Paricalcitol pretreatment attenuates renal ischemia/reperfusion injury by inhibiting p38 MAPK and activating PI3K/Akt signaling pathways
-
Zahide Cavdar
, Cemre Ural
, Sevki Arslan
Abstract
Objective
This study aimed to investigate the renoprotective effects of paricalcitol, a synhetic vitamin D analog, through its possible roles on p38 MAPK and PI3K/Akt signaling pathways to prevent oxidative stress, inflammation and apoptosis during renal I/R.
Materials and methods
Total 20 kidney tissues of sham (n = 6), subjected to renal I/R bilaterally for 45 min ischemia followed by 24 h reperfusion (n = 7) and paricalcitol (0.3 μg/kg, ip) pretreated Wistar albino rats (n =7) were used in this study. Interstitial inflammation and active caspase-3 expression were evaluated histologically. TNF-α, IL-1β, kidney injury molecule-1 (KIM-1), MDA and SOD activity in kidneys were analysed biochemically. Furthermore, activation of p38 MAPK, PI3K/Akt signaling pathways and NFκB p65 were evaluated by western blot.
Results
Paricalcitol pretreatment significantly reduced interstitial inflammation during renal I/R, which was consistent with decreased tumor TNF-α, IL-1β, active caspase-3 and KIM-1 expression. Paricalcitol also reduced MDA level and attenuated the reduction of SOD activity in the kidney during I/R. Moreover, paricalcitol could suppress the p38 MAPK and NFκB p65, and also activate PI3K/Akt signaling pathway during renal I/R.
Conclusion
All these findings indicate that paricalcitol may be an effective practical strategy to prevent renal I/R injury.
Öz
Amaç
Bu çalışmanın amacı vitamin D’nin bir sentetik analoğu olan parikalsitol’ün renal iskemi/reperfüzyon (I/R) süresince oksidatif stres, inflamasyon ve apoptozise karşı renoprotektif etkisini p38 MAPK ve PI3K/Akt sinyal ileti yolakları üzerine olası rolleri ile araştırmaktır.
Gereç ve yöntem
Sham (n = 6) grubu, bilateral renal iskemi/reperfüzyon (I/R) (45 dak iskemi, 24 saat reperfüzyon) (n = 7) ve renal I/R’den önce parikalsitol (0.3 μg/kg, i.p) (n = 7) uygulanan Wistar albino sıçanlardan alınan toplam 20 böbrek dokusu kullanıldı. İnterstisyel inflamasyon ve aktif kaspaz-3 histolojik analizler ile değerlendirildi. Böbrek dokusunda TNF-α, IL-1β, böbrek hasar molekülü-1 (KIM-1), MDA ve SOD biyokimyasal olarak analiz edildi. p38 MAPK, PI3K/Akt sinyal yolaklarının aktivasyonu ve NFκB p65 Western blot ile değerlendirildi.
Bulgular
Parikalsitol, renal I/R süresince oluşan interstisyel inflamasyonu anlamlı olarak azalttı. Bununla ilişkili olarak renal I/R süresince artan TNF-α, IL-1β, aktif kaspaz-3 ve KIM-1 parikalsitol ile anlamlı olarak azaldı. Parikalsitol ile aynı zamanda renal I/R süresince böbrek dokusunda artan MDA seviyesi anlamlı olarak azalırken, SOD aktivite düzeyi ise anlamlı olarak arttı. Ayrıca parikalsitolün renal I/R süresince artan p38 MAPK aktivasyonunu ve NFκB p65 protein ifadesini baskılayabildiği ve PI3K/Akt sinyal ileti yolağını da aktive edebildiği saptandı.
Sonuç
Tüm bu bulgular parikalsitol’ün renal I/R hasarına karşı geliştirilen stratejilerde aday olabileceğine işaret etmektedir.
Introduction
Renal ischemia/reperfusion (I/R) injury is the most important reason for acute kidney injury, which is related to high morbidity and mortality. It is resulted from blood deprivation followed by reperfusion and frequently encountered in shock, trauma, renal resection, and transplantation. The molecular mechanisms regarding renal I/R injury are very complex. It has been known that reactive oxygen species (ROS), necrosis, apoptosis and also inflammation are involved in renal I/R injury [1], [2], [3].
p38 Mitogen-activated protein kinase (MAPK) is a critical signaling pathway, which regulates various pathological condition, including renal I/R injury. The activation of p38MAPK by both oxidative stress and cytokines occurred following renal I/R leads to renal apoptosis and inflammatory responses [4], [5], [6]. The results of previous studies indicated that inhibition of p38 MAPK activation could prevent renal I/R injury in experimental models [7], [8]. In contrast, the phosphoinositide 3 kinase (PI3K/Akt) is an important cell survival pathway against various stressors. Also, it has been shown to decrease oxidative stress and inflammatory response in the kidney during I/R [9], [10]. Thus, both suppression of p38MAPK and activation of PI3K/Akt signaling pathways may be potential targets for therapeutic strategy in renal I/R injury.
Paricalcitol (19-nor-1,25-dihydroxyvitamin D2) is a vitamin D analog that exhibits similar property in terms of biological activity, however, it has fewer adverse effects [11]. Accumulating evidence have indicated that renoprotective effect of paricalcitol is associated with its antioxidant, anti-inflammatory and antiapoptotic action in various experimental models, including drug-induced kidney injuries [12], [13], [14], diabetic [15], obstructive nephropathy [16] and also renal I/R injury models [17], [18], [19], [20], [21]. However, information on the mechanism of renoprotective effects of paricalcitol is limited [19], [20]. Recently, we demonstrated that paricalcitol attenuated renal tubular injury by decreasing matrix metalloproteinase (MMP)-2 and MMP-9 expressions, which were responsible for proteolytic degradation of renal microvascular matrix during renal I/R [21]. Based on current information, this study was designed to demonstrate underlying mechanism of renoprotective effects of paricalcitol and we hypothesized that paricalcitol may exhibits renoprotective effects through its possible roles on p38 MAPK and PI3K/Akt signaling pathways to prevent oxidative stress, inflammation and apoptosis during renal I/R.
Materials and methods
Animals and experimental design
Experiments were performed on kidney tissues obtained from our previous study [21] with ethical approval (DEU-HADYEK, 60/2016). Twenty male Wistar albino rats were divided into three groups: sham (S; n=6), I/R (n=7) and I/R+Paricalcitol (P) (I/R+P; n=7). For the sham group, only laparotomy without renal I/R was performed and the kidney samples were taken without any further manipulation. For I/R group, bilateral clamping of renal pedicles was performed for 45 min followed by 24 h reperfusion and bilateral nephrectomy was performed after 24 h of reperfusion, as described in our previous study [21]. For the I/R+P group, rats recieved 0.3 μg/kg (0.1 mL) paricalcitol (P) (Abbvie Inc., North Chicago, IL, USA) by intraperitoneal (i.p) injection 24 h before ischemia.
Histological examination
Hematoxylin and eosin (H-E) staining was applied to paraffinized kidney tissue sections in order to evaluate interstitial inflammation. Interstitial inflammation was scored semiquantitavely by using an image analyser (Olympus BX-51 Tokyo, Japan) as: 0=none, 1=1–25%, 2=26–50%, 3=51–75%, 4=76–100% of tubules [22].
Immunohistochemical analysis of caspase-3
To evaluate apoptosis, active caspase-3 (cleaved caspase-3) expression was analysed by immunohistochemically. All sections were deparaffinized and rehydrated followed by treatment with 10 mM citrate buffer (pH 6) and 3% H2O2. After blocking, sections were incubated with active caspase-3 antibody (1:100, AB3623, Millipore, Temecula, CA, USA) and secondary antibody. After staining and counter-staining with DAB (Roche Diagnostics, Mannheim, Germany) and Mayer hematoxylin, respectively, active caspase-3 positive staining in the sections was scored semiquantitavely defined as 0=no immunoreactivity, 1=little positive staining, 2=moderate positive staining which is between grade 1 and grade 3, 3=strong positive staining.
Preparation for biochemical analyses
The kidney homogenates were prepared in 50 mM potassium phosphate buffer at pH 7.8, which contained 0.15 M NaCl, 1 μg/mL leupeptin, 1 μg/mL pepstatin, 0.5 mmol/L phenylmethanesulfonyl floride, 1 μg/mL aprotinin and 1 μg/mL sodium ortho vanadate by using Tissue-Lyser (TissueLyser II, Qiagen, UK). The homogenates were centrifuged for 10 min at 2500×g and the supernatants were used for MDA. Then, the homogenates were further centrifuged at 11,000×g for 15 min to obtain supernatants for other analyses related to SOD activity, KIM-1 protein level, p38 MAPK, PI3K/Akt and NFκB p65 protein expressions.
Tissue MDA level
High performance liquid chromatography method was used for MDA levels in the kidney [23]. It depends on formation of thiobarbituric acid (TBA)-MDA complex. The fluorescence intensity of this complex was evaluated at 515 nm excitation and 553 nm emission followed by injection onto a column (C18, Nucleosil 100-5, 250–4.6 mm; Macherey-Nagel Inc., Diiren, Germany). The calibration curve was constructed by using 1,1,3,3-tetraethoxypropane (TEP) (Sigma, MO, USA) as a standard. MDA levels were expressed as μmol/mg protein.
Tissue SOD activity
SOD activity levels in kidney tissue were analysed by using the colorimetric kit (Cayman, MI, USA). The results were expressed as U/mg protein.
Tissue KIM-1 protein level
Kidney KIM-1 levels were quantified by ELISA kit (Boster Biological Technology, Wuhan, China). The results were represented as pg/mg protein.
Quantitative real-time PCR (qRT-PCR)
Total RNA was obtained from kidney tissues by using RNeasy lipid tissue kit (Qiagen, Hilden, Germany) and converted to cDNA by using a reverse transcription kit (Easy Script plus, ABM, BC, Canada). qRT-PCR was established with SYBR Green PCR Master Mix (Applied Biosystems, Warrington, UK) by using the Lightcycler 1.5 Instrument (Roche Diagnostics, Germany). The primers used in the qRT-PCR are the following: TNF-α, 5′-AAA TGG GCT CCC TCT CAT CAG TTC-3′ (forward) and 5′-TCT GCT TGG TGG TTT GCT ACG AC-3′(reverse); IL-1β, 5′-GGA AGG CAG TGT CAC TCA TTG TG-3′ (forward) and 5′-GGG TTC CAT GGT GAA GTC AAC-3′ (reverse); KIM-1, 5′-ACT CCT GCA GAC TGG AAT GG-3′ (forward) and 5′-CAA AGC TCA GAG AGC CCA TC-3′ (reverse); β-actin, which was used for internal standard, 5′-TGC AGA AGG AGA TTA CTG CC-3′ (forward) and 5′-CGC AGC TCA GTA ACA GTC C-3′ (reverse). β-actin was used for normalization of mRNA expression for each gene. 2−ΔΔ Ct method was used to calculate the relative expression levels of mRNAs [24].
Western blot
Briefly, kidney homogenate containing 50 μg protein per well was applied to 10% SDS-polyacrylamide gel electrophoresis [25]. Then the proteins on the gel were transferred to a polyvinylidene difluoride membrane (Millipore, MA, USA). Blocking was performed with 5% non fat dry milk in phosphate-buffered saline. The membranes were incubated with the primary antibodies: p-p38 MAPK (Thr180/Tyr182) (1:1000, Cell Signaling), NF-κB p-p65 (Ser536) (1:500, Cell Signaling) and p-Akt (Ser473) (1:1000, Cell Signaling). After washing, the membrane was treated with secondary antibody (sc-2004; Santa Cruz Biotechnology Inc.). Stripping protocol was performed for reprobing the membranes with the primary antibody against p38 MAPK (1:1000, Cell Signaling, Danvers, MA, USA), NF-κB p65 (1:500, Cell Signaling), Akt (1:500, Thermo Fisher Scientific Inc., Rockford, IL, USA) and β-actin (1:500, Santa Cruz Biotechnology Inc.). ECL reagent (Thermo Fisher, Rockford, IL, USA) was used for visualization of the bands. Densitometric analysis was done by using Image Studio Digits Ver 4.0 (LI-COR Biosciences, Lincoln, NE, USA).
Protein assay
Protein levels in the kidney tissues were measured by using bicinchoninic acid (BCA) assay kit (Sigma, Munich, Germany) [26].
Statistical analysis
Statistical analyses were performed using SPSS version 22.0 for Windows. The differences among groups were assessed by the Kruskal-Wallis test. Mann-Whitney U-test was used to examine the differences between two groups. Spearman test was performed for the correlation between KIM-1 with p-p38 MAPK and p-Akt. All values are expressed as mean±SEM and median (IQR). Values for p<0.05 were considered to be statistically significant.
Results
Paricalcitol pretreatment decreases I/R induced oxidative stress
Oxidative stress was evaluated by MDA level and SOD activity, which are an indicator of ROS mediated lipid peroxidation and the principal antioxidant enzyme, respectively. Compared with sham group (0.826±0.05 μmol/g protein) (Median: 0.822, IQR: 0.256), MDA in the kidneys was significantly elevated in I/R group (1.307±0.07 μmol/g protein) (Median: 1.262, IQR: 0.315) (p=0.002) and was reversed in paricalcitol pretreatment group (0.928±0.08 μmol/g protein) (p=0.015) (Median: 0.902, IQR: 0.316) (Figure 1A). On the other hand, the SOD activity was found to be significantly decreased in the kidneys during I/R (0.016±0.005 U/mg protein) (Median: 0.117, IQR: 0.02) (p=0.017), compared to the the sham group (0.067±0.01 U/mg protein) (Median: 0.052, IQR: 0.07) and paricalcitol significantly increased the SOD activity (0.061±0.011 U/mg protein) (Median: 0.060, IQR: 0.04), (p=0.008) (Figure 1B).
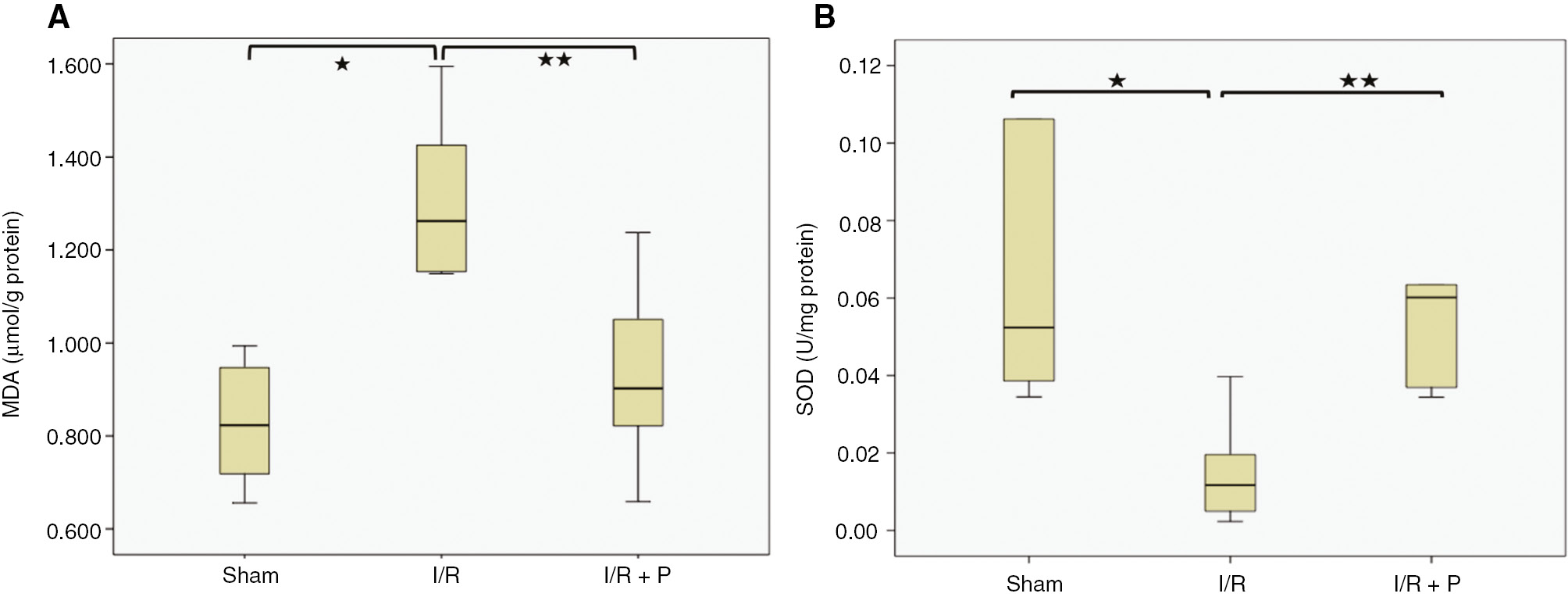
Box-plots with respect to effect of paricalcitol pretreatment on I/R induced oxidative stress.
MDA levels (A) and SOD activity (B) in kidneys were compared among study groups. S, sham (n=6); I/R, 45 min ischemia followed by 24 h reperfusion (n=7); I/R+P, paricalcitol administration (0.3 μg/kg) 24 h before I/R (n=7). *p<0.05 vs. S; **p<0.05 vs. I/R, Mann-Whitney U-test.
Paricalcitol pretreatment reduces I/R induced interstitial inflammation and mRNA expressions of TNF-α and IL1-β
As shown in Figure 2A and B, interstitial inflammation, which was detected in the glomerulus, capillaries and peritubular areas, was significantly increased in the I/R group, compared to the the sham group (p=0.029). However, paricalcitol significantly attenuated the interstitial inflammation (p=0.008). Additionally, TNF-α and IL1-β mRNA expressions in the kidneys were consistent with histological findings. TNF-α and IL1-β mRNA expressions in the I/R group increased significantly by 7.4 and 6.25-fold (p=0.004 and p=0.035, respectively), compared to the sham group. Paricalcitol pretreatment, however, decreased significantly mRNA expressions of both TNF-α and IL1-β, by 1.91 and 2.05-fold (p=0.041 and 0.017, respectively) (Figure 3).
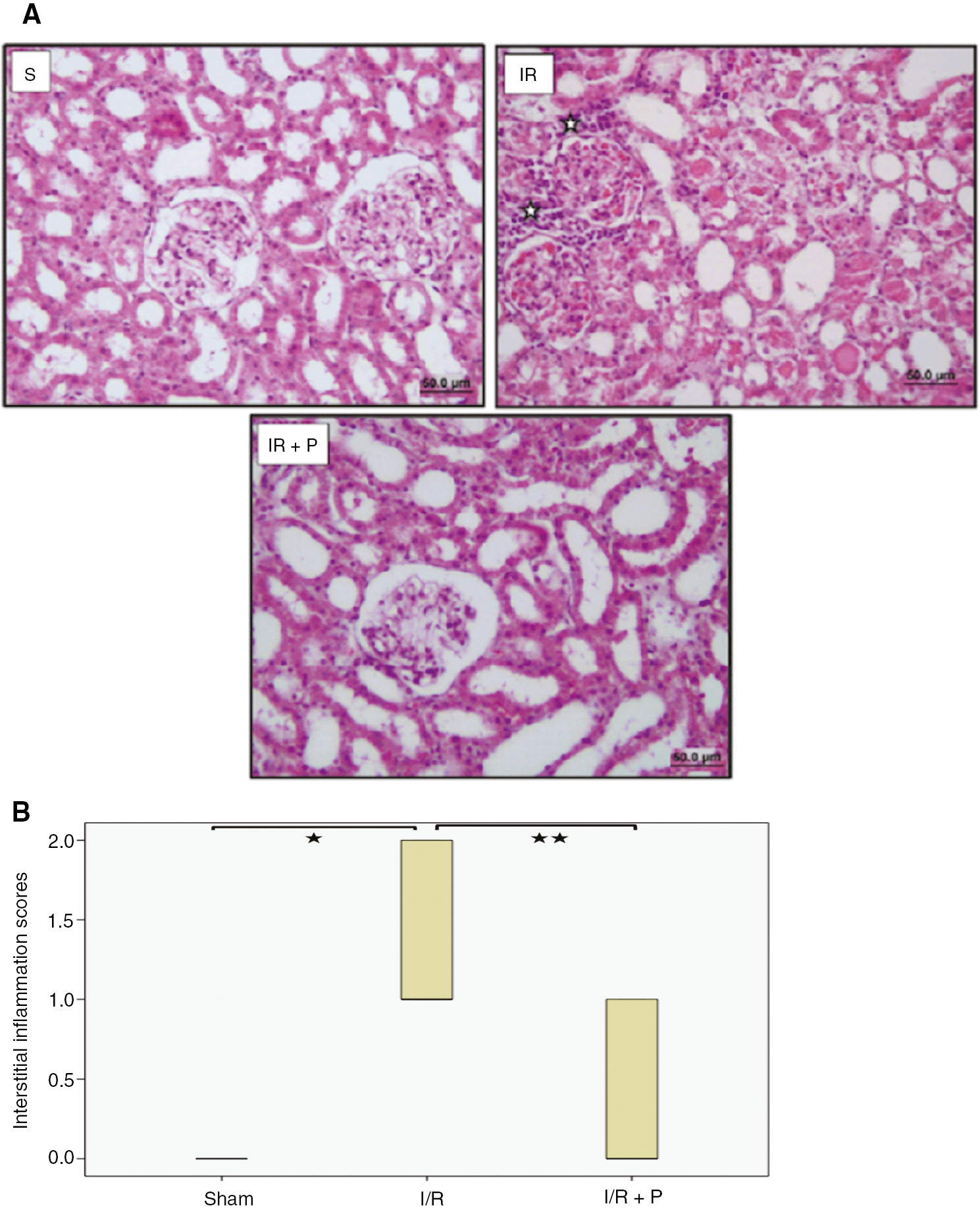
Effect of paricalcitol pretreatment on I/R induced interstitial inflammation.
Representative light microscopy images of H-E staining. ( ) indicate mononuclear cell infiltration (A). Box-plot representing the interstitial inflammation scores which were compared among study groups (B). S, sham (n=6); I/R, 45 min ischemia followed by 24 h reperfusion (n=7); I/R+P, paricalcitol administration (0.3 μg/kg) 24 h before I/R (n=7). *p<0.05 vs. S; **p<0.05 vs. I/R, Mann-Whitney U-test.
) indicate mononuclear cell infiltration (A). Box-plot representing the interstitial inflammation scores which were compared among study groups (B). S, sham (n=6); I/R, 45 min ischemia followed by 24 h reperfusion (n=7); I/R+P, paricalcitol administration (0.3 μg/kg) 24 h before I/R (n=7). *p<0.05 vs. S; **p<0.05 vs. I/R, Mann-Whitney U-test.
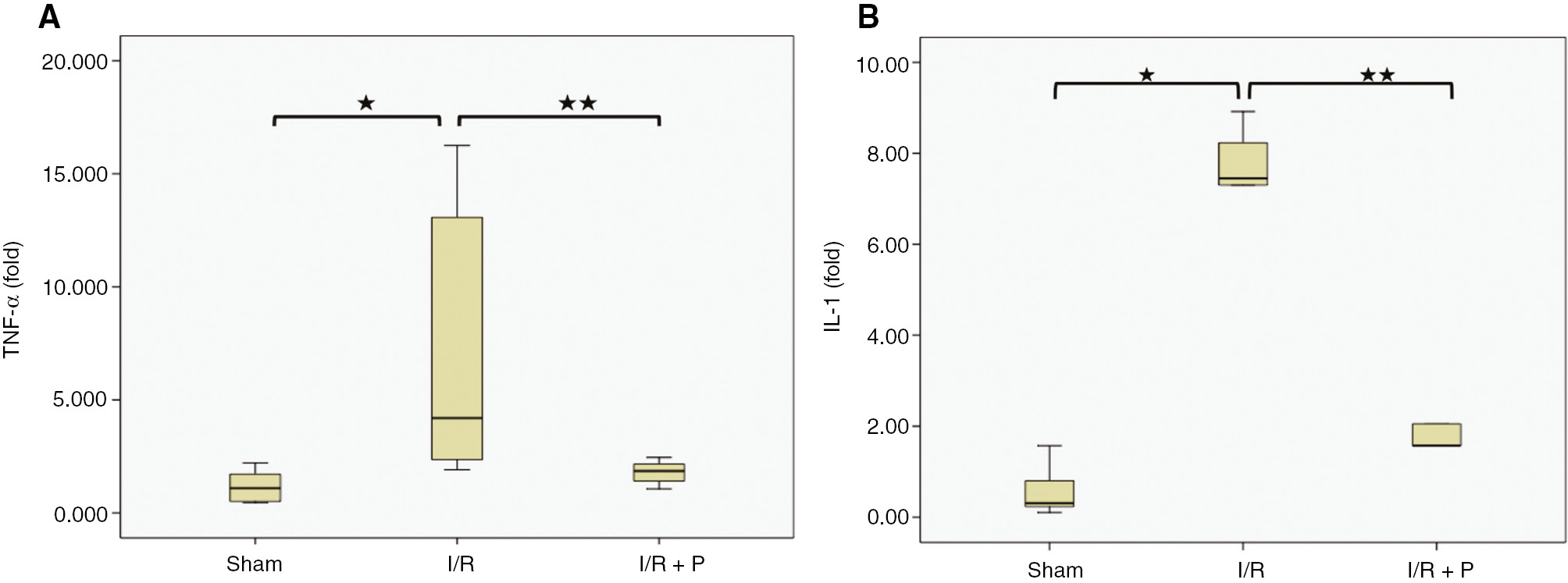
Box-plots with respect to effect of paricalcitol pretreatment on I/R induced pro-inflammatory cytokine expressions.
The expressions of TNF-α (A) and IL-1β mRNA (B) in kidneys were compared among study groups. S, sham (n=6); I/R, 45 min ischemia followed by 24 h reperfusion (n=7); I/R+P, paricalcitol administration (0.3 μg/kg) 24 h before I/R (n=7). *p<0.05 vs. S; **p<0.05 vs. I/R, Mann-Whitney U-test.
Paricalcitol pretreatment ameliorates I/R induced renal apoptosis
Active caspase-3 positive cells increased significantly in I/R group compared to sham group (p<0.001). However, paricalcitol administration resulted in significantly decreased the active caspase-3 positive cells (p=0.011) (Figure 4).
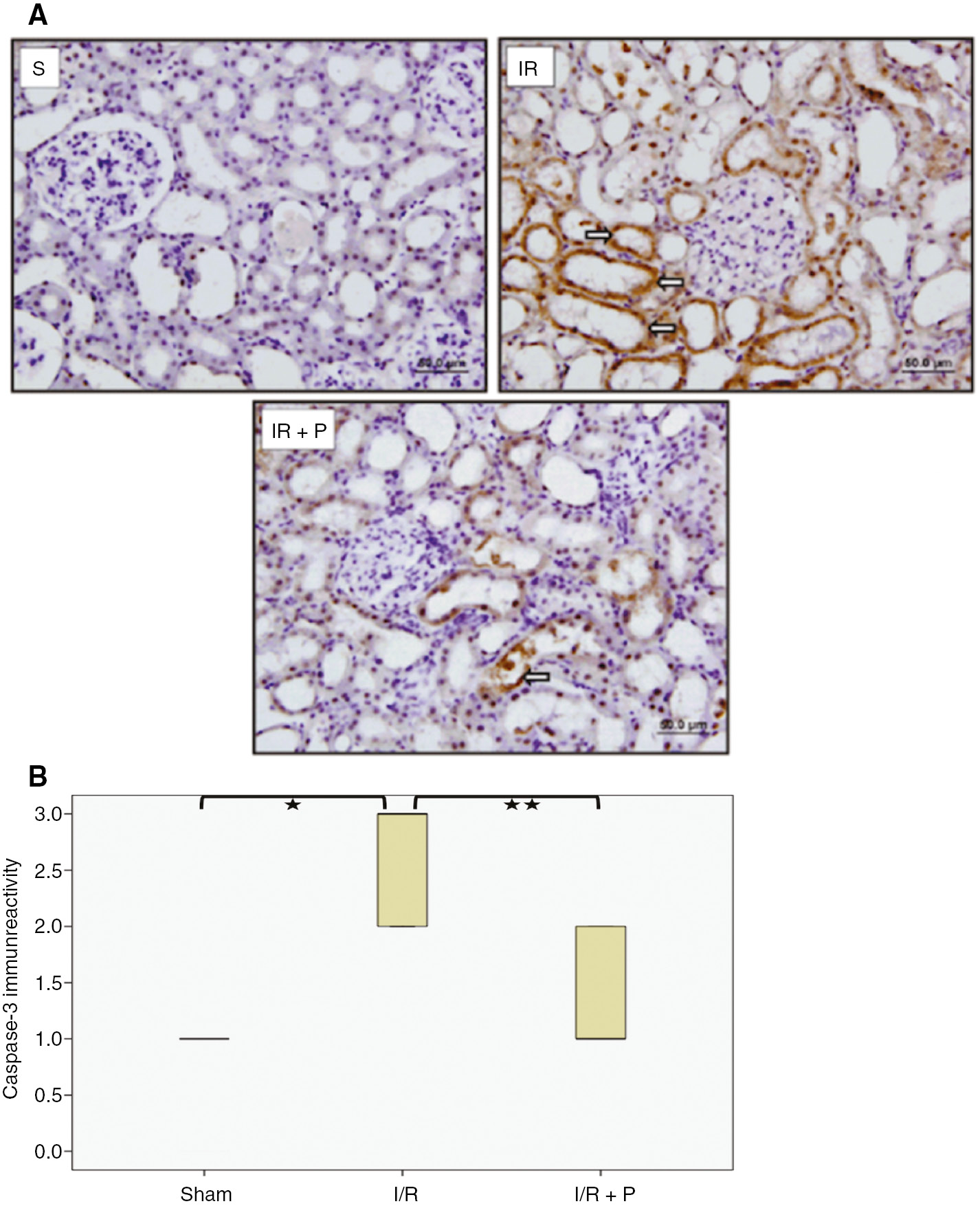
Effect of paricalcitol pretreatment on I/R induced tubular cell apoptosis.
Representative light-microscopic images of the active caspase-3 immunoreactivity in kidneys. Arrows ( ) indicate anti-active caspase-3 immunopositive cells (A). Box-plot representing the percent area of active caspase-3 immunopositive cells which were compared among study groups (B). S, sham (n=6); I/R, 45 min ischemia followed by 24 h reperfusion (n=7); I/R+P, paricalcitol administration (0.3 μg/kg) 24 h before I/R (n=7). *p<0.05 vs. S; **p<0.05 vs. I/R, Mann-Whitney U-test.
) indicate anti-active caspase-3 immunopositive cells (A). Box-plot representing the percent area of active caspase-3 immunopositive cells which were compared among study groups (B). S, sham (n=6); I/R, 45 min ischemia followed by 24 h reperfusion (n=7); I/R+P, paricalcitol administration (0.3 μg/kg) 24 h before I/R (n=7). *p<0.05 vs. S; **p<0.05 vs. I/R, Mann-Whitney U-test.
Paricalcitol pretreatment reduces I/R induced KIM-1 expression
KIM-1 mRNA expression in the kidneys was found to be significantly elevated in the I/R group (677-fold), compared to the sham group (p=0.016). Paricalcitol significantly reduced KIM-1 mRNA expression (218-fold) (p<0.001) (Figure 5A). KIM-1 protein levels in the kidneys also increased significantly in the I/R group (759±26.02 pg/mg protein) (Median: 759.612, IQR: 104.65), compared to the sham group (460.73±47.04 pg/mg protein) (Median: 453.458, IQR: 161.16) (p=0.0022). It was found to be significantly decreased by paricalcitol pretreatment (644.46±29.71 pg/mg protein) (Median: 634.877, IQR: 129.49) (p=0.015) (Figure 5B).
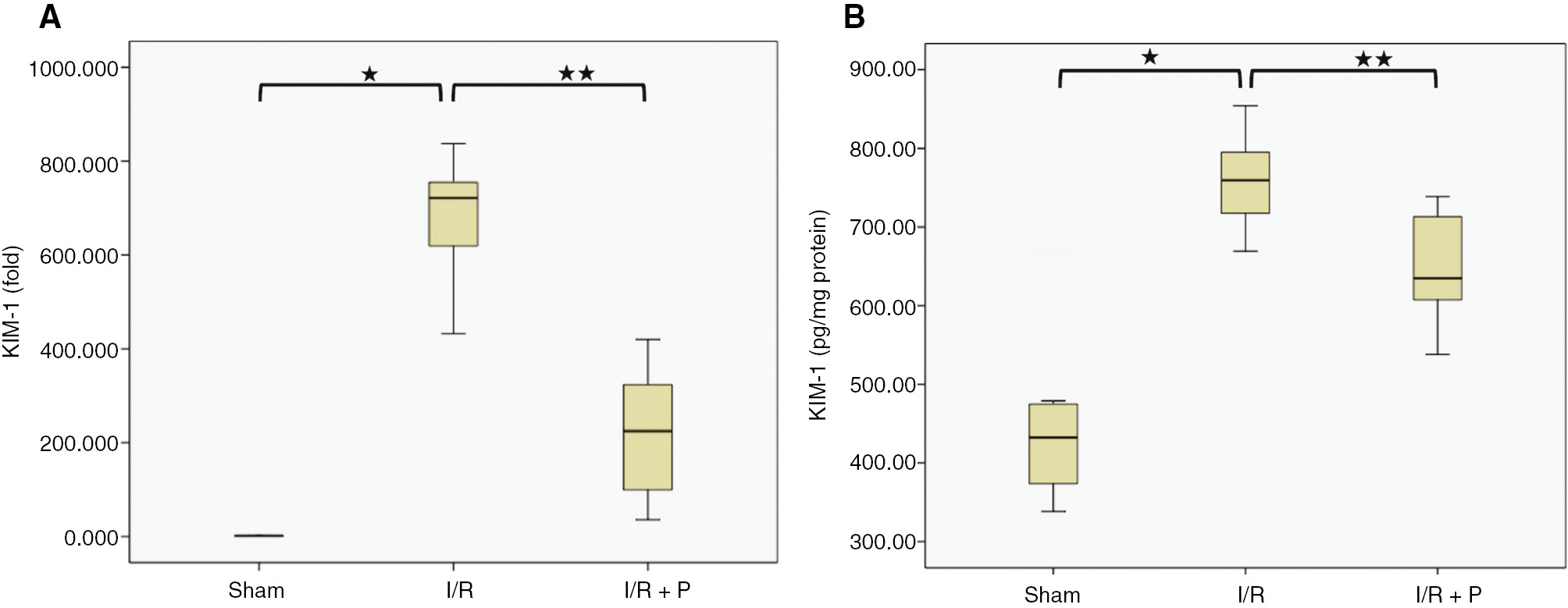
Box-plots with respect to effects of paricalcitol pretreatment on I/R induced KIM-1 expression.
The expression of KIM-1 mRNA (A) and protein (B) in the kidneys were compared among study groups. S, sham (n=6); I/R, 45 min ischemia followed by 24 h reperfusion (n=7); I/R+P, paricalcitol administration (0.3 μg/kg) 24 h before I/R (n=7). *p<0.05 vs. S; **p<0.05 vs. I/R, Mann-Whitney U-test.
Paricalcitol pretreatment suppresses not only p38 MAPK and NFκB activation but also activates PI3K/Akt during renal I/R
I/R was found to be significantly induced the phosphorylated p38 (p-p38) level, which reflects to activation of p38 MAPK signaling (p=0.015) (Figure 6A). However, paricalcitol suppressed significantly the levels of p-p38, compared to I/R group, (p=0.001). Activation of PI3K/Akt was evaluated by elevated levels of phosphorylated Akt, p-Akt, in the I/R group, compared to the sham group (p=0.031). It was found that paricalcitol increased significantly p-Akt during renal I/R (p=0.038) (Figure 6B). Additionally, both p-p38 MAPK and p-Akt were correlated positively with KIM-1 (r=0.410, p=0.023; r=0.624, p=0.019, respectively). For the determination of the effects of paricalcitol pretreatment on NFκB activation, total p65 and phosphorylated p65 (p-p65) expression levels were also evaluated in this study. As compared to sham group, I/R induced significantly p-p65 expression in the renal I/R group (p=0.007). However, pretreatment with paricalcitol significantly inhibited p-p65 expression in the kidney during renal I/R (p=0.01) (Figure 6C). Similar to p38 MAPK and total Akt, total p65 protein expressions were not changed significantly between the groups.
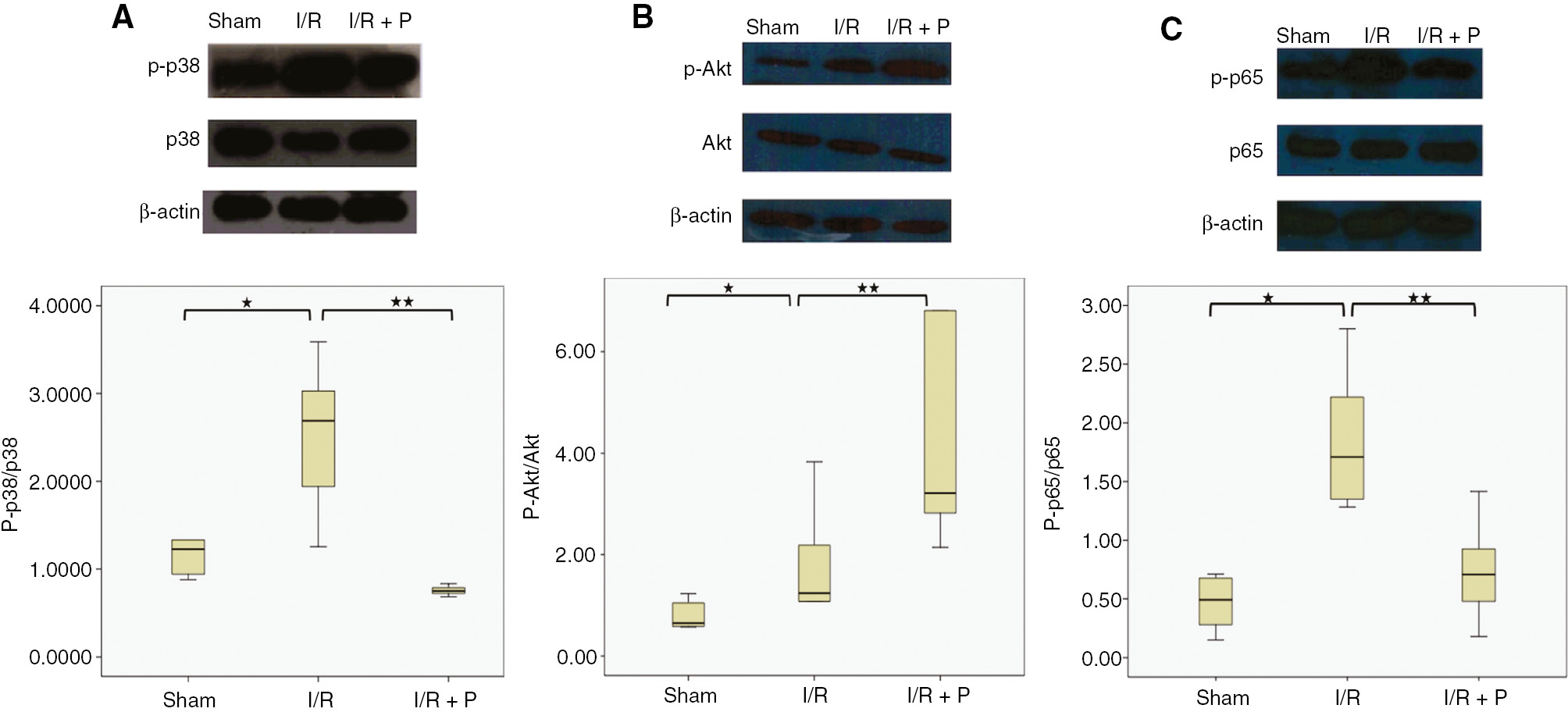
Effects of paricalcitol pretreatment on p38 MAPK, PI3K/Akt signaling and NFκB activation during renal I/R.
Western blot analysis was used for the protein expressions of p-p38, total p38 MAPK (A), pAkt, total Akt (B) NFκB p-p65 and total NFκB p65 in kidneys (C). β actin was used for normalization. Box-plot representing semi-quantitative analysis of protein expressions which were compared among study groups. S, sham (n=6); I/R, 45 min ischemia followed by 24 h reperfusion (n=7); I/R+P, paricalcitol administration (0.3 μg/kg) 24 h before I/R (n=7). *p<0.05 vs. S; **p<0.05 vs. I/R, Mann-Whitney U-test.
Discussion
The present study presented for the first time that paricalcitol pretreatment attenuated oxidative stress, inflammation, and apoptosis in the I/R induced kidney tissues, which could be related to its effects on both suppression of p38 MAPK and activation of PI3K/Akt signaling pathways during renal I/R.
It is well known that the excessive ROS generation results in tissue injury during renal I/R by attacking membrane lipids, DNA, and proteins [27]. The protective effect of paricalcitol on oxidative stress were investigated in various renal diseases [12], [13], [14], [15], [16] and also renal I/R injury models [17], [18], [19], [20], [21]. The results of our study regarding significantly decreased MDA level and elevated the activity of SOD in kidneys by paricalcitol during renal I/R was in line with the those studies.
Previous studies showed that p38 MAPK signaling has rapid response against oxidative stress and inflammation, which resulted in cell apoptosis as well as inflammatory responses in I/R injury [3], [4], [5]. Also, there is accumulating evidence that inhibition of p38MAPK is a promosing target for the treatment of renal I/R injury [7], [8]. Recently, a study performed on unilateral ureteral obstruction model in mice reported that paricalcitol treatment also markedly reduced p-p38 level in kidneys [28]. However, the effect of paricalcitol on p38MAPK signaling in renal I/R has not been reported yet. This is the first study to demonstrate paricalcitol pretreatment could suppress p38 MAPK activation, which reflects to decreased p-p38 MAPK expression. It has been shown that p38 MAPK pathway can also regulate NFκB activation. NFκB is one of the important transcription factor involved in regulation of inflammation and apoptosis. Its activation is mainly dependent on p65 phosphorylation [29]. There are few studies investigating the impact of paricalcitol pretreatment on NFκB activity in renal I/R. Lee et al. reported that paricalcitol decreased NFκB activation, however, the dose of paricalcitol was higher than that of our study. They suggested that the inhibition of NFκB activity caused by paricalcitol could be related to supression of Toll-like receptor 4 (TLR4) activation [19]. In another study performed on mice by using same dose as in our study, it was shown that paricalcitol inhibited NFκB via PGE2-receptor EP4 signaling pathway [20]. Similarly, we also showed that the dose of paricalcitol used for our experimental setting had capacity to inhibit NFκB activation. Subsequently, it was also determined that paricalcitol significantly decreased TNF-α and IL1-β mRNA expression in parallel to reduced I/R induced inflammatory cell infiltration as demonstrated by histological analysis. We also evaluated the activation of caspase-3, known as a final mediator in the accomplishment of apoptosis induced by oxidative stress and inflammatory response in the kidney [5], [30], [31]. Previously, it was reported that paricalcitol decreased the active caspase-3 expression in gentamycine induced kidney [32]. Herein, we first report that paricalcitol pretreatment inhibited significantly kidney expression of active caspase-3, which was induced during renal I/R. The renoprotective effect of paricalcitol was further confirmed by analysing the expression of kidney injury molecule-1 (KIM-1), known as a strong marker for proximal tubular injury. Normally, its expression is low in the kidney. However, it is highly increased in the proximal tubules after acute injury and rapidly decreases during remodeling phase. In fact, it mediates phagocytosis of apoptotic bodies since it is a phosphatidyl serine receptor. However, it was shown that its chronic expression resulted in chronic inflammation and fibrosis [33], [34], [35]. In this study, we reported, for the first time, that the rats pretreated with paricalcitol during renal I/R had significantly lower gene expression of KIM-1 in their kidneys which also reflected to its protein level, compared to renal I/R group. This finding is an another strong indication of reduced injury in the paricalcitol pretreatment group. It was also consistent with our previous study [21] and other publications [19], [20] which report that pretreatment with paricalcitol attenuated the elevation of serum creatinine level, which is a renal function parameter. On the other hand, a previous study showed that p38 MAPK signaling directly induced KIM-1 expression in HK-2 cells [34]. In this study, the ability of paricalcitol on downregulation of KIM-1 expression during I/R indicated that it has also the capacity to prevent the progression of acute kidney injury. Besides, activation of PI3K/Akt signaling has been known to inhibit renal apoptosis and inflammation [9], [10]. KIM-1 also has been reported to activate PI3K/Akt signaling [35]. In this regard, we also found that activation of p38 MAPK and Akt were significantly together with increased KIM-1 during renal I/R, indicating that there might be relation among them. Paricalcitol pretreatment also significantly induced Akt activation, compared with the I/R group. Studies investigating the impact of paricalcitol on activation of PI3K/Akt signaling during renal I/R are very limited. Hong et al. showed that induction of EP4 activation by paricalcitol mediated also activation of Akt, which is associated to attenuated inflammation and apoptosis [19]. By contrast, Suh et al. demonstrated that gentamicin-induced Akt activation was counteracted by paricalcitol co-treatment. They suggested that decreased activation of Akt by paricalcitol might be associated with protection from renal fibrosis, indicating that the conflincting roles of Akt activation in the kidney various stimuli [32]. Our results collectively indicate that paricalcitol would prevent renal injury by both suppressing the activation of p38 MAPK and inducing the activation of PI3K/Akt signaling. These results suggested that there may be a regulatory cross talk between these signaling pathways involved in inflammation and apoptosis.
In conclusion, we demonstrated that the renoprotective effects of paricalcitol in renal I/R injury model in rats could be related to inhibited p38 MAPK and activated PI3K/Akt signaling pathways. These actions of paricalcitol can play a critical role in its antioxidant, anti-inflammatory and antiapoptotic effects in renal I/R injury. However, the precise mechanisms of the effects of paricalcitol require further research extending the spectra of pathways involved. Nevertheless, all these findings indicate that paricalcitol may be an effective practical strategy to prevent renal I/R injury.
Acknowledgments
Our work was conducted partially at Dokuz Eylul University Medical School Learning Resources Center Research Laboratory (R-LAB). We thank Prof.Dr. Hülya Ellidokuz for statistical evaluation. This study was presented as a poster at the 42nd Federation of European Biochemical Societies (FEBS) Congress, Jerusalem, September, July 10–14, 2017.
Conflict of interest: The authors declare that they have no conflict of interest.
References
1. Chatterjee PK. Novel pharmacological approaches to the treatment of renal ischemia-reperfusion injury: a comprehensive review. Naunyn Schmiedebergs Arch Pharmacol 2007;376:1–43.10.1007/s00210-007-0183-5Search in Google Scholar PubMed
2. Reel B, Guzeloglu M, Bagriyanik A, Atmaca S, Aykut K, Albayrak G, et al. The effects of PPAR-γ agonist pioglitazone on renal ischemia/reperfusion injury in rats. J Surg Res 2013;182:176–84.10.1016/j.jss.2012.08.020Search in Google Scholar PubMed
3. Ahmadiasl N, Banaei S, Alihemati A, Baradaran B, Azimian E. Effect of a combined treatment with erythropoietin and melatonin on renal ischemia reperfusion injury in male rats. Clin Exp Nephrol 2014;18:855–64.10.1007/s10157-014-0937-6Search in Google Scholar PubMed
4. Son Y, Cheong YK, Kim NH, Chung HT, Kang DG, Pae HO, et al. Mitogen-activated protein kinases and reactive oxygen species: how can ROS activate MAPK pathways? J Signal Transduct 2011;2011:792639.10.1155/2011/792639Search in Google Scholar PubMed PubMed Central
5. Thurman JM. Triggers of inflammation after renal ischemia/reperfusion. Clin Immunol 2007;123:7–13.10.1016/j.clim.2006.09.008Search in Google Scholar PubMed PubMed Central
6. Luo F, Shi J, Shi Q, Xu X, Xia Y, He, X. Mitogen-activated protein kinases and hypoxic/ischemic nephropathy. Cell Physiol Biochem 2016;39:1051–67.10.1159/000447812Search in Google Scholar PubMed
7. Ye S, Zhu Y, Ming Y, She X, Liu H, Ye Q. Glycyrrhizin protects mice against renal ischemia-reperfusion injury through inhibition of apoptosis and inflammation by downregulating p38 mitogen-activated protein kinase signaling. Exp Ther Med 2014;7:1247–52.10.3892/etm.2014.1570Search in Google Scholar PubMed PubMed Central
8. Cavdar Z, Ural C, Celik A, Arslan S, Terzioglu G, Ozbal S, et al. Protective effects of taurine against renal ischemia/reperfusion injury in rats by inhibition of gelatinases, MMP-2 and MMP-9, and p38 mitogen-activated protein kinase signaling. Biotech Histochem 2017;92:524–35.10.1080/10520295.2017.1367033Search in Google Scholar PubMed
9. Xie L, Zheng X, Qin J, Chen Z, Jin Y, Ding W. Role of PI3-kinase/Akt signalling pathway in renal function and cell proliferation after renal ischaemia/reperfusion injury in mice. Nephrology (Carlton) 2006;11:207–12.10.1111/j.1440-1797.2006.00558.xSearch in Google Scholar PubMed
10. Zhang J, Zou YR, Zhong X, Deng HD, Pu L, Peng K, et al. Erythropoietin pretreatment ameliorates renal ischaemia-reperfusion injury by activating PI3K/Akt signalling. Nephrology (Carlton) 2015;20:266–72.10.1111/nep.12384Search in Google Scholar PubMed
11. Cunningham J, Zehnder D. New vitamin D analogs and changing therapeutic paradigms. Kidney Int 2011;79:702–7.10.1038/ki.2010.387Search in Google Scholar PubMed
12. Park JW, Bae EH, Kim IJ, Ma SK, Choi C, Lee J, et al. Paricalcitol attenuates cyclosporine-induced kidney injury in rats. Kidney Int 2010;77:1076–85.10.1038/ki.2010.69Search in Google Scholar PubMed
13. Park JW, Bae EH, Kim IJ, Ma SK, Choi C, Lee J, et al. Renoprotective effects of paricalcitol on gentamicin-induced kidney injury in rats. Am J Physiol Renal Physiol 2010;298:F301–13.10.1152/ajprenal.00471.2009Search in Google Scholar PubMed
14. Bulut G, Basbugan Y, Ari E, Erten R, Bektas H, Alp HH, et al. Paricalcitol may improve oxidative DNA damage on experimental amikacin-induced nephrotoxicity model. Ren Fail 2016;38:751–8.10.3109/0886022X.2016.1158071Search in Google Scholar PubMed
15. Sanchez-Niño MD, Bozic M, Córdoba-Lanús E, Valcheva P, Gracia O, Ibarz M, et al. Beyond proteinuria: VDR activation reduces renal inflammation in experimental diabetic nephropathy. Am J Physiol Renal Physiol 2012;302:F647–57.10.1152/ajprenal.00090.2011Search in Google Scholar PubMed
16. Tan X, Wen X, Liu Y. Paricalcitol inhibits renal inflammation by promoting vitamin D receptor-mediated sequestration of NF-kappaB signaling. J Am Soc Nephrol 2008;19:1741–52.10.1681/ASN.2007060666Search in Google Scholar PubMed PubMed Central
17. Azak A, Huddam B, Haberal N, Kocak G, Ortabozkoyun L, Şenes M, et al. Effect of novel vitamin D receptor activator paricalcitol on renal ischaemia/reperfusion injury in rats. Ann R Coll Surg Engl 2013;95:489–94.10.1308/003588413X13629960049117Search in Google Scholar PubMed PubMed Central
18. Hwang HS, Yang KJ, Park KC, Choi HS, Kim SH, Hong SY, et al. Pretreatment with paricalcitol attenuates inflammation in ischemia-reperfusion injury via the up-regulation of cyclooxygenase-2 and prostaglandin E2. Nephrol Dial Transplant 2013;28:1156–66.10.1093/ndt/gfs540Search in Google Scholar PubMed
19. Lee JW, Kim SC, Ko YS, Lee HY, Cho E, Kim MG, et al. Renoprotective effect of paricalcitol via a modulation of the TLR4-NF-κB pathway in ischemia/reperfusion-induced acute kidney injury. Biochem Biophys Res Commun 2014;444: 121–7.10.1016/j.bbrc.2014.01.005Search in Google Scholar PubMed
20. Hong YA, Yang KJ, Jung SY, Park KC, Choi H, Oh JM, et al. Paricalcitol pretreatment attenuates renal ischemia-reperfusion injury via prostaglandin E2 Receptor EP4 Pathway. Oxid Med Cell Longev 2017;2017:5031926.10.1155/2017/5031926Search in Google Scholar
21. Ersan S, Celik A, Tanrisev M, Kose I, Cavdar Z, Unlu M, et al. Pretreatment with paricalcitol attenuates level and expression of matrix metalloproteinases in a rat model of renal ischemia-reperfusion injury. Clin Nephrol 2017;88:231–8.10.5414/CN109121Search in Google Scholar PubMed
22. Yasuda H, Yuen PS, Hu X, Zhou H, Star RA. Simvastatin improves sepsis-induced mortality and acute kidney injury via renal vascular effects. Kidney Int 2006;69:1535–42.10.1038/sj.ki.5000300Search in Google Scholar
23. Lykkesfeldt J. Determination of malondialdehyde as dithiobarbituric acid adduct in biological samples by HPLC with fluorescence detection: comparison with ultraviolet-visible spectrophotometry. Clin Chem 2001;47:1725–7.10.1093/clinchem/47.9.1725Search in Google Scholar
24. Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 2008;3:1101–08.10.1038/nprot.2008.73Search in Google Scholar
25. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970;227:680–5.10.1038/227680a0Search in Google Scholar
26. Wiechelman KJ, Braun RD, Fitzpatrick JD. Investigation of the bicinchoninic acid protein assay: identification of the groups responsible for color formation. Anal Biochem 1988;175:231–7.10.1016/0003-2697(88)90383-1Search in Google Scholar
27. Dennis JM, Witting PK. Protective role for antioxidants in acute kidney disease. Nutrients 2017;9. pii: E718. DOI: 10.3390/nu9070718.10.3390/nu9070718Search in Google Scholar PubMed PubMed Central
28. Chung S, Kim S, Kim M, Koh ES, Shin SJ, Park CW, et al. Treatment combining aliskiren with paricalcitol is effective against progressive renal tubulointerstitial fibrosis via dual blockade of intrarenal renin. PLoS One 2017;28;12:e0181757.10.1371/journal.pone.0181757Search in Google Scholar PubMed PubMed Central
29. Guijarro C, Egido J. Transcription factor-kappa B (NF-kappa B) and renal disease. Kidney Int 2001;59:415–24.10.1046/j.1523-1755.2001.059002415.xSearch in Google Scholar PubMed
30. Yang B, Jain S, Ashra SY, Furness PN, Nicholson ML. Apoptosis and caspase-3 in long-term renal ischemia/reperfusion injury in rats and divergent effects of immunosuppressants. Transplantation 2006;81:1442–50.10.1097/01.tp.0000209412.77312.69Search in Google Scholar PubMed
31. Wang YP, Li G, Ma LL, Zheng Y, Zhang SD, Zhang HX, et al. Penehyclidine hydrochloride ameliorates renal ischemia-reperfusion injury in rats. J Surg Res 2014;186:390–7.10.1016/j.jss.2013.07.041Search in Google Scholar PubMed
32. Suh SH, Lee KE, Park JW, Kim IJ, Kim O, Kim CS, et al. Antiapoptotic effect of paricalcitol in gentamicin-induced kidney injury. Korean J Physiol Pharmacol 2013;17:435–40.10.4196/kjpp.2013.17.5.435Search in Google Scholar PubMed PubMed Central
33. Yang L, Brooks CR, Xiao S, Sabbisetti V, Yeung MY, Hsiao LL, et al. KIM-1-mediated phagocytosis reduces acute injury to the kidney. J Clin Invest 2015;125:1620–36.10.1172/JCI75417Search in Google Scholar PubMed PubMed Central
34. Xie H, Li J, Gao H, Wang J, Li C, Xu Y, et al. Total flavone of Desmodium styracifolium relieved apoptosis and autophagy of COM-induced HK-2 cells by regulating KIM-1 via p38/MAPK pathway. Mol Cell Biochem 2018;442:169–75.10.1007/s11010-017-3201-zSearch in Google Scholar PubMed
35. de Souza AJ, Oak JS, Jordanhazy R, DeKruyff RH, Fruman DA, Kane LP. T cell Ig and mucin domain-1-mediated T cell activation requires recruitment and activation of phosphoinositide 3-kinase. J Immunol 2008;180:6518–26.10.4049/jimmunol.180.10.6518Search in Google Scholar PubMed PubMed Central
©2019 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Research Articles
- Investigating the impact of polysomy 17 in breast cancer patients with HER2 amplification through meta-analysis
- Diagnostic performance of microRNAs in the circulation in differential diagnosis of BPH, chronic prostatitis and prostate cancer
- Enhanced anticancer effect of cetuximab combined with stabilized silver ion solution in EGFR-positive lung cancer cells
- CA125, YKL-40, HE-4 and Mesothelin: a new serum biomarker combination in discrimination of benign and malign epithelial ovarian tumor
- Paricalcitol pretreatment attenuates renal ischemia/reperfusion injury by inhibiting p38 MAPK and activating PI3K/Akt signaling pathways
- Identification of cytoplasmic sialidase NEU2-associated proteins by LC-MS/MS
- Investigation of tyrosinase inhibition by some 1,2,4 triazole derivative compounds: in vitro and in silico mechanisms
- Investigation of alanine, propionylcarnitine (C3) and 3-hydroxyisovalerylcarnitine (C5-OH) levels in patients with partial biotinidase deficiency
- The expression levels of miR-655-3p, miR127-5p, miR-369-3p, miR-544a in gastric cancer
- Evaluation of the JAK2 V617F gene mutation in myeloproliferative neoplasms cases: a one-center study from Eastern Anatolia
- Effects of Rituximab on JAK-STAT and NF-κB signaling pathways in acute lymphoblastic leukemia and chronic lymphocytic leukemia
- Analysis of the effect of DEK overexpression on the survival and proliferation of bone marrow stromal cells
- Serum fetuin-A levels and association with hematological parameters in chronic kidney disease and hemodialysis patients
- Investigation of relaxation times in 5-fluorouracil and human serum albumin mixtures
- Oxydative stress markers and cytokine levels in rosuvastatin-medicated hypercholesterolemia patients
- The protective effects of urapidil on lung tissue after intestinal ischemia-reperfusion injury
- Effects of SR-BI rs5888 and rs4238001 variations on hypertension
- Antioxidant and cytotoxic activity of three Turkish marine-derived fungi
- Is spectrophotometric enzymatic method a cost-effective alternative to indirect Ion Selective Electrode based method to measure electrolytes in small clinical laboratories?
- Plasma presepsin in determining gastric leaks following bariatric surgery
Articles in the same Issue
- Frontmatter
- Research Articles
- Investigating the impact of polysomy 17 in breast cancer patients with HER2 amplification through meta-analysis
- Diagnostic performance of microRNAs in the circulation in differential diagnosis of BPH, chronic prostatitis and prostate cancer
- Enhanced anticancer effect of cetuximab combined with stabilized silver ion solution in EGFR-positive lung cancer cells
- CA125, YKL-40, HE-4 and Mesothelin: a new serum biomarker combination in discrimination of benign and malign epithelial ovarian tumor
- Paricalcitol pretreatment attenuates renal ischemia/reperfusion injury by inhibiting p38 MAPK and activating PI3K/Akt signaling pathways
- Identification of cytoplasmic sialidase NEU2-associated proteins by LC-MS/MS
- Investigation of tyrosinase inhibition by some 1,2,4 triazole derivative compounds: in vitro and in silico mechanisms
- Investigation of alanine, propionylcarnitine (C3) and 3-hydroxyisovalerylcarnitine (C5-OH) levels in patients with partial biotinidase deficiency
- The expression levels of miR-655-3p, miR127-5p, miR-369-3p, miR-544a in gastric cancer
- Evaluation of the JAK2 V617F gene mutation in myeloproliferative neoplasms cases: a one-center study from Eastern Anatolia
- Effects of Rituximab on JAK-STAT and NF-κB signaling pathways in acute lymphoblastic leukemia and chronic lymphocytic leukemia
- Analysis of the effect of DEK overexpression on the survival and proliferation of bone marrow stromal cells
- Serum fetuin-A levels and association with hematological parameters in chronic kidney disease and hemodialysis patients
- Investigation of relaxation times in 5-fluorouracil and human serum albumin mixtures
- Oxydative stress markers and cytokine levels in rosuvastatin-medicated hypercholesterolemia patients
- The protective effects of urapidil on lung tissue after intestinal ischemia-reperfusion injury
- Effects of SR-BI rs5888 and rs4238001 variations on hypertension
- Antioxidant and cytotoxic activity of three Turkish marine-derived fungi
- Is spectrophotometric enzymatic method a cost-effective alternative to indirect Ion Selective Electrode based method to measure electrolytes in small clinical laboratories?
- Plasma presepsin in determining gastric leaks following bariatric surgery

