Crystal structure of poly[diaqua-μ 3-(5-(3,5-dicarboxy-2,4,6-trimethylbenzyl)-2,4,6-trimethylisophthalato)-κ 6O,O′:O″,O‴:O‴′,O‴″) terbium(III)-monohydrate], C23H28TbO12
Abstract
C23H28TbO12, orthorhombic, P212121 (no. 19), a = 8.5571(5) Å, b = 11.4427(7) Å, c = 24.8022(15) Å, V = 2428.5(3) Å3, Z = 4, R gt(F) = 0.0254, wR ref (F 2) = 0.0466, T = 293(2) K.
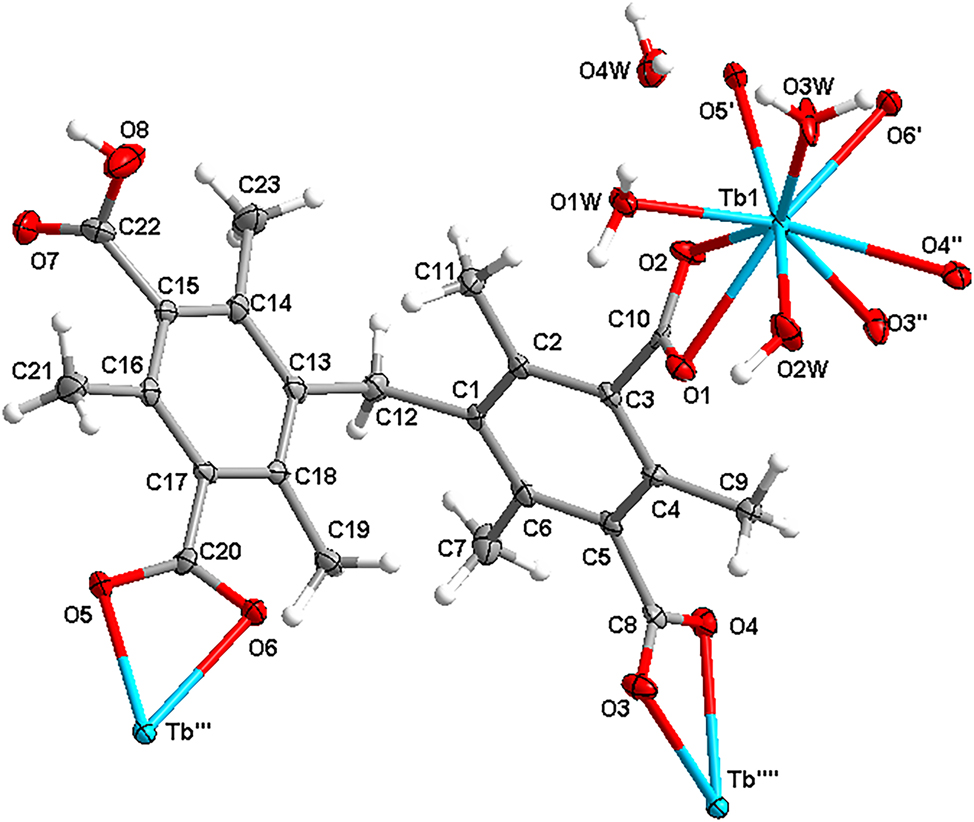
The molecular structure is shown in the figure. Table 1 contains the crystallographic data and the list of the atoms including atomic coordinates and displacement parameters can be found in the cif-file attached to this article.
Data collection and handling.
| Crystal: | Colourless rod |
| Size: | 0.35 × 0.10 × 0.10 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 2.98 mm−1 |
| Diffractometer, scan mode: | Bruker APEX2, φ and ω scans |
| θ max, completeness: | 27.5°, 100 % |
| N(hkl)measured, N(hkl)unique, R int: | 14354, 5506, 0.034 |
| Criterion for I obs, N(hkl)gt: | I obs > 2σ(I obs), 5150 |
| N(param)refined: | 337 |
| Programs: | Bruker, 1 Olex2, 2 SHELX, 3 , 4 Diamond 5 |
1 Source of materials
An aqueous solution (0.5 ml) containing Tb(NO3)3·6H2O (6.0 mg) and H3PW12O40·xH2O (4.0 mg) was added into a glass tube. Then, the aqueous solution of tetrabutylammonium bromide (4 mg/ml) 0.25 ml was added. Next, a solution of 5,5′-methylenebis (2,4,6-trimethylisophthalic acid) (H4BTMIPA) (4.0 mg) in cyclohexanol and toluene (1:1 v/v, 0.5 ml) was layered on the aqueous solution. The glass tube was heated slowly to 363 K from room temperature in 300 min, kept at 363 K for 3 min and then cooled slowly to 303 K over a period of 600 min. Colourless rod-shaped crystals formed suitable for single crystal X-ray structure.
2 Experimental details
The structure was solved by Direct Methods with the SHELX. 3 All H-atoms were positioned geometrically and refined using a riding model with d(C–H) = 0.93 Å, U iso = 1.2U eq(C) for aromatic, 0.97 Å, U iso = 1.2U eq (C) for CH2, 0.96 Å, U iso = 1.5U eq (C) for CH3 atoms and d(O–H) = 0.85 Å, U iso = 1.2U eq (O) for H2O.
3 Comment
In recent years, metal-organic frameworks have attracted extensive attention due to their controllable structures and a wide variety of potential applications. 6 , 7 , 8 , 9 As one special type of MOF materials, the lanthanide MOFs have outstanding performances in fluorescent and photochromic materials 10 , 11 and many excellent Ln–MOFs were used for sensing metal ions or organic molecules. 12 Our team has also developed an europium-organic framework which can sense Fe3+ and Al3+ through fluorescence quenching and enhancement, respectively. 13 The Ln–MOFs may have different properties depending on their ion centers. The Tb3+ and Tb–MOFs usually exhibit strong fluorescence emission in the visible light region. 14 , 15 This property may endow it with unique application value in fields such as luminescent materials and fluorescence sensing. In this work, we report the crystal structure of a terbium-based framework.
The asymmetric unit cell was found to contain one Tb3+ ion, an incompletely deprotonated HBTMIPA ligand, one hydroxyl group, two coordination water molecules and one lattice water molecule. The central Tb3+ is located within a subtly distorted nine-coordination sphere. This sphere is defined by six oxygen atoms stemming from three distinct HBTMIPA ligands, one coordinated hydroxyl group and two coordinated water molecules. The lengths of the Tb–O bonds span a narrow range from 2.303(3) to 2.640(3) Å. Each BTMIPA ligand connects three Tb3+ ions by means of its three carboxylate groups. Significantly, all these carboxylate groups assume a chelating coordination mode, which is different with the coordination mode in previously reported Ln-framwork. 13 Consequently, the central Tb3+ ions were interconnected via BTMIPA ligands, thereby generating a one - dimensional wavy structure oriented in the [010] direction. Adjacent wavy structures are linked to form a two – dimensional structure through O–H⋯O hydrogen bonds (O1W⋯O4$1 = 2.8149(2) Å and O1W–H1WB⋯O4$1 = 161.252(5)∘, symmetry code: $1 = 1 + X, Y, Z) in [100] direction. The extended three-dimensional supramolecular structure was formed by other O–H⋯O hydrogen bonds (O8⋯O2$2 = 2.6657(1) Å and O8–H8⋯O2$2 = 127.330(4)∘, symmetry code: $2 = −1/2 + X, −1/2−Y, −1−Z).
Funding source: Hebei Minzu Normal University Postgraduate Funding Project
Award Identifier / Grant number: HQ2025003
Funding source: S&T program of Chengde
Award Identifier / Grant number: HZLC2024020
Funding source: Hebei Provincial College Students’ Innovation and Entrepreneurship Training Program Project
Funding source: The Construction Project of High-Quality Experimental Courses of Hebei Minzu Normal University
Acknowledgments
We are grateful to the team of the High-Quality Experimental Courses of Hebei Minzu Normal University for their support in terms of venues and guidance provided for certain experiments.
-
Research funding: This research is funded by S&T program of Chengde (No. HZLC2024020), Hebei Provincial College Students’ Innovation and Entrepreneurship Training Program Project, Hebei Minzu Normal University Postgraduate Funding Project (No. HQ2025003) and the Construction Project of High-Quality Experimental Courses of Hebei Minzu Normal University.
References
1. Bruker. Sadabs, Saint and Smart; Bruker AXS Inc.: Madison, Wisconsin, USA, 2008.Suche in Google Scholar
2. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. Olex2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Suche in Google Scholar
3. Sheldrick, G. M. A Short History of Shelx. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Suche in Google Scholar PubMed
4. Sheldrick, G. M. Crystal Structure Refinement with Shelxl. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Suche in Google Scholar
5. Brandenburg, K. Diamond. Visual Crystal Structure Information System. Ver. 4.0; Crystal Impact: Bonn, Germany, 2015.Suche in Google Scholar
6. Zhou, H. C.; Long, J. R.; Yaghi, O. M. Introduction to Metal-Organic Frameworks. Chem. Rev. 2012, 112, 673–674; https://doi.org/10.1021/cr300014x.Suche in Google Scholar PubMed
7. Furukawa, H.; Cordova, K. E.; O’Keeffe, M.; Yaghi, O. M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444; https://doi.org/10.1126/science.1230444.Suche in Google Scholar PubMed
8. Yusuf, F. V.; Malek, N. I.; Kailasa, S. K. Review on Metal-Organic Framework Classification, Synthetic Approaches, and Influencing Factors: Applications in Energy, Drug Delivery, and Wastewater Treatment. ACS Omega 2022, 7, 44507–44531; https://doi.org/10.1021/acsomega.2c05310.Suche in Google Scholar PubMed PubMed Central
9. Yuan, S.; Feng, L.; Wang, K.; Pang, J.; Bosch, M.; Lollar, C.; Sun, Y. J.; Qin, J. S.; Yang, X. Y.; Zhang, P.; Wang, Q.; Zou, L. F.; Zhang, Y. M.; Zhang, L. L.; Fang, Y.; Li, J. L.; Zhou, H. C. Stable Metal-Organic Frameworks: Design, Synthesis, and Applications. Adv. Mater. 2018, 30, 1704303–1704337; https://doi.org/10.1002/adma.201704303.Suche in Google Scholar PubMed
10. Li, Q. P.; Du, S. W. A Family of 3D Lanthanide Organic Frameworks with Tunable Luminescence and Slow Magnetic Relaxation. RSC Adv. 2015, 5, 9898–9903; https://doi.org/10.1039/c4ra14939d.Suche in Google Scholar
11. Liu, Q. Y.; Wang, W. F.; Wang, Y. L.; Shan, Z. M.; Wang, M. S.; Tang, J. K. Diversity of Lanthanide(III)-Organic Extended Frameworks with a 4,8-Disulfonyl-2,6-Naphthalenedicarboxylic Acid Ligand: Syntheses, Structures, and Magnetic and Luminescent Properties. Inorg. Chem. 2012, 51, 2381–2392; https://doi.org/10.1021/ic2023727.Suche in Google Scholar PubMed
12. Lustig, W.; Mukherjee, S.; Rudd, N.; Desai, A.; Li, J.; Ghosh, S. Metal-Organic Frameworks: Functional Luminescent and Photonic Materials for Sensing Applications. Chem. Soc. Rev. 2017, 46, 3242; https://doi.org/10.1039/c6cs00930a.Suche in Google Scholar PubMed
13. Chen, Z.; Sun, Y. W.; Zhang, L. L.; Sun, D.; Liu, F. L.; Meng, Q. G.; Wang, R. M.; Sun, D. F. A Tubular Europium-Organic Framework Exhibiting Selective Sensing of Fe3+ and Al3+ Over Mixed Metal Ions. Chem. Commun. 2013, 49, 11557–11559; https://doi.org/10.1039/c3cc46613b.Suche in Google Scholar PubMed
14. Wang, X. F.; Chu, C. X.; Wu, Y. W.; Deng, Y. Y.; Zhou, J.; Yang, M.; Zhang, S. Y.; Huo, D. Q.; Hou, C. J. Synthesis of Yttrium(III)-Based Rare-Earth Metal-Organic Framework Nanoplates and its Applications for Sensing of Fluoride Ions and Ph. Sensors Actuators B: Chem. 2020, 321, 128455; https://doi.org/10.1016/j.snb.2020.128455.Suche in Google Scholar
15. Sun, Y.; Chen, Z.; Wang, X.; Wang, L.; Yang, X.; Liang, X.; Fan, S.; Zhang, P. Novel Ba2+ and Pb2+ Metal-Organic Frameworks Based on a Semi-Rigid Tetra-Carb-Oxy-Lic Acid: Syntheses, Structures, Topologies and Luminescence Properties. Acta Crystallogr., Sect. C: Struct. Chem. 2021, 77, 291; https://doi.org/10.1107/S2053229621005143.Suche in Google Scholar PubMed
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of (S)-N-(10-((2,2-dimethoxyethyl)amino)-1,2,3-trimethoxy-9-oxo-5,6,7,9-tetrahydrobenzo[a]heptalen-7-yl)acetamide, C25H32N2O7
- The crystal structure of 6,6′-difluoro-3,3′-dimethyl-5,5′-di(10H-phenoxazin-10-yl)- [1,1′-biphenyl]-2,2′-dicarbonitrile, C40H24F2N4O2
- Crystal structure of poly[(di-ethylenediamine-κ2N,N′)cadmium(II) tetradedocyloxidohexavanadate] (V4+/V5+ = 2/1), C4H16CdN4O14V6
- The crystal structure of poly[bis(dimethylformamide-κ1N)-(μ4-2′,3,3″,5′-tetrakis(trifluoromethyl)-[1,1′:4′,1″-terphenyl]-4,4″-dicarboxylato-κ4 O,O′: O″,O‴)dicadmium(II)], C27H15CdF12NO5
- Crystal structure of bis(μ2-ferrocenylcarboxylato-O,O′)-(μ3-oxido-κ3O:O:O)-bis(μ2-salicyladoximato-κ2N,O,O′)-(μ2-isopropoxo)-tris(isopropoxy-κ1O trititanium(IV)), C48H55N2O13Fe2Ti3
- Crystal structure of 3-(diethylamino)-7,9,11-trimethyl-8-phenyl-6H,13H-12λ4,13λ4-chromeno[3′,4′:4,5]pyrrolo[1,2-c]pyrrolo[2,1-f][1,3,2]diazaborinin-6-one, C28H26BF2N3O2
- The crystal structure of catena-poly[aqua-μ2-2-nitro-benzene-1,3-dicarboxylato-κ2O,O′)-(1,10-phenanthroline-κ2N,N′)-zinc(II)], C20H13N3O7Zn
- Crystal structure of poly[diaqua-{μ3-1-(3-carboxylatophenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylato-κ4O,O′:O′′:O′′′′}manganese(II)] hydrate
- Crystal structure of N′-((1-hydroxycyclohexyl)(phenyl)methyl)-2-methoxybenzohydrazide methanol solvate, C22H28N2O4
- The cocrystal of caffeic acid — progesterone — water (1/2/1), C51H70O9
- Crystal structure of (((oxido(quinolin-6-yl)methoxy)triphenyl-λ5-stibanyl)oxy)(quinolin-7-yl)methanolate
- Crystal structure of [(E)-6′-(diethylamino)-2-(2-(((E)-pyren-1-ylmethylene)amino)ethyl)-4′-(2-((E)-1,3,3-trimethylindolin-2-ylidene)ethylidene)-1′,2′,3′,4′-tetrahydrospiro[isoindoline-1,9′-xanthen]-3-one]-methanol, solvate C57H56N4O3
- The crystal structure of 1-(acridin-9-yl)pyrrolidine-2,5-dione, C17H22N2O2
- Crystal structure of N-(4-acetylphenyl)-2-(6-methoxynaphthalen-2-yl)propanamide, C22H21NO3
- The crystal structure of 5,10,15,20-tetrakis(4-(1H-1,2,4-triazol-1-yl)phenyl)porphyrin, C52H34N16
- Crystal structure of hexacarbonyl-μ2-[phenylmethanedithiolato-κ4S:S,S′:S′]diiron (Fe–Fe) C13H6Fe2O6S2
- Crystal structure of diiodo-bis(1-((2-propyl-1H-benzo[d]imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κ1N)cadmium(II), C34H34CdI2N10
- Crystal structure of (E)-(3-(3-bromophenyl)acryloyl)ferrocene, C19H15BrFeO
- Crystal structure of catena-poly(μ2-6-chloropyridine-2-carboxylato-κ3N,O:O′)(6-chloropyridine-2-carboxylato-κ2O,N)copper(II), C12H6Cl2N2O4Cu
- Crystal structure of poly[diaqua-μ 3-(5-(3,5-dicarboxy-2,4,6-trimethylbenzyl)-2,4,6-trimethylisophthalato)-κ 6O,O′:O″,O‴:O‴′,O‴″) terbium(III)-monohydrate], C23H28TbO12
- Crystal structure of (E)-2-(((5-chloro-3-methyl-1-phenyl-1H-pyrazol-4-yl)methylene)amino)-3′,6′-dihydroxyspiro[isoindoline-1,9′-xanthen]-3-one – ethanol (1/2), C35H33ClN4O6
- The crystal structure of 3-(5-amino-3-phenylisoxazol-4-yl)-4-chloro-3-hydroxyindolin-2-one, C17H12ClN3O3
- The crystal structure of dimethylammonium 4-[2-(4-fluorophenyl)-4, 5-diphenyl-1H-imidazol-1-yl]benzenesulfonate, C29H26FN3O3S
- Crystal structure of (R)-2-ammonio-3-((5-carboxypentyl)thio)propanoate
- Crystal structure of 4-cyclohexyl-5-(thiophen-2-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione, C12H15N3S2
- The crystal structure of 4,6-bis(dimethylamino)-2-fluoroisophthalonitrile, C12H13FN4
- Hydrogen bonding in the crystal structure of nicotin-1,1′-dium tetrabromidomanganate(II)
- The crystal structure of bis(2-bromobenzyl)(2-((2-oxybenzylidene)amino)-4-methylpentanoato-κ3N, O,O′)tin(IV), C27H27Br2NO3Sn
- Crystal structure of (E)-(3-(p-tolyl)acryloyl)ferrocene, C20H18FeO
- Crystal structure of (E)-7-fluoro-2-((5-(4-methylpiperazin-1-yl)pyridin-2-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C21H22FN3O
- Crystal structure of (E)-7-methoxy-2-((5-(4-methylpiperazin-1-yl)pyridin-2-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C22H25N3O2
- The crystal structure of poly(bis(μ2-1,3,5-tri(1H-imidazol-1-yl)benzene-κ2N:N′)-(μ2-2,3,5,6-tetrafluoroterephthalato-κ2O:O′)-manganese(II), C38H24F4N12O4Mn
- Crystal structure of (3,4-dimethoxybenzyl)triphenylphosphonium bromide ethanol solvate, C29H32BrO3P
- Crystal structure of tetraethylammonium hydrogencarbonate – (diaminomethylene)thiourea – water (2/1/3)
- Crystal structure of N, N-Dimethyl-N′-tosylformimidamide, C10H14N2O2S
- The crystal structure of ethyl 2-methyl-5-oxo-4-(2-methoxyphenyl)-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C20H23N2O4
- Crystal structure of bis(μ2-1,5-bis[(E)-1-(2-hydroxyphenyl)ethylidene] thiocarbonohydrazide)-bis(dimethylformamide)-dizinc(II) dimethylformamide solvate, C40H46N10O6S2Zn2⋅C3H7NO
- Crystal structure of azido-κ1N{hydridotris(3-tert-butyl-5-methylpyrazol-1-yl)borato-κ3N,N′,N″}copper(II), C24H40BCuN9
- The crystal structure of fac-tricarbonyl(1,10-phenanthroline-κ2N,N′)-(azido-κ1N)rhenium(I), C15H8N5O3Re
- Crystal structure of 4-((triphenylphosphonio)methyl)pyridin-1-ium tetrachloridozincate(II), C24H22Cl4NPZn
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of (S)-N-(10-((2,2-dimethoxyethyl)amino)-1,2,3-trimethoxy-9-oxo-5,6,7,9-tetrahydrobenzo[a]heptalen-7-yl)acetamide, C25H32N2O7
- The crystal structure of 6,6′-difluoro-3,3′-dimethyl-5,5′-di(10H-phenoxazin-10-yl)- [1,1′-biphenyl]-2,2′-dicarbonitrile, C40H24F2N4O2
- Crystal structure of poly[(di-ethylenediamine-κ2N,N′)cadmium(II) tetradedocyloxidohexavanadate] (V4+/V5+ = 2/1), C4H16CdN4O14V6
- The crystal structure of poly[bis(dimethylformamide-κ1N)-(μ4-2′,3,3″,5′-tetrakis(trifluoromethyl)-[1,1′:4′,1″-terphenyl]-4,4″-dicarboxylato-κ4 O,O′: O″,O‴)dicadmium(II)], C27H15CdF12NO5
- Crystal structure of bis(μ2-ferrocenylcarboxylato-O,O′)-(μ3-oxido-κ3O:O:O)-bis(μ2-salicyladoximato-κ2N,O,O′)-(μ2-isopropoxo)-tris(isopropoxy-κ1O trititanium(IV)), C48H55N2O13Fe2Ti3
- Crystal structure of 3-(diethylamino)-7,9,11-trimethyl-8-phenyl-6H,13H-12λ4,13λ4-chromeno[3′,4′:4,5]pyrrolo[1,2-c]pyrrolo[2,1-f][1,3,2]diazaborinin-6-one, C28H26BF2N3O2
- The crystal structure of catena-poly[aqua-μ2-2-nitro-benzene-1,3-dicarboxylato-κ2O,O′)-(1,10-phenanthroline-κ2N,N′)-zinc(II)], C20H13N3O7Zn
- Crystal structure of poly[diaqua-{μ3-1-(3-carboxylatophenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylato-κ4O,O′:O′′:O′′′′}manganese(II)] hydrate
- Crystal structure of N′-((1-hydroxycyclohexyl)(phenyl)methyl)-2-methoxybenzohydrazide methanol solvate, C22H28N2O4
- The cocrystal of caffeic acid — progesterone — water (1/2/1), C51H70O9
- Crystal structure of (((oxido(quinolin-6-yl)methoxy)triphenyl-λ5-stibanyl)oxy)(quinolin-7-yl)methanolate
- Crystal structure of [(E)-6′-(diethylamino)-2-(2-(((E)-pyren-1-ylmethylene)amino)ethyl)-4′-(2-((E)-1,3,3-trimethylindolin-2-ylidene)ethylidene)-1′,2′,3′,4′-tetrahydrospiro[isoindoline-1,9′-xanthen]-3-one]-methanol, solvate C57H56N4O3
- The crystal structure of 1-(acridin-9-yl)pyrrolidine-2,5-dione, C17H22N2O2
- Crystal structure of N-(4-acetylphenyl)-2-(6-methoxynaphthalen-2-yl)propanamide, C22H21NO3
- The crystal structure of 5,10,15,20-tetrakis(4-(1H-1,2,4-triazol-1-yl)phenyl)porphyrin, C52H34N16
- Crystal structure of hexacarbonyl-μ2-[phenylmethanedithiolato-κ4S:S,S′:S′]diiron (Fe–Fe) C13H6Fe2O6S2
- Crystal structure of diiodo-bis(1-((2-propyl-1H-benzo[d]imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κ1N)cadmium(II), C34H34CdI2N10
- Crystal structure of (E)-(3-(3-bromophenyl)acryloyl)ferrocene, C19H15BrFeO
- Crystal structure of catena-poly(μ2-6-chloropyridine-2-carboxylato-κ3N,O:O′)(6-chloropyridine-2-carboxylato-κ2O,N)copper(II), C12H6Cl2N2O4Cu
- Crystal structure of poly[diaqua-μ 3-(5-(3,5-dicarboxy-2,4,6-trimethylbenzyl)-2,4,6-trimethylisophthalato)-κ 6O,O′:O″,O‴:O‴′,O‴″) terbium(III)-monohydrate], C23H28TbO12
- Crystal structure of (E)-2-(((5-chloro-3-methyl-1-phenyl-1H-pyrazol-4-yl)methylene)amino)-3′,6′-dihydroxyspiro[isoindoline-1,9′-xanthen]-3-one – ethanol (1/2), C35H33ClN4O6
- The crystal structure of 3-(5-amino-3-phenylisoxazol-4-yl)-4-chloro-3-hydroxyindolin-2-one, C17H12ClN3O3
- The crystal structure of dimethylammonium 4-[2-(4-fluorophenyl)-4, 5-diphenyl-1H-imidazol-1-yl]benzenesulfonate, C29H26FN3O3S
- Crystal structure of (R)-2-ammonio-3-((5-carboxypentyl)thio)propanoate
- Crystal structure of 4-cyclohexyl-5-(thiophen-2-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione, C12H15N3S2
- The crystal structure of 4,6-bis(dimethylamino)-2-fluoroisophthalonitrile, C12H13FN4
- Hydrogen bonding in the crystal structure of nicotin-1,1′-dium tetrabromidomanganate(II)
- The crystal structure of bis(2-bromobenzyl)(2-((2-oxybenzylidene)amino)-4-methylpentanoato-κ3N, O,O′)tin(IV), C27H27Br2NO3Sn
- Crystal structure of (E)-(3-(p-tolyl)acryloyl)ferrocene, C20H18FeO
- Crystal structure of (E)-7-fluoro-2-((5-(4-methylpiperazin-1-yl)pyridin-2-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C21H22FN3O
- Crystal structure of (E)-7-methoxy-2-((5-(4-methylpiperazin-1-yl)pyridin-2-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C22H25N3O2
- The crystal structure of poly(bis(μ2-1,3,5-tri(1H-imidazol-1-yl)benzene-κ2N:N′)-(μ2-2,3,5,6-tetrafluoroterephthalato-κ2O:O′)-manganese(II), C38H24F4N12O4Mn
- Crystal structure of (3,4-dimethoxybenzyl)triphenylphosphonium bromide ethanol solvate, C29H32BrO3P
- Crystal structure of tetraethylammonium hydrogencarbonate – (diaminomethylene)thiourea – water (2/1/3)
- Crystal structure of N, N-Dimethyl-N′-tosylformimidamide, C10H14N2O2S
- The crystal structure of ethyl 2-methyl-5-oxo-4-(2-methoxyphenyl)-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C20H23N2O4
- Crystal structure of bis(μ2-1,5-bis[(E)-1-(2-hydroxyphenyl)ethylidene] thiocarbonohydrazide)-bis(dimethylformamide)-dizinc(II) dimethylformamide solvate, C40H46N10O6S2Zn2⋅C3H7NO
- Crystal structure of azido-κ1N{hydridotris(3-tert-butyl-5-methylpyrazol-1-yl)borato-κ3N,N′,N″}copper(II), C24H40BCuN9
- The crystal structure of fac-tricarbonyl(1,10-phenanthroline-κ2N,N′)-(azido-κ1N)rhenium(I), C15H8N5O3Re
- Crystal structure of 4-((triphenylphosphonio)methyl)pyridin-1-ium tetrachloridozincate(II), C24H22Cl4NPZn

