Abstract
C25H32N2O7, Tetragonal, P43212, a = 9.0226 (8) Å, c = 59.048 (6) Å, V = 4806.9(10) Å3, Z = 8, Rgt(F) = 0.043, wRref(F2) = 0.112, T = 100 K.
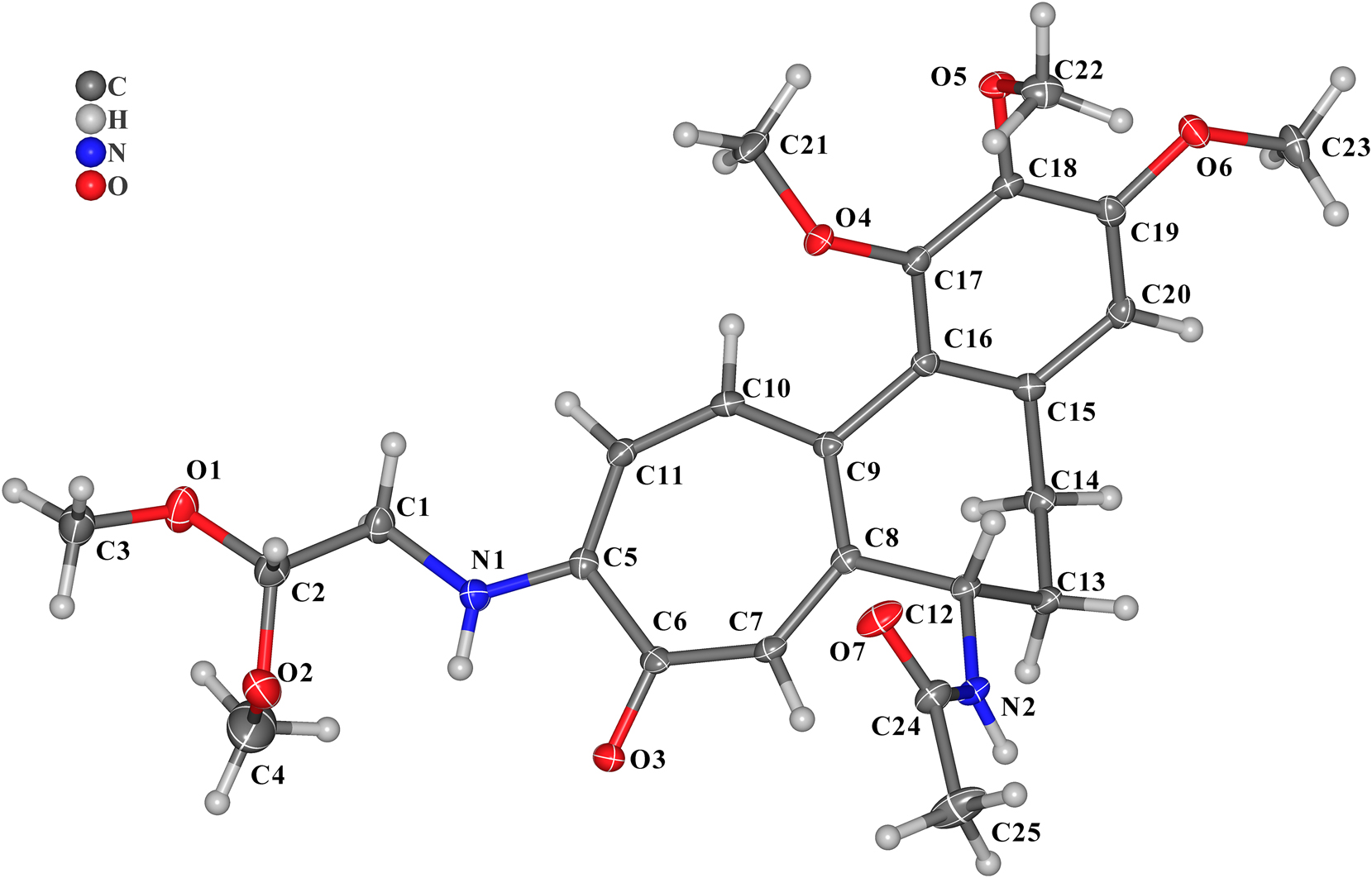
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless block |
| Size | 0.12 × 0.11 × 0.10 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.10 mm−1 |
| Diffractometer, scan mode: | Bruker PHOTON-II area detector, φ and ω scans |
| θmax, completeness: | 26.8°, 100 % |
| N(hkl)measured, N(hkl)unique, Rint: | 127638, 5134, 0.058 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 4,822 |
| N(param)refined: | 313 |
| Programs: | Bruker, 1 Olex2, 2 SHELX 3 , 4 |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| x | y | z | Uiso*/Ueq | |
|---|---|---|---|---|
| O1 | 1.1362 (2) | 1.0533 (2) | 0.32888 (4) | 0.0272 (5) |
| O2 | 0.9917 (3) | 1.0767 (3) | 0.29590 (4) | 0.0310 (5) |
| O3 | 0.7118 (2) | 0.7517 (2) | 0.26921 (3) | 0.0174 (4) |
| O4 | 0.5687 (2) | 0.3271 (2) | 0.36141 (3) | 0.0161 (4) |
| O5 | 0.4949 (2) | 0.0552 (2) | 0.38037 (3) | 0.0156 (4) |
| O6 | 0.4719 (2) | −0.1867 (2) | 0.35447 (3) | 0.0169 (4) |
| O7 | 0.2391 (2) | 0.5363 (2) | 0.30355 (3) | 0.0236 (5) |
| N1 | 0.8979 (3) | 0.7897 (3) | 0.30090 (4) | 0.0166 (5) |
| H1 | 0.873993 | 0.848915 | 0.289643 | 0.020* |
| N2 | 0.3277 (2) | 0.3967 (2) | 0.27457 (4) | 0.0138 (4) |
| H2 | 0.323223 | 0.378569 | 0.259943 | 0.017* |
| C1 | 1.0151 (3) | 0.8412 (3) | 0.31577 (5) | 0.0185 (6) |
| H1A | 1.112702 | 0.810875 | 0.309666 | 0.022* |
| H1B | 1.003254 | 0.796577 | 0.330978 | 0.022* |
| C2 | 1.0074 (3) | 1.0091 (3) | 0.31747 (5) | 0.0223 (6) |
| H2A | 0.919666 | 1.036293 | 0.326952 | 0.027* |
| C3 | 1.1330 (4) | 1.2042 (3) | 0.33578 (6) | 0.0277 (7) |
| H3A | 1.108166 | 1.266975 | 0.322784 | 0.042* |
| H3B | 1.230545 | 1.232512 | 0.341682 | 0.042* |
| H3C | 1.058212 | 1.217167 | 0.347650 | 0.042* |
| C4 | 1.1124 (5) | 1.0517 (5) | 0.28124 (7) | 0.0484 (11) |
| H4A | 1.113393 | 1.127753 | 0.269397 | 0.073* |
| H4B | 1.102674 | 0.953604 | 0.274270 | 0.073* |
| H4C | 1.205078 | 1.056321 | 0.289870 | 0.073* |
| C5 | 0.8217 (3) | 0.6625 (3) | 0.30230 (4) | 0.0133 (5) |
| C6 | 0.7092 (3) | 0.6512 (3) | 0.28423 (4) | 0.0131 (5) |
| C7 | 0.5996 (3) | 0.5382 (3) | 0.28323 (4) | 0.0140 (5) |
| H7 | 0.532036 | 0.551598 | 0.271055 | 0.017* |
| C8 | 0.5690 (3) | 0.4135 (3) | 0.29594 (4) | 0.0121 (5) |
| C9 | 0.6502 (3) | 0.3575 (3) | 0.31459 (4) | 0.0119 (5) |
| C10 | 0.7775 (3) | 0.4231 (3) | 0.32340 (4) | 0.0141 (5) |
| H10 | 0.822123 | 0.367530 | 0.335239 | 0.017* |
| C11 | 0.8522 (3) | 0.5535 (3) | 0.31836 (4) | 0.0144 (5) |
| H11 | 0.938000 | 0.571241 | 0.327289 | 0.017* |
| C12 | 0.4371 (3) | 0.3187 (3) | 0.28818 (4) | 0.0128 (5) |
| H12 | 0.386439 | 0.278587 | 0.301938 | 0.015* |
| C13 | 0.4955 (3) | 0.1868 (3) | 0.27416 (4) | 0.0151 (5) |
| H13A | 0.412577 | 0.117987 | 0.271022 | 0.018* |
| H13B | 0.532924 | 0.223950 | 0.259457 | 0.018* |
| C14 | 0.6201 (3) | 0.1016 (3) | 0.28621 (4) | 0.0152 (5) |
| H14A | 0.629548 | 0.001729 | 0.279416 | 0.018* |
| H14B | 0.715177 | 0.154364 | 0.283945 | 0.018* |
| C15 | 0.5898 (3) | 0.0866 (3) | 0.31140 (4) | 0.0136 (5) |
| C16 | 0.6026 (3) | 0.2135 (3) | 0.32494 (4) | 0.0122 (5) |
| C17 | 0.5689 (3) | 0.2012 (3) | 0.34815 (4) | 0.0130 (5) |
| C18 | 0.5247 (3) | 0.0662 (3) | 0.35748 (4) | 0.0128 (5) |
| C19 | 0.5140 (3) | −0.0596 (3) | 0.34367 (4) | 0.0143 (5) |
| C20 | 0.5460 (3) | −0.0489 (3) | 0.32067 (4) | 0.0147 (5) |
| H20 | 0.538051 | −0.133946 | 0.311261 | 0.018* |
| C21 | 0.6830 (3) | 0.3319 (4) | 0.37843 (5) | 0.0232 (6) |
| H21A | 0.780426 | 0.331262 | 0.371085 | 0.035* |
| H21B | 0.674076 | 0.245227 | 0.388337 | 0.035* |
| H21C | 0.672347 | 0.422491 | 0.387440 | 0.035* |
| C22 | 0.3394 (3) | 0.0645 (3) | 0.38558 (5) | 0.0204 (6) |
| H22A | 0.284658 | −0.006598 | 0.376222 | 0.031* |
| H22B | 0.303792 | 0.164988 | 0.382433 | 0.031* |
| H22C | 0.323926 | 0.041328 | 0.401617 | 0.031* |
| C23 | 0.4796 (3) | −0.3212 (3) | 0.34170 (5) | 0.0200 (6) |
| H23A | 0.404895 | −0.319228 | 0.329664 | 0.030* |
| H23B | 0.460887 | −0.405680 | 0.351728 | 0.030* |
| H23C | 0.578449 | −0.330791 | 0.334968 | 0.030* |
| C24 | 0.2328 (3) | 0.4959 (3) | 0.28364 (5) | 0.0179 (6) |
| C25 | 0.1182 (4) | 0.5555 (4) | 0.26727 (5) | 0.0322 (8) |
| H25A | 0.020261 | 0.516551 | 0.271296 | 0.048* |
| H25B | 0.143453 | 0.524597 | 0.251836 | 0.048* |
| H25C | 0.116574 | 0.664000 | 0.268082 | 0.048* |
1 Source of materials
A solution of colchicine (400 mg, 1.0 mmol) and 2,2-dimethoxyethan-1-amine (1.05 g, 10.0 mmol) was stirred in ethanol at reflux for 3 h. Following evaporation, the mixture was extracted with ethyl acetate (3 × 10 mL), and then the solvent was removed to obtain a yellow solid, which was subsequently dissolved in 90 % methanol and slowly evaporated to obtain crystals.
2 Experimental details
A suitable crystal was selected and tested on a Bruker D8 VENTURE diffractometer 1 . Using Olex2 1 , the structure was solved with the SHELXT 2 structure solution program Intrinsic Phasing and refined with the SHELX 3 refinement package using Least Squares minimisation.
3 Comment
Colchicine, one of the oldest drugs and one of the most famous natural molecules, is found mainly in plants of the genus Colchicine, the genus androcymbium, and the genus gloriosa. 4 Colchicine and its derivatives have anti-tumor, anti-gout and other biological activities. 5 We synthesized a colchicine derivative in this study.
A view on the structure of the title molecule is shown in the figure. The compound contains a three-methoxy modified benzene ring and a tropone. And the amine group of 2,2-dimethoxyethan-1-amine successfully replaced the methoxy ring of tropone. N–C single bond distance (N1–C5) is 1.340 (3) Å. N–C single bond distance (N1–C1) is 1.451(3) Å. The dihedral angle of benzene ring and the tropone is 55.635(75)°. All the bond lengths and angles are in the expected ranges. 6 , 7
-
Conflict of interest: The authors declare no conflicts of interest regarding this article.
-
Research funding: This work was financially supported by Jilin Provincial Science and Technology Development Plan Project (grant No. 20220204033YY).
-
Author contribution: All the authors have accepted responsibility for the manuscript and approved submission.
References
1. BRUKER. Saint, Apex2 and Sadabs; Bruker AXS Inc.: Madison, Wisconsin, USA, 2009.Search in Google Scholar
2. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. Olex2: a Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
3. Sheldrick, G. M. Crystal Structurer Refinement with Shelxl. Acta Crystallogr. 2015, C71, 3–8.10.1107/S2053229614024218Search in Google Scholar PubMed PubMed Central
4. Sheldrick, G. M. A Short History of Shelx (2008). Acta Crystallogr. 2008, A64, 112–122.10.1107/S0108767307043930Search in Google Scholar PubMed
5. Wallace, S. Benjamin Franklin and the Introduction of Colchicum into the United States. Bull Hist Med 1968, 42, 312–320.Search in Google Scholar
6. Gracheva, I. A.; Shchegravina, E. S.; Schmalz, H. –G.; Beletskaya, I. P.; Fedorov, A.Yu. Colchicine Alkaloids and Synthetic Analogues: Current Progress and Perspectives. J. Med. Chem. 2020, 63, 10618–10651; https://doi.org/10.1021/acs.jmedchem.0c00222.Search in Google Scholar PubMed
7. Czerwonka, D.; Sobczak, S.; Pedzinski, T.; Maj, E.; Wietrzyk, J.; Celewicz, L.; Katrusiak, A.; Huczyński, A. Photoinduced Skeletal Rearrangement of N-Substituted Colchicine Derivatives. J. Org. Chem. 2021, 86, 11029; https://doi.org/10.1021/acs.joc.0c02507.Search in Google Scholar PubMed PubMed Central
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of (S)-N-(10-((2,2-dimethoxyethyl)amino)-1,2,3-trimethoxy-9-oxo-5,6,7,9-tetrahydrobenzo[a]heptalen-7-yl)acetamide, C25H32N2O7
- The crystal structure of 6,6′-difluoro-3,3′-dimethyl-5,5′-di(10H-phenoxazin-10-yl)- [1,1′-biphenyl]-2,2′-dicarbonitrile, C40H24F2N4O2
- Crystal structure of poly[(di-ethylenediamine-κ2N,N′)cadmium(II) tetradedocyloxidohexavanadate] (V4+/V5+ = 2/1), C4H16CdN4O14V6
- The crystal structure of poly[bis(dimethylformamide-κ1N)-(μ4-2′,3,3″,5′-tetrakis(trifluoromethyl)-[1,1′:4′,1″-terphenyl]-4,4″-dicarboxylato-κ4 O,O′: O″,O‴)dicadmium(II)], C27H15CdF12NO5
- Crystal structure of bis(μ2-ferrocenylcarboxylato-O,O′)-(μ3-oxido-κ3O:O:O)-bis(μ2-salicyladoximato-κ2N,O,O′)-(μ2-isopropoxo)-tris(isopropoxy-κ1O trititanium(IV)), C48H55N2O13Fe2Ti3
- Crystal structure of 3-(diethylamino)-7,9,11-trimethyl-8-phenyl-6H,13H-12λ4,13λ4-chromeno[3′,4′:4,5]pyrrolo[1,2-c]pyrrolo[2,1-f][1,3,2]diazaborinin-6-one, C28H26BF2N3O2
- The crystal structure of catena-poly[aqua-μ2-2-nitro-benzene-1,3-dicarboxylato-κ2O,O′)-(1,10-phenanthroline-κ2N,N′)-zinc(II)], C20H13N3O7Zn
- Crystal structure of poly[diaqua-{μ3-1-(3-carboxylatophenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylato-κ4O,O′:O′′:O′′′′}manganese(II)] hydrate
- Crystal structure of N′-((1-hydroxycyclohexyl)(phenyl)methyl)-2-methoxybenzohydrazide methanol solvate, C22H28N2O4
- The cocrystal of caffeic acid — progesterone — water (1/2/1), C51H70O9
- Crystal structure of (((oxido(quinolin-6-yl)methoxy)triphenyl-λ5-stibanyl)oxy)(quinolin-7-yl)methanolate
- Crystal structure of [(E)-6′-(diethylamino)-2-(2-(((E)-pyren-1-ylmethylene)amino)ethyl)-4′-(2-((E)-1,3,3-trimethylindolin-2-ylidene)ethylidene)-1′,2′,3′,4′-tetrahydrospiro[isoindoline-1,9′-xanthen]-3-one]-methanol, solvate C57H56N4O3
- The crystal structure of 1-(acridin-9-yl)pyrrolidine-2,5-dione, C17H22N2O2
- Crystal structure of N-(4-acetylphenyl)-2-(6-methoxynaphthalen-2-yl)propanamide, C22H21NO3
- The crystal structure of 5,10,15,20-tetrakis(4-(1H-1,2,4-triazol-1-yl)phenyl)porphyrin, C52H34N16
- Crystal structure of hexacarbonyl-μ2-[phenylmethanedithiolato-κ4S:S,S′:S′]diiron (Fe–Fe) C13H6Fe2O6S2
- Crystal structure of diiodo-bis(1-((2-propyl-1H-benzo[d]imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κ1N)cadmium(II), C34H34CdI2N10
- Crystal structure of (E)-(3-(3-bromophenyl)acryloyl)ferrocene, C19H15BrFeO
- Crystal structure of catena-poly(μ2-6-chloropyridine-2-carboxylato-κ3N,O:O′)(6-chloropyridine-2-carboxylato-κ2O,N)copper(II), C12H6Cl2N2O4Cu
- Crystal structure of poly[diaqua-μ 3-(5-(3,5-dicarboxy-2,4,6-trimethylbenzyl)-2,4,6-trimethylisophthalato)-κ 6O,O′:O″,O‴:O‴′,O‴″) terbium(III)-monohydrate], C23H28TbO12
- Crystal structure of (E)-2-(((5-chloro-3-methyl-1-phenyl-1H-pyrazol-4-yl)methylene)amino)-3′,6′-dihydroxyspiro[isoindoline-1,9′-xanthen]-3-one – ethanol (1/2), C35H33ClN4O6
- The crystal structure of 3-(5-amino-3-phenylisoxazol-4-yl)-4-chloro-3-hydroxyindolin-2-one, C17H12ClN3O3
- The crystal structure of dimethylammonium 4-[2-(4-fluorophenyl)-4, 5-diphenyl-1H-imidazol-1-yl]benzenesulfonate, C29H26FN3O3S
- Crystal structure of (R)-2-ammonio-3-((5-carboxypentyl)thio)propanoate
- Crystal structure of 4-cyclohexyl-5-(thiophen-2-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione, C12H15N3S2
- The crystal structure of 4,6-bis(dimethylamino)-2-fluoroisophthalonitrile, C12H13FN4
- Hydrogen bonding in the crystal structure of nicotin-1,1′-dium tetrabromidomanganate(II)
- The crystal structure of bis(2-bromobenzyl)(2-((2-oxybenzylidene)amino)-4-methylpentanoato-κ3N, O,O′)tin(IV), C27H27Br2NO3Sn
- Crystal structure of (E)-(3-(p-tolyl)acryloyl)ferrocene, C20H18FeO
- Crystal structure of (E)-7-fluoro-2-((5-(4-methylpiperazin-1-yl)pyridin-2-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C21H22FN3O
- Crystal structure of (E)-7-methoxy-2-((5-(4-methylpiperazin-1-yl)pyridin-2-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C22H25N3O2
- The crystal structure of poly(bis(μ2-1,3,5-tri(1H-imidazol-1-yl)benzene-κ2N:N′)-(μ2-2,3,5,6-tetrafluoroterephthalato-κ2O:O′)-manganese(II), C38H24F4N12O4Mn
- Crystal structure of (3,4-dimethoxybenzyl)triphenylphosphonium bromide ethanol solvate, C29H32BrO3P
- Crystal structure of tetraethylammonium hydrogencarbonate – (diaminomethylene)thiourea – water (2/1/3)
- Crystal structure of N, N-Dimethyl-N′-tosylformimidamide, C10H14N2O2S
- The crystal structure of ethyl 2-methyl-5-oxo-4-(2-methoxyphenyl)-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C20H23N2O4
- Crystal structure of bis(μ2-1,5-bis[(E)-1-(2-hydroxyphenyl)ethylidene] thiocarbonohydrazide)-bis(dimethylformamide)-dizinc(II) dimethylformamide solvate, C40H46N10O6S2Zn2⋅C3H7NO
- Crystal structure of azido-κ1N{hydridotris(3-tert-butyl-5-methylpyrazol-1-yl)borato-κ3N,N′,N″}copper(II), C24H40BCuN9
- The crystal structure of fac-tricarbonyl(1,10-phenanthroline-κ2N,N′)-(azido-κ1N)rhenium(I), C15H8N5O3Re
- Crystal structure of 4-((triphenylphosphonio)methyl)pyridin-1-ium tetrachloridozincate(II), C24H22Cl4NPZn
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of (S)-N-(10-((2,2-dimethoxyethyl)amino)-1,2,3-trimethoxy-9-oxo-5,6,7,9-tetrahydrobenzo[a]heptalen-7-yl)acetamide, C25H32N2O7
- The crystal structure of 6,6′-difluoro-3,3′-dimethyl-5,5′-di(10H-phenoxazin-10-yl)- [1,1′-biphenyl]-2,2′-dicarbonitrile, C40H24F2N4O2
- Crystal structure of poly[(di-ethylenediamine-κ2N,N′)cadmium(II) tetradedocyloxidohexavanadate] (V4+/V5+ = 2/1), C4H16CdN4O14V6
- The crystal structure of poly[bis(dimethylformamide-κ1N)-(μ4-2′,3,3″,5′-tetrakis(trifluoromethyl)-[1,1′:4′,1″-terphenyl]-4,4″-dicarboxylato-κ4 O,O′: O″,O‴)dicadmium(II)], C27H15CdF12NO5
- Crystal structure of bis(μ2-ferrocenylcarboxylato-O,O′)-(μ3-oxido-κ3O:O:O)-bis(μ2-salicyladoximato-κ2N,O,O′)-(μ2-isopropoxo)-tris(isopropoxy-κ1O trititanium(IV)), C48H55N2O13Fe2Ti3
- Crystal structure of 3-(diethylamino)-7,9,11-trimethyl-8-phenyl-6H,13H-12λ4,13λ4-chromeno[3′,4′:4,5]pyrrolo[1,2-c]pyrrolo[2,1-f][1,3,2]diazaborinin-6-one, C28H26BF2N3O2
- The crystal structure of catena-poly[aqua-μ2-2-nitro-benzene-1,3-dicarboxylato-κ2O,O′)-(1,10-phenanthroline-κ2N,N′)-zinc(II)], C20H13N3O7Zn
- Crystal structure of poly[diaqua-{μ3-1-(3-carboxylatophenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylato-κ4O,O′:O′′:O′′′′}manganese(II)] hydrate
- Crystal structure of N′-((1-hydroxycyclohexyl)(phenyl)methyl)-2-methoxybenzohydrazide methanol solvate, C22H28N2O4
- The cocrystal of caffeic acid — progesterone — water (1/2/1), C51H70O9
- Crystal structure of (((oxido(quinolin-6-yl)methoxy)triphenyl-λ5-stibanyl)oxy)(quinolin-7-yl)methanolate
- Crystal structure of [(E)-6′-(diethylamino)-2-(2-(((E)-pyren-1-ylmethylene)amino)ethyl)-4′-(2-((E)-1,3,3-trimethylindolin-2-ylidene)ethylidene)-1′,2′,3′,4′-tetrahydrospiro[isoindoline-1,9′-xanthen]-3-one]-methanol, solvate C57H56N4O3
- The crystal structure of 1-(acridin-9-yl)pyrrolidine-2,5-dione, C17H22N2O2
- Crystal structure of N-(4-acetylphenyl)-2-(6-methoxynaphthalen-2-yl)propanamide, C22H21NO3
- The crystal structure of 5,10,15,20-tetrakis(4-(1H-1,2,4-triazol-1-yl)phenyl)porphyrin, C52H34N16
- Crystal structure of hexacarbonyl-μ2-[phenylmethanedithiolato-κ4S:S,S′:S′]diiron (Fe–Fe) C13H6Fe2O6S2
- Crystal structure of diiodo-bis(1-((2-propyl-1H-benzo[d]imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κ1N)cadmium(II), C34H34CdI2N10
- Crystal structure of (E)-(3-(3-bromophenyl)acryloyl)ferrocene, C19H15BrFeO
- Crystal structure of catena-poly(μ2-6-chloropyridine-2-carboxylato-κ3N,O:O′)(6-chloropyridine-2-carboxylato-κ2O,N)copper(II), C12H6Cl2N2O4Cu
- Crystal structure of poly[diaqua-μ 3-(5-(3,5-dicarboxy-2,4,6-trimethylbenzyl)-2,4,6-trimethylisophthalato)-κ 6O,O′:O″,O‴:O‴′,O‴″) terbium(III)-monohydrate], C23H28TbO12
- Crystal structure of (E)-2-(((5-chloro-3-methyl-1-phenyl-1H-pyrazol-4-yl)methylene)amino)-3′,6′-dihydroxyspiro[isoindoline-1,9′-xanthen]-3-one – ethanol (1/2), C35H33ClN4O6
- The crystal structure of 3-(5-amino-3-phenylisoxazol-4-yl)-4-chloro-3-hydroxyindolin-2-one, C17H12ClN3O3
- The crystal structure of dimethylammonium 4-[2-(4-fluorophenyl)-4, 5-diphenyl-1H-imidazol-1-yl]benzenesulfonate, C29H26FN3O3S
- Crystal structure of (R)-2-ammonio-3-((5-carboxypentyl)thio)propanoate
- Crystal structure of 4-cyclohexyl-5-(thiophen-2-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione, C12H15N3S2
- The crystal structure of 4,6-bis(dimethylamino)-2-fluoroisophthalonitrile, C12H13FN4
- Hydrogen bonding in the crystal structure of nicotin-1,1′-dium tetrabromidomanganate(II)
- The crystal structure of bis(2-bromobenzyl)(2-((2-oxybenzylidene)amino)-4-methylpentanoato-κ3N, O,O′)tin(IV), C27H27Br2NO3Sn
- Crystal structure of (E)-(3-(p-tolyl)acryloyl)ferrocene, C20H18FeO
- Crystal structure of (E)-7-fluoro-2-((5-(4-methylpiperazin-1-yl)pyridin-2-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C21H22FN3O
- Crystal structure of (E)-7-methoxy-2-((5-(4-methylpiperazin-1-yl)pyridin-2-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C22H25N3O2
- The crystal structure of poly(bis(μ2-1,3,5-tri(1H-imidazol-1-yl)benzene-κ2N:N′)-(μ2-2,3,5,6-tetrafluoroterephthalato-κ2O:O′)-manganese(II), C38H24F4N12O4Mn
- Crystal structure of (3,4-dimethoxybenzyl)triphenylphosphonium bromide ethanol solvate, C29H32BrO3P
- Crystal structure of tetraethylammonium hydrogencarbonate – (diaminomethylene)thiourea – water (2/1/3)
- Crystal structure of N, N-Dimethyl-N′-tosylformimidamide, C10H14N2O2S
- The crystal structure of ethyl 2-methyl-5-oxo-4-(2-methoxyphenyl)-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C20H23N2O4
- Crystal structure of bis(μ2-1,5-bis[(E)-1-(2-hydroxyphenyl)ethylidene] thiocarbonohydrazide)-bis(dimethylformamide)-dizinc(II) dimethylformamide solvate, C40H46N10O6S2Zn2⋅C3H7NO
- Crystal structure of azido-κ1N{hydridotris(3-tert-butyl-5-methylpyrazol-1-yl)borato-κ3N,N′,N″}copper(II), C24H40BCuN9
- The crystal structure of fac-tricarbonyl(1,10-phenanthroline-κ2N,N′)-(azido-κ1N)rhenium(I), C15H8N5O3Re
- Crystal structure of 4-((triphenylphosphonio)methyl)pyridin-1-ium tetrachloridozincate(II), C24H22Cl4NPZn

