Abstract
Polyurethane-acrylate/SiO2 composite (SPUA) emulsions were synthesized by in situ polymerization accompanying the sol-gel process, in which the hydrolysis reaction and polymerization of monomers could be accomplished at the same time. The particle morphology of the SPUA emulsions was observed by scanning electron microscopy and transmission electron microscopy. The particle diameter was measured on a laser particle size analyzer. The molecular structure of the copolymers was characterized by Fourier transform infrared spectrometer (FTIR). Furthermore, tensile testing machine, Shore durometer, and thermogravimetric analyzer were used to investigate the mechanical properties, hardness, and thermal properties. The results indicated that, by increasing the mass fraction of tetraethyl orthosilicate (TEOS), the mechanical properties of the films were improved remarkably. When the weight % of TEOS was 8% in the SPUA emulsion, the water absorption of obtained film was 2.1%, the tensile strength was 16.8 MPa, the shore hardness was 94, and the temperature for the maximal thermal mass-loss rate was 416°C.
1 Introduction
Waterborne polyurethane-acrylate (PUA) composite materials have been thought as “the third generation waterborne polyurethane” because the PUA films are wear resistant and corrosion resistant, in addition to the brightness, good water resistance, and good mechanical properties [1], [2], [3]. Nowadays, PUA composite materials are becoming the development trends in coatings. For example, they are very suitable to be applied as hood light lacquer in automobile industry [2], [3]. Moreover, many other products with different properties can be synthesized by alternating the structure and composition of raw materials and by changing the NCO/OH ratio of PUA composite materials. However, there is a big problem that the bending resistance of PUA coating is poor and the film surface is vulnerable to stain. In addition, the improvement of properties of PUA composite materials such as hardness and weather ability is limited.
To solve these problems, researchers are now trying new strategies including modification of PUA by nanosized materials [4], [5], [6]. In recent years, inorganic nanoparticles have been widely adopted in the fabrication of polymeric composites [7], [8], [9], [10]. Among them, nanosized silicon dioxide might be the most commonly used to improve the heat resistance, mechanical properties, and electrical properties of polymer materials [11], [12]. However, because of the tiny size and high surface energy, the SiO2 nanoparticles are of strong tendency to aggregate. The SiO2-incorporated PUA nanocomposites cannot have all their property improved. It is even worse that the stability of PUA emulsions might be destroyed after the incorporation of SiO2 nanoparticles. Therefore, the key to those problems is to uniformly disperse the nano-SiO2 into the PUA emulsions.
The incorporation of surface-modified SiO2 nanoparticles into waterborne PU or PUA is thought to be a good choice [13], [14]. Zhang et al. synthesized nano-SiO2 with surface double bonds from KH-570 via sol-gel method and incorporated them into waterborne polyurethane (WPU). The UV-curable WPU nanocomposites based on PEGMA surface-modified silica sol was also reported. Mechanical properties and thermal properties of these materials were remarkably improved by the nano-SiO2. Nevertheless, the dispersion of SiO2 nanoparticles in the PU composites was still not satisfying.
In this work, polyurethane-acrylate/SiO2 composite (SPUA) emulsions were prepared by in situ polymerization accompanying the sol-gel process. Tetraethyl orthosilicates (TEOS) and double bonds containing coupling agent γ-methacryloxypropyltrimethoxysilane (KH-570) were used simultaneously with the diols and isocyanates. In this way, the hydrolysis of the silane precursors and polymerization of the monomers could be controlled at the same time to uniformly disperse the in situ-obtained SiO2 nanoparticles in the emulsions. The results showed that the water resistance, tensile strength, hardness, and heat resistance of the obtained PUA/SiO2 composite films were all improved dramatically. It is believed that this composite film is able to be widely applied as adhesives, sealants, plastic coatings, wood finishes, and coatings of flexible substrates such as fabric, leather, and paper.
2 Materials and methods
2.1 Materials
Isophorone diisocyanate (IPDI, Bayer, Germany), polyether (N-210, Mn=1000, Haian Petroleum Chemical Plant, Jiangsu Province, China), dihydroxymethyl propionic acid (DMPA, Baihang Chemical Ltd. Co., Dongyang, Zhejiang Province, China), and acetone were industrial products. Diethylene glycol (DEG), stannous octoate (T-9), and dibutyltin dilaurate (T-12) were purchased from the third chemical factory of Dagang (Tianjin). Triethylamine (TEA) was supplied by QiangSheng Chemical Co. Ltd. of Jiangsu. Methyl methacrylate (MMA), butyl acrylate (BA), and potassium persulfate (KPS) were purchased from BoDi chemical Co. Ltd. of Tianjin. Hydroxyethyl acrylate (HEA, 97%) and TEOS were obtained from Aladdin Chemical Co. Ltd. γ-Methacryloxypropyltrimethoxysilane (KH-570, ≥98%) was supplied by the Sinopharm Chemical Reagents Co., Ltd.
2.2 Preparation of SPUA emulsions
2.2.1 Preparation of anionic WPUs
A 250-ml four-neck flask equipped with a thermometer, a condenser tube, and a mechanical stirrer was charged with N-210 (45.0 g, 0.045 mol) and IPDI (40.0 g, 0.18 mol). The reactants were let to react at 90°C for 2 h, and then the DMPA, DEG, T-9, and T-12 were added into the flask to extend the chains for another 4 h at 75°C. During that process, the proper amount of acetone was added to lower the viscosity. Then HEA (the molar ratio of HEA to the theoretically residual NCO was 1.2:1) was added in to block the chain ends for another 3 h at 70°C. The temperature was then reduced to 40°C. Later, TEA (the molar ratio of DMPA and TEA was 1:1) was added in to neutralize the carboxylic groups of DMPA, and then deionized water was poured into the flask to obtain emulsion under violent stirring. The anionic WPU ended by double bonds was obtained after the solvent was evaporated. The solid content was 29% by weighting method. The synthetic route of the WPU is shown in Figure 1.

Synthetic route of the WPU.
2.2.2 Preparation of SPUA emulsions
A 250-ml four-neck flask equipped with a thermometer, a condenser tube, a dropping funnel, and a mechanical stirrer was charged with 67.7 g WPU and half of the total mass of BA. The mixture was emulsified at high shearing rate for 30 min. TEOS and KH-570 were added to hydrolyze for 15 min at 40°C. KPS (0.06 g, dissolved in 5 g water) was added to induce polymerization for another 30 min at 80°C. The residual KPS (0.12 g, dissolved in 30 g water) and the residual BA and MMA were added into the flask drop by drop for another 3 h synchronically. The temperature was then increased to 85°C and kept at that temperature for 1 h. Lastly, the SPUA emulsion was obtained. The schematic diagram of the synthesis of SPUA composite emulsion is shown in Figure 2.

Schematic diagram of the synthesis of SPUA composite emulsion.
2.3 Measurements
Fourier transform infrared spectrometer (FTIR) analysis was performed on a NEXUS-870 spectrometer (Nicolet Instruments, USA). Particle diameter was measured on a laser particle size analyzer (Bluewave S3500; Microtrac, USA) in the range 0.01–2800 μm. After the emulsion was centrifuged at 6000 rpm for 10 min, the mechanical stability was judged by the appearance of precipitation or stratification. The morphology of nanoparticles inside the composite films was observed by transmission electron microscopy (TEM) (JEM-100 SX, JEOL, Japan) with an acceleration voltage of 200 kV. The surface morphology of the nanocomposites was characterized by a scanning electron microscopy (SEM) (S-4800, Hitachi, Japan) with an acceleration voltage of 5.0 kV. Tensile strength and elongation at break for all of the composite films were conducted on a tensile tester (XLW-500; Wuxi Jiangyi Experimental Equipment Co. Ltd., China) at room temperature with a running speed of 200 mm/min. The dumbbell-type specimen was 25 mm long and 4 mm wide at the neck. The hardness of the composite films was measured by a shore durometer (KYLX-A; Laizhou Huayin Test Machine Co. Ltd., China). Measurement was performed three times for each sample, and the average value was calculated. The thermogravimetric analysis (TGA) of the composite films was performed on a TG 209, (NETZSCH, Germany). The heating range was from 25°C to 600°C at a heating rate of 20°C/min under a nitrogen atmosphere. The dynamic mechanical analysis (DMA) of the composites was performed using DMA Q800 Analyzer (TA Instruments, USA). Measurements were taken in a temperature range of −50°C to +150°C at an operating frequency of 1 Hz and heating rate of 5°C/min. The samples were 3 mm thick, 10 mm wide, and 20 mm long. Storage modulus and glass transition temperature vs. temperature were determined. X-ray diffraction (XRD) patterns were recorded on XD-3 (Persee General Instruments Co. Ltd., China). The X-ray generator was run at 36 kV and 25 mA. All of the XRD measurements were performed at 2θ values between 5° and 30°. Water absorption was examined by the preparation of dried films (original weight designated as w1) immersed in water for 24 h at room temperature. The residual water was wiped quickly from the films using filter paper, and the weight (w2) was measured immediately. The water absorption (w) was calculated as follows:
3 Results and discussion
3.1 Feed composition and characterization of SPUA emulsions
The feed composition and the particle size of SPUA emulsions are shown in Table 1. It could be seen that the appearance of most SPUA emulsions was bluish white. The emulsion was milky white only when the TEOS content was the highest (8%). That is, the particle size of most of the SPUA emulsions was small. The particle size of emulsions is one of the most important factors influencing the emulsion stability, wet ability, and some other properties. Therefore, the appropriate emulsion particle size is very important to the final performance of the composite films. As shown in Figure 3, although the particle size of SPUA emulsions grew bigger and the distribution of SPUA emulsions became wider too by increasing the weight % of TEOS, the particle size of SPUA emulsions was within 20–180 nm. It suggests that the emulsions might be stable. Those changes in particle size and size distribution were attributed to the cross-linking between the polymer chains and the nano-SiO2 produced by the hydrolysis of TEOS. SPUA00, without TEOS and coupling agent, had the minimal average particle size and the narrowest distribution.
Feed composition and characterization of SPUA emulsions with different TEOS weight %.
| Samplesa | KH-570 (g) | TEOS (wt%b) | Particle size (nm) | Appearance | Mechanical stability |
|---|---|---|---|---|---|
| SPUA00 | 0 | 0 | 50 | Bluish white | Passed |
| SPUA0 | 1.6 | 0 | 67 | Bluish white | Passed |
| SPUA2 | 1.6 | 2 | 62 | Bluish white | Passed |
| SPUA4 | 1.6 | 4 | 60 | Bluish white | Passed |
| SPUA6 | 1.6 | 6 | 65 | Bluish white | Passed |
| SPUA8 | 1.6 | 8 | 79 | Milky white | Passed |
aIn SPUAs, m(PU):m(PA)=1:1; in PA, m(BA):m(MMA)=1:1.
bThe weight % of TEOS is relative to the total mass of PU and PA.

Particle size and size distribution of SPUA emulsions.
3.2 Dispersion of nano-SiO2 in the composite materials
SEM and TEM were used to visually evaluate the dispersion of silicon dioxide nanoparticles in PUA composite films. As an example, the micrograph of fracture surface of SPUA8 (A) and SPUA0 (B) films is shown in Figure 4. It could be seen that numerous white spots dispersed uniformly in the sample SPUA8. Those white spots were apparently the SiO2 nanoparticles. Compared with SPUA8, there was no white spot in the sample SPUA0 because no TEOS was added into SPUA0 emulsion. As indicated in Figure 5, there were black shades dispersed uniformly. Their size was approximately 30 nm. They were supposed to be the SiO2 nanoparticles prepared in situ. During the polymerization of acrylates, the TEOS hydrolyze spontaneously in the PUA emulsions. The double bonds from KH-570 linked the PUA and the SiO2 nanoparticles together, which was the key to the uniform distribution of SiO2 nanoparticles. From SEM and TEM observation, it could be concluded that PUA/SiO2 composite emulsions were successfully prepared by in situ polymerization accompanying the sol-gel process.
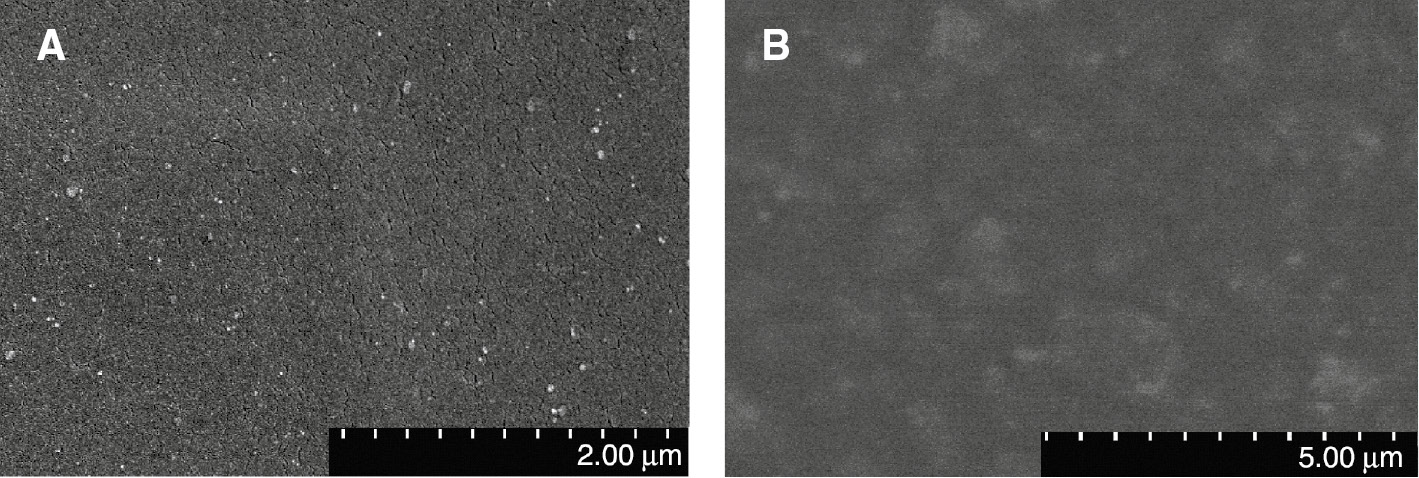
SEM micrographs of fracture surface of SPUA8 (A) and SPUA0 (B) films.

TEM micrograph of SPUA8 emulsion particles.
3.3 FT-IR analysis for the samples
Figure 6A displays the characteristic hydrocarbon stretching peaks of CH3 and CH2 at 2947 and 2845 cm−1. The stretching vibration of C=C, Si-O, and C-Si bonds could be detected at 1638, 1100, and 800 cm−1, respectively. Figure 6B shows that the peak of the –NCO band at 2270 cm−1 disappeared and the peak of the N–H bond at 3340 cm−1 appeared. These results indicated that the NCO had reacted with OH completely. The peaks at 3068, 1715, and 1168 cm−1 are characteristic stretching peaks of =C-H, C=O, and C-O. The characteristic deformation vibration peak of N-H occurs at 1538 cm−1. All these peaks showed that anionic polyurethane blocked by double bond was synthesized successfully. Compared with Figure 6B, the bending vibration peak of CH2 at 1450 cm−1 on Figure 6C was enhanced apparently. It was even stronger than the absorption peak of CH3 at 1380 cm−1, indicating that the CH2-abundant BA was copolymerized [15]. In Figure 6C, the stretching vibration peak of C=O at 1735 cm−1 moved to higher position. There were two possible reasons: (i) the hydrogen bonding between the hard segments of PU was weakened because of the dilute effect of copolymerized acrylate monomers and (ii) the vibration peak of C=O on acrylates became dominant as the acrylates were incorporated, as shown by Zhang et al. [16]. In Figure 6D, the peak at 1100 cm−1 was the stretching vibration peak of Si-O, which showed that KH-570 participated in the copolymerization reaction.

FTIR spectra of KH-570 (A), PU (B), SPUA00 (C), and SPUA0 (D).
Figure 7 shows the FTIR spectra of SPUA0, SPUA2, SPUA4, and SPUA6. Clearly, there was a strong absorption band from 1050 to 1120 cm−1 for the samples, showing the existence of a Si-O backbone. The hydrolysis of the TEOS formed the Si-O-Si network and generated an interpenetrating polymer network between the organic and the inorganic phases. Furthermore, the absorption peaks of Si-O became stronger and stronger by increasing the weight % of TEOS.

FTIR spectra of SPUA0 (A), SPUA2 (B), SPUA4 (C), and SPUA6 (D).
3.4 Mechanical properties and the water absorption of SPUA films
Figure 8 shows the stress-strain curves of SPUA films with different TEOS mass fractions. Tensile strength, elongation at break, shore hardness, and water absorption are listed in Table 2. The tensile strength and the shore hardness of SPUA films increased gradually by increasing the weight % of TEOS, whereas the elongation at break became smaller. The increase of tensile strength and hardness could be explained by the fact that the in situ-formed SiO2 nanoparticles enhanced the mechanical strengths of PUA resin. Moreover, the in situ-formed SiO2 nanoparticles might function as cross-linker, leading to an interpenetrating network inside the PUA-SiO2 composite films. The cross-linking structure could effectively dissipate the stress and, thus, improve the mechanical properties of composite materials.

Stress-strain curve of SPUA films with different TEOS weight %.
Mechanical properties and water absorption of SPUA films with different TEOS weight %.
| Samples | SPUA00 | SPUA0 | SPUA2 | SPUA4 | SPUA6 | SPUA8 |
|---|---|---|---|---|---|---|
| Tensile strength (MPa) | 10.8 | 11.6 | 13.9 | 14.6 | 15.9 | 16.8 |
| Elongation at break (%) | 248.2 | 187.3 | 173.2 | 135.6 | 128.7 | 113.5 |
| Shore hardness | 80 | 82 | 84 | 85 | 91 | 94 |
| Water absorption (%) | 12.1 | 15.4 | 8.2 | 5.9 | 3.2 | 2.1 |
DMA was also conducted for SPUA0 and SPUA8, as shown in Figure 9. It could be seen in Figure 9 that the storage modulus of SPUA8 was much higher than that of the SPUA0. Furthermore, the glass transition temperature of SPUA8 was higher than that of the SPUA0 too. The results from DMA test indicated that the in situ-formed SiO2 nanoparticles effectively restricted the molecular motion of PUA resin, especially in the glassy state. It could be attributed to the hard and rigid nature of the SiO2 nanoparticles. On the other hand, these results also hinted that there were strong interactions among the SiO2 nanoparticles and the PUA chains. According to the literature [13], the SiO2 nanoparticles synthesized with KH-570 were of double bonds on their surfaces, which might participate in the chemical reaction during the polymerization of acrylate monomers. It could be reasonably speculated that the PUA chains might be cross-linked by the double bonds containing SiO2 nanoparticles.
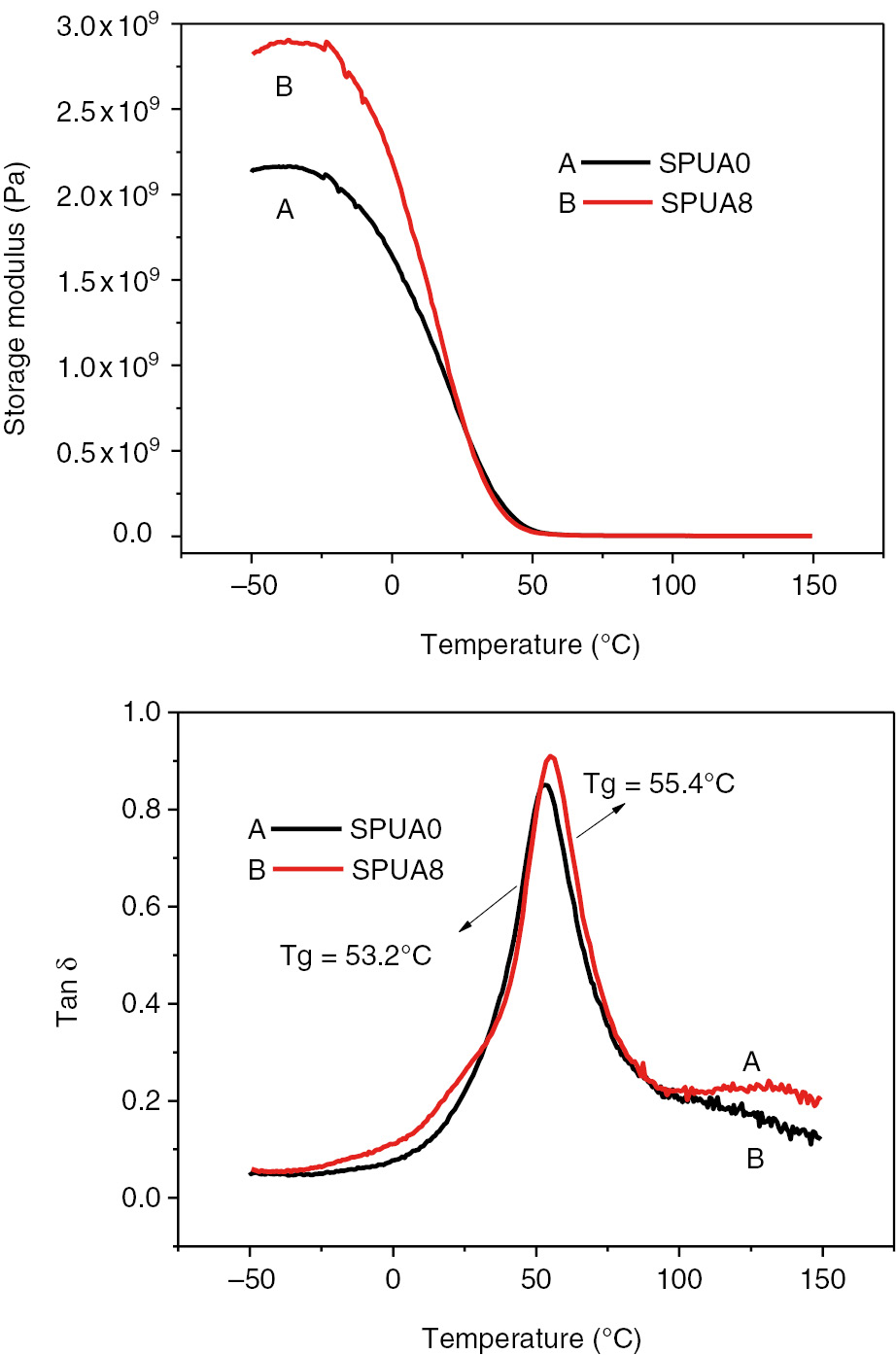
Storage modulus and tan δ curves for SPUA0 and SPUA8 composite films.
Table 2 also shows that the water absorption of SPUA films decreased gradually by increasing the weight % of TEOS. That is, the resistance to water of SPUA films was enhanced. The improvement of water resistance might also the result of cross-linking. With more TEOS applied, the structure of composite films was more compact.
3.5 Thermal properties of SPUA films
The TGA curves of SPUA0, SPUA4, and SPUA8 are shown in Figure 10. It was observed that these curves were similar, and all the samples showed three-step degradation profiles. The first weight loss occurred in the temperature range of 50°C to 250°C, the second weight loss was approximately from 250°C to 380°C, and the third weight loss was approximately from 380°C to 470°C. The first step of degradation could be attributed to the residual water and decomposition of oligomer, and it was almost negligible in Figure 10. The second weight loss could be assigned to the degradation of main chains of WPU, and the third loss was supposed to be the degradation of polyacrylate.

TGA curves of SPUA0 (A), SPUA4 (B), and SPUA8 (C).
In Figure 10, it was shown that the temperatures for the maximal thermal mass-loss rate of the second stage decomposition of SPUA0, SPUA4, and SPUA8 were 332°C, 361°C, and 370°C, respectively. The temperatures for the maximal thermal mass-loss rate of the third phase decomposition of SPUA0, SPUA4, and SPUA8 were 409°C, 411°C, and 416°C, respectively. These results indicated that the incorporation of SiO2 nanoparticles had slightly enhanced the thermal stability of SPUA, which was attributed to the uniform distribution of SiO2 nanoparticles and the cross-linking effect. Moreover, the residual mass fractions of SPUA0, SPUA4, and SPUA8 were 0.26%, 2.08%, and 4.06%, respectively, which confirmed the improvement of SiO2 nanoparticles on the thermal stability of the PUA resin.
3.6 XRD for samples
XRD analyses of the SPUA0, SPUA4, and SPUA8 hybrid films are shown in Figure 11. For the SPUA0 film, a broad diffraction peak was observed near 2θ=20°. It was attributed to the amorphous phase of SPUA0. The sample SPUA4 and SPUA8 also had diffraction peaks appearing near 20°. They slightly shifted to larger diffraction angles. It suggested that a higher degree of short-range-order arrangement occurred in the SiO2-incorporated PUA resin. This phenomenon further confirmed that there was a strong interaction between the polymer chain and the SiO2 nanoparticles [17].

XRD patterns of SPUA0 (A), SPUA4 (B), and SPUA8 (C).
4. Conclusions
A series of PUA/SiO2 (SPUA) composite emulsions were successfully prepared via in situ polymerization accompanying the sol-gel process. The average particle size of the SPUA emulsions increased with the TEOS content increasing. From SEM and TEM results, it was found that the SiO2 nanoparticles uniformly distributed in the SPUA resin. FTIR analysis indicated that the SiO2, polyacrylate, and PU were all incorporated together. With the aid of those in situ-formed SiO2, the tensile strength, hardness, water resistance, and thermal stability of PUA composite films were increased. The in situ polymerization of PUA accompanying the hydrolysis of TEOS proposed in this work shows to be very effective in incorporating inorganic nanoparticles into the polymer substrate. This novel idea to obtain organic-inorganic hybrids is a good reference to the other researchers working in such a field. The enhanced hydrophobicity, thermal stability, and mechanical properties will contribute to the extensive application of SPUA. Composite films prepared in this method are promising for application as adhesives, sealants, plastic coatings, wood finishes, and coatings of flexible substrates such as fabric, leather, and paper.
References
[1] Lin WC, Yang CH, Wang TL, Shieh YT, Chen WJ. Express Polym. Lett. 2012, 6, 2–13.10.3144/expresspolymlett.2012.2Search in Google Scholar
[2] Oh IS, Park NH. J. Appl. Polym. Sci. 2000, 75, 968–975.10.1002/(SICI)1097-4628(20000214)75:7<968::AID-APP14>3.0.CO;2-GSearch in Google Scholar
[3] Zhang SF, Wang RM, He YF, Song PF, Wu ZM. Prog. Org. Coat. 2013, 76, 729–735.10.1016/j.porgcoat.2013.01.003Search in Google Scholar
[4] Yang ZH, Ni AQ, Wang JH. J. Appl. Polym. Sci. 2013, 10, 2905–2909.10.1002/app.37698Search in Google Scholar
[5] Nasab RH, Mirabedini SM. Prog. Org. Coat. 2013, 76, 1016–1023.10.1016/j.porgcoat.2013.02.016Search in Google Scholar
[6] Duan YN, Jana SC, Lama B, Espe MP. Langmuir 2013, 29, 6156–6165.10.1021/la4007394Search in Google Scholar
[7] Gu HB, Guo J, Zhang X, He QL, Huang YD, Colorado HA, Haldolaarachchige N, Xin HL, Young DP, Wei SY, Guo ZH. J. Phys. Chem. C. 2013, 117, 6426–6436.10.1021/jp311471fSearch in Google Scholar
[8] Han WS. Polym. Comp. 2013, 10, 156–163.10.1002/pc.22388Search in Google Scholar
[9] Allauddin S, Narayan R, Raju KVSN. ACS Sustainable Chem. Eng. 2013, 1, 910.10.1021/sc3001756Search in Google Scholar
[10] Peng HS, Li XH, You FT, Teng F, Huang SH. Microchim. Act. 2013, 180, 807–812.10.1007/s00604-013-1003-xSearch in Google Scholar
[11] Zhang SW, Yu A, Song XQ, Liu XY. Prog. Org. Coat. 2013, 76, 1032–1039.10.1016/j.porgcoat.2013.02.019Search in Google Scholar
[12] Qiu FX, Xu HP, Wang YY, Wu WL, Yang DY, Guo Q. J. Coat Technol. Res. 2012, 9, 503–514.10.1007/s11998-012-9397-7Search in Google Scholar
[13] Zhang LH, Zhang H, Guo JS. Ind. Eng. Res. 2012, 51, 8434–8441.10.1021/ie3000248Search in Google Scholar
[14] Zhang SW, Yu AX, Liu SL, Zhao J, Jiang JQ, Liu XY. Polym. Bull. 2012, 68, 1469–1482.10.1007/s00289-011-0689-3Search in Google Scholar
[15] Li H, Chen GM, Chen W, Zhang MY, Xu GW, Huang YP. Chin. J. Appl. Chem. 2011, 28, 1135–1142 (in Chinese).10.3724/SP.J.1095.2011.00650Search in Google Scholar
[16] Zhang MY, Chen GM, Zhang XL, Wu LX, Huang YP. Fine Chem. Eng. 2011, 28, 1041–1045 (in Chinese).10.1016/j.cej.2011.01.079Search in Google Scholar
[17] Yang J, Han CR, Duan JF, Xu F, Sun RC. J. Phys. Chem. C. 2013, 117, 8223–8230.10.1021/jp400200sSearch in Google Scholar
©2018 Walter de Gruyter GmbH, Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Original articles
- Review of the mechanical performance of variable stiffness design fiber-reinforced composites
- Exact solution for bending analysis of functionally graded micro-plates based on strain gradient theory
- Synthesis, microstructure, and mechanical properties of in situ TiB2/Al-4.5Cu composites
- Microstructure and properties of W-Cu/1Cr18Ni9 steel brazed joint with different Ni-based filler metals
- Drilling studies on the prepared aluminum metal matrix composite from wet grinder stone dust particles
- Studies on mechanical properties of thermoplastic composites prepared from flax-polypropylene needle punched nonwovens
- Design of and with thin-ply non-crimp fabric as building blocks for composites
- Effect of coir fiber reinforcement on mechanical properties of vulcanized natural rubber composites
- Investigation and analysis of glass fabric/PVC composite laminates processing parameters
- Abrasive wear behavior of silane treated nanoalumina filled dental composite under food slurry and distilled water condition
- Finite element study into the effects of fiber orientations and stacking sequence on drilling induced delamination in CFRP/Al stack
- Preparation of PAA/WO3 composite films with enhanced electrochromism via layer-by-layer method
- Effect of alkali treatment on hair fiber as reinforcement of HDPE composites: mechanical properties and water absorption behavior
- Integration of nano-Al with one-step synthesis of MoO3 nanobelts to realize high exothermic nanothermite
- A time-of-flight revising approach to improve the image quality of Lamb wave tomography for the detection of defects in composite panels
- The simulation of the warpage rule of the thin-walled part of polypropylene composite based on the coupling effect of mold deformation and injection molding process
- Novel preparation method and the characterization of polyurethane-acrylate/ nano-SiO2 emulsions
- Microwave properties of natural rubber based composites containing carbon black-magnetite hybrid fillers
- Simulation on impact response of FMLs: effect of fiber stacking sequence, thickness, and incident angle
Articles in the same Issue
- Frontmatter
- Original articles
- Review of the mechanical performance of variable stiffness design fiber-reinforced composites
- Exact solution for bending analysis of functionally graded micro-plates based on strain gradient theory
- Synthesis, microstructure, and mechanical properties of in situ TiB2/Al-4.5Cu composites
- Microstructure and properties of W-Cu/1Cr18Ni9 steel brazed joint with different Ni-based filler metals
- Drilling studies on the prepared aluminum metal matrix composite from wet grinder stone dust particles
- Studies on mechanical properties of thermoplastic composites prepared from flax-polypropylene needle punched nonwovens
- Design of and with thin-ply non-crimp fabric as building blocks for composites
- Effect of coir fiber reinforcement on mechanical properties of vulcanized natural rubber composites
- Investigation and analysis of glass fabric/PVC composite laminates processing parameters
- Abrasive wear behavior of silane treated nanoalumina filled dental composite under food slurry and distilled water condition
- Finite element study into the effects of fiber orientations and stacking sequence on drilling induced delamination in CFRP/Al stack
- Preparation of PAA/WO3 composite films with enhanced electrochromism via layer-by-layer method
- Effect of alkali treatment on hair fiber as reinforcement of HDPE composites: mechanical properties and water absorption behavior
- Integration of nano-Al with one-step synthesis of MoO3 nanobelts to realize high exothermic nanothermite
- A time-of-flight revising approach to improve the image quality of Lamb wave tomography for the detection of defects in composite panels
- The simulation of the warpage rule of the thin-walled part of polypropylene composite based on the coupling effect of mold deformation and injection molding process
- Novel preparation method and the characterization of polyurethane-acrylate/ nano-SiO2 emulsions
- Microwave properties of natural rubber based composites containing carbon black-magnetite hybrid fillers
- Simulation on impact response of FMLs: effect of fiber stacking sequence, thickness, and incident angle

