Abstract
Background
Tyrosinase plays a central role in the biosynthesis pathway of melanin pigment. Melanin protects human skin against radiation and its unusual levels cause some skin disorders such as pregnancy scar, oldness spots and melanoma. Tyrosinase has also been linked to Parkinson’s and other neurodegenerative diseases. In addition, melanin plays a critical role as a defense molecule for insects during wound healing and is important for their life. Therefore, determination of inhibitor molecules for tyrosinase has a promising potential for therapies of some diseases and is an alternative method for keeping insects under control.
Material and methods
In this study, 1-hepthyl-3-(4-methoxybenzyl)-4H-1,2,4-triazole-5-one derivative (A6, A8, A15) and 3-(4-chlorophenyl)- 5-(4-methoxybenzyl)-4H-1,2,4-triazole (B5, B9, B13) derivative compounds were evaluated in terms of their potential for mushroom tyrosinase inhibition. IC50 values of these six molecules were determined.
Results
It was seen that B9 molecule was the most effective inhibitor. Docking studies also nearly supported this end result. Tyrosinase inhibition type and Ki value were found to be uncompetitive and 370.7±0.3 μM, respectively, in the presence of B9 compound.
Conclusion
These results suggest that B9 compound is a potential tyrosinase inhibitor.
Öz
Amaç
Tirozinaz, melanin pigmentinin biyosentez yolunda merkezi bir rol oynar. Melanin insan cildini radyasyona karşı korur ve artan miktarı hamilelik ve yaşlılık lekeleri ile melanoma gibi bazı cilt bozukluklarına neden olur. Tirozinaz ayrıca Parkinson ve diğer nörodejeneratif hastalıklarla da bağlantılıdır. Buna ek olarak, melanin böcekler için, yara iyileşmesi sırasında bir savunma molekülü olarak kritik bir rol oynar ve onların yaşamları için önemlidir. Bu nedenle, tirozinaz için inhibitör moleküllerin belirlenmesi, bazı hastalıkların tedavileri için ümit verici bir potansiyele sahiptir ve böceklerin kontrol altında tutulmasi için alternatif bir yöntemdir.
Gereç ve Yöntem
Bu çalışmada, 1-heptil-3-(4-metoksibenzil)-4H-1,2,4-triazol-5-on türevi (A6, A8, A15) ve 3-(4-klorofenil)-5-(4-metoksibenzil)-4H-1,2,4-triazol (B5, B9, B13) türevi bileşikler, mantar tirozinaz inhibisyonu açısından potansiyel olarak değerlendirildi ve bu altı molekülün IC50 değerleri belirlendi.
Bulgular
Çalışma sonucunda B9 molekülünün en etkili inhibitör olduğu görülmüştür. Doking çalışmaları da bu sonucu desteklemektedir. Tirozinaz inhibisyon tipinin ve Ki değerinin, B9 bileşiğinin varlığında sırasıyla unkompetetif ve 370.7±0.3 μM olduğu bulundu.
Sonuç
Bu sonuçlar, B9 bileşiğinin potansiyel bir tirozinaz inhibitörü olduğunu göstermektedir.
Introduction
Besides the fact that enzymes and their activities are extremely necessary for life, the selective inhibition of critical enzymes is also considerably important for chemotherapeutic intervention in some diseases. Unregulated high enzyme activity results in the formation of reaction products at abnormal levels which can cause specific pathologies. Nowadays, the strategy of selective enzyme inhibition gets attention in modern pharmacy and enzymes have become interesting targets in drug therapies [1]. For this reason, many organic molecules have been synthesized as specific enzyme inhibitors and continue to be synthesized. Modeling methods for the three-dimensional structure and topology of the enzyme, active site and the organic molecules help to researcher to design new drug molecules or evaluate synthesized molecules for their enzyme inhibition potentials.
Tyrosinase (Polyphenol oxidase, PPO, E.C.1.14.18.1) which is a copper-containing metalloenzyme, catalyzes two major reactions in the biosynthesis pathway of melanin pigment: (1) the hydroxylation and oxidation of monophenols to o-quinones (monophenolase activity) and (2) the oxidation of o-diphenols to o-quinones (diphenolase activity) [2]. Melanin is an important pigment which is found in the eyes, hair and skin of animals and especially protecting human skin against radiation [3]. The melanin pigment is produced in the melanocyte cells located in the basal layer of the human epidermis and is called melanogenesis. However, excessive production and hyperpigmentation of melanin causes of dermatological disorders such as melasma, ephelides, chloasma, freckles, melanoderma and senile lentigines and can induce inflammation such as eczema, irritant and allergic eczema contact dermatitis, which can result in critical and emotionally distressing trouble [4], [5], [6].
Melanoma resulted from abnormal accumulation of melanin is one of the fastest-spreading and deadly skin cancers among cancer types worldwide [7]. As the tyrosinase enzyme is found only in melanoma cells, inhibition of this enzyme will help to establish a highly specific treatment for skin cancer. So, in melanoma cells, a wide variety of inhibitors are used for the inhibition of tyrosinase enzyme [8]. However, it is expected that these inhibitors only target cancer cells and do not cause damage to DNA of healthy cells. Unfortunately, today’s cancer treatments are not long-lived. The greatest reason for this is that the chemotherapeutics applied for the treatment delay or prevent the definitive treatment of the disease by causing damage to the DNA of the healthy cells in the body [9]. This encourages the researchers in the world to design, synthesis and kinetically investigation of new organic molecules without side effect. Tyrosinase is not only associated with melanoma but also with Parkinson’s disease and some other neurodegenerative diseases [10]. The limited number of pharmacological agents used for Parkinson’s disease inhibitors are reported to show their effectiveness in reducing or preventing the progression of the disease. It is reported that the increased amount of enzyme also increases the amount of metabolites that are formed, leading to oxidative damage to the body in the long run. Therefore, synthesizing therapeutic drugs for Parkinson’s disease provides a vital way to treat this disease [8], [11].
Melanin plays a critical role as a defense molecule for insects during molting process and wound healing and is important for their survival. In addition, quinonoid intermediates generated by tyrosinase serve as defense molecules for insects. All of these make tyrosinase a potential target for developing effective inhibitors as insecticides for insect control [2].
Alkoxybenzoic acids, tropolone, arbutin, aromatic acids, aromatic aldehydes and kojic acid, and lately, dialkylphosphorylhydrazones have been reported as well-known tyrosinase inhibitors. However, due to the low activity and cellular toxicity of these inhibitors in clinical use, effective and feasible new anti-tyrosinase agents should be developed [12], [13].
Many heterocyclic compounds containing 1,2,4-triazole ring is known to have important pharmacological properties such as anticonvulsant, antifungal, antimicrobial, analgesic, antiviral, anti-inflammatory, antioxidant, antitumor, anti-HIV and antihypertensive [14]. In addition, many compounds containing the 1,2,4-triazole ring are used as medicines in the market [15]. In recent year, synthesis of new thiosemicarbazones, phenylbenzoic acid, apocarotenoids, fumaric acid, 4H-chromene analogs, oxaloacetic acid as inhibitors of mushroom tyrosinase were reported. These molecules have been evaluated in terms of tyrosinase inhibition potentials by studying kinetic mechanism and molecular docking [16], [17], [18], [19]. In another study, some novel synthesized hydroxybenzaldehyde based kojic acid analogs as potential tyrosinase inhibitors were evaluated by performing biological activity and kinetic mechanism [20]. Several studies have also been conducted with new scaffold type of phenylbenzoic acid derivatives synthesized as potential tyrosinase inhibitor [18]. Also, Lippia origanoides essential oils and neorauflavane were isolated from Campylotropis hirtella supported by kinetic studies in terms of tyrosinase inhibitory potency [5], [21]. Addition to in vitro and in silico studies, in vivo studies have been carried out by the researchers for declaring tyrosinase inhibition potential of some newly synthesized molecules. As an example of recent studies on this perspective, chalcone derivative 1-(2-cyclohexylmethoxy-6-hydroxy-phenyl)-3-(4-hydroxymethyl-phenyl)-propenone and Na7PMo11CuO40 investigated in terms of tyrosinase inhibitor effect with in vivo anticancer studies [22].
However, due to the wide pharmaceutical properties of the triazole compounds is well known, the purpose of this study is to examine tyrosinase inhibition potentials of some newly synthesized 1,2,4-triazole derivatives (Table 1) by performing biochemical, kinetic and molecular modeling studies.
Organic molecules evaluated for tyrosinase inhibition potentials.
| Code | Nomenclature | Formulas |
|---|---|---|
| A6 | 2-[3-(4-Methoxybenzyl)-1-heptyl-5-oxo-1,5-dihydro-4H-1,2,4-triazol-4-yl]-N′-[(5-chloro-2- hydroxyphenyl)methylidene] acetohydrazide | 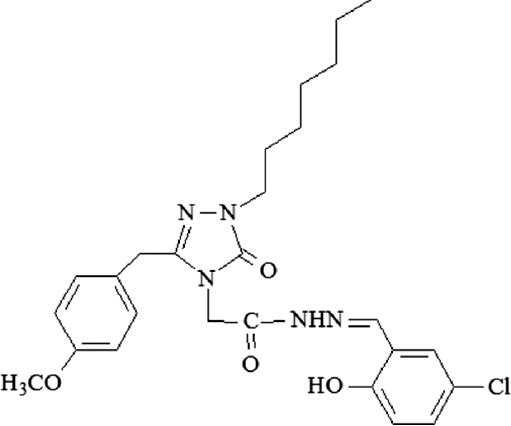 |
| A8 | 2-[3-(4-Methoxybenzyl)-1-heptyl-5-oxo-1,5-dihydro-4H-1,2,4-triazol-4-yl]-N′-[(2-hydroxy-5- nitrophenyl)methylidene] acetohydrazide | 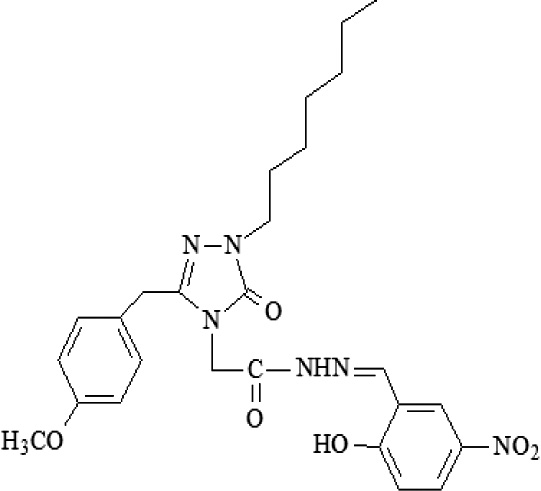 |
| A15 | 2-heptyl-4-[(4-phenyl-4,5-dihydro-5-thione-1H-1,2,4-triazol-3-yl)methyl]-5-(4-methoxybenzyl)-2,4 dihydro-3H-1,2,4-triazol-3-one | 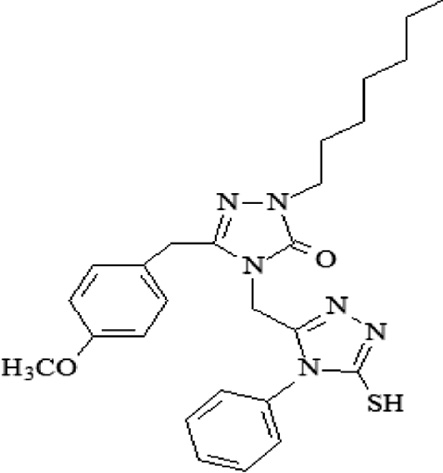 |
| B5 | 4-{[4-(fluorophenyl)methyl]amino}-5-{[3-(4-chlorophenyl)-5-(4-methoxybenzyl)-4H-1,2,4-triazol-4-yl]-methyl}-2,4-dihydro-3H-1,2,4-triazole-3-thione | 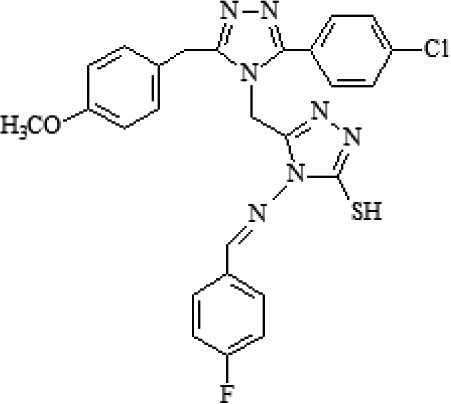 |
| B9 | 4-{[4-(fluorophenyl)methyl]amino}-2-(4-methylpiperazin-1-yl)-5-{[3-(4-chlorophenyl)-5- (4-methoxybenzyl)-4H-1,2,4-triazol-4-yl] methyl}-2,4-dihydro-3H-1,2,4-triazole-3-thione |  |
| B13 | 5-{[3-(4-chlorophenyl)-5-(4-methoxybenzyl)-4H-1,2,4-triazol-4-yl]methyl}-4-methyl-2,4-dihydro-3H-1,2,4-triazole-3-thione | 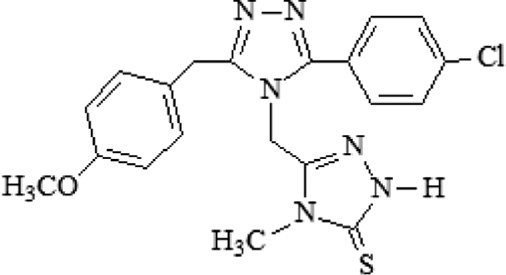 |
Materials and methods
Chemicals
The main chemicals used in this study were ethanol (Merck, Germany), mushroom tyrosinase (Sigma, T3824, ≥1000 unit/mg solid, USA), L-tyrosine (Sigma, T3754, USA), N,N-dimethylformamide (DMF, Sigma, 227056, USA), 3-Methyl-2-benzothiazolinone hydrazone hydrochloride hydrate (MBTH, Sigma, 129739, USA), Kojic acid (Sigma, K3125, USA).
Tyrosinase assay, activity optimization and inhibition
Tyrosinase (polyphenol oxidase, PPO) activity was determined according to the method described by Espin et al. [26] with slight modification and kojic acid was used as a standard inhibitor. Briefly, 100 μL L-tyrosine solution (0.14 mM), 720 μL citrate-phosphate buffer solution (50 mM, pH 5.0), 100 μL MBTH solution (1 mM), 20 μL DMF (anhydrous), 10 μL pH 5.0 citrate-phosphate buffer solution (in inhibiton studies, it replaces with equal volume of inhibitor solution) and 50 μL tyrosinase solution (43.5 μg/mL in citrate-phosphate buffer solution) were added to the reaction mixture, respectively.
Optimum pH, temperature and reaction time, and Km and Vmax values were separately determined before inhibition studies for avoiding errors. For inhibition studies, 10 μL of each inhibitor solutions (in ethanol) were added onto 50 μL tyrosinase solution and preincubated at 25°C for 15 min. The activity was determined in the presence of L-tyrosine as substrate by measuring the increase in absorbance due to the formation of quinonoid compound as reaction product at 500 nm.
One unit of PPO activity was defined as 1 μmol of product formed per min in 1 mL of reaction mixture. The inhibition efficiencies of examined organic molecules were expressed as the concentration which inhibits 50% of the enzyme activity (IC50).
Determination of Km, Vmax, Ki and inhibition type
After measuring tyrosinase activities in the absence and presence of inhibitor molecule (B9; 0 μM, 200 μM and 400 μM), Linewevar-Burk graphics were prepared in the 50–700 μM L-tyrosine concentration range for the estimation of inhibition type, Ki value and changes in Km and Vmax values.
Enzyme and compounds preparation for docking
Firstly, the inhibitor organic molecules given in Table 1 were optimized by using the Gaussian 03 program for docking studies. DFT 6-31G (d, p) was used as the optimization method and the most appropriate comformations of the compounds were identified [27], [28]. Crystalline form of the tyrosinase selected as the target receptor for which the docking of the compounds is to be performed was obtained in the PDB format (2Y9W for Agaricus bisporus tyrosinase) from the Protein Data Bank (PDB) website after crystalline form was found from literature [3]. Enzyme was purified using Discovery Studio 4.1 Client program and then ligand-protein interactions of the organic compounds in the binding pocket were investigated using AutoDock Vina 1.1.2 and PyMOL programs [29], [30], [31], [32]. The binding energies of organic molecules were calculated and the inhibition potentials were theoretically monitored.
Statistical analysis
Statistical analyses were performed using SPSS software (version 23), and the data were compared by one way ANOVA test. The differences between groups were analyzed by Post-hoc test. Tukey test p-values less than 0.05 were considered as significant.
Results
After optimization of tyrosinase activity (Table 2), inhibition studies were carried out in the presence of 0.14 mM (Km) L-tyrosine substrate as described above. Percentage relative activities were separately plotted versus inhibitor concentrations for each organic molecule and the inhibitor concentration at which the relative activity is reduced by 50% determined as IC50 value. Accordingly, B9 among the examined compounds was found as the most effective inhibitor for tyrosinase activity (Table 2). Changes in Km and Vmax values were observed by using Lineweaver-Burk graphs in the absence and presence of (0 μM, 200 μM and 400 μM) the B9 compound. The type of inhibition and Ki value were also identified for B9 compound (Figure 1, Table 3). According to this, inhibiton type was found to be uncompetetive and Ki value was calculated as 370.7±0.3 μM.
Tyrosinase inhibition potentials of organic compounds.
| IC50 (μM) | Maximum inhibition | Binding affinity (ΔG, kcal/mol) | ||
|---|---|---|---|---|
| % | [I] (μM) | |||
| A6 | >800 | 44.2±0.8 | 800 | −7.5 |
| A8 | >720 | 20.1±0.5 | 720 | −6.7 |
| A15 | >480 | 28.3±1.4 | 480 | −5.5 |
| B5 | >108 | 29.7±2.5 | 108 | −7.0 |
| B9 | 400 | 55.2±0.7 | 450 | −7.6 |
| B13 | 580 | 50.1±0.4 | 580 | −8.1 |
| Kojic acid | 18,000 | 97.3±0.2 | 1×106 | – |
![Figure 1: Plot of 1/[S] vs. 1/v.Determination of inhibiton type, and changes in Km and Vmax values in the presence of B9 (200 and 400 μM) or its absence.](/document/doi/10.1515/tjb-2018-0273/asset/graphic/j_tjb-2018-0273_fig_001.jpg)
Plot of 1/[S] vs. 1/v.
Determination of inhibiton type, and changes in Km and Vmax values in the presence of B9 (200 and 400 μM) or its absence.
Type of tyrosinase inhibition in the presence of B9 compound and some kinetic parameters.
| [Inhibitor] (μM) | Apparent Km (μM) | Vmax (μmol/min) | Type of inhibition | Ki (μM) |
|---|---|---|---|---|
| 0 | 257.9±1.4 | 149.3±1.2 | Uncompetetive | 370.7±0.3 |
| 200 | 192.8±0.9 | 109.9±0.8 | ||
| 400 | 71.0±0.7 | 41.2±0.4 |
The compounds were investigated by performing docking studies with the crystal structure of mushroom tyrosinase to observe the interaction of these compounds with the enzyme. The top 9 predicted conformations of organic compounds generated by DFT 6-31G(d,p) were retained for analyzing the binding affinities of them. Accordingly, the results of the docking studies nearly support the experimental results and show that the B9 compound among the evaluated molecules binds to the enzyme (Figure 2A) efficiently as an inhibitor (Table 2). Importance of inhibitor-metal ion interactions in tyrosinase binding site which is a Cu2+ containing metalloenzyme and its highlights were discussed in detail with different perspectives [33], [34]. The interactions of different groups of the B9 molecule with the amino acid side chains in the appropriate position in the three-dimensional structure of the enzyme are also important in the formation of the enzyme-inhibitor complex (Figure 2B). Some of these highlights are listed as follows; (1) π-donor and π-alkyl interactions of chlorophenyl ring of B9 with Ser146 and Ala202, respectively, (2) π-anion, π-anion, π-alkyl and conventional interactions of triazole ring with Asp99, Asp113, Ala110 and Ser96, respectively, (3) π-alkyl interaction of methoxyphenyl ring with Ala110.
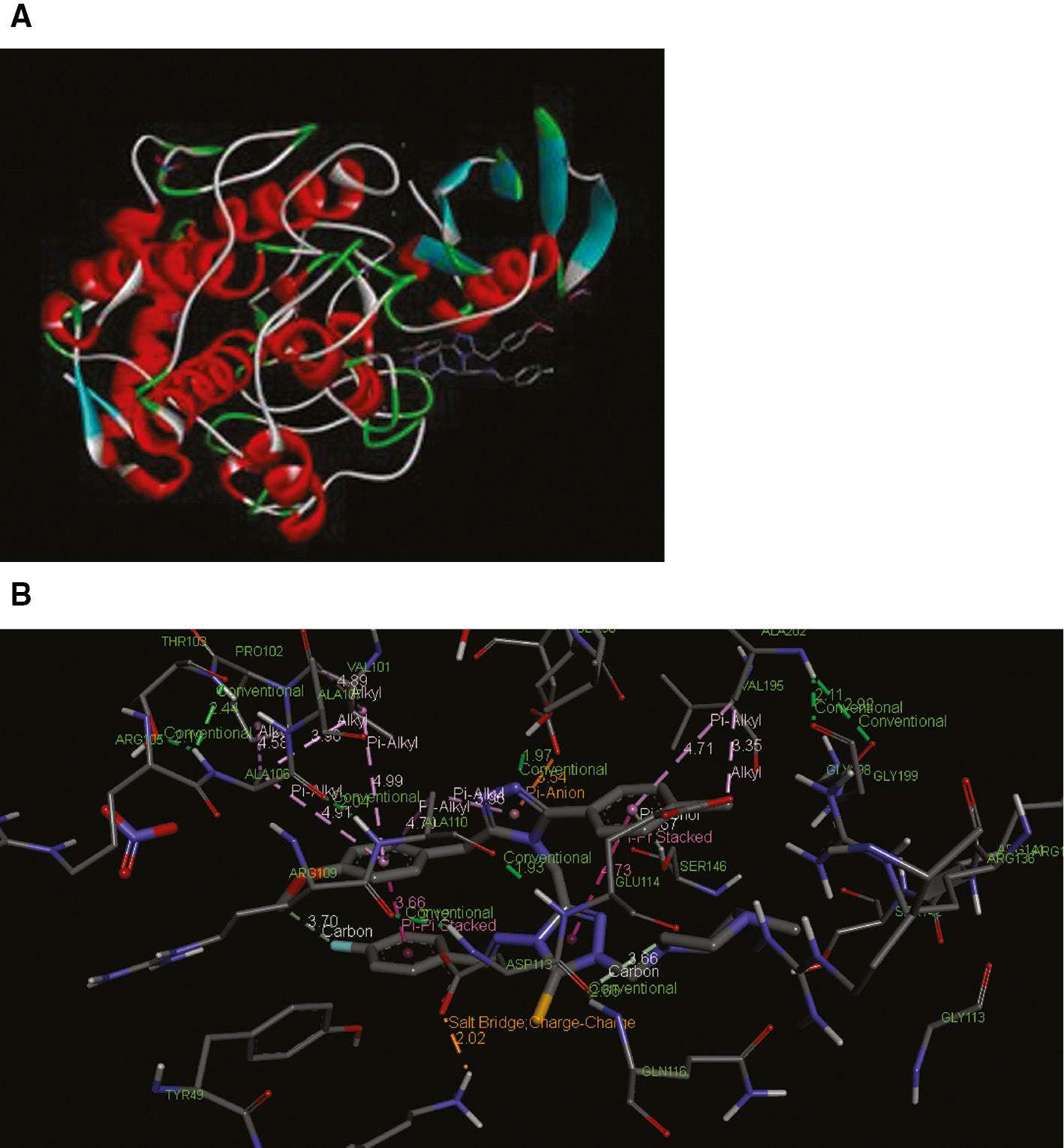
Predicted conformation of the compound inside the binding pocket of mushroom tyrosinase.
(A) general projection (B) micro environment which shows various types of interactions of the compounds atoms with the amino acid residues.
For the detection of tyrosinase inhibition, in the light of these explanations, 1-hepthyl-3-(4-methoxybenzyl)-4H-1,2,4-triazole-5-one derivative (A6, A8, A15) and 3-(4-chlorophenyl)-5-(4-methoxybenzyl)-4H-1,2,4-triazole derivative (B5, B9, B13) compounds shown in Table 1 were used and the inhibition studies were carried out in the presence of L-tyrosine substrate (0.14 mM). The IC50 value in the presence of the B9 compound was determined to be 400 μM. In the presence of other inhibitor molecules, inhibition of tyrosinase was observed in the range of 20–55% even at the highest inhibitor concentrations tested (Table 2).
To determine the inhibition type of tyrosinase activity, biochemical kinetic studies were performed at two different concentrations of the B9 compound. Likewise, it has been reported that inhibition efficiency and mechanisms of the molecules derived from a particular starting molecule are likely to be similar. Graphs of 1/[S]−1/V were plotted for each studied concentration of B9 compound. Accordingly, as a result of activity measurements in the absence of inhibitor, Km and Vmax values were determined to be 257.9 μM and 149.3 μmol/min, respectively. Whereas the concentration of B9 compound had an increase from 200 μM to 400 μM, both Km (192.8 μM and 71.0 μM, respectively) and Vmax (109.9 μmol/min and then 41.2 μmol/min) values decreased (Table 3).
Binding energies of inhibitor molecules were calculated in molecular docking studies for mushroom tyrosinase. The molecule with the lowest binding energy has the strongest interaction with the enzyme and thus it can be said that it is a potential molecule that causes inhibition. Accordingly, it was determined that among the studied six molecules, B13, B9 and A6 compounds were the most potent for tyrosinase inhibition (Table 3). But B9 had lowest IC50 value than the others and so our study focused on B9 compound. This demonstrates that in vitro and in silico study results almost support each other. It has been identified that the B9-tyrosinase complex is mainly consisting some kind weak interactions such as π-alkyl interaction, π-anion interaction, π-donor interaction and conventional interaction.
Discussion
Currently, many compounds used as drugs possess five member heterocyclic rings such as 1,2,4-triazole, 1,3,4-thiadiazole and 1,3,4-oxadiazole derivatives having various isomers in terms of the position of the heteroatoms and a broad spectrum of biological activity particularly anticancer, antibacterial, antifungal anti-HIV, antiviral, antidepressant, antiinflammatory, antituberculosis, diuretic, analgesic [35], [36]. In addition, the easily synthesis of Schiff and Mannich base derivatives of 1,2,4-triazoles and using in a variety of applications have led these compounds to attract a great deal of attention in biology and chemistry in the last decade [37], [38]. It has also been reported that some 1,3,4-thiadiazole, 1,3,4-oxadiazole, 1,2,4-triazole and substituted hydrazide compounds have been synthesized as alternative inhibitors to hyperpigmentation for clinical usage [2]. Some new synthesized triazole compounds are used in the market but they are not indicated to be suitable for clinical use due to their mild activity and safety concerns [39], [40]. So, in this study, some of the synthesized 1,2,4-triazole derivative compounds by Bekircan et al. [23], [24], [25] have been examined in terms of tyrosinase inhibition potentials.
Prior to initiation of inhibition studies, tyrosinase activity was determined in accordance with the literature and then optimized. So, optimum temperature and pH value of tyrosinase activity and the amount of enzyme and substrate to be used in the reaction mixture for inhibition studies were determined. We paid attention to some ordered critical points when performing inhibition studies [1]: (i) to determine the IC50 value of each inhibitor molecule, substrate concentration in reaction mixtures was adjusted to Km value calculated in optimization studies. Later, the type of inhibition and Ki values were tried to be determined in the presence of the inhibitor molecule for which IC50 value was found lowest. (ii) for this study, activity measurements were made at a series of substrate (in the concentration range of 0.2–5.0 Km) and inhibitor concentrations (range caused 10–75% tyrosinase inhibition), (iii) after adding inhibitor solutions (in ethanol) to the reaction mixture, the final concentration of the organic solvent in the reaction mixture was adjusted to not exceed 1%. As declared before, organic solvents start to inhibit enzymes in the presence of their higher concentration than 3% [1]. It was also observed that tyrosinase partly inhibited by ethanol in its high concentration. For this reason, suitable blank mixtures were prepared considering the inhibitory effect of the solvent and relative activities are calculated according to this fact. Besides that even if the organic compounds are prepared at a given concentration in a suitable organic solvent, it has been observed that there is occasional collapse after the addition to the reaction medium. It can be attributed to the fact that the inhibitor organic molecules can precipitate in reaction mixtures containing largely buffer solution. Because of each organic molecule used in the study has a different behavioral profile as stated above, the concentration range of inhibitor molecules used in the inhibition studies for the tyrosinase activity was also different from the molecule to molecule (Table 2).
Kojic acid was used as the reference inhibitor molecule in this study and its IC50 value was determined to be 18 mM. In this case, it can be said that the B9 compound used in the study is a more potent tyrosinase inhibitor than kojic acid. However, due to solubility problems, the B9 compound could not be studied for concentrations higher than 450 μM and a maximum inhibition (55.2%) was observed at this concentration. But, kojic acid that is a water-soluble molecule inhibited almost completely tyrosinase at a final concentration of 1 M. Oyama et al. [18] calculated IC50 values for three different phenylbenzoic acid derivative molecules in terms of tyrosinase inhibition and reported the highest and lowest IC50 values as >1000 μM and 6.97 μM, respectively. In another study, Gou et al. [17] observed tyrosinase inhibition in the presence of fumaric acid with the IC50 and Ki values 13.7±0.25 mM and 12.64±0.75 mM, respectively. Xie et al. [20] also found the highest and lowest IC50 values of novel 14 synthetic compounds which were hydroxybenzaldehyde based kojic acid analogs as 17.50±2.75 μM and 1.35±2.15 μM, respectively. According to these results, the inhibitor potential of B9 compound is more effective than the molecules reported by Oyama et al. [18] and Gou et al. [17] but they are not when compared Xie’s molecules.
It was determined that tyrosinase inhibition type was uncompetetive in the presence of B9 compound (Figure 1). Therefore, it can be said that B9 is caused an inhibition for tyrosinase activity by binding enzyme-substrate complex reversibly with weak interactions at a site other than the active site of enzyme. In addition, Ki value was determined as 370.7±0.3 μM as a result of this study. However, Soares et al. [19] investigated the effect of thiosemicarbazones derivatives (thio1-8) on tyrosinase inhibition and they reported the Ki as varying values between 7.3 mM and 0.06 mM and the inhibition patterns as uncompetitive mixed (thio-1,5), uncompetitive (thio-6) and competitive (thio-2-4,7-8) inhibition. Brasil et al. [16] also found the highest and lowest Ki values of 4H-Chromene analogs four synthetic compounds in order of 2.40 mM and 4.00 μM.
In conclusion, tyrosinase inhibition has a pharmacological importance. So, some newly synthesized triazole derivative molecules were evaluated in terms of their tyrosinase inhibition efficiencies as a first. It was determined that the B9 molecule among six molecules which was not studied before in terms of the tyrosinase inhibition potentials, caused tyrosinase inhibition at micromolar level. These in vitro studies have also been supported by molecular modeling studies. It can be speculated that further chemical and pharmacological studies on tyrosinase inhibition may perform for B9 molecule.
Acknowledgements
The authors are grateful to the Research Fund of the TUBITAK for this support with Project No 114Z711.
Conflict of interest: The authors have declared no conflict of interest.
References
1. Copeland RA. Evaluation of enzyme inhibitors in drug discovery. New Jersey: A John Wiley & Sons, Inc. Publication, 2005.Search in Google Scholar
2. Ghani U, Ullah N. New potent inhibitors of tyrosinase: novel clues to binding of 1,3,4-thiadizole-2 (3H)-thiones, 1,3,4-oxadiazole-2 (3H)-thiones, 4–amino-1,2,4-triazole-5(4H)-thiones, and substituted hydrazides to the dicopper active site. Bioorg Med Chem 2010;18:4042–8.10.1016/j.bmc.2010.04.021Search in Google Scholar PubMed
3. Ismaya WT, Rozeboom HJ, Weijn A, Mes JJ, Fusetti F, Wichers HJ, et al. Crystal structure of Agaricus bisporus mushroom tyrosinase: identity of the tetramer subunits and interaction with tropolone. Biochemistry 2011;50:5477–86.10.1021/bi200395tSearch in Google Scholar PubMed
4. Ando H, Kondoh H, Ichihashi M, Hearing VJ. Approaches to identify inhibitors of melanin biosynthesis via the quality control of tyrosinase. J Investig Dermatol 2007;127:751–61.10.1038/sj.jid.5700683Search in Google Scholar PubMed
5. Tan X, Song YH, Park C, Lee KW, Kim JY, Kim DW, et al. Highly potent tyrosinase inhibitor, neorauflavane from Campylotropis hirtella and inhibitory mechanism with molecular docking. Bioorgan Med Chem 2016;24:153–9.10.1016/j.bmc.2015.11.040Search in Google Scholar PubMed
6. Xue CB, Luo WC, Ding Q, Liu SZ, Gao XX. Quantitative structure-activity relationship studies of mushroom tyrosinase inhibitors. J Comput Aid Mol Des 2008;22:299–309.10.1007/s10822-008-9187-6Search in Google Scholar PubMed
7. Taylor JS. The dark side of sunlight and melanoma. Science 2015;347:824.10.1126/science.aaa6578Search in Google Scholar PubMed
8. Chen WF, Chakraborty C, Sung CS, Feng CW, Jean YH, Lin YY, et al. Neuroprotection by marine-derived compound, 11-dehydrosinulariolide, in an in vitro Parkinson’s model: a promising candidate for the treatment of Parkinson’s disease. N-S Arch Pharmacol 2012;385:265–75.10.1007/s00210-011-0710-2Search in Google Scholar PubMed
9. Goodspeed A, Heiser LM, Gray JW, Costello JC. Tumor-derived cell lines as molecular models of cancer pharmacogenomics. Mol Cancer Res 2016;14:3–13.10.1158/1541-7786.MCR-15-0189Search in Google Scholar PubMed PubMed Central
10. Pan TH, Li XQ, Jankovic J. The association between Parkinson’s disease and melanoma. Int J Cancer 2011;128:2251–60.10.1002/ijc.25912Search in Google Scholar PubMed
11. Serra PA, Esposito G, Enrico P, Mura MA, Migheli R, Delogu MR, et al. Manganese increases L-DOPA auto-oxidation in the striatum of the freely moving rat: potential implications to L-DOPA long-term therapy of Parkinson’s disease. Brit J Pharmacol 2000;130:937–45.10.1038/sj.bjp.0703379Search in Google Scholar PubMed PubMed Central
12. Ma D, Tu ZC, Wang H, Zhang L, He N, McClements DJ. Mechanism and kinetics of tyrosinase inhibition by glycolic acid: a study using conventional spectroscopy methods and hydrogen/deuterium exchange coupling with mass spectrometry. Food Funct 2017;8:122–31.10.1039/C6FO01384HSearch in Google Scholar
13. Kim JM, Ko RK, Jung DS, Kim SS, Lee NH. Tyrosinase inhibitory constituents from the stems of Maackia fauriei. Phytother Res 2010;24:70–5.10.1002/ptr.2870Search in Google Scholar PubMed
14. Maddila S, Pagadala R, Jonnalagadda SB. 1,2,4-Triazoles: a review of synthetic approaches and the biologicalactivity. Lett Org Chem 2013;10:693–714.10.2174/157017861010131126115448Search in Google Scholar
15. Wakale V, Pattan S, Tambe V. Therapeutic importance of 1,2,4-triazole: a review. Int J Res Pharm Biomed Sci 2013;4:1411–23.Search in Google Scholar
16. Brasil EM, Canavieira LM, Cardoso ÉT, Silva EO, Lameira J, Nascimento JL, et al. Inhibition of tyrosinase by 4H-chromene analogs: synthesis, kinetic studies, and computational analysis. Chem Biol Drug Des 2017;8:1–7.10.1111/cbdd.13001Search in Google Scholar
17. Gou L, Lee J, Yang JM, Park YD, Zhou HM, Zhan Y, et al. Inhibition of tyrosinase by fumaric acid: integration of inhibition kinetics with computational docking simulations. Int J Biol Macromol 2016;105:1663–9.10.1016/j.ijbiomac.2016.12.013Search in Google Scholar PubMed
18. Oyama T, Takahashi S, Yoshimori A, Yamamoto T, Sato A, Kamiya T, et al. Discovery of a new type of scaffold for the creation of novel tyrosinase inhibitors. Bioorgan Med Chem 2016;24:4509–15.10.1016/j.bmc.2016.07.060Search in Google Scholar PubMed
19. Soares MA, Almeida MA, Marins-Goulart C, Chaves OA, Echevarria A, de Oliveira MC. Thiosemicarbazones as inhibitors of tyrosinase enzyme. Bioorg Med Chem Lett 2017;27:3546–50.10.1016/j.bmcl.2017.05.057Search in Google Scholar PubMed
20. Xie WL, Zhang HL, He JJ, Zhang JG, Yu QY, Luo CX, et al. Synthesis and biological evaluation of novel hydroxybenzaldehyde-based kojic acid analogues as inhibitors of mushroom tyrosinase. Bioorg Med Chem Lett 2017;27:530–2.10.1016/j.bmcl.2016.12.027Search in Google Scholar PubMed
21. Silva AP. Optimization approaches to supervised classification. Eur J Oper Res 2017;261:772–88.10.1016/j.ejor.2017.02.020Search in Google Scholar
22. Xing R, Wang F, Dong L, Zheng AP, Wang L, Su WJ, et al. Inhibitory effects of Na7PMo11CuO40 on mushroom tyrosinase and melanin formation and its antimicrobial activities. Food Chem 2016;197:205–11.10.1016/j.foodchem.2015.10.119Search in Google Scholar PubMed
23. Bekircan O, Mentese E, Ulker S, Kucuk C. Synthesis of some new 1,2,4-triazole derivatives starting from 3-(4-chlorophenyl)-5-(4-methoxybenzyl)-4H-1,2,4-triazol with anti-lipase and anti-urease activities. Arch Pharm 2014;347:387–97.10.1002/ardp.201300344Search in Google Scholar PubMed
24. Bekircan O, Mentese E, Ulkerc S. Synthesis and pharmacological activities of some new 2-[1-heptyl-3-(4-methoxybenzyl)-5-oxo-1,5-dihydro-4H-1,2,4-triazol-4-yl]acetohydrazide Derivatives. Z Naturforsch 2014;69:969–81.10.5560/znb.2014-4126Search in Google Scholar
25. Bekircan O, Ulker S, Mentese E. Synthesis of some novel heterocylic compounds derived from 2-[3-(4-chlorophenyl)-5-(4-methoxybenzyl)-4H-1,2,4-triazol-4-yl]acetohydrazide and investigation of their lipase and alpha-glucosidase inhibition. J Enzym Inhib Med Ch 2015;30:1002–9.10.3109/14756366.2014.1003213Search in Google Scholar PubMed
26. Espin JC, Morales M, Varon R, Tudela J, Garciacanovas F. A continuous spectrophotometric method for determining the monophenolase and diphenolase activities of apple polyphenol oxidase. Anal Biochem 1995;231:237–46.10.1006/abio.1995.1526Search in Google Scholar PubMed
27. Hehre WJ, Ditchfield R, Pople JA. Self-consistent molecular-orbital methods.12. Further extensions of gaussian-type basis sets for use in molecular-orbital studies of organic-molecules. J Chem Phys 1972;56:2257.10.1063/1.1677527Search in Google Scholar
28. Lee CT, Yang WT, Parr RG. Development of the colle-salvetti correlation-energy formula into a functional of the electron-density. Phys Rev B 1988;37:785–9.10.1103/PhysRevB.37.785Search in Google Scholar PubMed
29. Trott O, Olson AJ. Software news and update autodock vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 2010;31:455–61.10.1002/jcc.21334Search in Google Scholar
30. Ringsdorf H, Simon J, Winnik FM. Hydrophobically-modified poly(N-isopropylacrylamides) in water – probing of the microdomain composition by nonradiative energy-transfer. Macromolecules 1992;25:5353–61.10.1021/ma00046a038Search in Google Scholar
31. Seeliger D, de Groot BL. Ligand docking and binding site analysis with PyMOL and Autodock/Vina. J Comput Aid Mol Des 2010;24:417–22.10.1007/s10822-010-9352-6Search in Google Scholar PubMed PubMed Central
32. Laskowski RA, Swindells MB. LigPlot: multiple ligand-protein interaction diagrams for drug discovery. J Chem Inf Model 2011;51:2778–86.10.1021/ci200227uSearch in Google Scholar PubMed
33. Xie J, Dong H, Yu Y, Cao S. Inhibitory effect of synthetic aromatic heterocycle thiosemicarbazone derivatives on muhroom tyrosinase: insights from fluorescence, 1H NMR titration and molecular docking studies. Food Chem 2016;190:709–16.10.1016/j.foodchem.2015.05.124Search in Google Scholar PubMed
34. Lam KW, Syahida A, Ul-Haq Z, Rahman MB, Lajis NH. Synthesis and biological activity of oxadiazole and triazolothiadiazole derivatives as tyrosinase inhibitors. Bioorgan Med Chem Lett 2010;20:3755–9.10.1016/j.bmcl.2010.04.067Search in Google Scholar PubMed
35. Bekircan O, Bektas H. Synthesis of new bis-1,2,4-triazole derivatives. Molecules 2006;11:469–77.10.3390/11060469Search in Google Scholar PubMed PubMed Central
36. Demirbas N, Demirbas A, Karaoglu SA. Synthesis and biological activities of new 1,2,4-triazol-3-one derivatives. Russ J Bioorg Chem 2005;31:387–97.10.1007/s11171-005-0054-0Search in Google Scholar PubMed
37. Karthikeyan MS, Prasad DJ, Poojary B, Bhat KS, Holla BS, Kumari NS. Synthesis and biological activity of Schiff and Mannich bases bearing 2,4-dichloro-5-fluorophenyl moiety. Bioorgan Med Chem 2006;14:7482–9.10.1016/j.bmc.2006.07.015Search in Google Scholar PubMed
38. Shi HJ, Wang ZY, Shi HX. Study on the intramolecular Mannich reaction of 4-amino-3-aryl-2,3-dihydrogen-5-mercapto-1,2,4-triazoles. Synthetic Commun 1998;28:3973–82.10.1002/chin.199902136Search in Google Scholar
39. Panich U, Kongtaphan K, Onkoksoong T, Jaemsak K, Phadungrakwittaya R, Thaworn A, et al. Modulation of antioxidant defense by Alpinia galanga and Curcuma aromatica extracts correlates with their inhibition of UVA-induced melanogenesis. Cell Biol Toxicol 2010; 26:103–16.10.1007/s10565-009-9121-2Search in Google Scholar PubMed
40. Rathmann SM, Janzen N, Valliant JF. Synthesis, radiolabelling, and biodistribution studies of triazole derivatives for targeting melanoma. Can J Chem 2016;94:773–80.10.1139/cjc-2016-0239Search in Google Scholar
©2019 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Research Articles
- Investigating the impact of polysomy 17 in breast cancer patients with HER2 amplification through meta-analysis
- Diagnostic performance of microRNAs in the circulation in differential diagnosis of BPH, chronic prostatitis and prostate cancer
- Enhanced anticancer effect of cetuximab combined with stabilized silver ion solution in EGFR-positive lung cancer cells
- CA125, YKL-40, HE-4 and Mesothelin: a new serum biomarker combination in discrimination of benign and malign epithelial ovarian tumor
- Paricalcitol pretreatment attenuates renal ischemia/reperfusion injury by inhibiting p38 MAPK and activating PI3K/Akt signaling pathways
- Identification of cytoplasmic sialidase NEU2-associated proteins by LC-MS/MS
- Investigation of tyrosinase inhibition by some 1,2,4 triazole derivative compounds: in vitro and in silico mechanisms
- Investigation of alanine, propionylcarnitine (C3) and 3-hydroxyisovalerylcarnitine (C5-OH) levels in patients with partial biotinidase deficiency
- The expression levels of miR-655-3p, miR127-5p, miR-369-3p, miR-544a in gastric cancer
- Evaluation of the JAK2 V617F gene mutation in myeloproliferative neoplasms cases: a one-center study from Eastern Anatolia
- Effects of Rituximab on JAK-STAT and NF-κB signaling pathways in acute lymphoblastic leukemia and chronic lymphocytic leukemia
- Analysis of the effect of DEK overexpression on the survival and proliferation of bone marrow stromal cells
- Serum fetuin-A levels and association with hematological parameters in chronic kidney disease and hemodialysis patients
- Investigation of relaxation times in 5-fluorouracil and human serum albumin mixtures
- Oxydative stress markers and cytokine levels in rosuvastatin-medicated hypercholesterolemia patients
- The protective effects of urapidil on lung tissue after intestinal ischemia-reperfusion injury
- Effects of SR-BI rs5888 and rs4238001 variations on hypertension
- Antioxidant and cytotoxic activity of three Turkish marine-derived fungi
- Is spectrophotometric enzymatic method a cost-effective alternative to indirect Ion Selective Electrode based method to measure electrolytes in small clinical laboratories?
- Plasma presepsin in determining gastric leaks following bariatric surgery
Articles in the same Issue
- Frontmatter
- Research Articles
- Investigating the impact of polysomy 17 in breast cancer patients with HER2 amplification through meta-analysis
- Diagnostic performance of microRNAs in the circulation in differential diagnosis of BPH, chronic prostatitis and prostate cancer
- Enhanced anticancer effect of cetuximab combined with stabilized silver ion solution in EGFR-positive lung cancer cells
- CA125, YKL-40, HE-4 and Mesothelin: a new serum biomarker combination in discrimination of benign and malign epithelial ovarian tumor
- Paricalcitol pretreatment attenuates renal ischemia/reperfusion injury by inhibiting p38 MAPK and activating PI3K/Akt signaling pathways
- Identification of cytoplasmic sialidase NEU2-associated proteins by LC-MS/MS
- Investigation of tyrosinase inhibition by some 1,2,4 triazole derivative compounds: in vitro and in silico mechanisms
- Investigation of alanine, propionylcarnitine (C3) and 3-hydroxyisovalerylcarnitine (C5-OH) levels in patients with partial biotinidase deficiency
- The expression levels of miR-655-3p, miR127-5p, miR-369-3p, miR-544a in gastric cancer
- Evaluation of the JAK2 V617F gene mutation in myeloproliferative neoplasms cases: a one-center study from Eastern Anatolia
- Effects of Rituximab on JAK-STAT and NF-κB signaling pathways in acute lymphoblastic leukemia and chronic lymphocytic leukemia
- Analysis of the effect of DEK overexpression on the survival and proliferation of bone marrow stromal cells
- Serum fetuin-A levels and association with hematological parameters in chronic kidney disease and hemodialysis patients
- Investigation of relaxation times in 5-fluorouracil and human serum albumin mixtures
- Oxydative stress markers and cytokine levels in rosuvastatin-medicated hypercholesterolemia patients
- The protective effects of urapidil on lung tissue after intestinal ischemia-reperfusion injury
- Effects of SR-BI rs5888 and rs4238001 variations on hypertension
- Antioxidant and cytotoxic activity of three Turkish marine-derived fungi
- Is spectrophotometric enzymatic method a cost-effective alternative to indirect Ion Selective Electrode based method to measure electrolytes in small clinical laboratories?
- Plasma presepsin in determining gastric leaks following bariatric surgery

