Abstract
C20H16N6, monoclinic, P21/c (no. 14), a = 7.6623(4) Å, b = 11.8206(5) Å, c = 8.8960(4) Å, β = 91.825(2)°,V = 805.33(7) Å3, Z = 2, R gt (F) = 0.0577, wR ref(F 2) = 0.1588, T = 200(2) K.
Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Prism, colorless |
| Size: | 0.35 × 0.18 × 0.16 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.09 mm−1 |
| Diffractometer, scan mode: | SCXmini, ω-scans |
| θ max, completeness: | 27.5°, >99% |
| N(hkl)measured, N(hkl)unique, R int: | 11,399, 1848, 0.042 |
| Criterion for I obs, N(hkl)gt: | I obs > 2 σ(I obs), 1504 |
| N(param)refined: | 119 |
| Programs: | CrystalClear [1], OLEX2 [2], SHELX [3, 4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| N2 | 0.17893 (18) | 0.90166 (11) | 0.49443 (14) | 0.0247 (4) |
| N1 | 0.3891 (2) | 0.76545 (13) | 0.49976 (16) | 0.0316 (4) |
| H1 | 0.422127 | 0.799670 | 0.417555 | 0.038* |
| N3 | 0.4950 (2) | 0.64946 (12) | 0.69759 (16) | 0.0303 (4) |
| C1 | 0.0235 (2) | 0.94368 (13) | 0.53295 (17) | 0.0234 (4) |
| C10 | 0.2317 (2) | 0.80296 (14) | 0.55461 (17) | 0.0253 (4) |
| C4 | 0.1321 (2) | 0.74258 (14) | 0.65720 (19) | 0.0291 (4) |
| H4 | 0.173922 | 0.674092 | 0.701095 | 0.035* |
| C2 | −0.0862 (2) | 0.88787 (15) | 0.62940 (19) | 0.0293 (4) |
| H2 | −0.197151 | 0.918115 | 0.652035 | 0.035* |
| C5 | 0.5019 (2) | 0.68280 (14) | 0.55401 (18) | 0.0265 (4) |
| C3 | −0.0283 (2) | 0.78606 (15) | 0.6920 (2) | 0.0314 (4) |
| H3 | −0.100128 | 0.746299 | 0.759236 | 0.038* |
| C6 | 0.6245 (2) | 0.63903 (15) | 0.4550 (2) | 0.0311 (4) |
| H6 | 0.628163 | 0.665418 | 0.354263 | 0.037* |
| C7 | 0.7385 (3) | 0.55759 (16) | 0.5067 (2) | 0.0346 (5) |
| H7 | 0.822917 | 0.526926 | 0.442220 | 0.042* |
| C8 | 0.7297 (3) | 0.52024 (16) | 0.6542 (2) | 0.0356 (5) |
| H8 | 0.806287 | 0.463001 | 0.691977 | 0.043* |
| C9 | 0.6076 (3) | 0.56813 (16) | 0.7439 (2) | 0.0348 (5) |
| H9 | 0.602091 | 0.542426 | 0.844852 | 0.042* |
Source of materials
About 2.0 mmol 6,6′-diamino-2,2′-bipyridine, 6.0 mmol 2-bromopyridine and 8.1 mmol KOtBu were weighed in a dry Schlenk tube, 0.040 mmol Pd2(dba)3, 0.08 mmol BINAP as catalyst and 30 mL of dry, degassed toluene as solvent were added. The mixture was heated to 80 °C and stirred under argon for three days before it was cooled to room temperature. After that 150 mL water were added and stirred vigorously for 1 h. The solid was filtered out and the title product was obtained by recrystallization from a hot mixture of methanol and DMF.
Experimental details
Hydrogen atoms were placed in their geometrically idealized positions and constrained to ride on their parent atoms.
Comment
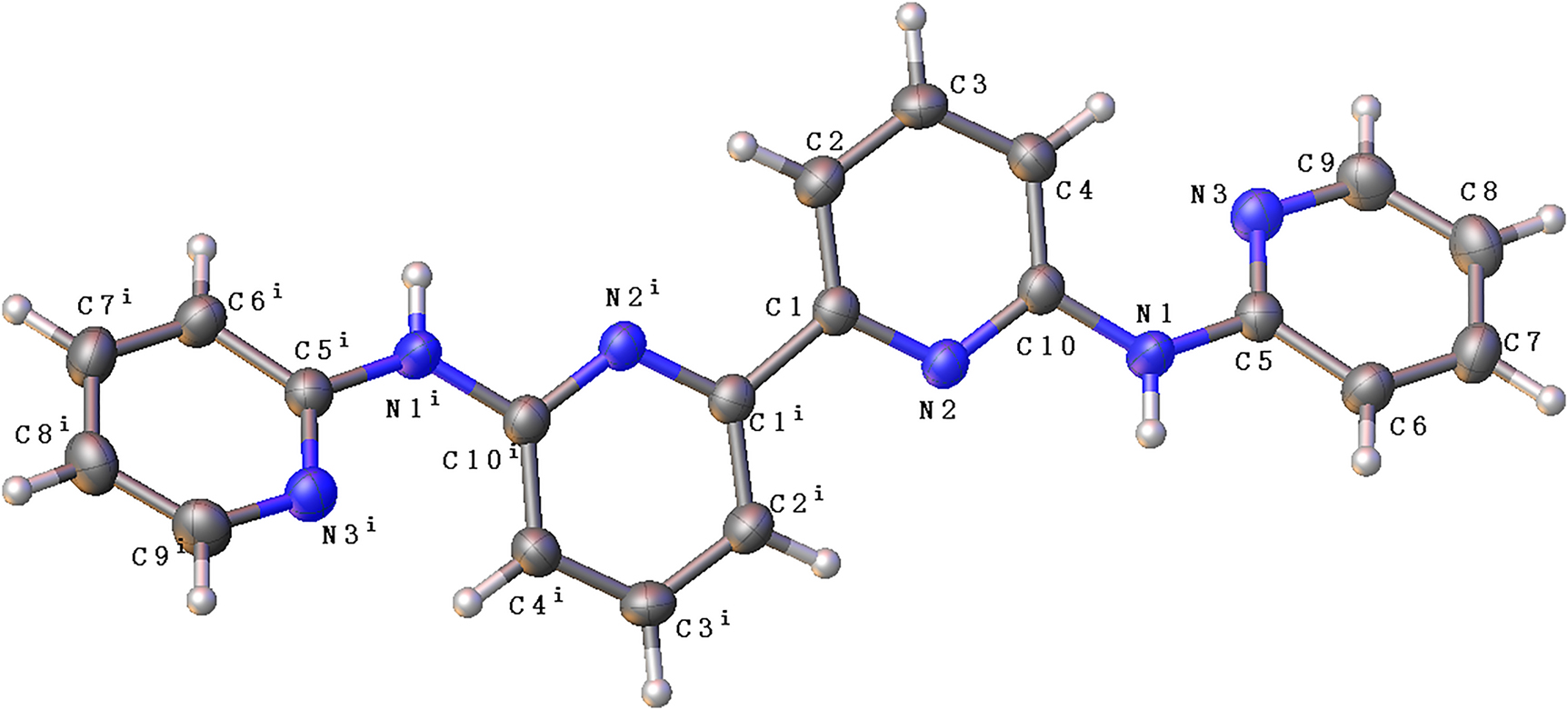
The title compound is an useful organic blocks for the synthesis of metal organic hybrid phase transition materials [5], [6], [7], which are used in nonvolatile memory storage [8], electronics [9], optics [10] and telecom shelters [11]. It is also an interesting work to try to detect metal ion pollutants using this compound [12]. In our exploration on multiferroic compounds and searching for new metal organic hybrid phase transition materials [13], we come across the crystal structure of the title compound. In the title molecule, all bond lengths are in normal ranges [14], [15], [16]. The molecular structure is centro-symmetric arranged around a inversion center (see the Figure). The dihedral angle between the pyridine rings of each half molecular is 33.8°. In the crystal, N–H⃛N hydrogen bonds stabilized the structure.
Funding source: Innovative Practice Project of Jiangsu Vocational Institute of Architectural Technology
Award Identifier / Grant number: JYSCZ19-13
Funding source: Key Research and Development Program of Xuzhou
Award Identifier / Grant number: KC18134
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This work was supported by ‘Innovative Practice Project of Jiangsu Vocational Institute of Architectural Technology (JYSCZ19-13)’ and ‘Key Research and Development Program of Xuzhou (KC18134)’.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Rigaku. CrystalClearSM Expert; Rigaku Corporation: Tokyo, Japan, 2005.Suche in Google Scholar
2. Bourhis, L. J., Dolomanov, O. V., Gildea, R. J., Howard, J. A. K., Puschmann, H. The anatomy of a comprehensive constrained, restrained refinement program for the modern computing environment-olex2 dissected. Acta Crystallogr. 2015, A71, 59–75; https://doi.org/10.1107/s2053273314022207.Suche in Google Scholar
3. Sheldrick, G. M. SHELXT – integrated space-group and crystal structure determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Suche in Google Scholar
4. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Suche in Google Scholar
5. Bonnet, S. G. M., Costa, J. S., Siegler, M. A., Spek, A. L., Bousseksou, A., Fu, W. T., Gamez, P., Reedijk, J. Influence of sample preparation, temperature, light, and pressure on the two-step spin crossover mononuclear compound [Fe(bapbpy)(NCS)2]. Chem. Mater. 2009, 21, 1123–1136; https://doi.org/10.1021/cm803414q.Suche in Google Scholar
6. Vincent, H. S., Anja, B., Mathijs, F. W., Samantha, L. H., Bianka, S., Corjan van de, G., Maxime, A. S., Tiziano, M., Francesco, P., Marta, F., Paola, G., Carla, B., Luigi, M., Sylvestre, B. Induction of a four-way junction structure in the DNA palindromic hexanucleotide 5′-d(CGTACG)-3′ by a mononuclear platinum complex. Angew. Chem. Int. Ed. 2019, 58, 9378–9382.10.1002/anie.201814532Suche in Google Scholar PubMed PubMed Central
7. Nicolas, Q., Sun, D. Y., Jennifer, F., Jacques, P., Martin, J. F., Murielle, C. K., Vincent, A. Electrocatalytic hydrogen evolution with a cobalt complex bearing pendant proton relays: acid strength and applied potential govern mechanism and stability. J. Am. Chem. Soc. 2020, 142, 274–282.10.1021/jacs.9b10407Suche in Google Scholar PubMed
8. Gamba, I., Mutikainen, I., Bouwman, E., Reedijk, J., Bonnet, S. Synthesis and characterization of copper complexes of a tetrapyridyl ligand, and their use in the catalytic aerobic oxidation of benzyl alcohol. Eur. J. Inorg. Chem. 2013, 1, 115–123; https://doi.org/10.1002/ejic.201200807.Suche in Google Scholar
9. Bonnet, S., Siegler, M. A., Costa, J. S., Molnar, G., Bousseksou, A., Spek, A. L., Gamez, P., Reedijk, J. A two-step spin crossover mononuclear iron(ii) complex with a [HS–LS–LS] intermediate phase. Chem. Commun. 2008, 5619–5621; https://doi.org/10.1039/b811746b.Suche in Google Scholar
10. Queyriaux, N., Abel, K., Fize, J., Pécaut, J., Orio, M., Hammarström, L. From non-innocent to guilty: on the role of redox-active ligands in the electro-assisted reduction of CO2 mediated by a cobalt(II)-polypyridyl complex. Sustain. Energy Fuel. 2020, 4, 3668–3676; https://doi.org/10.1039/d0se00570c.Suche in Google Scholar
11. Vincent, H. S., Geri, F. M., Maxime, A. S., Luigi, M., Sylvestre, B. Controlling with light the interaction between trans-tetrapyridyl ruthenium complexes and an oligonucleotide. Dalton Trans. 2018, 47, 507–516.10.1039/C7DT03613BSuche in Google Scholar PubMed
12. Molenbroek, E., Straathof, N., Duck, S., Rashid, Z., Lenthe, J. H., Lutz, M., Gandubert, A., Gebbink, R. J. M. K., Cola, L. D., Bonnet, S. Zinc coordination to the bapbpy ligand in homogeneous solutions and at liposomes: zinc detection via fluorescence enhancement. Dalton Trans. 2013, 42, 2973–2984; https://doi.org/10.1039/c2dt32488a.Suche in Google Scholar
13. Wang, W. X., Cai, H. L., Xiong, R. G. Hydrothermal synthesis method of 5-(4′-methylbiphenyl-2-yl)-1H-tetrazole. Chin. Chem. Lett. 2013, 24, 783–785; https://doi.org/10.1016/j.cclet.2013.04.041.Suche in Google Scholar
14. Queyriaux, N., Giannoudis, E., Windle, C. D., Roy, S., Pécaut, J., Coutsolelos, A. G., Artero, V., Chavarot-Kerlidou, M. A noble metal-free photocatalytic system based on a novel cobalt tetrapyridyl catalyst for hydrogen production in fully aqueous medium. Sustain. Energy Fuel. 2018, 2, 553–557; https://doi.org/10.1039/c7se00428a.Suche in Google Scholar
15. Zheng, S., Siegler, M. A., Sanchez, J. C., Fu, W. T., Bonnet, S. Effect of metal dilution on the thermal spin transition of [FexZn1−x(bapbpy)(NCS)2]. Eur. J. Inorg. Chem. 2013, 5, 1033–1042; https://doi.org/10.1002/ejic.201201183.Suche in Google Scholar
16. Wang, W. X. The crystal structure of ethyl 5-methyl-7-(4-(phenylthio)phenyl)-4,7-dihydrotetrazolo[1,5-a]pyrimidine-6-carboxylate, C20H19N5O2S. Z. Kristallogr. N. Cryst. Struct. 2021, 236, 781–783; https://doi.org/10.1515/ncrs-2021-0122.Suche in Google Scholar
© 2021 Yue Sun et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of (E)-7-hydroxy-2-((6-methoxypyridin-3-yl)methylene)-3, 4-dihydronaphthalen-1(2H)-one, C17H15NO3
- Crystal structure of (E)-7-methoxy-2-((2-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1 (2H)-one, C18H17NO3
- The crystal structure of N 6,N 6′-di(pyridin-2-yl)-[2,2′-bipyridine]-6,6′-diamine, C20H16N6
- The crystal structure of {N 1,N 2-bis[2,4-dimethyl-6-(4-(tert-butyl)phenyl)(phenyl)methyl]acenaphthylene-1,2-diimino-κ2 N, N′}-dibromido-nickel(II) – dichloromethane(1/2), C64H64Br2Cl4N2Ni
- Synthesis and crystal structure of nonacarbonyltris[(2-thia-1,3,5-triaza-7-phosphatricylco[3.3.1.1]decane-κ1 P)-2,2-dioxide]triruthenium(0) – acetonitrile (7/6), C25.71H32.57N9.86O15P3S3Ru3
- A new polymorph of 1-(4-nitrophenyl)-1H-benzimidazole (C13H9N3O2)
- The crystal structure of 2,2′-((1E,1′E)-(naphthalene-2,3 diylbis(azanylylidene)) bis(methanylylidene))bis(4-methylphenol), C26H22N2O2
- The crystal structure of bis(μ2-iodido)-bis(η6-benzene)-bis(iodido)-diosmium(II), C12H12I4Os2
- Redetermination of the crystal structure of bis{hydridotris(3,5-dimethylpyrazol-1-yl-κN 3)borato}copper(II), C30H44B2CuN12
- Crystal structure of (E)-3-((4-(tert-butyl)phenyl)thio)-4-hydroxypent-3-en-2-one, C15H20O2S
- Crystal structure of 2,2′-(p-tolylazanediyl)bis(1-phenylethan-1-one), C23H21NO2
- Redetermination of the crystal structure of the crystal sponge the poly[tetrakis(μ3-2,4,6-tris(pyridin-4-yl)-1,3,5-triazine)-dodecaiodidohexazinc(II) nitrobenzene solvate], C72H48I12N24Zn6⋅10(C6H5NO2)
- Crystal structure of (4′E)-6′-(diethylamino)-2-[(E)-[(6-methylpyridin-2-yl)methylidene]amino]-4′-{2-[(2E)-1,3,3-trimethyl-2,3-dihydro-1H-indol-2-ylidene]ethylidene}-1′,2,2′,3,3′,4′-hexahydrospiro[isoindole-1,9′-xanthene]-3-one, C44H45N5O2
- Crystal structure of (E)-7-fluoro-2-(3-fluorobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C17H12F2O1
- Crystal structure of tetrabutylammonium sulfanilate – 1-(diaminomethylene)thiourea (1/2)
- Crystal structure of [2,2′-{azanediyl)bis[(propane-3,1-diyl)(azanylylidene)methylylidene]} bis(3,5-dichlorophenolato)-κ2O,O′]-isothiocyanato-κN-iron(III), C21H19Cl4FeN4O2S
- Crystal structure of (4-chlorophenyl)(4-hydroxyphenyl)methanone, C13H9ClO2
- Crystal structure of 6,6′-((pentane-1,3-diylbis(azaneylylidene))bis(methaneylylidene))bis(2,4-dibromolphenolato-κ4 N,N′,O,O′)copper(II),) C19H16Br4CuN2O2
- Chlorido-(2,2′-(ethane-bis(5-methoxyphenolato))-κ4 N,N′,O,O′)manganese(III) monohydrate, C19H18Cl2CuN2O2
- Crystal structure of 2,6-di-tert-butyl-4-(4-methoxybenzylidene)cyclohexa-2,5-dien-1-one, C22H28O2
- Crystal structure of [6,6′-(((2,2-dimethylpropane-1,3-diyl)bis(azanylylidene))bis(methanylylidene))bis(2-chlorophenolato)-κ4N,N′,O,O′]copper(II)
- Crystal structure of 2-chloro-3-((thiophen-2-ylmethyl)amino)naphthalene-1,4-dione, C30H20O4N2Cl2S2
- Crystal structure of bis{hydridotris(3-trifluoromethyl-5-methylpyrazolyl-1-yl)borato-κN 3}manganese(II), C30H26B2F18MnN12
- Crystal structure of 1-(2-methylphenyl)-2-(2-methylbenzo[b]thienyl)-3,3,4,4,5,5-hexafluorocyclopent-ene, C21H14F6S
- Crystal structure of 2-(3-((carbamimidoylthio)methyl)benzyl)isothiouronium hexafluorophosphate monohydrate, C10H17F6N4OPS2
- Crystal structure of 4,5-diiodo-1,3-dimesityl-1H-1,2,3-triazol-3-ium chloride – chloroform (1/1), C21H23Cl4I2N3
- Crystal structure of azido-k1 N-{6,6′-((((methylazanediyl)bis(propane-3,1-diyl))bis(azanylylidene))bis(methanylylidene))bis(2,4-dibromophenolato)k5 N,N′,N″,O,O′}cobalt(III)-methanol (1/1)), C21H23Br4CoN6O3
- The crystal structure of 2-(4-((carbamimidoylthio)methyl)benzyl)isothiouronium hexafluorophosphate monohydrate, C10H17F6N4OPS2
- Crystal structure of 1,1′-(methane-1,1-diyl)bis(3-methyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C9H14F12N4P2
- Crystal structure of (4′E)-6′-(diethylamino)-2-[(E)-[(pyren-1-yl)methylidene]amino]-4′-{2-[(2E)-1,3,3-trimethyl-2,3-dihydro-1H-indol-2-ylidene]ethylidene}-1′,2,2′,3,3′,4′-hexahydrospiro[isoindole-1,9′-xanthene]-3-one, C54H48N4O2
- Crystal structure of poly[bis(μ2-2,6-bis(1-imidazoly)pyridine-κ2 N,N′)-bis(thiocyanato-κ1 N)copper(II)] dithiocyanate, C24H18CuN12S2
- Cones with a three-fold symmetry constructed from three hydrogen bonded theophyllinium cations that coat [FeCl4]− anions in the crystal structure of tris(theophyllinium) bis(tetrachloridoferrate(III)) chloride trihydrate, C21H33Cl9Fe2N12O9
- Crystal structure of 14-O-[(4-(4-hydroxypiperidine-1-yl)-6-methylpyrimidine-2-yl)thioacetyl]-mutilin monohydrate, C32H49N3O6S
- The crystal structure of (E)-3-chloro-2-(2-(4-methylbenzylidene)hydrazinyl)pyridine, C13H12ClN3
- The crystal structure of 4-phenyl-4-[2-(pyridine-4-carbonyl)hydrazinylidene]butanoic acid, C16H15N3O3
- The crystal structure of 6-amino-5-carboxypyridin-1-ium pentaiodide monohydrate C6H9I5N2O3
- Crystal structure of bis(μ3-oxido)-bis(μ2-2-formylbenzoato-k2O:O′)-bis(2-(dimethoxymethyl)-benzoato-κO)-oktakismethyl-tetratin(IV)
- Crystal structure of 2-((E)-(((E)-2-hydroxy-4-methylbenzylidene) hydrazineylidene)methyl)-4-methylphenol, C16H16N2O2
- Crystal structure of (E)-amino(2-((5-methylfuran-2-yl)methylene)hydrazinyl) methaniminium nitrate monohydrate, C14H26N10O10
- The crystal structure of N′-(2-chloro-6-hydroxybenzylidene)thiophene-2-carbohydrazide monohydrate, C12H11ClN2O3S
- Crystal structure of catena-poly[(μ2-1,1′-(biphenyl-4,4′-diyl)bis(1H-imidazol)-κ2N:N′)-bis(4-bromobenzoate-κ1O)zinc(II)], C64H44Br4N8O8Zn2
- The crystal structure of catena-poly[(1-(4-carboxybenzyl)pyridin-1-ium-4-carboxylato-κ1O)-(μ2-oxalato-κ4 O:O′:O″:O‴)dioxidouranium(VI)], C16H11NO10U
- Crystal structure of 3-allyl-4-(2-bromoethyl)-5-(4-methoxyphenyl)-2-phenylfuran, C22H21BrO2
- Halogen bonds in the crystal structure of 4,3′:5′,4″-terpyridine — 1,3-diiodotetrafluorobenzene (1/1), C21H11F4I2N3
- Crystal structure of 2-(1H-indol-3-yl)ethan-1-aminium 2-(4-acetylphenoxy)acetate, C20H22N2O4
- Chalcogen bonds in the crystal structure of 4,7-dibromo-2,1,3-benzoselenadiazole, C6H2Br2N2Se
- The crystal structure of 1,4-bis((1H-benzimidazol-2-yl)methyl)-piperazine-2,5-dione dihydrate, C20H22N6O4
- The crystal structure of C19H20O8
- The crystal structure of KNa3Te8O18·5H2O exhibiting a ∞2[Te4O9]2− layer
- Erratum
- Erratum to: Crystal structure of (Z)-3-(6-bromo-1H-indol-3-yl)-1,3-diphenylprop-2-en-1-one, C23H16BrNO
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of (E)-7-hydroxy-2-((6-methoxypyridin-3-yl)methylene)-3, 4-dihydronaphthalen-1(2H)-one, C17H15NO3
- Crystal structure of (E)-7-methoxy-2-((2-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1 (2H)-one, C18H17NO3
- The crystal structure of N 6,N 6′-di(pyridin-2-yl)-[2,2′-bipyridine]-6,6′-diamine, C20H16N6
- The crystal structure of {N 1,N 2-bis[2,4-dimethyl-6-(4-(tert-butyl)phenyl)(phenyl)methyl]acenaphthylene-1,2-diimino-κ2 N, N′}-dibromido-nickel(II) – dichloromethane(1/2), C64H64Br2Cl4N2Ni
- Synthesis and crystal structure of nonacarbonyltris[(2-thia-1,3,5-triaza-7-phosphatricylco[3.3.1.1]decane-κ1 P)-2,2-dioxide]triruthenium(0) – acetonitrile (7/6), C25.71H32.57N9.86O15P3S3Ru3
- A new polymorph of 1-(4-nitrophenyl)-1H-benzimidazole (C13H9N3O2)
- The crystal structure of 2,2′-((1E,1′E)-(naphthalene-2,3 diylbis(azanylylidene)) bis(methanylylidene))bis(4-methylphenol), C26H22N2O2
- The crystal structure of bis(μ2-iodido)-bis(η6-benzene)-bis(iodido)-diosmium(II), C12H12I4Os2
- Redetermination of the crystal structure of bis{hydridotris(3,5-dimethylpyrazol-1-yl-κN 3)borato}copper(II), C30H44B2CuN12
- Crystal structure of (E)-3-((4-(tert-butyl)phenyl)thio)-4-hydroxypent-3-en-2-one, C15H20O2S
- Crystal structure of 2,2′-(p-tolylazanediyl)bis(1-phenylethan-1-one), C23H21NO2
- Redetermination of the crystal structure of the crystal sponge the poly[tetrakis(μ3-2,4,6-tris(pyridin-4-yl)-1,3,5-triazine)-dodecaiodidohexazinc(II) nitrobenzene solvate], C72H48I12N24Zn6⋅10(C6H5NO2)
- Crystal structure of (4′E)-6′-(diethylamino)-2-[(E)-[(6-methylpyridin-2-yl)methylidene]amino]-4′-{2-[(2E)-1,3,3-trimethyl-2,3-dihydro-1H-indol-2-ylidene]ethylidene}-1′,2,2′,3,3′,4′-hexahydrospiro[isoindole-1,9′-xanthene]-3-one, C44H45N5O2
- Crystal structure of (E)-7-fluoro-2-(3-fluorobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C17H12F2O1
- Crystal structure of tetrabutylammonium sulfanilate – 1-(diaminomethylene)thiourea (1/2)
- Crystal structure of [2,2′-{azanediyl)bis[(propane-3,1-diyl)(azanylylidene)methylylidene]} bis(3,5-dichlorophenolato)-κ2O,O′]-isothiocyanato-κN-iron(III), C21H19Cl4FeN4O2S
- Crystal structure of (4-chlorophenyl)(4-hydroxyphenyl)methanone, C13H9ClO2
- Crystal structure of 6,6′-((pentane-1,3-diylbis(azaneylylidene))bis(methaneylylidene))bis(2,4-dibromolphenolato-κ4 N,N′,O,O′)copper(II),) C19H16Br4CuN2O2
- Chlorido-(2,2′-(ethane-bis(5-methoxyphenolato))-κ4 N,N′,O,O′)manganese(III) monohydrate, C19H18Cl2CuN2O2
- Crystal structure of 2,6-di-tert-butyl-4-(4-methoxybenzylidene)cyclohexa-2,5-dien-1-one, C22H28O2
- Crystal structure of [6,6′-(((2,2-dimethylpropane-1,3-diyl)bis(azanylylidene))bis(methanylylidene))bis(2-chlorophenolato)-κ4N,N′,O,O′]copper(II)
- Crystal structure of 2-chloro-3-((thiophen-2-ylmethyl)amino)naphthalene-1,4-dione, C30H20O4N2Cl2S2
- Crystal structure of bis{hydridotris(3-trifluoromethyl-5-methylpyrazolyl-1-yl)borato-κN 3}manganese(II), C30H26B2F18MnN12
- Crystal structure of 1-(2-methylphenyl)-2-(2-methylbenzo[b]thienyl)-3,3,4,4,5,5-hexafluorocyclopent-ene, C21H14F6S
- Crystal structure of 2-(3-((carbamimidoylthio)methyl)benzyl)isothiouronium hexafluorophosphate monohydrate, C10H17F6N4OPS2
- Crystal structure of 4,5-diiodo-1,3-dimesityl-1H-1,2,3-triazol-3-ium chloride – chloroform (1/1), C21H23Cl4I2N3
- Crystal structure of azido-k1 N-{6,6′-((((methylazanediyl)bis(propane-3,1-diyl))bis(azanylylidene))bis(methanylylidene))bis(2,4-dibromophenolato)k5 N,N′,N″,O,O′}cobalt(III)-methanol (1/1)), C21H23Br4CoN6O3
- The crystal structure of 2-(4-((carbamimidoylthio)methyl)benzyl)isothiouronium hexafluorophosphate monohydrate, C10H17F6N4OPS2
- Crystal structure of 1,1′-(methane-1,1-diyl)bis(3-methyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C9H14F12N4P2
- Crystal structure of (4′E)-6′-(diethylamino)-2-[(E)-[(pyren-1-yl)methylidene]amino]-4′-{2-[(2E)-1,3,3-trimethyl-2,3-dihydro-1H-indol-2-ylidene]ethylidene}-1′,2,2′,3,3′,4′-hexahydrospiro[isoindole-1,9′-xanthene]-3-one, C54H48N4O2
- Crystal structure of poly[bis(μ2-2,6-bis(1-imidazoly)pyridine-κ2 N,N′)-bis(thiocyanato-κ1 N)copper(II)] dithiocyanate, C24H18CuN12S2
- Cones with a three-fold symmetry constructed from three hydrogen bonded theophyllinium cations that coat [FeCl4]− anions in the crystal structure of tris(theophyllinium) bis(tetrachloridoferrate(III)) chloride trihydrate, C21H33Cl9Fe2N12O9
- Crystal structure of 14-O-[(4-(4-hydroxypiperidine-1-yl)-6-methylpyrimidine-2-yl)thioacetyl]-mutilin monohydrate, C32H49N3O6S
- The crystal structure of (E)-3-chloro-2-(2-(4-methylbenzylidene)hydrazinyl)pyridine, C13H12ClN3
- The crystal structure of 4-phenyl-4-[2-(pyridine-4-carbonyl)hydrazinylidene]butanoic acid, C16H15N3O3
- The crystal structure of 6-amino-5-carboxypyridin-1-ium pentaiodide monohydrate C6H9I5N2O3
- Crystal structure of bis(μ3-oxido)-bis(μ2-2-formylbenzoato-k2O:O′)-bis(2-(dimethoxymethyl)-benzoato-κO)-oktakismethyl-tetratin(IV)
- Crystal structure of 2-((E)-(((E)-2-hydroxy-4-methylbenzylidene) hydrazineylidene)methyl)-4-methylphenol, C16H16N2O2
- Crystal structure of (E)-amino(2-((5-methylfuran-2-yl)methylene)hydrazinyl) methaniminium nitrate monohydrate, C14H26N10O10
- The crystal structure of N′-(2-chloro-6-hydroxybenzylidene)thiophene-2-carbohydrazide monohydrate, C12H11ClN2O3S
- Crystal structure of catena-poly[(μ2-1,1′-(biphenyl-4,4′-diyl)bis(1H-imidazol)-κ2N:N′)-bis(4-bromobenzoate-κ1O)zinc(II)], C64H44Br4N8O8Zn2
- The crystal structure of catena-poly[(1-(4-carboxybenzyl)pyridin-1-ium-4-carboxylato-κ1O)-(μ2-oxalato-κ4 O:O′:O″:O‴)dioxidouranium(VI)], C16H11NO10U
- Crystal structure of 3-allyl-4-(2-bromoethyl)-5-(4-methoxyphenyl)-2-phenylfuran, C22H21BrO2
- Halogen bonds in the crystal structure of 4,3′:5′,4″-terpyridine — 1,3-diiodotetrafluorobenzene (1/1), C21H11F4I2N3
- Crystal structure of 2-(1H-indol-3-yl)ethan-1-aminium 2-(4-acetylphenoxy)acetate, C20H22N2O4
- Chalcogen bonds in the crystal structure of 4,7-dibromo-2,1,3-benzoselenadiazole, C6H2Br2N2Se
- The crystal structure of 1,4-bis((1H-benzimidazol-2-yl)methyl)-piperazine-2,5-dione dihydrate, C20H22N6O4
- The crystal structure of C19H20O8
- The crystal structure of KNa3Te8O18·5H2O exhibiting a ∞2[Te4O9]2− layer
- Erratum
- Erratum to: Crystal structure of (Z)-3-(6-bromo-1H-indol-3-yl)-1,3-diphenylprop-2-en-1-one, C23H16BrNO

