Abstract
C10H14N2O2S, Triclinic, P1̄ (no. 2), a = 7.622(3) Å, b = 7.949(2) Å, c = 10.868(3) Å, α = 74.179(4)°, β = 78.292(4)°, γ = 62.651(4)°, V = 560.4(3) Å3, Z = 2, Rgt(F) = 0.0498, wRref(F2) = 0.1424, T = 293(2) K.
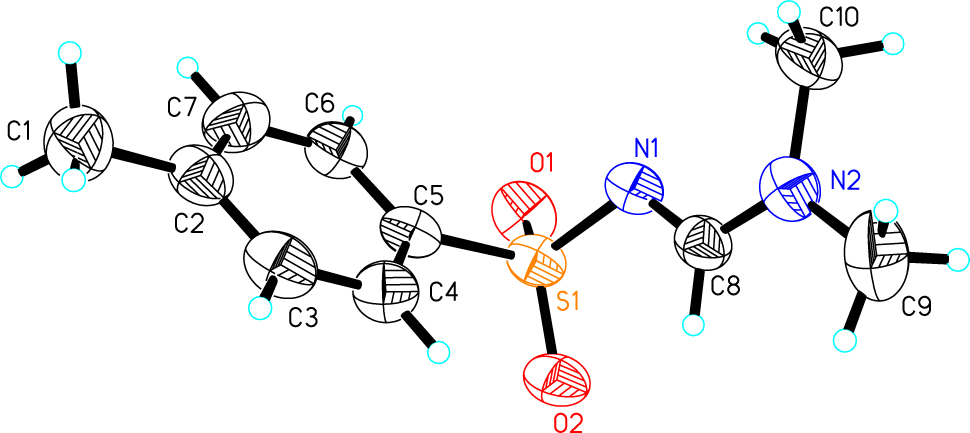
The molecular structure is shown in the figure. Table 1 contains the crystallographic data and the list of the atoms including atomic coordinates and displacement parameters can be found in the cif-file attached to this article.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.05 × 0.05 × 0.04 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.27 mm−1 |
| Diffractometer, scan mode: | Bruker Apex2, φ and ω scans |
| θmax, completeness: | 25.1°, 99 % |
| N(hkl)measured, N(hkl)unique, Rint: | 3988, 1966, 0.036 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 1,446 |
| N(param)refined: | 139 |
| Programs: | Bruker, 1 Olex2, 2 Shelx 3 |
1 Source of materials
According to the synthesis method in refs. [4], [5], [6, NBS (196 mg, 1.1 mmol) and water (3.6 μL) were added to a solution of 4-methylbenzenesulfonamide (171 mg, 1.0 mmol) in DMF (2 mL). The resulting solution was stirred at 80 °C for 8 h. After completion of the reaction, the mixture was extracted with dichloromethane (3 × 10 mL) and the combined extracts were dried with anhydrous sodium sulfate. The solvent was removed under reduced pressure, then the pure product was purified by silica gel column chromatography to obtain the pure white solid. The colorless crystals of N, N-Dimethyl-N′-tosylformimidamide were obtained by slow evaporation of a dichloromethane solution at room temperature.
2 Experimental details
Hydrogen atoms were placed in their geometrically idealized positions and refined isotropically using a riding model. For all C(H) groups, Uiso(H) = 1.2 Ueq(C); for all C(H, H, H) groups, Uiso(H) = 1.5 Ueq(C). Idealised Me was refined as rotating group.
3 Comment
The synthesis of N-sulfonylamidines has attracted a great deal of attention due to their widespread applications in organic synthesis and biochemistry. 7 , 8 , 9 , 10 , 11 Due to their unique structural motifs, they exhibit a range of biological activities like antibacterial, antifungal, and anticancer properties, 12 , 13 and also play an important role in delaying Alzheimer’s disease. 14
The title structure crystallized in the triclinic P1̄ space group, with one molecule in the asymmetric unit. In the crystal structure, a methyl formate group and a sulfamidine group are connected to the same benzene ring with C(1)–C(2) and C(5)–S(1). The bond lengths of S(1)–C(5), S(1)–O(1), S(1)–O(2), and S(1)–N(1) are 1.750(3), 1.429(2), 1.437(2), 1.611(2) Å, respectively. The bond lengths of N(1)–C(8), N(2)–C(8), N(2)–C(10) and N(2)–C(9) are 1.297(4), 1.310(4), 1.450(4) and 1.447(4) Å, respectively. The bond angles of C(5)–S(1)–N(1), C(5)–S(1)–O(2), C(5)–S(1)–O(1), N(1)–S(1)–O(1), N(1)–S(1)–O(2) and O(1)–S(1)–O(2) are 103.42(13), 107.94(13), 108.57(14), 107.26(13), 112.12(13) and 116.69(13)°. The bond angles of N(1)–C(8)–N(2), C(8)–N(2)–C(9) and C(9)–N(2)–C(10) are 122.9(3), 122.2(3) and 117.1(3)°. All geometrical parameters are comparable with those of structural analogs that were found in the Cambridge Structural Database. 15
Acknowledgments
We gratefully acknowledge support by Outstanding Young and Middle-aged Science and Technology Innovation Team Project for Colleges and Universities of Hubei Province, China (NO: T2023052).
References
1. BRUKER. Saint, Apex2 and Sadabs; Bruker AXS Inc.: Madison, Wisconsin, USA, 2009.Suche in Google Scholar
2. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. Olex2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Suche in Google Scholar
3. Sheldrick, G. M. A Short History of Shelx. Acta Cryst. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Suche in Google Scholar PubMed
4. Niu, Z.; Lin, S.; Dong, Z.; Sun, H.; Liang, F.; Zhang, J. Otherwise Inert Reaction of Sulfonamides/Carboxamides with Formamides via Proton Transfer-Enhanced Reactivity. Org. Biomol. Chem. 2013, 11, 2460–2465; https://doi.org/10.1039/c3ob27351b.Suche in Google Scholar PubMed
5. Gazvoda, M.; Marijan, K.; Polanc, S. In Situ Formation of Vilsmeier Reagents Mediated by Oxalyl Chloride: A Tool for the Selective Synthesis of N-Sulfonylformamidines. Eur. J. Org Chem. 2013, 24, 5381–5386.10.1002/ejoc.201300402Suche in Google Scholar
6. Chandna, N.; Chandak, N.; Kumar, P.; Kapoor, J. K.; Sharma, P. K. Metal- and Solvent-Free Synthesis of N-Sulfonylformamidines. Green Chem. 2013, 15, 2294–2301; https://doi.org/10.1039/c3gc40797g.Suche in Google Scholar
7. Yang, Y.; Bai, J.; Shen, R.; Brown, S. A. N.; Komissarova, E.; Huang, Y.; Jiang, N.; Alberts, G. F.; Costa, M.; Lu, L.; Winkles, J. A.; Dai, W. Polo-Like Kinase 3 Functions as a Tumor Suppressor and Is a Negative Regulator of Hypoxia-Inducible Factor-1α Under Hypoxic Conditions. Cancer Res. 2008, 68, 4077–4085; https://doi.org/10.1158/0008-5472.can-07-6182.Suche in Google Scholar
8. Stachulski, A. V.; Meng, X. L. Glucuronides from Metabolites to Medicines: A Survey of the in Vivo Generation, Chemical Synthesis and Properties of Glucuronides. Nat. Prod. Rep. 2013, 30, 806–848; https://doi.org/10.1039/c3np70003h.Suche in Google Scholar PubMed
9. Ethell, B. T.; Riedel, J.; Englert, H.; Jantz, H.; Oekonomopulos, R.; Burchell, B. Glucuronidation of HMR1098 in Human Microsomes: Evidence for the Involvement of UGT1A1 in the Formation of S-Glucuronides. Drug Metab. Dispos. 2003, 31, 1027–1034; https://doi.org/10.1124/dmd.31.8.1027.Suche in Google Scholar PubMed
10. Bekhit, A. A.; Ashour, H. M. A.; Ghany, Y. S. A.; Bekhit, A. E. A.; Baraka, A. Synthesis and Biological Evaluation of Some Thiazolyl and Thiadiazolyl Derivatives of 1H-pyrazole as Anti-Inflammatory Antimicrobial Agents. Eur. J. Med. Chem. 2008, 43, 456–463; https://doi.org/10.1016/j.ejmech.2007.03.030.Suche in Google Scholar PubMed
11. Goubet, A.; Chardon, A.; Kumar, P.; Pawan, K.; Rakesh, S. Synthesis of DNA Oligonucleotides Containing C5-Ethynylbenzenesulfon Amide-Modified Nucleotides (EBNA) by Polymerases Towards the Construction of Base Functionalized Nucleic Acids. Bioorg. Med. Chem. Lett. 2013, 23, 761–763; https://doi.org/10.1016/j.bmcl.2012.11.096.Suche in Google Scholar PubMed
12. Stolic, I.; Paljetak, H.; Peric, M.; Matijasic, M.; Stepanic, V.; Verbanac, D.; Bajic, M. Synthesis and Structure-Activity Relationship of Amidine Derivatives of 3,4-Ethylenedioxythiophene as Novel Antibacterial Agents. Eur. J. Med. Chem. 2015, 90, 68–81; https://doi.org/10.1016/j.ejmech.2014.11.003.Suche in Google Scholar PubMed
13. Wang, M.; Liu, Y.; Chang, L.; Kuo-Hsiung, L.; Zhao, Y. L.; Zhao, X. B.; Qian, K.; Nan, X.; Yang, L.; Yang, X. M.; Hung, H. Y.; Yang, J. S.; Kuo, D. H.; Goto, M.; Morris-Natschke, S. L.; Pan, S. L.; Teng, C. M.; Kuo, S. C.; Wu, T. S.; Wu, Y. C.; Lee, K. H. Design, Synthesis, Mechanisms of Action, and Toxicity of Novel 20(S)-Sulfonylamidine Derivatives of Camptothecin as Potent Antitumor Agents. J. Med. Chem. 2014, 57, 6008–6018; https://doi.org/10.1021/jm5003588.Suche in Google Scholar PubMed PubMed Central
14. Scott, J. D.; Li, S. W.; Brunskill, A. P. J.; Chen, X.; Cox, K.; Cumming, J. N.; Forman, M.; Gilbert, E. J.; Hodgson, R. A.; Hyde, L. A.; Jiang, Q.; Iserloh, U.; Kazakevich, I.; Kuvelkar, R.; Mei, H.; Meredith, J.; Misiaszek, J.; Orth, P.; Rossiter, L. M.; Slater, M.; Stone, J.; Strickland, C. O.; Voigt, J. H.; Wang, G.; Wang, H.; Wu, Y.; Greenlee, W. J.; Parker, E. M.; Kennedy, M. E.; Stamford, A. W. Discovery of the 3-Imino-1,2,4-thiadiazinane 1,1-Dioxide Derivative Verubecestat (MK-8931)–A β-Site Amyloid Precursor Protein Cleaving Enzyme 1 Inhibitor for the Treatment of Alzheimer’s Disease. J. Med. Chem. 2016, 59, 10435–10450; https://doi.org/10.1021/acs.jmedchem.6b00307.Suche in Google Scholar PubMed
15. Guo, C.-X.; Yogendra, S.; Gomila, R. M.; Frontera, A.; Hennersdorf, F.; Steup, J.; Schwedtmann, K.; Weigand, J. J. A Convenient Access to Fluorophosphonium Triflate Salts by Electrophilic Fluorination and Anion Exchange. Inorg. Chem. Front. 2021, 8, 2854–2864; https://doi.org/10.1039/D1QI00322D.Suche in Google Scholar
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of (S)-N-(10-((2,2-dimethoxyethyl)amino)-1,2,3-trimethoxy-9-oxo-5,6,7,9-tetrahydrobenzo[a]heptalen-7-yl)acetamide, C25H32N2O7
- The crystal structure of 6,6′-difluoro-3,3′-dimethyl-5,5′-di(10H-phenoxazin-10-yl)- [1,1′-biphenyl]-2,2′-dicarbonitrile, C40H24F2N4O2
- Crystal structure of poly[(di-ethylenediamine-κ2N,N′)cadmium(II) tetradedocyloxidohexavanadate] (V4+/V5+ = 2/1), C4H16CdN4O14V6
- The crystal structure of poly[bis(dimethylformamide-κ1N)-(μ4-2′,3,3″,5′-tetrakis(trifluoromethyl)-[1,1′:4′,1″-terphenyl]-4,4″-dicarboxylato-κ4 O,O′: O″,O‴)dicadmium(II)], C27H15CdF12NO5
- Crystal structure of bis(μ2-ferrocenylcarboxylato-O,O′)-(μ3-oxido-κ3O:O:O)-bis(μ2-salicyladoximato-κ2N,O,O′)-(μ2-isopropoxo)-tris(isopropoxy-κ1O trititanium(IV)), C48H55N2O13Fe2Ti3
- Crystal structure of 3-(diethylamino)-7,9,11-trimethyl-8-phenyl-6H,13H-12λ4,13λ4-chromeno[3′,4′:4,5]pyrrolo[1,2-c]pyrrolo[2,1-f][1,3,2]diazaborinin-6-one, C28H26BF2N3O2
- The crystal structure of catena-poly[aqua-μ2-2-nitro-benzene-1,3-dicarboxylato-κ2O,O′)-(1,10-phenanthroline-κ2N,N′)-zinc(II)], C20H13N3O7Zn
- Crystal structure of poly[diaqua-{μ3-1-(3-carboxylatophenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylato-κ4O,O′:O′′:O′′′′}manganese(II)] hydrate
- Crystal structure of N′-((1-hydroxycyclohexyl)(phenyl)methyl)-2-methoxybenzohydrazide methanol solvate, C22H28N2O4
- The cocrystal of caffeic acid — progesterone — water (1/2/1), C51H70O9
- Crystal structure of (((oxido(quinolin-6-yl)methoxy)triphenyl-λ5-stibanyl)oxy)(quinolin-7-yl)methanolate
- Crystal structure of [(E)-6′-(diethylamino)-2-(2-(((E)-pyren-1-ylmethylene)amino)ethyl)-4′-(2-((E)-1,3,3-trimethylindolin-2-ylidene)ethylidene)-1′,2′,3′,4′-tetrahydrospiro[isoindoline-1,9′-xanthen]-3-one]-methanol, solvate C57H56N4O3
- The crystal structure of 1-(acridin-9-yl)pyrrolidine-2,5-dione, C17H22N2O2
- Crystal structure of N-(4-acetylphenyl)-2-(6-methoxynaphthalen-2-yl)propanamide, C22H21NO3
- The crystal structure of 5,10,15,20-tetrakis(4-(1H-1,2,4-triazol-1-yl)phenyl)porphyrin, C52H34N16
- Crystal structure of hexacarbonyl-μ2-[phenylmethanedithiolato-κ4S:S,S′:S′]diiron (Fe–Fe) C13H6Fe2O6S2
- Crystal structure of diiodo-bis(1-((2-propyl-1H-benzo[d]imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κ1N)cadmium(II), C34H34CdI2N10
- Crystal structure of (E)-(3-(3-bromophenyl)acryloyl)ferrocene, C19H15BrFeO
- Crystal structure of catena-poly(μ2-6-chloropyridine-2-carboxylato-κ3N,O:O′)(6-chloropyridine-2-carboxylato-κ2O,N)copper(II), C12H6Cl2N2O4Cu
- Crystal structure of poly[diaqua-μ 3-(5-(3,5-dicarboxy-2,4,6-trimethylbenzyl)-2,4,6-trimethylisophthalato)-κ 6O,O′:O″,O‴:O‴′,O‴″) terbium(III)-monohydrate], C23H28TbO12
- Crystal structure of (E)-2-(((5-chloro-3-methyl-1-phenyl-1H-pyrazol-4-yl)methylene)amino)-3′,6′-dihydroxyspiro[isoindoline-1,9′-xanthen]-3-one – ethanol (1/2), C35H33ClN4O6
- The crystal structure of 3-(5-amino-3-phenylisoxazol-4-yl)-4-chloro-3-hydroxyindolin-2-one, C17H12ClN3O3
- The crystal structure of dimethylammonium 4-[2-(4-fluorophenyl)-4, 5-diphenyl-1H-imidazol-1-yl]benzenesulfonate, C29H26FN3O3S
- Crystal structure of (R)-2-ammonio-3-((5-carboxypentyl)thio)propanoate
- Crystal structure of 4-cyclohexyl-5-(thiophen-2-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione, C12H15N3S2
- The crystal structure of 4,6-bis(dimethylamino)-2-fluoroisophthalonitrile, C12H13FN4
- Hydrogen bonding in the crystal structure of nicotin-1,1′-dium tetrabromidomanganate(II)
- The crystal structure of bis(2-bromobenzyl)(2-((2-oxybenzylidene)amino)-4-methylpentanoato-κ3N, O,O′)tin(IV), C27H27Br2NO3Sn
- Crystal structure of (E)-(3-(p-tolyl)acryloyl)ferrocene, C20H18FeO
- Crystal structure of (E)-7-fluoro-2-((5-(4-methylpiperazin-1-yl)pyridin-2-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C21H22FN3O
- Crystal structure of (E)-7-methoxy-2-((5-(4-methylpiperazin-1-yl)pyridin-2-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C22H25N3O2
- The crystal structure of poly(bis(μ2-1,3,5-tri(1H-imidazol-1-yl)benzene-κ2N:N′)-(μ2-2,3,5,6-tetrafluoroterephthalato-κ2O:O′)-manganese(II), C38H24F4N12O4Mn
- Crystal structure of (3,4-dimethoxybenzyl)triphenylphosphonium bromide ethanol solvate, C29H32BrO3P
- Crystal structure of tetraethylammonium hydrogencarbonate – (diaminomethylene)thiourea – water (2/1/3)
- Crystal structure of N, N-Dimethyl-N′-tosylformimidamide, C10H14N2O2S
- The crystal structure of ethyl 2-methyl-5-oxo-4-(2-methoxyphenyl)-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C20H23N2O4
- Crystal structure of bis(μ2-1,5-bis[(E)-1-(2-hydroxyphenyl)ethylidene] thiocarbonohydrazide)-bis(dimethylformamide)-dizinc(II) dimethylformamide solvate, C40H46N10O6S2Zn2⋅C3H7NO
- Crystal structure of azido-κ1N{hydridotris(3-tert-butyl-5-methylpyrazol-1-yl)borato-κ3N,N′,N″}copper(II), C24H40BCuN9
- The crystal structure of fac-tricarbonyl(1,10-phenanthroline-κ2N,N′)-(azido-κ1N)rhenium(I), C15H8N5O3Re
- Crystal structure of 4-((triphenylphosphonio)methyl)pyridin-1-ium tetrachloridozincate(II), C24H22Cl4NPZn
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of (S)-N-(10-((2,2-dimethoxyethyl)amino)-1,2,3-trimethoxy-9-oxo-5,6,7,9-tetrahydrobenzo[a]heptalen-7-yl)acetamide, C25H32N2O7
- The crystal structure of 6,6′-difluoro-3,3′-dimethyl-5,5′-di(10H-phenoxazin-10-yl)- [1,1′-biphenyl]-2,2′-dicarbonitrile, C40H24F2N4O2
- Crystal structure of poly[(di-ethylenediamine-κ2N,N′)cadmium(II) tetradedocyloxidohexavanadate] (V4+/V5+ = 2/1), C4H16CdN4O14V6
- The crystal structure of poly[bis(dimethylformamide-κ1N)-(μ4-2′,3,3″,5′-tetrakis(trifluoromethyl)-[1,1′:4′,1″-terphenyl]-4,4″-dicarboxylato-κ4 O,O′: O″,O‴)dicadmium(II)], C27H15CdF12NO5
- Crystal structure of bis(μ2-ferrocenylcarboxylato-O,O′)-(μ3-oxido-κ3O:O:O)-bis(μ2-salicyladoximato-κ2N,O,O′)-(μ2-isopropoxo)-tris(isopropoxy-κ1O trititanium(IV)), C48H55N2O13Fe2Ti3
- Crystal structure of 3-(diethylamino)-7,9,11-trimethyl-8-phenyl-6H,13H-12λ4,13λ4-chromeno[3′,4′:4,5]pyrrolo[1,2-c]pyrrolo[2,1-f][1,3,2]diazaborinin-6-one, C28H26BF2N3O2
- The crystal structure of catena-poly[aqua-μ2-2-nitro-benzene-1,3-dicarboxylato-κ2O,O′)-(1,10-phenanthroline-κ2N,N′)-zinc(II)], C20H13N3O7Zn
- Crystal structure of poly[diaqua-{μ3-1-(3-carboxylatophenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylato-κ4O,O′:O′′:O′′′′}manganese(II)] hydrate
- Crystal structure of N′-((1-hydroxycyclohexyl)(phenyl)methyl)-2-methoxybenzohydrazide methanol solvate, C22H28N2O4
- The cocrystal of caffeic acid — progesterone — water (1/2/1), C51H70O9
- Crystal structure of (((oxido(quinolin-6-yl)methoxy)triphenyl-λ5-stibanyl)oxy)(quinolin-7-yl)methanolate
- Crystal structure of [(E)-6′-(diethylamino)-2-(2-(((E)-pyren-1-ylmethylene)amino)ethyl)-4′-(2-((E)-1,3,3-trimethylindolin-2-ylidene)ethylidene)-1′,2′,3′,4′-tetrahydrospiro[isoindoline-1,9′-xanthen]-3-one]-methanol, solvate C57H56N4O3
- The crystal structure of 1-(acridin-9-yl)pyrrolidine-2,5-dione, C17H22N2O2
- Crystal structure of N-(4-acetylphenyl)-2-(6-methoxynaphthalen-2-yl)propanamide, C22H21NO3
- The crystal structure of 5,10,15,20-tetrakis(4-(1H-1,2,4-triazol-1-yl)phenyl)porphyrin, C52H34N16
- Crystal structure of hexacarbonyl-μ2-[phenylmethanedithiolato-κ4S:S,S′:S′]diiron (Fe–Fe) C13H6Fe2O6S2
- Crystal structure of diiodo-bis(1-((2-propyl-1H-benzo[d]imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κ1N)cadmium(II), C34H34CdI2N10
- Crystal structure of (E)-(3-(3-bromophenyl)acryloyl)ferrocene, C19H15BrFeO
- Crystal structure of catena-poly(μ2-6-chloropyridine-2-carboxylato-κ3N,O:O′)(6-chloropyridine-2-carboxylato-κ2O,N)copper(II), C12H6Cl2N2O4Cu
- Crystal structure of poly[diaqua-μ 3-(5-(3,5-dicarboxy-2,4,6-trimethylbenzyl)-2,4,6-trimethylisophthalato)-κ 6O,O′:O″,O‴:O‴′,O‴″) terbium(III)-monohydrate], C23H28TbO12
- Crystal structure of (E)-2-(((5-chloro-3-methyl-1-phenyl-1H-pyrazol-4-yl)methylene)amino)-3′,6′-dihydroxyspiro[isoindoline-1,9′-xanthen]-3-one – ethanol (1/2), C35H33ClN4O6
- The crystal structure of 3-(5-amino-3-phenylisoxazol-4-yl)-4-chloro-3-hydroxyindolin-2-one, C17H12ClN3O3
- The crystal structure of dimethylammonium 4-[2-(4-fluorophenyl)-4, 5-diphenyl-1H-imidazol-1-yl]benzenesulfonate, C29H26FN3O3S
- Crystal structure of (R)-2-ammonio-3-((5-carboxypentyl)thio)propanoate
- Crystal structure of 4-cyclohexyl-5-(thiophen-2-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione, C12H15N3S2
- The crystal structure of 4,6-bis(dimethylamino)-2-fluoroisophthalonitrile, C12H13FN4
- Hydrogen bonding in the crystal structure of nicotin-1,1′-dium tetrabromidomanganate(II)
- The crystal structure of bis(2-bromobenzyl)(2-((2-oxybenzylidene)amino)-4-methylpentanoato-κ3N, O,O′)tin(IV), C27H27Br2NO3Sn
- Crystal structure of (E)-(3-(p-tolyl)acryloyl)ferrocene, C20H18FeO
- Crystal structure of (E)-7-fluoro-2-((5-(4-methylpiperazin-1-yl)pyridin-2-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C21H22FN3O
- Crystal structure of (E)-7-methoxy-2-((5-(4-methylpiperazin-1-yl)pyridin-2-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C22H25N3O2
- The crystal structure of poly(bis(μ2-1,3,5-tri(1H-imidazol-1-yl)benzene-κ2N:N′)-(μ2-2,3,5,6-tetrafluoroterephthalato-κ2O:O′)-manganese(II), C38H24F4N12O4Mn
- Crystal structure of (3,4-dimethoxybenzyl)triphenylphosphonium bromide ethanol solvate, C29H32BrO3P
- Crystal structure of tetraethylammonium hydrogencarbonate – (diaminomethylene)thiourea – water (2/1/3)
- Crystal structure of N, N-Dimethyl-N′-tosylformimidamide, C10H14N2O2S
- The crystal structure of ethyl 2-methyl-5-oxo-4-(2-methoxyphenyl)-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C20H23N2O4
- Crystal structure of bis(μ2-1,5-bis[(E)-1-(2-hydroxyphenyl)ethylidene] thiocarbonohydrazide)-bis(dimethylformamide)-dizinc(II) dimethylformamide solvate, C40H46N10O6S2Zn2⋅C3H7NO
- Crystal structure of azido-κ1N{hydridotris(3-tert-butyl-5-methylpyrazol-1-yl)borato-κ3N,N′,N″}copper(II), C24H40BCuN9
- The crystal structure of fac-tricarbonyl(1,10-phenanthroline-κ2N,N′)-(azido-κ1N)rhenium(I), C15H8N5O3Re
- Crystal structure of 4-((triphenylphosphonio)methyl)pyridin-1-ium tetrachloridozincate(II), C24H22Cl4NPZn

