Abstract
The polyacrylic acid/tungsten oxide (PAA/WO3) composite films with good electrochromic properties have been prepared by a layer-by-layer method. The porous PAA layers were used as a template for the deposition of WO3, and finally, the composite film showed a network structure with high porosity. The electrochromic performance of the PAA/WO3 composite film was investigated by means of cyclic voltammogram (CV), chronoamperometry (CA), and transmittance measurements. The PAA/WO3 film also exhibited a remarkable electrochromism ability with the reversible color change from transparent colorless to dark blue and the transmittance change from 83% to 24% at 620 nm. This research provided a cost-effective approach for the preparation of PAA/WO3 composite films with controllable microstructure and good electrochromic property.
1 Introduction
Tungsten oxide (WO3) has been extensively investigated for a series of applications [1, 2], such as smart window [3], alphanumeric display [4], variable-reflectance mirrors [5], camouflage materials [6], energy storage [7, 8], etc. W ions of WO3 exhibit different oxidation states, and the intervalence electron transfer from W (V) to W (VI) states produces a broad absorption. Thus, WO3 particles display a color change from transparency to blue when they were affected by a number of small cations: H+, Li+, K+ inserting into the oxide matrix [9]. Owing to the intense and stable electrochromic property, the WO3 films have been produced by a number of different deposition techniques including thermal evaporation in vacuum [10], electrochemical oxidation of tungsten metal [11], chemical vapor depositions (CVD) [12], sol-gel methods [13], and RF sputtering [14].
However, the electrochromic performance of WO3 is still not good enough for practical applications because of bad adhesion, short cycle life, and degradation. Various methods have been employed to improve the electrochromic properties of oxide-based materials by controlling the composition or the microstructure of the films. For example, Pang et al. [15] prepared Ag/WO3 composite films by cathodic electrodeposition of WO3 onto the surface of Ag films, which have a substantial enhancement in the electroactive and electrochromic performance. Lin et al. [16] made TiO2/WO3 thin films with good reversible electrochromic behavior and improved electrochromic properties by a spin-coating method. Polymers, such as poly (3,4-ethylenedioxythiophene) and poly(styrenesulfonate) [17] were also used to prepare WO3/polymer hybrid films. The enhanced coloring efficiency and larger electrochemical activity of the hybrid films were concerned with the porous surface morphology of the composite films.
In order to control the microstructure of the films, several structure-guide reagents were used during the preparation of WO3 films. Badilescu successfully prepared porous nano-structured WO3 thin films with the aid of polystyrene microspheres [18]. Yuan et al. reported that mesoporous tungsten oxide films were prepared with a new nonionic gemini surfactant structure-directing agent [19]. The mesostructured WO3 films have large specific surface area and exhibit high electrochromic performance.
Though these attempts have been made to improve the electrochromic properties of WO3, some problems remain to be resolved. The techniques for preparation of composite films are comparatively complicated, and the materials are expensive, which is a real hindrance for large-scale production and application. Therefore, it is necessary to find a simple process to prepare porous composite films.
Polyacrylic acid (PAA) is a normal polyelectrolyte, which is easy to make porous film with good elasticity and flexibility [20]. In this paper, it is demonstrated how the PAA/WO3 composite film is formed with a homogeneous hybrid structure in which the PAA provides a porous matrix for the electrochromic tungsten oxide films. The tungsten oxide films are prepared as normal sol-gel method, and the PAA/WO3 composite films are made by a layer-by-layer method. The morphology and the spectra of the PAA/WO3 composite films are characterized, and the influence of PAA on the optical and the electrochromic behavior of the composite films are detailedly investigated. Our research provides a cost-effective approach for the preparation of PAA/WO3 composite films with controllable microstructure and good electrochromic property.
2 Materials and methods
The PAA (Aldrich, Mv≈450,000) was dissolved in absolute ethanol with concentrations of 2.0 wt % by stirring for 10 h at room temperature to obtain a homogeneous PAA solution. The WO3 solution was prepared by adding 4 g of tungsten powder in 30 ml of H2O2 (30%) solution, stirred continuously for 4 h, and filtered. The absolute ethanol and glacial acetic acid were added to get a WO3 precursor solution.
The PAA layers were coated on the clean tin-doped indium oxide (ITO) glass using the PAA solution by the dip-coating technique at dipping rates of 5~10 mm/s employing a motor-driven dip-coating equipment. Then, the film was held at 80°C for 30 min to allow the residual organics and moisture to volatilize. Then, the PAA layers were dip coated again with WO3 precursor solution with the same withdrawal speed and dried at 100°C for 10 min. After the above layer by layer, films were rinsed with absolute ethanol and dried; the PAA/WO3 composite films were obtained.
The morphology and the microstructure of the samples were characterized by a field emission scanning electron microscope (FESEM, HITACHI, S-4800), a transmission electron microscope (TEM, JEM-2010), cyclic voltammograms (CV, and chronoamperometry (CA) was carried out in a three-electrode system containing 0.1 m LiClO4-PC as the electrolyte, the ITO glass covered with films as working electrode, Pt sheets as counter electrode, and an Ag/AgCl electrode as reference electrode on the CHI660B Electrochemical Workstation (Chenhua, Shanghai). The electrochemical reaction of the films was carried out in the potential range of −1.5~1.5 V with the scanning rate of 50 mV/s at room temperature. The changes in UV/Vis absorption spectra for the coloring and bleaching states were measured ex situ with a UV/Vis spectrophotometer (UV 2102pc, UNICO).
3 Results and discussion
3.1 Characterization and morphology of the PAA/WO3 films
The Raman spectra of the PAA/WO3 and WO3 films are shown in Figure 1. From the spectra of the WO3 film, a small area of broad peak emerged at 680~730 cm−1, which should be attributed to stretching O-W-O modes of the bridging oxygens. The band at around 934 cm−1 was assigned to the stretching mode of the terminal W=O bond, while in the spectra of the PAA/WO3 composite film, the strong band at around 967 cm−1 again can be assigned to the terminal W=O stretching mode, possibly on the surface of the cluster and in microvoid structures in the film [21]. The band at 815 cm−1 was attributed to the O-W-O stretching vibration, which had a blue shift and became weaker with the existence of PAA. The small peaks at around 1100 cm−1 and 1350 cm−1 in the attached spectra were corresponding to the characteristic band of the PAA [22].
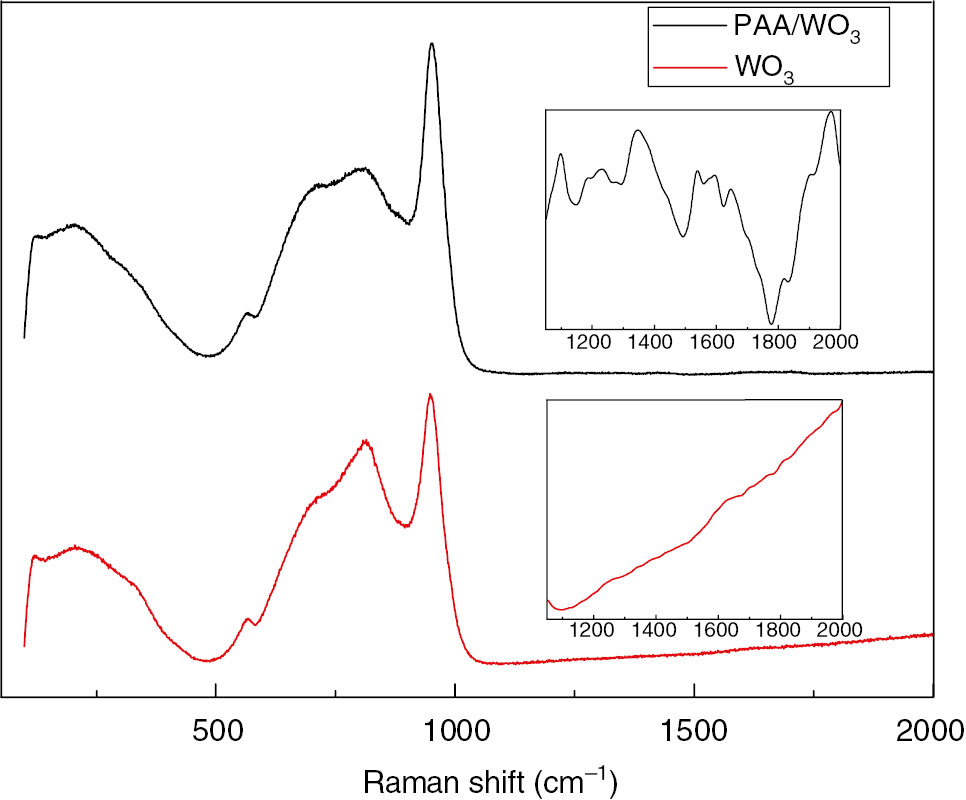
Raman spectra of the WO3 and PAA/WO3 films.
The surface morphological features of the PAA/WO3 film and the WO3 film are presented in Figure 2. The image of the WO3 film revealed a uniform amorphous surface mature, and the nanometer amorphous WO3 (Figure s) grains looked like they were well dispersed on the film. While in the image of PAA/WO3 composite film, the whole film presented a uniform, porous network structure with the PAA layer bringing the porous template. Such specific network structure had much bigger electrochemical activity and possessed electrochemical active point of ion absorption [23].

FE-SEM images of WO3 and PAA/WO3 films.
The crystal structure and the morphology of the PAA/WO3 hybrid film were studied by TEM. Figure 3 presented the TEM images of the PAA/WO3 films, which showed that tungsten oxide particles were embedded in PAA media with an average diameter of 5~10 nm. From the images, the catenulate grain clusters and the framework of PAA sol could be observed clearly. Small clusters collided and infiltrated each other by Brown movement, growing up to a bigger anomalous network. As the PAA showed a porous and amorphous matrix for WO3 crystallite, a homogeneous composite structure of PAA/WO3 could be obtained.
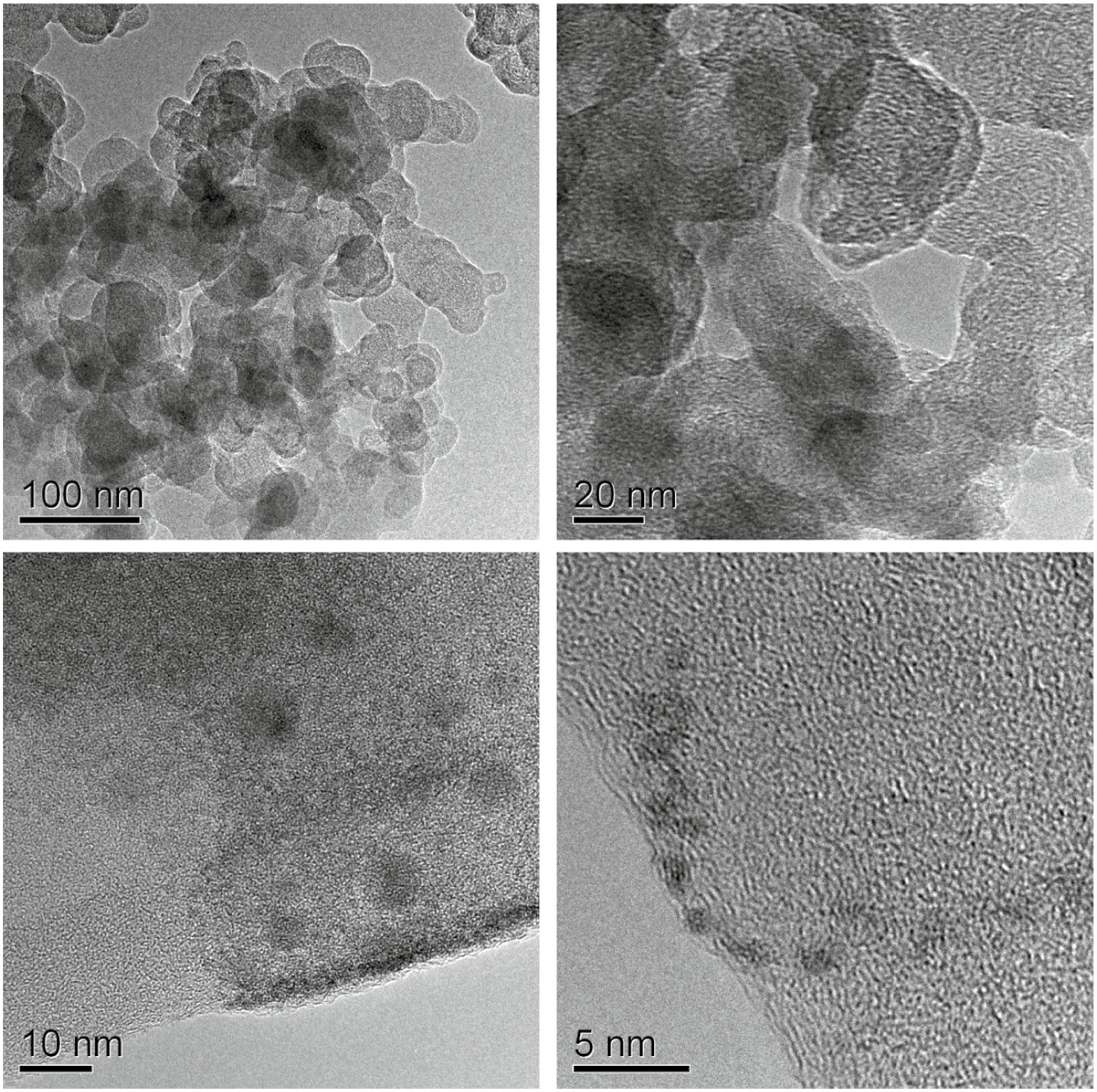
TEM images of PAA/WO3 films with different magnifications.
On the basis of the characterization and morphology results, the synthesis process of the PAA/WO3 films was illustrated by a schematic model in Figure 4. The PAA layer behaved as a porous matrix for WO3 crystallites, and the uniform network structure of the PAA/WO3 could be achieved. PAA has good electrochemical stability [24], and neither affects the electrical contacts between the WO3 component and the ITO stuff. Thus, the porous and amorphous layered structure of the PAA/WO3 films was favorable for the injection (extraction) of Li+ into (from) the films. Finally, the marvelous electrochromic property of the PAA/WO3 composite films had been obtained.

Synthesis scheme of the PAA/WO3 composite films.
3.2 Electrochromic and electrochemical properties
The optical transmittance spectra of the colored and bleached WO3 and PAA/WO3 composite films are shown in Figure 5. It was obvious that the maximum transmittance for the bleached PAA/WO3 films was about 83%, with a variation of 73~83% in the visible region. For the pure WO3 films, the bleached film also had a good transmittance property in the whole visible region, which was up to 80%. While in the colored state, the PAA/WO3 composite films showed a higher absorption property than the WO3 films, owning to the preferred colored effect on the films. The transmission modulation of the PAA/WO3 and WO3 films was 60% and 34%, respectively. Apparently the addition of the PAA improved the optical transmittance property of the WO3 films. The intense redox reaction could occur in the WO3/PAA composite films, which could bring on the increase in transmittance change.
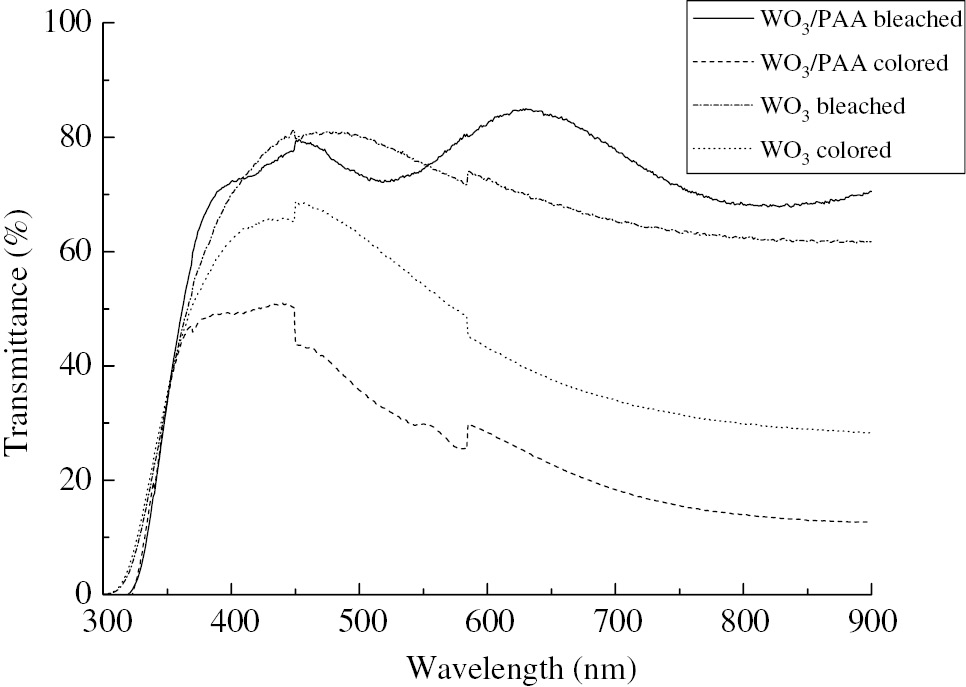
Optical transmittance spectra of WO3 and PAA/WO3 composite films.
Typical cyclic voltammograms were recorded at a scan rate of 50 mV/s for the PAA/WO3 and WO3 films, and the investigation is shown in Figure 6. During the cyclic voltammetry at the potential range of −1.5~1.5V, the films exhibited the well-known redox behavior of WVI→V, which accompanied a reversible color change between the transparent and the dark blue. In the PAA/WO3 curve, the open-circuit potentials of the pure WO3 films were 0.212 V and 0.253 V. The lower opening current revealed that the composite films had better electrical properties and brought electrochemical reactions easily. The reversibility during the electrochemical reactions could be estimated by the ratio of the charge densities (Qa/Qc): 66% and 78% for the WO3 and PAA/WO3 films, respectively. The negative movement of the oxidation peak indicated that the color bleaching of the PAA/WO3 composite films was faster than that of pure WO3 films, which was also confirmed in the response time. In this report, the coloration time and the bleaching time were defined as the time required for the cathodic/anodic current of the films to attain a steady state in the coloration/bleaching cycle. It had been found that bleaching kinetics was faster than coloration kinetics for both films, which was 5.2 s, 20 s, and 7.3 s, 26 s for PAA/WO3 films and WO3 films, respectively. It was evident that the microstructure of the PAA/WO3 films was more favorable for a rapid ion intercalation and deintercalation process as the films exhibited faster coloring-bleaching kinetics than pure WO3 films.
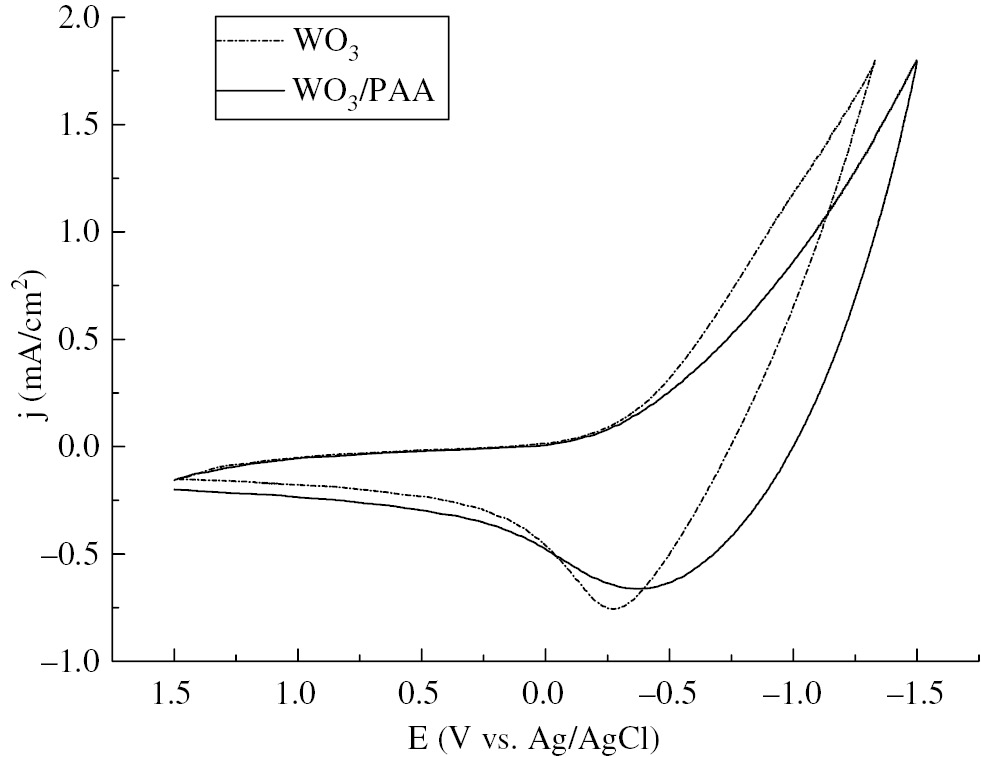
CV curves of PAA/WO3 films in a PC solution containing 0.1 m LiClO4.
4 Conclusion
The PAA/WO3 composite films were successfully prepared by the layer-by-layer method. The PAA layer acted as the template for the deposition of the WO3 films, and the composite films showed a porous network structure. The as-prepared PAA/WO3 composite films exhibited marvelous electrochromic properties. It was shown that the reversible color changed from transparent colorless to dark blue. The PAA/WO3 composite film presented a good color contrast with a transmittance variation up to 60% at 650 nm. Also, the composite films had good electrochemical reversibility with the ratio of charge densities of 77%. The results indicated that the attempt to get a cheaper and good for large-scale production of the PAA/WO3 composite films by the normal sol-gel method at room temperature was feasible. Furthermore, such kind of mechanism of preparation of the PAA/WO3 composite film could be a reference for other nanocrystalline electrochromic materials.
Acknowledgment
This study originated from the Industry Program of Science and Technology Support Project of Jiangsu Province (BE2014128), the Clinical Medical Special Program of Science and Technology Project of Jiangsu Province (BL2014074), the Prospective Joint Research Program of Jiangsu Province (BY2015005-01), the Major Program of Natural Science Fund in Colleges and Universities of Jiangsu Province (15KJA430005), China Postdoctoral Science Foundation (2015M570442), the Aeronautical Science Foundation of China (201452T4001), the Program for Innovative Research Team in the University of Ministry of Education of China (no. IRT_15R35), and the China Postdoctoral Science Foundation (2015M570442), Changjiang Scholars and innovative team development plan (IRT1146).
References
[1] Zheng H, Ou JZ, Strano MS, Kaner RB, Mitchell A, Kalantar-zadeh K. Adv. Funct. Mater. 2011, 21, 2175–2196.10.1002/adfm.201002477Search in Google Scholar
[2] Sydam R, Deepa M, Shivaprasad SM, Srivastava AK. Sol. Energy Mater. Sol. Cells 2015, 132, 148–161.10.1016/j.solmat.2014.08.034Search in Google Scholar
[3] Gaikwad DK, Mali SS, Hong CK, Kadam AV. J. Alloys Compd. 2016, 669, 240–245.10.1016/j.jallcom.2016.01.226Search in Google Scholar
[4] Feng LG, Yan L, Cui Z, Liu C, Xing W. J. Power Sources 2011, 196, 2469–2474.10.1016/j.jpowsour.2010.11.073Search in Google Scholar
[5] Richardson TJ. Solid State Ion. 2003, 165, 305–308.10.1016/j.ssi.2003.08.047Search in Google Scholar
[6] Niklasson GA, Granqvist CG. J. Mater. Chem. 2007, 17, 127–156.10.1039/B612174HSearch in Google Scholar
[7] Cai CA, Guan DS, Wang Y. J. Alloys Compd. 2011, 509, 909–915.10.1016/j.jallcom.2010.09.129Search in Google Scholar
[8] Zhou MJ, Zeng ZO, Zhong L. Corros. Sci. 2009, 51, 1386–1391.10.1016/j.corsci.2009.03.024Search in Google Scholar
[9] Patel KJ, Panchal CJ, Desai MS, Mehta PK. Mater. Chem. Phys. 2010, 124, 884–890.10.1016/j.matchemphys.2010.08.021Search in Google Scholar
[10] Patel KJ, Panchal CJ, Kheraj VA, Desai MS. Mater. Chem. Phys. 2009, 114, 475–478.10.1016/j.matchemphys.2008.09.071Search in Google Scholar
[11] da Costa NBD, Pazinato JCO, Sombrio G, Pereira MB, Boudinov H, Gündel A, Moreira EC, Garcia ITS. Thin Solid Films 2015, 578, 124–132.10.1016/j.tsf.2015.02.031Search in Google Scholar
[12] Houweling ZS, Geus JW, Schropp REI, Chem. Vap. Deposition 2010, 16, 179–184.10.1002/cvde.200906838Search in Google Scholar
[13] Wang KF, Zeng P, Zhai J, Liu Q. Electrochem. Commun. 2013, 26, 5–9.10.1016/j.elecom.2012.09.037Search in Google Scholar
[14] Yamada Y, Tabata K, Yashima T. Sol. Energy Mater. Sol. Cells 2007, 91, 29–37.10.1016/j.solmat.2005.11.014Search in Google Scholar
[15] Pang YH, Chen Q, Shen X, Tang L, Qian H. Thin Solid Films 2010, 518, 1920–1924.10.1016/j.tsf.2009.07.138Search in Google Scholar
[16] Hsu CS, Lin CK, Chan CC, Chang CC, Tsay CY. Thin Solid Films 2006, 494, 228–233.10.1016/j.tsf.2005.08.124Search in Google Scholar
[17] Deepa M, Srivastava AK, Sood KN, Murugan AV. J. Electrochem. Soc. 2008, 155, D703–D710.10.1149/1.2975388Search in Google Scholar
[18] Badilescu S, Ashrit PV. Solid State Ionics 2003, 158, 187–197.10.1016/S0167-2738(02)00764-6Search in Google Scholar
[19] Yuan JG, et al. Acta Chimica Sinica 2005, 63, 1884–1888.Search in Google Scholar
[20] Hashimoto S, Matsuoka H. J. Electrochem. Soc. 1991, 138, 2403–2408.10.1149/1.2085985Search in Google Scholar
[21] Lee SH, Cheong HM, Zhang J-G, Mascarenhas A, Benson DK, Deb SK. Appl. Phys. Lett. 1999, 74, 242–244.10.1063/1.123268Search in Google Scholar
[22] Dong J, Ozaki Y, Nakashima K. Macromolecules 1997, 30, 1111–1117.10.1021/ma960693xSearch in Google Scholar
[23] Choy JH, Kim YI, Kim BW, Park NG, Campet G, Grenier JC. Chem. Mater. 2000, 12, 2950–2956.10.1021/cm990718hSearch in Google Scholar
[24] Magasinski A, Zdyrko B, Kovalenko I, Hertzberg B, Burtovyy R, Huebner CF, Fuller TF, Luzinov I, Yushin G. ACS Appl. Mater. Interfaces 2010, 2, 3004–3010.10.1021/am100871ySearch in Google Scholar PubMed
Supplemental Material:
The online version of this article (DOI: https://doi.org/10.1515/secm-2016-0052) offers supplementary material, available to authorized users.
©2018 Walter de Gruyter GmbH, Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Original articles
- Review of the mechanical performance of variable stiffness design fiber-reinforced composites
- Exact solution for bending analysis of functionally graded micro-plates based on strain gradient theory
- Synthesis, microstructure, and mechanical properties of in situ TiB2/Al-4.5Cu composites
- Microstructure and properties of W-Cu/1Cr18Ni9 steel brazed joint with different Ni-based filler metals
- Drilling studies on the prepared aluminum metal matrix composite from wet grinder stone dust particles
- Studies on mechanical properties of thermoplastic composites prepared from flax-polypropylene needle punched nonwovens
- Design of and with thin-ply non-crimp fabric as building blocks for composites
- Effect of coir fiber reinforcement on mechanical properties of vulcanized natural rubber composites
- Investigation and analysis of glass fabric/PVC composite laminates processing parameters
- Abrasive wear behavior of silane treated nanoalumina filled dental composite under food slurry and distilled water condition
- Finite element study into the effects of fiber orientations and stacking sequence on drilling induced delamination in CFRP/Al stack
- Preparation of PAA/WO3 composite films with enhanced electrochromism via layer-by-layer method
- Effect of alkali treatment on hair fiber as reinforcement of HDPE composites: mechanical properties and water absorption behavior
- Integration of nano-Al with one-step synthesis of MoO3 nanobelts to realize high exothermic nanothermite
- A time-of-flight revising approach to improve the image quality of Lamb wave tomography for the detection of defects in composite panels
- The simulation of the warpage rule of the thin-walled part of polypropylene composite based on the coupling effect of mold deformation and injection molding process
- Novel preparation method and the characterization of polyurethane-acrylate/ nano-SiO2 emulsions
- Microwave properties of natural rubber based composites containing carbon black-magnetite hybrid fillers
- Simulation on impact response of FMLs: effect of fiber stacking sequence, thickness, and incident angle
Articles in the same Issue
- Frontmatter
- Original articles
- Review of the mechanical performance of variable stiffness design fiber-reinforced composites
- Exact solution for bending analysis of functionally graded micro-plates based on strain gradient theory
- Synthesis, microstructure, and mechanical properties of in situ TiB2/Al-4.5Cu composites
- Microstructure and properties of W-Cu/1Cr18Ni9 steel brazed joint with different Ni-based filler metals
- Drilling studies on the prepared aluminum metal matrix composite from wet grinder stone dust particles
- Studies on mechanical properties of thermoplastic composites prepared from flax-polypropylene needle punched nonwovens
- Design of and with thin-ply non-crimp fabric as building blocks for composites
- Effect of coir fiber reinforcement on mechanical properties of vulcanized natural rubber composites
- Investigation and analysis of glass fabric/PVC composite laminates processing parameters
- Abrasive wear behavior of silane treated nanoalumina filled dental composite under food slurry and distilled water condition
- Finite element study into the effects of fiber orientations and stacking sequence on drilling induced delamination in CFRP/Al stack
- Preparation of PAA/WO3 composite films with enhanced electrochromism via layer-by-layer method
- Effect of alkali treatment on hair fiber as reinforcement of HDPE composites: mechanical properties and water absorption behavior
- Integration of nano-Al with one-step synthesis of MoO3 nanobelts to realize high exothermic nanothermite
- A time-of-flight revising approach to improve the image quality of Lamb wave tomography for the detection of defects in composite panels
- The simulation of the warpage rule of the thin-walled part of polypropylene composite based on the coupling effect of mold deformation and injection molding process
- Novel preparation method and the characterization of polyurethane-acrylate/ nano-SiO2 emulsions
- Microwave properties of natural rubber based composites containing carbon black-magnetite hybrid fillers
- Simulation on impact response of FMLs: effect of fiber stacking sequence, thickness, and incident angle

