Taking a break in response to pain. An experimental investigation of the effects of interruptions by pain on subsequent activity resumption
-
Rena Gatzounis
, Martien G.S. Schrooten
Abstract
Background and aims
Interrupting ongoing activities with the intention to resume them again later is a natural response to pain. However, such interruptions might have negative consequences for the subsequent resumption and performance of the interrupted activity. Activity interruptions by pain may be more impairing than interruptions by non-painful stimuli, and also be subjectively experienced as such. These effects might be more pronounced in people high in pain catastrophizing. These hypotheses were investigated in two experiments.
Methods
In Experiment 1, healthy volunteers (n = 24) performed an ongoing task requiring a sequence of joystick movements. Occasionally, they received either a painful electrocutaneous or a non-painful vibrotactile stimulus, followed by suspension of the ongoing task and temporary engagement in a different task (interruption task). After performing the interruption task for 30 s, participants resumed the ongoing task. As the ongoing task of Experiment 1 was rather simple, Experiment 2 (n = 30) included a modified, somewhat more complex version of the task, in order to examine the effects of activity interruptions by pain.
Results
Participants made more errors and were slower to initiate movements (Experiment 1 & 2) and to complete movements (Experiment 2) when they resumed the ongoing task after an interruption, indicating that interruptions impaired subsequent performance. However, these impairments were not larger when the interruption was prompted by painful than by non-painful stimulation. Pain catastrophizing did not influence the results.
Conclusions
Results indicate that activity interruptions by pain have negative consequences for the performance of an activity upon its resumption, but not more so than interruptions by non-painful stimuli. Potential explanations and avenues for future research are discussed.
Implications
Interrupting ongoing activities is a common response to pain. In two experiments using a novel paradigm we showed that activity interruptions by pain impair subsequent activity resumption and performance. However, this effect seems to not be specific to pain.
1 Introduction
Pain is a signal of bodily threat that motivates action and urges us to interrupt ongoing activities in order to control the pain [1, 2]. Indeed, when feeling pain we often take a break from what we are doing, whilst planning to resume our activity later [3]. Despite the fact that such activity interruptions by pain are common, their effects on subsequent activity resumption remain unclear.
Although there is substantial research showing that task performance is impaired during pain [4, 5, 6, 7, 8], the research on whether task performance is impaired when pain forces the suspension of the activity for longer time is sparse [3]. People with pain complaints report continuing work outside working hours when their work-related goals were interrupted because of pain [9], indicating that various compensatory strategies may be used to counter the effects of interruptions by pain. Further, evidence suggests that healthy people scoring high in pain catastrophizing spend less time on a task when they are required to take breaks because of pain, compared to when they continue uninterrupted [10]. Systematic research regarding how activity interruptions by pain influence performance after the interruption, however, is missing.
Studies from the field of human factors and ergonomics have shown that interruptions caused by demands other than pain often impair performance of the interrupted task [11, 12], for instance by increasing completion time and error rate [13, 14]. The general premise is that, in order to resume a task successfully, one needs to encode task-related information in (prospective) memory when the interruption occurs and to further retain this information during the interruption [15, 16]. Just as with interruptions by non-painful external stimuli (e.g., [11, 12], interruptions by pain are expected to impair subsequent task resumption [3]. Further, given the biological relevance and urgency of pain, we expect that painful interruption cues interfere to a larger degree with the encoding of task-state information and are thus more disruptive than nonpainful interruption cues. Moreover, an enhanced threat value of pain enhances its attentional capture [2, 6] and might further impair the encoding at interruption and thus the subsequent resumption of the interrupted activity [3, 10].
The present manuscript describes two experiments aiming at shedding light on the effects of interruptions by pain on activity resumption. In both experiments, healthy volunteers were interrupted while performing an ongoing task. Participants were interrupted by either painful (electrocutaneous) stimulation or non-painful (vibrotactile) stimulation (within-subjects), followed by temporary engagement in a different task. We hypothesized that receiving painful stimuli as interruption cues would impair task performance after task resumption, and that this impairment would be greater than the impairment caused by non-painful stimuli. We expected to see negative effects of interruptions by pain in task performance, and in subjective ratings of resumption difficulty and resumption motivation. Differences were expected to be more pronounced when pain was perceived as threatening, which is the case in people high in pain catastrophizing. Task difficulty might be a factor determining interruption effects. Therefore, in Experiment 2 we used a more complex ongoing task than in Experiment 1.

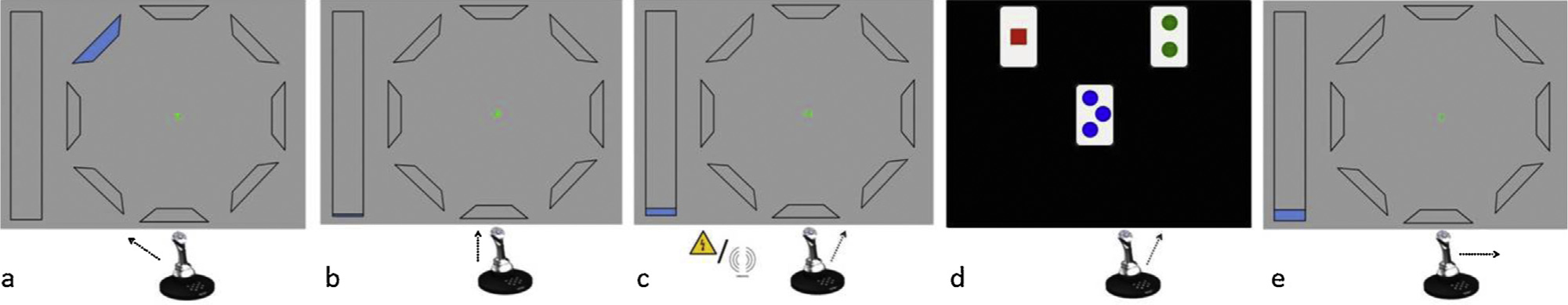
Schematic representation of the On going task and Interruption task trials (Experiment 1).
Four targets are presented on the screen. Participants are required to move a joystick towards the cued target in the first trial (panel a), and then continue making movements towards the targets in a way that follows a clockwise fashion (panel b). After each trial, avertical bar that is presented on the left side ofthe screen and which indicates the total length of the ongoing task gets coloured in such a speed, that it is only completely coloured at the end of the task. An interruption cue, i.e. a painful electrocutaneous stimulus or a non-painful vibrotactile stimulus, is delivered on the wrist of the participant’s dominant hand during randomly preselected trials (panel c). The interruption cue is followed by the suspension of the ongoing task and the initiation of the interruption task (panel d). On the first intertrial interval upon completion of 30s on the interruption task, the screen configuration of the ongoing task is presented again (panel e). Participants are then required to resume the ongoing task with the next movement.
2 Experiment 1
2.1 Methods
2.1.1 Participants
Twenty-four healthy volunteers participated in the study. Exclusion criteria were: pregnancy; history of psychiatric or neurological diagnosis; presence of (acute or chronic) pain, cardiovascular disease, or other serious medical conditions; use of electronic implants (e.g., cardiac pacemaker); use of anxiolytic and/or antidepressive medication; imperfect command of the Dutch language; and impaired (uncorrected) eyesight. Exclusion criteria were checked by means of self-report at the beginning of each experimental session. Participants were students from Maastricht University, who participated on an informed consent basis in return for monetary compensation (€20). The study protocol was approved by the Ethical Review Committee Psychology and Neuroscience (ERCPN) of Maastricht University (study number: ECP-127 11_04_2013).
2.1.2 Experimental task
Participants performed an ongoing joystick task (cf. [17]) during which they occasionally experienced interruptions, i.e. time intervals during which the task was suspended. Interruptions were prompted either by a painful or by a non-painful interruption cue (see below Interruption cues). During the interruptions, participants performed a different task (interruption task), which aimed at engaging them in a similar way during ongoing task suspension. After fixed time on the interruption task, participants resumed the ongoing task at the point where they had been interrupted. A detailed description follows (see also Fig. 1):
Ongoing task. Throughout the ongoing task, one blue circle was presented at each of four target locations (top, bottom, left, and right) on a grey computer screen background. Each target location corresponded to each of four possible joystick movements (to the screen, to the participant, to the left and to the right, respectively). The start of each trial was cued by a white cross appearing between the target locations. Participants were required to move the joystick with their dominant hand to one of the locations as fast and as accurately as possible. During the first trial, a red frame appeared around one circle and cued the correct direction of the first movement (Fig. 1a). In subsequent trials, participants were required to make movements in a clockwise fashion (Fig. 1b), but no locations were cued. After every completed movement, the cross disappeared, indicating movement registration. During the inter-trial interval (ITI; 2500 ms), participants returned the joystick to its centre point. A vertical bar that was presented on the left side of the screen got gradually filled with blue colour after every completed movement. Participants were informed that the task would be completed when the bar was completely coloured.
There were 288 ongoing task trials, 12 of which contained an interruption cue (“interruption trials”; randomly preselected, same for all participants) (Fig. 1c). The interruption cue started was administered during the joystick movement. When the movement had been registered and the interruption cue completed, the ongoing task was suspended and the interruption task started.
Interruption task. In a simplified version of the Wisconsin card sorting task [18], participants were required to categorize cards according to one known rule. During each trial, one card was presented in the centre of the screen, with two more cards either on its left and right (horizontal task version; 50% of interruptions; cf. Fig. 1d), or above and below it (vertical task version; 50% of interruptions). Screen background was black. Each card depicted one to four identical geometrical shapes, in one of four colours (e.g. four red circles). There were five kinds of shapes. Participants were requested to move the joystick as fast as possible towards the card that depicted the same shape as the one depicted on the middle card. The cards stayed on the screen until the participant responded, or for 4500 ms. During the ITI (1500 ms), participants returned the joystick to its centre point. On the first ITI after 30 s on the interruption task, the interruption task stopped and the next trial of the ongoing task (“resumption trial”) immediately started. During the resumption trial (Fig. 1e) the participant was required to perform the movement that should normally follow the movement performed during the interruption trial. No location was cued and no instructions were repeated on the resumption trials.
2.1.3 Interruption cues
Painful electrocutaneous stimuli. Electrocutaneous stimuli (square-wave; 700 ms duration; 10 Hz frequency) served as interruption cues in the Pain condition. These stimuli were generated by a DS5 constant current stimulator (Digitimer Limited, Hertfordshire, UK) and delivered through two 8 mm Ag/AgCl surface electrodes (Bilaney, Düsseldorf, Germany) placed on the dorsal side of the wrist of the participant’s dominant hand with an inter-electrode distance of ∼1 cm. Before applying the electrodes, the experimenter scrubbed the participant’s skin with a commercial scrub cream in order to reduce skin resistance, and filled the electrodes with electroconductive gel (K-Y gel, Johnson & Johnson).
After applying the electrodes, the stimulus intensity was individually determined. The experimenter administered a series of electrocutaneous stimuli, starting with an intensity of 0.2 mA and increasing in steps of 0.4 mA, until the participant did not wish to be administered stimuli of higher intensity, or until they had rated the last stimulus as a 9 on an 11-point “effort to tolerate” scale (0 =“no effort at all”; 10 =“maximum effort I can exert”). In order to better understand participants’ experience, they were also asked to rate the stimulus painfulness and unpleasantness (0 =“not at all painful/unpleasant”; 10 =“the most painful/unpleasant that I can imagine”) several times throughout the stimulus series, including upon the last stimulus. Mean intensity used in the experiment was 4.5 mA (SD 2.4, range 1.2–9.8).
Non-painful vibrotactile stimuli. Vibrotactile stimuli (700 ms duration) served as interruption cues in the Non-pain condition. These stimuli were generated and delivered by a custom-made device, made from a commercially available CE-certified eccentric motor that was electrically powered and controlled via software by means of a TTL signal. The motor was enclosed into a plastic case, which was inserted into an arm wallet typically used by joggers. The arm wallet was fastened around the wrist of the participant’s dominant hand in such a way that the motor was on its dorsal side. To keep the weight on the wrist similar across conditions, the arm wallet was also fastened on top of the electrodes in the Pain condition.
After securing the arm wallet, participants in the Non-pain condition were familiarized with the vibrotactile stimuli. The experimenter administered two stimuli of the same intensity. Each time, the participant was asked to rate the stimulus on the “effort to tolerate”, “painfulness” and “unpleasantness” scales (as above, but with adjusted wording to refer to the vibrotactile stimulus).
2.1.4 Measures
2.1.4.1 Behavioural measures
Our main outcome variables were the following indices of ongoing task performance: (1) accuracy of movement direction, i.e. percentage of trials in which the participant moved the joystick in the correct direction; (2) response latency, i.e. time from trial onset to movement onset; and (3) response duration, i.e. time from movement onset to movement offset. For each trial, correct movement direction was determined on the basis of movement direction in the previous trial given a clockwise order. Therefore, one error did not trigger a cascade of wrong responses, as long as the participant kept making movements in a clockwise fashion. For resumption trials, the previous trial was the one during which the interruption cue was delivered.
2.1.4.2 Self-report measures
Pain catastrophizing was measured by means of the Pain Catastrophizing Scale (PCS; [19, 20]), to assess whether it moderates the effects of activity interruptions by pain on subsequent resumption and performance. The PCS consists of thirteen items that measure three dimensions of pain catastrophizing, namely helplessness, magnification, and rumination. Each item is scored on a 0 (“not at all”) to 4 (“all the time”) scale. The Dutch version of the PCS has shown very good psychometric qualities previously [19], and a very high reliability in the present study (α = .93, n = 23).
Motivation to perform the tasks, and perceived difficulty to resume the ongoing task after an interruption were rated on 11-point numerical scales ranging from 0 (“not at all”) to 10 (“to a very high degree”). These ratings served as outcome variables.
Further, interruption cue characteristics (painfulness, unpleasantness, and threat value) were also rated on 11-point numerical scales ranging from 0 (“not at all”) to 10 (“to a very high degree”). These ratings allowed us to investigate whether our manipulation was successful.
2.1.5 Equipment
The computer task was programmed in Affect, version 4.0 [21]. Participants viewed the task on a standard computer screen set at a resolution of 1024×768 pixels, and performed it by means of an Attack™ 3 Joystick (Logitech International S. A., Lausanne, Switzerland). Limesurvey [22] was used for the online questionnaire battery.
2.1.6 Procedure
The study consisted of two lab sessions, planned seven days (±1 day) apart. Two days after the second lab session, participants completed online a battery of questionnaires that included the PCS and other questionnaires administered for exploratory reasons (not further discussed). Participants were randomly assigned to one of the two orders (either starting with the Pain condition followed by the Non-pain condition, or vice versa). During the lab sessions, participants were tested individually in a dimly lit room, as follows (unless mentioned otherwise, descriptions apply for both sessions):
Introduction
Upon arrival to the lab, participants read general information about the study (first session only) and about the session of that day, and signed the informed consent (first session only) and exclusion criteria form (both sessions).
Interruption cue calibration/familiarization
The experimenter attached the electrodes and/or vibrotactile stimuli device, and participants underwent a procedure either for the calibration of the painful stimulus intensity or for the familiarization with the vibrotactile stimuli (see Interruption cues).
Experimental task
Participants received oral and written instructions about the tasks. After reading on-screen instructions, participants practiced 12 ongoing task trials. Subsequently, they read on-screen instructions about the interruption task, and practiced 8 trials. After the instruction and practice phase, participants performed 288 trials of the ongoing task with interspersed 12 interruptions.
Self-report measures
After completing the experimental task, participants provided the task and interruption cue ratings, and the experimenter removed the electrodes and/or vibrotactile stimuli device. Participants received monetary compensation at the end of the second session, and were debriefed about the real purpose of the study when the whole sample had been tested.
2.1.7 Statistical analyses
Descriptive statistics were computed for the sample characteristics, questionnaire scores, and debriefing questions. The interruption cue ratings given at the end of each session were compared by means of paired samples t-tests.
Results focus on outcome variables that are directly relevant to the present hypotheses regarding impaired resumption of the ongoing task. This is considered to be reflected in (1) fewer correct movements, (2) longer response latency and (3) longer response duration in resumption trials (i.e., ongoing task trials immediately after an interruption) than in baseline trials (i.e. ongoing task trials with no task resumption or interruption cue). Responses of movements in the incorrect direction, responses faster than 100 ms (i.e., reflecting anticipation of the cross), and exceptionally fast and slow responses (2.5SDs above or below the individual mean within each condition and trial type) were excluded from analyses on response latency and duration. Accuracy of movement direction, response latency and response duration were subjected to separate Repeated Measures Analyses of Variance (RM ANOVAs) with interruption cue type (2: pain vs. non-pain) and trial type (2: baseline trial vs. resumption trial) as within-subjects factors, followed by simple contrasts. Trials containing an interruption cue were excluded from analyses for the sake of clarity. A separate set of analyses including all trials, however, did not yield essentially different results. Further, motivation ratings were subjected to a RM ANOVA with interruption cue type (2: pain vs. non-pain) and task type (2: ongoing task vs. interruption task) as within-subjects factors, with post hoc simple contrast analysis. Ratings of difficulty to resume the ongoing task were subjected to a RM ANOVA with interruption cue type (2: pain vs. non-pain) as within-subjects factor. In order to explore whether pain catastrophizing influences the effects of interruptions by pain, we centred the total PCS score [23] and included it in the above analyses as a covariate. When the ANCOVAs yielded significant PCS effects by means of correla-tional analyses. In the interest of brevity, we report the results of the (RM) ANCOVAs only when these yielded essentially different results.
Because sphericity was violated in some of the analyses of variance, we report Pillai’s trace multivariate results [24, 25]. Reported effect sizes are ηp2 and ηG2 [26, 27]. Where appropriate, we report corrected degrees of freedom and mean differences with their 95% confidence intervals (CIs). Missing values (one participant did not fill in the online questionnaires) were excluded listwise. Analyses were performed with SPSS version 22.0 [28].
2.2 Results
2.2.1 Sample characteristics
Table 1 presents the sample’s demographic characteristics and PCS score.
Demographic characteristics and Pain Catastrophizing Scale score (means, 95% Confidence Intervals in brackets, SDs and range in parentheses).
| Experiment 1 | Experiment 2 | |
|---|---|---|
| n = 24 | n = 30 | |
| Females/Males | 22/2 | 24/6 |
| Right-handed/Left-handed | 23/1 | 27/3 |
| Age | 21.2 [20.4, 22.0] | 23.2 [21.8, 24.5] |
| (SD 1.9, 18–26) | (SD 3.5, 18–34) | |
| Pain Catastrophizing Scale | 11.9 [8.3, 15.5] | 14.5 [11.0, 18.0] |
| (SD 8.4, 2–31) | (SD 9.3, 1–30) |
2.2.2 Manipulation check
Interruption cue characteristics Participants rated the vibrotactile stimulus as significantly less painful,t(23)=18.9,p <.001 (mean difference 6.9 95% CI [6.2, 7.7]), less unpleasant,t(23)=20.4,p <.001 (mean difference 7.3 95% CI [6.6, 8.1]) and less threatening, t(23)=11.8,p <.001 (mean difference 5.6, 95% CI [4.6, 6.6]) than the painful stimulus (see Table 2), thus indicating that it was an appropriate control stimulus.
Retrospective ratings of the interruption cue (means, 95% Confidence Intervals in brackets, SDs and range in parentheses).
| Pain | Non-pain | |
|---|---|---|
| Experiment 1 (n = 24) | ||
| Painfulness | 6.9 [6.2, 7.7] (1.8, 2–10) | 0 (constant) |
| Unpleasantness | 7.9 [7.1, 8.6] (1.8, 1–10) | 0.5 [0.3, 0.8] (0.7, 0–2) |
| Threat value | 5.9 [4.9, 6.9] (2.4, 0–10) | 0.3 [0,0.5] (0.7, 0–3) |
| Experiment 2 (n = 30) | ||
| Painfulness | 7 [6.5, 7.5] (1.3, 4–9) | 0.2 [0.1, 0.4] (0.5, 0–2) |
| Unpleasantness | 7.6 [7.0, 8.2] (1.7, 3–10) | 0.6 [0.4, 0.9] (0.7, 0–2) |
| Threat value | 5.1 [4.1, 6.1] (2.6, 0–10) | 0.5 [0.01, 0.8] (1, 0–4) |
-
Note. Interruption cue Painfulness/Unpleasantness/Threat value rated on 11-point numerical scales (0 = not at all; 10 = to a very high degree).
2.2.3 Interruption effects on ongoing task performance
The behavioural data can be seen in Table 3. Accuracy of movement direction was lower in resumption trials than in baseline trials, F(1, 23) = 13.60, p = .001, ηp2 = .372, ηc2 = .273, by approximately 18.72% (95% CI [−29.22,−8.22]). There was no effect of interruption cue type (main effect: F(1, 23) = 0.36, p = .553, ηp2 = .016, ηG2 = .002; interruption cue type × trial type: F(1, 23) = 0.64, p = .430, ηp2 = .027, ηG2 = .003).
Accuracy (percentage correct), response latency (milliseconds) and response duration (milliseconds) (means, 95% Confidence Intervals in brackets, SDs and range in parentheses) per condition and trial type.
| Pain | Non-pain | |||
|---|---|---|---|---|
|
|
|
|||
| Baseline trials | Resumption trials | Baseline trials | Resumption trials | |
| Experiment 1 (n = 24) | ||||
| Accuracy | 99.3 [99.0, 99.5] (0.7, 97.7–100) | 78.9 [67.2, 90.7] (27.9, 8.3–100) | 98.9 [98.3, 99.4] (1.3, 96.2–100) | 81.8 [70.9, 92.7] (25.9, 8.3–100) |
| Response Latency | 516.4 [493.9, 538.8] (53.2, 385.1–663.6) | 1150.1 [1001.6, 1298.7] (351.8, 759.2–2596.6) | 529.8 [501.1, 558.6] (68.0, 349.4–701.8) | 1064.1 [954.5, 1173.7] (259.6, 206.0–1537.5) |
| Response Duration | 326.8 [317.5, 336.0] (22.0, 281.3–366.1) | 343.1 [320.9, 365.4] (52.6, 271.0–543.7) | 339.3 [326.0, 352.5] (31.4, 286.6–433.5) | 325.7 [312.2, 339.2] (31.9, 250.0–388.0) |
| Experiment 2 (n = 30) | ||||
| Accuracy | 99.8 [99.6, 99.9] (0.4, 98.2–100) | 60.4 [46.5, 74.4] (37.4, 0–100) | 99.7 [99.5, 99.9] (0.6, 97.3–100) | 61.5 [50.1, 72.8] (30.5, 0–100) |
| Response Latency | 425.8 [407.2, 444.4] (49.8, 350.9–551.3) | 1184.6 [1009.3, 1359.9] (443.2, 665.0–2538.0) (n = 27) | 422.6 [407.1, 438.1] (41.4, 347.7–507.0) | 1120.8 [1009.0, 1232.6] (293.9, 600.0–1894.0) (n = 29) |
| Response Duration | 274.2 [223.5, 324.9] (135.8, 137.0–817.7) | 358.2 [296.4, 420.1] (146.5, 186.7–802.0) (n = 24) | 275.8 [221.4, 330.2] (145.7, 130.2–737.4) | 394.7 [317.9, 471.5] (198.0, 135.2–860.5) (n = 28) |
-
Note. In experiment 2, the n of resumption trials equals the number of participants who had at least one accurate resumption.
Further, response latency was significantly higher in resumption trials, as compared to baseline trials, F(1, 23) = 174.28, p <.001, ηp2 = .883, ηG2 = .692. On average, participants took 584 ms (95% CI [492.5, 675.5]) more to initiate a movement in the resumption trials. There was no effect of interruption cue type (main effect: F(1, 23) = 0.59, p = .449, ηp2 = .025, ηG2 = .009; interruption cue type × trial type: F(1, 23) = 1.32, p = .262, ηp2 = .054, ηG2 = .016).
The analysis on response duration yielded a statistically significant interruption cue type × trial type interaction, F(1, 23) = 5.76, p = .025, ηp2 = .200, ηG2 = .071. Participants completed the resumption trials somewhat faster than the baseline trials in the Non-pain condition, p = .042 (mean difference−13.6 ms, 95% CI [−26.6,−0.5]), but with similar speed in the Pain condition, p = .085. The analysis yielded no main effects of trial type, F(1, 23) = 0.09, p = .768, ηp2 = .004, ηG2 = .001, or interruption cue type, F(1, 23) = 0.18, p = .680, ηp2 = .008, ηG2 = .002 (see Table 3).
2.2.4 Interruption effects on self-reported motivation to perform and difficulty to resume
Ratings are shown in Table 4. Participants reported being more motivated to perform the interruption task, as compared to the ongoing task, in the Pain condition, p = .002 (mean difference 1.96, 95% CI [0.83, 3.09]), but not in the Non-pain condition, p = .366 (mean difference 0.25, 95% CI [−0.31, 0.81]) (interruption cue type × task type: F(1, 23) = 8.81, p = .007, ηp2 = .277, ηG2 = .065; main effect of interruption cue type,F(1, 23) = 0.14, p = .708, ηp2 = .006, ηG2 = .002; main effect of task type: F(1, 23) = 11.81, p = .002, ηp2 = .339, ηG2 = .104). Further, participants reported a similar degree of difficulty to resume the target task in the Pain condition and in the Non-pain condition,F(1, 22) = 2.41, p = .135, ηp2 = .099, ηG2 = .047.
Ratings of the motivation to perform the ongoing task and the interruption task, and of the perceived difficulty to resume the ongoing task after an interruption (means, 95% Confidence Intervals in brackets, SDs and range in parentheses).
| Pain | Non-pain | |
|---|---|---|
| Experiment 1 (n = 24) | ||
| Motivation to perform | 2.5 [1.7, 3.2] | 3.5 [2.5, 4.4] |
| ongoing task again | (1.8, 0–5) | (2.3, 0–8) |
| Motivation to perform | 4.4 [3.4, 5.5] | 3.7 [2.8, 4.7] |
| interruption task again | (2.5, 0–10) | (2.3, 0–8) |
| Difficulty to resume ongoing | 3.6[a] [2.3, 4.9] | 2.9 [2.1,3.7] |
| task | (3.0, 0–8) | (2.0, 0–7) |
| Experiment 2(n = 30) | ||
| Motivation to perform | 7.3 [6.7, 8.1] | 7.1 [6.3, 7.9] |
| ongoing task | (2.3, 2–10) | (2.1, 3–10) |
| Motivation to perform | 7.7 [7.0, 8.4] | 7.7 [7.0, 8.3] |
| interruption task | (1.9, 3–10) | (1.7, 3–10) |
| Difficulty to resume ongoing | 4.9 [3.8, 6.1] | 4.1 [3.0, 5.1] |
| task | (3.0, 0–10) | (2.9, 0–9) |
-
Note. In experiment 1, participants were asked to rate the degree to which they “would like to perform the (target/interruption) task again”, whereas in experiment 2, they were asked to rate the degree to which they “were motivated to perform the (target/interruption) task” on an 11-point numerical scale (0 = not at all; 10 = to a very high degree).
2.2.5 Influence of pain catastrophizing
Pain catastrophizing was included in the above analyses as a continuous variable, but was not found to essentially change the results.
2.3 Discussion
Participants made more errors and took longer to initiate responses when resuming the ongoing task after an interruption, as compared to other trials. These results are in line with task interruption research from outside the field of pain showing that, in most cases, task performance is impaired upon task resumption [11, 12]. The expected difference in response duration was not found. Further, contrary to our expectations, the costs in task performance were similar irrespective of whether participants received the painful or the non-painful interruption cue. When interrupted by pain, however, participants reported lower motivation to perform the ongoing task compared to the interruption task. It is possible that the expected difference in interruption effect between the two interruption cue conditions did not arise due to a ceiling effect in target task performance, especially regarding accuracy (cf.Table 3). To investigate this idea, we adapted experiment 1 using a more complex target task containing more targets.
Schematic representation of the Ongoing task and Interruption task trials (Experiment 2). Eight targets, arranged in a circle, are presented. Participants are required to move a joystick towards the cued location in the first trial (panel a), and continue making movements in a way that follows a clockwise fashion (panel b). The vertical bar on the left side of the screen gets filled with colour with every registered trial in such a way, that it is completely filled only at the end of the ongoing task, thus indicating to the participant their progress in the task. During randomly preselected trials, a painful electrocutaneous or a non-painful vibrotactile stimulus is delivered to the wrist of the participant’s dominant hand (panel c). Subsequently, the target task is suspended and the interruption task is initiated (panel d). On the first intertrial interval after 30 s on the interruption task, the interruption task is suspended and the target task is immediately initiated(panel e), requiring the participant to make a movement towards the next location.
3 Experiment 2
3.1 Methods
3.1.1 Participants
Thirty-five healthy volunteers participated. Exclusion criteria were the same as in experiment 1, plus the additional criterion of having received medical advice to avoid stressful situations. Five participants were excluded from the analyses due to a technical problem resulting in unreliable administration of the electrocutaneous stimulation. Participants were students of the University of Leuven, who participated on the basis of informed consent, in exchange for monetary compensation (€18). The study protocol was approved by the Social and Societal Ethics Committee and the Medical Ethics Committee of the University of Leuven (study number: ML 10825).
3.1.2 Experimental task
The experimental task of experiment 1 was modified as follows (see also Fig. 2):
Ongoing task. Participants were required to make eight possible joystick movements, each of which corresponded to one target location on the screen. The target locations were indicated by eight rounded trapeziums presented in a circle. In the centre of this circle, a cross was shown to indicate the start and end of a trial and ITI, as follows: When the cross turned green, participants were required to initiate a joystick movement to one of the target locations as fast and as accurately as possible. When the cross turned white (upon movement completion), participants were required to bring the joystick back to its centre point, visually indicated by the cross, and keep it there. Then the cross turned red, indicating that the ITI (duration: variable between 1000 and 3000 ms) began. Participants were not allowed to move the joystick cursor outside the area of the cross before the end of the ITI, and received feedback when they did so. The ongoing task consisted of 256 trials, 16 of which had been randomly preselected to contain an interruption cue (Fig. 2c).
Interruption task. The interruption task was the same as in experiment 1 except for card location and joystick movement. One card was always presented at the centre point and the other two cards randomly on two out of eight possible locations (i.e. the target locations of the ongoing task) (Fig. 2d). At the start of each trial, the three cards were presented, indicating to the participant that they had to choose the correct card as fast as possible. When the response was registered, the cards disappeared and the white cross appeared at the centre point. Participants had to bring the joystick cursor to the cross and keep it there during the ITI (duration: 3000 ms), which was signalled by the cross turning red and terminated by the presentation of the three card stimuli in the following trial.
3.1.3 Interruption cues
Electrocutaneous stimuli (mean intensity 4.4 mA, SD 1.9, range 1.0–9.8), vibrotactile stimuli, and the calibration and familiarization procedure were the same as in Experiment 1.
3.1.4 Measures
The same behavioural outcome variables as in Experiment 1 were used. Self-report measures were also the same as in Experiment 1, apart from a different phrasing of the task motivation questions (see Table 4).
3.1.5 Equipment
Similar equipment as in Experiment 1 was used, apart from the addition of a pair of standard computer speakers (Logitech International S. A., Lausanne, Switzerland) for the error feedback, and the use of a Hawk®force-feedback joystick with a hydraulic system (Paccus Interfaces, Almere, The Netherlands). This joystick allows for high user control and for movements also outside the two-axes area (i.e. front-back and left-right) covered by conventional joysticks.
3.1.6 Procedure
The procedure was similar to that of Experiment 1, except for the fact that the two within-subjects conditions were performed within one lab session, as follows: Upon arrival at the lab, participants read general information about the study, signed informed consent, and reported whether they fulfilled exclusion criteria. Subsequently, they received information about the condition they would perform first, underwent either the calibration (Pain condition) or the familiarization (Non-pain condition) procedure, and received instructions for and performed the experimental task. After task completion, participants provided task and interruption cue ratings, and the experimenter removed the electrodes and/or vibrotactile stimuli device. Subsequently, the same procedure was followed for the other condition. Two days after the lab session, participants filled in the online questionnaires (cf. Experiment 1). Participants were debriefed when the whole sample had been tested.
3.1.7 Statistical analyses
Expectations and statistical procedures were the same as in Experiment 1.
3.2 Results
3.2.1 Sample characteristics
The sample characteristics are presented in Table 1.
3.2.2 Manipulation check: Interruption cue characteristics, and difficulty to resume the ongoing task in relation to Experiment 1
Participants rated the electrocutaneous stimulus as significantly more painful,t(29) = 28.12, p <.001 (mean difference 6.7, 95% CI [6.2, 7.2]), unpleasant,t(29) = 19.88, p <.001 (mean difference 6.9, 95% CI [6.2, 7.6]), and threatening,t(29) = 8.95, p <.001 (mean difference 4.6, 95% CI [3.6, 5.7]) than the vibrotactile stimulus (see Table 2), thus providing additional evidence that the vibrotactile stimulus was a good control to pain. Further, participants rated the difficulty of resuming the ongoing task higher than in Experiment 1, by approximately 1.28 points (95% CI [-0.01, 2.65]) on the 0–10 scale (see Table 4). This difference was of marginal statistical significance, F(1, 51) = 3.47, p = .068, ηp2 = .064, as shown by a RM ANOVA with task type (2: ongoing task vs. interruption task) as the within-subjects factor and experiment (2: Experiment 1 vs. Experiment 2) as the between-subjects factor.
3.2.3 Interruption effects on ongoing task performance
The behavioural data can be seen in Table 3.
Accuracy of movement direction was lower in resumption trials as compared to baseline trials, F(1, 29) = 46.44, p <.001, ηp2 = .616, ηG2 = .494, by 38.81% on average (95% Cl [−50.46,−27.16]). There was no effect of interruption cue type (main effect:F(1, 29) = 0.04, p = .846, ηp2 = .001, pG2 = .0001; interruption cue type × trial type:F(1, 29) = 0.05, p = .828, pp 2 = .002, ηG2 = .0002).
Similarly, response latency was higher in resumption trials as compared to baseline trials, F(1, 26) = 158.58, p <.001, ηp2 = .859, ηG2 = .732, by 720.66 ms on average (95% Cl [603.02,838.29]).There was no effect of interruption cue type (main effect:F(1, 26) = 1.19, p = .285, ηp2 = .044, ηG2 = .010; interruption cue type × trial type:F(1, 26) = 1.22, p = .280, pp2 = .045, ηG2 = .010).
Response duration was also higher in resumption trials compared to baseline trials, F(1, 23) = 30.53, p <.001, ηp2 = .570, ηG2 = .229, by 104.39 ms on average (95% Cl [65.31, 143.47]) (see Table 3). There was no effect of interruption cue type (main effect:F(1, 23) = 0.36, p = .851, ηp2 = .002, ηG2 = .0003; interruption cue type × trial type:F(1, 23) = 0.10, p = .753, pp 2 = .004, ηG2 = .0006).
3.2.4 Interruption effects on self-reported motivation to perform and difficulty to resume the ongoing task
Table 4 presents the participants’ task-related ratings.There was a marginally significant effect of task type on the self-reported motivation to perform the two tasks,F(1, 29) = 4.07, p = .053, ηp2 = .123, ηG2 = .027. Participants reported having been somewhat more motivated to perform the interruption task, compared to the ongoing task (mean difference 0.5, 95% Cl [−0.007, 1.007]). There was no effect of interruption cue type (main effect interruption cue type:F(1, 29) = 0.18, p = .672, ηp2 = .006, ηG2 = .002; interruption cue type*task type:F(1, 29) = 0.06, p = .813, ηp2 = .002, ηG2 = .0005). Participants also reported a higher perceived degree of difficulty to resume the ongoing task in the Pain condition as compared to the Non-pain condition. This effect was of marginal statistical significance,F(1, 29) = 3.86, p = .059, pp 2 = .117, ηG2 = .037.
3.2.5 Influence of pain catastrophizing
The RM ANCOVA on response duration revealed an additional statistically significant interaction of interruption cue type*PCS,F(1, 22) = 5.07, p = .035, ηp2 = .187, ηG2 = .051. The PCS score, however, was not found to correlate significantly with the response duration in any of the trial types (–0.02<r < 0.34, all p’s>.069), pointing to the possibility that this finding might have been a statistical artefact. The other ANCOVAs yielded no essentially changed results and are thus omitted from the results.
3.3 Discussion
Experiment 2 replicates the findings of Experiment 1 in demonstrating that participants were significantly less accurate and slower to initiate movements when resuming the ongoing task after an interruption, as compared to other trials. Further, it extends these findings by demonstrating that participants were also slower to complete movements in resumption trials. Despite the more complex ongoing task, impairment upon resumption was not greater when the task was interrupted by pain, as compared to non-painful stimuli, and did not appear to depend on level of pain catastrophizing.
4 General Discussion
The two experiments described in this manuscript aimed at shedding light on the consequences of interruptions by pain for the subsequent resumption of the interrupted activity. Healthy volunteers performed an ongoing task requiring joystick movements. Occasionally, they received either painful electrocutaneous or non-painful vibrotactile stimulation. Crucially, these stimuli were followed by ongoing task suspension and initiation of a different task. After spending some time away from the ongoing task, participants resumed it. Our focus lay on performance indices upon resumption, but self-reported measures of task performance were also investigated.
Our first hypothesis, i.e. that interruptions by pain impair task performance upon resumption, was confirmed. Participants were less accurate and took longer to initiate a joystick movement on ongoing task trials immediately after an interruption (resumption trials) as compared to other trials (baseline trials). In experiment 2, they also took longer to complete a joystick movement in resumption trials, as compared to baseline trials. These findings support a theoretical model in which interruptions by pain have negative consequences for behaviour and goal pursuit [3], and are in line with a large body of research from the field of human factors and ergonomics (e.g., [12]) showing that interruptions by (pain-irrelevant) external demands have detrimental consequences for outcomes such as accuracy [29, 30, 31], resumption time [32, 33], and, for complex tasks, also quality of performance [34] (although see also [35]).
Our second hypothesis was that interruptions by pain would impair task performance more than by other somatic stimuli, presumably because pain would result in larger interference with attention, and subsequently also with the encoding of task-related information [2, 3]. In contrast to our expectation, we did not find that interruptions by pain have more detrimental consequences than interruptions by non-painful vibrotactile stimuli. The observed effects were of a similar magnitude in both conditions and are thus not specific to pain. One reason for the lack of the expected interruption cue type effect on task resumption might be the complexity or cognitive demand of the ongoing task. Interruptions of easy tasks may even facilitate performance instead of impairing it [36]. Although in Experiment 2 we attempted to increase ongoing task complexity, the task was probably still fairly easy for our sample. Higher error rates and error rate variance might leave more room for potential interruption cue effects to be observed. Further, low cognitive demand of the interruption task might have allowed participants to use compensation strategies [37], such as information rehearsal [38]. Compensation strategies might have been used to a different degree in the two conditions.
The absence of an interruption cue type effect may also be explained by a similar opportunity to encode task-related information (e.g., the last joystick movement) following the painful and non-painful interruption cues. According to stage models of interruptions [11, 15, 39], the interruption cue is followed by the interruption lag, a time interval during which the person has the opportunity to “wrap up” the activity and/or to encode information about its state before suspending it [39]. As a threat signal, pain might lead people to disengage from their activity promptly, resulting in a dramatic decrease of the interruption lag duration and therefore a decreased chance of successful activity resumption [3]. In the present experimental setup, however, the interruption lags following the painful and non-painful interruption cue were similar, thus offering a similar opportunity for encoding. Further research is needed to clarify the lack of an effect of interruption cue type on task resumption, taking into account task complexity and interruption lag duration.
Participants reported somewhat higher motivation to perform the interruption task rather than the ongoing task in the pain condition, but not in the non-pain condition, indicating that breaks from a pain-relevant task were more pleasant than breaks from a pain-irrelevant task. This result, however, was only found in Experiment 1 and was not further confirmed by Experiment 2. Future research may investigate the motivational effects of interruptions by pain.
Pain catastrophizing was not found to influence the consequences of interruptions by pain. This was in contrast to our third hypothesis [3], and to a previous study, showing that pain catastrophizing moderates the effects of interruptions by pain [10]. It must be noted, however, that the mean Pain Catastrophizing Scale [19, 20 scores presently obtained (Experiment 1: 11.9, SD = 8.4; Experiment 2:14.5, SD = 9.3) were somewhat lower than these previously found in similar samples (16.6, SD = 7.8; [19]). It is possible that the pain catastrophizing level of our samples was too low to further enhance the negative effects of interruptions by pain. Further research in whether and how pain catastrophizing amplifies the detrimental effects of interruptions by pain is therefore warranted.
Response duration after the interruption was increased only in Experiment 2, which might relate to the different joystick used in that study. Specifically, whereas the joystick of Experiment 1 required user control mainly with regards to initiating a movement (“pushing” the joystick beyond the centerpoint), after which it moved fairly easily on one of two axes, the joystick of Experiment 2 required high user control not just with regards to the initiation of a movement, but to its whole performance in a larger area.
Some study limitations should be noted. First, the experimental tasks were rather easy, as demonstrated by participants’ high accuracy. In Experiment 2 we attempted to increase ongoing task complexity by increasing the number of targets, thus expecting that memory load would also be increased. Although in relation to Experiment 1 we observed an increase in reported task difficulty, the difference was not statistically significant. Despite the decline in accuracy and rise in variance in the resumption trials, the target task might still have been easy for our high-functioning sample. Future research may investigate the effects of interruptions by pain on even more complex tasks.
Further, our samples consisted of healthy volunteers, who received experimentally induced pain in a controlled laboratory environment. Our results may thus not generalize to clinical samples. Also, different results might be yielded with a more tonic pain model (e.g. heat or ischaemic pain). Moreover, in order to answer our question regarding the effect of interruption cue type, our studies focused on experimenter-initiated and -paced interruptions. Future research might address the effects of self-initiated and - paced interruptions or, more generally, the motivational context of interruptions [40].
Taken together, the two experiments presented here suggest that interruptions by pain do impair the resumption of a simple task, but not more so than interruptions by non-painful somatic stimuli. To our knowledge, these experiments are the first to systematically investigate the effects of interruptions by pain on subsequent activity resumption, by using a controlled task interruption paradigm. Previous relevant studies have focused on impaired task performance during pain (e.g., [5, 6, 7, 8, 41] and on the effects of rapid switching between tasks in the context of pain [42]. Although these situations are highly related to interruptions for longer time, they are still placed on different ends of the multitasking continuum [43].
Activity interruptions by pain deserve to be systematically investigated because they are a common response to pain even when the pain has no adaptive value, as is the case with chronic pain [2]. Further, they constitute part of a (chronic) pain management technique called activity pacing, which (amongst others) includes breaking activities into smaller parts, taking breaks, and alternating activity with rest [44, 45]. Theoretical approaches to activity pacing vary [45], but when activity pacing is informed by the operant learning theory, patients are advised to take breaks contingent on reaching a preset goal instead of contingent on pain [45, 46], which is the most common scenario in “naturalistic” pacing [47]. Consistent findings for the effectiveness of activity pacing are lacking [45, 48] and, to our knowledge, activity pacing has not been experimentally investigated. Research on the effects of interruptions in the context of pain and the role of interruption characteristics (such as interruption contingency, duration, etc.) therein, may shed light on the type of breaks that could be most helpful for pain patients.
Highlights
Activity interruptions by pain impair subsequent resumption of the activity.
This impairment is similar to that caused by interruptions by non-painful stimuli.
Pain catastrophizing did not appear to influence the results.
-
Ethical issues: The studies described in the present manuscript were approved by the appropriate Ethical Boards (Experiment 1: Ethical Review Committee Psychology and Neuroscience (ERCPN) of Maastricht University, study number: ECP-127 11_04_2013; Experiment 2: Social and Societal Ethics Committee and Medical Ethics Committee of the University of Leuven, study number: ML 10825). Participants of both studies provided informed consent prior to participation.
-
Conflict of interest: The authors have no conflict of interest to report. All authors have discussed the results and commented on the manuscript.
-
Funding sources: The conductance of these studies and preparation of the manuscript were supported by a PhD “Aspirant” grant (PSG-C5007-Asp/12) funded by the Research Foundation – Flanders, Belgium (FWO Vlaanderen).
Acknowledgments
The authors wish to thank Jeroen Clarysse and Johan Gielissen for technical support.
References
[1] Van Damme S, Crombez G, Eccleston C. Coping with pain: a motivational perspective. Pain 2008;139:1–4.Search in Google Scholar
[2] Eccleston C, Crombez G. Pain demands attention: a cognitive-affective model of the interruptive function of pain. Psychol Bull 1999;125:356–66.Search in Google Scholar
[3] Gatzounis R, Schrooten MGS, Crombez G, Vlaeyen JWS. Interrupted by pain: an anatomy of pain-contingent activity interruptions. Pain 2014;155:1192–5.Search in Google Scholar
[4] Boselie JJLM, Vancleef LMG, Smeets T, Peters ML. Increasing optimism abolishes pain-induced impairments in executive task performance. Pain 2014;155:334–40.Search in Google Scholar
[5] Buhle J, Wager TD. Performance-dependent inhibition of pain by an executive working memory task. Pain 2010;149:19–26.Search in Google Scholar
[6] Crombez G, Eccleston C, Baeyens F, Eelen P. Attentional disruption is enhanced by the threat of pain. Behav Res Ther 1998;36:195–204.Search in Google Scholar
[7] Moore DJ, Keogh E, Eccleston C. The effect of threat on attentional interruption by pain. Pain 2013;154:82–8.Search in Google Scholar
[8] Vancleef LMG, Peters ML. Pain catastrophizing, but not injury/illness sensitivity or anxiety sensitivity, enhances attentional interference by pain. J Pain 2006;7:23–30.Search in Google Scholar
[9] Okun M, Karoly P, Mun CJ, Kim H. Pain-contingent interruption and resumption of work goals: a within-day diary analysis. J Pain 2016;17:65–75.Search in Google Scholar
[10] Schrooten MGS, Karsdorp PA, Vlaeyen JWS. Pain catastrophizing moderates the effects of pain-contingent task interruptions. Eur J Pain 2013;17:1082–92.Search in Google Scholar
[11] Boehm-Davis DA, Remington R. Reducing the disruptive effects of interruption: a cognitive framework for analysing the costs and benefits of intervention strategies. Accid Anal Prev 2009;41:1124–9.Search in Google Scholar
[12] Trafton GJ, Monk CA. Task interruptions. Rev Hum Factors Ergon 2007;3:111–26.Search in Google Scholar
[13] Bailey BP, Konstan JA. On the need for attention-aware systems: measuring effects of interruption on task performance, error rate, and affective state. Comput Human Behav 2006;22:685–708.Search in Google Scholar
[14] Westbrook JI, Woods A, Rob MI, Dunsmuir WTM, Day RO. Association of interruptions with an increased risk and severity of medication administration errors. Arch Intern Med 2010;170:683–90.Search in Google Scholar
[15] Altmann EM, Trafton JG. Memory for goals: an activation-based model. Cogn Sci 2002;26:39–83.Search in Google Scholar
[16] Dodhia RM, Dismukes RK. Interruptions create prospective memory tasks. Appl Cogn Psychol 2009;23:73–89.Search in Google Scholar
[17] Meulders A, Vansteenwegen D, Vlaeyen JWS. The acquisition of fear of movement-related pain and associative learning: a novel pain-relevant human fear conditioning paradigm. Pain 2011;152:2460–9.Search in Google Scholar
[18] Grant Da, Berg Ea. A behavioral analysis of degree of reinforcement and ease of shifting to new responses in a Weigl-type card-sorting problem. J Exp Psychol 1948;38:404–11.Search in Google Scholar
[19] Van Damme S, Crombez G, Bijttebier P, Goubert L, Van Houdenhove B. A confirmatory factor analysis of the Pain Catastrophizing Scale. Invariant factor structure across clinical and non-clinical populations. Pain 2002;96:319–24.Search in Google Scholar
[20] Sullivan MJL, Bishop SRS, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess 1995;7:524–32.Search in Google Scholar
[21] Spruyt A, Clarysse J, Vansteenwegen D, Baeyens F, Hermans D. Affect 4.0: A free software package for implementing psychological and psychophysiological experiments. Exp Psychol 2009;57:36–45.Search in Google Scholar
[22] LimeSurvey Project Team, Schmitz C. LimeSurvey: An Open Source survey tool; 2012.Search in Google Scholar
[23] Walton DM, Wideman TH, Sullivan MJL. A Rasch analysis of the pain catastrophizing scale supports its use as an interval-level measure. Clin J Pain 2013;29:499–506.Search in Google Scholar
[24] Howell DC. Statistical methods for psychology. Thomson Wadsworth; 2007.Search in Google Scholar
[25] McCall RB, Appelbaum MI. Bias in the analysis of repeated-measures designs: some alternative approaches. Child Dev 1973;44:401–15.Search in Google Scholar
[26] Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol 2013;4:1–12.Search in Google Scholar
[27] Olejnik S, Algina J. Generalized eta and omega squared statistics: measures of effect size for some common research designs. Psychol Methods 2003;8:434–47.Search in Google Scholar
[28] IBM. IBM SPSS Statistics for Windows; 2013.Search in Google Scholar
[29] Altmann EM, Trafton JG, Hambrick DZ. Momentary interruptions can derail the train of thought. J Exp Psychol Gen 2014;143:215–26.Search in Google Scholar
[30] Altmann EM, Trafton JG. Brief lags in interrupted sequential performance: evaluating a model and model evaluation method. Int J Hum Comput Stud 2015;79:51–65.Search in Google Scholar
[31] Trafton JG, Altmann EM, Ratwani RM. A memory for goals model of sequence errors. Cogn Syst Res 2011;12:134–43.Search in Google Scholar
[32] Hodgetts HM, Jones DM. Contextual cues aid recovery from interruption: the role of associative activation. J Exp Psychol Learn Mem Cogn 2006;32: 1120–32.Search in Google Scholar
[33] Hodgetts HM, Jones DM. Interruptions in the Tower of London task. Can preparation minimise disruption? Proc Hum Factors Ergon Soc Annu Meet. 2013. p. 1000–4.Search in Google Scholar
[34] Foroughi CK, Werner NE, Nelson ET, Boehm-Davis Da. Do interruptions affect the quality of work? Proc Hum Factors Ergon Soc Annu Meet 2013;57:154–7.Search in Google Scholar
[35] Zijlstra FRH, Roe RA, Leonora AB, Krediet I. Temporal factors in mental work. Effects of interrupted activities. J Occup Organ Psychol 1999;72:163–85.Search in Google Scholar
[36] Speier C, Valacich JS, Vessey I. The influence of task interruption on individual decision making. An information overload perspective. Decis Sci 1999;30:337–60.Search in Google Scholar
[37] Mark G, Gudith D, Klocke U. The cost of interrupted work: More speed and stress. In: CHI 2008 Proc SIGCHI Conf Hum Factors Comput Syst. 2008. p. 107–10.Search in Google Scholar
[38] Cades DM, Davis DAB, Trafton JG, Monk CA. Does the difficulty of an interruption affect our ability to resume? Proc Hum Factors Ergon Soc Annu Meet 2007;51:234–8.Search in Google Scholar
[39] Trafton JG, Altmann EM, Brock DP, Mintz FE. Preparing to resume an interrupted task. Effects of prospective goal encoding and retrospective rehearsal. Int J Hum Comput Stud 2003;58:583–603.Search in Google Scholar
[40] Vlaeyen JWS, Morley S, Crombez G. The experimental analysis of the interruptive, interfering, and identity-distorting effects of chronic pain. Behav Res Ther 2016.Search in Google Scholar
[41] Kuhajda MC, Thorn BE, Klinger MR, Rubin NJ. The effect of headache pain on attention (encoding) and memory (recognition). Pain 2002;97:213–21.Search in Google Scholar
[42] Van Ryckeghem DML, Crombez G, Eccleston C, Liefooghe B, Van Damme S. The interruptive effect of pain in a multitask environment: an experimental investigation. J Pain 2012;13:131–8.Search in Google Scholar
[43] Salvucci DD, Taatgen Na, Borst J. Toward a unified theory of the multitasking continuum. From concurrent performance to task switching, interruption, and resumption. Chi 2009:1819–28.Search in Google Scholar
[44] Birkholtz M, Aylwin L, Harman RM. Activity pacing in chronic pain management: one aim, but which method? Part two: National Activity Pacing Survey. Br J Occup Ther 2004;67:481–7.Search in Google Scholar
[45] Nielson WR, Jensen MP, Karsdorp PA, Vlaeyen JWS. Activity pacing in chronic pain: concepts, evidence, and future directions. Clin J Pain 2013;29:461–8.Search in Google Scholar
[46] Gatzounis R, Schrooten MGS, Crombez G, Vlaeyen JWS. Operant learning theory in pain and chronic pain rehabilitation. Curr Pain Headache Rep 2012;16:117–26.Search in Google Scholar
[47] Murphy SL, Kratz AL. Activity pacing in daily life: a within-day analysis. Pain 2014;155:2630–7.Search in Google Scholar
[48] Gill JR, Brown CA. A structured review of the evidence for pacing as a chronic pain intervention. Eur J Pain 2009;13:214–6.Search in Google Scholar
© 2017 Scandinavian Association for the Study of Pain
Articles in the same Issue
- Scandinavian Journal of Pain
- Editorial comment
- Glucocorticoids – Efficient analgesics against postherpetic neuralgia?
- Original experimental
- Effect of intrathecal glucocorticoids on the central glucocorticoid receptor in a rat nerve ligation model
- Editorial comment
- Important new insight in pain and pain treatment induced changes in functional connectivity between the Pain Matrix and the Salience, Central Executive, and Sensorimotor networks
- Original experimental
- Salience, central executive, and sensorimotor network functional connectivity alterations in failed back surgery syndrome
- Editorial comment
- Education and support strategies improve assessment and management of pain by nurses
- Clinical pain research
- Using education and support strategies to improve the way nurses assess regular and transient pain – A quality improvement study of three hospitals
- Editorial comment
- The interference of pain with task performance: Increasing ecological validity in research
- Original experimental
- The disruptive effects of pain on multitasking in a virtual errands task
- Editorial comment
- Analyzing transition from acute back pain to chronic pain with linear mixed models reveals a continuous chronification of acute back pain
- Observational study
- From acute to chronic back pain: Using linear mixed models to explore changes in pain intensity, disability, and depression
- Editorial comment
- NSAIDs relieve osteoarthritis (OA) pain, but cardiovascular safety in question even for diclofenac, ibuprofen, naproxen, and celecoxib: what are the alternatives?
- Clinical pain research
- Efficacy and safety of diclofenac in osteoarthritis: Results of a network meta-analysis of unpublished legacy studies
- Editorial comment
- Editorial comment on Nina Kreddig’s and Monika Hasenbring’s study on pain anxiety and fear of (re) injury in patients with chronic back pain: Sex as a moderator
- Clinical pain research
- Pain anxiety and fear of (re) injury in patients with chronic back pain: Sex as a moderator
- Editorial comment
- Intraoral QST – Mission impossible or not?
- Clinical pain research
- Multifactorial assessment of measurement errors affecting intraoral quantitative sensory testing reliability
- Editorial comment
- Objective measurement of subjective pain-experience: Real nociceptive stimuli versus pain expectation
- Clinical pain research
- Cerebral oxygenation for pain monitoring in adults is ineffective: A sequence-randomized, sham controlled study in volunteers
- Editorial comment
- Association between adolescent and parental use of analgesics
- Observational study
- The association between adolescent and parental use of non-prescription analgesics for headache and other somatic pain – A cross-sectional study
- Editorial comment
- Cancer-pain intractable to high-doses systemic opioids can be relieved by intraspinal local anaesthetic plus an opioid and an alfa2-adrenoceptor agonist
- Clinical pain research
- Spinal analgesia for severe cancer pain: A retrospective analysis of 60 patients
- Editorial comment
- Specific symptoms and signs of unstable back segments and curative surgery?
- Clinical pain research
- Symptoms and signs possibly indicating segmental, discogenic pain. A fusion study with 18 years of follow-up
- Editorial comment
- Local anaesthesia methods for analgesia after total hip replacement: Problems of anatomy, methodology and interpretation?
- Clinical pain research
- Local infiltration analgesia or femoral nerve block for postoperative pain management in patients undergoing total hip arthroplasty. A randomized, double-blind study
- Editorial
- Scientific presentations at the 2017 annual meeting of the Scandinavian Association for the Study of Pain (SASP)
- Abstracts
- Correlation between quality of pain and depression: A post-operative assessment of pain after caesarian section among women in Ghana
- Abstracts
- Dynamic and static mechanical pain sensitivity is associated in women with migraine
- Abstracts
- The number of active trigger points is associated with sensory and emotional aspects of health-related quality of life in tension type headache
- Abstracts
- Chronic neuropathic pain following oxaliplatin and docetaxel: A 5-year follow-up questionnaire study
- Abstracts
- Expression of α1 adrenergic receptor subtypes by afferent fibers that innervate rat masseter muscle
- Abstracts
- Buprenorphine alleviation of pain does not compromise the rat monoarthritic pain model
- Abstracts
- Association between pain, disability, widespread pressure pain hypersensitivity and trigger points in subjects with neck pain
- Abstracts
- Association between widespread pressure pain hypersensitivity, health history, and trigger points in subjects with neck pain
- Abstracts
- Neuromas in patients with peripheral nerve injury and amputation - An ongoing study
- Abstracts
- The link between chronic musculoskeletal pain and sperm quality in overweight orthopedic patients
- Abstracts
- Several days of muscle hyperalgesia facilitates cortical somatosensory excitability
- Abstracts
- Social stress, epigenetic changes and pain
- Abstracts
- Characterization of released exosomes from satellite glial cells under normal and inflammatory conditions
- Abstracts
- Cell-based platform for studying trigeminal satellite glial cells under normal and inflammatory conditions
- Abstracts
- Tramadol in postoperative pain – 1 mg/ml IV gave no pain reduction but more side effects in third molar surgery
- Abstracts
- Tempo-spatial discrimination to non-noxious stimuli is better than for noxious stimuli
- Abstracts
- The encoding of the thermal grill illusion in the human spinal cord
- Abstracts
- Effect of cocoa on endorphin levels and craniofacial muscle sensitivity in healthy individuals
- Abstracts
- The impact of naloxegol treatment on gastrointestinal transit and colonic volume
- Abstracts
- Preoperative downregulation of long-noncoding RNA Meg3 in serum of patients with chronic postoperative pain after total knee replacement
- Abstracts
- Painful diabetic polyneuropathy and quality of life in Danish type 2 diabetic patients
- Abstracts
- “What about me?”: A qualitative explorative study on perspectives of spouses living with complex chronic pain patients
- Abstracts
- Increased postural stiffness in patients with knee osteoarthritis who are highly sensitized
- Abstracts
- Efficacy of dry needling on latent myofascial trigger points in male subjects with neck/shoulders musculoskeletal pain. A case series
- Abstracts
- Identification of pre-operative of risk factors associated with persistent post-operative pain by self-reporting tools in lower limb amputee patients – A feasibility study
- Abstracts
- Renal function estimations and dose recommendations for Gabapentin, Ibuprofen and Morphine in acute hip fracture patients
- Abstracts
- Evaluating the ability of non-rectangular electrical pulse forms to preferentially activate nociceptive fibers by comparing perception thresholds
- Abstracts
- Detection of systemic inflammation in severely impaired chronic pain patients, and effects of a CBT-ACT-based multi-modal pain rehabilitation program
- Abstracts
- Fixed or adapted conditioning intensity for repeated conditioned pain modulation
- Abstracts
- Combined treatment (Norspan, Gabapentin and Oxynorm) was found superior in pain management after total knee arthroplasty
- Abstracts
- Effects of conditioned pain modulation on the withdrawal pattern to nociceptive stimulation in humans – Preliminary results
- Abstracts
- Application of miR-223 onto the dorsal nerve roots in rats induces hypoexcitability in the pain pathways
- Abstracts
- Acute muscle pain alters corticomotor output of the affected muscle stronger than a synergistic, ipsilateral muscle
- Abstracts
- The subjective sensation induced by various thermal pulse stimulation in healthy volunteers
- Abstracts
- Assessing Offset Analgesia through electrical stimulations in healthy volunteers
- Abstracts
- Metastatic lung cancer in patient with non-malignant neck pain: A case report
- Abstracts
- The size of pain referral patterns from a tonic painful mechanical stimulus is increased in women
- Abstracts
- Oxycodone and macrogol 3350 treatment reduces anal sphincter relaxation compared to combined oxycodone and naloxone tablets
- Abstracts
- The effect of UVB-induced skin inflammation on histaminergic and non-histaminergic evoked itch and pain
- Abstracts
- Topical allyl-isothiocyanate (mustard oil) as a TRPA1-dependent human surrogate model of pain, hyperalgesia, and neurogenic inflammation – A dose response study
- Abstracts
- Dissatisfaction and persistent post-operative pain following total knee replacement – A 5 year follow-up of all patients from a whole region
- Abstracts
- Paradoxical differences in pain ratings of the same stimulus intensity
- Abstracts
- Pain assessment and post-operative pain management in orthopedic patients
- Abstracts
- Combined electric and pressure cuff pain stimuli for assessing conditioning pain modulation (CPM)
- Abstracts
- The effect of facilitated temporal summation of pain, widespread pressure hyperalgesia and pain intensity in patients with knee osteoarthritis on the responds to Non-Steroidal Anti-Inflammatory Drugs – A preliminary analysis
- Abstracts
- How to obtain the biopsychosocial record in multidisciplinary pain clinic? An action research study
- Abstracts
- Experimental neck muscle pain increase pressure pain threshold over cervical facet joints
- Abstracts
- Are we using Placebo effects in specialized Palliative Care?
- Abstracts
- Prevalence and pattern of helmet-induced headache among Danish military personnel
- Abstracts
- Aquaporin 4 expression on trigeminal satellite glial cells under normal and inflammatory conditions
- Abstracts
- Preoperative synovitis in knee osteoarthritis is predictive for pain 1 year after total knee arthroplasty
- Abstracts
- Biomarkers alterations in trapezius muscle after an acute tissue trauma: A human microdialysis study
- Abstracts
- PainData: A clinical pain registry in Denmark
- Abstracts
- A novel method for investigating the importance of visual feedback on somatosensation and bodily-self perception
- Abstracts
- Drugs that can cause respiratory depression with concomitant use of opioids
- Abstracts
- The potential use of a serious game to help patients learn about post-operative pain management – An evaluation study
- Abstracts
- Modelling activity-dependent changes of velocity in C-fibers
- Abstracts
- Choice of rat strain in pre-clinical pain-research – Does it make a difference for translation from animal model to human condition?
- Abstracts
- Omics as a potential tool to identify biomarkers and to clarify the mechanism of chronic pain development
- Abstracts
- Evaluation of the benefits from the introduction meeting for patients with chronic non-malignant pain and their relatives in interdisciplinary pain center
- Observational study
- The changing face of acute pain services
- Observational study
- Chronic pain in multiple sclerosis: A10-year longitudinal study
- Clinical pain research
- Functional disability and depression symptoms in a paediatric persistent pain sample
- Observational study
- Pain provocation following sagittal plane repeated movements in people with chronic low back pain: Associations with pain sensitivity and psychological profiles
- Observational study
- A longitudinal exploration of pain tolerance and participation in contact sports
- Original experimental
- Taking a break in response to pain. An experimental investigation of the effects of interruptions by pain on subsequent activity resumption
- Clinical pain research
- Sex moderates the effects of positive and negative affect on clinical pain in patients with knee osteoarthritis
- Original experimental
- The effects of a brief educational intervention on medical students’ knowledge, attitudes and beliefs towards low back pain
- Observational study
- The association between pain characteristics, pain catastrophizing and health care use – Baseline results from the SWEPAIN cohort
- Topical review
- Couples coping with chronic pain: How do intercouple interactions relate to pain coping?
- Narrative review
- The wit and wisdom of Wilbert (Bill) Fordyce (1923 - 2009)
- Letter to the Editor
- Unjustified extrapolation
- Letter to the Editor
- Response to: “Letter to the Editor entitled: Unjustified extrapolation” [by authors: Supp G., Rosedale R., Werneke M.]
Articles in the same Issue
- Scandinavian Journal of Pain
- Editorial comment
- Glucocorticoids – Efficient analgesics against postherpetic neuralgia?
- Original experimental
- Effect of intrathecal glucocorticoids on the central glucocorticoid receptor in a rat nerve ligation model
- Editorial comment
- Important new insight in pain and pain treatment induced changes in functional connectivity between the Pain Matrix and the Salience, Central Executive, and Sensorimotor networks
- Original experimental
- Salience, central executive, and sensorimotor network functional connectivity alterations in failed back surgery syndrome
- Editorial comment
- Education and support strategies improve assessment and management of pain by nurses
- Clinical pain research
- Using education and support strategies to improve the way nurses assess regular and transient pain – A quality improvement study of three hospitals
- Editorial comment
- The interference of pain with task performance: Increasing ecological validity in research
- Original experimental
- The disruptive effects of pain on multitasking in a virtual errands task
- Editorial comment
- Analyzing transition from acute back pain to chronic pain with linear mixed models reveals a continuous chronification of acute back pain
- Observational study
- From acute to chronic back pain: Using linear mixed models to explore changes in pain intensity, disability, and depression
- Editorial comment
- NSAIDs relieve osteoarthritis (OA) pain, but cardiovascular safety in question even for diclofenac, ibuprofen, naproxen, and celecoxib: what are the alternatives?
- Clinical pain research
- Efficacy and safety of diclofenac in osteoarthritis: Results of a network meta-analysis of unpublished legacy studies
- Editorial comment
- Editorial comment on Nina Kreddig’s and Monika Hasenbring’s study on pain anxiety and fear of (re) injury in patients with chronic back pain: Sex as a moderator
- Clinical pain research
- Pain anxiety and fear of (re) injury in patients with chronic back pain: Sex as a moderator
- Editorial comment
- Intraoral QST – Mission impossible or not?
- Clinical pain research
- Multifactorial assessment of measurement errors affecting intraoral quantitative sensory testing reliability
- Editorial comment
- Objective measurement of subjective pain-experience: Real nociceptive stimuli versus pain expectation
- Clinical pain research
- Cerebral oxygenation for pain monitoring in adults is ineffective: A sequence-randomized, sham controlled study in volunteers
- Editorial comment
- Association between adolescent and parental use of analgesics
- Observational study
- The association between adolescent and parental use of non-prescription analgesics for headache and other somatic pain – A cross-sectional study
- Editorial comment
- Cancer-pain intractable to high-doses systemic opioids can be relieved by intraspinal local anaesthetic plus an opioid and an alfa2-adrenoceptor agonist
- Clinical pain research
- Spinal analgesia for severe cancer pain: A retrospective analysis of 60 patients
- Editorial comment
- Specific symptoms and signs of unstable back segments and curative surgery?
- Clinical pain research
- Symptoms and signs possibly indicating segmental, discogenic pain. A fusion study with 18 years of follow-up
- Editorial comment
- Local anaesthesia methods for analgesia after total hip replacement: Problems of anatomy, methodology and interpretation?
- Clinical pain research
- Local infiltration analgesia or femoral nerve block for postoperative pain management in patients undergoing total hip arthroplasty. A randomized, double-blind study
- Editorial
- Scientific presentations at the 2017 annual meeting of the Scandinavian Association for the Study of Pain (SASP)
- Abstracts
- Correlation between quality of pain and depression: A post-operative assessment of pain after caesarian section among women in Ghana
- Abstracts
- Dynamic and static mechanical pain sensitivity is associated in women with migraine
- Abstracts
- The number of active trigger points is associated with sensory and emotional aspects of health-related quality of life in tension type headache
- Abstracts
- Chronic neuropathic pain following oxaliplatin and docetaxel: A 5-year follow-up questionnaire study
- Abstracts
- Expression of α1 adrenergic receptor subtypes by afferent fibers that innervate rat masseter muscle
- Abstracts
- Buprenorphine alleviation of pain does not compromise the rat monoarthritic pain model
- Abstracts
- Association between pain, disability, widespread pressure pain hypersensitivity and trigger points in subjects with neck pain
- Abstracts
- Association between widespread pressure pain hypersensitivity, health history, and trigger points in subjects with neck pain
- Abstracts
- Neuromas in patients with peripheral nerve injury and amputation - An ongoing study
- Abstracts
- The link between chronic musculoskeletal pain and sperm quality in overweight orthopedic patients
- Abstracts
- Several days of muscle hyperalgesia facilitates cortical somatosensory excitability
- Abstracts
- Social stress, epigenetic changes and pain
- Abstracts
- Characterization of released exosomes from satellite glial cells under normal and inflammatory conditions
- Abstracts
- Cell-based platform for studying trigeminal satellite glial cells under normal and inflammatory conditions
- Abstracts
- Tramadol in postoperative pain – 1 mg/ml IV gave no pain reduction but more side effects in third molar surgery
- Abstracts
- Tempo-spatial discrimination to non-noxious stimuli is better than for noxious stimuli
- Abstracts
- The encoding of the thermal grill illusion in the human spinal cord
- Abstracts
- Effect of cocoa on endorphin levels and craniofacial muscle sensitivity in healthy individuals
- Abstracts
- The impact of naloxegol treatment on gastrointestinal transit and colonic volume
- Abstracts
- Preoperative downregulation of long-noncoding RNA Meg3 in serum of patients with chronic postoperative pain after total knee replacement
- Abstracts
- Painful diabetic polyneuropathy and quality of life in Danish type 2 diabetic patients
- Abstracts
- “What about me?”: A qualitative explorative study on perspectives of spouses living with complex chronic pain patients
- Abstracts
- Increased postural stiffness in patients with knee osteoarthritis who are highly sensitized
- Abstracts
- Efficacy of dry needling on latent myofascial trigger points in male subjects with neck/shoulders musculoskeletal pain. A case series
- Abstracts
- Identification of pre-operative of risk factors associated with persistent post-operative pain by self-reporting tools in lower limb amputee patients – A feasibility study
- Abstracts
- Renal function estimations and dose recommendations for Gabapentin, Ibuprofen and Morphine in acute hip fracture patients
- Abstracts
- Evaluating the ability of non-rectangular electrical pulse forms to preferentially activate nociceptive fibers by comparing perception thresholds
- Abstracts
- Detection of systemic inflammation in severely impaired chronic pain patients, and effects of a CBT-ACT-based multi-modal pain rehabilitation program
- Abstracts
- Fixed or adapted conditioning intensity for repeated conditioned pain modulation
- Abstracts
- Combined treatment (Norspan, Gabapentin and Oxynorm) was found superior in pain management after total knee arthroplasty
- Abstracts
- Effects of conditioned pain modulation on the withdrawal pattern to nociceptive stimulation in humans – Preliminary results
- Abstracts
- Application of miR-223 onto the dorsal nerve roots in rats induces hypoexcitability in the pain pathways
- Abstracts
- Acute muscle pain alters corticomotor output of the affected muscle stronger than a synergistic, ipsilateral muscle
- Abstracts
- The subjective sensation induced by various thermal pulse stimulation in healthy volunteers
- Abstracts
- Assessing Offset Analgesia through electrical stimulations in healthy volunteers
- Abstracts
- Metastatic lung cancer in patient with non-malignant neck pain: A case report
- Abstracts
- The size of pain referral patterns from a tonic painful mechanical stimulus is increased in women
- Abstracts
- Oxycodone and macrogol 3350 treatment reduces anal sphincter relaxation compared to combined oxycodone and naloxone tablets
- Abstracts
- The effect of UVB-induced skin inflammation on histaminergic and non-histaminergic evoked itch and pain
- Abstracts
- Topical allyl-isothiocyanate (mustard oil) as a TRPA1-dependent human surrogate model of pain, hyperalgesia, and neurogenic inflammation – A dose response study
- Abstracts
- Dissatisfaction and persistent post-operative pain following total knee replacement – A 5 year follow-up of all patients from a whole region
- Abstracts
- Paradoxical differences in pain ratings of the same stimulus intensity
- Abstracts
- Pain assessment and post-operative pain management in orthopedic patients
- Abstracts
- Combined electric and pressure cuff pain stimuli for assessing conditioning pain modulation (CPM)
- Abstracts
- The effect of facilitated temporal summation of pain, widespread pressure hyperalgesia and pain intensity in patients with knee osteoarthritis on the responds to Non-Steroidal Anti-Inflammatory Drugs – A preliminary analysis
- Abstracts
- How to obtain the biopsychosocial record in multidisciplinary pain clinic? An action research study
- Abstracts
- Experimental neck muscle pain increase pressure pain threshold over cervical facet joints
- Abstracts
- Are we using Placebo effects in specialized Palliative Care?
- Abstracts
- Prevalence and pattern of helmet-induced headache among Danish military personnel
- Abstracts
- Aquaporin 4 expression on trigeminal satellite glial cells under normal and inflammatory conditions
- Abstracts
- Preoperative synovitis in knee osteoarthritis is predictive for pain 1 year after total knee arthroplasty
- Abstracts
- Biomarkers alterations in trapezius muscle after an acute tissue trauma: A human microdialysis study
- Abstracts
- PainData: A clinical pain registry in Denmark
- Abstracts
- A novel method for investigating the importance of visual feedback on somatosensation and bodily-self perception
- Abstracts
- Drugs that can cause respiratory depression with concomitant use of opioids
- Abstracts
- The potential use of a serious game to help patients learn about post-operative pain management – An evaluation study
- Abstracts
- Modelling activity-dependent changes of velocity in C-fibers
- Abstracts
- Choice of rat strain in pre-clinical pain-research – Does it make a difference for translation from animal model to human condition?
- Abstracts
- Omics as a potential tool to identify biomarkers and to clarify the mechanism of chronic pain development
- Abstracts
- Evaluation of the benefits from the introduction meeting for patients with chronic non-malignant pain and their relatives in interdisciplinary pain center
- Observational study
- The changing face of acute pain services
- Observational study
- Chronic pain in multiple sclerosis: A10-year longitudinal study
- Clinical pain research
- Functional disability and depression symptoms in a paediatric persistent pain sample
- Observational study
- Pain provocation following sagittal plane repeated movements in people with chronic low back pain: Associations with pain sensitivity and psychological profiles
- Observational study
- A longitudinal exploration of pain tolerance and participation in contact sports
- Original experimental
- Taking a break in response to pain. An experimental investigation of the effects of interruptions by pain on subsequent activity resumption
- Clinical pain research
- Sex moderates the effects of positive and negative affect on clinical pain in patients with knee osteoarthritis
- Original experimental
- The effects of a brief educational intervention on medical students’ knowledge, attitudes and beliefs towards low back pain
- Observational study
- The association between pain characteristics, pain catastrophizing and health care use – Baseline results from the SWEPAIN cohort
- Topical review
- Couples coping with chronic pain: How do intercouple interactions relate to pain coping?
- Narrative review
- The wit and wisdom of Wilbert (Bill) Fordyce (1923 - 2009)
- Letter to the Editor
- Unjustified extrapolation
- Letter to the Editor
- Response to: “Letter to the Editor entitled: Unjustified extrapolation” [by authors: Supp G., Rosedale R., Werneke M.]

