Abstract
Background
In this study, the objective was to evaluate the diagnostic performance of some miRNAs, which were shown to have a diagnostic value for prostate cancer (PCa), and the effect of chronic prostatitis in distinguishing benign prostatic hyperplasia (BPH) and PCa.
Materials and methods
Serum levels of 11 miRNAs were investigated in BPH, chronic prostatitis and PCa patients. Measurements were performed using qRT-PCR.
Results
In the analysis, serum levels of miR-375, -125b-5p, -30c-5p, -26b-5p, and let-7c-5p were downregulated in cancer compared with non-cancer group and AUCs of these miRNAs in distinguishing PCa group from non-cancer group were calculated as 0.781, 0.782, 0.762, 0.874, and 0.845, respectively. AUC of the combination of miR-375 and miR-26b-5p in distinguishing PCa group from non-cancer group was 0.891, AUC of these two miRNAs in distinguishing PCa group from BPH group was 0.944.
Conclusion
In our study, 11 miRNAs were studied and 5 of these miRNAs were considered as biomarker candidates as these miRNAs, individually or combined, could be used to distinguish PCa from benign conditions. Furthermore, a higher specificity and sensitivity were obtained in distinguishing BPH and PCa when data for diagnostic potential of miRNAs were analyzed without including chronic prostatic group.
Öz
Amaç
Bu çalışma ile prostat kanserinde tanısal değeri olduğuna yönelik bilimsel literatürde çalışmalar bulunan bazı miRNA’ların prostat kanseri tanısındaki performansının ve kronik prostatitin, BPH ve prostat kanseri (PK) ayrımına etkisinin değerlendirmesi amaçlanmıştır.
Gereç ve Yöntem
Çalışma kapsamında toplam 11 miRNA’nın BPH’lı, kronik prostatitli ve prostat kanserli hasta serumlarındaki seviyeleri değerlendirilmiştir. Ölçümler, qRT-PCR kullanılarak gerçekleştirilmiştir.
Bulgular
Yapılan analizde, kanser olmayan gruba kıyasla kanser grubunda miR-375, -125b-5p, -30c-5p, -26b-5p ve let-7c-5p serum seviyelerinin downregüle olduğu görülmüş ve bu miRNA’ların kanser olmayan gruptan PK’nın ayrımındaki AUC’leri sırasıyla; 0.781, 0.782, 0.762, 0.874 ve 0.845 olarak hesaplanmıştır. miR-375 ve miR-26b-5p kombinasyonunu için kanser olmayan hasta grubundan kanser grubunun ayırımında AUC 0.891, BPH’tan kanser grubunun ayrımında AUC 0.944 olarak hesaplanmıştır.
Tartışma ve Sonuç
Çalışmamızda düzeyleri ölçülen 11 miRNA’nın 5’nin, PK’nın benign durumlardan ayrımında tek tek ya da kombine olarak güçlü birer biyobelirteç adayı olabilecekleri değerlendirilmiştir. Ayrıca, çalışmamızda analiz edilen miRNA’ların tanısal yeterliliğine ilişkin bulgular değerlendirildiğinde, kronik prostatit grubunun analize dahil edilmemesi ile BPH ve PK ayrımında daha yüksek spesifisite ve sensitivite değerleri elde edilmiştir.
Introduction
Prostate cancer (PCa) is the second most common cancer in males and is at fifth place in cancer-associated mortality [1]. Benign prostatic hyperplasia (BPH) and prostatitis are other diseases frequently observed in men. PSA test has important limitations although it is used commonly in PCa screenings and its levels may increase in many different conditions. BPH and prostatitis are leading causes for high PSA values [2], [3].
MicroRNAs (miRNAs), which are 20–22 nucleotides long and have a role in posttranscriptional regulation of genes, are non-coding RNA molecules. miRNAs are transported to the cytoplasm after their synthesis in the nucleus, they bind to mRNAs and then, they regulate protein synthesis and gene expression through the repression of translation or mRNA degradation depending on their match with mRNA [4].
In studies conducted in recent years, findings that they are noninvasive biomarker candidates for the diagnosis of miRNAs in PCa have been obtained [5]. At the same time, It has been shown that miRNAs are associated with the inflammatory processes on a variety of cells and tissues and it has been reported that they may play a role in the development of cancer from underlying inflammation via molecular pathways such as NF-κB [6]. Furthermore, in epidemiological studies, it has been revealed that the risk of PCa in men diagnosed with chronic prostatitis increase within a period of their lives [7]. However, the molecular mechanism of this increased risk has not been fully elucidated, and it is not known whether miRNAs contribute to this increased risk.
In this study, it has been aimed to evaluate the performance of 11 miRNAs (miR-375, -93-5p, -125b-5p, -30c-5p, -26b-5p, -181a2-3p, -221-3p, -222-3p -141-3p, -331-3p and let-7c-3p) with findings in terms of its diagnostic value in the diagnosis of PCa in the literature in the differential diagnosis of BPH, chronic prostatitis and PCa. In addition, it has also been tried to reveal whether miRNAs can be a prognostic marker in the development of cancer from the basis of chronic prostatitis by determining whether the miRNA profile is different compared to BPH and PCa in patient serums with chronic prostatitis or not. It has also been aimed at assessing the effect of chronic prostatitis on the performance of miRNAs on the differential diagnosis of BPH and PCa.
Materials and methods
Study groups
Study samples were obtained from patients of urology clinic of Ankara Dışkapı Yıldırım Beyazıt Education and Research Hospital in a thesis study of specialty in medicine in medical biochemistry. Serum were collected from patients who had prostate needle biopsy with transrectal ultrasonography (TRUS) due to high levels of PSA, lower urinary tract symptoms (LUTS) or a suspicious digital rectal examination and serum were stored at −80°C until the day of experiment. Patients were divided into three groups with respect to biopsy results. According to the biopsy result, those diagnosed with BPH were included in the BPH group, those diagnosed with chronic prostatitis were included in the chronic prostatitis group, and those diagnosed with cancer in the PCa group were included. Patients formerly diagnosed with another cancer type were excluded even if they were cured. An evaluation for metastasis was not performed for patients.
BPH group (n=25)
Asymptomatic prostatitis group (n=10)
PCa group (n=33)
It’s classified as asymptomatic prostatitis by National Institute of Health (NIH), because patients with chronic prostatitis are admitted to the hospital for reasons other than prostate problems and they are directed to urology clinic due to high levels of measured PSA. Gleason score was calculated for each patient with PCa diagnosis. All participants’ rights had been protected and a written informed consents had been obtained before the procedures according to the Helsinki Declaration during sample collection. Ethical approval was obtained from Eskişehir Osmangazi University Medical School Ethics Committee (ref no: 80558721/G-57).
Blood sampling
Suggestions of Clinical and Laboratory Standards Institute (CLSI) were taken into consideration in the process of blood sample collection and storage [8]. Detailed sample collection information is given in Supplementary 1.
Laboratory studies
In all patients, total PSA measurements were performed using immuno-chemiluminescence method in hormone auto-analyzer (Advia Centaur® XP, Siemens, Ireland). RNeasy MinElute spin column kit (Qiagen) was used for RNA isolation. miScript II RT Kit (Qiagen) was used for cDNA synthesis. miScript SYBR® Green PCR Kit (Qiagen) was used for PCR amplification and measurements of miRNAs in Agilent Aria MX device. All steps were performed by following commercial kit procedures. Detailed laboratory steps are given in Supplementary 1.
Statistical analysis
Statistical analysis was performed using PASW Statistics 18. Fold change calculations were performed online using Qiagen website. Ct value (threshold cycle) is a threshold value measured by quantitative real-time PCR by florescence. −Δct values were calculated by normalizing Ct values of miRNAs with respect to the Ct value of ce-miR-39. For this, −(Cttarget miRNA−Ctreference miRNA) formula was used. 2−∆∆Ct formula was used in fold change calculations. Fold changes >2 are considered significant. The association between Gleason score and miRNA serum levels was evaluated using a non-parametric test, Spearman correlation analysis since Gleason score is an ordinal variable. The significance of difference of miRNA levels between cancer group and non-cancer group was analyzed using unpaired t-test. The significance of difference among BPH, chronic prostatitis and cancer groups was analyzed by one-way ANOVA test. Tukey HSD test was used as a post-hoc test. Specificity, sensitivity, positive predictive value (PPV) and negative predictive value (NPV) were calculated by ROC analysis for diagnostic efficiency. The point of ROC curve, which is closest to left upper corner, was taken as a basis for the determination of cut-off values. p-Value <0.05 was considered significant in all calculations.
Results
Age and PSA data of patient groups
Mean age (years) was 64.3 (50.0–79.0) in non-cancer group and 69.4 (51.0–82.0) in cancer group. The difference in age between the two groups was found statistically significant (p=0.010). Only the average age of BPH group was found as 63.6 (50.0–79.0) and the average age of the chronic prostatitis group was also found as 66.0 (57.0–75.0). Age was different between BPH and cancer group (p=0.020). No significant difference was found between the other groups (p>0.05). Since PSA values are not normally distributed, median values were taken into consideration. Median of PSA (ng/mL) values in non-cancer group was 6.0 (2.2–137.9), it was 15.0 (2.8–1654.0) in cancer group. PSA values were different between the two groups (p<0.001). The PSA median was calculated as 5.3 (2.2–137.9) in the BPH group and calculated as 8.9 (4.7–22.6) in the chronic prostatitis group. There was no difference between BPH and chronic prostatitis groups in terms of PSA values (p=0.096). PSA values of PCa group were different from both BPH and chronic prostatitis groups (p<0.001 and p=0.024, respectively).
Investigation of differences of miRNA serum levels between cancer and non-cancer groups
All 11 miRNAs were downregulated in cancer group compared with non-cancer group. 13.01, 12.43, 4.48, 3.60 and 2.64-fold statistically significant downregulation was observed in levels of let-7c-5p, miR-26b-5p, -375, -125b-5p and -30c 5p, respectively.
Although serum levels of miR-93-5p were different between cancer and non-cancer patient groups, it was neglected since the difference was less than two-fold. Remaining five miRNAs (miR-181a2-3p, -221-3p, -222-3p, -141-3p and -331-3p) were not found to be different between groups (p>0.05).
−∆Ct values of miRNAs significantly differed between non-cancer and cancer groups were demonstrated in a box plot chart in Figure 1.
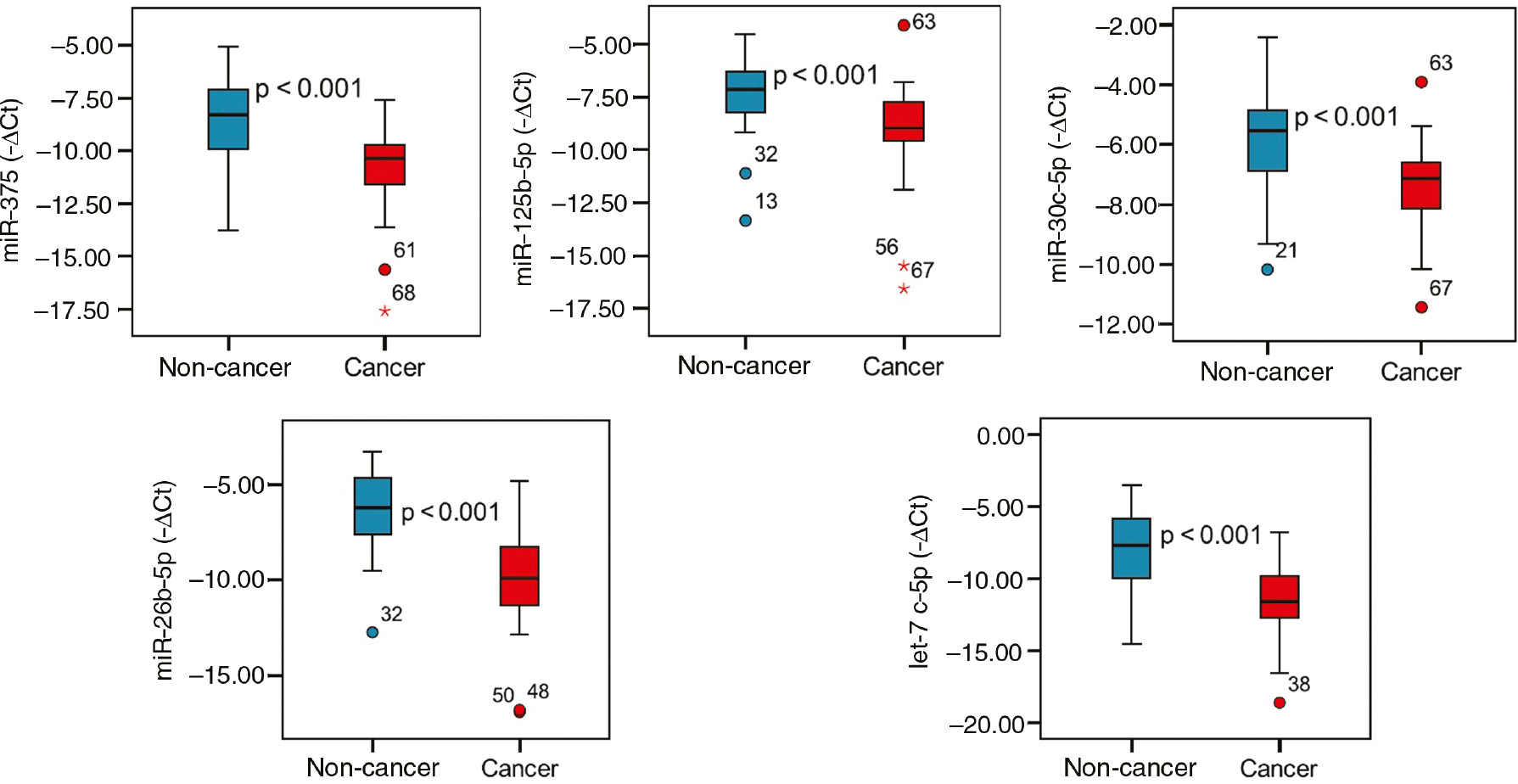
miRNA serum levels in non-cancer and cancer groups.
Evaluation of differences of miRNA measurements between subgroups
It was analyzed whether there is a difference of miRNA measurements between subgroups by one-way ANOVA. After certain miRNAs were found to be at different levels in different groups by variance analysis, Tukey HSD test and online fold change calculations were performed to determine groups that demonstrate difference for selected miRNAs.
miR-141 were amplified in less than two thirds of patients. In variance analysis, p-values for miR-375, -93-5p, -125b-5p, -26b-5p, -30c-5p, let-7c-5p were less than 0.05, therefore, it was accepted that there was a significant difference between groups for these miRNAs. There was not a significant difference between groups for miR-141, -181a2, -221, -222 and -331-3p (p>0.05).
In cancer group compared with BPH group, the expression levels miR-375, -125b-5p, -30c-5p, -26b-5p and let-7c-5p in serum were found to be downregulated 5.60, 3.79, 2.64, 18.53 and 20.67-fold, respectively. In cancer group compared with chronic prostatitis group, serum levels of miR-375, -93-5p, -30c-5p, -26b-5p and let-7c-5p were found to be downregulated 2.58, 2.78, 2.64, 4.59 and 4.09-fold, respectively. In chronic prostatitis group compared with BPH group, expression levels of miR-26b-5p and let-7c-5p were found to be downregulated 4.04 and 5.06, respectively.
Box plot charts for miRNAs that were found to have statistically different serum levels between BPH, chronic prostatitis and cancer patients were presented in Figure 2.
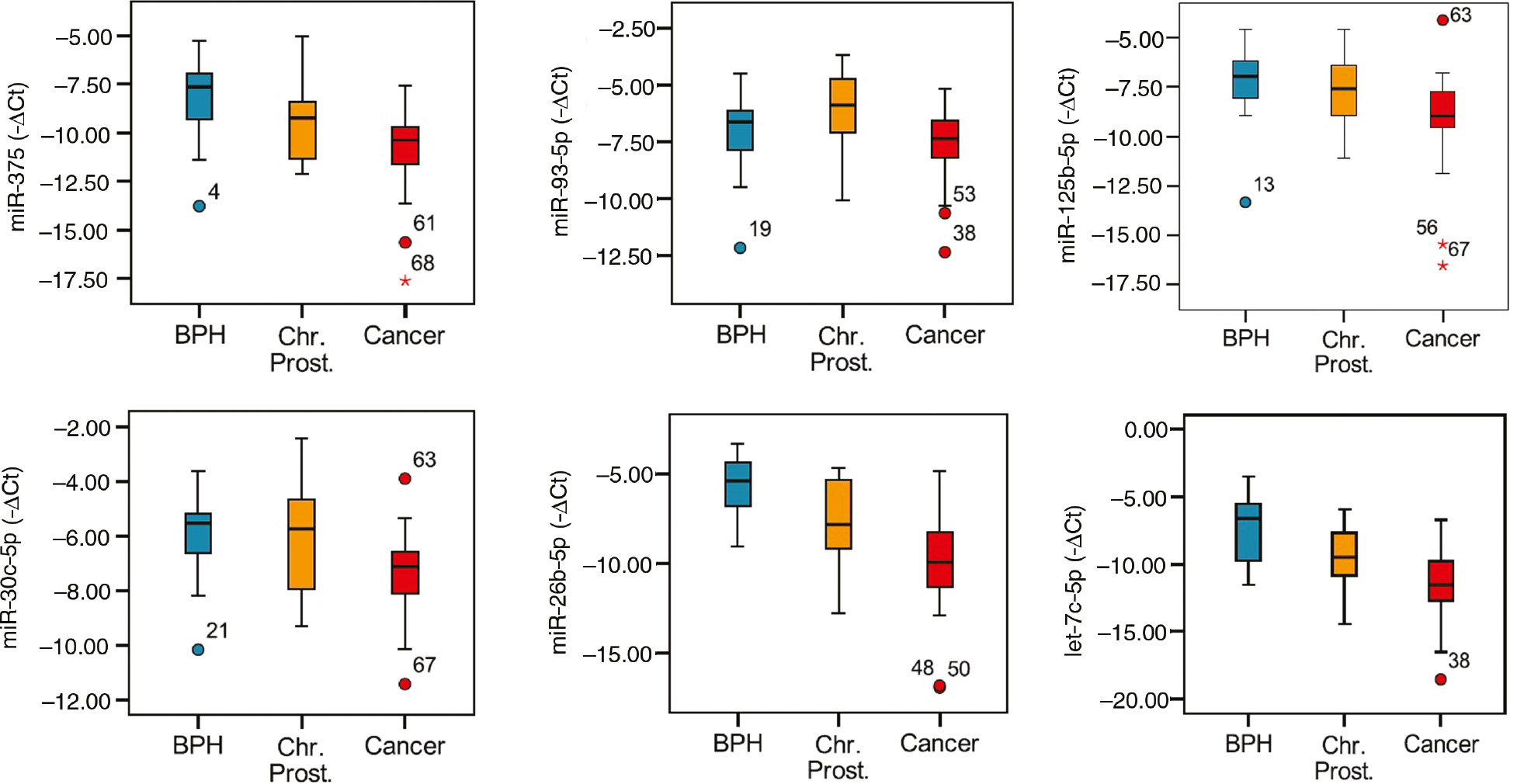
Demonstration of miRNA levels in subgroups using a box plot chart.
Differences of miRNA measurements between cancer subgroups and their association with Gleason score
PCa patients were divided into three groups as Gleason≤6, Gleason=7 and Gleason≥8 with respect to Gleason scores. In variance analysis, serum miRNA levels were not different between groups (p>0.05). Also, there was not a significant correlation between Gleason scores and serum levels of studied miRNAs (p>0.05).
Calculation of diagnostic efficiency of miRNAs in differential diagnosis of prostate cancer
In discriminating cancer group from non-cancer (BPH+chronic prostatitis) group, the highest area under the curves (AUCs) were calculated for miR-26b-5p with 0.874 and let-7c-5p with 0.845. The sensitivity and specificity of these miRNAs were 82% and 83% for miR-26b-5p, and 76% and 71% for let-7c-5p, respectively. AUCs obtained for miRNAs with a statistically significant diagnostic performance and sensitivity, specificity, PPV and NPV percentages were given in Table 1. ROC curves of miRNAs were presented in Figure 3.
Diagnostic efficiency of miRNAs in discriminating groups from each other.
| MicroRNA | Group | AUC | p-Value | SP (%) | SN (%) | PPD (%) | NPD (%) |
|---|---|---|---|---|---|---|---|
| miR-375 | Non-cancer – Cancer | 0.781 | <0.001 | 74 | 76 | 74 | 76 |
| BPH – Ch. Prostatitis | 0.694 | 0.077 | 64 | 80 | 44 | 84 | |
| BPH – Cancer | 0.829 | <0.001 | 80 | 76 | 83 | 71 | |
| Ch. Prostatitis – Cancer | 0.661 | 0.128 | 60 | 76 | 86 | 43 | |
| miR-93-5p | Non-cancer – Cancer | 0.662 | 0.022 | 74 | 61 | 69 | 67 |
| BPH – Ch. Prostatitis | 0.348 | 0.165 | 52 | 40 | 25 | 68 | |
| BPH – Cancer | 0.629 | 0.094 | 72 | 61 | 74 | 58 | |
| Ch. Prostatitis – Cancer | 0.744 | 0.021 | 80 | 61 | 91 | 38 | |
| miR-125b-5p | Non-cancer – Cancer | 0.782 | <0.001 | 80 | 67 | 76 | 72 |
| BPH – Ch. Prostatitis | 0.580 | 0.465 | 56 | 60 | 35 | 78 | |
| BPH – Cancer | 0.812 | <0.001 | 88 | 67 | 88 | 67 | |
| Ch. Prostatitis – Cancer | 0.709 | 0.047 | 60 | 67 | 85 | 35 | |
| miR-30c-5p | Non-cancer – Cancer | 0.762 | <0.001 | 71 | 73 | 71 | 74 |
| BPH – Ch. Prostatitis | 0.504 | 0.971 | 68 | 50 | 39 | 77 | |
| BPH – Cancer | 0.790 | <0.001 | 80 | 73 | 83 | 69 | |
| Ch. Prostatitis – Cancer | 0.691 | 0.070 | 60 | 88 | 91 | 60 | |
| miR-26b-5p | Non-cancer – Cancer | 0.874 | <0.001 | 83 | 82 | 82 | 83 |
| BPH – Ch. Prostatitis | 0.768 | 0.014 | 72 | 70 | 50 | 86 | |
| BPH – Cancer | 0.926 | <0.001 | 92 | 82 | 93 | 79 | |
| Ch. Prostatitis – Cancer | 0.745 | 0.020 | 60 | 82 | 87 | 50 | |
| let-7c-5p | Non-cancer – Cancer | 0.845 | <0.001 | 71 | 76 | 71 | 76 |
| BPH – Ch. Prostatitis | 0.754 | 0.020 | 72 | 70 | 50 | 86 | |
| BPH – Cancer | 0.888 | <0.001 | 72 | 91 | 81 | 86 | |
| Ch. Prostatitis – Cancer | 0.736 | 0.025 | 70 | 61 | 87 | 35 |
AUC, Area under the curve; SP, specificity; SN, sensitivity; PPD, positive predictive value; NPD, negative predictive value. Bold values denote statistical significance at the p<0.05 level.
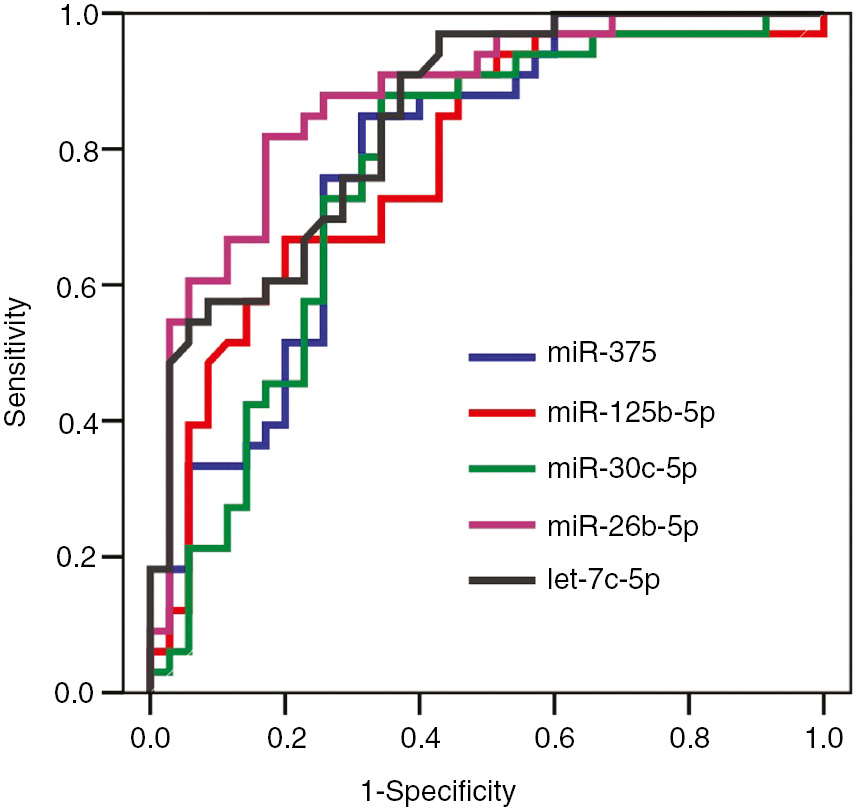
ROC curves of miRNAs in discriminating prostate cancer from non-cancer group.
In discriminating subgroups from each other having chronic prostatitis as a separate group, diagnostic performances of mentioned miRNAs were analyzed with ROC analysis. Results were given in Table 1.
In discriminating BPH group from chronic prostatitis group, AUCs calculated for miR-26b-5p and let-7c-5 were statistically significant. AUCs for miR-26b-5p and let-7c-5p were 0.768 and 0.754, respectively. In discriminating BPH group from chronic prostatitis group, sensitivity and specificity values for miR-26b-5p were 70% and 72%, for let-7c-5p were 70% and 72%, respectively (Figure 4A).
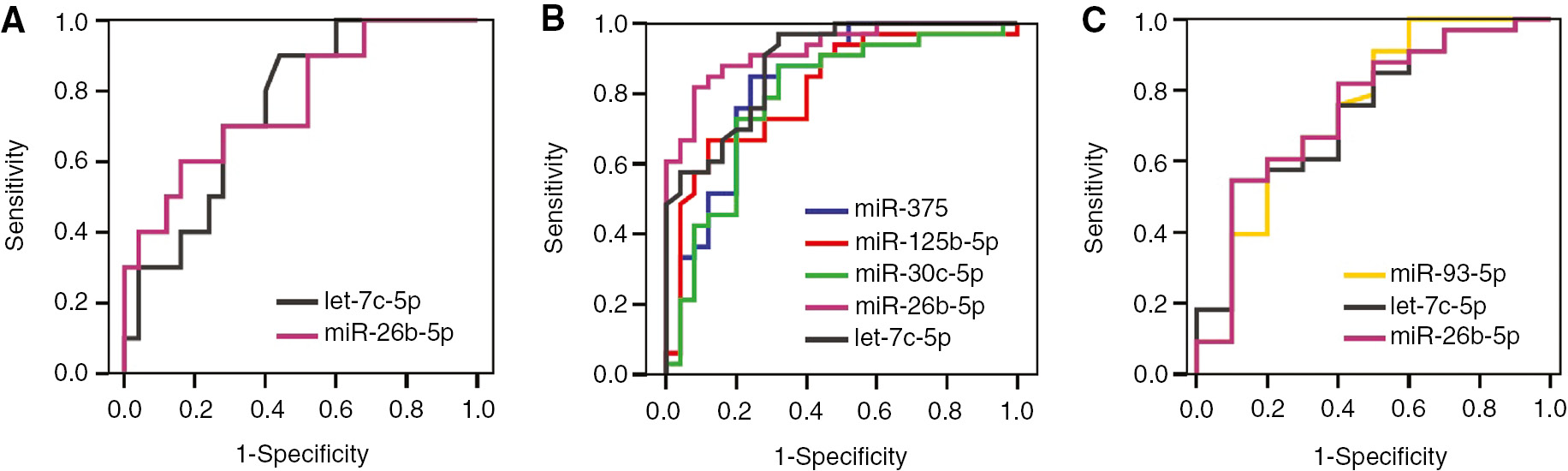
ROC curves of miRNAs in discriminating subgroups from each other.
(A) Chronic prostatitis group vs. BPH group. (B) Prostate cancer group vs. BPH group. (C) Prostate cancer group vs. chronic prostatitis group.
In discriminating BPH group from cancer group, the highest AUCs were 0.926 for miR-26b-5p and 0.888 for let-7c-5p. Sensitivity and specificity values for miR-26b-5p were 82% and 92%, for let-7c-5p were 91% and 72%, respectively (Figure 4B).
In discriminating chronic prostatitis group from cancer group, AUCs for miR-93-5p, miR-26b-5p and let-7c-5p were statistically significant. In distinguishing these two groups, AUCs were 0.745, 0.744 and 0.736 for miR-26b-5p, miR-93-5p and let-7c-5p, respectively. Sensitivity and specificity values for miR-26b-5p were 82% and 60%, for miR-93-5p were 61% and 80%, and for let-7c-5p were 61% and 70%, respectively (Figure 4C).
Logistic regression and ROC analysis were performed to investigate combined diagnostic performance of miRNAs. Results were shown in Table 2 and Figure 5. Among different combinations, the highest AUC belonged to the combination of miR-375 and miR-26b-5p. Adding other miRNAs to this combination or combining all miRNAs together did not result a higher AUC. Accordingly, AUC of the combination of miR-375 and -26b-5p in discriminating cancer group form non-cancer group was 0.891, its sensitivity was 91%, its specificity was 83%, PPV and NPV were 83% and 91%, respectively. AUC of this combination increased to 0.944 in discriminating cancer group from BPH. In this case, the sensitivity was 91%, specificity was 92%, PPV and NPV were 94% and 89%, respectively.
Diagnostic efficiency of the combination of miRNAs in discriminating groups.
| MicroRNA | Group | AUC | p-Value | SP (%) | SN (%) | PPD (%) | NPD (%) |
|---|---|---|---|---|---|---|---|
| miR-375+miR-26b | Non-cancer – Cancer | 0.891 | <0.001 | 83 | 91 | 83 | 91 |
| BPH – Cancer | 0.944 | <0.001 | 92 | 91 | 94 | 89 |
AUC, Area under the curve; SP, specificity; SN, sensitivity; PPD, positive predictive value; NPD, negative predictive value. Bold values denote statistical significance at the p<0.05 level.
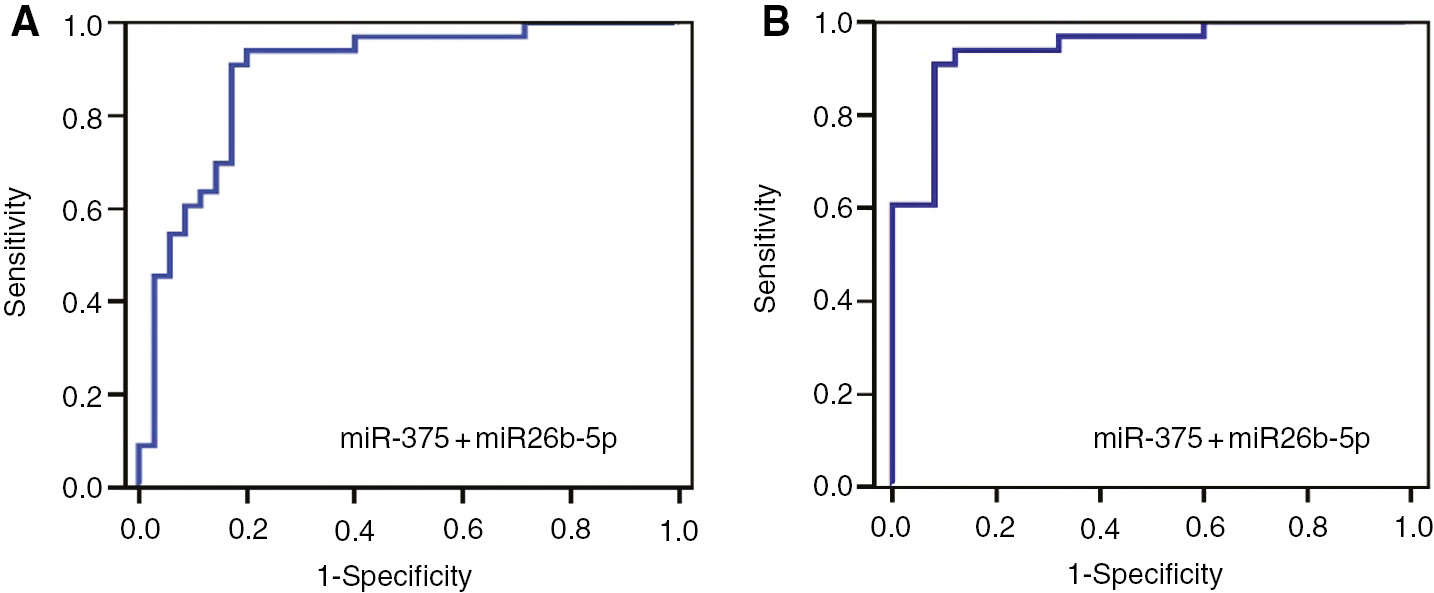
Demonstration of individual and combined diagnostic efficiency of miR-375 and miR-26b-5p by ROC curves in discriminating cancer group and non-cancer group.
(A) Cancer group vs. non-cancer group. (B) Cancer group vs. BPH group.
Discussion and conclusion
In our study, the downregulation of miR-125b-5p, -30c-5p, -26b-5p and let-7c-5p serum levels in PCa group compared with non-cancer group is in accordance with the literature. In a study of Mihelich et al., miR-30c and -223 serum levels were found to be significantly downregulated in high risk PCa group compared with BPH and low risk patients [9]. In the study of Moltzahn et al., serum levels of miR-30c and -26b were found to be downregulated in cancer group patients compared with healthy controls [10]. Serum levels of miR-125b were found to be upregulated in recurrent PCa group compared with non-recurrent PCa patients. Interestingly, it was reported that decreased levels of miR-125b in tumor tissues of the same patients could be used to predict recurrent PCa [11]. In the study conducted by Akbayır et al., it has been reported that a tPSA level of miR-125b-5p is downregulated in the PCa group with greater than 10 ng/mL compared to the benign patient group [12].
In our study, miR-141-3p were amplified over the threshold in only 38 of 68 samples (21 BPH, five chronic prostatitis and 12 PCa samples), it could not be detected in 30 samples. When a statistical analysis was performed considering samples, in which the amplification was successful, serum levels of miR-141-3p were not different between samples.
In several studies that investigate circulatory levels of miR-141, it was shown to be upregulated in metastatic PCa patients compared with healthy controls [13], [14], [15]. However, there are studies which report that circulatory levels of miR-141 are similar in PCa patients and healthy controls and miR-141 could not be amplified in some patients as in our study [16], [17]. When these studies were examined carefully, miR-141 was generally found to be upregulated in metastatic castration-resistant prostate cancer (mCRPC) patients. Although metastasis was not evaluated in our PCa patients, these patients were not treated, medically castrated and therefore did not have resistance to the castration at the time of our sample collection. Therefore, we probably did not find difference for miR-141 serum levels between our non-cancer and PCa patients, because, in the literature, miR-141 was found to be upregulated during androgen deprivation therapy or in metastasis stage of the disease. Hence, a finding supporting this idea came from Xiao et al. In this study, miR-141 was found to enhance the transcription of androgen receptor (AR) by targeting a co-repressor of AR, small heterodimer partner (shp), in androgen sensitive PCa cell lines [18].
There are different findings in the literature regarding circulating levels of miR-375 in PCa. In more than one study it has been reported that circulating levels of miR-375 are upregulated in metastatic PCa compared to localized PCa [19], [20], [21]. However, Kachakova et al. have findings in accordance with our study. In this study, miR-375, let-7c and miR-30c levels have been found to be downregulated in PCa compared with BPH, the highest downregulation has been observed for miR-375 [17]. Besides Sapre et al. have reported that a miRNA panel including miR-375 show no difference for plasma levels between the PCa patients of high risk and low risk [22].
Until now, the only study published recently and assessed of chronic prostatitis miRNA levels has been a study in which the levels of miR-141-3p, -21-5p, -30a-5p, -30d-5p and -103a-3p in the prostatic secretory fluid in patients with prostatitis/chronic pelvic pain syndrome (CP/CPPS) was identified as upregulated compared to healthy controls [23]. For this reason, as we know our study is the first study to evaluate circulating miRNA levels in patients with chronic prostatitis. In our study, the demonstration of its downregulation in the levels of miR-26b-5p and let-7c-5p in the chronic prostatitis group similarly PCa compared to BPH makes think that these miRNAs may be a prognostic marker candidate in the development of cancer from the basis of chronic prostatitis. However, we are in the opinion that prospective cohort studies in which patients with chronic prostatitis are monitored for the development of cancer is need in order to be able to better comment on this issue.
In our study, diagnostic performances of miR-375, 93-5p, -125b-5p, -30c-5p, -26b-5p, and let-7c-5p in discriminating PCa from non-cancer group were found to be statistically significant in the analysis (Table 1). When looked through the studies in the literature, in the study of Cheng et al. AUCs in discriminating healthy control and PCa groups were 0.842 for miR-141 and 0.66 for miR-375 [15]. In a study of Kachakova et al., in discriminating PCa from BPH, AUC was 0.809, specificity was 73% and sensitivity was 81% for miR375; AUC was 0.757, specificity was 61% and sensitivity was 75% for let-7c; AUC was 0.630, specificity was 42% and sensitivity was 63% for miR-30c, AUC was 0.510, specificity was 71% and sensitivity was 50% for miR-141 [17].
In discriminating PCa from non-cancer group, the combination of miR-375 and -26b-5p had a higher AUC compared with any of individual miRNAs (Table 2). In the literature, there are some studies on diagnostic performances of the combination of several miRNAs. In the study of Chen et al. AUC of the combination of miR-622, -1285, -30c, let-7c and let-7e was 0.860 in distinguishing PCa from healthy controls, 0.924 in distinguishing from BPH [24]. In a study of Mihelich et al. 14 miRNAs including miR-26b, -223, -30c and -93, was found to be downregulated in high risk PCa compared with low risk PCa and BPH. The combination of these 14 miRNAs had PPV of 59% and NPV of 100% in distinguishing high risk PCa from low risk PCa [9].
When chronic prostatitis group was not included in the analysis, AUCs of miRNAs except miR-93-5p generally increased in discriminating PCa from BPH. In discriminating PCa from BPH, the highest AUCs were calculated for miR-26b-5p and let-7c-5p, respectively. Also, the highest AUC in discriminating PCa from BPH belonged to the combination of miR-375 and miR-26b-5p.
In conclusion, it was evaluated that miRNAs, whose circulatory levels were identified in our study, were good biomarker candidates individually or in combination in discriminating PCa from benign conditions. Also, when findings for diagnostic efficiency of analyzed miRNAs in our study were evaluated, higher specificity and sensitivity values were obtained in discriminating PCa and BPH without including chronic prostatitis group in the analysis. Thus, it was demonstrated in this study that chronic prostatitis might be an important interference factor in the evaluation of diagnostic performances of miRNAs in discriminating PCa and benign diseases of prostate. Having a gradual change in measured levels of studied miRNAs in chronic prostatitis and PCa compared with BPH, suggests that the relationship of inflammation and cancer transition is reflected also in circulatory miRNA profile. However, we think that further studies need to be carried out in this regard.
Conflict of interest statement: None of the authors have any conflict of interest, financial or otherwise.
Funding: Bilecik Seyh Edebali University, grant number: 2017-01.BSEÜ.25-01.
References
1. GLOBOCAN. “Prostate Cancer Estimated Incidence, Mortality and Prevalence Worldwide in 2012”, International Agency for Research on Cancer, http://globocan.iarc.fr/old/FactSheets/cancers/prostate-new.asp. Accessed: 3 Aug 2018.Search in Google Scholar
2. Bailey SV, Brewster SF. Prostate cancer: to screen or not to screen. Arch Esp Urol 2011;64:406–18.Search in Google Scholar
3. Lim KB. Epidemiology of clinical benign prostatic hyperplasia. Asian J Urol 2017;4:148–51.10.1016/j.ajur.2017.06.004Search in Google Scholar PubMed PubMed Central
4. Peng Y, Croce CM. The role of microRNAs in human cancer. Signal Transduct Target Ther 2016;1:15004.10.1038/sigtrans.2015.4Search in Google Scholar PubMed PubMed Central
5. Vanacore D, Boccellino M, Rossetti S, Cavaliere C, D’Aniello C, Di Franco R. Micrornas in prostate cancer: an overview. Oncotarget 2017;8:50240–51.10.18632/oncotarget.16933Search in Google Scholar PubMed PubMed Central
6. Markopoulos GS, Roupakia E, Tokamani M, Alabasi G, Sandaltzopoulos R, Marcu KB, et al. Roles of NF-кB signaling in the regulation of miRNAs impacting on inflammation in cancer. Biomedicines 2018;6:E40.10.3390/biomedicines6020040Search in Google Scholar PubMed PubMed Central
7. Cheng I, Witte JS, Jacobsen SJ, Haque R, Quinn VP, Quesenberry CP. Prostatitis, sexually transmitted diseases, and prostate cancer: the California Men’s Health Study. PLoS One 2010;5:e8736.10.1371/journal.pone.0008736Search in Google Scholar PubMed PubMed Central
8. Kiechle FL, Betsou F, Blakeney J, Calam RR, Catalasan IM, Raj P, et al. Procedures for the handling and processing of blood specimens for common laboratory tests; Approved Guideline, Fourth Edition. CLSI Document 2010;30:H18–A4.Search in Google Scholar
9. Mihelich BL, Maranville JC, Nolley R, Peehl DM, Nonn L. Elevated serum microRNA levels associate with absence of high-grade prostate cancer in a retrospective cohort. PLoS One 2015;10:e0124245.10.1371/journal.pone.0124245Search in Google Scholar PubMed PubMed Central
10. Moltzahn F, Olshen AB, Baehner L, Peek A, Fong L, Stöppler H, et al. Microfluidic-based multiplex qRT-PCR identifies diagnostic and prognostic microRNA signatures in the serum of prostate cancer patients. Cancer Res 2011;71:550–60.10.1158/0008-5472.CAN-10-1229Search in Google Scholar PubMed PubMed Central
11. Singh PK, Preus L, Hu Q, Yan L, Long MD, Morrison CD, et al. Serum microRNA expression patterns that predict early treatment failure in prostate cancer patients. Oncotarget 2014;5:824–40.10.18632/oncotarget.1776Search in Google Scholar PubMed PubMed Central
12. Akbayır S, Muslu N, Erden S, Bozlu M. Diagnostic value of microRNAs in prostate cancer patients with prostate specific antigen (PSA) levels between 2, and 10 ng/mL. Turk J Urol 2016;42:247–55.10.5152/tud.2016.52463Search in Google Scholar PubMed PubMed Central
13. Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci USA 2008;105:10513–8.10.1073/pnas.0804549105Search in Google Scholar PubMed PubMed Central
14. Selth LA, Townley S, Gillis JL, Ochnik AM, Murti K, Macfarlane RJ, et al. Discovery of circulating microRNAs associated with human prostate cancer using a mouse model of disease. Int J Cancer 2012;131:652–61.10.1002/ijc.26405Search in Google Scholar PubMed
15. Cheng HH, Mitchell PS, Kroh EM, Dowell AE, Chéry L, Siddiqui J, et al. Circulating microRNA profiling identifies a subset of metastatic prostate cancer patients with evidence of cancer-associated hypoxia. PLoS One 2013;8:e69239.10.1371/journal.pone.0069239Search in Google Scholar PubMed PubMed Central
16. Agaoglu FY, Kovancılar M, Dizdar Y, Darendeliler E, Holdenrieder S, Dalay N, et al. Investigation of miR-21, miR-141, and miR-221 in blood circulation of patients with prostate cancer. Tumour Biol 2011;32:583–8.10.1007/s13277-011-0154-9Search in Google Scholar PubMed
17. Kachakova D, Mitkova A, Popov E, Popov I, Vlahova A, Dikov T, et al. Combinations of serum prostate-specific antigen and plasma expression levels of let-7c, miR-30c, miR-141, and miR-375 as potential better diagnostic biomarkers for prostate cancer. DNA Cell Biol 2015;34:189–200.10.1089/dna.2014.2663Search in Google Scholar PubMed PubMed Central
18. Xiao J, Gong AY, Eischeid AN, Chen D, Deng C, Young CY, et al. miR-141 modulates androgen receptor transcriptional activity in human prostate cancer cells through targeting the small heterodimer partner protein. Prostate 2012;72:1514–22.10.1002/pros.22501Search in Google Scholar PubMed
19. Bryant RJ, Pawlowski T, Catto JW, Marsden G, Vessella RL, Rhees B, et al. Changes in circulating microRNA levels associated with prostate cancer. Br J Cancer 2012;106:768–74.10.1038/bjc.2011.595Search in Google Scholar PubMed PubMed Central
20. Brase JC, Johannes M, Schlomm T, Fälth M, Haese A, Steuber T, et al. Circulating miRNAs are correlated with tumor progression in prostate cancer. Int J Cancer 2011;128:608–16.10.1002/ijc.25376Search in Google Scholar PubMed
21. Nguyen HC, Xie W, Yang M, Hsieh C-L, Drouin S, Lee G-S, et al. Expression differences of circulating microRNAs in metastatic castration resistant prostate cancer and low-risk, localized prostate cancer. Prostate 2013;73:346–54.10.1002/pros.22572Search in Google Scholar PubMed PubMed Central
22. Sapre N, Hong MK, Macintyre G, Lewis H, Kowalczyk A, Costello AJ, et al. Curated microRNAs in urine and blood fail to validate as predictive biomarkers for high-risk prostate cancer. PLoS One 2014;9:e91729.10.1371/journal.pone.0091729Search in Google Scholar PubMed PubMed Central
23. Chen Y, Chen S, Zhang J, Wang Y, Jia Z, Zhang X, et al. Expression profile of microRNAs in expressed prostatic secretion of healthy men and patients with IIIA chronic prostatitis/chronic pelvic pain syndrome. Oncotarget 2018;9:12186–200.10.18632/oncotarget.24069Search in Google Scholar PubMed PubMed Central
24. Chen Z-H, Zhang G-L, Li H-R, Luo J-D, Li Z-X, Chen G-M, et al. A panel of five circulating microRNAs as potential biomarkers for prostate cancer. Prostate 2012;72:1443–52.10.1002/pros.22495Search in Google Scholar PubMed
Supplementary Material
The online version of this article offers supplementary material (https://doi.org/10.1515/tjb-2018-0198).
©2019 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Research Articles
- Investigating the impact of polysomy 17 in breast cancer patients with HER2 amplification through meta-analysis
- Diagnostic performance of microRNAs in the circulation in differential diagnosis of BPH, chronic prostatitis and prostate cancer
- Enhanced anticancer effect of cetuximab combined with stabilized silver ion solution in EGFR-positive lung cancer cells
- CA125, YKL-40, HE-4 and Mesothelin: a new serum biomarker combination in discrimination of benign and malign epithelial ovarian tumor
- Paricalcitol pretreatment attenuates renal ischemia/reperfusion injury by inhibiting p38 MAPK and activating PI3K/Akt signaling pathways
- Identification of cytoplasmic sialidase NEU2-associated proteins by LC-MS/MS
- Investigation of tyrosinase inhibition by some 1,2,4 triazole derivative compounds: in vitro and in silico mechanisms
- Investigation of alanine, propionylcarnitine (C3) and 3-hydroxyisovalerylcarnitine (C5-OH) levels in patients with partial biotinidase deficiency
- The expression levels of miR-655-3p, miR127-5p, miR-369-3p, miR-544a in gastric cancer
- Evaluation of the JAK2 V617F gene mutation in myeloproliferative neoplasms cases: a one-center study from Eastern Anatolia
- Effects of Rituximab on JAK-STAT and NF-κB signaling pathways in acute lymphoblastic leukemia and chronic lymphocytic leukemia
- Analysis of the effect of DEK overexpression on the survival and proliferation of bone marrow stromal cells
- Serum fetuin-A levels and association with hematological parameters in chronic kidney disease and hemodialysis patients
- Investigation of relaxation times in 5-fluorouracil and human serum albumin mixtures
- Oxydative stress markers and cytokine levels in rosuvastatin-medicated hypercholesterolemia patients
- The protective effects of urapidil on lung tissue after intestinal ischemia-reperfusion injury
- Effects of SR-BI rs5888 and rs4238001 variations on hypertension
- Antioxidant and cytotoxic activity of three Turkish marine-derived fungi
- Is spectrophotometric enzymatic method a cost-effective alternative to indirect Ion Selective Electrode based method to measure electrolytes in small clinical laboratories?
- Plasma presepsin in determining gastric leaks following bariatric surgery
Articles in the same Issue
- Frontmatter
- Research Articles
- Investigating the impact of polysomy 17 in breast cancer patients with HER2 amplification through meta-analysis
- Diagnostic performance of microRNAs in the circulation in differential diagnosis of BPH, chronic prostatitis and prostate cancer
- Enhanced anticancer effect of cetuximab combined with stabilized silver ion solution in EGFR-positive lung cancer cells
- CA125, YKL-40, HE-4 and Mesothelin: a new serum biomarker combination in discrimination of benign and malign epithelial ovarian tumor
- Paricalcitol pretreatment attenuates renal ischemia/reperfusion injury by inhibiting p38 MAPK and activating PI3K/Akt signaling pathways
- Identification of cytoplasmic sialidase NEU2-associated proteins by LC-MS/MS
- Investigation of tyrosinase inhibition by some 1,2,4 triazole derivative compounds: in vitro and in silico mechanisms
- Investigation of alanine, propionylcarnitine (C3) and 3-hydroxyisovalerylcarnitine (C5-OH) levels in patients with partial biotinidase deficiency
- The expression levels of miR-655-3p, miR127-5p, miR-369-3p, miR-544a in gastric cancer
- Evaluation of the JAK2 V617F gene mutation in myeloproliferative neoplasms cases: a one-center study from Eastern Anatolia
- Effects of Rituximab on JAK-STAT and NF-κB signaling pathways in acute lymphoblastic leukemia and chronic lymphocytic leukemia
- Analysis of the effect of DEK overexpression on the survival and proliferation of bone marrow stromal cells
- Serum fetuin-A levels and association with hematological parameters in chronic kidney disease and hemodialysis patients
- Investigation of relaxation times in 5-fluorouracil and human serum albumin mixtures
- Oxydative stress markers and cytokine levels in rosuvastatin-medicated hypercholesterolemia patients
- The protective effects of urapidil on lung tissue after intestinal ischemia-reperfusion injury
- Effects of SR-BI rs5888 and rs4238001 variations on hypertension
- Antioxidant and cytotoxic activity of three Turkish marine-derived fungi
- Is spectrophotometric enzymatic method a cost-effective alternative to indirect Ion Selective Electrode based method to measure electrolytes in small clinical laboratories?
- Plasma presepsin in determining gastric leaks following bariatric surgery

