Abstract
Background
Cytotoxic, antiproliferative, cell cycle inhibitive, oxidative and apoptotic effects of cetuximab [antibody for epidermal growth factor receptor (EGFR)] alone and together with stabilized silver ion solution (St-Ag) on P-H1299, R-H1299, A-431 and A-549 cells were investigated.
Materials and methods
Cytotoxic effects of cetuximab alone and together with St-Ag on cells were determined by Cell Titer-Blue® Cell Viability and Lactate Dehydrogenase Activity tests. Cell cycle distributions and apoptosis were detected by reverse transcription polymerase chain reaction (RT-PCR).
Results
St-Ag enhanced cetuximab cytotoxic effect on all cells. LDH activity, as a result of cell death, was found the highest level at treatment of cetuximab with St-Ag in all cells. Both treatment increased caspase-3/7 activity which is apoptotic enzyme was found higher in A-549 cells than other cells. Also, treatment of cetuximab with St-Ag caused increasing Bax/Bcl-2 ratio in all cells. Cetuximab with St-Ag treatment increased glutathione peroxidase activity in all cells generating oxidative stress. Proliferating Cell Nuclear Antigen (PCNA), topoisomerase II-alpha (except R-H1299), cyclin D1 and D2 genes expression were decreased in all cells which explain the cell cycle inhibition effect.
Conclusion
These findings suggest that treatment of cetuximab combined with St-Ag exhibit more carcinogenesis reducing potential than cetuximab alone.
Öz
Amaç
Cetuximab’ın (epidermal büyüme faktörü reseptörü antikoru) tek başına ve stabilize gümüş iyon çözeltisiyle (St-Ag) birlikte sitotoksik, antiproliferatif, hücre döngüsü engelleyici, oksidatif ve apoptotik etkileri P-H1299, R-H1299, A-431 ve A-549 hücrelerinde incelenmiştir.
Gereç ve Yöntem
Cetuximab’ın tek başına ve St-Ag ile birlikte hücreler üzerindeki sitotoksik etkileri Cell Titer-Blue® Hücre Canlılık testi ve Laktat dehidrogenaz aktivite testleri ile belirlenmiştir. Hücre döngüsü dağılımları ve apoptoz ters transkriptaz polimeraz zincir reaksiyonu (Rt-PCR) ile belirlenmiştir.
Bulgular
St-Ag, cetuximab’ın sitotoksik etkisini tüm hücrelerde arttırmıştır. Her iki uygulamanın apoptotik enzim olan kaspaz-3/7 aktivitesini A-549 hücrelerinde diğer hücrelere göre daha fazla arttırdığı bulunmuştur. Ayrıca, cetuximab’ın St-Ag ile birlikte uygulanması tüm hücrelerde Bax/Bcl-2 oranının artmasına neden olmuştur. Cetuximab’ın St-Ag ile birlikte uygulanması tüm hücrelerde oksidatif stres yaratarak glutatyon peroksidaz aktivitesini arttırmıştır. Hücre döngüsü inhibisyon etkisini açıklayan Prolifere Hücre Nükleer Antijeni (PCNA), topoizomeraz II-alfa (R-H1299 hariç), siklin D1 ve D2 genlerinin ekspresyonu tüm hücrelerde azalmıştır.
Sonuç
Bu bulgular, cetuximab’ın St-Ag ile birlikte uygulanmasının, tek başına cetuximab uygulamasından daha fazla karsinogenezisi azaltma potansiyelinin olduğunu göstermektedir.
Introduction
Non-small cell lung cancer (NSCLC) remains one of the most fatal cancer related malignancies. NSCLC cells have drug resistance mechanisms, which are the biggest obstacles before pulmonary cancer treatment, and contributing to success achieved in cancer treatment [1], [2]. This acquired resistance to anti-cancer drugs necessitated the search for different treatment modalities. Over expression of epidermal growth factor receptor (EGFR) on cancer cells and its role in metastasis, malignancy and drug resistance in many human cancers lead to its selection as a promising target for cancer treatment [3], [4]. Recently, the oncology studies has focused to targeted therapy. Several studies have investigated the effectiveness of EGFR inhibitors for therapy [5], [6]. Among these inhibitors, one of the most important monoclonal antibody against EGFR which is overexpressed in NSCLC cells is cetuximab. Antitumor effects of cetuximab have been reported in various studies [7], [8], [9]. EGFR is expressed in most NSCLC [10], [11], [12], [13]. The anti-cancer effect of cetuximab in NSCLC has yet to be established [14]. On the other hand, most of the targeted drugs had anti-cancer effect at high concentration with serious adverse effects [15]. Combine therapy can cause increase of anti-cancer effect of cetuximab with low concentration.
In the recent years, one of the most important goals for cancer therapy has become discovery of new compounds with antitumor activity. An interesting group of silver complexes agents used in cancer therapy comprises molecules that interact with DNA after passed cell membrane [16]. The research in this area has revealed a range of DNA recognizing molecules that act as antitumor agents, including oxidizing agents and intercalator compounds. Ag(I) mixed ligand complexes showed excellent anti-cancer activity against Ehrlich’s ascites tumor cells (EACs) [17]. Silver and hydrogen peroxide acted synergistically on the viability of E. coli. It appears that the combined toxic effect of silver and hydrogen peroxide may be related with damage to cellular proteins [16], [18]. In one study silver ion-doped transparent thin films synthesized successfully using the sol–gel method. One percent silver-doped coated thin films were found to have an efficient antibacterial activity against the both gram-positive (S. aureus) and gram-negative (E. coli) colonies [19]. So, the stabilized-silver (St-Ag) solution we use in our work can cause DNA and membrane damages by oxidation and display cytotoxic effect on cancer cells. The St-Ag can enhance the anti-cancer activity of cetuximab in NSCLC cells.
In this present work, we explored the cytotoxic, antiproliferative, inhibition of cell cycle progression, oxidative stress generation and apoptotic effects and effect mechanisms of cetuximab, which is a monoclonal antibody targeted to EGFR alone and combined with St-Ag treatments on P-H1299, R-H1299, A-431 and A-549 cells. These cells are EGFR-positive lung cancer cells which have different levels of EGFR expressions.
Materials and methods
Cell culture
The EGFR-positive lung cancer cell lines A-549 (NSCLC), H1299 (NSCLC) and A-431 (human epithelial carcinoma) were purchased from the American Type Culture Collection (ATCC). All cells were maintained in RPMI1640 media contained 10% fetal bovine serum (FBS) and 100 units/mL of Pen-Strep.
Epirubicin was purchased from Calbiochem and dissolved in water under sterile condition. The epirubicin-resistant H1299 tumor cells (R-H1299) were derived from the parental cell line (P-H1299) by stepwise selection in increasing concentrations of epirubicin until the cells were capable of propagating 220 ng/mL drugs, as described previously [2]. Typically, the resistant cells were grown in medium lacking epirubicin as minimum four passages before they were used in experiment. A-549 has functional p53 and EGFR copy number as 3.4, H1299 has null p53 and EGFR copy number as 3.5 and A-431 cell line has mutant p53 and was used as positive control for expressing EGFR in high amounts. In this study, we would like to evaluate the cellular responses given by cells with different EGFR expression levels to cetuximab alone and with silver ion solution (St-Ag) treatments.
Preparation of stabilized-silver ion solution
The stabilization of silver ion solution (St-Ag) had been optimized previously [19]. For prepare the St-Ag, silver nitrate (AgNO3) was dissolved in an isopropyl alcohol:ethyl alcohol:acetone mixture (1140 mL:340 mL:70 mL by volume) to yield a final concentration of 0.65 M followed by dropwise addition of N-(2-aminoethyl)-3-aminopropyltrimethoxysilane (DIAMO, 97% Aldrich). The molar ratio of AgNO3 to DIAMO was 1:5. This mixture was stirred for 2 h at room temperature.
Cell viability assays
The cells (104 cells/well) were plated in a 96 well plate. The next day the cells were treated with different concentrations of cetuximab (Erbutix-MerckSerono) (5–7000 μg/mL) and St-Ag (1.63 mM–84.5 mM) for 72 h (h). The cytotoxicity of cetuximab and St-Ag on cancer cells was determined by the Cell Titer-Blue® Cell Viability Assay (Promega). The Cell Titer-Blue® Cell Viability Assay is based on the ability of living cells to convert a redox dye (resazurin) into a fluorescent end product (resorufin). Nonviable cells rapidly lose metabolic capacity and thus do not generate a fluorescent signal [20]. Following cellular reduction, fluorescence was recorded at 560 nm (excitation) and 590 nm (emission) spectrofluorometrically. The data were expressed as average values obtained from three wells for each concentration. The IC50 concentration for cetuximab treatment and IC5, IC10, IC20, IC30 and IC40 (<IC50) concentrations for St-Ag were calculated. In this study, we also wanted to investigate whether St-Ag concentrations lower than IC50 increased the cytotoxic effect of cetuximab (IC50). Therefore, each cell line treated with cetuximab (IC50) combined with each of St-Ag concentrations (IC5, IC10, IC20, IC30 and IC40). As a result of this treatment, which combination concentration have the highest cytotoxic effect for each cell line was calculated. For the calculation of these values, Microsoft Excel software was used. The control group was used as a 100% viability value.
Lactate dehydrogenase (LDH) assay
LDH activity were determined after the cells were exposed to cetuximab alone (IC50), St-Ag alone (St-Ag concentration in which showed the most effective cytotoxic effect when co-administration of cetuximab and St-Ag in each cell line) and combination of cetuximab and St-Ag concentrations showing the most effective cytotoxic effects in each cell line for 72 h. Cell supernatant was collected and LDH activity in the medium was measured using LDH Activity Assay Kit (MAK066, Sigma-Aldrich) according to the manufacturer’s instruction. In this kit, LDH reduces NAD to NADH, which is specifically detected by colorimetric (450 nm) assay. LDH activity measurements were made as three replicates. LDH activity was calculated using the following formula provided by the instructions [21], [22]. The results are given in mU/mL. One unit of LDH activity is defined as the amount of enzyme that catalyzes the conversion of lactate into pyruvate to generate 1.0 μmol of NADH per minute at 37°C.
Glutathione peroxidase (GPx) activity
Glutathione peroxidase (GPx) activity were determined after the cells were exposed to cetuximab alone (IC50), St-Ag alone (St-Ag concentration in which showed the most effective cytotoxic effect when co-administration of cetuximab and St-Ag in each cell line) and combination of cetuximab and St-Ag concentrations showing the most effective cytotoxic effects in each cell line for 72 h. Cells were scraped off culture plates with culture medium and were centrifuged 600×g for 10 min. The cell pellets were washed with phosphate buffered saline and then sonicated (3×15 s) in 50 mM potassium phosphate, pH 7.2, containing 1 mM fluoride and 1 μg/mL of leupeptin and centrifuged at 150,000×g for 45 min. The supernatant was used for the determination of GPx activity. GPx was determined according to Flohe and Gunzler [23] with tert-butyl hydroperoxidase as substrate. One unit of enzyme activity results in the oxidation of 1 μmol GSH/min. GPx activity measurements were made as three replicates. Protein was determined by the Bradford method [24] with bovine serum as a standard.
Caspase-3/7 activity
Caspase-3/7 activity were investigated after by applying cetuximab alone (IC50), St-Ag alone (St-Ag concentration in which showed the most effective cytotoxic effect when co-administration of cetuximab and St-Ag in each cell line) and combination of cetuximab and St-Ag concentrations showing the most effective cytotoxic effects in each cell line for 72 h. Caspase-3/7 activity were determined using the fluorometric ApoTox-Glo™ Triplex Assay kit (Promega) [25]. After adding100 μL of caspase-Glo 3/7 to all wells, the samples were briefly mixed by orbital shaking (500 rpm for 30 s). After incubating for 1 h at room temperature, luminescence was measured using a microplate reader to assess apoptosis. Caspase-3/7 activity measurements were made as three replicates. Caspase-3/7 activity was measured in relative light unit (RLU).
RNA extraction and study of gene expression
The cells were incubated in presence of either cetuximab alone (IC50) and combined with St-Ag (the most effective combination of concentrations showing cytotoxic effects). RNA expression were investigated after by applying cetuximab alone (IC50) and combination of cetuximab and St-Ag concentrations showing the most effective cytotoxic effects in each cell line. Gene expression studies were made as three trials. Total RNA were isolated from the cells by using RN easy mini kit (Qiagen) following the manufacturer’s instructions after 72 h incubation. cDNA was synthesized from 1 mg of total RNA using Titan One Tube RT-PCR System kit (Roche Applied Science) following the manufacturer’s instructions.
To analyze RNA expression by reverse transcription-PCR (RT-PCR), 1 mg of total RNA from each sample was used for RT-PCR with Taq DNA polymerase. The primers for the gene-specific RT-PCR analysis were as follows: PCNA primer 1, CGCGCAGAGGGTTGGTAGTT and 2, AAGCCTTCGGAGCGCAGAGT [26], Bcl-2 primer 1, TGCACCTGACGCCCTTCAC and 2, AGACAGCCAGGAGAAATCAAACAG [26], Bax primer 1, ACCAAGAAGCTGAGCGAGT GTC and 2, ACAAAGATGGTCACGGTCTGCC [26], GAPDH primer 1, TTCATTGACCTC AACTACAT and 2, GAGGGGCCATCCACAGTCTT [26], Cyclin D1 primer 1, CCG TCC ATG CGG AAG ATC and 2, ATG GCC AGC GGG AAG AC [27], Cyclin D2 primer 1, TAC TTC AAG TGC GTG CAG AAG GAC and 2, TCCCACACTTCCAGTTGCGATCAT [28], Topoisomerase II-α primer 1, CAG ATC ATG GAA AATGCTGA and 2, GCAGCATCATCTTCAGGACC [29]. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a references.
The following experimental protocol for RT-PCR reaction (30 cycles was used: denaturation: 1 min; 94°C; annealing: 1 min, at 55°C (GAPDH and Bax), 58°C (Cyclin D1) and 59°C (PCNA), respectively; elongation: 2 min, 72°C, final elongation: 72°C, 90 s. Touchdown PCR was used for Bcl-2 gene. It was first kept at 73°C and lowered to 63°C by reducing 1°C in each cycle (10 cycles). After 63°C, the PCR was completed by making 25 cycles. PCR products were analyzed by electrophoresis on 2.5% agarose in TBE (Tris 40 mM, EDTA 1 mM, boric acid 44 mM) containing 1 μg/mL ethidium bromide for 30 min at 120 V (constant voltage) with 100 bp ladder as molecular weight markers. Adobe Photoshop CS4 programmed was used for band density analyzing. The density of the PCR bands were divided by that of the housekeeping gene and expressed as percent of the control band density.
Data analysis
The results of the replicates were pooled and expressed as mean±standard deviation (SD). Analysis of variance (ANOVA) was carried out. The two-way ANOVA was used to determine whether there are any significant differences between groups on variables in cell viability assays (cytotoxicity of cetuximab alone, St-Ag alone and combination of cetuximab and St-Ag). The three-way ANOVA was used to determine whether there are any significant differences between groups on variables in LDH activity, GPx activity and caspase 3/7 activity. Dunnett (used for all experiments except investigation of cytotoxic effect of combination of cetuximab and St-Ag) and Duncan (used for the experiment as investigation of cytotoxic effect of combination of cetuximab and St-Ag) multiple comparisons tests were used. Statistical differences were considered significant at p<0.05. Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) [30].
Results
Effect of cetuximab combined with St-Ag on the viability of lung cancer cells
In this study, cytotoxic effects of cetuximab alone and combined with St-Ag on P-H1299, R-H1299 and A-549 lung cancer cells having different level of EGFR expression and A-431 human epithelial carcinoma cells (positive control for EGFR) cells were investigated. As shown in Figures 1 and 2, the viability of the cells was decreased treating with cetuximab and St-Ag. Statistically significant difference (p<0.05) was observed for cytotoxic effects of cetuximab and St-Ag between the cell lines. Hundred and eighty samples were used in both cetuximab alone and St-Ag alone treated groups. The effect of each of cell line differences and concentrations alone on both cetuximab and St-Ag cytotoxicity was found to be statistically significant (p<0.05). Also, the effect of cell line differences and concentrations together on both cetuximab and St-Ag cytotoxicity was found to be statistically significant (p<0.05). The cytotoxic effect observed after all cetuximab concentrations applied in all cell lines was statistically different from the control groups (treated with only the medium-untreated cells) (p<0.05). The cytotoxic effect of cetuximab alone and St-Ag alone in R-H1299 was statistically different from the cytotoxic effect of cetuximab alone and St-Ag alone in other cell lines (p<0.05) and A-431 statistically were found to be the most different (p<0.05). Cetuximab and St-Ag decreased cancer cell viability at higher concentration. While IC50 values of cetuximab were calculated 3300, 2000, 1800 and 1000 μg/mL (Figure 1) for R-H1299, A-549, P-H1299 and A-431, St-Ag IC50 values were found 18.53 mM, 16.25 mM, 13 mM and 11.38 mM for A-549, R-H1299, P-H1299 and A-431 cells, respectively (Figure 2). The tested sensitivity of cells against cetuximab cytotoxicity were found to follow the order of A-431>P-H1299> A-549>R-H1299 and against St-Ag cytotoxicity were found A-431>P-H1299>R-H1299>A-549.
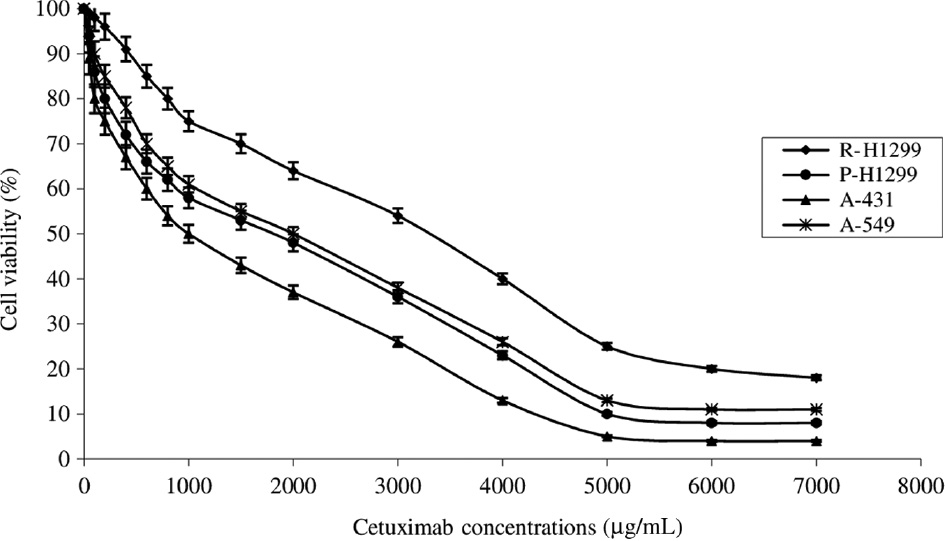
The cytotoxic effects of cetuximab (5–7000 μg/mL) for 72 h on parental H1299 (P-H1299), drug-resistant H1299 (R-H1299), A-549, and A-431 cells as measured by Cell Titer-Blue cell viability assay.
Results are presented as viability ratio compared with the control group (treated with only the medium-untreated cells). Values are expressed as the mean of three separate trials with three replications±standard deviation (SD) (ANOVA with Dunnett test, p<0.05).
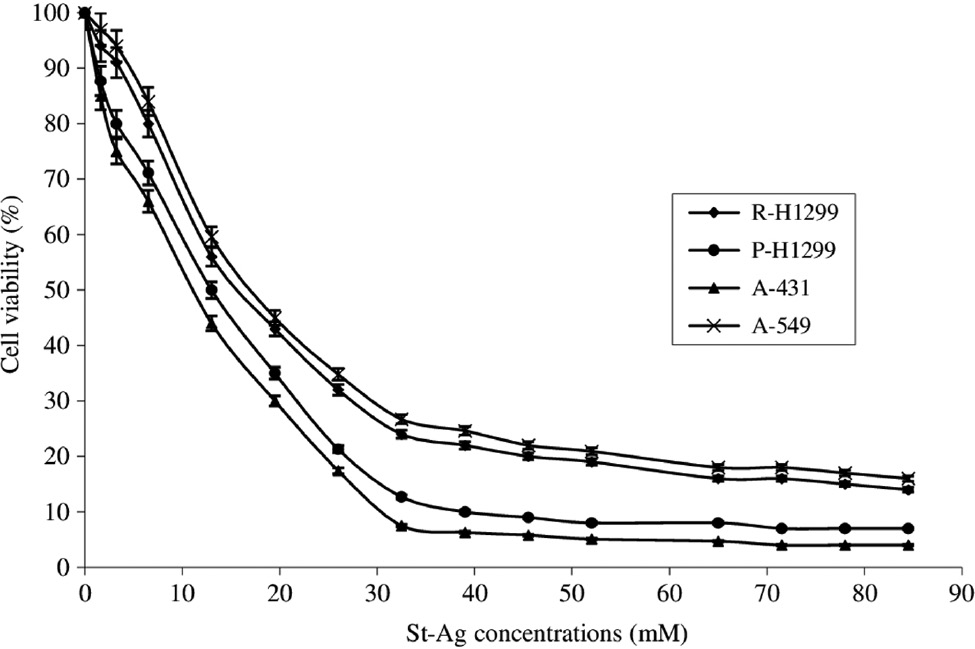
The cytotoxic effects of St-Ag solution (1.63–84.5 mM) for 72 h on parental H1299 (P-H1299), drug-resistant H1299 (R-H1299), A-549, and A-431 cells as measured by Cell Titer-Blue cell viability assay.
Results are presented as viability ratio compared with the control group (treated with only the medium-untreated cells). Values are expressed as the mean of three separate trials with three replications±standard deviation (SD) (ANOVA with Dunnett test, p<0.05).
The cells were treated with cetuximab alone and combined with each of St-Ag concentrations for 72 h. The highest cytotoxic effect of cetuximab (IC50) combined with each St-Ag concentration (IC40 IC30, IC20, IC10 IC5) were found IC50+IC40 for P-H1299 (IC40>IC30>IC10>IC5=IC20), IC50+IC10 for R-H1299 cells (IC10>IC30>IC20>IC40>IC5), IC50+IC10 for A-549 cells (IC10>IC20>IC5>IC30>IC40) and IC50+IC10 for A-431 (IC10>IC40>IC30>IC20>IC50) (Figure 3). Sixty samples were used. The effect of each of cell line differences and combination concentrations on cytotoxicity was found to be statistically significant (p<0.05). Also, the effect of cell line differences and combination concentrations together on cytotoxicity was found to be statistically significant (p<0.05). The cytotoxic effect observed in each cell line was statistically different from each other (p<0.05). Also, the cytotoxic effects of all combination concentrations applied were statistically different from each other in each cell line (p<0.05). As a result that, cetuximab combined with St-Ag treatment enhanced cytotoxic effect in P-H1299, R-H1299, A-431 and A-549 cells compared to cetuximab alone treatment.
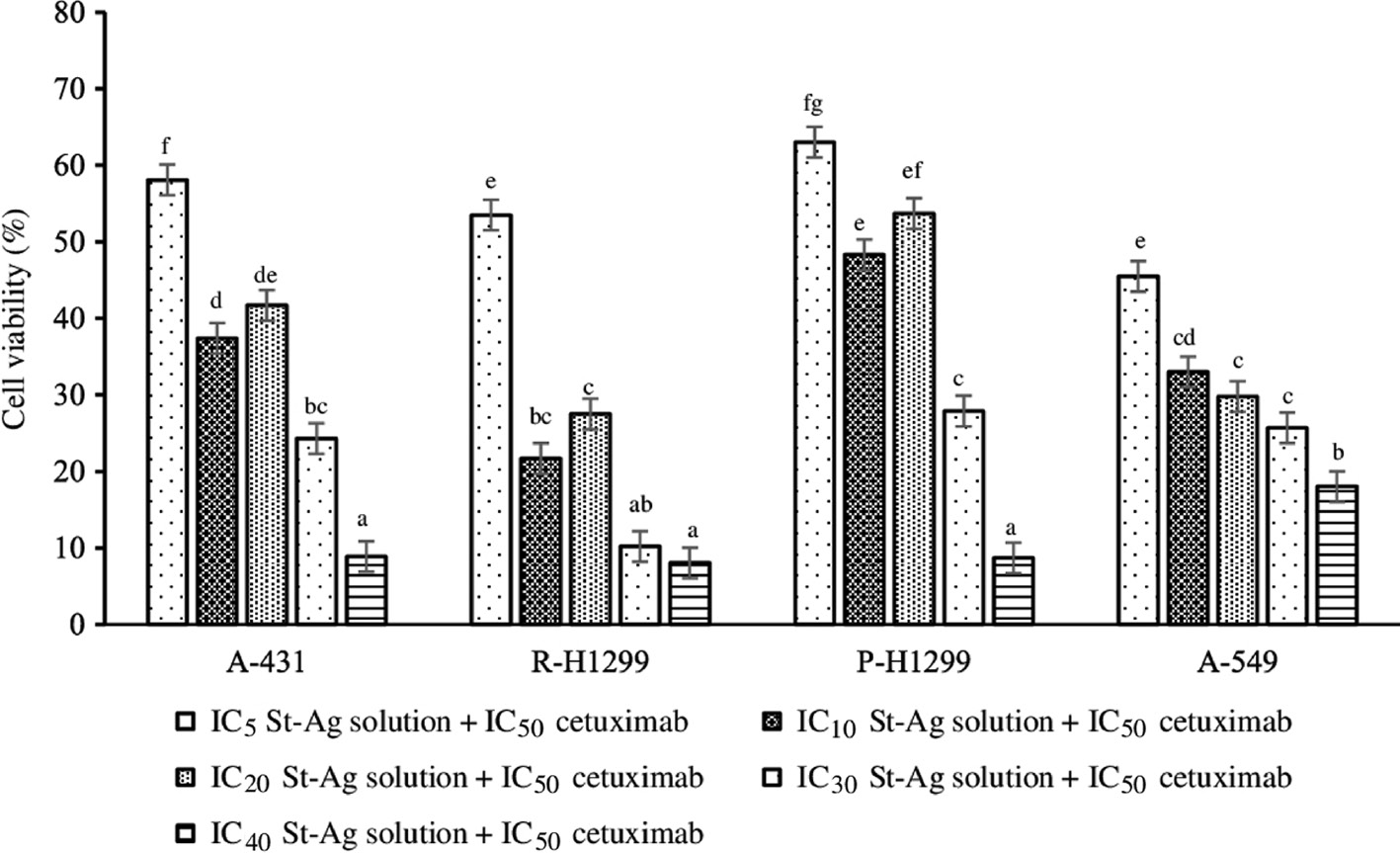
Combined cytotoxic effects of cetuximab and St-Ag (IC5, IC10, IC20, IC30, IC40) solution for 72 h on parental H1299 (P-H1299), drug-resistant H1299 (R-H1299), A-549, and A-431 cells.
Results are presented as viability ratio compared with the control group (treated with only the medium-untreated cells). Values are expressed as the mean of three separate trials with three replications±standard deviation (SD). Bars with the same letter indicate no significant difference (ANOVA with Duncan test, p<0.05). Different letters represent significant differences among treatments (p<0.05).
Effect of cetuximab combined with St-Ag on lactate dehydrogenase activity in lung cancer cells
Lactate dehydrogenase (LDH) is a cytoplasmic enzyme released as a result of the disruption of the cell membrane integrity, it has been widely used to evaluate the presence of damage and toxicity of cells. To test the effect of cetuximab alone and combined with St-Ag on cellular permeability, release of LDH from the cells into the medium was measured, as an indicator of early cell apoptosis [21]. The result is shown in Table 1. Incubation with either cetuximab or cetuximab together with St-Ag for 72 h caused a significant increase (p<0.05) in LDH release by 3.3 fold and 5.1 fold for P-H1299 cells, 1.5 fold and 4.2 fold for R-H1299 cells, 6 fold and 12.3 fold for A-431 cells and 2.7 fold and 5.6 fold for A-549 cells, respectively compare to control. At the same time, statistical analysis showed an extremely significant difference (p<0.05) in LDH activity in the culture medium between the cetuximab and cetuximab combined with St-Ag treatment groups (Table 1). The LDH activity were found higher in cetuximab combined with St-Ag treatment than cetuximab treatment alone for all cell lines compare to control group. This indicated that cetuximab combined with St-Ag treatment could result in the more significant change in cell permeability than cetuximab treatment alone.
Effect of cetuximab alone and combine with St-Ag on lactate dehydrogenase activity, caspase-3/7 activity and glutathione peroxidase activity in P-H1299, R-H1299, A549 and A431 cells.
| Concentrations | LDH activity (mU/mL) X±SD | Caspase-3/7 activity (RLUa103) X±SD | GPx activity (U/mg protein) X±SD |
|---|---|---|---|
| Cetuximab (IC50), P-H1299a,b | 619.4±0.66 | 2.2 ± 0.77 | 9.3±1.23 (increased by 15% compared to control) |
| St-Ag (IC40), P-H1299a,b | 231.3±0.44 | 1.7±0.55 | 3.2±0.90 (decreased by 60% compared to control) |
| Cetuximab+St-Ag, P-H1299a,b | 956.6±0.33 | 3±0.55 | 10.2±0.70 (increased by 26% compared to control) |
| Control, P-H1299 | 186.5±0.22 | 1±0.33 | 8.07±1.43 |
| Cetuximab (IC50), R-H1299a,b | 137.3±0.26 | 2.0±0.37 | 9.4±1.36 (increased by 9% compared to control) |
| St-Ag (IC10), R-H1299a,b | 132.2±0.48 | 1.2±0.44 | 9.5±1.10 (increased by 10% compared to control) |
| Cetuximab+St-Ag, R-H1299a,b | 500±0.55 | 3±0.51 | 11.2±0.86 (increased by 30% compared to control) |
| Control, R-H1299 | 119±0.70 | 1±0.22 | 8.6±1.19 |
| Cetuximab (IC50), A-431a,b | 830±0.70 | 2±0.44 | 9.1±0.95 (increased by 28% compared to control) |
| St-Ag (IC10), A-431a,b | 154±0.48 | 1.3±0.33 | 7.9±0.66 (increased by 11% compared to control) |
| Cetuximab+St-Ag, A-431a,b | 1700±0.95 | 3±0.42 | 10.13±0.59 (increased by 42% compared to control) |
| Control, A-431 | 138.4±0.46 | 1±0.26 | 7.1±1.32 |
| Cetuximab (IC50), A-549a | 626.7±0.60 | 2±0.46 | 8.3±1.25 (increased by 15% compared to control) |
| St-Ag (IC10), A-549a | 255.6±0.48 | 1.3±0.33 | 8±0.73 (increased by 11% compared to control) |
| Cetuximab+St-Ag, A-549a | 1300±0.68 | 5±0.59 | 9.1±1.08 (increased by 26% compared to control) |
| Control, A-549 | 230±0.55 | 1±0.22 | 7.2±1.17 |
A difference was considered significant at p<0.05 (three-way ANOVA with Dunnett test). All activity were investigated after by applying cetuximab alone (IC50), St-Ag alone (St-Ag concentration in which showed the most effective cytotoxic effect when co-administration of cetuximab and St-Ag in each cell line) and combination of cetuximab and St-Ag concentrations showing the most effective cytotoxic effects in each cell line for 72 h. One hundred and forty four samples were used. Data are expressed as mean±standard deviation (SD). aSignificantly different from control (p<0.05). bSignificantly different from LDH, GPx and caspase 3/7 activity in A-549 cells (p<0.05). All activity measurements were made as three replicates. P-H1299, parental H1299 cells. R-H1299, resistant H1299 cells. LDH, lactate dehydrogenase. GPx, glutathione peroxidase. X, each value is an average of three repetitions of LDH, caspase-3/7 and GPx activities. One unit of LDH activity is defined as the amount of enzyme that catalyzes the conversion of lactate into pyruvate to generate 1.0 μmol of NADH per minute at 37°C. RLU, relative light unit.
Effect of cetuximab combined with St-Ag on glutathione peroxidase activity
Glutathione peroxidase (GPx) is the general name of an enzyme family with peroxidase activity whose main biological role is to protect the organism from oxidative damage. In this study, statistical analysis revealed significantly (p<0.05) higher activity of GPx by 15% and 26% for P-H1299 and A-549 cells, 9% and 30% for R-H1299, 28% and 42% for A-431 cell incubated with cetuximab alone and cetuximab combined with St-Ag for 72 h, respectively than control cells (Table 1).
Thus, these data suggest that cetuximab and cetuximab combined with St-Ag induced oxidative stress producing reactive oxygen species in the cells. Also, this results indicated that oxidative effect of cetuximab were enhanced in EGRF positive cells by treatment of cetuximab combined with St-Ag.
Cetuximab combined with St-Ag induces caspase-3/7 mediated apoptosis in lung cancer cells
Caspase 3/7 activity is one of the key enzymes of apoptotic pathway. Therefore, the activation of caspase-3 is considered a reliable marker for cells undergoing apoptosis [31]. To examine the apoptotic effect of cetuximab and cetuximab combined with St-Ag, we measured the bioluminescent intensities of caspase-3/7 activities of cetuximab and cetuximab combined with St-Ag treated cells at 72 h. As shown in the Table 1 significant increase in caspase-3/7 activity were detected after 72 h of cetuximab and cetuximab together with St-Ag treatment. Incubation with either cetuximab alone or cetuximab combined with St-Ag for 72 h caused a significant increase (p<0.05) in caspase 3/7 activity by 2 fold and 3 fold for P-H1299, R-H1299 and A-431 cells, 2 fold and 5 fold for A-549 cells, respectively compare to control. At the same time, statistical analysis showed an extremely significant difference (p<0.05) in caspase 3/7 activity in in the cells between the cetuximab alone and cetuximab combined with St-Ag treatment groups (Table 1).
The effect of each of cell line differences and cetuximab alone and cetuximab combined with St-Ag treatments on LDH, GPx and caspase 3/7 activity was found to be statistically significant (p<0.05). One hundred and forty four samples were used. The effect of cell line differences and cetuximab alone and cetuximab combined with St-Ag treatments together on LDH, GPx and caspase 3/7 activity was found to be statistically significant (p<0.05). Also, interactions of together with cell line differences, cetuximab alone and cetuximab combined with St-Ag treatments and enzyme type have been found to be statistically significant (p<0.05). The LDH, GPx and caspase 3/7 activity observed after all treatments (only cetuximab, only St-Ag and combination concentrations) was statistically different from the control groups (treated with only the medium-untreated cells) in all cell lines (p<0.05). The LDH, GPx and caspase 3/7 activity of all treatments (only cetuximab, only St-Ag and combination concentrations) in P-H1299, R-H1299 and A-431 was statistically different from the LDH, GPx and caspase 3/7 activity of all treatments (only cetuximab, only St-Ag and combination concentrations) in A-549 (p<0.05).
Effect of cetuximab alone and combined with St-Ag on PCNA, topoisomerase II-alpha, cyclin D1, cyclin D2, Bax and Bcl-2 mRNA expression
Proliferating cell nuclear antigen (PCNA) is a DNA clamp that acts as a processivity factor for DNA polymerase δ in eukaryotic cells and is essential for replication [32]. The changes in PCNA mRNA expression were investigated to show the antiproliferative effect of cetuximab alone and the combination treatment (cetuximab+St-Ag) in the cells. In all cells (except R-H1299) the combined treatment was found to be more effective in reducing PCNA expression compared to cetuximab alone treatment. When cetuximab was applied alone, the greatest reduction in PCNA expression was seen in R-H1299 cells, decreased by 31.5% compared to the control, while the greatest reduction in combined treatment was seen in P-H1299 cells, decreased by 23.6% compared to the control. The maximum reduction in PCNA expression among all treatments was seen in P-H1299 cells in combination treatment (Figure 4).
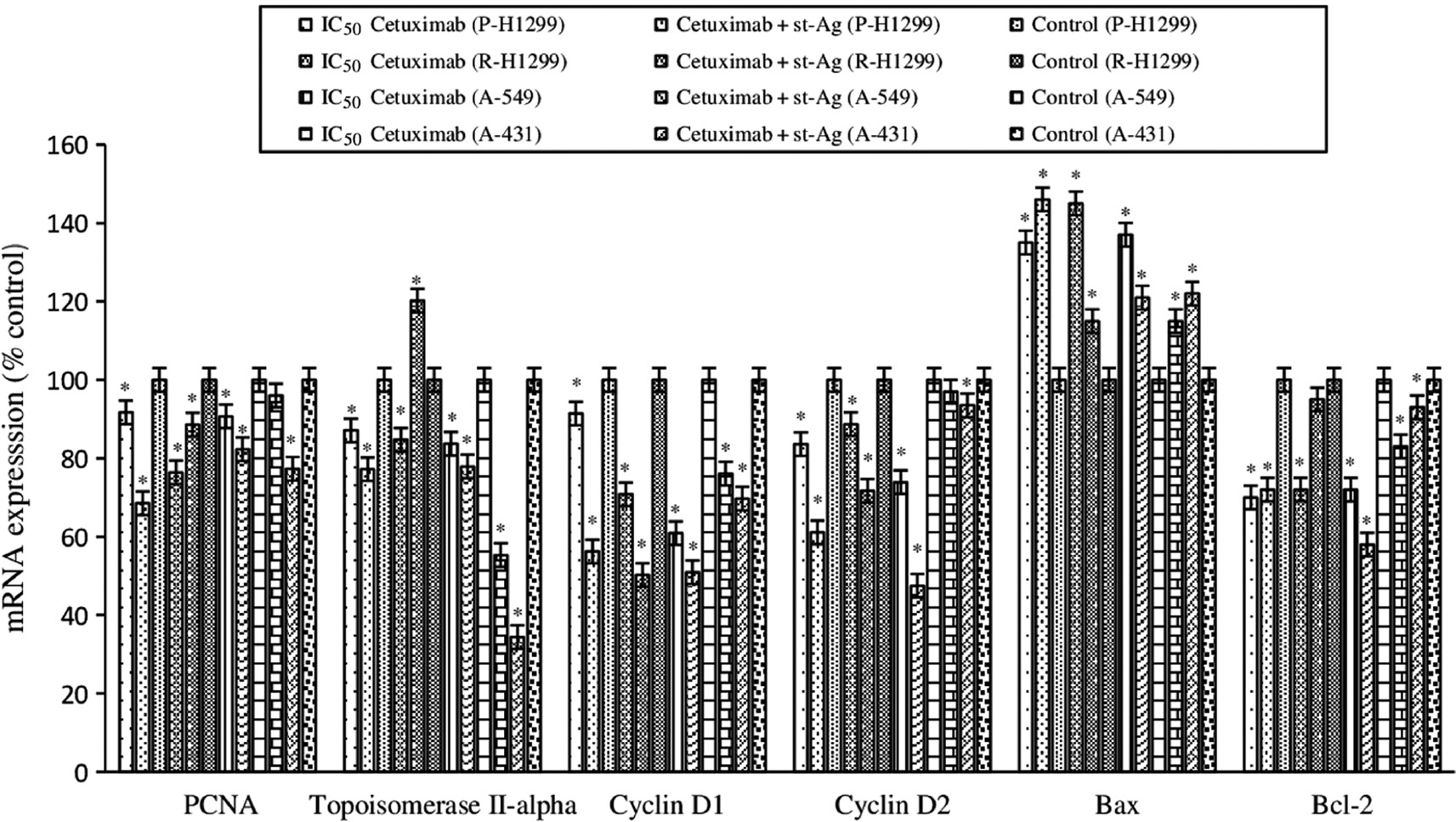
Effect of cetuximab alone and combine with St-Ag on PCNA, topoisomerase II-alpha, cyclin D1, cyclin D2, Bax and Bcl-2 mRNA expression.
The intensities of the bands were quantized by densitometric scanning of agarose gel bands and expressed as percent of mRNA expression respect to the control. Results are from three independent trials. Significantly different from controls *p<0.05.
Topoisomerase II-alpha controls and alters the topologic states of DNA during transcription. The highest reduction in topoisomerase II-alpha mRNA expression in both cetuximab alone and combined treatments was seen in A-431 cells. Cetuximab treatment alone caused 44.7% decrease compared to the control in topoisomerase II-alpha expression in A-431 cells, while the combined treatment caused a reduction of 65.7% compared to control (Figure 4).
Cyclin D1 and D2 are important regulators of cell cycle progression as a transcriptional co-regulator. We assessed the effect of cetuximab alone and combined treatments on cell cycle by determining the changes in mRNA expression of the cyclin D1 and cyclin D2. When cetuximab was applied alone, the greatest reduction in cyclin D1 expression was seen in A-549 cells, decreased by 39.1% compared to the control, while the greatest reduction in combined treatment was seen in R-H1299 cells, decreased by 49.8% compared to the control. The greatest reduction in cyclin D2 expression in both cetuximab alone and combined treatments was observed in A-549 cells. When cetuximab was applied alone cyclin D2 expression decreased 39.1% compared to the control, while combined treatment caused 49.8% decrease compared to the control in A431 cells. The combined treatment was found to be more effective in reducing both cyclin D1 and cyclin D2 expressions compared to cetuximab alone treatment (Figure 4).
We evaluated changes in Bax/Bcl-2 ratio to determine the effect of cetuximab alone and the combined treatments on apoptosis. When cetuximab was applied alone, the greatest increase in Bax mRNA expression was seen in R-H1299 cells, increased by 45% compared to the control, while the greatest increase in combined treatment was seen in P-H1299 cells, increased by 46% compared to the control. When cetuximab was applied alone, the greatest reduction in Bcl-2 expression was seen in P-1299 cells, decreased by 30% compared to the control, while the greatest reduction in combined treatment was seen in A-549 cells, decreased by 42% compared to the control (Figure 4).
Discussion
Lung cancer is of one the most leading causes of cancer-associated mortality across the world with nearly 1.4 million deaths per year. Also, 1.6 million new cases of lung cancer are diagnosed each year [33]. NSCLC pathological types is responsible for approximately 85% of all diagnosed cases of the disease [34], [35], [36]. Conventional chemotherapy has proven efficacy in preventing tumor recurrence and prolonging patients overall survival. However, the lack of tumor-specific delivery of chemotherapeutic agents results in limited efficiency, increased toxicity, and severe side effects. Therefore, novel strategies for the selective delivery of anti-cancer drugs to lung malignant cells are highly desired.
One of the new treatment strategies developed recently is the co-treatment of targeted chemotherapeutics with conventional chemotherapeutics. One of the most important targets of targeted chemotherapeutics (antibodies and small molecule inhibitors) in cancer treatment is the EGFR, which is abnormally and continuously exaggerated in many cancer cells. Several studies have shown that EGFR contributed to the aggressive growth properties of tumors are frequently expressed in lung cancer [37]. A lot of work was done about serious side effects cetuximab alone application on patients. In these studies, it has been shown that cetuximab inhibited the proliferation of various human cancer cells such as breast, colon, lung, kidney and prostate by inhibiting phosphorylation of EGFR, AKT and mitogen-activated protein kinases (MAPK) by inducing apoptosis [38], [39], [40].
Recently, promising results have been obtained in cancer treatment by utilizing two or three anti-cancer agents in combination [41], [42]. It is known that targeted chemotherapeutic agents lead to various side effects, primarily dermal allergy, when administered alone in high concentrations. However, use of new cancer treatment strategies may be devised by administering them combined with anti-cancer agents that are not toxic to healthy cells.
We chose to use St-Ag solution in combination with cetuximab due to the fact that it has a low toxic effect on human health and the environment, can be produced at low costs, and it remains intact without any decomposition for a prolonged period of time [43]. These are reasons which indicate that it may possibly make a positive contribution to national economy.
On the other hand, drug resistance developed in cancer patients leads to the prolongation of treatment and the use of high-dose drugs, leading to increased side effects in patients and making treatment more difficult [44]. Therefore, we consider the need to develop new treatment strategies that will kill parental cancer cells (P-H1299) as well as resistant cancer cells (R-H1299), when dealing with increasing therapeutic efficacy of existing drugs.
Our aim in this study is to shed light on treatment strategies that will overcome the EGFR positive and drug resistance cancer cells, and at the same time reduce cetuximab side effect in the treatment of lung cancer.
In one study, LDH test showed that cetuximab inhibited the proliferation of eight head and neck squamous carcinoma cells (PE/CA-PJ-15, PE/CA-PJ-34, PE/CA-PJ-41, PE/CA-PJ-49, Cal-27, Kyse-140, CLS-354, UM-SCC-14C) depending on time (24, 48, 72 h) and dose [7], [8]. Furthermore, cetuximab had antiproliferative effect on A-431, PJ 15, PE/CA-PJ 41, Cal-27 and Kyse-140 cells and also cetuximab combined with bortezomib caused more LDH release than treatment of cetuximab alone [7], [8], [9]. But, another observation was that cetuximab suppressed lactate dehydrogenase A (LDH-A) and inhibited glycolysis in cetuximab sensitive head and neck squamous cell carcinoma (HNSCC) [45]. The insulin-like growth factor 1 receptor (IGF-1 R) tyrosine kinase inhibitor (TKI)-NVP-AEW541 combined with cetuximab was found to cause an increase in LDH release in human colorectal adenocarcinoma cell lines (HT29 and HCT116) in comparison to control cells [46].
In our study cetuximab and St-Ag decreased cancer cell viability at higher concentrations. R-H1299 cells which have drug resistance property and EGFR, were found more resistant to cetuximab cytotoxicity with higher IC50 value than other cells. A-431 cells which is positive control were found more sensitive to cetuximab as we expected. On the other hand, A431 cells were found more sensitive to St-Ag cytotoxicity with lowest IC50 value than other cells. After, treatment of cetuximab (IC50) combined with different St-Ag concentrations (IC40, IC30, IC20, IC10, IC5), we calculated combined concentration which is enhanced cytotoxicity for each cells. As a result that, treatment of cetuximab combined with St-Ag enhanced cytotoxic effect in P-H1299, R-H1299, A-431 and A-549 cells compare to treatment of cetuximab alone. The lower St-Ag concentrations may have shown better cytotoxic effects in cell lines with cetuximab as those concentrations may have stimulated the antioxidant mechanisms of cells more effectively and also the St-Ag concentration, which showed the most effective cytotoxic effect in combination, may have changed as according the drug resistance properties of the cells.
Besides the cell viability, the release of LDH is also a vital index of cytotoxicity. LDH, a stable cytoplasmic enzyme inside cells, is released into the culture medium after the damage of cell membrane [47]. Changes in LDH enzyme activity were measured after the each cell line was exposed to IC50 cetuximab and IC50 cetuximab combined with St-Ag concentration which is combination concentration showing the most potent cytotoxic effect for 72 h. It was found that the LDH enzyme activity increased around 2 fold in P-H1299, R-H1299, A-431 and A-549 cells subjected to combination concentrations compare to alone cetuximab treatment. Our LDH activity result supports cell viability results.
The fact that cells feature differing levels of sensitivity toward treatment methods may be attributable to the differences in their natural antioxidant protection systems. Antioxidant systems protect cells against oxidative damage. It was shown that HepG2 cells (human hepatoma cancer cell line) that have developed epirubicin-HCl resistance causing oxidative stresses have higher glutathione peroxidase and glutathione-S-transferase (GST) enzyme activities, GST-pi, p-glcoprotein and NADPH-cytochrome P-450 reductase (3A4) expressions than parental cells [48]. It was shown that the activity of superoxide dismutase (SOD) was 3 times, the activity of selenium-dependent glutathione peroxidase (GPx) was 13 times, and the activity of glutathion-S-transferase was 12 times higher than parental cells in epirubicin-HCl resistant MCF-7 cells (human breast tumor cell line). Furthermore, the activity of NADPH-cytochrome P-450 reductase, known to activate epirubicin-HCl, was found to be 2-fold higher in resistant cells [49]. In another study, it was shown that M38K mesothelioma cells were more resistant to epirubicin-HCl than M14K cells, while at the same time Mn SOD and catalase activities and mRNA levels were higher, glutathione peroxidase and glutathione reductase levels were not significantly different [50]. Some of the research indicates that the deposition of AgNOs in the liver induced cytotoxicity through oxidative damage [51]. There are also researches showing that Ag nanoparticles caused membrane damage and reduced GSH levels by increasing oxidative stress in two mammalian cell lines HeLa and HaCaT [52], [53].
In our study, treatments of cetuximab, either alone or combined with St-Ag solution, were found to increase GPx activity in the cell lines. Because, cetuximab and cetuximab combined with St-Ag induced oxidative stress producing reactive oxygen species in cells. The activities of glutathione peroxidase in P-H1299, R-H1299, A-431 and A-549 cells treated with cetuximab combined with St-Ag were found to be at least 1.5 times greater than the activity of glutathione peroxidase in cells treated with cetuximab alone.
Caspase-3 is responsible for the breakdown of key cellular proteins, such as skeletal proteins, which cause typical morphological changes observed in apoptotic cells. For this reason, apoptosis is a critical player. Our results suggest that cetuximab and cetuximab+St-Ag induced apoptosis in the cell. Also, apoptotic effect of cetuximab were enhanced by combined treatment cetuximab+St-Ag in EGRF positive cells. Many chemotherapeutic agents exhibit cytotoxic effects by inducing apoptosis. Treatment of the combination of 5-fluorouracil (5-FU), leucovorin and oxaliplatin (FOLFOX) with cetuximab to SW480 (colorectal cancer) cells for 48 h increased caspase-3 proteolytic degradation required for caspase-3 activation compared to controls [54]. Epirubicin-HCl and mapatumumab, which is a DR4-specific human agonist monoclonal antibody, combination was shown to activate caspase-8, -9 and -3, which are downstream molecules of death receptors in human bladder cancer cells (T24, KU7 and RT112) [41]. In another research, it was displayed that human head and necks squamous cell carcinoma (UMSCC1) xenografts responded better to treatment of cetuximab after gemsitabin in comparison to vice versa, whereas there was no difference between the two applications in terms of caspase-3 activity [42].
In our study, cetuximab alone and combined with St-Ag treatments led to higher caspase-3 activity, thus inducing more intense apoptotic effect. According to our results, treatment of cetuximab combined with St-Ag in cells enhanced apoptosis compare to treatment of cetuximab alone.
mRNA expressions of the proliferating cell nuclear antigen (PCNA), which exhibits antiproliferative effect and is one of the DNA polymerase components involved in replication and mRNA expressions of cyclin D1 and cyclin D2 genes which are active in the transition from the G1 phase to the S phase of the cell cycle and exhibit cell cycle arresting effect were examined by RT-PCR. In one study, sorafenib was shown to reduce PCNA expression synergistically with EGFR inhibitors gefitinib and erlotinib in Hep 3B cells, which are hepatoma cells [55]. In HSC3, an oral squamous cell carcinoma cell line, cetuximab alone was not shown to alter PCNA expression relative to control cells, but co-administration with genistein (isoflavone and a strong protein tyrosine kinase inhibitor) was shown to reduce PCNA expression [56]. One particular research explains that expression of c-myc, Bcl-2, Bcl-XL and cyclin D1 genes are not changed in RAS-activated HNSCC cells after treatment of cetuximab which shows that RAS-activation in turn plays an important role in the resistance of cetuximab [57]. In tumor tissue xenograft models with primary colon carcinoma which have lymphatic and hepatic metastasis, it was seen that PCNA expression was significantly suppressed [58]. Another finding was that the combination of bevacizumab, cetuximab and cisplatine treatment reduced Bcl-2 expression in mice with neck squamous cell carcinoma [59].
After both treatments, our results revealed that, PCNA mRNA expressions decreased in P-H1299, A549 and A431 cells compare to control cells. But when cetuximab was applied alone, the greatest reduction in PCNA expression was seen in R-H1299 cells. The highest reduction in topoisomerase II-alpha mRNA expression in both cetuximab alone and combined treatments was seen in A-431 cells. The combined treatment was found to be more effective in reducing both cyclin D1 and cyclin D2 expressions compared to cetuximab alone treatment in R-H1299, P-H1299, A-549 and A-431 cells. The treatment of cetuximab with St-Ag was found to be more effective in increasing Bax mRNA expression, a pro-apoptotic gene, and decreasing Bcl-2 mRNA expression, an anti-apoptotic gene, than cetuximab alone treatment in cells. When cetuximab was applied alone, the greatest increase in Bax mRNA expression was seen in R-H1299 cells, while the greatest increase in combined treatment was seen in P-H1299 cells compared to the control. When cetuximab was applied alone, the greatest reduction in Bcl-2 expression was seen in P-1299 cells, while the greatest reduction in combined treatment was seen in A-549 cells compared to the control (Figure 4). Both cetuximab alone and cetuximab combine treatment resulted in an increase in Bax/Bcl-2 in cells. Increasing this ratio in cells showed that both treatment induced apoptosis in R-H1299, P-H1299, A-549 and A-431 cells.
Our study has been the first one to examine and observe the differences between responses of cells which have different expression of EGFR levels and drug-resistant cell to cetuximab alone and combined with St-Ag treatments in comparison to control cells. The fact that cetuximab was observed to increase the apoptotic effect in some cells is coherent with other studies. Effect of combined treatment on cancer cells changed depend of cells properties such as EGFR expression, drug resistance.
Acknowledgements
The authors wish to thanks to Akdeniz University Scientific Research Projects Unit (Funder Id: http://dx.doi.org/10.13039/501100005703, 2013.01.0115.002) for financial support of this work.
Conflict of interests: The authors declare that there are no conflicts of interests.
Ethical approval: This article does not contain any studies with human participants or animals performed by any of the authors.
Funding: This study was funded by 2013.01.0115.002.
References
1. Rihova B, Strohalm J, Kobackova K. Acquired and specific immunological mechanisms co-responsible for efficacy of polymer-bound drugs. J Control Release 2002;7:97–114.10.1016/S0168-3659(01)00489-8Search in Google Scholar
2. Ozkan A. Lymphokine-activated killer cell susceptibility in epirubicin resistant and parental human non-small cell lung cancer (NSCLC). Biologia 2007;62:232–7.10.2478/s11756-007-0040-5Search in Google Scholar
3. Franovic A, Gunaratnam L, Smith K, Robert I, Patten D, Lee S. Translational up-regulation of the EGFR by tumor hypoxia provides a nonmutational explanation for its overexpression in human cancer. Proc Natl Acad Sci USA 2007;104:13092–7.10.1073/pnas.0702387104Search in Google Scholar PubMed PubMed Central
4. Maya S, Sarmento B, Lakshmanan VK, Menon D, Jayakumar R. Actively targeted cetuximab conjugated gamma-poly(glutamic acid)-docetaxel nanomedicines for epidermal growth factor receptor over expressing colon cancer cells. J Biomed Nanotechnol 2014;10:1416–28.10.1166/jbn.2014.1841Search in Google Scholar PubMed
5. Kris MG, Natale RB, Herbst RS, Lynch Jr TJ, Prager D, Belani CP, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. J Am Med Assoc 2003;290:2149–58.10.1001/jama.290.16.2149Search in Google Scholar PubMed
6. Perez-Soler R, Chachoua A, Hammond LA, Rowinsky EK, Huberman M, Karp D, et al. Determinants of tumor response and survival with erlotinib in patients with non – small-cell lung cancer. Clin Oncol 2004;22:3238–47.10.1200/JCO.2004.11.057Search in Google Scholar PubMed
7. Wagenblast J, Baghi M, Mortel S, Hirth D, Thron L, Arnoldner C, et al. Does dexamethasone inhibit anti-cancer activity of cetuximab in squamous cell carcinoma cell lines of the head and neck. Oncol Rep 2009;22:171–6.10.3892/or_00000421Search in Google Scholar PubMed
8. Wagenblast J, Baghi M, Arnoldner C, Bisdas S, Gstottner W, Ackermann H, et al. Cetuximab enhances the efficacy of bortezomib in squamous cell carcinoma cell lines. J Cancer Res Clin Oncol 2009;135:387–93.10.1007/s00432-008-0477-0Search in Google Scholar PubMed
9. Wagenblast J, Baghi M, Arnoldner C, Bisdas S, Gstttner W, Ackermann H, et al. Effect of bortezomib and cetuximab in EGF-stimulated HNSCC. Anticancer Res 2008;28:2239–43.Search in Google Scholar
10. Raben D, Helfrich B, Chan DC, Ciardiello F, Zhao L, Franklin W, et al. The effects of cetuximab alone and in combination with radiation and/or chemotherapy in lung cancer. Clin Cancer Res 2005;11:795–805.10.1158/1078-0432.795.11.2Search in Google Scholar
11. Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380–8.10.1056/NEJMoa0909530Search in Google Scholar PubMed
12. Jänne PA, Yang JC, Kim DV, Planchard D, Ohe Y, Ramalingam SS, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med 2015;372:1689–99.10.1056/NEJMoa1411817Search in Google Scholar PubMed
13. Sequist LV, Soria JC, Goldman JW, Wakelee HA, Gadgeel SM, Varga A, et al. Rociletinib in EGFR-mutated non-small-cell lung cancer. N Engl J Med 2015;372:1700–9.10.1056/NEJMoa1413654Search in Google Scholar
14. Ettinger DS. Clinical implications of EGFR expression in the development and progression of solid tumors: focus on non-small cell lung cancer. Oncologist 2006;11:358–73.10.1634/theoncologist.11-4-358Search in Google Scholar
15. Birnbaum A, Dipetrillo T, Rathore R, Anderson E, Wanebo H, Puthwala Y, et al. Cetuximab, paclitaxel, carboplatin, and radiation for head and neck cancer: a toxicity analysis. Am J Clin Oncol 2010;33:144–7.10.1097/COC.0b013e3181979093Search in Google Scholar
16. Erdoğan A, Özkan Ö, Kiraz N, Özkan A. Comparison of cellular responses of parental and epirubicin-resistant non-small cell lung cancer cells against stabilized-Ag ion solution induced injury. Cukurova Med J 2016;41:74–81.10.17826/cutf.147192Search in Google Scholar
17. Shukla S, Mishra AP. Synthesis, structure, and anti-cancerous properties of silver complexes. J Chem 2013;2013:1–6.10.1155/2013/527123Search in Google Scholar
18. Pedahzur R, Shuval HI, Ulitzur S. Silver and hydrogen peroxide as potential drinking water disinfectants: their bactericidal effects and possible modes of action. Water Sci Tech 1997;35:87–93.10.2166/wst.1997.0715Search in Google Scholar
19. Tatar P, Kiraz N, Asiltürk M, Sayılkan F, Sayılkan H, Arpaç E. Antibacterial thin films on glass substrate by sol gel process. J Inorg Organomet Polym Mater 2007;3:127–32.10.1007/s10904-007-9142-3Search in Google Scholar
20. Gloeckner H, Jonuleit T, Lemke HD. Monitoring of cell viability and cell growth in a hollow-fiber bioreactor by use of the dye Alamar Blue (TM). J Immunol Methods 2001;252:131–8.10.1016/S0022-1759(01)00347-7Search in Google Scholar
21. Nelson DL, Cox MM. Lehninger Biyokimyanın İlkeleri (Çev. Edit. Kılıç N.). Ankara: Palme yayıncılık, 2005:130–52.Search in Google Scholar
22. Decker T, Lohmann-Matthes ML. A quick and simple method for the quantitation of lactate dehydrogenase release in measurements of cellular cytotoxicity and tumor necrosis factor (TNF) activity. J Immunol Methods 1988;115:61–9.10.1016/0022-1759(88)90310-9Search in Google Scholar
23. Flohe L, Gunzler WA. Glutathione peroxidase. Methods Enzymol 1984;105:115–21.Search in Google Scholar
24. Bradford MM. A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 1976;72:248–54.10.1016/0003-2697(76)90527-3Search in Google Scholar
25. Cummings BS, Schnellmann RG. Cisplatin-induced renal cell apoptosis: caspase 3-dependent and-independent pathways. J Pharmacol Exp Ther 2002;302:8–17.10.1124/jpet.302.1.8Search in Google Scholar
26. Huang ST, Yang RC, Yang LJ, Lee PN, Pang JH. Phyllanthus urinaria triggers the apoptosis and Bcl-2 down regulation in Lewis lung carcinoma cells. Life Sci 2003;72:1705–16.10.1016/S0024-3205(03)00016-XSearch in Google Scholar
27. Bijwaard KE, Aguilera NS, Monczak YT, Taubenberger JK, Jack H, Lichy JH. Quantitative Real-Time Reverse Transcription-PCR assay for cyclin D1 expression: utility in the diagnosis of mantle cell lymphoma. Clin Chem 2001;47:195–201.10.1093/clinchem/47.2.195Search in Google Scholar
28. Choi D, Yoon S, Lee E, Seongsoo HS, Song S, Kim J, et al. Characterization of cyclin D2 expression in human endometrium. J Soc Gynecol Invest 2002;9:41–6.10.1177/107155760200900109Search in Google Scholar
29. Mirski SE, Voskoglou-Nomikos T, Young LC, Deeley RG, Campling BG, Gerlach JH, et al. Simultaneous quantitation of topoisomerase II a and b isoform mRNAs in lung tumor cells and normal and malignant lung tissue. Lab Invest 2000;80:787–95.10.1038/labinvest.3780083Search in Google Scholar
30. IBM SPSS software. www.ibm.com/analytics/spss-statistics-software (Last accessed: October 2018).Search in Google Scholar
31. Shi Y. Mechanisms of caspase activation and inhibition during apoptosis. Mol Cell 2002;9:459–70.10.1016/S1097-2765(02)00482-3Search in Google Scholar
32. Strzalka W, Ziemienowicz A. Proliferating cell nuclear antigen (PCNA): a key factor in DNA replication and cell cycle regulation. Ann Bot 2011;107:1127–40.10.1093/aob/mcq243Search in Google Scholar PubMed PubMed Central
33. Ramalingam SS, Owonikoko TK, Khuri FR. Lung cancer: new biological insights and recent therapeutic advances. CA Cancer J Clin 2011;61:91–112.10.3322/caac.20102Search in Google Scholar PubMed
34. Ettinger DS, Akerley W, Borghaei H, Chang AC, Cheney RT, Chirieac LR, et al. Non-small cell lung cancer. J Natl Compr Cancer Netw 2012;10:1236–71.10.6004/jnccn.2012.0130Search in Google Scholar PubMed
35. Lemjabbar-Alaoui H, Hassan OU, Yang YW, Buchanan P. Lung cancer: biology and treatment options. Biochim Biophys Acta 2015;1856:189–210.10.1016/j.bbcan.2015.08.002Search in Google Scholar PubMed PubMed Central
36. Sekido Y, Fong KM, Minna JD. Molecular genetics of lung cancer. Annu Rev Med 2003;54:73–87.10.1146/annurev.med.54.101601.152202Search in Google Scholar PubMed
37. Prabhakar CN. Epidermal growth factor receptor in non-small cell lung cancer. Transl Lung Cancer Res 2015;4:110–8.Search in Google Scholar
38. D’angelo D, Mussnich P, Rosa R, Bianco R, Tortora G, Fusca A. High mobility group A1 protein expression reduces the sensitivity of colon and thyroid cancer cells to antineoplastic drugs. BMC Cancer 2014;14:851.10.1186/1471-2407-14-851Search in Google Scholar PubMed PubMed Central
39. Chen W, Hu QD, Xia XF, Liand C, Liu H, Zhang Q, et al. Rapamycin enhances cetuximab cytotoxicity by inhibiting mTOR-mediated drug resistance in mesenchymal hepatoma cells. Cancer Biol Ther 2014;15:992.10.4161/cbt.29113Search in Google Scholar PubMed PubMed Central
40. Mao C, Liao RY, Chen Q. BRAF mutation predicts resistance to anti-EGFR monoclonal antibodies in wild-type KRAS metastatic colorectal cancer. J Cancer Res Clin Oncol 2010;136:1293.10.1007/s00432-010-0922-8Search in Google Scholar PubMed
41. Ahmed SM, Wu X, Jin X, Zhang X, Togo Y, Suzuki T, et al. Synergistic induction of apoptosis by mapatumumab and anthracyclines in human bladder cancer cells. Oncol Rep 2015;33:566.10.3892/or.2014.3654Search in Google Scholar PubMed
42. Maseki S, Ijichi K, Nakanishi H, Hasegawa Y, Ogawa T, Murakami S. Efficacy of gemcitabine and cetuximab combination treatment in head and neck squamous cell carcinoma. Mol Clin Oncol 2013;1:918–24.10.3892/mco.2013.159Search in Google Scholar PubMed PubMed Central
43. Chandra Ray P, Hongtao Y, Peter PF. Toxicity and environmental risks of nanomaterials: challenges and future needs. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 2009;27:1–35.10.1080/10590500802708267Search in Google Scholar PubMed PubMed Central
44. Gao AM, Ke ZP, Shi F, Sun GC, Chen H. Chrysin enhances sensitivity of BEL-7402/ADM cells to doxorubicin by suppressing PI3K/Akt/Nrf2 and ERK/Nrf2 pathway. Chem Biol Interact 2013;206:100–8.10.1016/j.cbi.2013.08.008Search in Google Scholar PubMed
45. Lu H, Li X, Luo Z, Liu J, Fan Z. Cetuximab reverses the Warburg effect by inhibiting HIF-1-regulated LDH-A. Mol Cancer Ther 2013;12:2187–99.10.1158/1535-7163.MCT-12-1245Search in Google Scholar PubMed PubMed Central
46. Hopfner M, Sutter AP, Huether A, Baradari V, Scherubl H. Tyrosine kinase of insulin-like growth factor receptor as target for novel treatment and prevention strategies of colorectal cancer. World J Gastroenterol 2006;12:5635–43.10.3748/wjg.v12.i35.5635Search in Google Scholar PubMed PubMed Central
47. Ramalingam M, Kim SJ. Insulin on hydrogen peroxide-induced oxidative stress involves ROS/Ca2+ and Akt/Bcl-2 signaling pathways. Free Radic Res 2014;48:347.10.3109/10715762.2013.869588Search in Google Scholar PubMed
48. Ozkan A, Fışkın K. Investigation of effects of Epirubicin HCI and LAK on antioxidant mechanism and free radicals scavenger enzymes in hepatoma G2 cell line. Akdeniz University, Doctor’s thesis, 2002.Search in Google Scholar
49. Özkan A, Ayhan A, Fışkın K. Combined effect of epirubicin and lymphokine-activated killer cells on the resistant human breast cancer cells. Cell Biol Toxicol 2004;20:261.10.1007/s10565-004-3471-6Search in Google Scholar PubMed
50. Kahlos K, Anttila S, Asikainen T, Kinnula K, Raivio KO, Mattson K, et al. Manganese superoxide dismutase in healthy human pleural mesothelium and in malignant pleural mesothelioma. Am J Respir Cell Mol Biol 1998;18:570.10.1165/ajrcmb.18.4.2943Search in Google Scholar PubMed
51. Hsin YH, Chen CF, Huang S, Shih TS, Lai PS, Chueh PJ. The apoptotic effect of nanosilver is mediated by a ROS- and JNK-dependent mechanism involving the mitochondrial pathway in NIH3T3 cells. Toxicol Lett 2008;179:130–9.10.1016/j.toxlet.2008.04.015Search in Google Scholar PubMed
52. Kim YS, Kim JK, Cho HS, Rha DS, Kim JM, Park JD, et al. Twenty-eight-day oral toxicity, genotoxicity, and gender-related tissue distribution of silver nanoparticles in Sprague-Dawley rats. Inhal Toxicol 2008;20:575–83.10.1080/08958370701874663Search in Google Scholar PubMed
53. Mukherjee SG, O’claonadh N, Casey A, Chambers G. Comparative in vitro cytotoxicity study of silver nanoparticle on two mammalian cell lines. Toxicol In Vitro 2012;26:238–51.10.1016/j.tiv.2011.12.004Search in Google Scholar PubMed
54. Roh SA, Choi EY, Cho DH, Yon YS, Kim TW, Kim YS, et al. Characterization of biological responses of colorectal cancer cells to anti-cancer regimens. J Korean Surg Soc 2012;83:21.10.4174/jkss.2012.83.1.21Search in Google Scholar PubMed PubMed Central
55. Ezzoukhry Z, Louandre C, Trécherel E, Godin C, Chauffert B, Dupont S, et al. EGFR activation is a potential determinant of primary resistance of hepatocellular carcinoma cells to sorafenib. Int J Cancer 2012;131:2961.10.1002/ijc.27604Search in Google Scholar PubMed
56. Park SJ, Kim MJ, Kim YK, Kim SM, Park JY, Myoung H. Combined cetuximab and genistein treatment shows additive anti-cancer effect on oral squamous cell carcinoma. Cancer Lett 2010;292:54.10.1016/j.canlet.2009.11.004Search in Google Scholar PubMed
57. Bhojani MS, Nyati MK, Zhao L, Normolle DP, Ross BD, Lawrence TS, et al. Molecular ımaging of Akt enables early prediction of response to molecular targeted therapy. Transl Oncol 2011;4:122–5.10.1593/tlo.11112Search in Google Scholar PubMed PubMed Central
58. Dong X, Jin K, Hu X, Du F, Lan H, Han N, et al. Antitumor effect of FP3 in combination with cetuximab on patient-derived tumor tissue xenograft models of primary colon carcinoma and related lymphatic and hepatic metastases. Int J Mol Med 2012;30:126–32.Search in Google Scholar
59. Wang Y, Dong L, Bi Q, Li X, Wu D, Ge X, et al. Investigation of the efficacy of a bevacizumab-cetuximab-cisplatin regimen in treating head and neck squamous cell carcinoma in mice. Target Oncol 2010;5:237–43.10.1007/s11523-010-0164-3Search in Google Scholar PubMed
©2019 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Research Articles
- Investigating the impact of polysomy 17 in breast cancer patients with HER2 amplification through meta-analysis
- Diagnostic performance of microRNAs in the circulation in differential diagnosis of BPH, chronic prostatitis and prostate cancer
- Enhanced anticancer effect of cetuximab combined with stabilized silver ion solution in EGFR-positive lung cancer cells
- CA125, YKL-40, HE-4 and Mesothelin: a new serum biomarker combination in discrimination of benign and malign epithelial ovarian tumor
- Paricalcitol pretreatment attenuates renal ischemia/reperfusion injury by inhibiting p38 MAPK and activating PI3K/Akt signaling pathways
- Identification of cytoplasmic sialidase NEU2-associated proteins by LC-MS/MS
- Investigation of tyrosinase inhibition by some 1,2,4 triazole derivative compounds: in vitro and in silico mechanisms
- Investigation of alanine, propionylcarnitine (C3) and 3-hydroxyisovalerylcarnitine (C5-OH) levels in patients with partial biotinidase deficiency
- The expression levels of miR-655-3p, miR127-5p, miR-369-3p, miR-544a in gastric cancer
- Evaluation of the JAK2 V617F gene mutation in myeloproliferative neoplasms cases: a one-center study from Eastern Anatolia
- Effects of Rituximab on JAK-STAT and NF-κB signaling pathways in acute lymphoblastic leukemia and chronic lymphocytic leukemia
- Analysis of the effect of DEK overexpression on the survival and proliferation of bone marrow stromal cells
- Serum fetuin-A levels and association with hematological parameters in chronic kidney disease and hemodialysis patients
- Investigation of relaxation times in 5-fluorouracil and human serum albumin mixtures
- Oxydative stress markers and cytokine levels in rosuvastatin-medicated hypercholesterolemia patients
- The protective effects of urapidil on lung tissue after intestinal ischemia-reperfusion injury
- Effects of SR-BI rs5888 and rs4238001 variations on hypertension
- Antioxidant and cytotoxic activity of three Turkish marine-derived fungi
- Is spectrophotometric enzymatic method a cost-effective alternative to indirect Ion Selective Electrode based method to measure electrolytes in small clinical laboratories?
- Plasma presepsin in determining gastric leaks following bariatric surgery
Articles in the same Issue
- Frontmatter
- Research Articles
- Investigating the impact of polysomy 17 in breast cancer patients with HER2 amplification through meta-analysis
- Diagnostic performance of microRNAs in the circulation in differential diagnosis of BPH, chronic prostatitis and prostate cancer
- Enhanced anticancer effect of cetuximab combined with stabilized silver ion solution in EGFR-positive lung cancer cells
- CA125, YKL-40, HE-4 and Mesothelin: a new serum biomarker combination in discrimination of benign and malign epithelial ovarian tumor
- Paricalcitol pretreatment attenuates renal ischemia/reperfusion injury by inhibiting p38 MAPK and activating PI3K/Akt signaling pathways
- Identification of cytoplasmic sialidase NEU2-associated proteins by LC-MS/MS
- Investigation of tyrosinase inhibition by some 1,2,4 triazole derivative compounds: in vitro and in silico mechanisms
- Investigation of alanine, propionylcarnitine (C3) and 3-hydroxyisovalerylcarnitine (C5-OH) levels in patients with partial biotinidase deficiency
- The expression levels of miR-655-3p, miR127-5p, miR-369-3p, miR-544a in gastric cancer
- Evaluation of the JAK2 V617F gene mutation in myeloproliferative neoplasms cases: a one-center study from Eastern Anatolia
- Effects of Rituximab on JAK-STAT and NF-κB signaling pathways in acute lymphoblastic leukemia and chronic lymphocytic leukemia
- Analysis of the effect of DEK overexpression on the survival and proliferation of bone marrow stromal cells
- Serum fetuin-A levels and association with hematological parameters in chronic kidney disease and hemodialysis patients
- Investigation of relaxation times in 5-fluorouracil and human serum albumin mixtures
- Oxydative stress markers and cytokine levels in rosuvastatin-medicated hypercholesterolemia patients
- The protective effects of urapidil on lung tissue after intestinal ischemia-reperfusion injury
- Effects of SR-BI rs5888 and rs4238001 variations on hypertension
- Antioxidant and cytotoxic activity of three Turkish marine-derived fungi
- Is spectrophotometric enzymatic method a cost-effective alternative to indirect Ion Selective Electrode based method to measure electrolytes in small clinical laboratories?
- Plasma presepsin in determining gastric leaks following bariatric surgery

