Abstract
MoO3 nanobelts were prepared by a one-step hydrothermal method and then assembled with aluminum nanoparticles using polyvinylpyrrolidone as a binder. The physicochemical properties of the as-prepared samples were carefully characterized by scanning electron microscopy, transmission electron microscopy, X-ray diffraction, thermogravimetric analysis/differential scanning calorimetry, and drop weight impact test. The results showed that the Tonset of Mo-Al-0, Mo-Al-1, Mo-Al-2, and Fe-Al-0 are 474.8°C, 484.2°C, 478.5°C, and 514.8°C, respectively, which are 66.0°C, 56.6°C, 62.3°C, and 26.0°C lower than that of pure Al-NPs (540.8°C). The total exothermic heat of self-assembled MoO3/Al during DSC test is about 2626.9 J/g, which is 229.6, 420.8, and 11.1 J/g higher than that of Al/commercial MoO3, Al/hydrothermal MoO3, and Al/referenced Fe2O3. Furthermore, the high exothermic self-assembled MoO3/Al thermite is totally insensitive to impact.
1 Introduction
Because of the high energy densities and reaction temperatures, energetic materials composed of fuel and oxidizer have received considerable attention. Aluminum as fuel has been the principal ingredient in most fuel-rich solid rocket propellant and other propulsive systems, owing to its high oxide reducing potential, availability, low vapor pressure, and low melting temperature [1], [2], [3], [4], [5]. Reducing the structure size to nanoscale has some critical advances in many important materials, including energetic materials. Nanocomposite energetic materials containing aluminum nanoparticles (Al-NPs) have been studied extensively, which show significantly lower ignition temperature, higher reactivity, and faster propagation rate than that of the conventional aluminum [6], [7], [8], [9], due to their shorter diffusion distance, larger contact areas, and better homogeneity of fuel and oxidizer particles. Among the candidates for the metal oxides, such as Fe2O3, CuO, NiO, PbO2, Co3O4, Bi2O3, WoO3, CuO, TiO2, Cr2O3, and MoO3, the exothermic enthalpy of stoichiometric Al/MoO3 thermite reaction is the highest in the following thermite mixtures: Fe2O3/Al, CuO/Al, NiO/Al, PbO2/Al, Co3O4/Al, Bi2O3/Al, WoO3/Al, CuO/Al, TiO2/Al, and Cr2O3. Researches indicate that an Al/MoO3 nanothermite can provide favorable ignition property compared to pure aluminum in oxidizing atmospheres because any reduced molybdenum metal could be reoxidized at high temperature. After that, MoO3 still acts as an oxidizer in oxygen-containing atmospheres. If less MoO3 content was used in the thermite, the composite could potentially provide the majority of high-energy aluminum with superior ignition characteristics [10], [11], [12], [13], [14], [15]. Nowadays, various methods have been developed to prepare nanothermites, including physical mixing, sol-gel, ultrasonic method, and arrested reactive milling [16], [17], [18], [19], [20], [21].
In this work, we used a one-step hydrothermal method to prepare MoO3 nanobelts, and the as-synthesized MoO3 nanobelts were used to integrate with Al-NPs through two different approaches. The first way is to mix Al-NPs with MoO3 nanobelts by ultrasonication, and another way is to combine Al-NPs with MoO3 through a self-assembly method using polyvinylpyrrolidone (PVP) as a stabilizer. The nanoaluminum can be arranged around metallic oxidizers in an ordered manner, and these thermites can provide more active sites and a higher rate of energy release and increase the contact areas between Al-NPs and MoO3 nanobelts. Commercial Fe2O3 nanoparticles mixed with 100 nm Al-NPs by the ultrasonic method were used as reference. The results show that the thermite consisting of Al and MoO3 by the self-assembled method exhibited higher performance than Al-NPs, Al/hydrothermal MoO3, and Al/referenced Fe2O3 prepared by the ultrasonic method in thermal test. The drop weight impact test was conducted to measure the sensitivity of Al/MoO3 thermite, and the self-assembled Al/MoO3 thermite is insensitive to impact.
2 Materials and methods
2.1 Materials and reagents
All the chemicals were of analytical grade and used as purchased without any further treatment. Al-NPs were obtained from Beijing Nachen Technology Co., Ltd., with an average size of 100 nm and a purity of 72%. Referenced MoO3 was purchased from Kaituo Muye Co., Ltd., with an average size of 100 nm and a purity of 99.5%, and was marked as MoO3-0. Referenced Fe2O3 was from Beijing Nachen Technology Co., Ltd., with an average size of 100 nm and a purity of 99.9%.
2.2 Sample preparation
2.2.1 Synthesis of MoO3 nanobelts
MoO3 nanobelts were synthesized according to the reported literature [22]. The typical process is described as follows.
MoO3-1 nanobelts were synthesized through a hydrothermal method. Then, 0.8 mmol (0.9885 g) (NH4)6Mo7O24·4H2O was added to 20 ml deionized water, and 2 mmol (0.7278 g) cetyltrimethylammonium bromide (CTAB) was added to the above solution with constant magnetic stirring. Next, 20 ml HNO3 (2.2 m) was added to the solution in a dropwise manner, and subsequently, a white precipitate was formed. Finally, the suspension was transferred to a 100 ml Teflon-lined autoclave and heated at 180°C for 20 h. After the hydrothermal reaction, the light blue product was washed twice with ethanol and acetone, respectively, and dried at 80°C.
The preparation process of MoO3-2 nanobelt is similar to that of MoO3-1 by changing the adding order of deionized water and HNO3.
The MoO3-3 sample was obtained by a hydrothermal method as well. Then, a 25 ml saturated water solution of (NH4)6Mo7O24·4H2O was ultrasonicated for 10 min, and 2.2 mol/l HNO3 was added to the solution with magnetic stirring. The molar ratio of CTAB to (NH4)6Mo7O24·4H2O was 1:2. After the reaction, the suspension was transferred into a Teflon-lined autoclave and kept at 180°C for 40 h in a hot oven. The precipitate was separated by centrifugation, washed with deionized water, ethanol, and acetone several times, and dried at 80°C.
2.2.2 Synthesis of nanothermites
The Mo-Al-0 composite was prepared by adding MoO3-0 and 100 nm Al-NPs (MoO3/Al molar ratio 1:3) to 20 ml hexane by the ultrasonic method for 1 h. This formulation contains 36.0 wt% Al-NPs and 64.0 wt% MoO3-0 nanoparticles.
The Mo-Al-1 composite was obtained by mixing MoO3-1 and 100 nm Al-NPs (MoO3/Al molar ratio 1:3) in 20 ml hexane by the ultrasonic method for 1 h.
The Mo-Al-2 composite was synthesized by a self-assembly method. First, 0.1 g PVP was dissolved in 100 ml isopropanol. Then, MoO3-1 was added to the above solution and the mixture was ultrasonicated for 4 h. Next, the mixture was washed by isopropanol three times to remove the excess PVP and dried at 120°C for 1.5 h. Finally, the dried PVP-coated MoO3 was mixed with Al-NPs in hexane by ultrasonication for 1.5 h.
The Fe-Al-0 composite was fabricated by typically the ultrasonic method. Then, 100 nm Fe2O3 and 100 nm Al-NPs were added to 20 ml hexane in a sonic bath for 1 h. The composite is composed of 33.6 wt% Al-NPs and 66.4 wt% Fe2O3.
2.3 Characterization
The phase structures of the as-synthesized samples were determined on a Bruker D8 Advance X-ray diffractometer (40 kV, 40 mA) with CuKα radiation (λ=0.1542 nm). The morphology and microstructure were observed by field-emission scanning electron microscopy (FE-SEM; Quanta™ 250) and transmission electron microscopy (TEM; JEOL JEM-2100). An SDT Q600 V8.1 Build 99 thermal analyzer was applied to measure the thermal property of the thermite. The measurement was conducted from 40°C to 1000°C in nitrogen atmosphere with an increasing temperature rate of 20°C/min. A sensitivity calibration was done using a sapphire sample with a known specific heat for differential scanning calorimetry (DSC) data. A temperature calibration was conducted based on the melting onset temperature of pure metal samples of indium, tin, aluminum, and zinc. The drop weight impact test was used to obtain the characteristic height (h50%) of thermite by a custom-built drop weight impact tester with a 5 kg hammer.
It should be noted that all the reactions are performed in a nitrogen environment such that nitrogen from the surroundings may contribute to the oxidation of Al-NPs. The contribution from the reaction of Al-NPs and nitrogen may shift this optimum point from fuel rich toward stoichiometric. Unfortunately, the precise contribution of nitrogen in the reaction is yet unknown in this work, and the method for determining the equivalence ratio was solely based on the global reaction from Eq. (1):
3 Results and discussion
3.1 Characterization of MoO3
3.1.1 Crystal structure
X-ray diffraction (XRD) analysis was used to determine the phase structure of as-synthesized products. Figure 1 represents the typical XRD patterns of MoO3 samples. All the samples show similar XRD patterns and all the diffraction peaks from 10° to 80° can be attributed to monoclinic MoO3 [Joint Committee on Powder Diffraction Standards (JCPDS) No. 05-0508] [23], [24]. The main peaks at 2θ=12.9°, 23.1°, 25.8°, 27.4°, and 39.0° correspond to the diffractions of (0 2 0), (1 1 0), (0 4 0), (0 2 1), and (0 6 0) planes of MoO3, respectively.
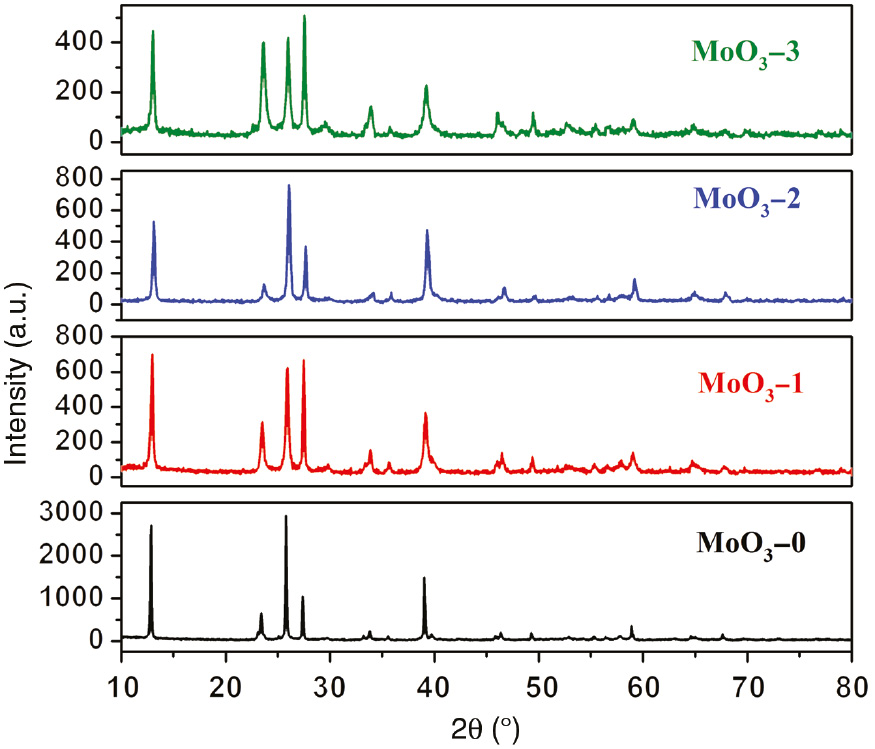
XRD patterns of MoO3-0, MoO3-1, MoO3-2, and MoO3-3.
3.1.2 Morphology of MoO3
The morphology evolution of MoO3 prepared under different conditions was observed by TEM. As shown in Figure 2A and B, the commercial MoO3-0 particles are micron-sized belts with a width size from 1 to 5 μm and length from 3 to 20 μm. The MoO3-1 nanobelts have a mean width of about 60 nm (Figure 2C and D). The shape of MoO3-2 and MoO3-3 is not a regular nanobelt with a large length range (Figure 2E–H). Therefore, the morphology of MoO3 is influenced by the adding order of raw materials and the concentration of the (NH4)6Mo7O24·4H2O solution. The selected area electron diffraction (SAED) images in the insets of Figure 2D, F, and H show a single crystal pattern, indicating that the MoO3-1, MoO3-2, and MoO3-3 nanobelts grow along one special direction. These results show that the MoO3-1 is the best belt and the following discussion is mainly focused on MoO3-1-based thermite.

TEM images of MoO3-0 (A and B), MoO3-1 (C and D), MoO3-2 (E and F), and MoO3-3 (G and H).
The inset images in D, F, and H are the SAED of MoO3-1, MoO3-2, and MoO3-3.
3.2 Characterization of Al/MoO3 thermites
3.2.1 Crystal structure of composite thermites
The XRD pattern of 100 nm Al-NPs is displayed in Figure 3. Typically, the diffraction peaks at 2θ=38.5°, 44.7°, 65.1°, and 78.2° can be indexed to (1 1 1), (2 0 0), (2 2 0), and (3 1 1) diffraction of Al (JCPDS No. 65-2869), respectively [25]. The XRD patterns of Mo-Al-0, Mo-Al-1, and Mo-Al-2 can be considered as the superimposition of MoO3 (JCPDS No. 05-0508) and Al (JCPDS No. 65-2869), which indicates the coexistence of MoO3 and Al in the composite thermites. No diffraction peaks of any other impurity were detected in the XRD patterns of Mo-Al-0 and Mo-Al-1, suggesting that Al-NPs were stable during the preparation process of composite thermites. As we can see from the XRD pattern of Mo-Al-2, the small broad peak from 10° to 15° is attributed to the existence of the PVP polymer [26].
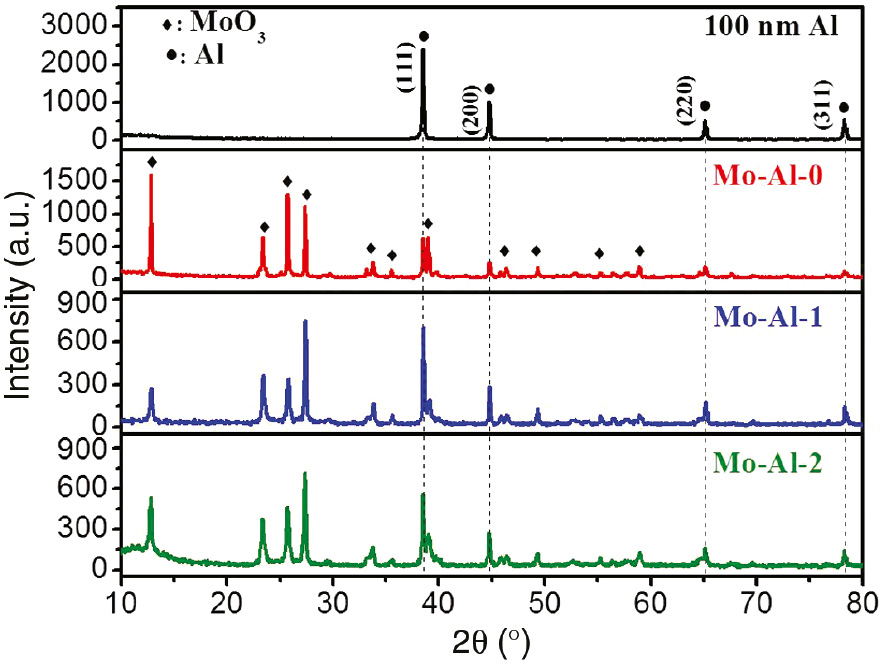
XRD patterns of 100 nm Al-NPs, Mo-Al-0, Mo-Al-1, and Mo-Al-2.
3.2.2 Morphology of Al/MoO3 thermite
Figure 4 shows the typical SEM and TEM images of Al/MoO3 composite thermites. As presented in Figure 4A and B, most of the Al-NPs in the Mo-Al-0 composite are agglomerated and there is no efficient contact between Al-NPs and MoO3 nanobelts due to the big size of MoO3-0 and the weak interaction. The dispersion of Al-NPs in Mo-Al-1 was highly improved (Figure 4B) because of the smaller size of MoO3-1 belts. The TEM images of Al-NPs (Figure 4D) show that the Al-NPs have a size distribution from 40 to 180 nm with a spherical shape. Compared to the samples of Mo-Al-0 and Mo-Al-1, Al-NPs in Mo-Al-2 (Figure 4C) have better dispersion and homogeneity around MoO3-1 nanobelts, which can be further confirmed by the results of TEM images (Figure 4E and F). This is mainly attributed to the assistance of PVP, which can provide binding sites for Al-NPs on the surface of MoO3 nanobelts.

SEM images of the Mo-Al-0 (A), Mo-Al-1 (B), and Mo-Al-2 (C) composites and TEM images of Al-NPs (D) and Mo-Al-2 (E and F).
The inset in (C) is the formula of PVP. The molar ratios of MoO3 to Al-NPs are kept at 1:3 in different samples.
3.2.3 Thermal properties of Mo-Al thermites
Thermogravimetric analysis (TGA)/DSC was applied to measure the thermal properties of Al-NPs and Mo-Al composite thermites. Figure 5 depicts the TGA/DSC curves of Al-NPs, Mo-Al-0, Mo-Al-1, Mo-Al-2, and Fe-Al-0. To compare the difference between Al-NPs and thermites, the onset temperatures and released heat are listed in Table 1.

TGA/DSC curves of 100 nm Al and composite thermites: (A) 100 nm Al-NPs, (B) Mo-Al-0, (C) Mo-Al-1, (D) Mo-Al-2, and (E) Fe-Al-0.
DSC parameters of 100 nm Al-NPs and thermites.
| Samples | H365.2–663.2 (J/g)a | H663.2–912.7 (J/g)b | Tp1 (°C) | Tp2 (°C) | Tp3 (°C) | Tonset (°C) | Remark |
|---|---|---|---|---|---|---|---|
| Al-NPs | 2173.8 | 3597.7 | 603.0 | 830.0 | — | 540.8 | N2, 20 K/min, 100 nm Al-NPs |
| Mo-Al-0 | 275.2 | 2122.3 | 572.6 | 672.3 | 783.7 | 474.8 | MoO3-0, ultrasonic method, N2, 20 K/min, 100 nm Al-NPs |
| Mo-Al-1 | 380.0 | 1826.1 | 586.4 | 678.0 | 783.8 | 484.2 | MoO3-1, ultrasonic method, N2, 20 K/min, 100 nm Al-NPs |
| Mo-Al-2 | 237.0 | 2389.9 | 572.3 | 647.7 | 783.1 | 478.5 | MoO3-1, self-assembly method, N2, 20 K/min, 100 nm Al-NPs |
| Fe-Al-0 | 958.8 | 1657.0 | 599.4 | 818.0 | – | 514.8 | 100 nm Fe2O3, ultrasonic method, N2, 20 K/min, 100 nm Al-NPs |
aFor samples Al-NPs and Fe-Al-0, the temperature range is from 365.2°C to 663.2°C; for samples Mo-Al-0, Mo-Al-1, and Mo-Al-2, the temperature range is from 400°C to 600°C.
bFor samples Al-NPs and Fe-Al-0, the temperature range is from 663.2°C to 912.7°C; for samples Mo-Al-0, Mo-Al-1, and Mo-Al-2, the temperature range is from 600°C to 912.7°C.
As shown in Figure 5, the TGA% represents the percent mass change occurring at a given temperature and the DSC scale means the energy change in mW/mg. As seen in Figure 5A, there is a mass loss (3.6%) roughly from 41°C to 365.2°C in the TGA curve of Al-NPs, owing to the desorption of O2, H2O, and CO2 on the surface of the Al-NPs. There are two exotherms during the oxidization of Al-NPs. The first exothermic reaction is from 365.2°C to 663.2°C, in which solid Al-NPs react with N2 with ~2173.8 J/g heat released and 27.6% weight gained (Figure 5A). The weak endothermic peak at 663.2°C resulting from the melting of Al-NPs may overlap with the major exothermic peaks. The second exotherm is attributed to the reaction between the remaining melted Al-NPs and N2 from 663.2°C to 912.7°C. The released heat during this step is about 3597.7 J/g. The onset temperature (540.8°C) was obtained using the tangent lines of TGA curves.
The DSC curves of Mo-Al-0, Mo-Al-1, and Mo-Al-2 (Figure 5B–D) show an initial exothermic reaction from 400.0°C to 600°C, which is associated with the reaction between solid Al-NPs and N2, and the latter two exothermic peaks correspond to the reactions between melted Al-NPs and N2, as well as the reactions of melted Al-NPs and MoO3, respectively. The weak endothermic peak that overlapped with the major exothermic peaks at about 600°C may result from the fusion of Al-NPs. TGA curves show a 20% mass up from 400°C to 800°C, which is associated with the reaction of Al-NPs and N2. The thermites show a sharp mass loss at 825°C, with a residual mass of 50%, implying the sublimation of MoO3 (http://webbook.nist.gov/chemistry/). As displayed in Figure 5, the Tonset of Mo-Al-0, Mo-Al-1, Mo-Al-2, and Fe-Al-0 are 474.8°C, 484.2°C, 478.5°C, and 514.8°C, respectively, which are 66.0°C, 56.6°C, 62.3°C, and 26.0°C lower than that of pure Al-NPs (540.8°C) and the thermites reported in Refs. [3], [12], [15]. This may correspond to the catalytic function of MoO3 and Fe2O3. Therefore, MoO3 can be used as a catalyst to improve the combustion and ignition of Al-NPs. The exothermic reaction temperature for Al-NPs decreases from 830°C to 783.7°C, 783.8°C, and 783.1°C in Mo-Al-0, Mo-Al-1, and Mo-Al-2 composites, respectively. An interesting phenomenon is found that there is an additional exothermic peak at about 830°C in the DSC curves of Mo-Al-1 and Mo-Al-2 composites. This may be caused by the reaction between newly formed MoO3 (oxidization of reduced Mo during thermite reaction) and residual melted Al-NPs in the thermite system. It is worth noting that there is no exothermic peak in the DSC curve of Mo-Al-0 because of the large particle size of MoO3-0, the agglomeration of Al-NPs, and the long diffusion distance between Al-NPs and MoO3 (Figure 4A).
By the integration of the exothermic peaks during the DSC test of Mo-Al-2, the exothermic heat from 400°C to 912.7°C is about 2626.9 J/g, whereas the theoretical reaction heat is 5548.9 J/g according to reaction (1) with the fuel-rich equivalence ratio of 1.5. (The reaction heat was calculated based on the data from http://webbook.nist.gov/chemistry/.) The heat released during DSC of Mo-Al-0, Mo-Al-1, and Fe-Al-0 are shown to be 2397.5, 2206.1, and 2615.8 J/g, respectively. As a result, the heat sequence from high to low is Mo-Al-2, Fe-Al-0, Mo-Al-0, and Mo-Al-1. Furthermore, the released heat of Mo-Al-2 is also high compared to the Fe2O3- and MoO3-based thermites in the reported references [3], [27]. The reason for the best thermal property of Mo-Al-2 thermite is that the self-assembly method can hinder the precombustion sintering between Al-NPs, thereby improving the interaction between Al-NPs and MoO3 nanobelt due to the lone pair of electrons of the pyrrole group in the PVP. It can donate to form a covalent bond with metals or undergo a hydrogen bonding with polar species [28]. Therefore, the Al-NPs can be arranged around MoO3 nanobelts in an ordered manner, and these thermites can provide more active sites and higher rate of energy release and increase the contact areas between Al-NPs and MoO3 nanobelts. Based on the research results of Zachariah’s group, the reaction between metal and oxidizer occurs at the surface of particles, and Al-NPs will melt and coalesce to bigger particles before reacting with the oxidizer on a fast timescale, which is called the pre-combustion sintering process [29], [30]. Therefore, the initial size and morphology of Al-NPs in Mo-Al-0 and Mo-Al-1 were dramatically changed before the redox reaction, suggesting a possible reason why nanostructured particles cannot react as fast as expected.
3.3 Impact sensitivity of the Mo-Al-2 composite
In the report of Aumann et al. [10], Al/MoO3 powder mixtures are highly sensitive to ignition by electrical spark and fraction and the storage and transportation of Al/MoO3 thermite will be a challenge. Therefore, the impact sensitivity of Mo-Al-2 was tested by the drop weight impact test. This is a common and convenient method to assess sensitivities, and the results can indicate the ignitability of the propellant, explosive, and thermite. In the test, 3 mg of a thermite sample were placed between a flat tool steel anvil and a polished surface of a tool striker. Typically, a 2.5 kg weight was dropped from a predetermined height to the striker (in this test, we used a 5 kg hammer owing to the insensitivity of as-prepared Mo-Al-2). The result of the event was determined by a combination of sound, smell, and visual inspection of the thermite. A sequence of 25 tests was carried out and the result was quoted as characteristic height (h50%), at which 50% of the tests result in explosion [31], [32], [33]. The result shows that the h50% value of Mo-Al-2 is higher than 100 cm, and we cannot increase the height of the hammer because 100 cm is the limited height for our hammer apparatus. This means that the thermite of Mo-Al-2 has low impact sensitivity [34], [35]. It is easy to store and transport for the application of Al/MoO3 thermite. Because of the inaccurate and irreproducible results of the drop weight impact test, three experiments were repeated to calculate the average h50% of Mo-Al-2 at 3 different days.
4 Conclusion
The additive sequence of HNO3 and deionized water and the concentration of (NH4)6Mo7O24·4H2O solution have great effects on the structure and morphology of MoO3 nanobelts. The self-assembly method can be used to obtain highly dispersed thermites. The onset temperature of Al-NPs decreased by 56.6°C–66°C in the presence of MoO3 nanobelt. MoO3 nanobelt can be used as a catalyst for the ignition and combustion of Al-NPs. Mo-Al-2 releases much higher heat than Mo-Al-0 and Mo-Al-1 because the self-assembly method can reduce the distance between Al-NPs and MoO3 nanobelts and decrease energy loss.
References
[1] Wang LL, Munir ZA, Maximov YM. J. Mater. Sci. 1993, 28, 3693–3708.10.1007/BF00353167Search in Google Scholar
[2] Bezmelnitsyn A, Thiruvengadathan R, Barizuddin S, Tappmeyer D, Apperson S, Gangopadhyay K, Gangopadhyay S, Redner P, Donadio M, Kapoor D, Nicolich S. Propell. Explos. Pyrotechn. 2010, 35, 384–394.10.1002/prep.200800077Search in Google Scholar
[3] Wang X, Zhang L, Zhu S, Zhao J. Chin. J. Inorg. Chem. 2012, 28, 2313–2320.Search in Google Scholar
[4] Blobaum KJ, Reiss ME, Plitzko JM, Weihs TP. J. Appl. Phys. 2003, 94, 2915–2922.10.1063/1.1598296Search in Google Scholar
[5] Illunga K, Fabbro OD, Yapi L, Focke WW. Powder Technol. 2011, 205, 97–102.10.1016/j.powtec.2010.08.071Search in Google Scholar
[6] Cervantes OG, Kuntz JD, Gash AE, Munir ZA. Combust. Flame 2010, 157, 2326–2332.10.1016/j.combustflame.2010.07.002Search in Google Scholar
[7] Bockmon BS, Pantoya ML, Son SF, Asay BW, Mang JT. J. Appl. Phys. 2005, 98, 064903–064903-7.10.1063/1.2058175Search in Google Scholar
[8] Patzke GR, Alexej M, Frank K, Nesper R, Grunwaldt JD, Baiker A. Chem. Mater. 2004, 16, 1126–1134.10.1021/cm031057ySearch in Google Scholar
[9] Lin H, Li H, Chen X, Yang M, Qi Y. J. Mol. Catal. 2010, 24, 99–104.Search in Google Scholar
[10] Aumann CE, Skofronick GL, Martin JA. J. Vacuum Sci. Technol. B 1995, 13, 1178–1183.10.1116/1.588232Search in Google Scholar
[11] Bazyn T, Lynch P, Krier H, Glumac N. Propell. Explos. Pyrotechn. 2010, 35, 93–99.10.1002/prep.200900016Search in Google Scholar
[12] Pantoya ML, Granier JJ. Propell. Explos. Pyrotechn. 2005, 30, 53–62.10.1002/prep.200400085Search in Google Scholar
[13] Yuma O, Liu SY, Rao PM, Zheng X. Proc. Combust. Inst. 2011, 33, 1909–1915.10.1016/j.proci.2010.05.048Search in Google Scholar
[14] Atuchin VV, Gavrilova TA, Kostrovsky VG, Pokrovsky LD, Troitskaia IB. Inorg. Mater. 2008, 44, 622–627.10.1134/S0020168508060149Search in Google Scholar
[15] Manesh NA. Heat Transfer in Multi-Layer Energtic Nanofilm on Composites Substrate. University of Central Florida: Florida, 2010, 1–15.Search in Google Scholar
[16] Zhou R, Han Y, Chen X. Nano Materials Technology. National Defence Industry Press: Beijing, 2003 (in Chinese).Search in Google Scholar
[17] Adachi M, Lockwood DJ. Nanostructure Science and Technology Series: Self-Organized Nanoscale Materials. Springer Science+Business Media, Inc., New York, 2006.10.1007/b137255Search in Google Scholar
[18] Cervantes OG, Kuntz JD, Gash AE, Munir ZA. Combust. Flame 2011, 158, 117–122.10.1016/j.combustflame.2010.07.023Search in Google Scholar
[19] Wang J, Hu A, Persic J, Wen JZ, Zhou YN. J. Phys. Chem. Solids 2010, 72, 620–625.10.1016/j.jpcs.2011.02.006Search in Google Scholar
[20] Chen SJ, Garitaonandia JS, Ortega D, Suzuki K. J. Alloys Compd. 2012, 536S, S287–S290.10.1016/j.jallcom.2011.11.033Search in Google Scholar
[21] Stamatis D, Dreizin EL. Combust. Flame 2011, 158, 1631–1637.10.1016/j.combustflame.2010.12.024Search in Google Scholar
[22] Wang ST, Zhang YG, Ma XC, Wang WZ, Li XB, Zhang ZD. Solid State Commun. 2005, 136, 283–287.10.1016/j.ssc.2005.08.002Search in Google Scholar
[23] Zhou J, Deng SZ, Xu NS, Chen J, She JC. Appl. Phys. Lett. 2003, 83, 2653–2655.10.1063/1.1613992Search in Google Scholar
[24] Reddya Subba ChV, Walker EH, Chen W, Mho S. J. Power Sources 2008, 183, 330–333.10.1016/j.jpowsour.2008.05.005Search in Google Scholar
[25] Xu D, Yang Y, Cheng H, Li Y, Zhang K. Combust. Flame 2012, 159 2202–2209.10.1016/j.combustflame.2012.01.022Search in Google Scholar
[26] Zheng M, Jin Y, Jin G, Gu M. J. Mater. Science Lett. 2000, 19, 433–436.10.1023/A:1006703224379Search in Google Scholar
[27] Umbrajkar SM, Schoenitz M, Dreizin EL. Propell. Explos. Pyrotechn. 2006, 31, 382–389.10.1002/prep.200600052Search in Google Scholar
[28] Wang H, Qiao X, Chen J, Wang X, Ding S. Mater. Chem. Phys. 2005, 94, 449–453.10.1016/j.matchemphys.2005.05.005Search in Google Scholar
[29] Sullivan KT, Piekiel NW, Wu C, Chowdhury S, Kelly ST, Hufnagel TC, Fezzaa K, Zachariah MR. Combust. Flame 2012, 159, 2–15.10.1016/j.combustflame.2011.07.015Search in Google Scholar
[30] Jacob RJ, Jian GQ, Guerieri PM, Zachariah MR. Combust. Flame 2015, 162, 258–264.10.1016/j.combustflame.2014.07.002Search in Google Scholar
[31] Rice BM, Hare JJ. J. Phys. Chem. A 2002, 106, 1770–1783.10.1021/jp012602qSearch in Google Scholar
[32] Chen Z, Xiao H. Int. J. Quant. Chem. 2000, 79, 350–357.10.1002/1097-461X(2000)79:6<350::AID-QUA3>3.0.CO;2-TSearch in Google Scholar
[33] Wilson WS, Bliss DE, Christian SL, Knight DJ. Explosive Properties of Polynitroaromatics. Naval Weapons Center China Lake, California, 1990.10.21236/ADA229627Search in Google Scholar
[34] Shi X, Wang J, Li X, An C. Cent. Eur. J. Energetic Mater. 2014, 11, 433–442.Search in Google Scholar
[35] Li H, Wang J, An C. Cent. Eur. J. Energetic Mater. 2014, 11, 237–255.Search in Google Scholar
©2018 Walter de Gruyter GmbH, Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Original articles
- Review of the mechanical performance of variable stiffness design fiber-reinforced composites
- Exact solution for bending analysis of functionally graded micro-plates based on strain gradient theory
- Synthesis, microstructure, and mechanical properties of in situ TiB2/Al-4.5Cu composites
- Microstructure and properties of W-Cu/1Cr18Ni9 steel brazed joint with different Ni-based filler metals
- Drilling studies on the prepared aluminum metal matrix composite from wet grinder stone dust particles
- Studies on mechanical properties of thermoplastic composites prepared from flax-polypropylene needle punched nonwovens
- Design of and with thin-ply non-crimp fabric as building blocks for composites
- Effect of coir fiber reinforcement on mechanical properties of vulcanized natural rubber composites
- Investigation and analysis of glass fabric/PVC composite laminates processing parameters
- Abrasive wear behavior of silane treated nanoalumina filled dental composite under food slurry and distilled water condition
- Finite element study into the effects of fiber orientations and stacking sequence on drilling induced delamination in CFRP/Al stack
- Preparation of PAA/WO3 composite films with enhanced electrochromism via layer-by-layer method
- Effect of alkali treatment on hair fiber as reinforcement of HDPE composites: mechanical properties and water absorption behavior
- Integration of nano-Al with one-step synthesis of MoO3 nanobelts to realize high exothermic nanothermite
- A time-of-flight revising approach to improve the image quality of Lamb wave tomography for the detection of defects in composite panels
- The simulation of the warpage rule of the thin-walled part of polypropylene composite based on the coupling effect of mold deformation and injection molding process
- Novel preparation method and the characterization of polyurethane-acrylate/ nano-SiO2 emulsions
- Microwave properties of natural rubber based composites containing carbon black-magnetite hybrid fillers
- Simulation on impact response of FMLs: effect of fiber stacking sequence, thickness, and incident angle
Articles in the same Issue
- Frontmatter
- Original articles
- Review of the mechanical performance of variable stiffness design fiber-reinforced composites
- Exact solution for bending analysis of functionally graded micro-plates based on strain gradient theory
- Synthesis, microstructure, and mechanical properties of in situ TiB2/Al-4.5Cu composites
- Microstructure and properties of W-Cu/1Cr18Ni9 steel brazed joint with different Ni-based filler metals
- Drilling studies on the prepared aluminum metal matrix composite from wet grinder stone dust particles
- Studies on mechanical properties of thermoplastic composites prepared from flax-polypropylene needle punched nonwovens
- Design of and with thin-ply non-crimp fabric as building blocks for composites
- Effect of coir fiber reinforcement on mechanical properties of vulcanized natural rubber composites
- Investigation and analysis of glass fabric/PVC composite laminates processing parameters
- Abrasive wear behavior of silane treated nanoalumina filled dental composite under food slurry and distilled water condition
- Finite element study into the effects of fiber orientations and stacking sequence on drilling induced delamination in CFRP/Al stack
- Preparation of PAA/WO3 composite films with enhanced electrochromism via layer-by-layer method
- Effect of alkali treatment on hair fiber as reinforcement of HDPE composites: mechanical properties and water absorption behavior
- Integration of nano-Al with one-step synthesis of MoO3 nanobelts to realize high exothermic nanothermite
- A time-of-flight revising approach to improve the image quality of Lamb wave tomography for the detection of defects in composite panels
- The simulation of the warpage rule of the thin-walled part of polypropylene composite based on the coupling effect of mold deformation and injection molding process
- Novel preparation method and the characterization of polyurethane-acrylate/ nano-SiO2 emulsions
- Microwave properties of natural rubber based composites containing carbon black-magnetite hybrid fillers
- Simulation on impact response of FMLs: effect of fiber stacking sequence, thickness, and incident angle

