after total knee arthroplasty
- Abstracts
- Biomarkers alterations in trapezius muscle after an acute tissue trauma: A human microdialysis study
- Abstracts
- PainData: A clinical pain registry in Denmark
- Abstracts
- A novel method for investigating the importance of visual feedback on somatosensation and bodily-self perception
- Abstracts
- Drugs that can cause respiratory depression with concomitant use of opioids
- Abstracts
- The potential use of a serious game to help patients learn about post-operative pain management – An evaluation study
- Abstracts
- Modelling activity-dependent changes of velocity in C-fibers
- Abstracts
- Choice of rat strain in pre-clinical pain-research – Does it make a difference for translation from animal model to human condition?
- Abstracts
- Omics as a potential tool to identify biomarkers and to clarify the mechanism of chronic pain development
- Abstracts
- Evaluation of the benefits from the introduction meeting for patients with chronic non-malignant pain and their relatives in interdisciplinary pain center
- Observational study
- The changing face of acute pain services
- Observational study
- Chronic pain in multiple sclerosis: A10-year longitudinal study
- Clinical pain research
- Functional disability and depression symptoms in a paediatric persistent pain sample
- Observational study
- Pain provocation following sagittal plane repeated movements in people with chronic low back pain: Associations with pain sensitivity and psychological profiles
- Observational study
- A longitudinal exploration of pain tolerance and participation in contact sports
- Original experimental
- Taking a break in response to pain. An experimental investigation of the effects of interruptions by pain on subsequent activity resumption
- Clinical pain research
- Sex moderates the effects of positive and negative affect on clinical pain in patients with knee osteoarthritis
- Original experimental
- The effects of a brief educational intervention on medical students’ knowledge, attitudes and beliefs towards low back pain
- Observational study
- The association between pain characteristics, pain catastrophizing and health care use – Baseline results from the SWEPAIN cohort
- Topical review
- Couples coping with chronic pain: How do intercouple interactions relate to pain coping?
- Narrative review
- The wit and wisdom of Wilbert (Bill) Fordyce (1923 - 2009)
- Letter to the Editor
- Unjustified extrapolation
- Letter to the Editor
- Response to: “Letter to the Editor entitled: Unjustified extrapolation” [by authors: Supp G., Rosedale R., Werneke M.]
Articles in the same Issue
- Scandinavian Journal of Pain
- Editorial comment
- Glucocorticoids – Efficient analgesics against postherpetic neuralgia?
- Original experimental
- Effect of intrathecal glucocorticoids on the central glucocorticoid receptor in a rat nerve ligation model
- Editorial comment
- Important new insight in pain and pain treatment induced changes in functional connectivity between the Pain Matrix and the Salience, Central Executive, and Sensorimotor networks
- Original experimental
- Salience, central executive, and sensorimotor network functional connectivity alterations in failed back surgery syndrome
- Editorial comment
- Education and support strategies improve assessment and management of pain by nurses
- Clinical pain research
- Using education and support strategies to improve the way nurses assess regular and transient pain – A quality improvement study of three hospitals
- Editorial comment
- The interference of pain with task performance: Increasing ecological validity in research
- Original experimental
- The disruptive effects of pain on multitasking in a virtual errands task
- Editorial comment
- Analyzing transition from acute back pain to chronic pain with linear mixed models reveals a continuous chronification of acute back pain
- Observational study
- From acute to chronic back pain: Using linear mixed models to explore changes in pain intensity, disability, and depression
- Editorial comment
- NSAIDs relieve osteoarthritis (OA) pain, but cardiovascular safety in question even for diclofenac, ibuprofen, naproxen, and celecoxib: what are the alternatives?
- Clinical pain research
- Efficacy and safety of diclofenac in osteoarthritis: Results of a network meta-analysis of unpublished legacy studies
- Editorial comment
- Editorial comment on Nina Kreddig’s and Monika Hasenbring’s study on pain anxiety and fear of (re) injury in patients with chronic back pain: Sex as a moderator
- Clinical pain research
- Pain anxiety and fear of (re) injury in patients with chronic back pain: Sex as a moderator
- Editorial comment
- Intraoral QST – Mission impossible or not?
- Clinical pain research
- Multifactorial assessment of measurement errors affecting intraoral quantitative sensory testing reliability
- Editorial comment
- Objective measurement of subjective pain-experience: Real nociceptive stimuli versus pain expectation
- Clinical pain research
- Cerebral oxygenation for pain monitoring in adults is ineffective: A sequence-randomized, sham controlled study in volunteers
- Editorial comment
- Association between adolescent and parental use of analgesics
- Observational study
- The association between adolescent and parental use of non-prescription analgesics for headache and other somatic pain – A cross-sectional study
- Editorial comment
- Cancer-pain intractable to high-doses systemic opioids can be relieved by intraspinal local anaesthetic plus an opioid and an alfa2-adrenoceptor agonist
- Clinical pain research
- Spinal analgesia for severe cancer pain: A retrospective analysis of 60 patients
- Editorial comment
- Specific symptoms and signs of unstable back segments and curative surgery?
- Clinical pain research
- Symptoms and signs possibly indicating segmental, discogenic pain. A fusion study with 18 years of follow-up
- Editorial comment
- Local anaesthesia methods for analgesia after total hip replacement: Problems of anatomy, methodology and interpretation?
- Clinical pain research
- Local infiltration analgesia or femoral nerve block for postoperative pain management in patients undergoing total hip arthroplasty. A randomized, double-blind study
- Editorial
- Scientific presentations at the 2017 annual meeting of the Scandinavian Association for the Study of Pain (SASP)
- Abstracts
- Correlation between quality of pain and depression: A post-operative assessment of pain after caesarian section among women in Ghana
- Abstracts
- Dynamic and static mechanical pain sensitivity is associated in women with migraine
- Abstracts
- The number of active trigger points is associated with sensory and emotional aspects of health-related quality of life in tension type headache
- Abstracts
- Chronic neuropathic pain following oxaliplatin and docetaxel: A 5-year follow-up questionnaire study
- Abstracts
- Expression of α1 adrenergic receptor subtypes by afferent fibers that innervate rat masseter muscle
- Abstracts
- Buprenorphine alleviation of pain does not compromise the rat monoarthritic pain model
- Abstracts
- Association between pain, disability, widespread pressure pain hypersensitivity and trigger points in subjects with neck pain
- Abstracts
- Association between widespread pressure pain hypersensitivity, health history, and trigger points in subjects with neck pain
- Abstracts
- Neuromas in patients with peripheral nerve injury and amputation - An ongoing study
- Abstracts
- The link between chronic musculoskeletal pain and sperm quality in overweight orthopedic patients
- Abstracts
- Several days of muscle hyperalgesia facilitates cortical somatosensory excitability
- Abstracts
- Social stress, epigenetic changes and pain
- Abstracts
- Characterization of released exosomes from satellite glial cells under normal and inflammatory conditions
- Abstracts
- Cell-based platform for studying trigeminal satellite glial cells under normal and inflammatory conditions
- Abstracts
- Tramadol in postoperative pain – 1 mg/ml IV gave no pain reduction but more side effects in third molar surgery
- Abstracts
- Tempo-spatial discrimination to non-noxious stimuli is better than for noxious stimuli
- Abstracts
- The encoding of the thermal grill illusion in the human spinal cord
- Abstracts
- Effect of cocoa on endorphin levels and craniofacial muscle sensitivity in healthy individuals
- Abstracts
- The impact of naloxegol treatment on gastrointestinal transit and colonic volume
- Abstracts
- Preoperative downregulation of long-noncoding RNA Meg3 in serum of patients with chronic postoperative pain after total knee replacement
- Abstracts
- Painful diabetic polyneuropathy and quality of life in Danish type 2 diabetic patients
- Abstracts
- “What about me?”: A qualitative explorative study on perspectives of spouses living with complex chronic pain patients
- Abstracts
- Increased postural stiffness in patients with knee osteoarthritis who are highly sensitized
- Abstracts
- Efficacy of dry needling on latent myofascial trigger points in male subjects with neck/shoulders musculoskeletal pain. A case series
- Abstracts
- Identification of pre-operative of risk factors associated with persistent post-operative pain by self-reporting tools in lower limb amputee patients – A feasibility study
- Abstracts
- Renal function estimations and dose recommendations for Gabapentin, Ibuprofen and Morphine in acute hip fracture patients
- Abstracts
- Evaluating the ability of non-rectangular electrical pulse forms to preferentially activate nociceptive fibers by comparing perception thresholds
- Abstracts
- Detection of systemic inflammation in severely impaired chronic pain patients, and effects of a CBT-ACT-based multi-modal pain rehabilitation program
- Abstracts
- Fixed or adapted conditioning intensity for repeated conditioned pain modulation
- Abstracts
- Combined treatment (Norspan, Gabapentin and Oxynorm) was found superior in pain management after total knee arthroplasty
- Abstracts
- Effects of conditioned pain modulation on the withdrawal pattern to nociceptive stimulation in humans – Preliminary results
- Abstracts
- Application of miR-223 onto the dorsal nerve roots in rats induces hypoexcitability in the pain pathways
- Abstracts
- Acute muscle pain alters corticomotor output of the affected muscle stronger than a synergistic, ipsilateral muscle
- Abstracts
- The subjective sensation induced by various thermal pulse stimulation in healthy volunteers
- Abstracts
- Assessing Offset Analgesia through electrical stimulations in healthy volunteers
- Abstracts
- Metastatic lung cancer in patient with non-malignant neck pain: A case report
- Abstracts
- The size of pain referral patterns from a tonic painful mechanical stimulus is increased in women
- Abstracts
- Oxycodone and macrogol 3350 treatment reduces anal sphincter relaxation compared to combined oxycodone and naloxone tablets
- Abstracts
- The effect of UVB-induced skin inflammation on histaminergic and non-histaminergic evoked itch and pain
- Abstracts
- Topical allyl-isothiocyanate (mustard oil) as a TRPA1-dependent human surrogate model of pain, hyperalgesia, and neurogenic inflammation – A dose response study
- Abstracts
- Dissatisfaction and persistent post-operative pain following total knee replacement – A 5 year follow-up of all patients from a whole region
- Abstracts
- Paradoxical differences in pain ratings of the same stimulus intensity
- Abstracts
- Pain assessment and post-operative pain management in orthopedic patients
- Abstracts
- Combined electric and pressure cuff pain stimuli for assessing conditioning pain modulation (CPM)
- Abstracts
- The effect of facilitated temporal summation of pain, widespread pressure hyperalgesia and pain intensity in patients with knee osteoarthritis on the responds to Non-Steroidal Anti-Inflammatory Drugs – A preliminary analysis
- Abstracts
- How to obtain the biopsychosocial record in multidisciplinary pain clinic? An action research study
- Abstracts
- Experimental neck muscle pain increase pressure pain threshold over cervical facet joints
- Abstracts
- Are we using Placebo effects in specialized Palliative Care?
- Abstracts
- Prevalence and pattern of helmet-induced headache among Danish military personnel
- Abstracts
- Aquaporin 4 expression on trigeminal satellite glial cells under normal and inflammatory conditions
- Abstracts
- Preoperative synovitis in knee osteoarthritis is predictive for pain 1 year after total knee arthroplasty
- Abstracts
- Biomarkers alterations in trapezius muscle after an acute tissue trauma: A human microdialysis study
- Abstracts
- PainData: A clinical pain registry in Denmark
- Abstracts
- A novel method for investigating the importance of visual feedback on somatosensation and bodily-self perception
- Abstracts
- Drugs that can cause respiratory depression with concomitant use of opioids
- Abstracts
- The potential use of a serious game to help patients learn about post-operative pain management – An evaluation study
- Abstracts
- Modelling activity-dependent changes of velocity in C-fibers
- Abstracts
- Choice of rat strain in pre-clinical pain-research – Does it make a difference for translation from animal model to human condition?
- Abstracts
- Omics as a potential tool to identify biomarkers and to clarify the mechanism of chronic pain development
- Abstracts
- Evaluation of the benefits from the introduction meeting for patients with chronic non-malignant pain and their relatives in interdisciplinary pain center
- Observational study
- The changing face of acute pain services
- Observational study
- Chronic pain in multiple sclerosis: A10-year longitudinal study
- Clinical pain research
- Functional disability and depression symptoms in a paediatric persistent pain sample
- Observational study
- Pain provocation following sagittal plane repeated movements in people with chronic low back pain: Associations with pain sensitivity and psychological profiles
- Observational study
- A longitudinal exploration of pain tolerance and participation in contact sports
- Original experimental
- Taking a break in response to pain. An experimental investigation of the effects of interruptions by pain on subsequent activity resumption
- Clinical pain research
- Sex moderates the effects of positive and negative affect on clinical pain in patients with knee osteoarthritis
- Original experimental
- The effects of a brief educational intervention on medical students’ knowledge, attitudes and beliefs towards low back pain
- Observational study
- The association between pain characteristics, pain catastrophizing and health care use – Baseline results from the SWEPAIN cohort
- Topical review
- Couples coping with chronic pain: How do intercouple interactions relate to pain coping?
- Narrative review
- The wit and wisdom of Wilbert (Bill) Fordyce (1923 - 2009)
- Letter to the Editor
- Unjustified extrapolation
- Letter to the Editor
- Response to: “Letter to the Editor entitled: Unjustified extrapolation” [by authors: Supp G., Rosedale R., Werneke M.]

