Abstract
Objectives
Since studies regarding the effect of polysomy 17 (P17) in breast cancer cases with some specific clinical findings are few in number and are in small sample sizes, meta-analysis was implemented to exhibit the effects of P17 in patients with Human Epidermal growth factor Receptor 2 (HER2) amplification on lymph node involvement and tumor grade.
Materials and methods
Pubmed literature database was scanned up to June 2017 by using the keywords “polysomy 17 breast cancer” and 141 studies were accessed. Ultimately four of the reviewed papers have been found to be appropriate for examining the effect of P17 on lymph node involvement and tumor grade. Prior to meta-analysis, publication bias and heterogeneity of the studies was examined.
Results
Meta-analysis in the examining the effect of polysomy 17 on lymph node involvement (OR=1.708, 95% CI: 1.068–2.733), on grade [3]/[3,1] (OR=3.402, 95% CI: 1.726–6.707), on grade [3]/[3, 2] (OR=2.581, 95% CI: 0.778–8.559) and on grade [2]/[2,1] (OR=1.854, 95% CI: 0.531–6.468) was determined in those with HER2 amplification.
Conclusion
It was observed that in terms of lymph node involvement, P17 was a risk factor in patients and with regard to tumor grade, P17 was a risk factor when grade increased in patients with amplification.
Öz
Amaç
Bazı spesifik klinik bulgulara sahip meme kanseri vakalarında polisomi 17’nin (P17) etkisine ilişkin çalışmalar az sayıda ve küçük örneklem boyutlarında olması nedeniyle, İnsan Epidermal Büyüme Faktörü Reseptör 2 (HER2) amplifikasyonu olan hastalarda P17’nin lenf nodu tutulumu ve tümör evresi üzerindeki etkilerini ortaya koymak amacıyla meta-analiz uygulanmıştır.
Gereç ve Yöntem
Pubmed literatür veri tabanı, 2017 yılının haziran ayına kadar “polysomy 17 meme kanseri” anahtar sözcükleri kullanılarak tarandı ve 141 çalışmaya erişildi. Gözden geçirilen çalışmalardan dördünün P17’nin lenf nodu tutulumu ve tümör evresi üzerindeki etkisini incelemek için uygun olduğu tespit edildi. Meta-analizden önce, çalışmaların yayın yanlılığı ve heterojenitesi incelendi.
Bulgular
Yapılan meta-analiz sonucunda HER2 amplifikasyonu olan hastalarda, P17’nin lenf nodu tutulumu (OR=1.708, 95% CI: 1.068–2.733), evre [3]/[3,1] (OR=3.402, 95% CI: 1.726–6.707), evre [3]/[3,2] (OR=2.581, 95% CI: 0.778–8.559) ve evre [2]/[2,1] (OR=1.854, 95% CI: 0.531–6.468) üzerindeki etkisi belirlendi.
Sonuç
Lenf nodu tutulumu açısından P17’nin amplifikasyonu olan hastalarda risk faktörü olduğu, tümör evresi göz önüne alındığında ise, amplifikasyonu olan hastalarda P17’nin evre yükseldikçe bir risk faktörü olduğu tespit edilmiştir.
Introduction
Breast cancer is the most frequent type of cancer in women and the second most common type of cancer in the worldwide [1]. Targeted treatment for breast cancer has now become part of primary clinical protocols all over the world. Trastuzumab is routinely used monoclonal antibody to treat patients with breast carcinoma overexpressing human epidermal growth factor receptor 2 (HER2) that markedly improves response rate and overall survival when combined to chemotherapy [2], [3], [4]. Approximately 20% of breast cancers overexpress HER2 which is associated with poor prognosis and responsiveness to trastuzumab, hence the evaluation of HER2 status has become an important tool in patient management [5], [6], [7].
HER2 is a crucial breast cancer oncogene, which is located on the long arm of chromosome 17 and encodes a 185-KDa cell surface receptor with tyrosine kinase activity [8]. Abnormalities of chromosome 17 are all important molecular genetic occasions since it harbors several important oncogenes (HER2, TOP2A, TAU), tumor-suppressive genes (p53, BRCA1, HIC-1), DNA double-strand break repair and recombination genes that play an essential role in the development and progression of breast cancer [9]. Two methods available for clinical use to assess HER2 status in breast cancer are the fluorescence in situ hybridization (FISH) for HER2 gene amplification and the immunohistochemistry (IHC) for HER2 protein overexpression [10]. IHC analysis is usually used as the primary assay and confirmatory FISH is performed for a specific subset of IHC results (e.g. 1+ or 2+) [11]. In the most commonly used FISH assay fluorescently labeled dual-probe is used; one that hybridizes to the target HER2 gene and the other that hybridizes to the centromeric region of chromosome 17 (CEP17) [12]. Above a certain threshold value of CEP17 signals that are used as a marker for chromosome 17 number indicates the presence of polysomy 17 [13]. It is reported that P17 is a common finding in breast cancer, about 5–50% [6], [14].
Based on the 2013 ASCO/CAP (American Society of Clinical Oncology/College of American Pathologists) guideline, tumors containing >10% of cells with complete and intense membrane staining by IHC are characterized HER2-positive breast carcinoma. FISH-positive carcinoma is defined considering the ratio of HER2 to CEP17 signals for maximum accuracy. When HER2:CEP17 ratio ≥2 or mean HER2 copy number ≥6, when the HER2:CEP17 ratio <2 are termed as HER2 gene amplification [15].
In cases of uncommon illnesses and their related features, meta-analysis applications are frequently-consulted systematic methods of evaluation. Studies regarding the effect of P17 in breast cancer cases in some clinical findings are few in number and are in small sample sizes. For this reason it is aimed to study, the effects of P17 in patients with amplification on lymph node involvement and grade have been examined.
Materials and methods
With the aim of researching the effects of P17 on lymph node involvement and tumor grade the Pubmed literature database was scanned up by using the keywords “polysomy 17 breast cancer”, until June 2017 and 141 studies were accessed. For research the impact of P17 on lymph node involvement and tumor grade in breast cancer patients through meta-analysis, 4 of the publications examined were found to be suitable for the purpose of our research. Among these studies, the lymph node involvement and grade having findings with HER2 amplification were included in the study.
In the analysis, while the impact of P17 was being researched, lymph node involvement and lacking were determined as “+” and “−”, respectively. When the impact of P17 on grade was being researched, meta-analysis was conducted by separation of cases into three categories; grade1–grade2, grade1–grade3 and grade2–grade3.
Prior to meta-analysis, publication bias of the studies was examined with Begg and Egger tests. In cases of publication bias, the trim and fill method was applied. Heterogeneity of the studies was evaluated according to the Cochran Q test, while to determine degree of heterogeneity, the I2 statistic was employed. In the meta-analyses, the lowest number of studies taken for analysis was 3. In the studies, the value of α was taken as 0.10 for the homogeneity and publication bias tests.
In cases where heterogeneity was determined in the publications following Cochran’s Q test, the DerSimonian-Laird method was carried out using the random effects model, while when there was homogeneity in the publications, the Mantel Haenszel method was applied using the fixed effects model [16]. In the statistical analyses, the MedCalc version 16.4 and Stata/SE 14.0 programs were used.
Results
Examination of impact of polysomy 17 on lymph node involvement in patients with amplification
With the aim of examining the impact of P17 on lymph node involvement, following the literature review of studies on patients with polysomy 17 and amplification, three studies were found. As a result of the Egger test (p=0.790) and Begg’s test (p=0.602), it was determined that there was no publication bias. Cochran’s Q test revealed that there was no heterogeneity (p=0.688; I2=0%). The results of the meta-analysis carried out to examine the impact of P17 on lymph node involvement are given in Table 1 and the forest graph is presented in Figure 1.
Relevant statistics for meta-analysis in examining the effect of polysomy 17 on lymph node involvement in those with amplification.
| Study | Polysomy 17a | Her 2 Nega | Odds ratio | %95 C.I. | z | p-Value | Weights (%) SEM |
|---|---|---|---|---|---|---|---|
| Watters et al. 2003 | 51/89 | 40/84 | 1.476 | 0.810–2.690 | 61.71 | ||
| Nakopoulou et al. 2002 | 9/16 | 4/9 | 1.607 | 0.310–8.322 | 8.21 | ||
| Tsukamoto et al. 2000 | 27/42 | 20/46 | 2.340 | 0.991–5.525 | 30.08 | ||
| Fixed effects | 87/147 | 64/139 | 1.708 | 1.068–2.733 | 2.234 | 0.025 | 100 |
aa/n, (number of lymph node involvement positive)/(total number of cases).
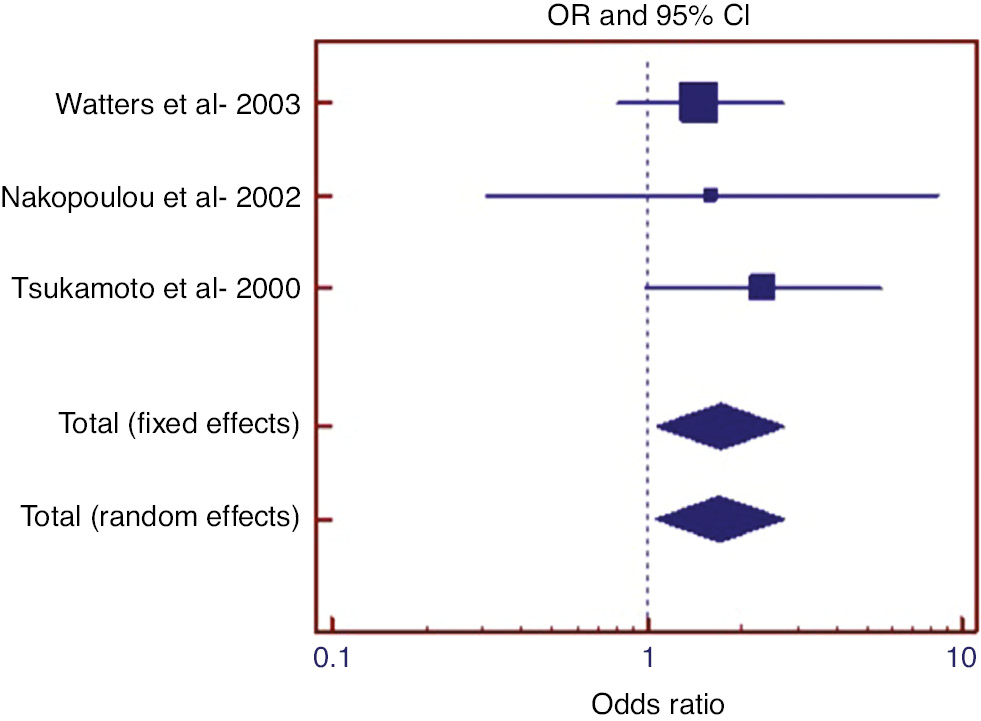
Forest graph in examining the effect of polysomy 17 on lymph node involvement in those with amplification.
Examination of impact of polysomy 17 on grade in patients with amplification
With the aim of examining the impact of P17 for grade 3 cases compared to grade 1 cases, following the literature review of studies on patients with P17 and amplification, three studies were found. As a result of the Egger test (p=0.847) and Begg’s test (p=0.602), it was determined that there was no publication bias. Cochran’s Q test revealed that there was no heterogeneity (p=0.440; I2=0%). The results of the meta-analysis carried out to examine the impact of P17 on grade are given in Table 2 and the forest graph is presented in Figure 2.
Relevant statistics for meta-analysis in examining the effect of polysomy 17 on grade (grade [3]/grade [3, 1]) in those with amplification.
| Study | Polysomy 17a | Her 2 Nega | Odds ratio | %95 C.I. | z | p-Value | Weights (%) SEM |
|---|---|---|---|---|---|---|---|
| Watters et al. 2003 | 50/56 | 38/62 | 5.263 | 1.957–14.151 | 48.75 | ||
| Nakopoulou et al. 2002 | 5/11 | 1/4 | 2.500 | 0.194–32.195 | 7.30 | ||
| Tsukamoto et al. 2000 | 20/29 | 17/33 | 2.092 | 0.738–5.927 | 43.95 | ||
| Fixed effects | 75/96 | 56/99 | 3.402 | 1.726–6.707 | 3.536 | <0.001 | 100 |
aa/n, (number of grade 3)/(total number of cases grade 3 and grade 1).
![Figure 2: Forest graph in examining the effect of polysomy 17 on grade (grade [3]/grade [3, 1]) in those with amplification.](/document/doi/10.1515/tjb-2018-0448/asset/graphic/j_tjb-2018-0448_fig_002.jpg)
Forest graph in examining the effect of polysomy 17 on grade (grade [3]/grade [3, 1]) in those with amplification.
With the aim of examining the impact of P17 for grade 3 cases compared to grade 2 cases, following the literature review of studies on patients with P17 and amplification, four studies were found. As a result of the Egger test (p=0.347) and Begg’s test (p=0.174), it was determined that there was no publication bias. Cochran’s Q test revealed that there was heterogeneity (p=0.003; I2=78.35%). The results of the meta-analysis carried out to examine the impact of polysomy 17 on grade are given in Table 3 and the forest graph is presented in Figure 3.
Relevant statistics for meta-analysis in examining the effect of polysomy 17 on grade (grade [3]/grade [3, 2]) in those with amplification.
| Study | Polysomy 17a | Her 2 Nega | Odds ratio | %95 C.I. | z | p-Value | Weights (%) REM |
|---|---|---|---|---|---|---|---|
| Watters et al. 2003 | 50/91 | 38/71 | 1.059 | 0.568–1.974 | 31.80 | ||
| Nakopoulou et al. 2002 | 5/10 | 1/7 | 6.000 | 0.516–69.757 | 14.16 | ||
| Tsukamoto et al. 2000 | 20/33 | 17/30 | 1.176 | 0.431–3.212 | 27.96 | ||
| Visscher et al. 2000 | 43/66 | 4/28 | 11.217 | 3.470–36.265 | 26.09 | ||
| Random effects | 118/200 | 60/136 | 2.581 | 0.778–8.559 | 1.550 | 0.121 | 100 |
aa/n, (number of grade 3)/(total number of cases grade 3 and grade 2).
![Figure 3: Forest graph in examining the effect of polysomy 17 on grade (grade [3]/grade [3, 2]) in those with amplification.](/document/doi/10.1515/tjb-2018-0448/asset/graphic/j_tjb-2018-0448_fig_003.jpg)
Forest graph in examining the effect of polysomy 17 on grade (grade [3]/grade [3, 2]) in those with amplification.
With the aim of examining the impact of P17 for grade 2 cases compared to grade 1 cases, following the literature review of studies on patients with P17 and amplification, three studies were found. As a result of the Egger test (p=0.249) and Begg’s test (p=0.117), it was determined that there was no publication bias. Cochran’s Q test revealed that there was heterogeneity (p=0.05; I2=65.66%). The results of the meta-analysis carried out to examine the impact of P17 on grade are given in Table 4 and the forest graph is presented in Figure 4.
Relevant statistics for meta-analysis in examining the effect of polysomy 17 on grade (grade [2]/grade [2, 1]) in those with amplification.
| Study | Polysomy 17a | Her 2 Nega | Odds ratio | %95 C.I. | z | p-Value | Weights (%) REM |
|---|---|---|---|---|---|---|---|
| Watters et al. 2003 | 41/47 | 33/57 | 4.970 | 1.819–13.580 | 38.81 | ||
| Nakopoulou et al. 2002 | 5/11 | 6/9 | 0.417 | 0.0672–2.584 | 24.62 | ||
| Tsukamoto et al. 2000 | 13/22 | 13/29 | 1.778 | 0.579–5.457 | 36.56 | ||
| Random effects | 59/80 | 52/95 | 1.854 | 0.531–6.468 | 0.968 | 0.333 | 100 |
aa/n, (number of grade 2)/(total number of cases grade 2 and grade 1).
![Figure 4: Forest graph in examining the effect of polysomy 17 on grade (grade [2]/grade [2, 1]) in those with amplification.](/document/doi/10.1515/tjb-2018-0448/asset/graphic/j_tjb-2018-0448_fig_004.jpg)
Forest graph in examining the effect of polysomy 17 on grade (grade [2]/grade [2, 1]) in those with amplification.
Discussion
Breast cancer is one of the cancer types seen in women worldwide and is the leading cause of cancer deaths [17]. Since it is a heterogeneous disease, it is characterized by multiple subtypes due to different gene expression profiles. This leads to the emergence of multiple prognostic and predictive tumor markers [18]. Accurate detection of human epidermal growth factor receptor 2 (HER2/neu) changes in tumors is crucial in assessing patient prognosis, predicting standard chemotherapy response, and determining compliance to HER2-tailored treatments [19].
Several related studies usually guide most clinical decisions and these studies often differ within in terms of their design; methodological quality; population studied; and the intervention, test, or condition considered. Clinical decision-making necessitates ongoing compromise of studies that ensures different answers to the same question so that combining available information to generate an integrated result seems more coherent. Meta-analysis is an application which enables a more general and representative value with regard to population parameter to be achieved in a particular topic [20], [21]. There are studies in the literature investigating the various effects of P17 and variable HER 2 amplification status in breast cancer patients, but no meta-analysis study is available based on our criteria [22], [23], [24]. In this study, too, due to these that in the literature there are few studies related with P17 and that the studies were made with a small number of cases in terms of the subgroups, impact of amplification on prognosis for breast cancer was examined through meta-analysis. In this study, the aim was to examine the impact of P17 on the lymph node involvement and grade factors namely for cases with HER2 amplification.
For cases with amplification, the literature review carried out with regard to the impact of P17 on lymph node involvement revealed three studies [25], [26], [27]. Although P17 was not found to be an important risk factor for lymph node involvement in each of these studies, it was determined that P17 had a risk factor 1.7 times more significant in cases with positive lymph node involvement, than cases with negative lymph node involvement.
When studies investigating the effect of P17 on tumor grade in patients with amplification were screened, three studies comparing grade 1 and grade 3 were found in the literature. Whilst the two of studies revealed that P17 was not a significant risk factor [25], [26], the other stated that it was a significant risk factor [27]. As a result of the meta-analysis, it was revealed that, P17 had a risk factor 3.4 times greater in grade 3 cases compared to grade 1 cases in patients with amplification.
When studies investigating the effect of P17 on tumor grade in patients with amplification were screened, four studies comparing grade 3 and grade 2 were found in the literature. Whilst the three studies revealed that P17 was not a significant risk factor [25], [26], [27], the other stated that it was a significant risk factor [28]. As a result of the meta-analysis, it was revealed that P17 was not a significant risk factor for grade 3 cases compared to grade 2 cases in patients with amplification.
When studies investigating the effect of P17 on tumor grade in patients with amplification were screened, three studies comparing grade 2 and grade 1 were found in the literature. Whilst the two studies revealed that P17 was not a significant risk factor [25], [26], one of them stated that it was a significant risk factor [27]. As a result of the meta-analysis, it was revealed that P17 was not a significant risk factor for grade 2 cases compared to grade 1 cases in patients with amplification.
In conclusion, when meta-analysis results are evaluated generally, it was observed that in terms of lymph node involvement, P17 was a risk factor in patients with HER2 amplification. With regard to grade, P17 was a risk factor when grade increased in patients with amplification.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interests regarding the publication of this article.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7–30.10.3322/caac.21387Search in Google Scholar
2. Farrell RJ, Peppercorn MA. Ulcerative colitis. Lancet 2002;359:331–40.10.1016/S0140-6736(02)07499-8Search in Google Scholar
3. Van Limbergen J, Russell RK, Drummond HE, Aldhous MC, Round NK, Nimmo ER, et al. Definition of phenotypic characteristics of childhood-onset inflammatory bowel disease. Gastroenterology 2008;135:1114–22.10.1053/j.gastro.2008.06.081Search in Google Scholar PubMed
4. Brant SR. Update on the heritability of inflammatory bowel disease: the importance of twin studies. Inflamm Bowel Dis 2011;17:1–5.10.1002/ibd.21385Search in Google Scholar PubMed
5. Jennette JC, Wilkman AS, Falk RJ. Anti-neutrophil cytoplasmic autoantibody-associated glomerulonephritis and vasculitis. Am J Pathol 1989;135:921–30.Search in Google Scholar
6. Noel LH, Geffriaud C, Chauveau D, Houhou S, Landais P, Kirhaoui F, et al. Antineutrophil cytoplasm antibodies: diversity and clinical applications. Adv Nephrol Necker Hosp 1993;22:237–67.Search in Google Scholar
7. Ramasundara M, Leach ST, Lemberg DA, Day AS. Defensins and inflammation: the role of defensins in inflammatory bowel disease. J Gastroenterol Hepatol 2009;24:202–8.10.1111/j.1440-1746.2008.05772.xSearch in Google Scholar PubMed
8. Rizzo A, Pallone F, Monteleone G, Fantini MC. Intestinal inflammation and colorectal cancer: a double-edged sword? World J Gastroenterol 2011;17:3092–100.Search in Google Scholar
9. Liang P, Pardee AB. Analysing differential gene expression in cancer. Nat Rev Cancer 2003;3:869–76.10.1038/nrc1214Search in Google Scholar PubMed
10. Dudley JT, Tibshirani R, Deshpande T, Butte AJ. Disease signatures are robust across tissues and experiments. Mol Syst Biol 2009;5:307.10.1038/msb.2009.66Search in Google Scholar PubMed PubMed Central
11. Mesko B, Poliska S, Szegedi A, Szekanecz Z, Palatka K, Papp M, et al. Peripheral blood gene expression patterns discriminate among chronic inflammatory diseases and healthy controls and identify novel targets. BMC Med Genomics 2010;3:15.10.1186/1755-8794-3-15Search in Google Scholar PubMed PubMed Central
12. Chang KC, Komm B, Arnold NB, Korc M. The application of differential display as a gene profiling tool. Methods Mol Biol 2007;383:31–40.10.1007/978-1-59745-335-6_3Search in Google Scholar PubMed
13. Zuo L, Ogle CK, Fischer JE, Nussbaum MS. mRNA differential display of colonic mucosa cells in ulcerative colitis. J Surg Res 1997;69:119–27.10.1006/jsre.1997.5041Search in Google Scholar PubMed
14. Bai VU, Kaseb A, Tejwani S, Divine GW, Barrack ER, Menon M, et al. Identification of prostate cancer mRNA markers by averaged differential expression and their detection in biopsies, blood, and urine. Proc Natl Acad Sci U S A 2007;104:2343–8.10.1073/pnas.0610504104Search in Google Scholar
15. Stein J, Liang P. Differential display technology: a general guide. Cell Mol Life Sci 2002;59:1235–40.10.1007/s00018-002-8501-zSearch in Google Scholar
16. Mirza AH, Kaur S, Brorsson CA, Pociot F. Effects of GWAS-associated genetic variants on lncRNAs within IBD and T1D candidate loci. PLoS One 2014;9:e105723.10.1371/journal.pone.0105723Search in Google Scholar
17. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74–108.10.3322/canjclin.55.2.74Search in Google Scholar
18. Turaga K, Acs G, Laronga C. Gene expression profiling in breast cancer. Cancer Control 2010;17:177–82.10.1177/107327481001700306Search in Google Scholar
19. Limentani SA, Brufsky AM, Erban JK, Jahanzeb M, Lewis D. Phase II study of neoadjuvant docetaxel, vinorelbine, and trastuzumab followed by surgery and adjuvant doxorubicin plus cyclophosphamide in women with human epidermal growth factor receptor 2-overexpressing locally advanced breast cancer. J Clin Oncol 2007;25:1232–8.10.1200/JCO.2005.05.3306Search in Google Scholar
20. Juni P, Egger M. PRISMAtic reporting of systematic reviews and meta-analyses. Lancet 2009;374:1221–3.10.1016/S0140-6736(09)61765-7Search in Google Scholar
21. da Costa BR, Juni P. Systematic reviews and meta-analyses of randomized trials: principles and pitfalls. Eur Heart J 2014;35:3336–45.10.1093/eurheartj/ehu424Search in Google Scholar PubMed
22. Petroni S, Addati T, Mattioli E, Caponio MA, Quero C, Rubini V, et al. Centromere 17 copy number alteration: negative prognostic factor in invasive breast cancer? Arch Pathol Lab Med 2012;136:993–1000.10.5858/arpa.2011-0327-OASearch in Google Scholar PubMed
23. Orsaria M, Khelifa S, Buza N, Kamath A, Hui P. Chromosome 17 polysomy: correlation with histological parameters and HER2NEU gene amplification. J Clin Pathol 2013;66:1070–5.10.1136/jclinpath-2013-201506Search in Google Scholar PubMed
24. Tas EO, Sag S, Ercan I. Investigating the impact of polysomy 17 in breast cancer patients without amplification through meta-analysis. Int J Hematol Oncol 2018;28:95–103.10.4999/uhod.182508Search in Google Scholar
25. Tsukamoto F, Miyoshi Y, Egawa C, Kasugai T, Takami S, Inazawa J, et al. Clinicopathologic analysis of breast carcinoma with chromosomal aneusomy detected by fluorescence in situ hybridization. Cancer 2001;93:165–70.10.1002/cncr.9024Search in Google Scholar
26. Nakopoulou L, Giannopoulou I, Trafalis D, Gakiopoulou H, Keramopoulos A, Davaris P. Evaluation of numeric alterations of chromosomes 1 and 17 by in situ hybridization in invasive breast carcinoma with clinicopathologic parameters. Appl Immunohistochem Mol Morphol 2002;10:20–8.10.1097/00129039-200203000-00004Search in Google Scholar
27. Watters AD, Going JJ, Cooke TG, Bartlett JM. Chromosome 17 aneusomy is associated with poor prognostic factors in invasive breast carcinoma. Breast Cancer Res Treat 2003;77: 109–14.10.1023/A:1021399923825Search in Google Scholar
28. Visscher D, Jimenez RE, Grayson M, 3rd Mendelin J, Wallis T. Histopathologic analysis of chromosome aneuploidy in ductal carcinoma in situ. Hum Pathol 2000;31:201–7.10.1016/S0046-8177(00)80220-8Search in Google Scholar
©2019 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Research Articles
- Investigating the impact of polysomy 17 in breast cancer patients with HER2 amplification through meta-analysis
- Diagnostic performance of microRNAs in the circulation in differential diagnosis of BPH, chronic prostatitis and prostate cancer
- Enhanced anticancer effect of cetuximab combined with stabilized silver ion solution in EGFR-positive lung cancer cells
- CA125, YKL-40, HE-4 and Mesothelin: a new serum biomarker combination in discrimination of benign and malign epithelial ovarian tumor
- Paricalcitol pretreatment attenuates renal ischemia/reperfusion injury by inhibiting p38 MAPK and activating PI3K/Akt signaling pathways
- Identification of cytoplasmic sialidase NEU2-associated proteins by LC-MS/MS
- Investigation of tyrosinase inhibition by some 1,2,4 triazole derivative compounds: in vitro and in silico mechanisms
- Investigation of alanine, propionylcarnitine (C3) and 3-hydroxyisovalerylcarnitine (C5-OH) levels in patients with partial biotinidase deficiency
- The expression levels of miR-655-3p, miR127-5p, miR-369-3p, miR-544a in gastric cancer
- Evaluation of the JAK2 V617F gene mutation in myeloproliferative neoplasms cases: a one-center study from Eastern Anatolia
- Effects of Rituximab on JAK-STAT and NF-κB signaling pathways in acute lymphoblastic leukemia and chronic lymphocytic leukemia
- Analysis of the effect of DEK overexpression on the survival and proliferation of bone marrow stromal cells
- Serum fetuin-A levels and association with hematological parameters in chronic kidney disease and hemodialysis patients
- Investigation of relaxation times in 5-fluorouracil and human serum albumin mixtures
- Oxydative stress markers and cytokine levels in rosuvastatin-medicated hypercholesterolemia patients
- The protective effects of urapidil on lung tissue after intestinal ischemia-reperfusion injury
- Effects of SR-BI rs5888 and rs4238001 variations on hypertension
- Antioxidant and cytotoxic activity of three Turkish marine-derived fungi
- Is spectrophotometric enzymatic method a cost-effective alternative to indirect Ion Selective Electrode based method to measure electrolytes in small clinical laboratories?
- Plasma presepsin in determining gastric leaks following bariatric surgery
Articles in the same Issue
- Frontmatter
- Research Articles
- Investigating the impact of polysomy 17 in breast cancer patients with HER2 amplification through meta-analysis
- Diagnostic performance of microRNAs in the circulation in differential diagnosis of BPH, chronic prostatitis and prostate cancer
- Enhanced anticancer effect of cetuximab combined with stabilized silver ion solution in EGFR-positive lung cancer cells
- CA125, YKL-40, HE-4 and Mesothelin: a new serum biomarker combination in discrimination of benign and malign epithelial ovarian tumor
- Paricalcitol pretreatment attenuates renal ischemia/reperfusion injury by inhibiting p38 MAPK and activating PI3K/Akt signaling pathways
- Identification of cytoplasmic sialidase NEU2-associated proteins by LC-MS/MS
- Investigation of tyrosinase inhibition by some 1,2,4 triazole derivative compounds: in vitro and in silico mechanisms
- Investigation of alanine, propionylcarnitine (C3) and 3-hydroxyisovalerylcarnitine (C5-OH) levels in patients with partial biotinidase deficiency
- The expression levels of miR-655-3p, miR127-5p, miR-369-3p, miR-544a in gastric cancer
- Evaluation of the JAK2 V617F gene mutation in myeloproliferative neoplasms cases: a one-center study from Eastern Anatolia
- Effects of Rituximab on JAK-STAT and NF-κB signaling pathways in acute lymphoblastic leukemia and chronic lymphocytic leukemia
- Analysis of the effect of DEK overexpression on the survival and proliferation of bone marrow stromal cells
- Serum fetuin-A levels and association with hematological parameters in chronic kidney disease and hemodialysis patients
- Investigation of relaxation times in 5-fluorouracil and human serum albumin mixtures
- Oxydative stress markers and cytokine levels in rosuvastatin-medicated hypercholesterolemia patients
- The protective effects of urapidil on lung tissue after intestinal ischemia-reperfusion injury
- Effects of SR-BI rs5888 and rs4238001 variations on hypertension
- Antioxidant and cytotoxic activity of three Turkish marine-derived fungi
- Is spectrophotometric enzymatic method a cost-effective alternative to indirect Ion Selective Electrode based method to measure electrolytes in small clinical laboratories?
- Plasma presepsin in determining gastric leaks following bariatric surgery

