Crystal structure of (4′E)-6′-(diethylamino)-2-[(E)-[(6-methylpyridin-2-yl)methylidene]amino]-4′-{2-[(2E)-1,3,3-trimethyl-2,3-dihydro-1H-indol-2-ylidene]ethylidene}-1′,2,2′,3,3′,4′-hexahydrospiro[isoindole-1,9′-xanthene]-3-one, C44H45N5O2
Abstract
C44H45N5O2, triclinic
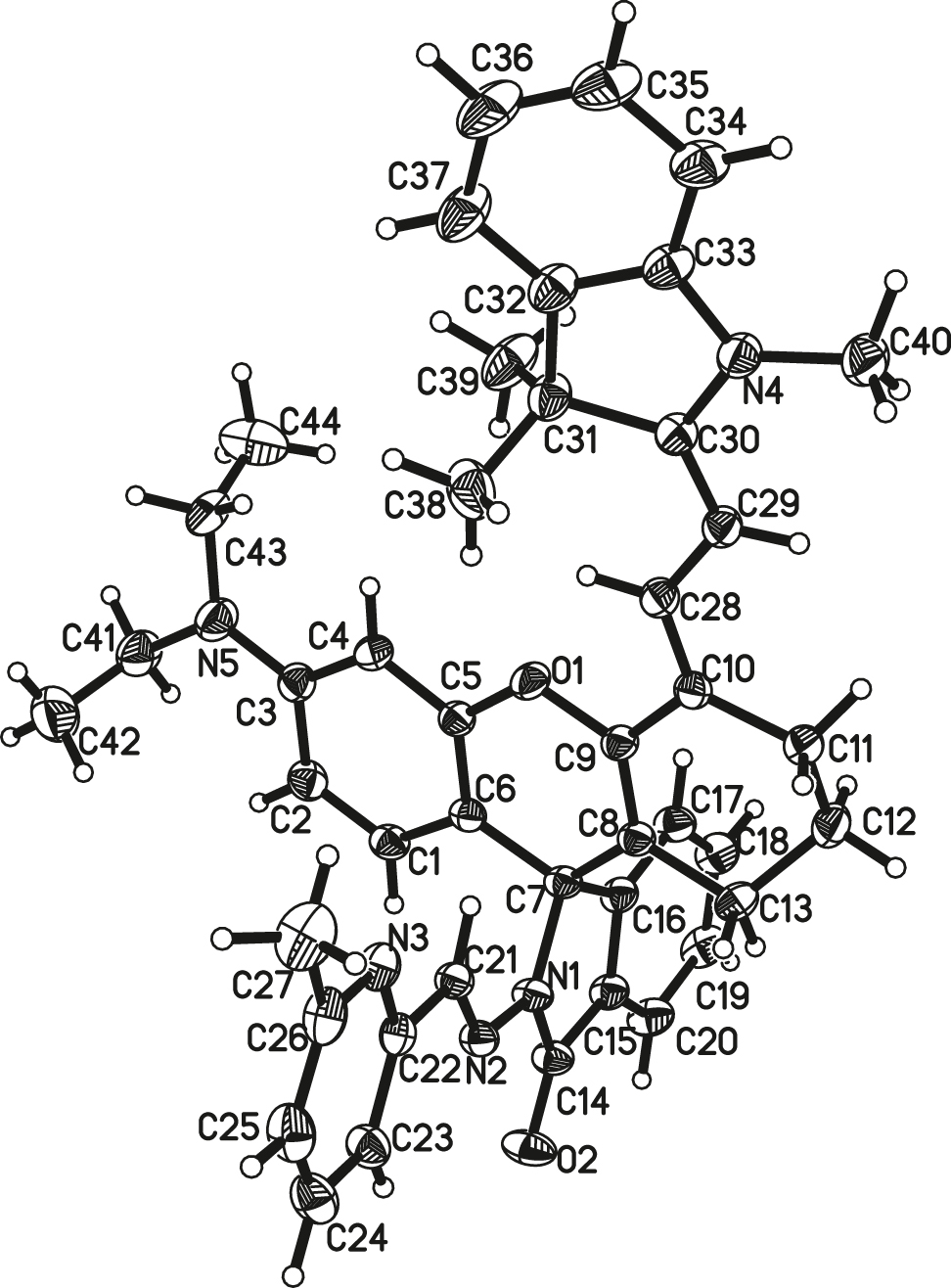
The asymmetric unit of the molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Yellow block |
| Size: | 0.13 × 0.1 × 0.08 mm |
| Wavelength: | Cu Kα radiation (1.54184 Å) |
| μ: | 0.59 mm−1 |
| Diffractometer, scan mode: | Xcalibur, ω-scans |
| θ max, completeness: | 67.1°, >99% |
| N(hkl)measured, N(hkl)unique, R int: | 13,570, 6625, 0.026 |
| Criterion for I obs, N(hkl)gt: | I obs > 2 σ(I obs), 5090 |
| N(param)refined: | 476 |
| Programs: | CrysAlisPRO [1], OLEX2 [2], SHELX [3, 4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| Cl1 | −0.32807 (8) | −0.58567 (6) | −0.37764 (7) | 0.01860 (19) |
| S1 | −0.08434 (7) | −0.51774 (6) | −0.83804 (6) | 0.01273 (18) |
| O1 | 0.76362 (15) | 0.76100 (12) | 0.06274 (10) | 0.0535 (4) |
| O2 | 0.85795 (16) | 1.10183 (16) | 0.46464 (11) | 0.0687 (5) |
| N1 | 0.79305 (15) | 0.98582 (14) | 0.31579 (12) | 0.0444 (4) |
| N2 | 0.68178 (15) | 0.95996 (14) | 0.33397 (12) | 0.0441 (4) |
| N3 | 0.40401 (16) | 0.80511 (15) | 0.22073 (14) | 0.0528 (4) |
| N4 | 0.44883 (18) | 0.68512 (15) | −0.35587 (13) | 0.0563 (5) |
| N5 | 0.9405 (2) | 0.46757 (17) | 0.22652 (16) | 0.0699 (6) |
| C1 | 0.92517 (19) | 0.77190 (18) | 0.31349 (15) | 0.0489 (5) |
| H1 | 0.9508 | 0.8226 | 0.3709 | 0.059* |
| C2 | 0.9486 (2) | 0.6599 (2) | 0.31324 (16) | 0.0545 (5) |
| H2 | 0.9880 | 0.6366 | 0.3705 | 0.065* |
| C3 | 0.9139 (2) | 0.57940 (18) | 0.22766 (16) | 0.0503 (5) |
| C4 | 0.8517 (2) | 0.61967 (17) | 0.14512 (15) | 0.0478 (5) |
| H4 | 0.8261 | 0.5697 | 0.0873 | 0.057* |
| C5 | 0.82739 (18) | 0.73266 (16) | 0.14808 (14) | 0.0424 (4) |
| C6 | 0.86455 (17) | 0.81227 (16) | 0.23110 (13) | 0.0410 (4) |
| C7 | 0.84398 (17) | 0.93617 (16) | 0.22998 (14) | 0.0414 (4) |
| C8 | 0.76765 (18) | 0.95574 (16) | 0.13445 (14) | 0.0425 (4) |
| C9 | 0.73562 (18) | 0.87235 (16) | 0.05851 (14) | 0.0425 (4) |
| C10 | 0.66598 (19) | 0.88652 (17) | −0.03697 (14) | 0.0444 (4) |
| C11a | 0.6399 (3) | 1.0058 (4) | −0.0550 (3) | 0.0500 (9) |
| H11Aa | 0.5561 | 1.0202 | −0.0512 | 0.060* |
| H11Ba | 0.6489 | 1.0127 | −0.1201 | 0.060* |
| C12a | 0.7250 (3) | 1.0940 (2) | 0.0179 (2) | 0.0592 (8) |
| H12Aa | 0.6969 | 1.1688 | 0.0108 | 0.071* |
| H12Ba | 0.8065 | 1.0904 | 0.0049 | 0.071* |
| C13a | 0.7291 (3) | 1.0738 (3) | 0.1218 (3) | 0.0506 (9) |
| H13Aa | 0.7863 | 1.1288 | 0.1668 | 0.061* |
| H13Ba | 0.6490 | 1.0838 | 0.1366 | 0.061* |
| C14 | 0.87521 (19) | 1.05826 (18) | 0.38669 (15) | 0.0477 (5) |
| C15 | 0.98309 (19) | 1.07136 (17) | 0.34444 (15) | 0.0469 (5) |
| C16 | 0.96482 (18) | 1.00598 (16) | 0.25407 (15) | 0.0449 (4) |
| C17 | 1.0477 (2) | 1.01039 (19) | 0.19607 (17) | 0.0549 (5) |
| H17 | 1.0349 | 0.9671 | 0.1345 | 0.066* |
| C18 | 1.1515 (2) | 1.0818 (2) | 0.2325 (2) | 0.0648 (6) |
| H18 | 1.2082 | 1.0875 | 0.1941 | 0.078* |
| C19 | 1.1722 (2) | 1.1444 (2) | 0.3245 (2) | 0.0645 (6) |
| H19 | 1.2435 | 1.1899 | 0.3479 | 0.077* |
| C20 | 1.0885 (2) | 1.1404 (2) | 0.38217 (18) | 0.0590 (6) |
| H20 | 1.1019 | 1.1825 | 0.4442 | 0.071* |
| C21 | 0.60486 (18) | 0.89185 (16) | 0.27227 (14) | 0.0440 (4) |
| H21 | 0.6250 | 0.8557 | 0.2156 | 0.053* |
| C22 | 0.48291 (18) | 0.87134 (16) | 0.29230 (15) | 0.0441 (4) |
| C23 | 0.4531 (2) | 0.9201 (2) | 0.37763 (17) | 0.0550 (5) |
| H23 | 0.5105 | 0.9659 | 0.4251 | 0.066* |
| C24 | 0.3372 (2) | 0.9001 (2) | 0.39181 (19) | 0.0654 (6) |
| H24 | 0.3149 | 0.9322 | 0.4488 | 0.078* |
| C25 | 0.2562 (2) | 0.8323 (2) | 0.3205 (2) | 0.0681 (7) |
| H25 | 0.1777 | 0.8169 | 0.3288 | 0.082* |
| C26 | 0.2905 (2) | 0.7865 (2) | 0.2363 (2) | 0.0605 (6) |
| C27 | 0.2009 (3) | 0.7154 (3) | 0.1537 (3) | 0.0877 (9) |
| H27A | 0.1378 | 0.7616 | 0.1269 | 0.132* |
| H27B | 0.1652 | 0.6550 | 0.1775 | 0.132* |
| H27C | 0.2428 | 0.6844 | 0.1042 | 0.132* |
| C28 | 0.62053 (19) | 0.79443 (17) | −0.10326 (15) | 0.0467 (5) |
| H28 | 0.6341 | 0.7251 | −0.0825 | 0.056* |
| C29 | 0.55418 (19) | 0.78962 (17) | −0.20126 (15) | 0.0475 (5) |
| H29 | 0.5438 | 0.8568 | −0.2262 | 0.057* |
| C30 | 0.50555 (19) | 0.69196 (18) | −0.25982 (15) | 0.0468 (5) |
| C31 | 0.4975 (2) | 0.57305 (19) | −0.23282 (17) | 0.0567 (6) |
| C32 | 0.4280 (2) | 0.5057 (2) | −0.32771 (18) | 0.0601 (6) |
| C33 | 0.4040 (2) | 0.57556 (19) | −0.39718 (17) | 0.0563 (6) |
| C34 | 0.3449 (2) | 0.5340 (2) | −0.49186 (19) | 0.0703 (7) |
| H34 | 0.3297 | 0.5806 | −0.5389 | 0.084* |
| C35 | 0.3091 (3) | 0.4192 (3) | −0.5136 (2) | 0.0839 (9) |
| H35 | 0.2689 | 0.3889 | −0.5765 | 0.101* |
| C36 | 0.3316 (3) | 0.3502 (2) | −0.4449 (3) | 0.0910 (10) |
| H36 | 0.3068 | 0.2740 | −0.4615 | 0.109* |
| C37 | 0.3913 (3) | 0.3930 (2) | −0.3506 (2) | 0.0811 (9) |
| H37 | 0.4063 | 0.3463 | −0.3036 | 0.097* |
| C38 | 0.4262 (3) | 0.5702 (3) | −0.1531 (2) | 0.0877 (10) |
| H38A | 0.4709 | 0.6139 | −0.0937 | 0.132* |
| H38B | 0.4144 | 0.4935 | −0.1439 | 0.132* |
| H38C | 0.3482 | 0.6014 | −0.1717 | 0.132* |
| C39 | 0.6239 (3) | 0.5242 (2) | −0.2061 (2) | 0.0848 (10) |
| H39A | 0.6714 | 0.5377 | −0.2523 | 0.127* |
| H39B | 0.6140 | 0.4443 | −0.2069 | 0.127* |
| H39C | 0.6649 | 0.5601 | −0.1423 | 0.127* |
| C40 | 0.4376 (3) | 0.7785 (2) | −0.40894 (19) | 0.0762 (8) |
| H40A | 0.3669 | 0.8186 | −0.3990 | 0.114* |
| H40B | 0.4294 | 0.7506 | −0.4769 | 0.114* |
| H40C | 0.5092 | 0.8285 | −0.3863 | 0.114* |
| C41 | 1.0005 (3) | 0.4261 (2) | 0.3139 (2) | 0.0766 (8) |
| H41A | 1.0610 | 0.4831 | 0.3525 | 0.092* |
| H41B | 1.0429 | 0.3591 | 0.2957 | 0.092* |
| C42 | 0.9129 (4) | 0.3977 (3) | 0.3746 (3) | 0.1156 (14) |
| H42A | 0.8570 | 0.3368 | 0.3387 | 0.173* |
| H42B | 0.8682 | 0.4628 | 0.3907 | 0.173* |
| H42C | 0.9576 | 0.3753 | 0.4332 | 0.173* |
| C43 | 0.9177 (3) | 0.3855 (2) | 0.1378 (2) | 0.0679 (7) |
| H43A | 0.8492 | 0.4081 | 0.0922 | 0.081* |
| H43B | 0.8950 | 0.3127 | 0.1523 | 0.081* |
| C44 | 1.0243 (4) | 0.3734 (4) | 0.0915 (3) | 0.1162 (14) |
| H44A | 1.0063 | 0.3139 | 0.0365 | 0.174* |
| H44B | 1.0938 | 0.3556 | 0.1374 | 0.174* |
| H44C | 1.0418 | 0.4431 | 0.0704 | 0.174* |
| C12Ab | 0.6581 (17) | 1.0873 (12) | 0.0434 (11) | 0.0592 (8) |
| H12Cb | 0.5822 | 1.0633 | 0.0585 | 0.071* |
| H12Db | 0.6516 | 1.1647 | 0.0316 | 0.071* |
| C13Ab | 0.763 (2) | 1.0818 (15) | 0.129 (2) | 0.0506 (9) |
| H13Cb | 0.8395 | 1.1111 | 0.1180 | 0.061* |
| H13Db | 0.7476 | 1.1241 | 0.1887 | 0.061* |
| C11Ab | 0.678 (2) | 1.012 (2) | −0.0473 (17) | 0.0500 (9) |
| H11Cb | 0.7590 | 1.0293 | −0.0575 | 0.060* |
| H11Db | 0.6187 | 1.0267 | −0.1033 | 0.060* |
-
aOccupancy: 0.830(5), bOccupancy: 0.170(5).
Source of materials
All reagents and starting materials were commercially available and used as received. The title compound was synthesized according to the literature method [5], [6], [7]. The title compound was obtained from cyclohexanone by a four-step reaction.
First step: Cyclohexanone (1.98 mL) was added dropwise to concentrated sulfuric acid (20.0 mL) under ice-bath condition. Then, 2-(4-diethylamino-hydroxybenzoyl) benzoic acid (3.00 g, 9.6 mmol) was added in portions under vigorous stirring. After the addition was completed, the reaction mixture was heated at 90 °C for 3 h, cooled down and poured onto ice (150.0 g). Perchloric acid (2.0 mL, 70%) was then added and the resulting precipitate was filtered, washed several times with cold water and dried in the air to give a red solid. Yield: ca. 94%.
Both the red solid (2.5 g, 6.65 mmol) obtained in the first step and equal number of moles of fisher aldehyde (1.34 g, 6.65 mmol) were dissolved directly in acetic anhydride (35.0 mL), refluxed for 0.5 h under stirring. After that, the resulting mixture was put into the refrigerator immediately to stop the reaction and then distilled under reduced pressure to obtain a dark-green solid. The dark-green solid was further purified by silica gel chromatography (CH2Cl2/CH3CH2OH = 200:1, 20:1 v/v) to give green microcrystalline, the intermediate of the second step. Yield: ca. 50%.
The green microcrystalline (1.2 g, 2.15 mmol), PyBOP (1.4 g, 2.68 mmol) and hydrazine hydrate (2.4 mL) were dispersed in CH2Cl2 (25 mL), and stirred at room temperature for 4 h. Then, the resulting mixture was distilled under reduced pressure to give a crude product, which was further purified by neutral alumina column chromatography (CH2Cl2/CH3CH2OH = 200:1, 50:1 v/v) to yield yellow powder, the intermediate of the third step. Yield: ca. 45%.
The ethanol solution of the yellow powder (0.5 g, 0.87 mmol) obtained from the above step and the ethanol solution of 6-methyl-2-pyridine carboxaldehyde (0.16 g, 1.31 mmol) were mixed and refluxed for 10 h. Then, the resulting mixture was concentrated by vacuum distillation to give the crude product of the title compound, which was further purified by neutral alumina column chromatography (CH2Cl2/CH3CH2OH = 200:1, 50:1 v/v) to give a yellow powder product, the title compound. Yield: ca. 20%.
Single crystals of the title compound were grown from a methanol solution by slow evaporation at room temperature. About one week later, light yellow block crystals were appeared. Yield: ca. 75%.
Experimental details
The H atoms were added using riding models. Their U iso values were set to 1.2 U eq of the parent atoms.
Comment
Rhodamine fluorescent dyes based on xanthene are widely used as fluorescent probes to track target molecules, because of the advantages of large molar extinction coefficient, high fluorescence quantum efficiency and good optical stability [8, 9]. At present, most of fluorescent probes based on xanthene are mainly concentrated in the visible region. Visible light imaging technology has some problems such as strong background fluorescence, photobleaching, phototoxicity and limited tissue penetration, which limits the application of fluorescent probes in chemical biology and clinical diagnostics. While near-infrared fluorescent probes have many advantages, such as long emission wavelength, little damage to cells, strong tissue penetration and low spontaneous emission background, which is widely used in detection, tracing and imaging of biomolecules in complex biological systems such as cells and tissues [10–13]. In this work, a near-infrared rhodamine-derived compound was reported, which may be a potential near-infrared fluorescent probe for the good coordination ability to transition metal ions and rare-earth metal ions [14, 15].
The asymmetric unit of the title compound contains one molecule in a ring-closed form (see the figure). The amide C=O bond distance is 1.209(3) Å, indicative of the keto form of the amide. The bond length of C21–N2 is 1.273(3) Å, which shows the existence of the Schiff base C=N [16]. The dihedral angle between the aroylhydrozone plane and the pyridyl plane is 8.62°. While, the xanthene least-square plane and pyridylaroyl hydrozone group are almost perpendicular, the dihedral angle is 82.97°. All geometric parameters are in the expected ranges.
Funding source: National Natural Science Foundation of China
Award Identifier / Grant number: 22101076
Award Identifier / Grant number: 21771115
Funding source: Natural Science Foundation of Henan
Award Identifier / Grant number: 202300410261
Funding source: Henan University of Chinese Medicine
Award Identifier / Grant number: BSJJ2020-01
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This work was supported by the National Natural Science Foundation of China (Project Nos. 22101076 and 21771115), the Natural Science Foundation of Henan, China (Project No. 202300410261) and the Startup Foundation for Doctors of Henan University of Chinese Medicine (Project No. BSJJ2020-01).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. CrysAlisPro Software system. version 1. 171. 38. 43f; Rigaku Corporation: Oxford, UK, 2015.Suche in Google Scholar
2. Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Suche in Google Scholar
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Suche in Google Scholar
4. Sheldrick, G. M. SHELXT-integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Suche in Google Scholar
5. Xu, Z., Wang, H., Chen, Z. Near-infrared fluorescent probe for selective detection of Cu2+ in living cells and in Vivo. Spectrochim. Acta Mol. Biomol. Spectrosc. 2019, 216, 404–410; https://doi.org/10.1016/j.saa.2019.03.062.Suche in Google Scholar
6. Xie, J.-Y., Li, C.-Y., Li, Y.-F. A near-infrared chemosensor for determination of trivalent aluminum ions in living cells and tissues. Dyes Pigments 2017, 136, 817–824; https://doi.org/10.1016/j.dyepig.2016.09.046.Suche in Google Scholar
7. Xie, J.-Y., Li, C.-Y., Li, Y.-F. A near-infrared fluorescent probe with high quantum yield and its application in the selective detection of glutathione in living cells and tissues. Anal. Chem. 2016, 19, 9746–9752; https://doi.org/10.1021/acs.analchem.6b02646.Suche in Google Scholar
8. Kim, H. N., Lee, M. H., Kim, H. J. A new trend in rhodamine-based chemosensors: application of spirolactam ring-opening to sensing ions. Chem. Soc. Rev. 2008, 19, 1465–1472; https://doi.org/10.1039/b802497a.Suche in Google Scholar
9. Chen, X.-Q., Pradhan, T., Wang, F. Fluorescent chemosensors based on spiroring-opening of xanthenes and related derivatives. Chem. Rev. 2012, 112, 1910–1956; https://doi.org/10.1021/cr200201z.Suche in Google Scholar
10. Zhang, W.-T., Yu, C.-W., Yang, M. Characterization of a Hg2+-selective fluorescent probe based on rhodamine B and its imaging in living cells. Molecules 2021, 11, 3385–3386; https://doi.org/10.3390/molecules26113385.Suche in Google Scholar
11. Gong, J., Liu, C., Jiao, X.-J. A novel near-infrared fluorescent probe with an improved Stokes shift for specific detection of Hg2+ in mitochondria. Org. Biomol. Chem. 2020, 27, 5238–5244; https://doi.org/10.1039/d0ob00507j.Suche in Google Scholar
12. Hilderbrand, S. A., Weissleder, R. Near-infrared fluorescence: application to in vivo molecular imaging. Curr. Opin. Chem. Biol. 2010, 14, 71–79; https://doi.org/10.1016/j.cbpa.2009.09.029.Suche in Google Scholar
13. Karaoglu, K., Kaya, K., Yilmaz, I. New chromenylium– cyanine based dual channel chemosensors for copper and hypochlorite sensing. Dyes Pigments 2020, 180, 108445; https://doi.org/10.1016/j.dyepig.2020.108445.Suche in Google Scholar
14. Huang, W., Wu, D., Guo, D., Zhu, X., He, C., Meng, Q., Duan, C. Efficient near-infrared emission of a Ytterbium(III) compound with a green light rhodamine donor. Dalton Trans. 2009, 2081–2084; https://doi.org/10.1039/b820131e.Suche in Google Scholar
15. Huang, W., Xu, J., Wu, D., Huang, X., Jiang, J. Rhodamine-based field-induced single molecule magnets in Yb(III) and Dy(III) series. New J. Chem. 2015, 39, 8650–8657; https://doi.org/10.1039/c5nj01724f.Suche in Google Scholar
16. Yuan, J., Cheng, D. Crystal structure of (E)-3′,6′-bis(ethylamino)-2′,7′-dimethyl-2-((pyridin-2-ylmethylene)amino)spiro[isoindoline-1,9′-xanthen]-3-one, C32H31N5O2. Z. Kristallogr.NCS 2018, 234, 149–151; https://doi.org/10.1515/ncrs-2018-0239.Suche in Google Scholar
© 2021 Kaihao Li et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of (E)-7-hydroxy-2-((6-methoxypyridin-3-yl)methylene)-3, 4-dihydronaphthalen-1(2H)-one, C17H15NO3
- Crystal structure of (E)-7-methoxy-2-((2-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1 (2H)-one, C18H17NO3
- The crystal structure of N 6,N 6′-di(pyridin-2-yl)-[2,2′-bipyridine]-6,6′-diamine, C20H16N6
- The crystal structure of {N 1,N 2-bis[2,4-dimethyl-6-(4-(tert-butyl)phenyl)(phenyl)methyl]acenaphthylene-1,2-diimino-κ2 N, N′}-dibromido-nickel(II) – dichloromethane(1/2), C64H64Br2Cl4N2Ni
- Synthesis and crystal structure of nonacarbonyltris[(2-thia-1,3,5-triaza-7-phosphatricylco[3.3.1.1]decane-κ1 P)-2,2-dioxide]triruthenium(0) – acetonitrile (7/6), C25.71H32.57N9.86O15P3S3Ru3
- A new polymorph of 1-(4-nitrophenyl)-1H-benzimidazole (C13H9N3O2)
- The crystal structure of 2,2′-((1E,1′E)-(naphthalene-2,3 diylbis(azanylylidene)) bis(methanylylidene))bis(4-methylphenol), C26H22N2O2
- The crystal structure of bis(μ2-iodido)-bis(η6-benzene)-bis(iodido)-diosmium(II), C12H12I4Os2
- Redetermination of the crystal structure of bis{hydridotris(3,5-dimethylpyrazol-1-yl-κN 3)borato}copper(II), C30H44B2CuN12
- Crystal structure of (E)-3-((4-(tert-butyl)phenyl)thio)-4-hydroxypent-3-en-2-one, C15H20O2S
- Crystal structure of 2,2′-(p-tolylazanediyl)bis(1-phenylethan-1-one), C23H21NO2
- Redetermination of the crystal structure of the crystal sponge the poly[tetrakis(μ3-2,4,6-tris(pyridin-4-yl)-1,3,5-triazine)-dodecaiodidohexazinc(II) nitrobenzene solvate], C72H48I12N24Zn6⋅10(C6H5NO2)
- Crystal structure of (4′E)-6′-(diethylamino)-2-[(E)-[(6-methylpyridin-2-yl)methylidene]amino]-4′-{2-[(2E)-1,3,3-trimethyl-2,3-dihydro-1H-indol-2-ylidene]ethylidene}-1′,2,2′,3,3′,4′-hexahydrospiro[isoindole-1,9′-xanthene]-3-one, C44H45N5O2
- Crystal structure of (E)-7-fluoro-2-(3-fluorobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C17H12F2O1
- Crystal structure of tetrabutylammonium sulfanilate – 1-(diaminomethylene)thiourea (1/2)
- Crystal structure of [2,2′-{azanediyl)bis[(propane-3,1-diyl)(azanylylidene)methylylidene]} bis(3,5-dichlorophenolato)-κ2O,O′]-isothiocyanato-κN-iron(III), C21H19Cl4FeN4O2S
- Crystal structure of (4-chlorophenyl)(4-hydroxyphenyl)methanone, C13H9ClO2
- Crystal structure of 6,6′-((pentane-1,3-diylbis(azaneylylidene))bis(methaneylylidene))bis(2,4-dibromolphenolato-κ4 N,N′,O,O′)copper(II),) C19H16Br4CuN2O2
- Chlorido-(2,2′-(ethane-bis(5-methoxyphenolato))-κ4 N,N′,O,O′)manganese(III) monohydrate, C19H18Cl2CuN2O2
- Crystal structure of 2,6-di-tert-butyl-4-(4-methoxybenzylidene)cyclohexa-2,5-dien-1-one, C22H28O2
- Crystal structure of [6,6′-(((2,2-dimethylpropane-1,3-diyl)bis(azanylylidene))bis(methanylylidene))bis(2-chlorophenolato)-κ4N,N′,O,O′]copper(II)
- Crystal structure of 2-chloro-3-((thiophen-2-ylmethyl)amino)naphthalene-1,4-dione, C30H20O4N2Cl2S2
- Crystal structure of bis{hydridotris(3-trifluoromethyl-5-methylpyrazolyl-1-yl)borato-κN 3}manganese(II), C30H26B2F18MnN12
- Crystal structure of 1-(2-methylphenyl)-2-(2-methylbenzo[b]thienyl)-3,3,4,4,5,5-hexafluorocyclopent-ene, C21H14F6S
- Crystal structure of 2-(3-((carbamimidoylthio)methyl)benzyl)isothiouronium hexafluorophosphate monohydrate, C10H17F6N4OPS2
- Crystal structure of 4,5-diiodo-1,3-dimesityl-1H-1,2,3-triazol-3-ium chloride – chloroform (1/1), C21H23Cl4I2N3
- Crystal structure of azido-k1 N-{6,6′-((((methylazanediyl)bis(propane-3,1-diyl))bis(azanylylidene))bis(methanylylidene))bis(2,4-dibromophenolato)k5 N,N′,N″,O,O′}cobalt(III)-methanol (1/1)), C21H23Br4CoN6O3
- The crystal structure of 2-(4-((carbamimidoylthio)methyl)benzyl)isothiouronium hexafluorophosphate monohydrate, C10H17F6N4OPS2
- Crystal structure of 1,1′-(methane-1,1-diyl)bis(3-methyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C9H14F12N4P2
- Crystal structure of (4′E)-6′-(diethylamino)-2-[(E)-[(pyren-1-yl)methylidene]amino]-4′-{2-[(2E)-1,3,3-trimethyl-2,3-dihydro-1H-indol-2-ylidene]ethylidene}-1′,2,2′,3,3′,4′-hexahydrospiro[isoindole-1,9′-xanthene]-3-one, C54H48N4O2
- Crystal structure of poly[bis(μ2-2,6-bis(1-imidazoly)pyridine-κ2 N,N′)-bis(thiocyanato-κ1 N)copper(II)] dithiocyanate, C24H18CuN12S2
- Cones with a three-fold symmetry constructed from three hydrogen bonded theophyllinium cations that coat [FeCl4]− anions in the crystal structure of tris(theophyllinium) bis(tetrachloridoferrate(III)) chloride trihydrate, C21H33Cl9Fe2N12O9
- Crystal structure of 14-O-[(4-(4-hydroxypiperidine-1-yl)-6-methylpyrimidine-2-yl)thioacetyl]-mutilin monohydrate, C32H49N3O6S
- The crystal structure of (E)-3-chloro-2-(2-(4-methylbenzylidene)hydrazinyl)pyridine, C13H12ClN3
- The crystal structure of 4-phenyl-4-[2-(pyridine-4-carbonyl)hydrazinylidene]butanoic acid, C16H15N3O3
- The crystal structure of 6-amino-5-carboxypyridin-1-ium pentaiodide monohydrate C6H9I5N2O3
- Crystal structure of bis(μ3-oxido)-bis(μ2-2-formylbenzoato-k2O:O′)-bis(2-(dimethoxymethyl)-benzoato-κO)-oktakismethyl-tetratin(IV)
- Crystal structure of 2-((E)-(((E)-2-hydroxy-4-methylbenzylidene) hydrazineylidene)methyl)-4-methylphenol, C16H16N2O2
- Crystal structure of (E)-amino(2-((5-methylfuran-2-yl)methylene)hydrazinyl) methaniminium nitrate monohydrate, C14H26N10O10
- The crystal structure of N′-(2-chloro-6-hydroxybenzylidene)thiophene-2-carbohydrazide monohydrate, C12H11ClN2O3S
- Crystal structure of catena-poly[(μ2-1,1′-(biphenyl-4,4′-diyl)bis(1H-imidazol)-κ2N:N′)-bis(4-bromobenzoate-κ1O)zinc(II)], C64H44Br4N8O8Zn2
- The crystal structure of catena-poly[(1-(4-carboxybenzyl)pyridin-1-ium-4-carboxylato-κ1O)-(μ2-oxalato-κ4 O:O′:O″:O‴)dioxidouranium(VI)], C16H11NO10U
- Crystal structure of 3-allyl-4-(2-bromoethyl)-5-(4-methoxyphenyl)-2-phenylfuran, C22H21BrO2
- Halogen bonds in the crystal structure of 4,3′:5′,4″-terpyridine — 1,3-diiodotetrafluorobenzene (1/1), C21H11F4I2N3
- Crystal structure of 2-(1H-indol-3-yl)ethan-1-aminium 2-(4-acetylphenoxy)acetate, C20H22N2O4
- Chalcogen bonds in the crystal structure of 4,7-dibromo-2,1,3-benzoselenadiazole, C6H2Br2N2Se
- The crystal structure of 1,4-bis((1H-benzimidazol-2-yl)methyl)-piperazine-2,5-dione dihydrate, C20H22N6O4
- The crystal structure of C19H20O8
- The crystal structure of KNa3Te8O18·5H2O exhibiting a ∞2[Te4O9]2− layer
- Erratum
- Erratum to: Crystal structure of (Z)-3-(6-bromo-1H-indol-3-yl)-1,3-diphenylprop-2-en-1-one, C23H16BrNO
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of (E)-7-hydroxy-2-((6-methoxypyridin-3-yl)methylene)-3, 4-dihydronaphthalen-1(2H)-one, C17H15NO3
- Crystal structure of (E)-7-methoxy-2-((2-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1 (2H)-one, C18H17NO3
- The crystal structure of N 6,N 6′-di(pyridin-2-yl)-[2,2′-bipyridine]-6,6′-diamine, C20H16N6
- The crystal structure of {N 1,N 2-bis[2,4-dimethyl-6-(4-(tert-butyl)phenyl)(phenyl)methyl]acenaphthylene-1,2-diimino-κ2 N, N′}-dibromido-nickel(II) – dichloromethane(1/2), C64H64Br2Cl4N2Ni
- Synthesis and crystal structure of nonacarbonyltris[(2-thia-1,3,5-triaza-7-phosphatricylco[3.3.1.1]decane-κ1 P)-2,2-dioxide]triruthenium(0) – acetonitrile (7/6), C25.71H32.57N9.86O15P3S3Ru3
- A new polymorph of 1-(4-nitrophenyl)-1H-benzimidazole (C13H9N3O2)
- The crystal structure of 2,2′-((1E,1′E)-(naphthalene-2,3 diylbis(azanylylidene)) bis(methanylylidene))bis(4-methylphenol), C26H22N2O2
- The crystal structure of bis(μ2-iodido)-bis(η6-benzene)-bis(iodido)-diosmium(II), C12H12I4Os2
- Redetermination of the crystal structure of bis{hydridotris(3,5-dimethylpyrazol-1-yl-κN 3)borato}copper(II), C30H44B2CuN12
- Crystal structure of (E)-3-((4-(tert-butyl)phenyl)thio)-4-hydroxypent-3-en-2-one, C15H20O2S
- Crystal structure of 2,2′-(p-tolylazanediyl)bis(1-phenylethan-1-one), C23H21NO2
- Redetermination of the crystal structure of the crystal sponge the poly[tetrakis(μ3-2,4,6-tris(pyridin-4-yl)-1,3,5-triazine)-dodecaiodidohexazinc(II) nitrobenzene solvate], C72H48I12N24Zn6⋅10(C6H5NO2)
- Crystal structure of (4′E)-6′-(diethylamino)-2-[(E)-[(6-methylpyridin-2-yl)methylidene]amino]-4′-{2-[(2E)-1,3,3-trimethyl-2,3-dihydro-1H-indol-2-ylidene]ethylidene}-1′,2,2′,3,3′,4′-hexahydrospiro[isoindole-1,9′-xanthene]-3-one, C44H45N5O2
- Crystal structure of (E)-7-fluoro-2-(3-fluorobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C17H12F2O1
- Crystal structure of tetrabutylammonium sulfanilate – 1-(diaminomethylene)thiourea (1/2)
- Crystal structure of [2,2′-{azanediyl)bis[(propane-3,1-diyl)(azanylylidene)methylylidene]} bis(3,5-dichlorophenolato)-κ2O,O′]-isothiocyanato-κN-iron(III), C21H19Cl4FeN4O2S
- Crystal structure of (4-chlorophenyl)(4-hydroxyphenyl)methanone, C13H9ClO2
- Crystal structure of 6,6′-((pentane-1,3-diylbis(azaneylylidene))bis(methaneylylidene))bis(2,4-dibromolphenolato-κ4 N,N′,O,O′)copper(II),) C19H16Br4CuN2O2
- Chlorido-(2,2′-(ethane-bis(5-methoxyphenolato))-κ4 N,N′,O,O′)manganese(III) monohydrate, C19H18Cl2CuN2O2
- Crystal structure of 2,6-di-tert-butyl-4-(4-methoxybenzylidene)cyclohexa-2,5-dien-1-one, C22H28O2
- Crystal structure of [6,6′-(((2,2-dimethylpropane-1,3-diyl)bis(azanylylidene))bis(methanylylidene))bis(2-chlorophenolato)-κ4N,N′,O,O′]copper(II)
- Crystal structure of 2-chloro-3-((thiophen-2-ylmethyl)amino)naphthalene-1,4-dione, C30H20O4N2Cl2S2
- Crystal structure of bis{hydridotris(3-trifluoromethyl-5-methylpyrazolyl-1-yl)borato-κN 3}manganese(II), C30H26B2F18MnN12
- Crystal structure of 1-(2-methylphenyl)-2-(2-methylbenzo[b]thienyl)-3,3,4,4,5,5-hexafluorocyclopent-ene, C21H14F6S
- Crystal structure of 2-(3-((carbamimidoylthio)methyl)benzyl)isothiouronium hexafluorophosphate monohydrate, C10H17F6N4OPS2
- Crystal structure of 4,5-diiodo-1,3-dimesityl-1H-1,2,3-triazol-3-ium chloride – chloroform (1/1), C21H23Cl4I2N3
- Crystal structure of azido-k1 N-{6,6′-((((methylazanediyl)bis(propane-3,1-diyl))bis(azanylylidene))bis(methanylylidene))bis(2,4-dibromophenolato)k5 N,N′,N″,O,O′}cobalt(III)-methanol (1/1)), C21H23Br4CoN6O3
- The crystal structure of 2-(4-((carbamimidoylthio)methyl)benzyl)isothiouronium hexafluorophosphate monohydrate, C10H17F6N4OPS2
- Crystal structure of 1,1′-(methane-1,1-diyl)bis(3-methyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C9H14F12N4P2
- Crystal structure of (4′E)-6′-(diethylamino)-2-[(E)-[(pyren-1-yl)methylidene]amino]-4′-{2-[(2E)-1,3,3-trimethyl-2,3-dihydro-1H-indol-2-ylidene]ethylidene}-1′,2,2′,3,3′,4′-hexahydrospiro[isoindole-1,9′-xanthene]-3-one, C54H48N4O2
- Crystal structure of poly[bis(μ2-2,6-bis(1-imidazoly)pyridine-κ2 N,N′)-bis(thiocyanato-κ1 N)copper(II)] dithiocyanate, C24H18CuN12S2
- Cones with a three-fold symmetry constructed from three hydrogen bonded theophyllinium cations that coat [FeCl4]− anions in the crystal structure of tris(theophyllinium) bis(tetrachloridoferrate(III)) chloride trihydrate, C21H33Cl9Fe2N12O9
- Crystal structure of 14-O-[(4-(4-hydroxypiperidine-1-yl)-6-methylpyrimidine-2-yl)thioacetyl]-mutilin monohydrate, C32H49N3O6S
- The crystal structure of (E)-3-chloro-2-(2-(4-methylbenzylidene)hydrazinyl)pyridine, C13H12ClN3
- The crystal structure of 4-phenyl-4-[2-(pyridine-4-carbonyl)hydrazinylidene]butanoic acid, C16H15N3O3
- The crystal structure of 6-amino-5-carboxypyridin-1-ium pentaiodide monohydrate C6H9I5N2O3
- Crystal structure of bis(μ3-oxido)-bis(μ2-2-formylbenzoato-k2O:O′)-bis(2-(dimethoxymethyl)-benzoato-κO)-oktakismethyl-tetratin(IV)
- Crystal structure of 2-((E)-(((E)-2-hydroxy-4-methylbenzylidene) hydrazineylidene)methyl)-4-methylphenol, C16H16N2O2
- Crystal structure of (E)-amino(2-((5-methylfuran-2-yl)methylene)hydrazinyl) methaniminium nitrate monohydrate, C14H26N10O10
- The crystal structure of N′-(2-chloro-6-hydroxybenzylidene)thiophene-2-carbohydrazide monohydrate, C12H11ClN2O3S
- Crystal structure of catena-poly[(μ2-1,1′-(biphenyl-4,4′-diyl)bis(1H-imidazol)-κ2N:N′)-bis(4-bromobenzoate-κ1O)zinc(II)], C64H44Br4N8O8Zn2
- The crystal structure of catena-poly[(1-(4-carboxybenzyl)pyridin-1-ium-4-carboxylato-κ1O)-(μ2-oxalato-κ4 O:O′:O″:O‴)dioxidouranium(VI)], C16H11NO10U
- Crystal structure of 3-allyl-4-(2-bromoethyl)-5-(4-methoxyphenyl)-2-phenylfuran, C22H21BrO2
- Halogen bonds in the crystal structure of 4,3′:5′,4″-terpyridine — 1,3-diiodotetrafluorobenzene (1/1), C21H11F4I2N3
- Crystal structure of 2-(1H-indol-3-yl)ethan-1-aminium 2-(4-acetylphenoxy)acetate, C20H22N2O4
- Chalcogen bonds in the crystal structure of 4,7-dibromo-2,1,3-benzoselenadiazole, C6H2Br2N2Se
- The crystal structure of 1,4-bis((1H-benzimidazol-2-yl)methyl)-piperazine-2,5-dione dihydrate, C20H22N6O4
- The crystal structure of C19H20O8
- The crystal structure of KNa3Te8O18·5H2O exhibiting a ∞2[Te4O9]2− layer
- Erratum
- Erratum to: Crystal structure of (Z)-3-(6-bromo-1H-indol-3-yl)-1,3-diphenylprop-2-en-1-one, C23H16BrNO

