Abstract
C22H28N2O4, monoclinic, P21/n (no. 14), a = 10.0094(5) Å, b = 17.4999(8) Å, c = 11.9872(6) Å, β = 96.926(2)°, V = 2084.40(18) Å3, Z = 4, Rgt(F) = 0.0542 wRref(F2) = 0.1607, T = 296 K.
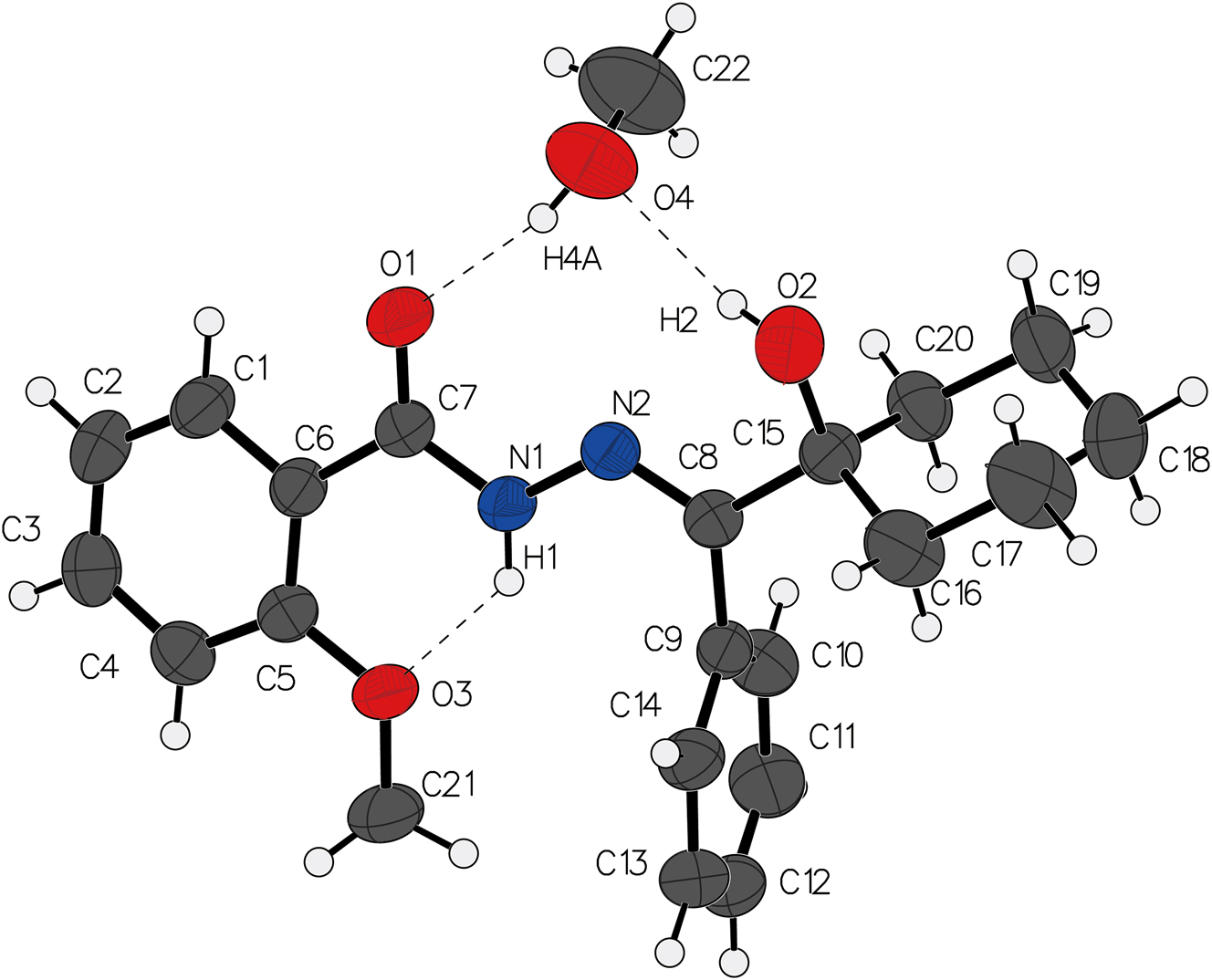
The molecular structure is shown in the figure. Table 1 contains the crystallographic data and the list of the atoms including atomic coordinates and displacement parameters can be found in the cif-file attached to this article.
Data collection and handling.
| Crystal: | Colorless block |
| Size: | 0.66 × 0.59 × 0.48 mm |
| Wavelength: μ: |
Mo Kα radiation (0.71073 Å) 0.08 mm−1 |
| Diffractometer, scan mode: θmax, completeness: |
Rigaku SuperNova, ω scans 28.3°, 100 % |
| N(hkl)measured, N(hkl)unique, Rint: | 77493, 5171, 0.131 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2σ(Iobs), 3827 |
| N(param)refined: | 257 |
| Programs: | Rigaku, 1 SHELX, 2 , 3 Olex2 4 |
1 Source of materials
Equimolar amounts (0.5 mmol) of 2-methoxybenzohydrazide (0.083 g) and 1-hydroxycyclohexyl phenyl ketone (0.102 g) were combined in anhydrous ethanol (20 mL) within a 50 mL round-bottom flask. The mixture was acidified with glacial acetic acid (0.25 mL, 5 drops) and heated under reflux at 50 °C with continuous stirring for 3 h. Reaction progression was tracked via TLC analysis using pre-coated silica gel plates (60 F254) with a mixture of ethyl acetate and hexanes (1:3 v/v) as the mobile phase. Upon confirmation of starting material consumption, the system was cooled to room temperature. The resulting solution was concentrated under reduced pressure to afford the crude product as a white powder. A portion (0.05 g) of the crude material was dissolved in methanol (5 mL) for further purification. The resulting solution was transferred to an open crystallization dish and subjected to gradual solvent evaporation under ambient fume hood conditions. Transparent block crystals formed over 72 h through slow volatilization.
2 Experimental details
Diffraction data was acquired at 296 K using a Bruker D8 Venture system with Mo Kα irradiation. 1 Structural determination was initiated via intrinsic phasing (ShelXT 2 ), followed by successive full-matrix least-squares refinement (ShelXL 3 ) executed through the Olex2 platform. 4 Anisotropic refinement was applied to non-hydrogen atoms, while hydrogen atoms were geometrically constrained and incorporated as riding contributions with fixed isotropic thermal parameters.
3 Comment
Benzohydrazide derivatives have garnered significant interest in structural chemistry due to their diverse biological activities and versatile coordination properties. 5 , 6 , 7 Single-crystal X-ray diffraction (SCXRD) serves as a cornerstone for elucidating their precise molecular conformations, intermolecular interactions, and supramolecular packing motifs of such compounds. 8 , 9 , 10 , 11 Such structural insights are critical for rationalizing structure-activity relationships, particularly in pharmaceutical and materials science contexts. The presence of functional groups like methoxy substituents and hydrazide linkages in these compounds often induces distinct hydrogen-bonding networks or π-stacking interactions, which govern crystallization behavior and stability. 12 , 13 , 14 , 15 , 16 Investigations into solvent-inclusive crystalline forms, such as methanol solvates, further illuminate the role of lattice solvents in modulating crystal packing and physicochemical properties.
In the title compound, as can be seen in the figure, the near-planar arrangement of non-hydrogen atoms within the 2-methoxybenzohydrazide moiety suggests effective π-conjugation across the hydrazide-carbonylic system, a characteristic known to enhance electronic delocalization and stabilize specific tautomeric forms in analogous derivatives. Notably, the cyclohexyl ring adopts a classical chair conformation, with minimal puckering deviations (θ < 2°), consistent with the steric preference for low-energy cyclic alkane geometries.
Distinct C–O bond lengths are observed for hydroxyl (C15–O2: 1.4167(16) Å), acyl (C7–O1: 1.2247(17) Å), and ether (C5–O3: 1.3628(16) Å) functionalities. The shortened acyl C–O bond aligns with partial double-bond character arising from resonance with the adjacent carbonyl group, while the elongated hydroxyl C–O bond likely reflects localized lone-pair electron density at O2. The intermediate ether C–O distance corresponds to typical values for methoxy substituents in aromatic systems.
Crucially, the methanol solvent molecule occupies a central cavity within the crystal lattice, engaging the parent molecule through a robust O4–H4A⋯O1 hydrogen bond (1.9763(11) Å, 165.1°). The directional preference of this interaction suggests it serves as the primary driving force for methanol inclusion, effectively templating the supramolecular assembly through bifurcated hydrogen-bond networks.
Acknowledgments
This work was financially supported by the projects of Social Development in Shaanxi Province Science and Technology Department (2023-YBSF-036), the 2024 Key Scientific Research Program Projects of the Shaanxi Provincial Department of Education (Key Laboratory Projects, 24JS004), the projects of Natural Science Foundation of Shannxi Province (2025JC–YBMS-1076), Key Laboratory of Molecular Imaging and Drug Synthesis of Xianyang city (2021QXNL-PT-0008), School-level Scientific and Technological Innovation Team for Design, Synthesis and Structural Modification of Drug Molecules (2024KCTD04).
References
1. Rigaku, O. D. CrysAlisPRO; Rigaku Oxford Diffraction Ltd: Yarnton, Oxfordshire, England, 2017.Suche in Google Scholar
2. Sheldrick, G. M. Shelxt–Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr., Sect. A: Found. Crystallogr. 2015, 71, 3–8; https://doi.org/10.1107/s2053273314026370.Suche in Google Scholar PubMed PubMed Central
3. Sheldrick, G. M. Crystal Structure Refinement with Shelxl. Acta Crystallogr., Sect. C: Struct. Chem. 2015, 71, 3–8; https://doi.org/10.1107/s2053229614024218.Suche in Google Scholar
4. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A.; Puschmann, H. OLEX2: a Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Suche in Google Scholar
5. Liu, B.; Liu, H.; Zhang, H.; Di, Q.; Zhang, H. Crystal Engineering of a Hydrazone Molecule Toward High Elasticity and Bright Luminescence. J. Phys. Chem. Lett. 2020, 11, 9178–9183; https://doi.org/10.1021/acs.jpclett.0c02623.Suche in Google Scholar PubMed
6. Alifu, Z.; Nizhamu, M.; Ablajan, K. Efficient Synthesis of N′-benzylidene-2-hydroxymethylbenzohydrazides from the One-Pot Reaction of Phthalide, Hydrazine and Aldehydes. Res. Chem. Intermed. 2019, 45, 4779–4788; https://doi.org/10.1007/s11164-019-03863-8.Suche in Google Scholar
7. Bai, B.; Zhang, M.; Ji, N.; Wei, J.; Wang, H.; Li, M. E–Z Isomerization of the –C=N-bond in Anthracene-based Acylhydrazone Derivatives Under Visible Light. Chem. Commun. 2017, 53, 2693–2696; https://doi.org/10.1039/c6cc08403f.Suche in Google Scholar PubMed
8. Peng, S.-J. Synthesis, Crystal Structures, and Antibacterial Activity of Two Hydrazone Derivatives 3-Methoxy-N′-(3,5-Dibromo-2-Hydroxybenzylidene) Benzohydrazide Methanol Solvate and 3-Methoxy-N′-(2,4-diclorobenzylidene) benzohydrazide. J. Chem. Crystallogr. 2011, 41, 280–285; https://doi.org/10.1007/s10870-010-9873-9Suche in Google Scholar
9. Xue, L.-W.; Han, Y.-J.; Zhao, G.-Q.; Feng, Y.-X. Synthesis, Characterization and Crystal Structures of N′-(5-Bromo-2-hydroxybenzylidene)-2-fluorobenzohydrazide in Unsolvated and Monohydrate Forms. J. Chem. Crystallogr. 2011, 41, 1599–1603; https://doi.org/10.1007/s10870-011-0146-z.Suche in Google Scholar
10. Zhu, H.-Y. Synthesis, Characterization, and Crystal Structures of 3-bromo–N′-(2-methoxybenzylidene)benzohydrazide and N′-(2-methoxybenzylidene)-3,4-methylenedioxybenzohydrazide. J. Chem. Crystallogr. 2011, 41, 1785–1789; https://doi.org/10.1007/s10870-011-9982-0.Suche in Google Scholar
11. Zhu, M.-A.; Qiu, X.-Y. Synthesis and Crystal Structures of N′-(5-Bromo-2-hydroxy-3-methoxybenzylidene)-4-methoxybenzohydrazide and 4-{[1-(5-bromo-2-hydroxy3-methoxyphenyl)methylidene]amino}-1-methyl-2-phenyl-1, 2-dihydropyrazol-3-one. J. Chem. Crystallogr. 2011, 41, 69–72; https://doi.org/10.1007/s10870-010-9839-y.Suche in Google Scholar
12. Bai, Z.-C.; Li, H.; Liu, Y.; Jing, Z.-L. N′-(2,4-Dichlorobenzylidene)-2-methoxybenzohydrazide. Acta Crystallogr. E 2006, 62, o2295–o2296; https://doi.org/10.1107/s1600536806017053.Suche in Google Scholar
13. Fun, H.-K.; Horkaew, J.; Chantrapromma, S. (E)-4-Hydroxy-N′-(3-hydroxy-4-methoxybenzylidene)benzohydrazide. Acta Crystallogr. E 2011, 67, o2644–o2645; https://doi.org/10.1107/s1600536811036579.Suche in Google Scholar PubMed PubMed Central
14. Yang, J.-G.; Pan, F.-Y. 2′-(2-Fluorobenzylidene)-2-hydroxybenzohydrazide. Acta Crystallogr. E 2004, 60, o2009–o2010; https://doi.org/10.1107/s1600536804024614.Suche in Google Scholar
15. Hao, Y.-M. Crystal Structure of 2-Chloro-N′-(2-methoxybenzylidene) benzohydrazide, C15H13ClN2O2. Z. Kristallogr. N. Cryst. Struct. 2011, 226, 11–12; https://doi.org/10.1524/ncrs.2011.0006.Suche in Google Scholar
16. Hao, Y.-M. Crystal Structure of 2-Chloro-N′-(2-hydroxy-3, 5-dichlorobenzylidene)-benzohydrazide-methanol (1:1), C14H9Cl3N2O2.CH3OH. Z. Kristallogr. N. Cryst. Struct. 2011, 226, 47–48; https://doi.org/10.1524/ncrs.2011.0023.Suche in Google Scholar
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of (S)-N-(10-((2,2-dimethoxyethyl)amino)-1,2,3-trimethoxy-9-oxo-5,6,7,9-tetrahydrobenzo[a]heptalen-7-yl)acetamide, C25H32N2O7
- The crystal structure of 6,6′-difluoro-3,3′-dimethyl-5,5′-di(10H-phenoxazin-10-yl)- [1,1′-biphenyl]-2,2′-dicarbonitrile, C40H24F2N4O2
- Crystal structure of poly[(di-ethylenediamine-κ2N,N′)cadmium(II) tetradedocyloxidohexavanadate] (V4+/V5+ = 2/1), C4H16CdN4O14V6
- The crystal structure of poly[bis(dimethylformamide-κ1N)-(μ4-2′,3,3″,5′-tetrakis(trifluoromethyl)-[1,1′:4′,1″-terphenyl]-4,4″-dicarboxylato-κ4 O,O′: O″,O‴)dicadmium(II)], C27H15CdF12NO5
- Crystal structure of bis(μ2-ferrocenylcarboxylato-O,O′)-(μ3-oxido-κ3O:O:O)-bis(μ2-salicyladoximato-κ2N,O,O′)-(μ2-isopropoxo)-tris(isopropoxy-κ1O trititanium(IV)), C48H55N2O13Fe2Ti3
- Crystal structure of 3-(diethylamino)-7,9,11-trimethyl-8-phenyl-6H,13H-12λ4,13λ4-chromeno[3′,4′:4,5]pyrrolo[1,2-c]pyrrolo[2,1-f][1,3,2]diazaborinin-6-one, C28H26BF2N3O2
- The crystal structure of catena-poly[aqua-μ2-2-nitro-benzene-1,3-dicarboxylato-κ2O,O′)-(1,10-phenanthroline-κ2N,N′)-zinc(II)], C20H13N3O7Zn
- Crystal structure of poly[diaqua-{μ3-1-(3-carboxylatophenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylato-κ4O,O′:O′′:O′′′′}manganese(II)] hydrate
- Crystal structure of N′-((1-hydroxycyclohexyl)(phenyl)methyl)-2-methoxybenzohydrazide methanol solvate, C22H28N2O4
- The cocrystal of caffeic acid — progesterone — water (1/2/1), C51H70O9
- Crystal structure of (((oxido(quinolin-6-yl)methoxy)triphenyl-λ5-stibanyl)oxy)(quinolin-7-yl)methanolate
- Crystal structure of [(E)-6′-(diethylamino)-2-(2-(((E)-pyren-1-ylmethylene)amino)ethyl)-4′-(2-((E)-1,3,3-trimethylindolin-2-ylidene)ethylidene)-1′,2′,3′,4′-tetrahydrospiro[isoindoline-1,9′-xanthen]-3-one]-methanol, solvate C57H56N4O3
- The crystal structure of 1-(acridin-9-yl)pyrrolidine-2,5-dione, C17H22N2O2
- Crystal structure of N-(4-acetylphenyl)-2-(6-methoxynaphthalen-2-yl)propanamide, C22H21NO3
- The crystal structure of 5,10,15,20-tetrakis(4-(1H-1,2,4-triazol-1-yl)phenyl)porphyrin, C52H34N16
- Crystal structure of hexacarbonyl-μ2-[phenylmethanedithiolato-κ4S:S,S′:S′]diiron (Fe–Fe) C13H6Fe2O6S2
- Crystal structure of diiodo-bis(1-((2-propyl-1H-benzo[d]imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κ1N)cadmium(II), C34H34CdI2N10
- Crystal structure of (E)-(3-(3-bromophenyl)acryloyl)ferrocene, C19H15BrFeO
- Crystal structure of catena-poly(μ2-6-chloropyridine-2-carboxylato-κ3N,O:O′)(6-chloropyridine-2-carboxylato-κ2O,N)copper(II), C12H6Cl2N2O4Cu
- Crystal structure of poly[diaqua-μ 3-(5-(3,5-dicarboxy-2,4,6-trimethylbenzyl)-2,4,6-trimethylisophthalato)-κ 6O,O′:O″,O‴:O‴′,O‴″) terbium(III)-monohydrate], C23H28TbO12
- Crystal structure of (E)-2-(((5-chloro-3-methyl-1-phenyl-1H-pyrazol-4-yl)methylene)amino)-3′,6′-dihydroxyspiro[isoindoline-1,9′-xanthen]-3-one – ethanol (1/2), C35H33ClN4O6
- The crystal structure of 3-(5-amino-3-phenylisoxazol-4-yl)-4-chloro-3-hydroxyindolin-2-one, C17H12ClN3O3
- The crystal structure of dimethylammonium 4-[2-(4-fluorophenyl)-4, 5-diphenyl-1H-imidazol-1-yl]benzenesulfonate, C29H26FN3O3S
- Crystal structure of (R)-2-ammonio-3-((5-carboxypentyl)thio)propanoate
- Crystal structure of 4-cyclohexyl-5-(thiophen-2-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione, C12H15N3S2
- The crystal structure of 4,6-bis(dimethylamino)-2-fluoroisophthalonitrile, C12H13FN4
- Hydrogen bonding in the crystal structure of nicotin-1,1′-dium tetrabromidomanganate(II)
- The crystal structure of bis(2-bromobenzyl)(2-((2-oxybenzylidene)amino)-4-methylpentanoato-κ3N, O,O′)tin(IV), C27H27Br2NO3Sn
- Crystal structure of (E)-(3-(p-tolyl)acryloyl)ferrocene, C20H18FeO
- Crystal structure of (E)-7-fluoro-2-((5-(4-methylpiperazin-1-yl)pyridin-2-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C21H22FN3O
- Crystal structure of (E)-7-methoxy-2-((5-(4-methylpiperazin-1-yl)pyridin-2-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C22H25N3O2
- The crystal structure of poly(bis(μ2-1,3,5-tri(1H-imidazol-1-yl)benzene-κ2N:N′)-(μ2-2,3,5,6-tetrafluoroterephthalato-κ2O:O′)-manganese(II), C38H24F4N12O4Mn
- Crystal structure of (3,4-dimethoxybenzyl)triphenylphosphonium bromide ethanol solvate, C29H32BrO3P
- Crystal structure of tetraethylammonium hydrogencarbonate – (diaminomethylene)thiourea – water (2/1/3)
- Crystal structure of N, N-Dimethyl-N′-tosylformimidamide, C10H14N2O2S
- The crystal structure of ethyl 2-methyl-5-oxo-4-(2-methoxyphenyl)-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C20H23N2O4
- Crystal structure of bis(μ2-1,5-bis[(E)-1-(2-hydroxyphenyl)ethylidene] thiocarbonohydrazide)-bis(dimethylformamide)-dizinc(II) dimethylformamide solvate, C40H46N10O6S2Zn2⋅C3H7NO
- Crystal structure of azido-κ1N{hydridotris(3-tert-butyl-5-methylpyrazol-1-yl)borato-κ3N,N′,N″}copper(II), C24H40BCuN9
- The crystal structure of fac-tricarbonyl(1,10-phenanthroline-κ2N,N′)-(azido-κ1N)rhenium(I), C15H8N5O3Re
- Crystal structure of 4-((triphenylphosphonio)methyl)pyridin-1-ium tetrachloridozincate(II), C24H22Cl4NPZn
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of (S)-N-(10-((2,2-dimethoxyethyl)amino)-1,2,3-trimethoxy-9-oxo-5,6,7,9-tetrahydrobenzo[a]heptalen-7-yl)acetamide, C25H32N2O7
- The crystal structure of 6,6′-difluoro-3,3′-dimethyl-5,5′-di(10H-phenoxazin-10-yl)- [1,1′-biphenyl]-2,2′-dicarbonitrile, C40H24F2N4O2
- Crystal structure of poly[(di-ethylenediamine-κ2N,N′)cadmium(II) tetradedocyloxidohexavanadate] (V4+/V5+ = 2/1), C4H16CdN4O14V6
- The crystal structure of poly[bis(dimethylformamide-κ1N)-(μ4-2′,3,3″,5′-tetrakis(trifluoromethyl)-[1,1′:4′,1″-terphenyl]-4,4″-dicarboxylato-κ4 O,O′: O″,O‴)dicadmium(II)], C27H15CdF12NO5
- Crystal structure of bis(μ2-ferrocenylcarboxylato-O,O′)-(μ3-oxido-κ3O:O:O)-bis(μ2-salicyladoximato-κ2N,O,O′)-(μ2-isopropoxo)-tris(isopropoxy-κ1O trititanium(IV)), C48H55N2O13Fe2Ti3
- Crystal structure of 3-(diethylamino)-7,9,11-trimethyl-8-phenyl-6H,13H-12λ4,13λ4-chromeno[3′,4′:4,5]pyrrolo[1,2-c]pyrrolo[2,1-f][1,3,2]diazaborinin-6-one, C28H26BF2N3O2
- The crystal structure of catena-poly[aqua-μ2-2-nitro-benzene-1,3-dicarboxylato-κ2O,O′)-(1,10-phenanthroline-κ2N,N′)-zinc(II)], C20H13N3O7Zn
- Crystal structure of poly[diaqua-{μ3-1-(3-carboxylatophenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylato-κ4O,O′:O′′:O′′′′}manganese(II)] hydrate
- Crystal structure of N′-((1-hydroxycyclohexyl)(phenyl)methyl)-2-methoxybenzohydrazide methanol solvate, C22H28N2O4
- The cocrystal of caffeic acid — progesterone — water (1/2/1), C51H70O9
- Crystal structure of (((oxido(quinolin-6-yl)methoxy)triphenyl-λ5-stibanyl)oxy)(quinolin-7-yl)methanolate
- Crystal structure of [(E)-6′-(diethylamino)-2-(2-(((E)-pyren-1-ylmethylene)amino)ethyl)-4′-(2-((E)-1,3,3-trimethylindolin-2-ylidene)ethylidene)-1′,2′,3′,4′-tetrahydrospiro[isoindoline-1,9′-xanthen]-3-one]-methanol, solvate C57H56N4O3
- The crystal structure of 1-(acridin-9-yl)pyrrolidine-2,5-dione, C17H22N2O2
- Crystal structure of N-(4-acetylphenyl)-2-(6-methoxynaphthalen-2-yl)propanamide, C22H21NO3
- The crystal structure of 5,10,15,20-tetrakis(4-(1H-1,2,4-triazol-1-yl)phenyl)porphyrin, C52H34N16
- Crystal structure of hexacarbonyl-μ2-[phenylmethanedithiolato-κ4S:S,S′:S′]diiron (Fe–Fe) C13H6Fe2O6S2
- Crystal structure of diiodo-bis(1-((2-propyl-1H-benzo[d]imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κ1N)cadmium(II), C34H34CdI2N10
- Crystal structure of (E)-(3-(3-bromophenyl)acryloyl)ferrocene, C19H15BrFeO
- Crystal structure of catena-poly(μ2-6-chloropyridine-2-carboxylato-κ3N,O:O′)(6-chloropyridine-2-carboxylato-κ2O,N)copper(II), C12H6Cl2N2O4Cu
- Crystal structure of poly[diaqua-μ 3-(5-(3,5-dicarboxy-2,4,6-trimethylbenzyl)-2,4,6-trimethylisophthalato)-κ 6O,O′:O″,O‴:O‴′,O‴″) terbium(III)-monohydrate], C23H28TbO12
- Crystal structure of (E)-2-(((5-chloro-3-methyl-1-phenyl-1H-pyrazol-4-yl)methylene)amino)-3′,6′-dihydroxyspiro[isoindoline-1,9′-xanthen]-3-one – ethanol (1/2), C35H33ClN4O6
- The crystal structure of 3-(5-amino-3-phenylisoxazol-4-yl)-4-chloro-3-hydroxyindolin-2-one, C17H12ClN3O3
- The crystal structure of dimethylammonium 4-[2-(4-fluorophenyl)-4, 5-diphenyl-1H-imidazol-1-yl]benzenesulfonate, C29H26FN3O3S
- Crystal structure of (R)-2-ammonio-3-((5-carboxypentyl)thio)propanoate
- Crystal structure of 4-cyclohexyl-5-(thiophen-2-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione, C12H15N3S2
- The crystal structure of 4,6-bis(dimethylamino)-2-fluoroisophthalonitrile, C12H13FN4
- Hydrogen bonding in the crystal structure of nicotin-1,1′-dium tetrabromidomanganate(II)
- The crystal structure of bis(2-bromobenzyl)(2-((2-oxybenzylidene)amino)-4-methylpentanoato-κ3N, O,O′)tin(IV), C27H27Br2NO3Sn
- Crystal structure of (E)-(3-(p-tolyl)acryloyl)ferrocene, C20H18FeO
- Crystal structure of (E)-7-fluoro-2-((5-(4-methylpiperazin-1-yl)pyridin-2-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C21H22FN3O
- Crystal structure of (E)-7-methoxy-2-((5-(4-methylpiperazin-1-yl)pyridin-2-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C22H25N3O2
- The crystal structure of poly(bis(μ2-1,3,5-tri(1H-imidazol-1-yl)benzene-κ2N:N′)-(μ2-2,3,5,6-tetrafluoroterephthalato-κ2O:O′)-manganese(II), C38H24F4N12O4Mn
- Crystal structure of (3,4-dimethoxybenzyl)triphenylphosphonium bromide ethanol solvate, C29H32BrO3P
- Crystal structure of tetraethylammonium hydrogencarbonate – (diaminomethylene)thiourea – water (2/1/3)
- Crystal structure of N, N-Dimethyl-N′-tosylformimidamide, C10H14N2O2S
- The crystal structure of ethyl 2-methyl-5-oxo-4-(2-methoxyphenyl)-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C20H23N2O4
- Crystal structure of bis(μ2-1,5-bis[(E)-1-(2-hydroxyphenyl)ethylidene] thiocarbonohydrazide)-bis(dimethylformamide)-dizinc(II) dimethylformamide solvate, C40H46N10O6S2Zn2⋅C3H7NO
- Crystal structure of azido-κ1N{hydridotris(3-tert-butyl-5-methylpyrazol-1-yl)borato-κ3N,N′,N″}copper(II), C24H40BCuN9
- The crystal structure of fac-tricarbonyl(1,10-phenanthroline-κ2N,N′)-(azido-κ1N)rhenium(I), C15H8N5O3Re
- Crystal structure of 4-((triphenylphosphonio)methyl)pyridin-1-ium tetrachloridozincate(II), C24H22Cl4NPZn

