Abstract
C132H98I12N34O20Zn6, monoclinic, P21 c, a = 34.97800(15) Å, b = 15.02541(6) Å, c = 30.13247(12) Å, β = 101.9033(4)°, V = 15,495.86(11) Å3, Z = 4, R gt (F) = 0.0501, wR ref(F 2) = 0.1329, T = 95 K.
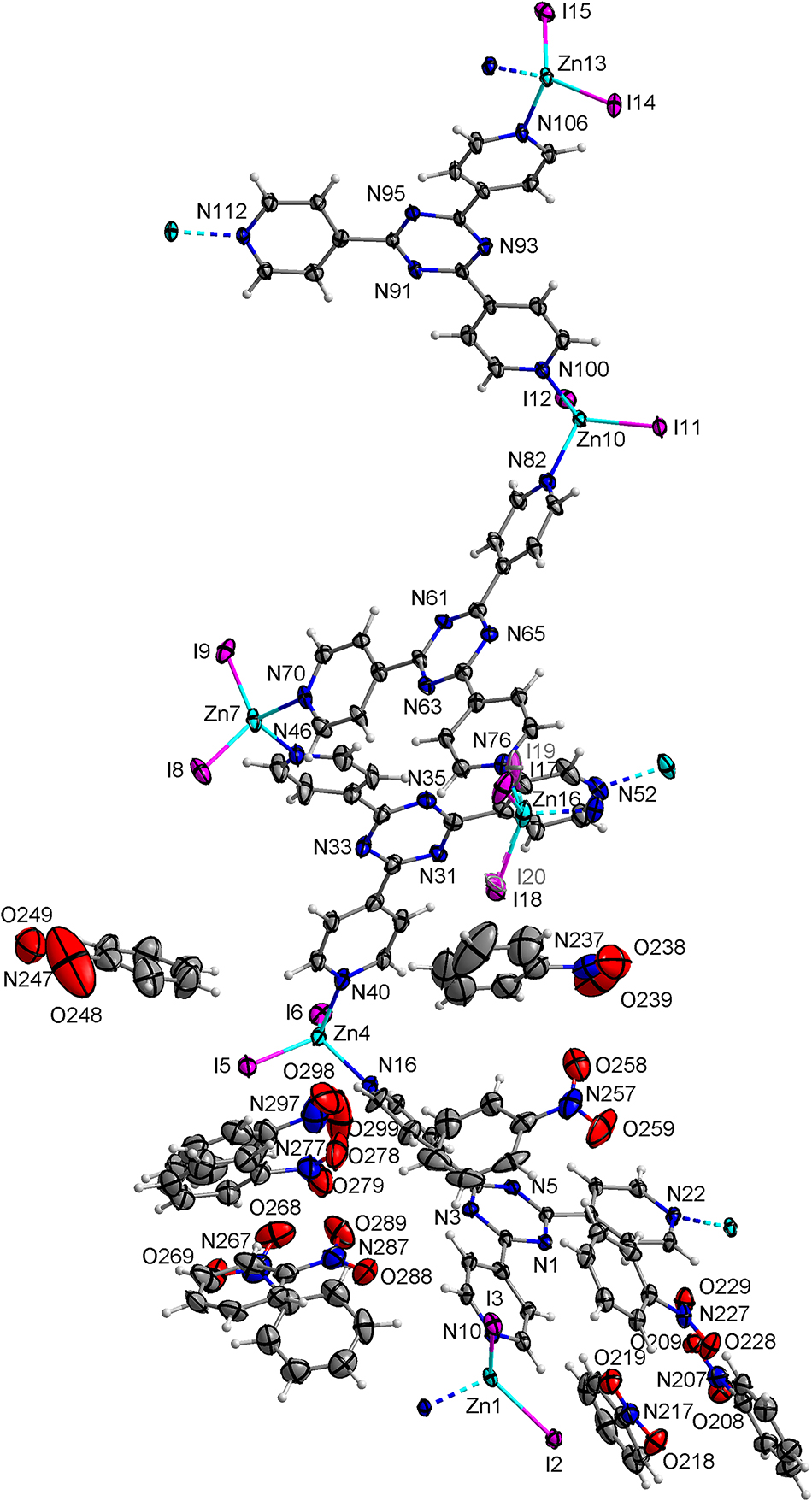
Asymmetric unit of the title crystal structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Prism, colourless |
| Size: | 0.21 × 0.15 × 0.08 mm |
| Wavelength: | Cu Kα radiation (1.54184 Å) |
| μ: | 20.39 mm−1 |
| Diffractometer, scan mode: | SuperNova, φ and ω-scans |
| θ max, completeness: | 74.3°, >99% |
| N(hkl)measured, N(hkl)unique, R int: | 268,201, 31,402, 0.043 |
| Criterion for I obs, N(hkl) gt: | I obs > 2 σ(I obs), 30257 |
| N(param)refined: | 1857 |
| Programs: | CrysAlisPRO[1], SUPERFLIP [2], CRYSTALS [3], DIAMOND [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| Zn1 | 0.91298 (2) | 0.14033 (5) | 0.26527 (3) | 0.0187 |
| I2 | 0.973586 (12) | 0.04184 (3) | 0.288425 (14) | 0.0242 |
| I3 | 0.867226 (13) | 0.12167 (3) | 0.188376 (14) | 0.0266 |
| Zn4 | 0.61592 (3) | 0.32508 (6) | 0.46251 (3) | 0.0246 |
| I5 | 0.627886 (14) | 0.47197 (3) | 0.424449 (17) | 0.0337 |
| I6 | 0.600615 (14) | 0.30765 (4) | 0.540701 (15) | 0.0342 |
| Zn7 | 0.33546 (3) | 0.35761 (6) | 0.09556 (3) | 0.0253 |
| I8 | 0.367867 (17) | 0.47845 (3) | 0.057323 (18) | 0.0410 |
| I9 | 0.275953 (14) | 0.38338 (3) | 0.130870 (15) | 0.0322 |
| Zn10 | 0.13381 (2) | −0.34865 (6) | −0.06260 (3) | 0.0200 |
| I11 | 0.151423 (13) | −0.49301 (3) | −0.098684 (16) | 0.0284 |
| I12 | 0.118202 (13) | −0.33546 (3) | 0.015898 (14) | 0.0263 |
| Zn13 | −0.16209 (2) | −0.38479 (5) | −0.42105 (3) | 0.0182 |
| I14 | −0.130337 (13) | −0.49828 (3) | −0.464480 (14) | 0.0265 |
| I15 | −0.213739 (13) | −0.40804 (3) | −0.373365 (14) | 0.0269 |
| Zn16 | 0.44177 (3) | −0.09036 (7) | −0.22754 (4) | 0.0356 |
| I17a | 0.41454 (7) | −0.05478 (17) | −0.30916 (5) | 0.0494 |
| I18a | 0.50263 (6) | −0.01339 (11) | −0.17795 (9) | 0.0458 |
| I19b | 0.3998 (4) | −0.0833 (4) | −0.31403 (17) | 0.0466 |
| I20b | 0.4943 (3) | −0.0153 (5) | −0.1955 (4) | 0.0495 |
| N1 | 0.81681 (15) | −0.0221 (4) | 0.43007 (18) | 0.0187 |
| C2 | 0.80715 (18) | 0.0520 (4) | 0.4056 (2) | 0.0173 |
| N3 | 0.77796 (15) | 0.1070 (4) | 0.40936 (18) | 0.0199 |
| C4 | 0.75995 (18) | 0.0863 (4) | 0.4430 (2) | 0.0186 |
| N5 | 0.76838 (15) | 0.0168 (4) | 0.47145 (18) | 0.0190 |
| C6 | 0.79574 (17) | −0.0370 (4) | 0.4613 (2) | 0.0162 |
| C7 | 0.83145 (19) | 0.0745 (4) | 0.3723 (2) | 0.0198 |
| C8 | 0.86822 (18) | 0.0381 (4) | 0.3769 (2) | 0.0191 |
| C9 | 0.89128 (19) | 0.0613 (4) | 0.3469 (2) | 0.0194 |
| N10 | 0.87908 (16) | 0.1177 (4) | 0.31234 (17) | 0.0197 |
| C11 | 0.8430 (2) | 0.1523 (4) | 0.3072 (2) | 0.0223 |
| C12 | 0.8183 (2) | 0.1333 (4) | 0.3366 (2) | 0.0221 |
| C13 | 0.72701 (18) | 0.1447 (4) | 0.4490 (2) | 0.0208 |
| C14 | 0.7063 (2) | 0.1926 (6) | 0.4129 (2) | 0.0324 |
| C15 | 0.6750 (2) | 0.2426 (6) | 0.4190 (3) | 0.0363 |
| N16 | 0.66355 (17) | 0.2478 (4) | 0.45855 (19) | 0.0255 |
| C17 | 0.68401 (19) | 0.2020 (5) | 0.4937 (2) | 0.0224 |
| C18 | 0.71564 (19) | 0.1491 (4) | 0.4904 (2) | 0.0229 |
| C19 | 0.80298 (18) | −0.1221 (4) | 0.4869 (2) | 0.0169 |
| C20 | 0.83105 (19) | −0.1808 (4) | 0.4778 (2) | 0.0201 |
| C21 | 0.83804 (19) | −0.2596 (4) | 0.5027 (2) | 0.0206 |
| N22 | 0.81867 (15) | −0.2792 (4) | 0.53568 (17) | 0.0194 |
| C23 | 0.79103 (19) | −0.2232 (4) | 0.5437 (2) | 0.0218 |
| C24 | 0.78239 (18) | −0.1439 (4) | 0.5200 (2) | 0.0183 |
| N31 | 0.47994 (17) | 0.0446 (4) | 0.32453 (19) | 0.0240 |
| C32 | 0.4820 (2) | 0.1335 (5) | 0.3218 (2) | 0.0257 |
| N33 | 0.46189 (18) | 0.1849 (4) | 0.2892 (2) | 0.0303 |
| C34 | 0.4374 (2) | 0.1409 (5) | 0.2572 (2) | 0.0260 |
| N35 | 0.43248 (17) | 0.0524 (4) | 0.25536 (19) | 0.0253 |
| C36 | 0.4553 (2) | 0.0079 (5) | 0.2895 (2) | 0.0252 |
| C37 | 0.5114 (2) | 0.1791 (5) | 0.3575 (2) | 0.0257 |
| C38 | 0.5317 (2) | 0.1337 (5) | 0.3952 (2) | 0.0267 |
| C39 | 0.5611 (2) | 0.1779 (5) | 0.4246 (2) | 0.0272 |
| N40 | 0.57116 (17) | 0.2630 (4) | 0.41790 (19) | 0.0256 |
| C41 | 0.5509 (2) | 0.3057 (5) | 0.3815 (3) | 0.0308 |
| C42 | 0.5206 (2) | 0.2675 (5) | 0.3513 (3) | 0.0328 |
| C43 | 0.4145 (2) | 0.1918 (5) | 0.2184 (2) | 0.0276 |
| C44 | 0.4037 (3) | 0.2776 (6) | 0.2241 (3) | 0.0499 |
| C45 | 0.3816 (3) | 0.3212 (6) | 0.1876 (3) | 0.0459 |
| N46 | 0.37206 (18) | 0.2867 (4) | 0.1454 (2) | 0.0294 |
| C47 | 0.3814 (2) | 0.2019 (5) | 0.1417 (3) | 0.0342 |
| C48 | 0.4024 (2) | 0.1513 (5) | 0.1769 (3) | 0.0334 |
| C49 | 0.4532 (2) | −0.0899 (5) | 0.2866 (2) | 0.0260 |
| C50 | 0.4320 (2) | −0.1306 (5) | 0.2480 (3) | 0.0357 |
| C51 | 0.4305 (3) | −0.2215 (6) | 0.2451 (3) | 0.0406 |
| N52 | 0.4495 (2) | −0.2746 (4) | 0.2782 (2) | 0.0323 |
| C53 | 0.4707 (2) | −0.2352 (5) | 0.3151 (3) | 0.0364 |
| C54 | 0.4735 (3) | −0.1457 (5) | 0.3208 (3) | 0.0369 |
| N61 | 0.27940 (16) | −0.0237 (4) | −0.03647 (19) | 0.0215 |
| C62 | 0.30616 (19) | 0.0360 (4) | −0.0422 (2) | 0.0198 |
| N63 | 0.32769 (16) | 0.0343 (4) | −0.07355 (17) | 0.0194 |
| C64 | 0.32147 (19) | −0.0376 (4) | −0.1007 (2) | 0.0199 |
| N65 | 0.29434 (15) | −0.1002 (4) | −0.09967 (18) | 0.0203 |
| C66 | 0.27429 (18) | −0.0890 (4) | −0.0670 (2) | 0.0206 |
| C67 | 0.31135 (19) | 0.1140 (4) | −0.0112 (2) | 0.0199 |
| C68 | 0.28425 (19) | 0.1342 (4) | 0.0148 (2) | 0.0229 |
| C69 | 0.2906 (2) | 0.2071 (5) | 0.0435 (2) | 0.0232 |
| N70 | 0.32279 (18) | 0.2575 (4) | 0.04785 (18) | 0.0231 |
| C71 | 0.3491 (2) | 0.2382 (5) | 0.0224 (2) | 0.0296 |
| C72 | 0.3446 (2) | 0.1676 (5) | −0.0076 (2) | 0.0260 |
| C73 | 0.34706 (19) | −0.0483 (4) | −0.1337 (2) | 0.0202 |
| C74 | 0.3712 (2) | 0.0212 (5) | −0.1412 (2) | 0.0250 |
| C75 | 0.3971 (2) | 0.0058 (5) | −0.1692 (2) | 0.0275 |
| N76 | 0.39949 (17) | −0.0725 (4) | −0.18991 (19) | 0.0250 |
| C77 | 0.3746 (2) | −0.1377 (5) | −0.1839 (2) | 0.0254 |
| C78 | 0.3484 (2) | −0.1289 (4) | −0.1564 (2) | 0.0235 |
| C79 | 0.24220 (18) | −0.1527 (4) | −0.0654 (2) | 0.0196 |
| C80 | 0.2306 (2) | −0.2145 (5) | −0.1000 (2) | 0.0258 |
| C81 | 0.1991 (2) | −0.2686 (4) | −0.0980 (2) | 0.0242 |
| N82 | 0.18009 (16) | −0.2662 (4) | −0.06376 (19) | 0.0220 |
| C83 | 0.1921 (2) | −0.2071 (5) | −0.0302 (2) | 0.0257 |
| C84 | 0.2224 (2) | −0.1497 (5) | −0.0300 (2) | 0.0261 |
| N91 | −0.01728 (15) | −0.0862 (3) | −0.18907 (17) | 0.0176 |
| C92 | −0.00901 (17) | −0.1724 (4) | −0.19574 (19) | 0.0149 |
| N93 | −0.02679 (15) | −0.2223 (3) | −0.23036 (17) | 0.0175 |
| C94 | −0.05460 (18) | −0.1822 (4) | −0.2596 (2) | 0.0171 |
| N95 | −0.06598 (15) | −0.0968 (4) | −0.25678 (18) | 0.0191 |
| C96 | −0.04565 (17) | −0.0523 (4) | −0.2212 (2) | 0.0159 |
| C97 | 0.02370 (18) | −0.2148 (4) | −0.16310 (19) | 0.0164 |
| C98 | 0.0467 (2) | −0.1661 (4) | −0.1287 (2) | 0.0229 |
| C99 | 0.0779 (2) | −0.2086 (4) | −0.1004 (2) | 0.0229 |
| N100 | 0.08682 (15) | −0.2936 (3) | −0.10606 (17) | 0.0182 |
| C101 | 0.0643 (2) | −0.3405 (4) | −0.1396 (2) | 0.0247 |
| C102 | 0.03235 (19) | −0.3037 (4) | −0.1684 (2) | 0.0232 |
| C103 | −0.07523 (18) | −0.2326 (4) | −0.3001 (2) | 0.0163 |
| C104 | −0.07391 (18) | −0.3249 (4) | −0.3026 (2) | 0.0195 |
| C105 | −0.09645 (19) | −0.3677 (4) | −0.3395 (2) | 0.0193 |
| N106 | −0.11880 (16) | −0.3219 (3) | −0.37427 (17) | 0.0181 |
| C107 | −0.1175 (2) | −0.2329 (4) | −0.3728 (2) | 0.0237 |
| C108 | −0.09659 (19) | −0.1858 (4) | −0.3371 (2) | 0.0204 |
| C109 | −0.05497 (19) | 0.0444 (4) | −0.2204 (2) | 0.0196 |
| C110 | −0.08863 (19) | 0.0760 (4) | −0.2490 (2) | 0.0235 |
| C111 | −0.0961 (2) | 0.1663 (4) | −0.2502 (2) | 0.0236 |
| N112 | −0.07208 (15) | 0.2262 (3) | −0.22618 (18) | 0.0194 |
| C113 | −0.03946 (19) | 0.1952 (4) | −0.1982 (2) | 0.0213 |
| C114 | −0.03016 (19) | 0.1051 (5) | −0.1943 (2) | 0.0221 |
| C201 | 0.9811 (2) | −0.3426 (5) | 0.5336 (3) | 0.0302 |
| C202 | 1.0185 (2) | −0.3715 (6) | 0.5513 (3) | 0.0349 |
| C203 | 1.0403 (2) | −0.4044 (6) | 0.5222 (3) | 0.0424 |
| C204 | 1.0251 (3) | −0.4071 (6) | 0.4756 (3) | 0.0419 |
| C205 | 0.9871 (3) | −0.3768 (6) | 0.4587 (3) | 0.0372 |
| C206 | 0.9647 (2) | −0.3441 (5) | 0.4877 (3) | 0.0329 |
| N207 | 0.95749 (19) | −0.3061 (4) | 0.5644 (2) | 0.0324 |
| O208 | 0.97070 (16) | −0.3098 (4) | 0.60555 (17) | 0.0364 |
| O209 | 0.92586 (15) | −0.2720 (4) | 0.54765 (18) | 0.0339 |
| C211 | 0.9731 (2) | −0.1075 (5) | 0.4824 (2) | 0.0270 |
| C212 | 0.9421 (2) | −0.0712 (5) | 0.4982 (3) | 0.0366 |
| C213 | 0.9467 (3) | −0.0576 (6) | 0.5446 (3) | 0.0511 |
| C214 | 0.9811 (4) | −0.0806 (6) | 0.5734 (3) | 0.0548 |
| C215 | 1.0115 (3) | −0.1172 (6) | 0.5571 (3) | 0.0487 |
| C216 | 1.0082 (2) | −0.1301 (5) | 0.5112 (3) | 0.0354 |
| N217 | 0.96967 (19) | −0.1200 (4) | 0.4337 (2) | 0.0300 |
| O218 | 0.99626 (19) | −0.1567 (5) | 0.4207 (2) | 0.0492 |
| O219 | 0.93966 (17) | −0.0934 (4) | 0.40822 (19) | 0.0401 |
| C221 | 0.8724 (2) | −0.1916 (5) | 0.3352 (2) | 0.0251 |
| C222 | 0.8896 (2) | −0.1578 (5) | 0.3013 (2) | 0.0268 |
| C223 | 0.8673 (2) | −0.1018 (5) | 0.2694 (2) | 0.0302 |
| C224 | 0.8290 (2) | −0.0828 (5) | 0.2713 (2) | 0.0309 |
| C225 | 0.8128 (2) | −0.1168 (5) | 0.3054 (3) | 0.0309 |
| C226 | 0.8343 (2) | −0.1725 (5) | 0.3384 (3) | 0.0310 |
| N227 | 0.89567 (19) | −0.2513 (4) | 0.3690 (2) | 0.0298 |
| O228 | 0.92387 (18) | −0.2892 (4) | 0.3597 (2) | 0.0411 |
| O229 | 0.88564 (18) | −0.2601 (4) | 0.40567 (19) | 0.0405 |
| C231 | 0.5974 (3) | −0.1173 (8) | 0.6068 (4) | 0.0579 |
| C232 | 0.5772 (6) | −0.1012 (13) | 0.5646 (6) | 0.1113 |
| C233 | 0.5712 (8) | −0.006 (2) | 0.5556 (7) | 0.1630 |
| C234 | 0.5840 (6) | 0.0555 (11) | 0.5898 (9) | 0.1113 |
| C235 | 0.6049 (5) | 0.0308 (11) | 0.6340 (7) | 0.0957 |
| C236 | 0.6093 (3) | −0.0570 (9) | 0.6402 (5) | 0.0659 |
| N237 | 0.6052 (3) | −0.2166 (9) | 0.6181 (5) | 0.0848 |
| O238 | 0.6037 (4) | −0.2607 (9) | 0.5829 (7) | 0.1623 |
| O239 | 0.6149 (4) | −0.2334 (12) | 0.6575 (6) | 0.1503 |
| C241 | 0.5215 (4) | 0.6896 (9) | 0.4909 (5) | 0.0757 |
| C242 | 0.5420 (5) | 0.6211 (9) | 0.4728 (4) | 0.0782 |
| C243 | 0.5523 (4) | 0.5488 (10) | 0.5013 (5) | 0.0818 |
| C244 | 0.5434 (4) | 0.5426 (9) | 0.5427 (4) | 0.0672 |
| C245 | 0.5257 (4) | 0.6090 (10) | 0.5599 (5) | 0.0794 |
| C246 | 0.5149 (3) | 0.6844 (9) | 0.5345 (5) | 0.0677 |
| N247 | 0.5108 (5) | 0.7647 (9) | 0.4599 (8) | 0.1356 |
| O248 | 0.5160 (8) | 0.7653 (12) | 0.4214 (4) | 0.2356 |
| O249 | 0.4984 (4) | 0.8302 (9) | 0.4802 (6) | 0.1367 |
| C251 | 0.7099 (2) | −0.0120 (6) | 0.2412 (3) | 0.0390 |
| C252 | 0.6905 (2) | 0.0391 (6) | 0.2677 (3) | 0.0350 |
| C253 | 0.7000 (3) | 0.1276 (7) | 0.2724 (3) | 0.0520 |
| C254 | 0.7265 (3) | 0.1668 (9) | 0.2532 (4) | 0.0652 |
| C255 | 0.7459 (3) | 0.1178 (10) | 0.2268 (4) | 0.0689 |
| C256 | 0.7387 (3) | 0.0274 (10) | 0.2202 (3) | 0.0592 |
| N257 | 0.7011 (3) | −0.1060 (6) | 0.2357 (3) | 0.0545 |
| O258 | 0.6706 (3) | −0.1325 (5) | 0.2437 (2) | 0.0635 |
| O259 | 0.7254 (3) | −0.1546 (7) | 0.2225 (4) | 0.1007 |
| C261 | 0.8254 (3) | 0.4816 (6) | 0.4036 (3) | 0.0369 |
| C262 | 0.8253 (3) | 0.3902 (7) | 0.4008 (3) | 0.0556 |
| C263 | 0.8562 (4) | 0.3501 (7) | 0.3843 (3) | 0.0587 |
| C264 | 0.8830 (3) | 0.4042 (8) | 0.3689 (3) | 0.0523 |
| C265 | 0.8819 (3) | 0.4944 (8) | 0.3728 (4) | 0.0603 |
| C266 | 0.8528 (3) | 0.5331 (7) | 0.3899 (4) | 0.0560 |
| N267 | 0.7942 (3) | 0.5273 (7) | 0.4216 (3) | 0.0523 |
| O268 | 0.7700 (2) | 0.4795 (7) | 0.4342 (3) | 0.0776 |
| O269 | 0.7937 (3) | 0.6086 (6) | 0.4216 (3) | 0.0740 |
| C271 | 0.7070 (3) | 0.5067 (6) | 0.1251 (3) | 0.0432 |
| C272 | 0.6913 (3) | 0.5286 (7) | 0.0810 (3) | 0.0508 |
| C273 | 0.6885 (3) | 0.6171 (7) | 0.0693 (3) | 0.0533 |
| C274 | 0.7005 (3) | 0.6815 (7) | 0.1013 (4) | 0.0540 |
| C275 | 0.7153 (3) | 0.6610 (7) | 0.1460 (3) | 0.0506 |
| C276 | 0.7190 (3) | 0.5720 (6) | 0.1581 (3) | 0.0436 |
| N277 | 0.7104 (3) | 0.4130 (6) | 0.1380 (3) | 0.0550 |
| O278 | 0.7002 (3) | 0.3567 (6) | 0.1086 (3) | 0.0881 |
| O279 | 0.7233 (3) | 0.3937 (6) | 0.1785 (3) | 0.0814 |
| C281 | 0.7968 (2) | 0.4865 (6) | 0.2519 (3) | 0.0422 |
| C282 | 0.8133 (3) | 0.5123 (6) | 0.2178 (4) | 0.0464 |
| C283 | 0.8178 (3) | 0.6023 (8) | 0.2108 (4) | 0.0551 |
| C284 | 0.8059 (3) | 0.6640 (7) | 0.2392 (4) | 0.0579 |
| C285 | 0.7888 (3) | 0.6374 (8) | 0.2735 (4) | 0.0643 |
| C286 | 0.7841 (3) | 0.5474 (7) | 0.2813 (3) | 0.0523 |
| N287 | 0.7917 (2) | 0.3907 (6) | 0.2600 (3) | 0.0531 |
| O288 | 0.8070 (2) | 0.3386 (6) | 0.2389 (3) | 0.0732 |
| O289 | 0.7726 (3) | 0.3686 (6) | 0.2879 (3) | 0.0709 |
| C291 | 0.6809 (3) | 0.4711 (7) | 0.2924 (4) | 0.0569 |
| C292 | 0.6746 (3) | 0.5264 (8) | 0.2567 (4) | 0.0605 |
| C293 | 0.6819 (4) | 0.6142 (9) | 0.2635 (5) | 0.0687 |
| C294 | 0.6960 (4) | 0.6482 (8) | 0.3047 (6) | 0.0729 |
| C295 | 0.7036 (4) | 0.5921 (13) | 0.3413 (4) | 0.0860 |
| C296 | 0.6960 (4) | 0.4993 (10) | 0.3351 (4) | 0.0756 |
| N297 | 0.6726 (4) | 0.3727 (8) | 0.2841 (4) | 0.0806 |
| O298 | 0.6603 (3) | 0.3469 (8) | 0.2479 (4) | 0.1029 |
| O299 | 0.6803 (5) | 0.3265 (7) | 0.3189 (5) | 0.1357 |
| H91 | 0.9168 | 0.0368 | 0.3508 | 0.0267* |
| H81 | 0.8774 | −0.0031 | 0.4006 | 0.0234* |
| H181 | 0.7293 | 0.1169 | 0.5159 | 0.0296* |
| H171 | 0.6765 | 0.2062 | 0.5222 | 0.0280* |
| H411 | 0.5582 | 0.3651 | 0.3763 | 0.0391* |
| H421 | 0.5062 | 0.3003 | 0.3264 | 0.0415* |
| H381 | 0.5251 | 0.0740 | 0.4009 | 0.0335* |
| H391 | 0.5754 | 0.1468 | 0.4502 | 0.0361* |
| H481 | 0.4083 | 0.0905 | 0.1728 | 0.0404* |
| H471 | 0.3733 | 0.1740 | 0.1131 | 0.0422* |
| H691 | 0.2716 | 0.2218 | 0.0608 | 0.0297* |
| H681 | 0.2616 | 0.0986 | 0.0131 | 0.0277* |
| H721 | 0.3635 | 0.1552 | −0.0253 | 0.0323* |
| H711 | 0.3718 | 0.2743 | 0.0252 | 0.0384* |
| H781 | 0.3314 | −0.1765 | −0.1526 | 0.0297* |
| H771 | 0.3754 | −0.1923 | −0.1995 | 0.0320* |
| H531 | 0.4846 | −0.2724 | 0.3385 | 0.0429* |
| H541 | 0.4893 | −0.1212 | 0.3474 | 0.0465* |
| H501 | 0.4190 | −0.0954 | 0.2232 | 0.0432* |
| H511 | 0.4150 | −0.2481 | 0.2189 | 0.0484* |
| H751 | 0.4140 | 0.0526 | −0.1742 | 0.0331* |
| H741 | 0.3699 | 0.0775 | −0.1272 | 0.0301* |
| H801 | 0.2437 | −0.2192 | −0.1245 | 0.0342* |
| H811 | 0.1906 | −0.3098 | −0.1219 | 0.0300* |
| H991 | 0.0931 | −0.1763 | −0.0759 | 0.0264* |
| H981 | 0.0415 | −0.1049 | −0.1247 | 0.0294* |
| H1021 | 0.0164 | −0.3386 | −0.1913 | 0.0304* |
| H1011 | 0.0707 | −0.4009 | −0.1439 | 0.0301* |
| H1141 | −0.0072 | 0.0852 | −0.1742 | 0.0272* |
| H1131 | −0.0224 | 0.2368 | −0.1804 | 0.0272* |
| H1111 | −0.1197 | 0.1871 | −0.2688 | 0.0300* |
| H1101 | −0.1060 | 0.0362 | −0.2677 | 0.0299* |
| H1081 | −0.0964 | −0.1226 | −0.3374 | 0.0245* |
| H1071 | −0.1321 | −0.2006 | −0.3978 | 0.0311* |
| H211 | 0.8572 | −0.3000 | 0.4965 | 0.0272* |
| H201 | 0.8451 | −0.1674 | 0.4548 | 0.0255* |
| H241 | 0.7627 | −0.1054 | 0.5264 | 0.0230* |
| H231 | 0.7770 | −0.2378 | 0.5666 | 0.0263* |
| H1051 | −0.0966 | −0.4309 | −0.3403 | 0.0252* |
| H1041 | −0.0574 | −0.3577 | −0.2793 | 0.0262* |
| H831 | 0.1789 | −0.2050 | −0.0056 | 0.0332* |
| H841 | 0.2299 | −0.1080 | −0.0061 | 0.0321* |
| H451 | 0.3722 | 0.3790 | 0.1924 | 0.0555* |
| H441 | 0.4112 | 0.3063 | 0.2526 | 0.0545* |
| H151 | 0.6606 | 0.2745 | 0.3938 | 0.0468* |
| H141 | 0.7138 | 0.1914 | 0.3843 | 0.0405* |
| H121 | 0.7931 | 0.1595 | 0.3322 | 0.0269* |
| H111 | 0.8341 | 0.1912 | 0.2824 | 0.0297* |
| H2121 | 0.9185 | −0.0553 | 0.4778 | 0.0465* |
| H2131 | 0.9259 | −0.0325 | 0.5563 | 0.0630* |
| H2141 | 0.9840 | −0.0708 | 0.6051 | 0.0668* |
| H2151 | 1.0348 | −0.1341 | 0.5776 | 0.0624* |
| H2161 | 1.0294 | −0.1537 | 0.4997 | 0.0423* |
| H2021 | 1.0289 | −0.3681 | 0.5830 | 0.0436* |
| H2031 | 1.0659 | −0.4266 | 0.5340 | 0.0547* |
| H2041 | 1.0405 | −0.4288 | 0.4554 | 0.0536* |
| H2051 | 0.9766 | −0.3784 | 0.4270 | 0.0461* |
| H2061 | 0.9388 | −0.3237 | 0.4768 | 0.0424* |
| H2221 | 0.9156 | −0.1735 | 0.2996 | 0.0335* |
| H2231 | 0.8786 | −0.0754 | 0.2465 | 0.0383* |
| H2241 | 0.8138 | −0.0457 | 0.2489 | 0.0373* |
| H2251 | 0.7865 | −0.1029 | 0.3063 | 0.0393* |
| H2261 | 0.8233 | −0.1958 | 0.3622 | 0.0376* |
| H2661 | 0.8513 | 0.5961 | 0.3921 | 0.0669* |
| H2621 | 0.8049 | 0.3557 | 0.4087 | 0.0673* |
| H2631 | 0.8590 | 0.2872 | 0.3844 | 0.0713* |
| H2521 | 0.6715 | 0.0140 | 0.2824 | 0.0435* |
| H2531 | 0.6868 | 0.1629 | 0.2906 | 0.0657* |
| H2541 | 0.7313 | 0.2288 | 0.2573 | 0.0787* |
| H2551 | 0.7651 | 0.1462 | 0.2134 | 0.0884* |
| H2561 | 0.7526 | −0.0064 | 0.2020 | 0.0749* |
| H2761 | 0.7300 | 0.5555 | 0.1885 | 0.0535* |
| H2751 | 0.7223 | 0.7062 | 0.1682 | 0.0611* |
| H2741 | 0.6992 | 0.7420 | 0.0920 | 0.0700* |
| H2731 | 0.6774 | 0.6330 | 0.0388 | 0.0627* |
| H2721 | 0.6828 | 0.4836 | 0.0590 | 0.0603* |
| H2861 | 0.7725 | 0.5273 | 0.3055 | 0.0614* |
| H2821 | 0.8213 | 0.4694 | 0.1984 | 0.0561* |
| H2831 | 0.8294 | 0.6214 | 0.1866 | 0.0677* |
| H2921 | 0.6652 | 0.5037 | 0.2270 | 0.0747* |
| H2931 | 0.6774 | 0.6529 | 0.2380 | 0.0803* |
| H2461 | 0.5037 | 0.7340 | 0.5466 | 0.0827* |
| H2641 | 0.9024 | 0.3772 | 0.3554 | 0.0627* |
| H2841 | 0.8102 | 0.7255 | 0.2348 | 0.0704* |
| H2361 | 0.6212 | −0.0797 | 0.6692 | 0.0772* |
| H2941 | 0.7000 | 0.7105 | 0.3083 | 0.0807* |
| H2851 | 0.7797 | 0.6808 | 0.2917 | 0.0763* |
| H2651 | 0.9013 | 0.5299 | 0.3634 | 0.0713* |
| H2961 | 0.7014 | 0.4588 | 0.3598 | 0.0860* |
| H2431 | 0.5648 | 0.5001 | 0.4902 | 0.0887* |
| H2421 | 0.5491 | 0.6277 | 0.4442 | 0.0938* |
| H2441 | 0.5500 | 0.4898 | 0.5600 | 0.0780* |
| H2351 | 0.6155 | 0.0736 | 0.6564 | 0.1046* |
| H2451 | 0.5207 | 0.6047 | 0.5896 | 0.0933* |
| H2951 | 0.7141 | 0.6155 | 0.3706 | 0.1030* |
| H2341 | 0.5771 | 0.1156 | 0.5823 | 0.1336* |
| H2321 | 0.5693 | −0.1455 | 0.5421 | 0.1407* |
| H2331 | 0.5576 | 0.0111 | 0.5262 | 0.1727* |
Source of materials
The crystals were prepared according to the published procedure for preparation of poly(bis(μ3-2,4,6-tris(4-pyridyl)-1,3,5-triazine)-hexakisiodo-trizinc nitrobenzene solvate) [5]. The resulting material is a mixture of expected crystals [5] and title compound.
Experimental details
The H atoms were refined with soft restraints (C–H 0.93–0.98 Å) and Uiso(H) (1.2–1.5 Ueq C), and then refined with riding constraints. The disordered iodide anions attached to Zn16 were refined using thermal similarity and occupancy sum restraints, resulting in final occupancy ratio of 820(7):180(7). The structure is a redetermination of crystal structure deposited with the CCDC [6]. The structure was a part of a thesis work not a peer reviewed journal. Therefore, the information on sample preparation and model refinement is lacking. Furthermore the disorder of iodide anions was missing in the structure and nitrobenzene molecules were severely distorted. The overall R factor was > 9%; therefore, the structure model presented here is a significant improvement related to the deposited structure.
Comment
Since their discovery [7], the crystal sponges have received a lot of attention worldwide for their ability to provide structural information on hard to crystallize or non-crystalline compounds. The method has seen success in the field of natural compounds [8, 9], and pharmaceutical substances [10]. The crystal structure of title compound is presented in the Figure. The asymmetric unit consists of six ZnI2 nodes connecting four TPT ligands forming the crystal framework and 10 nitrobenzene molecules. The non-hydrogen atoms forming the asymmetric unit are depicted with APDs drawn at 50% probability level. The single bonds within TPT ligands allow rotation of the pyridine rings, resulting in dihedral angles between mean planes through 1,3,5-triazine and pyridine rings ranging from 4.4(3) to 28.0(3)°. The tilting is closely tied to formation of non-covalent interactions. The Zn(II) cations were found to be in distorted tetrahedral coordination. The distortions are caused by the presence of two iodide anions within the coordination sphere. The Zn–I bond distances do not significantly deviate from the average value 2.55 Å, only in case of disordered iodides differences up to 0.056 Å were observed. The Zn–N bonds were found to be within 0.016 Å from the average value of 2.06 Å. The most significant angular distortions from ideal geometry were observed in I–Zn–I and N–Zn–N angles with average values of 125.06 and 101.93° respectively. Larger variance from the average values was observed with differences of up to 4.23 and 4.20° for I–Zn–I and N–Zn–N respectively. In absence of good hydrogen bond donors and abundance of good hydrogen bond acceptors the non-covalent interactions are dominated by weaker C–H···O and C–H···I hydrogen bonds. All nitrobenzene molecules are acceptors of at least one C–H···O hydrogen bond, while six out of 10 molecules also serve as hydrogen bond donors.
Funding source: Czech Science Foundation
Award Identifier / Grant number: 20-14770Y
-
Author contributions: The author has accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This research was supported by the project 20-14770Y of the Czech Science Foundation.
-
Conflict of interest statement: The author declares no conflicts of interest regarding this article.
References
1. Rigaku OD. CrysAlisPRO; Rigaku Oxford Diffraction Ltd: Yarnton, Oxfordshire, England, 2020. Suche in Google Scholar
2. Palatinus, L., Chapuis, G. SUPERFLIP, a computer program for the solution of crystal structures by charge flipping in arbitrary dimensions. J. Appl. Crystallogr. 2007, 40, 786–790; https://doi.org/10.1107/s0021889807029238.Suche in Google Scholar
3. Betteridge, P. W., Carruthers, J. R., Cooper, R. I., Prout, K., Watkin, D. J. CRYSTALS version 12: software for guided crystal structure analysis. J. Appl. Crystallogr. 2003, 36, 1487; https://doi.org/10.1107/s0021889803021800.Suche in Google Scholar
4. Brandenburg, K. DIAMOND; Crystal Impact GbR: Bonn, Germany, 1999. Suche in Google Scholar
5. Inokuma, Y., Yoshioka, S., Ariyoshi, J., Arai, T., Fujita, M. Preparation and guest-uptake protocol for a porous complex useful for crystal-free crystallography. Nat. Protoc. 2014, 9, 246–252; https://doi.org/10.1038/nprot.2014.007.Suche in Google Scholar
6. Pritchard, R. Fatima Attumi CCDC 1977262: experimental crystal structure determination, 2020; https://doi.org/10.5517/ccdc.csd.cc24chnp.Suche in Google Scholar
7. Inokuma, Y., Yoshioka, S., Ariyoshi, J., Arai, T., Hitora, Y., Takada, K., Matsunaga, S., Rissanen, K., Fujita, M. X-ray analysis on the nanogram to microgram scale using porous complexes. Nature 2013, 495, 461–466; https://doi.org/10.1038/nature11990.Suche in Google Scholar
8. Matsuda, Y., Mitsuhashi, T., Lee, S., Hoshino, M., Mori, T., Okada, M., Zhang, H., Hayashi, F., Fujita, M., Abe, I. Astellifadiene: structure determination by NMR spectroscopy and crystalline sponge method, and elucidation of its biosynthesis. Angew. Chem. Int. Ed. 2016, 55, 5785–5788; https://doi.org/10.1002/anie.201601448.Suche in Google Scholar
9. Kersten, R. D., Lee, S., Fujita, D., Pluskal, T., Kram, S., Smith, J. E., Iawi, T., Noel, J. P., Fujita, M., Weng, J. K. A red algal bourbonane sesquiterpene synthase defined by microgram-scale NMR-coupled crystalline sponge X-ray diffraction analysis. J. Am. Chem. Soc. 2017, 139, 16838–16844; https://doi.org/10.1021/jacs.7b09452.Suche in Google Scholar
10. Sakurai, F., Khutia, A., Kikuchi, T., Fujita, M. X-ray Structure Analysis of N-containing nucleophilic compounds by the crystalline sponge method. Chem. Eur J. 2017, 23, 15035–15040; https://doi.org/10.1002/chem.201704176.Suche in Google Scholar
© 2021 Václav Eigner, published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of (E)-7-hydroxy-2-((6-methoxypyridin-3-yl)methylene)-3, 4-dihydronaphthalen-1(2H)-one, C17H15NO3
- Crystal structure of (E)-7-methoxy-2-((2-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1 (2H)-one, C18H17NO3
- The crystal structure of N 6,N 6′-di(pyridin-2-yl)-[2,2′-bipyridine]-6,6′-diamine, C20H16N6
- The crystal structure of {N 1,N 2-bis[2,4-dimethyl-6-(4-(tert-butyl)phenyl)(phenyl)methyl]acenaphthylene-1,2-diimino-κ2 N, N′}-dibromido-nickel(II) – dichloromethane(1/2), C64H64Br2Cl4N2Ni
- Synthesis and crystal structure of nonacarbonyltris[(2-thia-1,3,5-triaza-7-phosphatricylco[3.3.1.1]decane-κ1 P)-2,2-dioxide]triruthenium(0) – acetonitrile (7/6), C25.71H32.57N9.86O15P3S3Ru3
- A new polymorph of 1-(4-nitrophenyl)-1H-benzimidazole (C13H9N3O2)
- The crystal structure of 2,2′-((1E,1′E)-(naphthalene-2,3 diylbis(azanylylidene)) bis(methanylylidene))bis(4-methylphenol), C26H22N2O2
- The crystal structure of bis(μ2-iodido)-bis(η6-benzene)-bis(iodido)-diosmium(II), C12H12I4Os2
- Redetermination of the crystal structure of bis{hydridotris(3,5-dimethylpyrazol-1-yl-κN 3)borato}copper(II), C30H44B2CuN12
- Crystal structure of (E)-3-((4-(tert-butyl)phenyl)thio)-4-hydroxypent-3-en-2-one, C15H20O2S
- Crystal structure of 2,2′-(p-tolylazanediyl)bis(1-phenylethan-1-one), C23H21NO2
- Redetermination of the crystal structure of the crystal sponge the poly[tetrakis(μ3-2,4,6-tris(pyridin-4-yl)-1,3,5-triazine)-dodecaiodidohexazinc(II) nitrobenzene solvate], C72H48I12N24Zn6⋅10(C6H5NO2)
- Crystal structure of (4′E)-6′-(diethylamino)-2-[(E)-[(6-methylpyridin-2-yl)methylidene]amino]-4′-{2-[(2E)-1,3,3-trimethyl-2,3-dihydro-1H-indol-2-ylidene]ethylidene}-1′,2,2′,3,3′,4′-hexahydrospiro[isoindole-1,9′-xanthene]-3-one, C44H45N5O2
- Crystal structure of (E)-7-fluoro-2-(3-fluorobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C17H12F2O1
- Crystal structure of tetrabutylammonium sulfanilate – 1-(diaminomethylene)thiourea (1/2)
- Crystal structure of [2,2′-{azanediyl)bis[(propane-3,1-diyl)(azanylylidene)methylylidene]} bis(3,5-dichlorophenolato)-κ2O,O′]-isothiocyanato-κN-iron(III), C21H19Cl4FeN4O2S
- Crystal structure of (4-chlorophenyl)(4-hydroxyphenyl)methanone, C13H9ClO2
- Crystal structure of 6,6′-((pentane-1,3-diylbis(azaneylylidene))bis(methaneylylidene))bis(2,4-dibromolphenolato-κ4 N,N′,O,O′)copper(II),) C19H16Br4CuN2O2
- Chlorido-(2,2′-(ethane-bis(5-methoxyphenolato))-κ4 N,N′,O,O′)manganese(III) monohydrate, C19H18Cl2CuN2O2
- Crystal structure of 2,6-di-tert-butyl-4-(4-methoxybenzylidene)cyclohexa-2,5-dien-1-one, C22H28O2
- Crystal structure of [6,6′-(((2,2-dimethylpropane-1,3-diyl)bis(azanylylidene))bis(methanylylidene))bis(2-chlorophenolato)-κ4N,N′,O,O′]copper(II)
- Crystal structure of 2-chloro-3-((thiophen-2-ylmethyl)amino)naphthalene-1,4-dione, C30H20O4N2Cl2S2
- Crystal structure of bis{hydridotris(3-trifluoromethyl-5-methylpyrazolyl-1-yl)borato-κN 3}manganese(II), C30H26B2F18MnN12
- Crystal structure of 1-(2-methylphenyl)-2-(2-methylbenzo[b]thienyl)-3,3,4,4,5,5-hexafluorocyclopent-ene, C21H14F6S
- Crystal structure of 2-(3-((carbamimidoylthio)methyl)benzyl)isothiouronium hexafluorophosphate monohydrate, C10H17F6N4OPS2
- Crystal structure of 4,5-diiodo-1,3-dimesityl-1H-1,2,3-triazol-3-ium chloride – chloroform (1/1), C21H23Cl4I2N3
- Crystal structure of azido-k1 N-{6,6′-((((methylazanediyl)bis(propane-3,1-diyl))bis(azanylylidene))bis(methanylylidene))bis(2,4-dibromophenolato)k5 N,N′,N″,O,O′}cobalt(III)-methanol (1/1)), C21H23Br4CoN6O3
- The crystal structure of 2-(4-((carbamimidoylthio)methyl)benzyl)isothiouronium hexafluorophosphate monohydrate, C10H17F6N4OPS2
- Crystal structure of 1,1′-(methane-1,1-diyl)bis(3-methyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C9H14F12N4P2
- Crystal structure of (4′E)-6′-(diethylamino)-2-[(E)-[(pyren-1-yl)methylidene]amino]-4′-{2-[(2E)-1,3,3-trimethyl-2,3-dihydro-1H-indol-2-ylidene]ethylidene}-1′,2,2′,3,3′,4′-hexahydrospiro[isoindole-1,9′-xanthene]-3-one, C54H48N4O2
- Crystal structure of poly[bis(μ2-2,6-bis(1-imidazoly)pyridine-κ2 N,N′)-bis(thiocyanato-κ1 N)copper(II)] dithiocyanate, C24H18CuN12S2
- Cones with a three-fold symmetry constructed from three hydrogen bonded theophyllinium cations that coat [FeCl4]− anions in the crystal structure of tris(theophyllinium) bis(tetrachloridoferrate(III)) chloride trihydrate, C21H33Cl9Fe2N12O9
- Crystal structure of 14-O-[(4-(4-hydroxypiperidine-1-yl)-6-methylpyrimidine-2-yl)thioacetyl]-mutilin monohydrate, C32H49N3O6S
- The crystal structure of (E)-3-chloro-2-(2-(4-methylbenzylidene)hydrazinyl)pyridine, C13H12ClN3
- The crystal structure of 4-phenyl-4-[2-(pyridine-4-carbonyl)hydrazinylidene]butanoic acid, C16H15N3O3
- The crystal structure of 6-amino-5-carboxypyridin-1-ium pentaiodide monohydrate C6H9I5N2O3
- Crystal structure of bis(μ3-oxido)-bis(μ2-2-formylbenzoato-k2O:O′)-bis(2-(dimethoxymethyl)-benzoato-κO)-oktakismethyl-tetratin(IV)
- Crystal structure of 2-((E)-(((E)-2-hydroxy-4-methylbenzylidene) hydrazineylidene)methyl)-4-methylphenol, C16H16N2O2
- Crystal structure of (E)-amino(2-((5-methylfuran-2-yl)methylene)hydrazinyl) methaniminium nitrate monohydrate, C14H26N10O10
- The crystal structure of N′-(2-chloro-6-hydroxybenzylidene)thiophene-2-carbohydrazide monohydrate, C12H11ClN2O3S
- Crystal structure of catena-poly[(μ2-1,1′-(biphenyl-4,4′-diyl)bis(1H-imidazol)-κ2N:N′)-bis(4-bromobenzoate-κ1O)zinc(II)], C64H44Br4N8O8Zn2
- The crystal structure of catena-poly[(1-(4-carboxybenzyl)pyridin-1-ium-4-carboxylato-κ1O)-(μ2-oxalato-κ4 O:O′:O″:O‴)dioxidouranium(VI)], C16H11NO10U
- Crystal structure of 3-allyl-4-(2-bromoethyl)-5-(4-methoxyphenyl)-2-phenylfuran, C22H21BrO2
- Halogen bonds in the crystal structure of 4,3′:5′,4″-terpyridine — 1,3-diiodotetrafluorobenzene (1/1), C21H11F4I2N3
- Crystal structure of 2-(1H-indol-3-yl)ethan-1-aminium 2-(4-acetylphenoxy)acetate, C20H22N2O4
- Chalcogen bonds in the crystal structure of 4,7-dibromo-2,1,3-benzoselenadiazole, C6H2Br2N2Se
- The crystal structure of 1,4-bis((1H-benzimidazol-2-yl)methyl)-piperazine-2,5-dione dihydrate, C20H22N6O4
- The crystal structure of C19H20O8
- The crystal structure of KNa3Te8O18·5H2O exhibiting a ∞2[Te4O9]2− layer
- Erratum
- Erratum to: Crystal structure of (Z)-3-(6-bromo-1H-indol-3-yl)-1,3-diphenylprop-2-en-1-one, C23H16BrNO
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of (E)-7-hydroxy-2-((6-methoxypyridin-3-yl)methylene)-3, 4-dihydronaphthalen-1(2H)-one, C17H15NO3
- Crystal structure of (E)-7-methoxy-2-((2-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1 (2H)-one, C18H17NO3
- The crystal structure of N 6,N 6′-di(pyridin-2-yl)-[2,2′-bipyridine]-6,6′-diamine, C20H16N6
- The crystal structure of {N 1,N 2-bis[2,4-dimethyl-6-(4-(tert-butyl)phenyl)(phenyl)methyl]acenaphthylene-1,2-diimino-κ2 N, N′}-dibromido-nickel(II) – dichloromethane(1/2), C64H64Br2Cl4N2Ni
- Synthesis and crystal structure of nonacarbonyltris[(2-thia-1,3,5-triaza-7-phosphatricylco[3.3.1.1]decane-κ1 P)-2,2-dioxide]triruthenium(0) – acetonitrile (7/6), C25.71H32.57N9.86O15P3S3Ru3
- A new polymorph of 1-(4-nitrophenyl)-1H-benzimidazole (C13H9N3O2)
- The crystal structure of 2,2′-((1E,1′E)-(naphthalene-2,3 diylbis(azanylylidene)) bis(methanylylidene))bis(4-methylphenol), C26H22N2O2
- The crystal structure of bis(μ2-iodido)-bis(η6-benzene)-bis(iodido)-diosmium(II), C12H12I4Os2
- Redetermination of the crystal structure of bis{hydridotris(3,5-dimethylpyrazol-1-yl-κN 3)borato}copper(II), C30H44B2CuN12
- Crystal structure of (E)-3-((4-(tert-butyl)phenyl)thio)-4-hydroxypent-3-en-2-one, C15H20O2S
- Crystal structure of 2,2′-(p-tolylazanediyl)bis(1-phenylethan-1-one), C23H21NO2
- Redetermination of the crystal structure of the crystal sponge the poly[tetrakis(μ3-2,4,6-tris(pyridin-4-yl)-1,3,5-triazine)-dodecaiodidohexazinc(II) nitrobenzene solvate], C72H48I12N24Zn6⋅10(C6H5NO2)
- Crystal structure of (4′E)-6′-(diethylamino)-2-[(E)-[(6-methylpyridin-2-yl)methylidene]amino]-4′-{2-[(2E)-1,3,3-trimethyl-2,3-dihydro-1H-indol-2-ylidene]ethylidene}-1′,2,2′,3,3′,4′-hexahydrospiro[isoindole-1,9′-xanthene]-3-one, C44H45N5O2
- Crystal structure of (E)-7-fluoro-2-(3-fluorobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C17H12F2O1
- Crystal structure of tetrabutylammonium sulfanilate – 1-(diaminomethylene)thiourea (1/2)
- Crystal structure of [2,2′-{azanediyl)bis[(propane-3,1-diyl)(azanylylidene)methylylidene]} bis(3,5-dichlorophenolato)-κ2O,O′]-isothiocyanato-κN-iron(III), C21H19Cl4FeN4O2S
- Crystal structure of (4-chlorophenyl)(4-hydroxyphenyl)methanone, C13H9ClO2
- Crystal structure of 6,6′-((pentane-1,3-diylbis(azaneylylidene))bis(methaneylylidene))bis(2,4-dibromolphenolato-κ4 N,N′,O,O′)copper(II),) C19H16Br4CuN2O2
- Chlorido-(2,2′-(ethane-bis(5-methoxyphenolato))-κ4 N,N′,O,O′)manganese(III) monohydrate, C19H18Cl2CuN2O2
- Crystal structure of 2,6-di-tert-butyl-4-(4-methoxybenzylidene)cyclohexa-2,5-dien-1-one, C22H28O2
- Crystal structure of [6,6′-(((2,2-dimethylpropane-1,3-diyl)bis(azanylylidene))bis(methanylylidene))bis(2-chlorophenolato)-κ4N,N′,O,O′]copper(II)
- Crystal structure of 2-chloro-3-((thiophen-2-ylmethyl)amino)naphthalene-1,4-dione, C30H20O4N2Cl2S2
- Crystal structure of bis{hydridotris(3-trifluoromethyl-5-methylpyrazolyl-1-yl)borato-κN 3}manganese(II), C30H26B2F18MnN12
- Crystal structure of 1-(2-methylphenyl)-2-(2-methylbenzo[b]thienyl)-3,3,4,4,5,5-hexafluorocyclopent-ene, C21H14F6S
- Crystal structure of 2-(3-((carbamimidoylthio)methyl)benzyl)isothiouronium hexafluorophosphate monohydrate, C10H17F6N4OPS2
- Crystal structure of 4,5-diiodo-1,3-dimesityl-1H-1,2,3-triazol-3-ium chloride – chloroform (1/1), C21H23Cl4I2N3
- Crystal structure of azido-k1 N-{6,6′-((((methylazanediyl)bis(propane-3,1-diyl))bis(azanylylidene))bis(methanylylidene))bis(2,4-dibromophenolato)k5 N,N′,N″,O,O′}cobalt(III)-methanol (1/1)), C21H23Br4CoN6O3
- The crystal structure of 2-(4-((carbamimidoylthio)methyl)benzyl)isothiouronium hexafluorophosphate monohydrate, C10H17F6N4OPS2
- Crystal structure of 1,1′-(methane-1,1-diyl)bis(3-methyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C9H14F12N4P2
- Crystal structure of (4′E)-6′-(diethylamino)-2-[(E)-[(pyren-1-yl)methylidene]amino]-4′-{2-[(2E)-1,3,3-trimethyl-2,3-dihydro-1H-indol-2-ylidene]ethylidene}-1′,2,2′,3,3′,4′-hexahydrospiro[isoindole-1,9′-xanthene]-3-one, C54H48N4O2
- Crystal structure of poly[bis(μ2-2,6-bis(1-imidazoly)pyridine-κ2 N,N′)-bis(thiocyanato-κ1 N)copper(II)] dithiocyanate, C24H18CuN12S2
- Cones with a three-fold symmetry constructed from three hydrogen bonded theophyllinium cations that coat [FeCl4]− anions in the crystal structure of tris(theophyllinium) bis(tetrachloridoferrate(III)) chloride trihydrate, C21H33Cl9Fe2N12O9
- Crystal structure of 14-O-[(4-(4-hydroxypiperidine-1-yl)-6-methylpyrimidine-2-yl)thioacetyl]-mutilin monohydrate, C32H49N3O6S
- The crystal structure of (E)-3-chloro-2-(2-(4-methylbenzylidene)hydrazinyl)pyridine, C13H12ClN3
- The crystal structure of 4-phenyl-4-[2-(pyridine-4-carbonyl)hydrazinylidene]butanoic acid, C16H15N3O3
- The crystal structure of 6-amino-5-carboxypyridin-1-ium pentaiodide monohydrate C6H9I5N2O3
- Crystal structure of bis(μ3-oxido)-bis(μ2-2-formylbenzoato-k2O:O′)-bis(2-(dimethoxymethyl)-benzoato-κO)-oktakismethyl-tetratin(IV)
- Crystal structure of 2-((E)-(((E)-2-hydroxy-4-methylbenzylidene) hydrazineylidene)methyl)-4-methylphenol, C16H16N2O2
- Crystal structure of (E)-amino(2-((5-methylfuran-2-yl)methylene)hydrazinyl) methaniminium nitrate monohydrate, C14H26N10O10
- The crystal structure of N′-(2-chloro-6-hydroxybenzylidene)thiophene-2-carbohydrazide monohydrate, C12H11ClN2O3S
- Crystal structure of catena-poly[(μ2-1,1′-(biphenyl-4,4′-diyl)bis(1H-imidazol)-κ2N:N′)-bis(4-bromobenzoate-κ1O)zinc(II)], C64H44Br4N8O8Zn2
- The crystal structure of catena-poly[(1-(4-carboxybenzyl)pyridin-1-ium-4-carboxylato-κ1O)-(μ2-oxalato-κ4 O:O′:O″:O‴)dioxidouranium(VI)], C16H11NO10U
- Crystal structure of 3-allyl-4-(2-bromoethyl)-5-(4-methoxyphenyl)-2-phenylfuran, C22H21BrO2
- Halogen bonds in the crystal structure of 4,3′:5′,4″-terpyridine — 1,3-diiodotetrafluorobenzene (1/1), C21H11F4I2N3
- Crystal structure of 2-(1H-indol-3-yl)ethan-1-aminium 2-(4-acetylphenoxy)acetate, C20H22N2O4
- Chalcogen bonds in the crystal structure of 4,7-dibromo-2,1,3-benzoselenadiazole, C6H2Br2N2Se
- The crystal structure of 1,4-bis((1H-benzimidazol-2-yl)methyl)-piperazine-2,5-dione dihydrate, C20H22N6O4
- The crystal structure of C19H20O8
- The crystal structure of KNa3Te8O18·5H2O exhibiting a ∞2[Te4O9]2− layer
- Erratum
- Erratum to: Crystal structure of (Z)-3-(6-bromo-1H-indol-3-yl)-1,3-diphenylprop-2-en-1-one, C23H16BrNO

