Green synthesis of silver nanoparticles using Ginkgo biloba seed extract: Evaluation of antioxidant, anticancer, antifungal, and antibacterial activities
-
Jungang Luo
, Bin Fang
und Bing Wu
Abstract
In the past decade, the use of green synthesis methods to prepare nanoparticles (NPs) has become a promising alternative to traditional chemical and physical methods. The synthesis of silver nanoparticles (AgNPs) using biological resources is not only simple, economical, and fast, but also environmentally friendly, effectively reducing energy consumption and pollution. This study developed an eco-friendly and non-toxic method for the green biosynthesis of AgNPs using Ginkgo biloba seed extract (GBSE), as a reducing and stabilizing agent. This method was optimized through single factor experiments and response surface methodology. The optimal reaction temperature, ratio, and time for GBSE-AgNPs were determined to be 94.725°C, 23.165 (v/v), and 235.890 min, respectively. The synthesized NPs with uniform distribution had a size of 17.95 ± 1.17 nm. The atomic percentage of Ag element is 19.87%, and the mass percentage is 65.53%. It has been proven that the Ag crystal structure of GBSE-AgNPs is face centered cubic, and the silver element is in a zero-valence state. Further research was conducted on the antibacterial properties of GBSE-AgNPs against Staphylococcus aureus and Escherichia coli and demonstrated good antibacterial performance. Meanwhile, the GBSE-AgNPs also exhibit excellent inhibitory ability against common pathogenic fungi (Aspergillus fumigatus, Aspergillus niger, Aspergillus flavus, and Penicillium marneffei). Compared with GBSE, GBSE-AgNPs possess better antioxidant potential. In addition, it has been also demonstrated that GBSE-AgNPs exhibit excellent inhibitory effects on gastric cancer cell line SGC-7901, causing apoptosis of SGC-7901. Therefore, the green synthesis and eco-friendly GBSE-AgNPs have significant antioxidant, antibacterial, antifungal, and anticancer properties, providing new possibilities for the widespread application of GBSE-AgNPs.
Abbreviations
- AgNPs
-
silver nanoparticles
- DLS
-
dynamic light scattering
- DPPH
-
diphenyl-2-trinitrophenylhydrazine
- DSC
-
differential scanning calorimetry
- E. coli
-
Escherichia coli
- EDS
-
energy dispersive X-ray spectroscopy
- FCC
-
face centered cubic
- FRAP
-
ferric reducing antioxidant power
- GBSE
-
Ginkgo biloba seed extract
- HPLC
-
high-performance liquid chromatography
- HRTEM
-
high-resolution transmission electron microscopy
- ICP-OES
-
inductively coupled plasma emission spectrometry
- MBC
-
minimum bactericidal concentration
- MNP
-
metal nanoparticles
- MIC
-
minimum inhibitory concentration
- PMP
-
1-phenyl-3-methyl-5-pyrazolone
- RSM
-
response surface methodology
- Sa
-
Staphylococcus aureus
- SEM
-
scanning electron microscopy
- SFA
-
single factor analysis
- SPR
-
surface plasmon resonance
- TFA
-
trifluoroacetic acid
- TG
-
thermogravimetric
- XPS
-
X-ray photoelectron spectroscopy
- XRD
-
X-ray diffraction
1 Introduction
Nanoscience and nanotechnology have attracted researchers and engineers to simulate the synthesis and testing of NPs through computational and experimental programs, which is crucial for promoting scientific and technological progress [1]. Metal nanoparticles (MNPs) have received widespread attention due to their highly controllable surface plasmon resonance (SPR) effect, high absorption coefficient, and high surface area to volume ratio [2]. Silver nanoparticles (AgNPs) have better biocompatibility compared to other MNPs, which has attracted research and commercial applications [3,4]. Currently, AgNPs can be synthesized in three different ways: physical, chemical, and biological methods. Physical and chemical synthesis methods require high temperatures (>300°C) and release gases and chemicals, which are highly toxic, severely polluting, and potentially harmful to the environment and organisms [5,6,7].
In recent years, the concept of green environmental protection has received increasing attention [8,9]. Green synthesis of AgNPs, using plant extracts as reducing and stabilizing agents, provides a sustainable and eco-friendly alternative [10]. Previous studies have demonstrated the versatility of green-synthesized NPs in various applications. For instance, Ru-template gold NPs exhibited potential in anticancer applications [11], while bromelain-loaded AgNPs showed potent antibacterial activity [12]. Furthermore, green synthesis strategies have also been used to design multifunctional nanomaterials with enhanced stability and reduced environmental impact [13]. While these studies highlight the potential of green synthesis, they primarily focus on single functionalities or specific agents. There remains a significant gap in developing multifunctional NPs by leveraging underutilized natural resources. AgNPs, in particular, are known for their broad-spectrum biological activities, including antibacterial, antifungal, antioxidant, and anticancer properties [14,15,16,17,18]. Their anticancer effects have been reported in various tumors, such as lung, colon, and liver cancers [19,20,21], primarily through mechanisms such as induction of reactive oxygen species (ROS), mitochondrial dysfunction, and apoptosis. While these findings highlight the versatility of AgNPs, there remains a gap in integrating such functionalities into green-synthesized systems, particularly those leveraging underutilized natural resources. To address this, we focus on the green synthesis of AgNPs using Ginkgo biloba seed extracts (GBSEs), a minimally exploited but phytochemically rich resource.
Ginkgo biloba seeds (GBS) are a rich plant resource known for their medicinal and nutritional values, exhibiting antioxidant, anticancer, and antibacterial activities [22]. However, their application is limited by the presence of toxic components such as ginkgo toxin, 4′-O-methylpyridoxine, and cyanides [23]. Studies have shown that heat treatment can effectively reduce these toxic compounds, yielding safer GBSEs [23,24]. GBSE, enriched with carbohydrates, proteins, and phytochemicals, represents a promising multifunctional reducing and stabilizing agent for green synthesis. Based on this potential, this study focuses on utilizing GBSE to develop an efficient green synthesis method for AgNPs and explores their applications in multifunctional domains such as antibacterial, antifungal, and antioxidant materials.
This research proposes an economic, eco-friendly, and multifunctional green nanotechnology method leveraging underutilized natural resources, addressing a significant gap in current green synthesis approaches. By utilizing GBSE as a unique reducing and stabilizing agent, this study explores an innovative application of a minimally exploited yet phytochemically rich resource. Unlike previous studies that primarily focus on single functionalities or extensively studied plant extracts, our approach integrates antibacterial, antifungal, antioxidant, and anticancer properties into a single NP system, thus advancing the multifunctionality of green-synthesized AgNPs. By optimizing the synthesis conditions through single-factor experiments and response surface methodology (RSM), stable and uniformly distributed AgNPs were successfully prepared. Their physical and chemical properties were systematically characterized using ultraviolet visible (UV-Vis) spectroscopy, scanning electron microscopy (SEM)/energy dispersive X-ray spectroscopy (EDS), transmission electron microscopy (TEM), X-ray diffraction (XRD), and Fourier transform infrared (FTIR). Functional evaluations demonstrated that GBSE-AgNPs exhibit significant antibacterial activity, with minimum inhibitory concentrations (MIC) and minimum bactericidal concentrations (MBC) confirming their efficacy against Staphylococcus aureus (Sa) and Escherichia coli (E. coli). Furthermore, GBSE-AgNPs showed potent antifungal activity against common pathogenic fungi, including Aspergillus fumigatus, Aspergillus niger, Aspergillus flavus, and Penicillium marneffei. Antioxidant assessments using the diphenyl-2-trinitrophenylhydrazine (DPPH) method revealed superior free radical scavenging capabilities compared to GBSE alone. Notably, anticancer evaluations indicated that GBSE-AgNPs exhibited significant antiproliferative effects against cancer cell lines, inducing ROS generation, mitochondrial dysfunction, and apoptosis. These findings highlight the potential of GBSE-AgNPs in the development of antibacterial, antifungal, antioxidant, and anticancer agents. By leveraging underutilized natural resources and integrating multiple functionalities into a single green-synthesized system, this study not only provides new insights into the application of plant resources in green nanotechnology but also underscores the sustainability and practical applications of GBSE-AgNPs in biomedical and environmental fields.
2 Materials and methods
2.1 Materials
GBS was provided by Guosheng Biotechnology (Shaanxi, China). 1.1 DPPH (>97%), silver nitrate (0.5 M), 1-phenyl-3-methyl-5-pyrazolone (PMP, ≥99%), trifluoroacetic acid (TFA, ≥97%), and anhydrous ethanol were purchased from China National Pharmaceutical Group Chemical Reagent Co., Ltd (Shanghai, China). The Milli-Q system was used to obtain deionized water. Staphylococcus aureus, Escherichia coli, Aspergillus niger, Aspergillus fumigatus, Aspergillus flavus, and Penicillium marneffei are all provided by the Microbiology Laboratory of Shaoxing Central Hospital.
2.2 Collection of plant extracts
GBS was purchased from Guosheng Biotechnology. The specific method is as follows: Take fresh ginkgo biloba fruit, remove excess flesh, and rinse thoroughly. 600 mL of deionized water and 40 g of crushed ginkgo biloba fruit were added to a round-bottom flask, and connected to a condenser tube to heat at 70–100°C. The water extract was extracted for 2 h each time for three consecutive times, and three separate water extracts were collected, concentrated to 1/20 of the original volume through a rotary evaporator, and then the concentrated liquid was spray dried, screened with a 100 μm screen, and the powder was collected for use and dried for storage.
2.3 Green synthesis of GBSE-AgNPs
100 mg GBSE was mixed with 100 mL deionized water to obtain 1 mg/mL GBSE solution. 5 mL AgNO3 (0.5 M) and 95 mL deionized water were added to a brown flask to obtain 0.025 M AgNO3 diluent, which was stored away from light. And then, 1 mg/mL GBSE solution and 0.025 M AgNO3 solution were mixed in a 50 mL beaker. The reaction was performed at 90°C with continuous magnetic stirring for 3 h.
2.4 Single factor analysis (SFA)
To optimize the green synthesis of GBSE-AgNPs, single-factor analysis was performed to investigate the effects of reaction time, reaction temperature, and reaction ratio on the synthesis process [25].
2.4.1 Reaction time
1 mg/mL of GBSE solution and 0.025 M of AgNO3 diluent were mixed in a 40:1 ratio and placed in a 50 mL beaker with a reaction system of 40 mL. The reaction temperature was 90°C, and the absorbance changes were observed at 30, 60, 120, 180, and 240 min, respectively.
2.4.2 Reaction temperature
1 mg/mL of GBSE solution and 0.025 M of AgNO3 diluent were mixed in a 40:1 ratio (GBSE:AgNO3, v/v) and placed in a 50 mL beaker with a reaction system of 40 mL. The reaction time was 180 min, and the absorbance changes were observed at 60, 70, 80, 90, and 95°C, respectively.
2.4.3 Reaction ratio
1 mg/mL of GBSE solution and 0.025 M of AgNO3 diluent are divided into 10, 20, 40, 80, 160: The volume ratio (GBSE:AgNO3, v/v) of 1 is mixed (GBSE concentration is 2 mg/mL at 80:1, GBSE concentration is 4 mg/mL at 160:1), and placed in a 50 mL beaker with a reaction system of 40 mL. The reaction temperature was 90°C, and the absorbance change was observed in 180 min.
2.5 RSM
The green synthesis conditions of GBSE-AgNPs include time (30, 60, 120, 180, 240 min), temperature (60, 70, 80, 90, 95°C), and volume ratio (10, 20, 40, 80, 160:1). These conditions were optimized by SFA. Then, by using Design-Expert 10 software, Box-Behnken method was used to optimize the response surface with three factors and three levels [26].
2.6 Verification of optimal conditions
By using Design-Expert 10 software, the response surface method was used to optimize the optimal value of the predicted synthesis conditions. The absorbance of GBSE-AgNPs under different conditions can be predicted by using the prediction formula. To verify the accuracy of the predicted results, three independent experiments were conducted to observe whether the actual absorbance of GBSE-AgNPs was statistically different from the predicted value.
2.7 Characterization of GBSE-AgNPs
2.7.1 UV-Vis spectrum
The synthetic GBSE-AgNPs were validated by UV-Vis in the scanning wavelength range of 300–600 nm. The GBSE-AgNPs solution was diluted to different concentrations with deionized water, and the characteristic peaks were observed. Then, the silver ion concentration at different concentrations was measured by inductively coupled plasma emission spectrometry (ICP-OES), and the concentration–absorbance curve was drawn for subsequent detection.
2.7.2 SEM
A small amount of freeze-dried GBSE-AgNPs samples were directly glued to the conductive adhesive, and gold was sprayed for 45 s with 10 mA by Quorum SC7620 sputtering coater. Then, a ZEISS Sigma 300 SEM was used to photograph the morphology of the sample and spot scan of the energy spectrum. The accelerated voltage during the morphology shooting was 3 kV, and the accelerated voltage during the energy spectrum shooting was 15 kV. The detector was a SE2 secondary electron detector.
2.7.3 XRD and X-ray photoelectron spectroscopy (XPS)
The crystal structure of GBSE-AgNPs was verified by XRD (Bruker D8 Advance, Germany) and characterized at a working voltage of 40 kV, a tube current of 40 mA, a scanning range of 10–80°, Cu-kα as a ray source, and a scanning speed of 2°/min. The GBSE-AgNPs solution was freeze-dried to obtain the powder, 100 mg of the powder was placed in the center of a fluted glass substrate, diluted with a slide, and then tested.
After taking an appropriate amount of sample, paste it on the sample tray, and put the sample into the sample chamber of XPS (Thermo Scientific K-Alpha) instrument. When the pressure of the sample chamber is less than 2.0 × 10−7 mbar, the sample is sent to the analysis chamber, the spot size is 400 μm, and the working voltage is 12 kV. Filament current 6 mA; Full spectrum scanning energy 150 eV, step size 1 eV; Fine spectrum scanning has a throughput of 50 eV and a step size of 0.1 eV.
2.7.4 High-resolution transmission electron microscopy (HRTEM)
The particle size and morphology of GBSE-AgNPs were analyzed by HRTEM (JEOL JEM-F200, Japan), and the elemental composition of GBSE-AgNPs surface was analyzed by energy spectrometer. Part of the sample was dispersed into ethanol solution for ultrasound, and then a few drops of the dispersed liquid were added to the copper net one by one. After drying, the accelerated voltage was 200 kV and the energy spectrum model was JED-2300T. The morphology and energy spectrum line scanning were taken.
2.7.5 FTIR spectroscopy
The organic functional groups of GBSE-AgNPs were analyzed by FTIR spectrometer (Nicolet iS5 FTIR spectrometer, Thermo Fisher scientific Co. Ltd, USA). The absorption bands were studied in the range of 4,000–400 cm−1. The GBSE-AgNPs powder 1 mg obtained from the lyophilized solution was mixed with KBr powder 100–200 mg and pressed into a transparent sheet. The slices were scanned by infrared spectroscopy with a scanning range of 4,000–400 cm−1.
2.7.6 Monosaccharide composition analysis
Set Mannose, Ribose, Rhamnose, Glucuronic acid, Galacturonic acid, N-acetyl-glucosamine, Glucose, and N-acetyl-galactose aminosus, Galactose, Xylose, Arabinose, Fucose are weighed and dissolved in ultra-pure water to prepare a mixed reference solution. The final concentration of the mixed reference solution was 0.4 mg/mL. GBSE-AgNPs (10.0 mg) and trifluoroacetic acid (TFA, 2 M, 5.0 mL) were added into a 10 mL hydrolysis tube, sealed with N2 (10 L/min, 1 min), and then hydrolyzed in a 110°C oven for 2 h. After cooling, open the lid, take out 1 mL and add 1 mL methanol, then dry with N2 in a water bath at 70°C, repeat adding methanol and dry with N2 twice to remove TFA. Adding 1 mL of 0.3 mol/L NaOH solution to fully dissolve the residue, the polysaccharide hydrolysate was determined after some dilution.
400 μL of mixed monosaccharide standard solution or polysaccharide hydrolysate was taken into 5 mL stoppered test tube, and 400 μL of PMP (1-phenyl-3-methyl-5-pyrazolone) methanol solution was added, and then mixed in swirl. Reaction was carried out in 70°C water bath for 2 h. Remove and allow it to cool to room temperature. Add 400 μL of 0.3 M of HCl to neutralize (pH 6–7). Add 1,200 μL of water and an equal volume of chloroform, mix thoroughly by swirling and shaking, then allow the mixture to stand. Discard the chloroform phase. Repeat the extraction twice. The aqueous phase was filtered by 0.45μm microporous membrane (water system) for high-performance liquid chromatography (HPLC) sampling analysis.
2.7.7 Analysis of particle size and zeta potential
The particle size distribution and zeta potential of GBSE-AgNPs were measured by dynamic light scattering (DLS) (Malvern Zetasizer Nano ZS90, DLS) analyzer. First, 1 mL of GBSE-AgNPs solution was ultrasounded for 5 min, then placed in a specific sample pool to determine the particle size distribution and zeta potential, then examined at 25°C and repeated three times.
2.7.8 Synchronous thermal analysis (using thermogravimetric [TG] and differential scanning calorimetry [DSC])
The thermal stability of the synthesized GBSE-AgNPs was evaluated by TG and DSC characterization using a TGA (German Netzsch STA 449 F3). GBSE-AgNPs obtained after lyophilization were used for TG and DSC analysis. The 20 mg GBSE-AgNPs was placed in the crucible from 30 to 800°C at a rate of 10°C/min and the nitrogen flow rate was 40 mL/min. Nitrogen levels were checked periodically during the analysis.
2.7.9 Time stability and pH stability
10 mL of GBSE-AgNPs diluent was prepared by deionized water and set aside at 4°C. At a specific time, the spectral morphology of GBSE-AgNPs at 300–600 nm and the absorbance at the characteristic wavelength were detected by UV-Vis to verify the time stability.
10 mL of GBSE-AgNPs concentrate was prepared by high-speed cryogenic centrifuge at 12,000 rpm for 10 min. 10 μL GBSE-AgNPs concentrated liquid was fully mixed with 90 μL deionized water with different pH (1–10), and the spectral morphology and absorbance of GBSE-AgNPs at 300–600 nm and characteristic wavelength were detected to verify pH stability.
2.8 Biological application of GBSE-AgNPs
2.8.1 Bacterial resistance performance
Sa and E. coli came from the microbiology laboratory of Shaoxing Central Hospital. The above strains were inoculated in LB liquid medium at 37°C overnight in a shaker. Then, the bacterial precipitation was obtained by centrifuge at low temperature and high speed at 4°C and 4,000 rpm. We diluted the bacterial precipitation with sterile normal saline and made the bacterial solution concentration to 1 × 106 CFU/mL, and set aside.
The paper method was used to verify the bacteriostatic zone [27]. The method is as follows: the heated sterile agar medium was poured into the bacterial culture dish and allowed to cool at room temperature, during which it solidified to form an agar plate. Take a sterile cotton swab with a concentration of 1 × 106 CFU/mL and evenly apply it three times on the agar plate, each time in a different direction. Sterile blank drug-sensitive paper with a diameter of 6 mm was immersed in different concentrations of GBSE-AgNPs, and then added to the AGAR dish containing bacteria, cultured at 37°C for 16–18 h. The size of the sterile area treated by GBSE-AgNPs to bacteria was observed at different concentrations.
Minimal inhibitory concentration (MIC) was investigated by a 96-well plate. First, 100 μL of bacterial solution (1 × 106 CFU/mL) was added into the 96-well plate, and then GBSE-AgNPs of different concentrations were added, so that the final concentration was 0, 0.25, 0.5, 1, 2, 4, 8, 16, 32, 64, and 128 μg/mL at 37°C for 16–18 h. Part of the solution could be seen in the turbid pore chamber. And 5 μL of Azurin indicator liquid (0.016 g dissolved in 100 mL of sterile distilled water) was added and incubated in the dark [28]. Observe the color change of the whole plate, pink is the positive result, and cyan is the running result. A cyan-colored sample located immediately before a sample showing a pink result is identified as the minimum inhibitory concentration (MIC). The minimal bactericidal concentration (MBC) verification was subsequently performed on an agar plate. The cyan result sample before the indicator color was pink was selected, and the sample was coated on an agar plate with a sampling needle dipped in bacterial solution, and cultured at 37°C for 16–18 h. Colony formation was observed [29]. The concentration of the colony-free group was MBC, and all operations were performed three times.
Mix 1 mL of bacterial solution (1 × 106 CFU/mL) with GBSE-AgNPs (128 μg) at 37°C and place overnight in a rocking bed. Then, the bacterial precipitation was obtained by centrifuge at low temperature and high speed at 4°C and 4,000 rpm. The precipitation was collected, washed twice with PBS, the supernatant was discarded, and 2.5% glutaraldehyde fixing solution at room temperature was slowly added along the tube wall and fixed overnight. A few samples were added to the copper net, dried naturally, and observed by TEM.
2.8.2 Antifungal activity
Four common environmental fungi, such as Aspergillus fumigatus, Aspergillus niger, Aspergillus flavus, and Penicillium marneffei, were selected for study. A few fungi were selected with sampling needle and inoculated on Sabouraud’s agar plate, cultured at 37°C for 48 h. Next we added 20 μL of GBSE-AgNPs (50, 100, and 200 μg/mL) with different concentrations on the Sabouraud’s agar plate and evenly applied it with a coating stick. Taking the edge of the fungal colony as the target, 1.5 × 1.5 mm2 bacteria cake was selected with the inoculum gun and inoculated on the Sabouraud’s agar plate containing GBSE-AgNPs at 37°C for 48 h. The change in fungal size was observed [30].
2.8.3 Anticancer ability
SGC-7901 cells at the logarithmic growth stage were inoculated in 96-well plates at a density of 3 × 103 cells/well, and cultured in a humid environment composed of 5% CO2 at 37°C for 24 h. GBSE-AgNPs solutions of different concentrations (0, 1, 2, 3, 4, 5, 6, 7, and 8 μg/mL) prepared with DEME complete medium were added to each well at 100 μL/well. Each group was tested in triplicate. After culture for 24 h, discard the old medium, add 100 μL of 10% CCK-8 solution in the dark, and continue to culture for 1 h. Absorbance at 450 nm was measured with a microplate reader. Cell survival rate (%) was calculated [31,32]. The following formula is used to measure the percentage of rejection:
where A 0 is the negative control group and A 1 is the GBSE-AgNPs group.
Apoptosis experiments were performed with Annexin V-FITC/7-AAD fluorescent double-stain apoptosis detection kit [32]. SGC-7901 cells in the logarithmic growth phase were inoculated in 24-well plates at a density of 5 × 104 cells/well, and cultured in a humid environment composed of 5% CO2 at 37°C for 24 h. The cells were digested with 0.25% pancreatic enzyme without EDTA, centrifuged at 300 × g for 5 min, the supernatant was discarded, the cells were collected, washed once with PBS, and the cells were gently suspended, centrifuged at 300g for 5 min, and the supernatant was discarded. The cells were washed with PBS once, the supernatant was discarded after centrifugation, and the cells were re-suspended by adding 500 μL of diluted 1× Annexin V Binding Buffer. The cell suspension was added with 5 μL Annexin V-FITC stain and 5 μL 7-AAD stain (100 μg/mL). After gentle swirl mixing, incubated at room temperature and away from light for 15–20 min and then immediately studied with the machine.
2.8.4 Free radical scavenging detection
The bleaching rate of 1.1-diphenyl-2-trinitrohydrazine (DPPH) was detected at characteristic wavelengths indicating free radical scavenging tests [33]. The hydrogen atom or electron donor potential of the sample was determined by bleaching the purple DPPH ethyl alcohol solution. The DPPH solution containing 5, 10, 20, 30, 40, 50 μg/mL of GBSE-AgNPs was obtained by adding different concentrations of GBSE-AgNPs to 1 mL of DPPH (0.4 mM) ethanol solution. The combination was vigorously stirred in the dark for 30 min, then the absorbance of the resulting solution was measured using a 517 nm spectrophotometer. Ascorbic acid was the positive control group, deionized water was the negative control group. The following formula is used to measure the percentage inhibition of DPPH free radical scavenging activity:
2.8.5 Ferric reducing antioxidant power (FRAP) assay
The ferric reducing power was measured as described by Cherian et al. [34]. To 190 µL of FRAP solution (TPTZ [2,4,6-tri(2-pyridyl)-1,3,5-triazine]; 10 mM), acetate buffer (300 mM, pH 3.6), and ferric chloride hexahydrate (FeCl3·6H2O; 20 mM) in a 10:1:1 (v/v/v) ratio], 10 µL of GBSE-AgNPs was added and incubated at room temperature for 15 min. The absorbance at 630 nm was recorded, and the reducing antioxidant effect was determined as Trolox equivalent antioxidant capacity (TEAC).
2.8.6 ABTS antioxidant assay
The ABTS assay was conducted according to the protocol outlined by Cherian et al. [34]. The reaction mixture consisted of equal proportions of 7 mM ABTS salt and 2.5 mM potassium persulfate, which was kept in the dark for 14–16 h. Before adding the GBSE-AgNPs, the absorbance was measured at 734 nm and adjusted to 0.7. Different concentrations of GBSE-AgNPs were then added to the reaction mixture and incubated at room temperature for 15 min under dark conditions. The antioxidant effect was assessed using TEAC, and the absorbance was measured at 734 nm (Trolox C equivalent antioxidant capacity, mM).
2.9 Statistical analysis
Data are expressed as mean value ± standard deviation. The data were statistically analyzed by one-way ANOVA and paired T-test. The sample size for each statistical analysis was n = 3, and significance was defined as p < 0.05.
3 Results and discussion
3.1 Green synthetic GBSE-AgNPs
The GBSE obtained from GBS was prepared into GBSE solution with a concentration of 1 mg/mL. Subsequently, the AgNO3 solution with a concentration of 0.5 M is configured to a concentration of 0.025 M. The conditions for the first attempt are as follows: volume ratio 40:1 (GBSE:AgNO3, v/v), temperature 90°C, reaction time 3 h. As exhibited in Figure 1a, through UV-Vis spectroscopy, we found that obvious characteristic absorption peaks were visible in the range of 300–600 nm, and SPR appeared at ∼415 nm. To prepare the samples, the GBSE-AgNPs were subjected to high-speed centrifugation at 12,000 rpm for 10 min. Following centrifugation, the samples were diluted to various concentrations. Notably, there was no significant shift in the SPR peak across the different concentrations. Then, we collected solutions (GBSE-AgNPs) of different concentrations, analyzed the silver concentration by ICP-OES, and plotted a “concentration–absorbance” curve (Figure 1b). In addition, we also observed that the color gets darker and darker over time during the synthesis process, indicating the successful formation of GBSE-AgNPs, (Figure 1c). We speculate that GBSE-AgNPs, have been preliminarily synthesized. Subsequently, we demonstrated the SPR of H2O, AgNO3, GBSE, and GBSE-AgNPs by UV-Vis spectra in Figure 1d. We put forward the possible mechanism of AgNPs synthesis from plant extracts in Figure 1e. Silver ions (Ag+) are reduced to silver atoms (Ag0) and gradually aggregate into small AgNPs. During this process, phytochemicals restrict the growth of AgNPs, preventing silver from coming close to each other at the nanoscale, resulting in the formation of small AgNPs [17].

Synthesis diagram and optimization of GBSE-AgNPs. (a) UV-vis spectra of preliminary synthesized AgNPs. (b) Absorbance of AgNPs at different concentrations. (c) Color and absorbance values of AgNPs at various time points. (d) UV-vis spectra of the synthesized precursors. (e) Schematic illustration of the AgNP synthesis mechanism. (f)–(h) SFA. (i) Response surface plots showing the interaction effects on absorbance value between two factors: time and ratio, temperature and ratio, and time and temperature. Time and ratio (i a), temperature and ratio (i b), and time and temperature (i c).
3.2 Single factor experimental analysis
3.2.1 Reaction time
The effect of reaction time on the synthesis process was studied by measuring the absorbance change at different times (30, 60, 120, 180, and 240 min). The remaining conditions are set as follows: temperature 90°C and volume ratio 40:1 (GBSE:AgNO3, v/v). As presented in Figure 1f, the formation of GBSE-AgNPs was confirmed by detecting the change in absorbance at ∼415 nm. The results proved that the absorbance changes rapidly with the extension of time (30–240 min). The absorbance changes slowly within 30–60 min. In the period of 60–180 min, the absorbance increased significantly, and the fastest speed was achieved at 120 min, while the value of absorbance increased slowly at 180 min, which seemed to reach a plateau. This may be the reason that in the beginning, there is rapid nucleation, forming many small particles. Over time, these particles grow by consuming more Ag ions, and the number of available ions decreases, slowing the rate at which new particles form and grow. As small particles form, this can lead to an initial increase in particle concentration, followed by a slower growth phase as larger particles grow at the expense of smaller ones [35,36]. At time point of 240 min, the absorbance further improved, and a significant decrease in the volume of the solution was observed compared to 0 min. Considering the decrease in the solution, there was a concentration phenomenon, which affected the absorbance value. It may also be due to the dissolution of smaller NPs after sufficient nucleation, while larger NPs grow faster, which may lead to an observed increase in absorbance [37]. Therefore, considering various factors, the optimal reaction time for RSM is chosen as 180 min.
3.2.2 Reaction temperature
The effect of reaction temperature on synthesis was studied by measuring the absorbance change at different temperatures (60, 70, 80, 90, and 95 min). The other synthesis conditions are fixed. As shown in Figure 1g, when the temperature is 60–80°C, no obvious absorption wavelength can be seen, so the reaction can be considered incomplete. With the increase in reaction temperature, the absorbance value increases gradually, and the absorption peak appears. When the temperature level is 90°C, the absorbance value increases significantly, which shows the dependence of reaction temperature in the preparation process of GBSE-AgNPs. This acceleration is mainly due to the enhancement of the kinetics of the reduction reaction. When heated, the energy provided increases the reaction rate, promoting faster reduction of silver ions (Ag0) to silver atoms, and the subsequent nucleation and growth of NPs [38]. However, at 95°C, the maximum absorption peak occurs blue shift, indicating that higher temperature will increase the reaction rate of silver ion reduction to form NPs. This faster reaction rate can lead to smaller particle sizes and result in a blue shift of the surface plasmon resonance (SPR) peak. This indicates that the size of small particles is larger and may still be in the process of nucleation [39,40]. Therefore, the optimal reaction temperature of RSM is 90°C.
3.2.3 Reaction ratio
The effect of reaction ratio on synthesis was studied by detecting the absorbance change at different ratios (10, 20, 40, 80, and 160:1). The other conditions are fixed. As demonstrated in Figure 1h, no obvious SPR was observed when the ratio was 80:1 and 160:1, which may be due to the excessive concentration of GBSE, which covered up the peak of GBSE-AgNPs. Because when the ratio is increased to 80:1 and 160:1, the solubility of GBSE is exceeded, and there will be powder precipitation after standing. At the ratio of 10:1, 20:1, and 40:1, the absorbance values show a proportional dependence, and when the ratio is set at 40:1, the absorbance value is the largest. In addition, the absorbance of 40:1 ratio is larger than that of 80:1 ratio. Therefore, the optimal response ratio for RSM is 40:1.
Therefore, based on the above results, the experimental conditions for RSM are as follows: reaction time was 180 min, reaction temperature was 90°C, and reaction ratio (GBSE:AgNO3, v/v) was 40:1.
3.3 RSM
As the SFE above shows, only one variable can be studied at a time, and this approach fails to account for synergies between variables. In order to solve above problem, this study adopts response surface analysis (RSM) based on SFE, and optimizes the experimental conditions according to the actual situation. The conditions of RSM experiment design include reaction time, reaction temperature, and the volume ratio of GBSE to AgNO3. Based on the results of SFE, the initial reaction conditions were set as follows: reaction time 180 min, temperature 90°C, and ratio (GBSE:AgNO3, v/v) 40:1.
3.3.1 Box-Behnken design
According to the SFE results, three-factor and three-level analysis was carried out through the RSM design, and the design was depicted in Table S1. The reaction ratio is 20:1 in low level, 80:1 in high water, and 50:1 in the middle as system generation. The reaction temperature is 85°C in low level, 95°C in high water, and 90°C in the middle for system generation. The reaction time was 120 min in low level, 240 min in high water, and 180 min in the middle of system generation. With the absorbance value as the response value, 17 tests were analyzed, and the results were exhibited in Table S2.
3.3.2 Model fitting and variance analysis
Regression analysis was performed on the data in Table S2, and the prediction equation was obtained as follows:
where A represents the reaction volume ratio, B represents the reaction temperature, and C represents the reaction time. According to the absolute value of the coefficient before the factor in the equation [41], it is shown that the changes affecting the absorbance value are as follows: reaction temperature > reaction time > reaction proportion.
Through the quadratic model variance analysis on the data in Table S2, the results are demonstrated in Table S3, F-value = 10.41, p < 0.01, and the model is not significant. The Lack of Fit P-value of 0.1167 suggests that the Lack of Fit is not significant relative to the pure error, indicating that the model fits the experimental data well. The R-squared value of 0.9305 indicates that 93.05% of the variability in the response can be explained by the model. The adjusted R-squared value of 0.841 accounts for the number of predictors and suggests a very good fit of the model. Therefore, the model can replace the actual detection to study and predict the synthesis of GBSE-AgNPs process. In addition, according to Table S3, B, C, AC, and B2 were significant factors (p < 0.05). The high F-values of B (40.51) and C (21.41) indicate that these factors have a significant effect on the variability of absorbance values. B has the greatest influence on the absorbance value, F-value is 40.51, p-value is 0.0004, and the significance level is the highest. C is second, F-value is 21.41, p-value is 0.0024, which is also significant. A has a certain effect, but it is not as significant as B and C. F-value is 3.80 and p-value is 0.0923, which is close to the significant level. In addition, the steepness of the 3D graph can be used to predict the influence of two factors on the response value [42]. As can be seen from the 3D response surface and contour map in Figure 1Ia, the slope of the surface is small and the color gradient changes gently. This suggests that the interaction between time and proportion has a relatively small effect on the response value. As can be seen from the 3D response surface and contours in Figure 1Ib, the slope of the surface is large, and the color gradient changes rapidly, especially in the region of high temperature and high proportion. This indicates that the interaction between temperature and proportion has a large influence on the response value. As can be seen from the 3D response surface and contour diagram of Figure 1Ic, the slope of the surface is small, and the color gradient changes gently. This suggests that the interaction between temperature and time has a relatively small effect on the response value. Through comprehensive analysis of the 3D response surface map and contour map, as well as the results of variance analysis, the following conclusions can be drawn: reaction temperature > reaction time > reaction proportion. In the interaction, the interaction between temperature and proportion (AC) has a significant effect on the response value. In the quadratic term, temperature (B²) has a significant effect on the response value. Therefore, in the process of RSM and experimental optimization, we should focus on the control of temperature and time to optimize the response value.
3.3.3 Verification of optimal conditions
According to the prediction equation given by the Design-Expert 10 software, the optimal synthesis conditions can be obtained: reaction temperature, reaction proportion, and reaction time are 94.725°C, 23.165 (v/v), and 235.890 min, respectively. According to the actual situation, the synthesis conditions were adjusted to the reaction temperature, proportion, and time of 94.7°C, 23.2 (v/v), and 236 min, respectively. Three experiments were conducted under these conditions, and the absorbance value was 0.9707. The predicted value is 0.9611, which is basically consistent with the actual value, which indicates the feasibility and accuracy of the model in predicting the optimal conditions for GBSE-AgNPs synthesis.
3.4 Characterization of GBSE-AgNPs
The synthesis of GBSE-AgNPs was exhibited in Figure 2a. The morphology and elemental composition of the synthesized GBSE-AgNPs were investigated by SEM and EDS. SEM imaging shows that the spherical NPs have a uniform size distribution, as demonstrated in Figure 2b. EDS analysis confirmed the elemental purity of the synthesized GBSE-AgNPs. The EDS spectra exhibited a characteristic peak corresponding to silver (Ag), which has an atomic percentage (%) of 19.87% and a weight percentage (%) of 65.53%. Elements such as C, N, O, S, and P were also detected, indicating that there may be GBSE components on the GBSE-AgNPs. Combined with SEM and EDS results, the morphology and elemental composition of the synthesized GBSE-AgNPs were characterized in detail, which confirmed that there were multiple elements in the NPs, and silver elements accounted for a relatively large proportion, and the morphology of the NPs was regular.
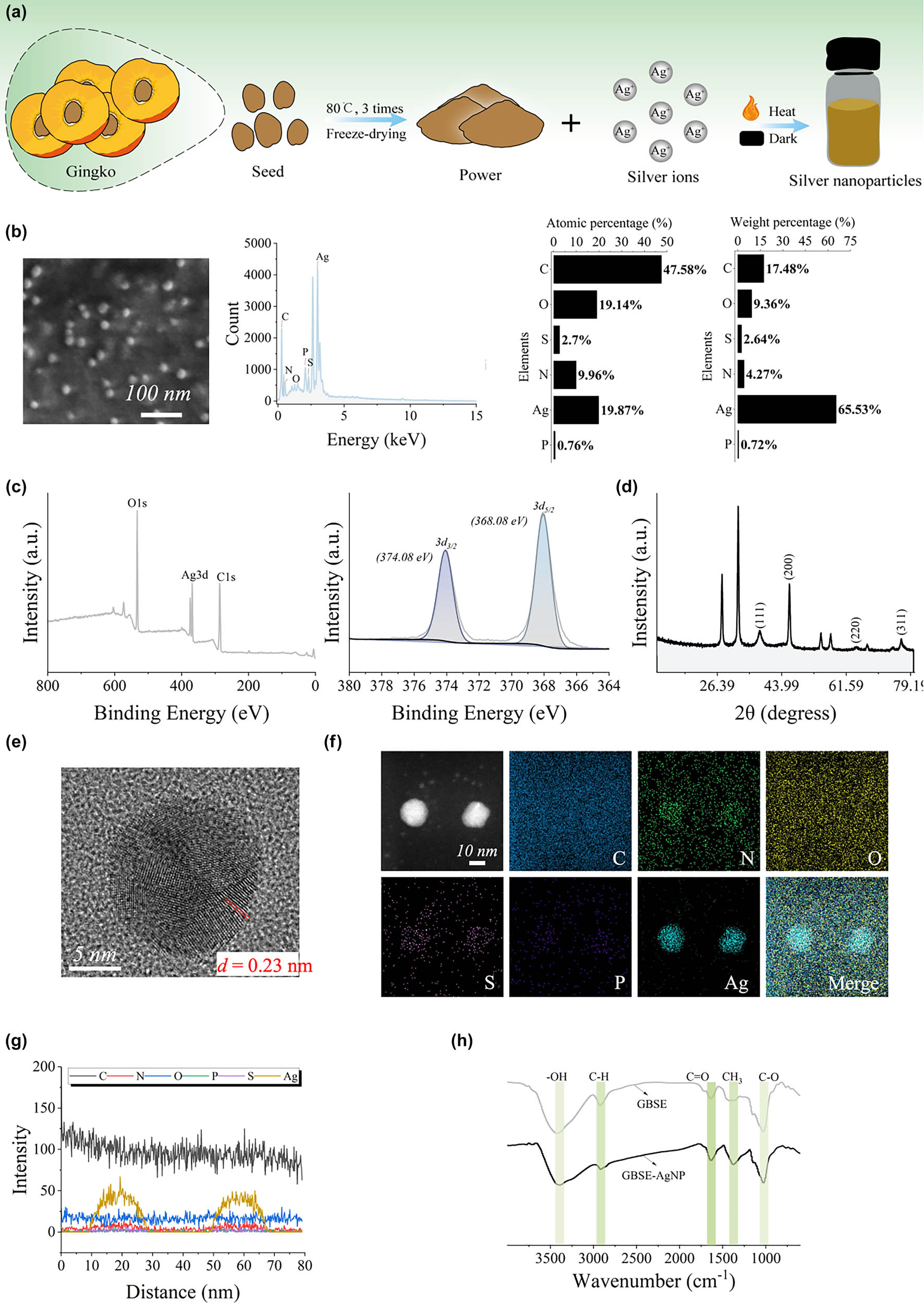
Characterization and stability of GBSE-AgNPs. (a) Synthesis diagram of GBSE-AgNPs. (b) SEM/EDS of AgNPs. (c) Full spectrum and high-resolution XPS spectra. (d) XRD pattern. (e) Lattice fringes observed under HRTEM. (f) EDS mapping mode. (g) Line scan mode. (h) FTIR spectrum.
Subsequently, the chemical composition and crystalline properties of the synthesized AgNPs (GBSE-AgNPs) were studied by XRD and XPS. XPS analysis revealed the elemental composition and chemical state of the synthesized GBSE-AgNPs. As demonstrated in Figure 2c, the spectra exhibited characteristic peaks corresponding to Ag 3d. The fineness XPS spectra of Ag 3d proved that the binding energy has two distinct peaks at ∼368.08 and ∼374.08 eV, corresponding to Ag 3d5/2 and Ag 3d2/3, respectively. The observed binding energy is consistent with that of the metal silver, confirming the metallic properties of the synthesized GBSE-AgNPs [27]. The crystallographic properties of the synthesized GBSE-AgNPs were studied by XRD. The XRD pattern demonstrated characteristic diffraction peaks at 2θ values of 37.94°, 46.10°, 64.56°, and 76.59°, corresponding to the (111), (200), (220), and (311) crystal planes of face-centered cubic (FCC) silver, respectively (Figure 2d). Among these planes, the (111) diffraction peak exhibited the highest intensity, indicating a preferential growth orientation along this plane, which is commonly observed in FCC AgNPs. The combination of XPS and XRD analyses unequivocally confirmed the metallic state, high purity, and well-defined crystallinity of GBSE-AgNPs. These findings highlight the efficiency of GBSE in facilitating the green synthesis of stable and crystalline AgNPs [43].
HRTEM combined with EDS was employed to characterize the structural features and elemental composition of the synthesized AgNPs (GBSE-AgNPs). The HRTEM images (Figure 2e) revealed well-defined lattice fringes on the NP surface, with an interplanar spacing of d = 0.23 nm, determined via fast Fourier transform analysis. This value is consistent with the lattice spacing reported for FCC silver, further confirming the crystalline structure of the GBSE-AgNPs [44]. Furthermore, elemental mapping conducted using EDS (Figure 2f) demonstrated the uniform distribution of silver throughout the NP samples. In addition to silver, minor traces of other elements, such as carbon (C), nitrogen (N), phosphorus (P), and sulfur (S), were detected, likely originating from the plant extract used in the synthesis process. Energy spectrum line scanning analysis (Figure 2g) further verified the homogeneous distribution of silver, emphasizing the high purity and uniformity of the synthesized NPs.
FTIR spectroscopy was employed to investigate the functional groups and chemical bonds of the synthesized GBSE-AgNPs. The FTIR spectra of both GBSE and GBSE-AgNPs are presented in Figure 2h. Several characteristic peaks were observed, providing insight into the chemical interactions between GBSE and the synthesized AgNPs [45,46]: Hydroxyl Groups (−OH): A broad absorption band at 3,200–3,500 cm⁻¹ in both GBSE and GBSE-AgNPs corresponds to the stretching vibrations of hydroxyl (−OH) groups. This suggests the presence of hydroxyl-containing compounds in GBSE that might have interacted with the silver ions during the reduction and stabilization process. A peak observed near 2,900 cm⁻¹ indicates the stretching vibrations of aliphatic and aromatic C–H bonds, confirming the presence of organic compounds from GBSE on the surface. A prominent peak at 1,600–1,700 cm⁻¹ corresponds to the stretching vibrations of carbonyl (−C═O) groups. These groups, likely derived from carboxylic acids, aldehydes, or ketones in GBSE, play a crucial role in the reduction of silver ions to AgNPs. Peaks in the region of 1,000–1,300 cm⁻¹ are attributed to the stretching vibrations of C–O and C═O bonds, which further confirm the involvement of carbohydrates or proteins in the synthesis process. The FTIR spectra of GBSE-AgNPs exhibit many peaks similar to those in GBSE, indicating the retention of functional groups from GBSE. However, slight shifts in peak positions and intensities (e.g., in the −OH and −C═O regions) suggest interactions between GBSE components and the AgNPs, confirming the role of GBSE as both a reducing and stabilizing agent. The FTIR analysis demonstrates the successful incorporation of GBSE components on the AgNP surface. The observed functional groups indicate the involvement of hydroxyl, carboxyl, and other organic functionalities in the reduction and stabilization process. These findings corroborate the multifunctionality of GBSE-AgNPs, where the retained organic groups contribute to their biological activities, such as antibacterial, antifungal, and antioxidant properties.
At present, studies on GBS polysaccharides are limited, and their products are often composed of single sugars, such as d-mannose, which are extracted in low yields (approximately 0.9%) through complex processes [47]. To simplify the synthesis process and maximize the retention of bioactive components, GBSE was chosen over isolated polysaccharides. The monosaccharide composition of the synthesized GBSE-AgNPs was analyzed using HPLC. As shown in Figure 3a and b, glucose was identified as the most abundant monosaccharide (95.2%), followed by minor components such as mannose (0.5%), ribose (0.4%), rhamnose (0.5%), glucuronic acid (2.9%), galactose (0.2%), and arabinose (0.2%). These findings are further detailed in Table S4, which provides a comprehensive breakdown of the monosaccharide composition. The high glucose content aligns with the hydrolysis of starches present in GBS, contributing a significant amount of reducing sugars essential for the reduction of silver ions [48]. The HPLC data are consistent with FTIR analysis (Figure 2h), which identified key functional groups such as hydroxyl (−OH) and carbonyl (−C═O) on the surface of GBSE-AgNPs. These groups, derived from carbohydrates and other phytochemicals, are instrumental in both the reduction of Ag⁺ to Ag0 and the stabilization of the resulting NPs. Notably, polysaccharides, as indicated by the glucose and mannose contents, play dual roles as reducing agents and stabilizers, which enhance the stability and bioactivity of the synthesized AgNPs. By utilizing GBSE, this study achieves a dual advantage: simplifying the synthesis process compared to isolated polysaccharides and leveraging the multifunctional phytochemical composition of GBSE, which includes proteins, carbohydrates, and secondary metabolites. These components synergistically contribute to the green synthesis process, as well as to the antibacterial, antioxidant, and antifungal properties of the synthesized AgNPs.
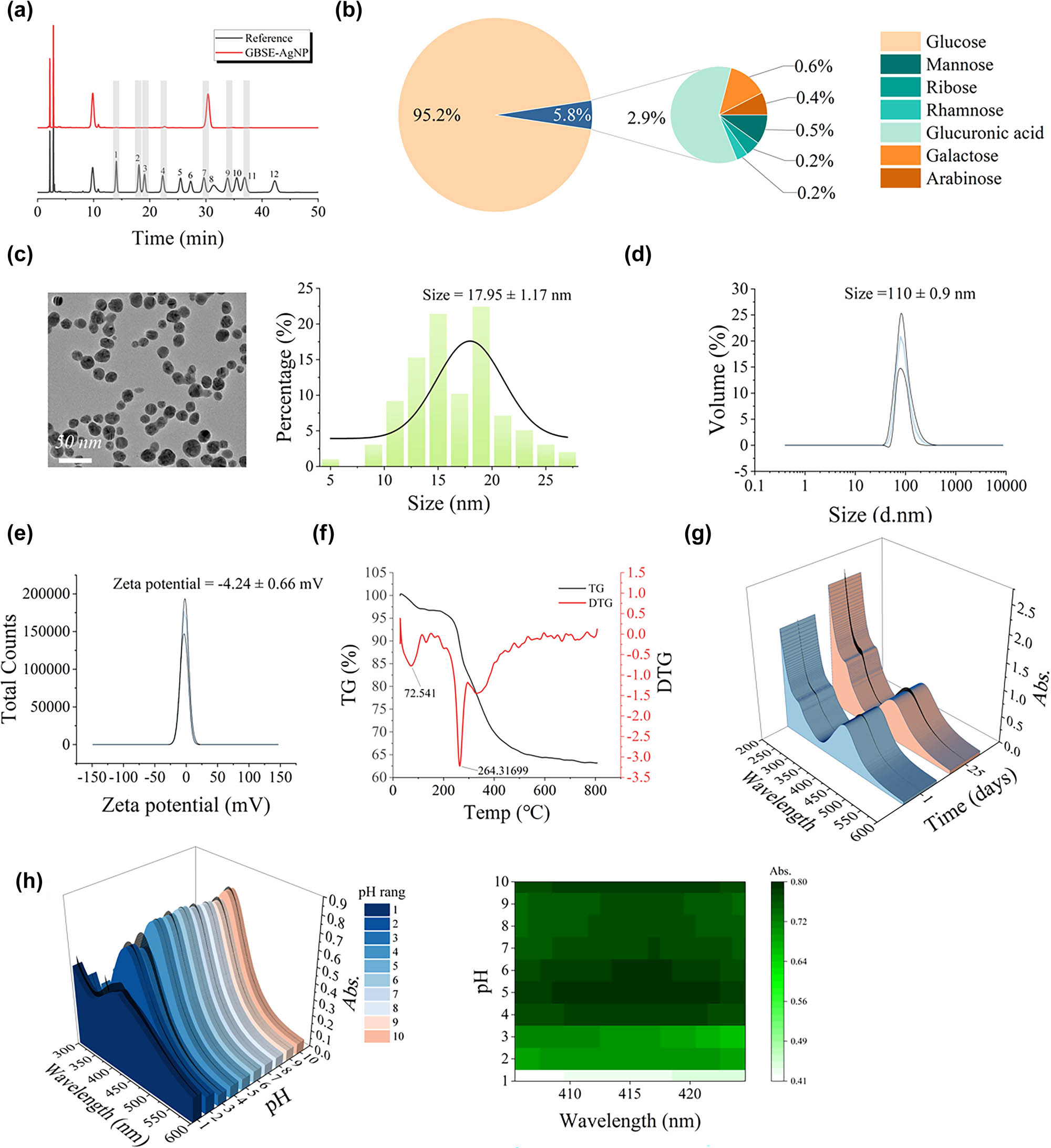
Characterization and stability of GBSE-AgNPs. (a) and (b) HPLC analysis of monosaccharide components. (c) Particle size observed under HRTEM. (d) DLS analysis of zeta potential. (e) DLS analysis of particle size. (f)–(h) TG analysis, time stability, and pH stability of AgNPs.
HRTEM analysis revealed that the synthesized GBSE-AgNPs possess a uniform spherical morphology with a well-defined size distribution (Figure 3c). The average core particle size, as determined by HRTEM, was approximately 17.95 ± 1.17 nm, confirming the nanometer-scale dimensions of the AgNPs. DLS measurements further provided insights into the hydrodynamic behavior of GBSE-AgNPs in solution. The DLS analysis revealed a unimodal particle size distribution, with an average hydrodynamic diameter of 110 ± 0.9 nm (Figure 3d). The larger hydrodynamic diameter compared to the HRTEM-measured size is attributed to the hydration shell and potential interactions with surface-bound biomolecules, such as proteins and polysaccharides derived from GBSE. The ζ-potential of GBSE-AgNPs was measured as −4.24 ± 0.66 mV (Figure 3e), indicating a moderately negative surface charge. This negative charge is primarily attributed to the presence of functional groups such as carboxylic (−COOH) and hydroxyl (−OH) groups on the NP surface. These functional groups not only enhance colloidal stability by providing electrostatic repulsion but also play a key role in the biofunctionalization and stabilization of the NPs during the green synthesis process.
To investigate the thermal stability and decomposition behavior of GBSE-AgNPs, TG analysis was performed. The TG curve presents a gradual mass loss curve in the temperature range from 25 to 800°C (Figure 3f). Below 200°C, a slow weight loss (∼5%) is observed, which can be attributed to the removal of physically adsorbed water molecules and volatile plant extract components. In the temperature range of 200–300°C, a more pronounced weight loss (∼15%) occurs, primarily due to the thermal decomposition of organic compounds from GBSE that serve as stabilizing and reducing agents in the synthesis process. At ∼264°C, the DTG curve exhibits a maximum decomposition rate, reflecting the breakdown of hydrogen bonds and the cleavage of carbon chains in GBSE-derived organic matter. Above 300°C, the mass loss rate stabilizes, with residual weight attributed to the thermally stable metallic Ag core, indicating the high thermal stability of the synthesized GBSE-AgNP. Notably, the absence of any significant exothermic peak in the DSC curve further supports the conclusion that GBSE-AgNPs exhibit high purity and stability.
The time stability and pH stability of GBSE-AgNPs were analyzed by UV-VIS spectroscopy. As exhibited in Figure 3g, the SPR peak of GBSE-AgNPs was monitored throughout day 1 and day 25, and its position and intensity were found to be basically unchanged. Consistent SPR peak properties indicate that the optical properties of GBSE-AgNPs remain constant over time, reflecting its stability to aggregation or degradation.
A previous study demonstrated that when the human body is infected, the pH of the environment in the body changes subtly [46], with blood gas analysis measuring a pH of 6.1 ± 0.6. In the spectral results and heat map analysis of pH = 3.0–10.0 (Figure 3h), the maximum absorbance value is basically unchanged. When pH < 3, the maximum absorbance value decreased significantly, which may be due to the protonation and aggregation of carboxyl groups on the surface of GBSE-AgNPs under acidic conditions. This suggests that GBSE-AgNPs can maintain stable performance in both normal and inflammatory pH ranges.
3.5 Biological performance of GBSE-AgNPs
3.5.1 Antioxidant activities of biosynthesized GBSE-AgNPs
In Figure 4a, DPPH free radical scavenging experiments proved that GBSE-AgNPs possess the dose-dependent scavenging activity on DPPH free radicals. With the increase in GBSE-AgNPs concentration, the inhibitory percentage of DPPH free radical scavenging activity also increased. The scavenging activity of GBSE-AgNPs was lower than that of positive control ascorbate, but higher than that of GBSE, indicating its effective oxygen free radical scavenging ability. Evaluation of oxygen free radical scavenging activity is essential to evaluate the antioxidant potential of GBSE-AgNPs. The ability of GBSE-AgNPs to scavenge DPPH free radicals highlights their potential applications as antioxidants in various industries, including pharmaceuticals, cosmetics, and food preservation.
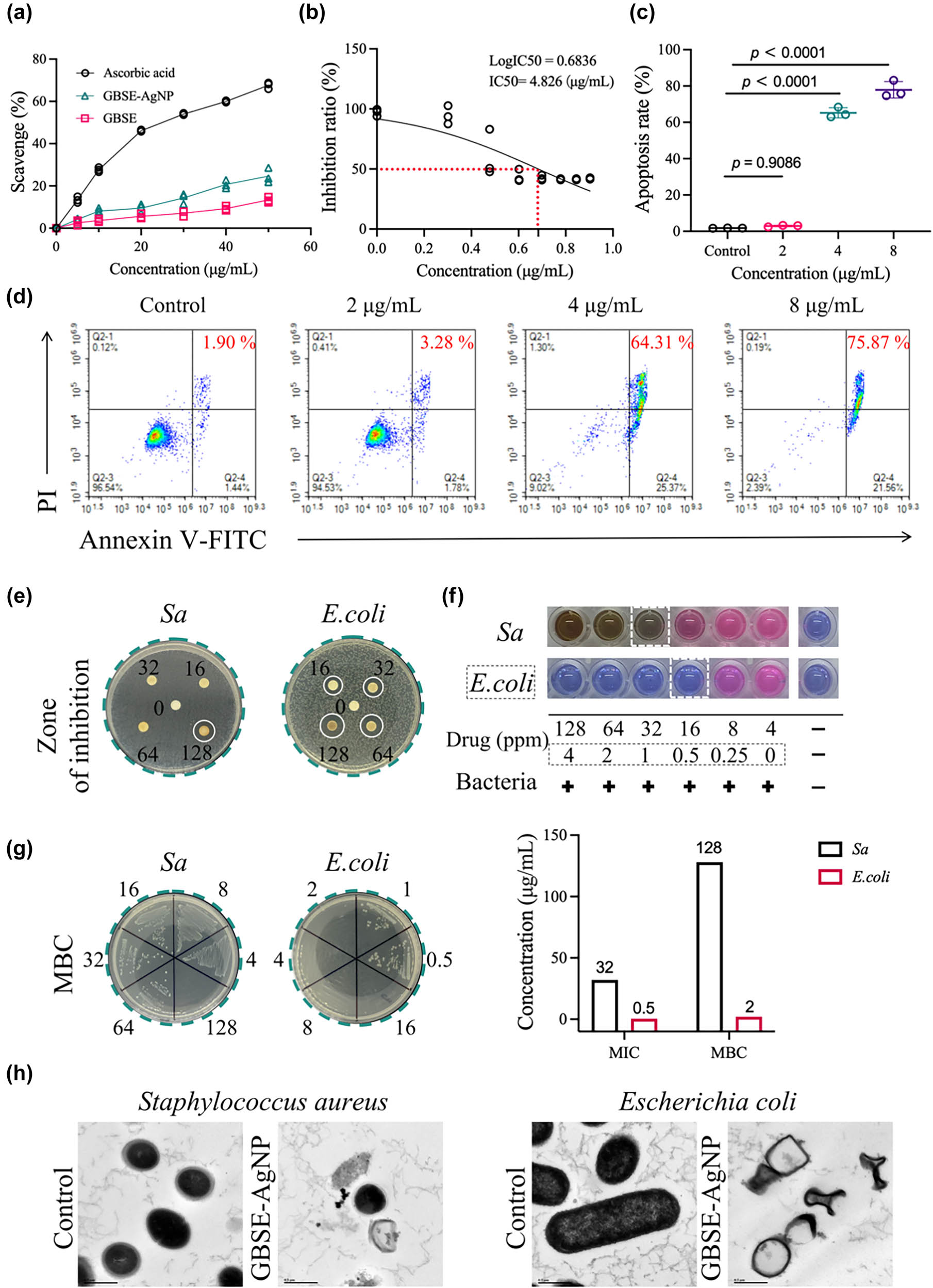
Bioactivity of GBSE-AgNPs. (a) Antioxidant activity measured using the DPPH assay. (b) IC50 value for gastric cancer cell line 7901. (c) and (d) Analysis of apoptosis. (e) Antibacterial activity assessed using the inhibition zone method. (f) MIC. (g) MBC. (h) Bacterial viability observed under HRTEM.
FRAP assay results (Figure S1) demonstrate the concentration-dependent ferric reducing antioxidant activity (TEAC values) of GBSE, GBSE-AgNPs, and ascorbic acid (positive control). Across all tested concentrations (5–50 µg/mL), ascorbic acid consistently exhibited the highest antioxidant activity, with TEAC values increasing up to approximately 600 µM at the highest concentration (50 µg/mL). In contrast, the TEAC values of GBSE and GBSE-AgNPs were significantly lower. At 50 µg/mL, the TEAC value of GBSE-AgNPs reached approximately 150 µM, while GBSE only achieved 70 µM. This indicates that GBSE-AgNPs demonstrated a modest but statistically significant improvement in antioxidant activity compared to GBSE (**p < 0.01). However, both GBSE and GBSE-AgNPs were markedly less potent than ascorbic acid (# p < 0.0001). Notably, the enhancement in antioxidant activity observed for GBSE-AgNPs can be attributed to the synergistic interaction between AgNPs and the bioactive phytochemicals in GBSE, which likely enhances their reducing power.
The antioxidant activity of GBSE, GBSE-AgNPs, and ascorbic acid was evaluated using the ABTS radical scavenging assay, and the results are presented in Figure S2. The data demonstrate that GBSE-AgNPs exhibit a dose-dependent antioxidant activity, with increasing concentrations (5–50 μg/mL) resulting in a significant increase in TEAC values (μM). The activity of GBSE-AgNPs was lower than that of the positive control (ascorbic acid), but surpasses that of the GBSE extract alone. And this indicated the enhancement of antioxidant properties. The statistical significance (*p > 0.05, # p > 0.0001) clearly illustrates that ascorbic acid remains the most potent antioxidant among the tested groups. GBSE-AgNPs exhibit significantly higher antioxidant activity compared to GBSE, supporting the hypothesis that the bioreduction and capping process involving GBSE contributes to the enhancement of the free radical scavenging potential. The superior antioxidant activity of GBSE-AgNPs can be attributed to the synergistic interaction between AgNPs and the bioactive phytochemicals present in GBSE. These bioactive compounds, including phenolics and flavonoids, are known to donate electrons, stabilizing free radicals, and enhancing antioxidant activity. The dose-dependent increase in activity underscores the potential of GBSE-AgNPs as a promising antioxidant agent [49,50], which could be explored further for applications in oxidative stress-related disorders.
3.5.2 Anti-cancer ability
As exhibited in Figure 4b, CCK-8 detection showed that GBSE-AgNPs had a dose-dependent inhibitory effect on cell viability in SGC-7901 cells. With the increase in GBSE-AgNPs concentration, the percentage of cell viability inhibition also increased, indicating that AgNPs has cytotoxic effect on cancer cells, IC = 4.826 μg/mL. Similarly, apoptosis assay exhibited a significant increase in apoptotic cells after treatment with GBSE-AgNPs compared to the control group, suggesting that induction of apoptosis is the mechanism of action for the anticancer activity of AgNPs (Figure 4c and d). The ability of GBSE-AgNPs to inhibit cell proliferation and induce apoptosis highlights their promise as anticancer agents for further preclinical and clinical studies. Similar anticancer mechanisms have been reported for AgNPs synthesized using other plant extracts, such as A. acuminata leaf which also demonstrated dose-dependent inhibition and apoptosis induction in gastric cancer cells [51]. However, compared to previously reported IC50 values for AgNPs synthesized using other biological agents (IC50 = 21.05 μg/mL) [52], GBSE-AgNPs exhibit superior potency (IC50 = 4.826 μg/mL), suggesting the unique contribution of the GBSE phytochemicals in enhancing anticancer activity. This underscores the potential advantage of using underutilized resources like GBSE for AgNP synthesis.
3.5.3 Antibacterial activity
A disk assay was used to evaluate the antibacterial activity of GBSE-AgNPs against bacterial strains (Sa and E. coli). As exhibited in Figure 4e, in the bacterial Petri dish containing Sa, obvious bacteria-free zone was observed only around the paper with a concentration of 128 μg/mL GBSE-AgNPs, indicating the antibacterial effect of GBSE-AgNPs on Sa. However, no significant bacteria-free zone was observed around the other concentrations (16, 32, 64 μg/mL). In the bacterial Petri dish containing E. coli, the clear bacteria-free zone was observed around the paper with the concentration of 16, 32, 64,128 μg/mL of GBSE-AgNPs, indicating that GBSE-AgNPs possess a strong antibacterial effect on E. coli.
The MIC and MBC values of GBSE-AgNPs were determined by half dilution method. Specifically, MIC was defined as the lowest concentration of GBSE-AgNPs that completely inhibited visible bacterial growth, while MBC was determined to be the lowest concentration that caused bacterial death. The MIC and MBC values of GBSE-AgNPs for Sa were 32 and 128 μg/mL, respectively, and the MIC and MBC values of GBSE-AgNPs for E. coli were 0.5 and 2 μg/mL, respectively. These results are comparable to or exceed those reported for AgNPs synthesized using Flos Sophorae Immaturus extract and tansy extract, where MIC values for Sa and E. coli ranged between 31.25 and 31.3 μg/mL [53,54]. Interestingly, the significantly lower MIC for GBSE-AgNPs against E. coli suggests that the unique composition of GBSE, particularly the presence of functional groups such as carboxyl or hydroxyl groups [1], may enhance the interaction of NPs with Gram-negative bacteria. This highlights the potential of GBSE as an efficient reducing and stabilizing agent for producing potent antibacterial NPs. The above results demonstrated that GBSE-AgNPs possess strong antibacterial and bactericidal activities against both Gram-positive and Gram-negative bacteria (Figure 4f and g).
Subsequently, we employed HRTEM to examine the morphological changes in bacteria exposed to GBSE-AgNPs. It can be seen that after treatment with GBSE-AgNPs, the bacterial morphology underwent significant changes, including cell membrane damage, cytoplasmic leakage, and cell deformation (Figure 4h). These findings elucidate the antimicrobial mechanism of GBSE-AgNPs, which predominantly involves the disruption of bacterial cell membranes and intracellular components. Such mechanisms are consistent with previous reports on AgNPs synthesized using alternative green methods, further highlighting the enhanced efficacy of GBSE-derived AgNPs [1]. The interaction between GBSE-AgNPs and bacterial cells could be attributed to the presence of functional groups identified in the FTIR analysis, such as hydroxyl and carboxyl groups, which facilitate strong adhesion to bacterial membranes and subsequent cell damage [1].
Furthermore, the morphological changes of bacterial cells exposed to GBSE-AgNPs were observed by SEM. As demonstrated in Figure 5a, the uniform analysis of silver elements on the surface of Staphylococcus aureus proved that atomic percentage (%) was 0.2% and weight percentage (%) was 0.8%. Energy spectrum line scans demonstrated a homogenous distribution of silver across the bacterial surface, supporting the hypothesis that GBSE-AgNPs effectively adhere to the bacterial membrane, facilitating their antibacterial mechanism through membrane disruption and intracellular component leakage. Further analysis by energy spectrum line sweep indicate that the distribution of silver element is uniform. Similarly, EDS data of E. coli are revealed in Figure 5b. Scattered silver elements can be analyzed in E. coli, with atomic percentage (%) of 0.4% and weight percentage (%) of 1.2%. Energy spectrum line scanning further confirmed the uniform distribution of silver elements on E. coli surfaces, emphasizing the ability of GBSE-AgNPs to achieve effective and consistent silver deposition on diverse bacterial species. These findings suggest that the uniform adhesion and deposition of GBSE-AgNPs on bacterial surfaces play a crucial role in their bactericidal activity.
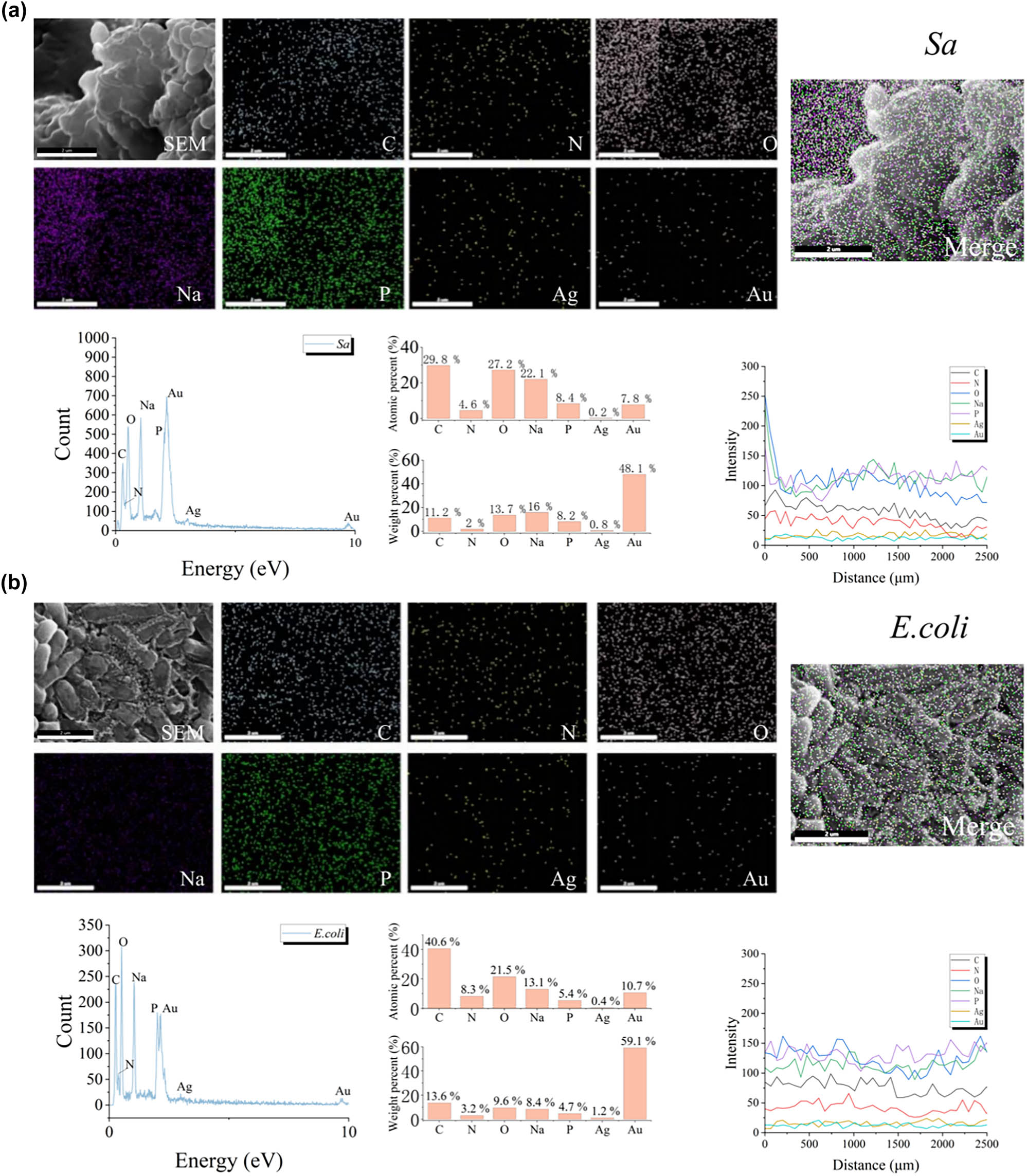
SEM/EDS analysis of Sa (a) and E. coli (b) showing the distribution of silver elements on their surfaces after reaction with GBSE-AgNPs.
3.5.4 Antifungal activity
Four common environmental fungal strains, including Aspergillus niger, Aspergillus fumigatus, Aspergillus flavus, and Penicillium marneffei, were selected to evaluate the antifungal activity of silver nanoparticles. All four fungi are widely present in the environment and are the most common pathogenic fungi in humans [55]. The antifungal activity of GBSE-AgNPs was evaluated by agar diffusion method. As shown in Figure 6, the growth of fungal colonies on Sabouraud’s agar plates containing GBSE-AgNPs was visually observed and compared with control plates without GBSE-AgNPs. Changes in fungal size, morphology, and growth inhibition were qualitatively assessed. GBSE-AgNPs exhibited dose-dependent inhibition of fungal growth. Smaller fungal volumes were observed around the pores containing 50 μg/mL GBSE-AgNPs than in the control group, but were not statistically significant. The fungal volume of 100 and 200 μg/mL GBSE-AgNPs treated pores was significantly reduced compared to the control group, indicating that they had effective antifungal activity against all fungal strains tested. The diameter of the inhibition zone was positively correlated with the concentration of GBSE-AgNPs.
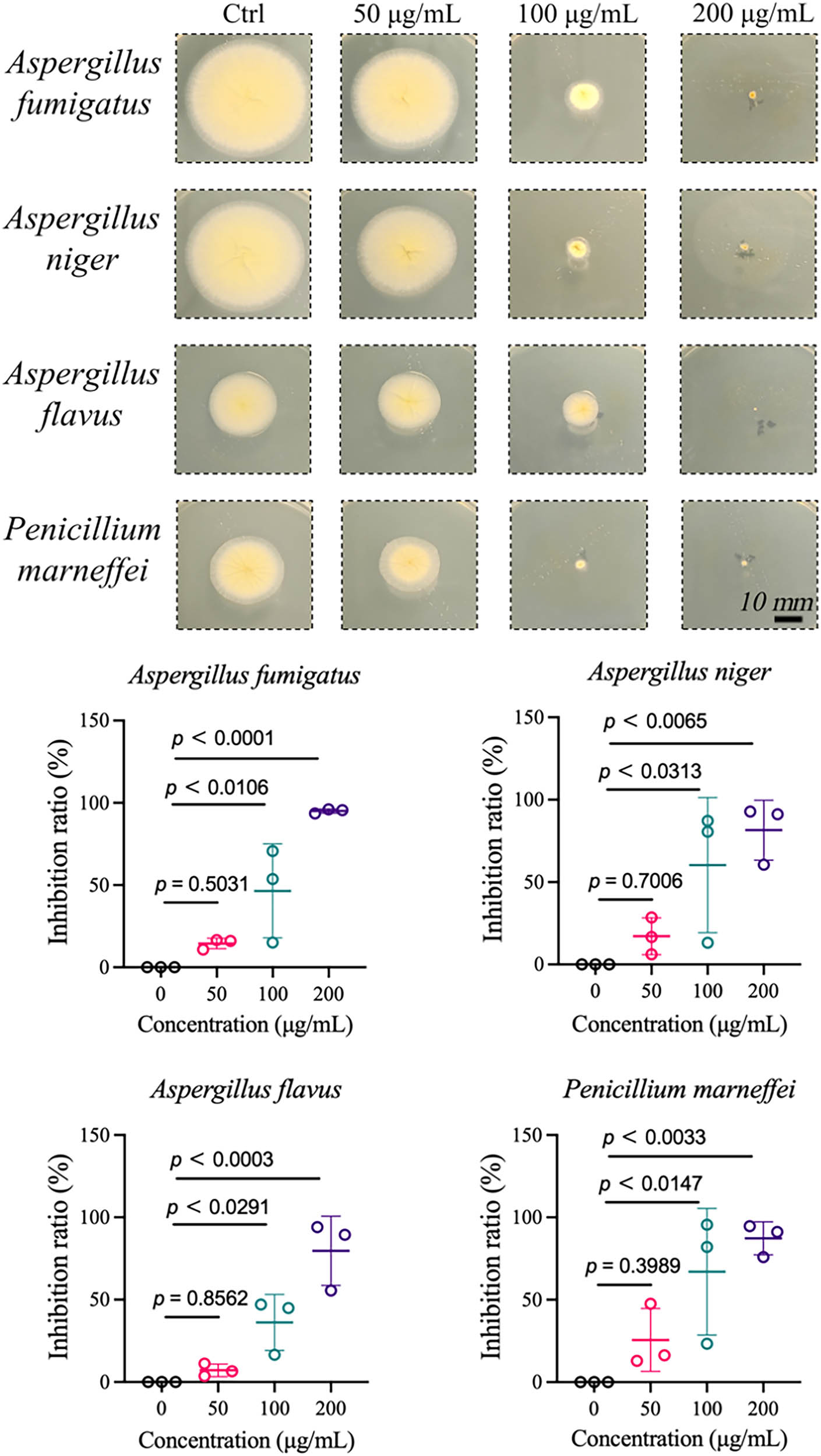
Antifungal activity of GBSE-AgNPs.
Evaluating the antifungal activity of GBSE-AgNPs against common environmental fungal strains is critical for developing effective antifungal strategies applicable in various settings, including indoor environments, healthcare facilities, and agricultural practices. Understanding the antifungal properties of GBSE-AgNPs provides valuable insights into their potential to mitigate fungal contamination and prevent fungus-related diseases.
Silver-containing NPs are widely recognized for their broad-spectrum and potent antimicrobial activities [56]. Even at low concentrations, they exhibit efficacy against diverse pathogenic microorganisms, including bacteria such as E. coli, Klebsiella pneumoniae, and Sa, as well as fungi such as Candida albicans and Aspergillus niger. In this study, the synthesized GBSE-AgNPs demonstrated exceptional antibacterial and antifungal activities, expanding the application scope of AgNPs as antimicrobial agents.
Unlike conventional antibiotics, which are increasingly associated with microbial resistance, AgNPs exhibit multiple antimicrobial mechanisms, leading to limited reports of resistance development [57]. This property highlights their advantage over conventional antimicrobials and underscores their importance in addressing the growing challenge of antimicrobial resistance. Currently, several proposed mechanisms explain the antimicrobial effects of AgNPs. As illustrated in Figure 7, 1. AgNPs continuously release silver ions, which inhibit microbial growth by interfering with key cellular processes [58]. 2. Silver ions denature ribosomes in the cytoplasm, inhibiting protein synthesis [59]. 3. Silver ions adhere to the bacterial cell wall and plasma membrane via electrostatic attraction and their affinity for thiol-containing proteins, increasing membrane permeability and causing cellular envelope destruction [60]. 4. Once internalized, silver ions inactivate respiratory enzymes, produce ROS, and disrupt ATP production, leading to oxidative stress and cellular damage [61]. 5. Silver ions interact with sulfur and phosphorus in DNA, inhibiting DNA replication, causing abnormal cell division, and ultimately leading to cell death. These multifaceted mechanisms explain the observed potent antimicrobial effects of GBSE-AgNPs and their potential to overcome microbial resistance.
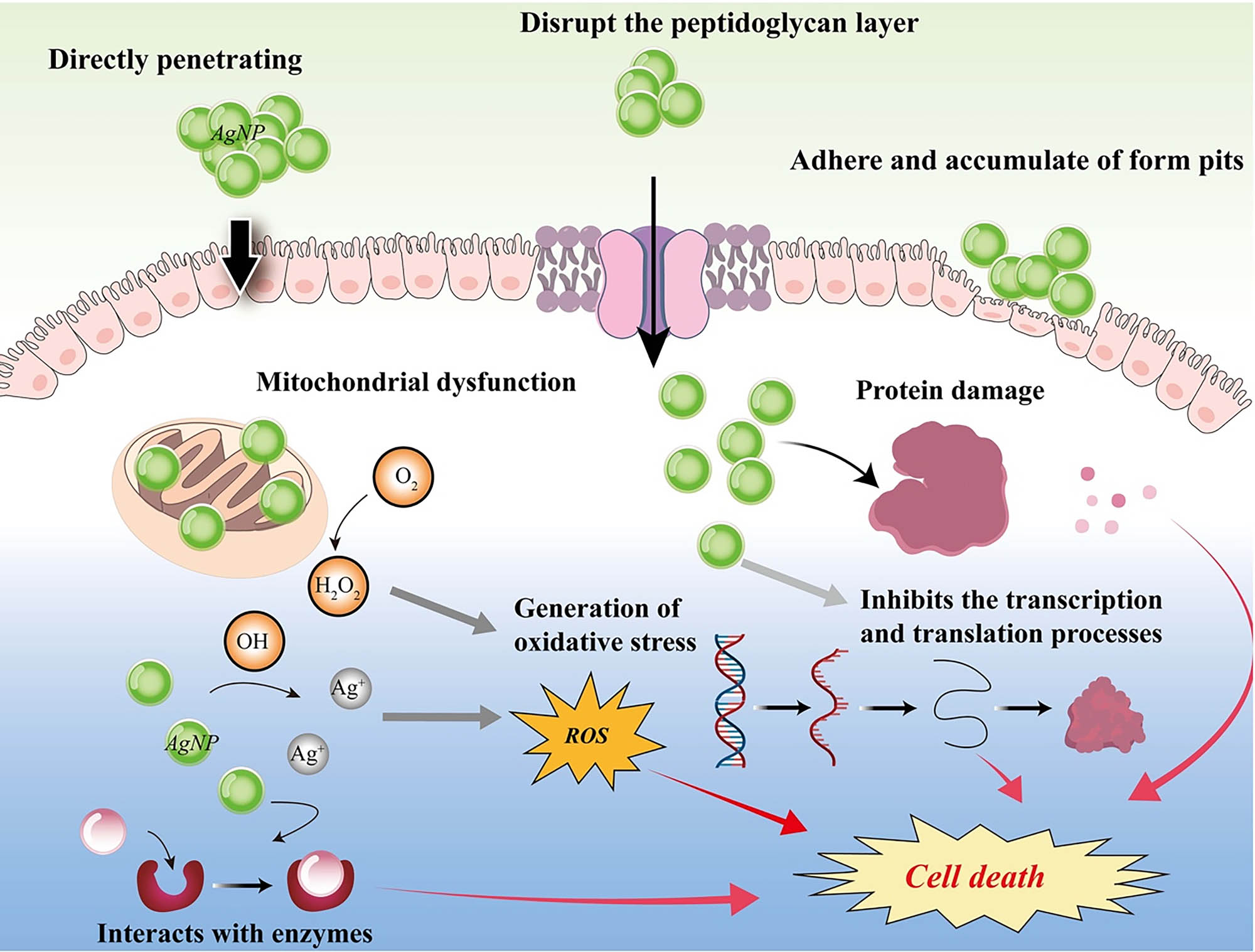
Proposed antibacterial mechanism of GBSE-AgNPs.
Previous studies on GBS are limited due to their low polysaccharide content, predominantly composed of mannose, and the low extraction efficiency of these bioactive compounds. In this study, we directly obtained GBSE via a simple water extraction method and synthesized GBSE-AgNPs. Mannose, the primary polysaccharide component, was confirmed through HPLC analysis. By optimizing the preparation process of GBSE-AgNPs using RSM, this study expands the research scope of GBS and highlights their potential as a novel source of bioactive compounds.
4 Conclusion
This study demonstrates the potential of GBSE as a reducing and stabilizing agent for the green synthesis of AgNPs (GBSE-AgNPs). Using RSM, the synthesis was optimized to achieve NPs with an average size of 17.95 ± 1.17 nm under optimal conditions. Characterization confirmed the involvement of hydroxyl (−OH) and carbonyl (−C═O) groups from GBSE, underscoring its multifunctionality. Compared to GBSE, GBSE-AgNPs exhibited enhanced antioxidant capacity, further showcasing their potential in scavenging free radicals. The GBSE-AgNPs exhibited strong antibacterial activity, with MIC of 0.5 μg/mL for E. coli and 32 μg/mL for Sa. Additionally, significant antifungal inhibition was observed at 100 µg/mL against common environmental fungi (Aspergillus fumigatus, Aspergillus niger, Aspergillus flavus, and Penicillium marneffei). Furthermore, GBSE-AgNPs demonstrated potent anticancer activity by inducing apoptosis in gastric cancer cells (SGC-7901) with an IC50 value of 4.826 µg/mL, highlighting their potential for biomedical applications. Though this study provides a sustainable and cost-effective synthesis method, limitations remain. Further studies are required to elucidate the precise molecular mechanisms involved in the formation of GBSE-AgNPs, which is essential for better control over NP size, monodispersity, and reproducibility. Moreover, additional investigations into the long-term stability and in vivo toxicity of GBSE-AgNPs are necessary to fully validate their biomedical applications. Nonetheless, this research offers valuable insights into the development of eco-friendly, bioactive NPs for biomedical and environmental applications.
Acknowledgments
The authors sincerely thank Dr. Beibei Fan from Henan University and Ms. Rutting Yang from Shaoxing Central Hospital for their valuable technical support.
-
Funding information: This work was supported by the Natural Science Foundation of Zhejiang Province (Grant Nos LBY22H180007 and LBY22H270004), the medical and health research project of Zhejiang province (Grant Nos 2022KY416 and 2023KY374), and Keqiao District Science and Technology Bureau (Grant No. 2023KZ18).
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: All data generated or analyzed during this study are included in this published article.
References
[1] Gong X, Jadhav ND, Lonikar VV, Kulkarni AN, Zhang H, Sankapal BR, et al. An overview of green synthesized silver nanoparticles towards bioactive antibacterial, antimicrobial and antifungal applications. Adv Colloid Interface Sci. 2024;323:103053.10.1016/j.cis.2023.103053Suche in Google Scholar PubMed
[2] Kumar H, Venkatesh N, Bhowmik H, Kuila A. Metallic nanoparticle: a review. Biomed J Sci Tech Res. 2018;4(2):3765–75.Suche in Google Scholar
[3] Xu L, Wang YY, Huang J, Chen CY, Wang ZX, Xie H. Silver nanoparticles: Synthesis, medical applications and biosafety. Theranostics. 2020;10(20):8996–9031.10.7150/thno.45413Suche in Google Scholar PubMed PubMed Central
[4] Vinci G, Rapa M. Noble metal nanoparticles applications: recent trends in food control. Bioeng (Basel). 2019;6(1):10.10.3390/bioengineering6010010Suche in Google Scholar PubMed PubMed Central
[5] Parveen K, Banse V, Ledwani L. Green synthesis of nanoparticles: Their advantages and disadvantages. AIP Conf Proc. 2016;1724(1):020048.10.1063/1.4945168Suche in Google Scholar
[6] Bahrulolum H, Nooraei S, Javanshir N, Tarrahimofrad H, Mirbagheri VS, Easton AJ, et al. Green synthesis of metal nanoparticles using microorganisms and their application in the agrifood sector. J Nanobiotechnol. 2021;19(1):86.10.1186/s12951-021-00834-3Suche in Google Scholar PubMed PubMed Central
[7] Arshad F, Naikoo GA, Hassan IU, Chava SR, El-Tanani M, Aljabali AA, et al. Bioinspired and green synthesis of silver nanoparticles for medical applications: a green perspective. Appl Biochem Biotechnol. 2024;196(6):3636–69.10.1007/s12010-023-04719-zSuche in Google Scholar PubMed PubMed Central
[8] Donga S, Chanda S. Facile green synthesis of silver nanoparticles using Mangifera indica seed aqueous extract and its antimicrobial, antioxidant and cytotoxic potential (3-in-1 system). Artif Cell Nanomed Biotechnol. 2021;49(1):292–302.10.1080/21691401.2021.1899193Suche in Google Scholar PubMed
[9] Wasilewska A, Klekotka U, Zambrzycka M, Zambrowski G, Święcicka I, Kalska-Szostko B. Physico-chemical properties and antimicrobial activity of silver nanoparticles fabricated by green synthesis. Food Chem. 2023;400:133960.10.1016/j.foodchem.2022.133960Suche in Google Scholar PubMed
[10] Sarli S, Kalani MR, Moradi A. A potent and safer anticancer and antibacterial taxus-based green synthesized silver nanoparticle. Int J Nanomed. 2020;15:3791–801.10.2147/IJN.S251174Suche in Google Scholar PubMed PubMed Central
[11] Shabani L, Kasaee SR, Chelliapan S, Abbasi M, Khajehzadeh H, Dehghani FS, et al. An investigation into green synthesis of Ru template gold nanoparticles and the in vitro photothermal effect on the MCF-7 human breast cancer cell line. Appl Phys A. 2023;129:564.10.1007/s00339-023-06832-6Suche in Google Scholar
[12] Gheisari F, Kasaee SR, Mohamadian P, Chelliapan S, Gholizadeh R, Zareshahrabadi Z, et al. Bromelain-loaded silver nanoparticles: formulation, characterization and biological activity. Inorg Chem Commun. 2024;161:112006.10.1016/j.inoche.2023.112006Suche in Google Scholar
[13] Abbasi M, Kasaee SR, Kamyab H, Chelliapan S, Kirpichnikova I, Mussa ZH, et al. Allium hooshidaryae (Alliaceae)-based green-synthesized Fe3O4@ MoS2 core–shell nanoparticles coated with chitosan and investigating their biological properties. Appl Phys A. 2024;130:284.10.1007/s00339-024-07440-8Suche in Google Scholar
[14] Siddiqi KS, Husen A, Rao RAK. A review on biosynthesis of silver nanoparticles and their biocidal properties. J Nanobiotechnol. 2018;16(1):14.10.1186/s12951-018-0334-5Suche in Google Scholar PubMed PubMed Central
[15] Ye M, Yang W, Zhang M, Huang H, Huang A, Qiu B. Biosynthesis, characterization, and antifungal activity of plant-mediated silver nanoparticles using Cnidium monnieri fruit extract. Front Microbiol. 2023;14:1291030.10.3389/fmicb.2023.1291030Suche in Google Scholar PubMed PubMed Central
[16] Slavin YN, Ivanova K, Hoyo J, Perelshtein I, Owen G, Haegert A, et al. Novel lignin-capped silver nanoparticles against multidrug-resistant bacteria. ACS Appl Mater Interfaces. 2021;13(19):22098–109.10.1021/acsami.0c16921Suche in Google Scholar PubMed
[17] Maťátková O, Michailidu J, Miškovská A, Kolouchová I, Masák J, Čejková A. Antimicrobial properties and applications of metal nanoparticles biosynthesized by green methods. Biotechnol Adv. 2022;58:107905.10.1016/j.biotechadv.2022.107905Suche in Google Scholar PubMed
[18] Singh N, Sen Gupta R, Bose S. A comprehensive review on singlet oxygen generation in nanomaterials and conjugated polymers for photodynamic therapy in the treatment of cancer. Nanoscale. 2024;16(7):3243–68.10.1039/D3NR05801HSuche in Google Scholar
[19] Chota A, George BP, Abrahamse H. Apoptotic efficiency of Dicoma anomala biosynthesized silver nanoparticles against A549 lung cancer cells. Biomed Pharmacother. 2024;176:116845.10.1016/j.biopha.2024.116845Suche in Google Scholar PubMed
[20] Bao J, Jiang Z, Ding W, Cao Y, Yang L, Liu J. Silver nanoparticles induce mitochondria-dependent apoptosis and late non-canonical autophagy in HT-29 colon cancer cells. Nanotechnol Rev. 2022;11(1):1911–26.10.1515/ntrev-2022-0114Suche in Google Scholar
[21] Azhar NA, Ghozali SZ, Abu Bakar SA, Lim V, Ahmad NH. Suppressing growth, migration, and invasion of human hepatocellular carcinoma HepG2 cells by Catharanthus roseus-silver nanoparticles. Toxicol Vitro. 2020;67:104910.10.1016/j.tiv.2020.104910Suche in Google Scholar PubMed
[22] Liu XG, Lu X, Gao W, Li P, Yang H. Structure, synthesis, biosynthesis, and activity of the characteristic compounds from Ginkgo biloba L. Nat Prod Rep. 2022;39(3):474–511.10.1039/D1NP00026HSuche in Google Scholar PubMed
[23] Zhang W, Shi M, Wang J, Cao F, Su E. A comprehensive review of ginkgotoxin and ginkgotoxin-5′-glucoside in Ginkgo biloba L. seeds. J Food Compos Anal. 2024;125:105726.10.1016/j.jfca.2023.105726Suche in Google Scholar
[24] Wang H, Shi M, Cao F, Su E. Ginkgo biloba seed exocarp: A waste resource with abundant active substances and other components for potential applications. Food Res Int. 2022;160:111637.10.1016/j.foodres.2022.111637Suche in Google Scholar PubMed
[25] Li H-F, Pan Z-C, Chen J-M, Zeng L-X, Xie H-J, Liang Z-Q, et al. Green synthesis of silver nanoparticles using Phlebopus portentosus polysaccharide and their antioxidant, antidiabetic, anticancer, and antimicrobial activities. Int J Biol Macromol. 2024;254:127579.10.1016/j.ijbiomac.2023.127579Suche in Google Scholar PubMed
[26] Wirwis A, Sadowski Z. Green synthesis of silver nanoparticles: optimizing green tea leaf extraction for enhanced physicochemical properties. ACS Omega. 2023;8:30532–49.10.1021/acsomega.3c03775Suche in Google Scholar PubMed PubMed Central
[27] Chen S, Cai D, Dong Q, Ma G, Xu C, Bao X, et al. Silver nanoparticles-decorated extracellular matrix graft: fabrication and tendon reconstruction performance. Biomater Res. 2023;27(1):85.10.1186/s40824-023-00428-0Suche in Google Scholar PubMed PubMed Central
[28] De Bleeckere A, Van den Bossche S, De Sutter PJ, Beirens T, Crabbé A, Coenye T. High throughput determination of the biofilm prevention concentration for Pseudomonas aeruginosa biofilms using a synthetic cystic fibrosis sputum medium. Biofilm. 2023;5:100106.10.1016/j.bioflm.2023.100106Suche in Google Scholar PubMed PubMed Central
[29] Chen S, Yao J, Huo S, Xu C, Yang R, Tao D, et al. Designing injectable dermal matrix hydrogel combined with silver nanoparticles for methicillin-resistant Staphylococcus aureus infected wounds healing. Nano Converg. 2024;11:41.10.1186/s40580-024-00447-0Suche in Google Scholar PubMed PubMed Central
[30] Ashraf H, Anjum T, Riaz S, Naseem S. Microwave-assisted green synthesis and characterization of silver nanoparticles using Melia azedarach for the management of fusarium wilt in tomato. Front Microbiol. 2020;11:238.10.3389/fmicb.2020.00238Suche in Google Scholar PubMed PubMed Central
[31] Peng L, Sang H, Wei S, Li Y, Jin D, Zhu X, et al. circCUL2 regulates gastric cancer malignant transformation and cisplatin resistance by modulating autophagy activation via miR-142-3p/ROCK2. Mol Cancer. 2020;19:156.10.1186/s12943-020-01270-xSuche in Google Scholar PubMed PubMed Central
[32] Wang Y, He Q, Rong K, Zhu M, Zhao X, Zheng P, et al. Vitamin D3 promotes gastric cancer cell autophagy by mediating p53/AMPK/mTOR signaling. Front Pharmacol. 2023;14:1338260.10.3389/fphar.2023.1338260Suche in Google Scholar PubMed PubMed Central
[33] Al Baloushi KSY, Senthilkumar A, Kandhan K, Subramanian R, Kizhakkayil J, Ramachandran T, et al. Green synthesis and characterization of silver nanoparticles using moringa peregrina and their toxicity on MCF-7 and Caco-2 human cancer cells. Int J Nanomed. 2024;19:3891–905.10.2147/IJN.S451694Suche in Google Scholar PubMed PubMed Central
[34] Cherian T, Ragavendran C, Remesh RKV, Jacob J, Jamal W, Kamaraj C, et al. Bio-functionalization, stabilization and potential functionalities of hyaluronate macromolecules capped copper oxide nanoparticles. J Environ Chem Eng. 2023;11:111051.10.1016/j.jece.2023.111051Suche in Google Scholar
[35] Amirjani A, Firouzi F, Haghshenas DF. Predicting the size of silver nanoparticles from their optical properties. Plasmonics. 2020;15(4):1077–82.10.1007/s11468-020-01121-xSuche in Google Scholar
[36] Pacioni NL, Borsarelli CD, Rey V, Veglia AV. Synthetic routes for the preparation of silver nanoparticles. In: Alarcon EI, Griffith M, Udekwu KI, editors. Silver nanoparticle applications: in the fabrication and design of medical and biosensing devices. Cham: Springer International Publishing; 2015. p. 13–46.10.1007/978-3-319-11262-6_2Suche in Google Scholar
[37] Mahiuddin M, Saha P, Ochiai B. Green synthesis and catalytic activity of silver nanoparticles based on Piper chaba stem extracts. Nanomaterials (Basel). 2020;10(9):1777.10.3390/nano10091777Suche in Google Scholar PubMed PubMed Central
[38] Asefian S, Ghavam M. Green and environmentally friendly synthesis of silver nanoparticles with antibacterial properties from some medicinal plants. BMC Biotechnol. 2024;24(1):5.10.1186/s12896-023-00828-zSuche in Google Scholar PubMed PubMed Central
[39] Adewumi OD, Folahan AA, Oluyomi SA, Oluwasesan MB, Adetunji CO, Oluwakemi JA, et al. Exploring the effect of operational factors and characterization imperative to the synthesis of silver nanoparticles. In: Khan M, editor. Silver nanoparticles. Rijeka: IntechOpen; 2018. p. 9.Suche in Google Scholar
[40] Quintero-Quiroz C, Acevedo N, Zapata-Giraldo J, Botero LE, Quintero J, Zárate-Triviño D, et al. Optimization of silver nanoparticle synthesis by chemical reduction and evaluation of its antimicrobial and toxic activity. Biomater Res. 2019;23:27.10.1186/s40824-019-0173-ySuche in Google Scholar PubMed PubMed Central
[41] Ebba M, Asaithambi P, Alemayehu E. Development of electrocoagulation process for wastewater treatment: optimization by response surface methodology. Heliyon. 2022;8(5):e09383.10.1016/j.heliyon.2022.e09383Suche in Google Scholar PubMed PubMed Central
[42] Liu J, Bai J, Shao C, Yao S, Xu R, Duan S, et al. Optimization of ultrasound-assisted aqueous two-phase extraction of polysaccharides from seabuckthorn fruits using response methodology, physicochemical characterization and bioactivities. J Sci Food Agric. 2023;103(6):3168–83.10.1002/jsfa.12283Suche in Google Scholar PubMed
[43] Sharma NK, Vishwakarma J, Rai S, Alomar TS, AlMasoud N, Bhattarai A. Green route synthesis and characterization techniques of silver nanoparticles and their biological adeptness. ACS Omega. 2022;7:27004–20.10.1021/acsomega.2c01400Suche in Google Scholar PubMed PubMed Central
[44] Mohamad Sukri SNA, Shameli K, Teow SY, Chew J, Ooi LT, Lee-Kiun Soon M, et al. Enhanced antibacterial and anticancer activities of plant extract mediated green synthesized zinc oxide-silver nanoparticles. Front Microbiol. 2023;14:1194292.10.3389/fmicb.2023.1194292Suche in Google Scholar PubMed PubMed Central
[45] Ghaffari-Moghaddam M, Hadi-Dabanlou R, Khajeh M, Rakhshanipour M, Shameli K. Green synthesis of silver nanoparticles using plant extracts. Korean J Chem Eng. 2014;31:548–57.10.1007/s11814-014-0014-6Suche in Google Scholar
[46] Fang B, Qiu P, Xia C, Cai D, Zhao C, Chen Y, et al. Extracellular matrix scaffold crosslinked with vancomycin for multifunctional antibacterial bone infection therapy. Biomaterials. 2021;268:120603.10.1016/j.biomaterials.2020.120603Suche in Google Scholar PubMed
[47] Boateng ID, Yang X-M. Ginkgo biloba L. seed; A comprehensive review of bioactives, toxicants, and processing effects. Ind Crop Prod. 2022;176:114281.10.1016/j.indcrop.2021.114281Suche in Google Scholar
[48] Hassabo AG, Nada AA, Ibrahim HM, Abou-Zeid NY. Impregnation of silver nanoparticles into polysaccharide substrates and their properties. Carbohydr Polym. 2015;122:343–50.10.1016/j.carbpol.2014.03.009Suche in Google Scholar PubMed
[49] Coman NA, Nicolae-Maranciuc A, Berța L, Nicolescu A, Babotă M, Man A, et al. Green synthesis of metallic nanoparticles from Quercus Bark extracts: characterization and functional properties. Antioxid (Basel). 2024;13:822.10.3390/antiox13070822Suche in Google Scholar PubMed PubMed Central
[50] Ahmad S, Ahmad S, Ali S, Esa M, Khan A, Yan H. Recent advancements and unexplored biomedical applications of green synthesized Ag and Au nanoparticles: A review. Int J Nanomed. 2024;19:3187–215.10.2147/IJN.S453775Suche in Google Scholar PubMed PubMed Central
[51] Yuan J, Khan SU, Luo J, Jiang Y, Yang Y, Yan J, et al. Biosynthetic silver nanoparticles inhibit the malignant behavior of gastric cancer cells and enhance the therapeutic effect of 5-fluorouracil by promoting intracellular ROS generation and apoptosis. Pharmaceutics. 2022;14:2109.10.3390/pharmaceutics14102109Suche in Google Scholar PubMed PubMed Central
[52] Salehi S, Shandiz SA, Ghanbar F, Darvish MR, Ardestani MS, Mirzaie A, et al. Phytosynthesis of silver nanoparticles using Artemisia marschalliana Sprengel aerial part extract and assessment of their antioxidant, anticancer, and antibacterial properties. Int J Nanomed. 2016;11:1835–46.10.2147/IJN.S99882Suche in Google Scholar PubMed PubMed Central
[53] Cheng Z, Tang S, Feng J, Wu Y. Biosynthesis and antibacterial activity of silver nanoparticles using Flos Sophorae Immaturus extract. Heliyon. 2022;8:e10010.10.1016/j.heliyon.2022.e10010Suche in Google Scholar PubMed PubMed Central
[54] Radzikowska-Büchner E, Flieger W, Pasieczna-Patkowska S, Franus W, Panek R, Korona-Głowniak I, et al. Antimicrobial and apoptotic efficacy of plant-mediated silver nanoparticles. Molecules. 2023;28:5519.10.3390/molecules28145519Suche in Google Scholar PubMed PubMed Central
[55] Kosmidis C, Denning DW. The clinical spectrum of pulmonary aspergillosis. Thorax. 2015;70(3):270–7.10.1136/thoraxjnl-2014-206291Suche in Google Scholar PubMed
[56] Otari SV, Patil RM, Ghosh SJ, Thorat ND, Pawar SH. Intracellular synthesis of silver nanoparticle by actinobacteria and its antimicrobial activity. Spectrochim Acta A Mol Biomol Spectrosc. 2015;136:1175–80.10.1016/j.saa.2014.10.003Suche in Google Scholar PubMed
[57] Panáček A, Kvítek L, Smékalová M, Večeřová R, Kolář M, Röderová M, et al. Bacterial resistance to silver nanoparticles and how to overcome it. Nat Nanotechnol. 2018;13(1):65–71.10.1038/s41565-017-0013-ySuche in Google Scholar PubMed
[58] Bapat RA, Chaubal TV, Joshi CP, Bapat PR, Choudhury H, Pandey M, et al. An overview of application of silver nanoparticles for biomaterials in dentistry. Mater Sci Eng: C. 2018;91:881–98.10.1016/j.msec.2018.05.069Suche in Google Scholar PubMed
[59] Durán N, Nakazato G, Seabra AB. Antimicrobial activity of biogenic silver nanoparticles, and silver chloride nanoparticles: an overview and comments. Appl Microbiol Biotechnol. 2016;100:6555–70.10.1007/s00253-016-7657-7Suche in Google Scholar PubMed
[60] Khorrami S, Zarrabi A, Khaleghi M, Danaei M, Mozafari MR. Selective cytotoxicity of green synthesized silver nanoparticles against the MCF-7 tumor cell line and their enhanced antioxidant and antimicrobial properties. Int J Nanomed. 2018;13:8013–24.10.2147/IJN.S189295Suche in Google Scholar PubMed PubMed Central
[61] Ramkumar VS, Pugazhendhi A, Gopalakrishnan K, Sivagurunathan P, Saratale GD, Dung TNB, et al. Biofabrication and characterization of silver nanoparticles using aqueous extract of seaweed Enteromorpha compressa and its biomedical properties. Biotechnol Rep. 2017;14:1–7.10.1016/j.btre.2017.02.001Suche in Google Scholar PubMed PubMed Central
© 2025 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- MHD radiative mixed convective flow of a sodium alginate-based hybrid nanofluid over a convectively heated extending sheet with Joule heating
- Experimental study of mortar incorporating nano-magnetite on engineering performance and radiation shielding
- Multicriteria-based optimization and multi-variable non-linear regression analysis of concrete containing blends of nano date palm ash and eggshell powder as cementitious materials
- A promising Ag2S/poly-2-amino-1-mercaptobenzene open-top spherical core–shell nanocomposite for optoelectronic devices: A one-pot technique
- Biogenic synthesized selenium nanoparticles combined chitosan nanoparticles controlled lung cancer growth via ROS generation and mitochondrial damage pathway
- Fabrication of PDMS nano-mold by deposition casting method
- Stimulus-responsive gradient hydrogel micro-actuators fabricated by two-photon polymerization-based 4D printing
- Physical aspects of radiative Carreau nanofluid flow with motile microorganisms movement under yield stress via oblique penetrable wedge
- Effect of polar functional groups on the hydrophobicity of carbon nanotubes-bacterial cellulose nanocomposite
- Review in green synthesis mechanisms, application, and future prospects for Garcinia mangostana L. (mangosteen)-derived nanoparticles
- Entropy generation and heat transfer in nonlinear Buoyancy–driven Darcy–Forchheimer hybrid nanofluids with activation energy
- Green synthesis of silver nanoparticles using Ginkgo biloba seed extract: Evaluation of antioxidant, anticancer, antifungal, and antibacterial activities
- A numerical analysis of heat and mass transfer in water-based hybrid nanofluid flow containing copper and alumina nanoparticles over an extending sheet
- Investigating the behaviour of electro-magneto-hydrodynamic Carreau nanofluid flow with slip effects over a stretching cylinder
- Electrospun thermoplastic polyurethane/nano-Ag-coated clear aligners for the inhibition of Streptococcus mutans and oral biofilm
- Investigation of the optoelectronic properties of a novel polypyrrole-multi-well carbon nanotubes/titanium oxide/aluminum oxide/p-silicon heterojunction
- Novel photothermal magnetic Janus membranes suitable for solar water desalination
- Green synthesis of silver nanoparticles using Ageratum conyzoides for activated carbon compositing to prepare antimicrobial cotton fabric
- Activation energy and Coriolis force impact on three-dimensional dusty nanofluid flow containing gyrotactic microorganisms: Machine learning and numerical approach
- Machine learning analysis of thermo-bioconvection in a micropolar hybrid nanofluid-filled square cavity with oxytactic microorganisms
- Research and improvement of mechanical properties of cement nanocomposites for well cementing
- Thermal and stability analysis of silver–water nanofluid flow over unsteady stretching sheet under the influence of heat generation/absorption at the boundary
- Cobalt iron oxide-infused silicone nanocomposites: Magnetoactive materials for remote actuation and sensing
- Magnesium-reinforced PMMA composite scaffolds: Synthesis, characterization, and 3D printing via stereolithography
- Bayesian inference-based physics-informed neural network for performance study of hybrid nanofluids
- Numerical simulation of non-Newtonian hybrid nanofluid flow subject to a heterogeneous/homogeneous chemical reaction over a Riga surface
- Enhancing the superhydrophobicity, UV-resistance, and antifungal properties of natural wood surfaces via in situ formation of ZnO, TiO2, and SiO2 particles
- Synthesis and electrochemical characterization of iron oxide/poly(2-methylaniline) nanohybrids for supercapacitor application
- Impacts of double stratification on thermally radiative third-grade nanofluid flow on elongating cylinder with homogeneous/heterogeneous reactions by implementing machine learning approach
- Synthesis of Cu4O3 nanoparticles using pumpkin seed extract: Optimization, antimicrobial, and cytotoxicity studies
- Cationic charge influence on the magnetic response of the Fe3O4–[Me2+ 1−y Me3+ y (OH2)] y+(Co3 2−) y/2·mH2O hydrotalcite system
- Pressure sensing intelligent martial arts short soldier combat protection system based on conjugated polymer nanocomposite materials
- Magnetohydrodynamics heat transfer rate under inclined buoyancy force for nano and dusty fluids: Response surface optimization for the thermal transport
- Fly ash and nano-graphene enhanced stabilization of engine oil-contaminated soils
- Enhancing natural fiber-reinforced biopolymer composites with graphene nanoplatelets: Mechanical, morphological, and thermal properties
- Performance evaluation of dual-scale strengthened co-bonded single-lap joints using carbon nanotubes and Z-pins with ANN
- Computational works of blood flow with dust particles and partially ionized containing tiny particles on a moving wedge: Applications of nanotechnology
- Hybridization of biocomposites with oil palm cellulose nanofibrils/graphene nanoplatelets reinforcement in green epoxy: A study of physical, thermal, mechanical, and morphological properties
- Design and preparation of micro-nano dual-scale particle-reinforced Cu–Al–V alloy: Research on the aluminothermic reduction process
- Spectral quasi-linearization and response optimization on magnetohydrodynamic flow via stenosed artery with hybrid and ternary solid nanoparticles: Support vector machine learning
- Ferrite/curcumin hybrid nanocomposite formulation: Physicochemical characterization, anticancer activity, and apoptotic and cell cycle analyses in skin cancer cells
- Enhanced therapeutic efficacy of Tamoxifen against breast cancer using extra virgin olive oil-based nanoemulsion delivery system
- A titanium oxide- and silver-based hybrid nanofluid flow between two Riga walls that converge and diverge through a machine-learning approach
- Enhancing convective heat transfer mechanisms through the rheological analysis of Casson nanofluid flow towards a stagnation point over an electro-magnetized surface
- Intrinsic self-sensing cementitious composites with hybrid nanofillers exhibiting excellent piezoresistivity
- Research on mechanical properties and sulfate erosion resistance of nano-reinforced coal gangue based geopolymer concrete
- Impact of surface and configurational features of chemically synthesized chains of Ni nanostars on the magnetization reversal process
- Porous sponge-like AsOI/poly(2-aminobenzene-1-thiol) nanocomposite photocathode for hydrogen production from artificial and natural seawater
- Multifaceted insights into WO3 nanoparticle-coupled antibiotics to modulate resistance in enteric pathogens of Houbara bustard birds
- Synthesis of sericin-coated silver nanoparticles and their applications for the anti-bacterial finishing of cotton fabric
- Enhancing chloride resistance of freeze–thaw affected concrete through innovative nanomaterial–polymer hybrid cementitious coating
- Review Articles
- A comprehensive review on hybrid plasmonic waveguides: Structures, applications, challenges, and future perspectives
- Nanoparticles in low-temperature preservation of biological systems of animal origin
- Fluorescent sulfur quantum dots for environmental monitoring
- Nanoscience systematic review methodology standardization
- Nanotechnology revolutionizing osteosarcoma treatment: Advances in targeted kinase inhibitors
- AFM: An important enabling technology for 2D materials and devices
- Carbon and 2D nanomaterial smart hydrogels for therapeutic applications
- Principles, applications and future prospects in photodegradation systems
- Do gold nanoparticles consistently benefit crop plants under both non-stressed and abiotic stress conditions?
- An updated overview of nanoparticle-induced cardiovascular toxicity
- Arginine as a promising amino acid for functionalized nanosystems: Innovations, challenges, and future directions
- Advancements in the use of cancer nanovaccines: Comprehensive insights with focus on lung and colon cancer
- Membrane-based biomimetic delivery systems for glioblastoma multiforme therapy
- The drug delivery systems based on nanoparticles for spinal cord injury repair
- Green synthesis, biomedical effects, and future trends of Ag/ZnO bimetallic nanoparticles: An update
- Application of magnesium and its compounds in biomaterials for nerve injury repair
- Micro/nanomotors in biomedicine: Construction and applications
- Hydrothermal synthesis of biomass-derived CQDs: Advances and applications
- Research progress in 3D bioprinting of skin: Challenges and opportunities
- Review on bio-selenium nanoparticles: Synthesis, protocols, and applications in biomedical processes
- Gold nanocrystals and nanorods functionalized with protein and polymeric ligands for environmental, energy storage, and diagnostic applications: A review
- An in-depth analysis of rotational and non-rotational piezoelectric energy harvesting beams: A comprehensive review
- Advancements in perovskite/CIGS tandem solar cells: Material synergies, device configurations, and economic viability for sustainable energy
- Deep learning in-depth analysis of crystal graph convolutional neural networks: A new era in materials discovery and its applications
- Review of recent nano TiO2 film coating methods, assessment techniques, and key problems for scaleup
- Antioxidant quantum dots for spinal cord injuries: A review on advancing neuroprotection and regeneration in neurological disorders
- Rise of polycatecholamine ultrathin films: From synthesis to smart applications
- Advancing microencapsulation strategies for bioactive compounds: Enhancing stability, bioavailability, and controlled release in food applications
- Corrigendum
- Corrigendum to “Synthesis and characterization of smart stimuli-responsive herbal drug-encapsulated nanoniosome particles for efficient treatment of breast cancer”
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part III
- Efficiency optimization of quantum dot photovoltaic cell by solar thermophotovoltaic system
- Exploring the diverse nanomaterials employed in dental prosthesis and implant techniques: An overview
- Electrochemical investigation of bismuth-doped anode materials for low‑temperature solid oxide fuel cells with boosted voltage using a DC-DC voltage converter
- Synthesis of HfSe2 and CuHfSe2 crystalline materials using the chemical vapor transport method and their applications in supercapacitor energy storage devices
- Special Issue on Green Nanotechnology and Nano-materials for Environment Sustainability
- Influence of nano-silica and nano-ferrite particles on mechanical and durability of sustainable concrete: A review
- Surfaces and interfaces analysis on different carboxymethylation reaction time of anionic cellulose nanoparticles derived from oil palm biomass
- Processing and effective utilization of lignocellulosic biomass: Nanocellulose, nanolignin, and nanoxylan for wastewater treatment
- Retraction
- Retraction of “Aging assessment of silicone rubber materials under corona discharge accompanied by humidity and UV radiation”
Artikel in diesem Heft
- Research Articles
- MHD radiative mixed convective flow of a sodium alginate-based hybrid nanofluid over a convectively heated extending sheet with Joule heating
- Experimental study of mortar incorporating nano-magnetite on engineering performance and radiation shielding
- Multicriteria-based optimization and multi-variable non-linear regression analysis of concrete containing blends of nano date palm ash and eggshell powder as cementitious materials
- A promising Ag2S/poly-2-amino-1-mercaptobenzene open-top spherical core–shell nanocomposite for optoelectronic devices: A one-pot technique
- Biogenic synthesized selenium nanoparticles combined chitosan nanoparticles controlled lung cancer growth via ROS generation and mitochondrial damage pathway
- Fabrication of PDMS nano-mold by deposition casting method
- Stimulus-responsive gradient hydrogel micro-actuators fabricated by two-photon polymerization-based 4D printing
- Physical aspects of radiative Carreau nanofluid flow with motile microorganisms movement under yield stress via oblique penetrable wedge
- Effect of polar functional groups on the hydrophobicity of carbon nanotubes-bacterial cellulose nanocomposite
- Review in green synthesis mechanisms, application, and future prospects for Garcinia mangostana L. (mangosteen)-derived nanoparticles
- Entropy generation and heat transfer in nonlinear Buoyancy–driven Darcy–Forchheimer hybrid nanofluids with activation energy
- Green synthesis of silver nanoparticles using Ginkgo biloba seed extract: Evaluation of antioxidant, anticancer, antifungal, and antibacterial activities
- A numerical analysis of heat and mass transfer in water-based hybrid nanofluid flow containing copper and alumina nanoparticles over an extending sheet
- Investigating the behaviour of electro-magneto-hydrodynamic Carreau nanofluid flow with slip effects over a stretching cylinder
- Electrospun thermoplastic polyurethane/nano-Ag-coated clear aligners for the inhibition of Streptococcus mutans and oral biofilm
- Investigation of the optoelectronic properties of a novel polypyrrole-multi-well carbon nanotubes/titanium oxide/aluminum oxide/p-silicon heterojunction
- Novel photothermal magnetic Janus membranes suitable for solar water desalination
- Green synthesis of silver nanoparticles using Ageratum conyzoides for activated carbon compositing to prepare antimicrobial cotton fabric
- Activation energy and Coriolis force impact on three-dimensional dusty nanofluid flow containing gyrotactic microorganisms: Machine learning and numerical approach
- Machine learning analysis of thermo-bioconvection in a micropolar hybrid nanofluid-filled square cavity with oxytactic microorganisms
- Research and improvement of mechanical properties of cement nanocomposites for well cementing
- Thermal and stability analysis of silver–water nanofluid flow over unsteady stretching sheet under the influence of heat generation/absorption at the boundary
- Cobalt iron oxide-infused silicone nanocomposites: Magnetoactive materials for remote actuation and sensing
- Magnesium-reinforced PMMA composite scaffolds: Synthesis, characterization, and 3D printing via stereolithography
- Bayesian inference-based physics-informed neural network for performance study of hybrid nanofluids
- Numerical simulation of non-Newtonian hybrid nanofluid flow subject to a heterogeneous/homogeneous chemical reaction over a Riga surface
- Enhancing the superhydrophobicity, UV-resistance, and antifungal properties of natural wood surfaces via in situ formation of ZnO, TiO2, and SiO2 particles
- Synthesis and electrochemical characterization of iron oxide/poly(2-methylaniline) nanohybrids for supercapacitor application
- Impacts of double stratification on thermally radiative third-grade nanofluid flow on elongating cylinder with homogeneous/heterogeneous reactions by implementing machine learning approach
- Synthesis of Cu4O3 nanoparticles using pumpkin seed extract: Optimization, antimicrobial, and cytotoxicity studies
- Cationic charge influence on the magnetic response of the Fe3O4–[Me2+ 1−y Me3+ y (OH2)] y+(Co3 2−) y/2·mH2O hydrotalcite system
- Pressure sensing intelligent martial arts short soldier combat protection system based on conjugated polymer nanocomposite materials
- Magnetohydrodynamics heat transfer rate under inclined buoyancy force for nano and dusty fluids: Response surface optimization for the thermal transport
- Fly ash and nano-graphene enhanced stabilization of engine oil-contaminated soils
- Enhancing natural fiber-reinforced biopolymer composites with graphene nanoplatelets: Mechanical, morphological, and thermal properties
- Performance evaluation of dual-scale strengthened co-bonded single-lap joints using carbon nanotubes and Z-pins with ANN
- Computational works of blood flow with dust particles and partially ionized containing tiny particles on a moving wedge: Applications of nanotechnology
- Hybridization of biocomposites with oil palm cellulose nanofibrils/graphene nanoplatelets reinforcement in green epoxy: A study of physical, thermal, mechanical, and morphological properties
- Design and preparation of micro-nano dual-scale particle-reinforced Cu–Al–V alloy: Research on the aluminothermic reduction process
- Spectral quasi-linearization and response optimization on magnetohydrodynamic flow via stenosed artery with hybrid and ternary solid nanoparticles: Support vector machine learning
- Ferrite/curcumin hybrid nanocomposite formulation: Physicochemical characterization, anticancer activity, and apoptotic and cell cycle analyses in skin cancer cells
- Enhanced therapeutic efficacy of Tamoxifen against breast cancer using extra virgin olive oil-based nanoemulsion delivery system
- A titanium oxide- and silver-based hybrid nanofluid flow between two Riga walls that converge and diverge through a machine-learning approach
- Enhancing convective heat transfer mechanisms through the rheological analysis of Casson nanofluid flow towards a stagnation point over an electro-magnetized surface
- Intrinsic self-sensing cementitious composites with hybrid nanofillers exhibiting excellent piezoresistivity
- Research on mechanical properties and sulfate erosion resistance of nano-reinforced coal gangue based geopolymer concrete
- Impact of surface and configurational features of chemically synthesized chains of Ni nanostars on the magnetization reversal process
- Porous sponge-like AsOI/poly(2-aminobenzene-1-thiol) nanocomposite photocathode for hydrogen production from artificial and natural seawater
- Multifaceted insights into WO3 nanoparticle-coupled antibiotics to modulate resistance in enteric pathogens of Houbara bustard birds
- Synthesis of sericin-coated silver nanoparticles and their applications for the anti-bacterial finishing of cotton fabric
- Enhancing chloride resistance of freeze–thaw affected concrete through innovative nanomaterial–polymer hybrid cementitious coating
- Review Articles
- A comprehensive review on hybrid plasmonic waveguides: Structures, applications, challenges, and future perspectives
- Nanoparticles in low-temperature preservation of biological systems of animal origin
- Fluorescent sulfur quantum dots for environmental monitoring
- Nanoscience systematic review methodology standardization
- Nanotechnology revolutionizing osteosarcoma treatment: Advances in targeted kinase inhibitors
- AFM: An important enabling technology for 2D materials and devices
- Carbon and 2D nanomaterial smart hydrogels for therapeutic applications
- Principles, applications and future prospects in photodegradation systems
- Do gold nanoparticles consistently benefit crop plants under both non-stressed and abiotic stress conditions?
- An updated overview of nanoparticle-induced cardiovascular toxicity
- Arginine as a promising amino acid for functionalized nanosystems: Innovations, challenges, and future directions
- Advancements in the use of cancer nanovaccines: Comprehensive insights with focus on lung and colon cancer
- Membrane-based biomimetic delivery systems for glioblastoma multiforme therapy
- The drug delivery systems based on nanoparticles for spinal cord injury repair
- Green synthesis, biomedical effects, and future trends of Ag/ZnO bimetallic nanoparticles: An update
- Application of magnesium and its compounds in biomaterials for nerve injury repair
- Micro/nanomotors in biomedicine: Construction and applications
- Hydrothermal synthesis of biomass-derived CQDs: Advances and applications
- Research progress in 3D bioprinting of skin: Challenges and opportunities
- Review on bio-selenium nanoparticles: Synthesis, protocols, and applications in biomedical processes
- Gold nanocrystals and nanorods functionalized with protein and polymeric ligands for environmental, energy storage, and diagnostic applications: A review
- An in-depth analysis of rotational and non-rotational piezoelectric energy harvesting beams: A comprehensive review
- Advancements in perovskite/CIGS tandem solar cells: Material synergies, device configurations, and economic viability for sustainable energy
- Deep learning in-depth analysis of crystal graph convolutional neural networks: A new era in materials discovery and its applications
- Review of recent nano TiO2 film coating methods, assessment techniques, and key problems for scaleup
- Antioxidant quantum dots for spinal cord injuries: A review on advancing neuroprotection and regeneration in neurological disorders
- Rise of polycatecholamine ultrathin films: From synthesis to smart applications
- Advancing microencapsulation strategies for bioactive compounds: Enhancing stability, bioavailability, and controlled release in food applications
- Corrigendum
- Corrigendum to “Synthesis and characterization of smart stimuli-responsive herbal drug-encapsulated nanoniosome particles for efficient treatment of breast cancer”
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part III
- Efficiency optimization of quantum dot photovoltaic cell by solar thermophotovoltaic system
- Exploring the diverse nanomaterials employed in dental prosthesis and implant techniques: An overview
- Electrochemical investigation of bismuth-doped anode materials for low‑temperature solid oxide fuel cells with boosted voltage using a DC-DC voltage converter
- Synthesis of HfSe2 and CuHfSe2 crystalline materials using the chemical vapor transport method and their applications in supercapacitor energy storage devices
- Special Issue on Green Nanotechnology and Nano-materials for Environment Sustainability
- Influence of nano-silica and nano-ferrite particles on mechanical and durability of sustainable concrete: A review
- Surfaces and interfaces analysis on different carboxymethylation reaction time of anionic cellulose nanoparticles derived from oil palm biomass
- Processing and effective utilization of lignocellulosic biomass: Nanocellulose, nanolignin, and nanoxylan for wastewater treatment
- Retraction
- Retraction of “Aging assessment of silicone rubber materials under corona discharge accompanied by humidity and UV radiation”

