Abstract
C48H55N2O13Fe2Ti3, monoclinic, P21/n, a = 19.1705(9) Å, b = 13.8600(4) Å, c = 20.7161(8) Å, β = 112.445(5)°, V = 5087.4(4) Å3, Z = 4, Rgt(F) = 0.0751, wRref(F2) = 0.1798, T = 293(2) K.
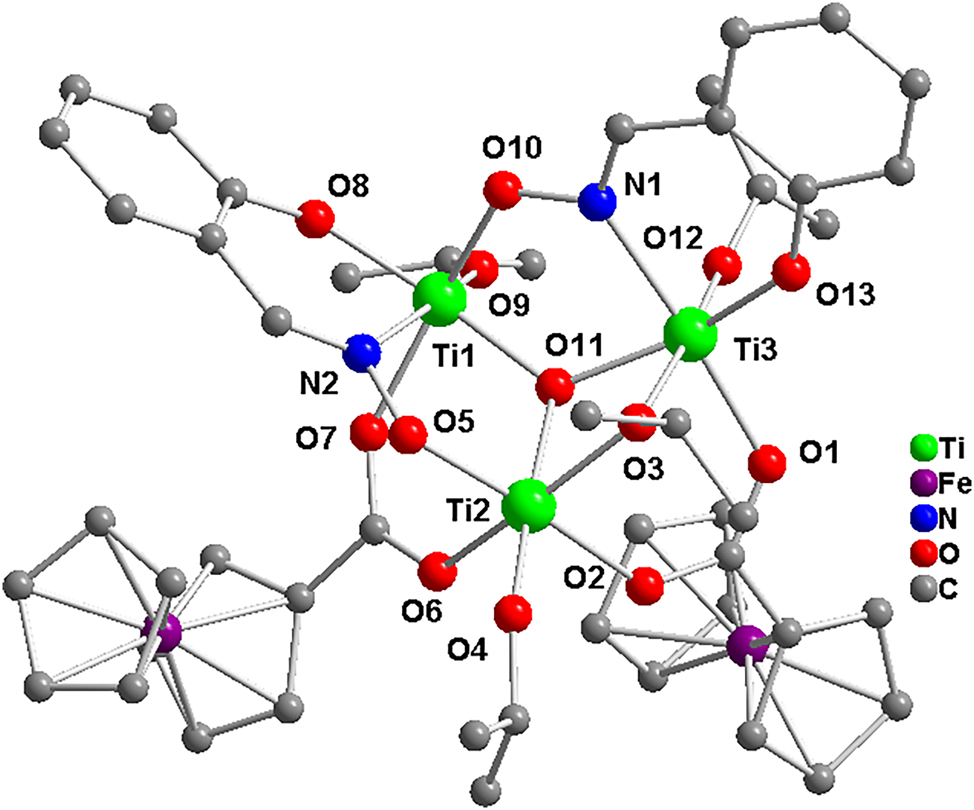
The molecular structure is shown in the figure. Table 1 contains the crystallographic data and the list of the atoms including atomic coordinates and displacement parameters can be found in the cif-file attached to this article.
Data collection and handling.
| Crystal: | Dark-red block |
| Size: | 0.26 × 0.25 × 0.20 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 1.07 mm−1 |
| Diffractometer, scan mode: | Bruker Apex2, φ and ω scans |
| θmax, completeness: | 28.3°, 100 % |
| N(hkl)measured, N(hkl)unique, Rint: | 49831, 12606, 0.040 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 8,030 |
| N(param)refined: | 620 |
| Programs: | Bruker, 1 Shelx, 2 Diamond 3 |
1 Source of material
All reagents and solvents employed in this work were commercially available and used without further purification.
In the first step, a mixture of salicylaldoxime (H2Saox) (1 mmol, 0.137 g) and ferrocenecarboxylic acid (FcCO2H) (0.87 mmol, 0.201 g) were placed in a Teflon-lined stainless vessel (15 mL). Secondly, isopropanol (2 mL) and acetonitrile (6 mL) as solvents were added. The above mixture was stirred for 5 min and then Ti(O i Pr)4 (1.63 mmol, 0.5 mL) was added. The resulting mixture was finally sealed and heated at 353 K for 96 h under autogenous pressure. After cooling to room temperature at a rate of 5 K·h−1, red and block crystals were obtained and washed with acetonitrile. The yield was 0.151 g (31 %, based on FcCO2H). Elemental analysis calcd. (found) for the title compound (%): C, 51.33 (51.28); H, 4.94 (4.89); N, 2.49 (2.54). IR (KBr, pellet, cm−1): 2970 (m), 2920 (w), 2864 (w), 1694 (m), 1542 (m), 1478 (s), 1391 (s), 1295 (s), 1199 (m), 1128 (s), 1006 (s), 915 (m), 844 (m), 753 (w), 683 (s) and 627 (s).
2 Experimental details
All the non-H atoms were refined anisotropically. H atoms were subsequently treated as riding atoms with distances C–H = 0.98 (CH3), 0.99 (CH) and 0.95 (ArH) Å. The H atom isotropic displacement parameters were fixed; Uiso(H) = 1.2 Ueq(C) for aromatic atoms, Uiso(H) = 1.2 Ueq(C) for saturated CH atoms, Uiso(H) = 1.5 Ueq(C) for CH3 atoms, allowing them to ride on the parent atom.
3 Discussion
Crystalline titanium oxo clusters with accurate structures have drawn much interest in recent years. 4 , 5 , 6 Titanium oxo clusters not only can provide structure and reactivity model for further study on the photocatalytic mechanism of titanium dioxide nanoparticles 7 but also have important potential applications in photocatalysis, such as photocatalytic CO2 reduction, 8 degradation 9 and hydrogen production. 10 Utilization multidentate and dye-functional as ligands are of great importance for titanium oxo clusters to increase hydrolytic stability and broaden light absorption, which is crucial for photocatalysis applications. 11 Both ferrocenecarboxylic acid and salicylaldoxime belong to multidentate and dye-functional ligands, and have proven to be effective ligands for construction of titanium oxo clusters with wide-ranged light absorption. 12 , 13 Simultaneously application of ferrocenecarboxylic acid and salicylaldoxime as ligands for titanium oxo clusters construction is appealing but has not been reported. In this work, a Ti3 core-based titanium oxo cluster protected by the above two ligands has been successfully synthesized and structurally analyzed.
The title titanium oxo cluster formulates as [Ti3(μ3O)(O2CFc)2(Saox)2(OiPr)4], crystallizes in the monoclinic system and P21/n space group. There are three Ti(IV) ions, one μ3O atom, two ferrocenecarboxylates, two salicylaldoxime and four isopropoxide groups in the molecular structure (the figure). The two salicylaldoxime groups adopt μ2-η1:η1:η1 coordination mode which are different from that of μ3-η1:η1:η2 in the literature. 14 , 15 , 16 The three Ti(IV) ions are all six-coordinated, among which two Ti(IV) ions present octahedral TiO5N coordination environments and one Ti(IV) ion shows TiO6 mode. The μ3O atom connects the three Ti(IV) ions together forming Ti3O core with a nearly flat mode. The bond lengths of Ti–O range from 1.765 to 2.127 Å. The band lengths of Ti(1)–N(1) and Ti(2)–N(2) are 2.153 and 2.236 Å, respectively. Both the bonds of Ti–O and the bonds of Ti–N are consistent with the literature. 14 , 17 , 18 No classical intermolecular and intramolecular hydrogen bonds are found in the structure. However, the weak intermolecular interactions of C(22)–H(22)⋯O(13) with length 3.089(8) Å could be found in the adjacent moleculars. In summary, this work will not only enrich the types of titanium oxide clusters, but also provide a facile synthetic route to crystalline titanium oxide clusters using mixed dye-functional ligands.
-
Research funding: This work was financially supported by the Foundation of Anyang Institute of Technology (YPY2021005), the National Natural Science Foundation of China (62104005), the International science and technology cooperation project of Henan Province (242102521001), the Teaching Reform Research and Practice Project of Higher Education in Henan Province (2023SJGLX356Y), the Key Research and Development and Promotion Projects of Anyang City (2023C01GX031), the Doctor Foundation of Anyang Institute of Technology (BSJ2022024), and the Postgraduate Education Reform and Quality Improvement Project of Henan Province (Grant No. YJS2023JD60), the Foundation of Anyang Institute of Technology (YPY2019003).
References
1. Bruker. Apex2; Bruker AXS Inc.: Madison, Wisconsin, USA, 2005.Suche in Google Scholar
2. Sheldrick, G. M. A Short History of Shelx. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Suche in Google Scholar PubMed
3. Brandenburg, K. Diamond. Visual Crystal Structure Information System. Version 3.2i. Crystal Impact: Bonn, Germany, 2012.Suche in Google Scholar
4. Fang, W. H.; Zhang, L.; Zhang, J. Synthetic Strategies, Diverse Structures and Tuneable Properties of polyoxo-titanium Clusters. Chem. Soc. Rev. 2018, 47, 404–421; https://doi.org/10.1039/c7cs00511c.Suche in Google Scholar PubMed
5. Liu, Y.-J.; Fang, W.-H.; Zhang, L.; Zhang, J. Recent Advances in Heterometallic Polyoxotitanium Clusters. Coord. Chem. Rev. 2020, 404, 213099–213106; https://doi.org/10.1016/j.ccr.2019.213099.Suche in Google Scholar
6. Zhu, Q.-Y.; Dai, J. Titanium oxo/alkoxyl Clusters Anchored with Photoactive Ligands. Coord. Chem. Rev. 2021, 430, 213664–213677; https://doi.org/10.1016/j.ccr.2020.213664.Suche in Google Scholar
7. Liu, J. X.; Gao, M. Y.; Fang, W. H.; Zhang, L.; Zhang, J. Bandgap Engineering of Titanium-Oxo Clusters: Labile Surface Sites Used for Ligand Substitution and Metal Incorporation. Angew. Chem., Int. Ed. 2016, 55, 5160–5165; https://doi.org/10.1002/anie.201510455.Suche in Google Scholar PubMed
8. Li, N.; Liu, J.-J.; Sun, J.-W.; Dong, B.-X.; Dong, L.-Z.; Yao, S.-J.; Xin, Z.; Li, S.-L.; Lan, Y.-Q. Calix[8]arene-constructed Stable polyoxo-titanium Clusters for Efficient CO2 Photoreduction. Green Chem. 2020, 22, 5325–5332; https://doi.org/10.1039/d0gc01497d.Suche in Google Scholar
9. Yu, Y. Z.; Guo, Y.; Zhang, Y. R.; Liu, M. M.; Feng, Y. R.; Geng, C. H.; Zhang, X. M. A Series of Silver Doped Butterfly-like Ti8Ag2 Clusters with Two Ag Ions Panelled on a Ti8 Surface. Dalton Trans. 2019, 48, 13423–13429; https://doi.org/10.1039/c9dt02508a.Suche in Google Scholar PubMed
10. Fang, W. H.; Zhang, L.; Zhang, J. A 3.6 Nm Ti52-Oxo Nanocluster with Precise Atomic Structure. J. Am. Chem. Soc. 2016, 138, 7480–7483; https://doi.org/10.1021/jacs.6b03489.Suche in Google Scholar PubMed
11. Guo, Y.-H.; Yu, Y.-Z.; Shen, Y.-H.; Yang, L.-G.; Liu, N.-N.; Zhou, Z.-Y.; Niu, Y.-S. “Three-in-One” structural-building-mode-based Ti16-Type Titanium Oxo Cluster Entirely Protected by the Ligands Benzoate and Salicylhydroxamate. Inorg. Chem. 2022, 61, 8685–8693; https://doi.org/10.1021/acs.inorgchem.2c00327.Suche in Google Scholar PubMed
12. Fan, Y.; Cui, Y.; Zou, G. D.; Duan, R. H.; Zhang, X.; Dong, Y. X.; Lv, H. T.; Cao, J. T.; Jing, Q. S. A ferrocenecarboxylate-functionalized titanium-oxo-cluster: The Ferrocene Wheel as a Sensitizer for Photocurrent Response. Dalton Trans. 2017, 46, 8057–8064; https://doi.org/10.1039/c7dt01756a.Suche in Google Scholar PubMed
13. Yu, Y.-Z.; Zhang, Y.-R.; Geng, C.-H.; Sun, L.; Guo, Y.; Feng, Y.-R.; Wang, Y.-X.; Zhang, X.-M. Precise and Wide-Ranged Band-Gap Tuning of Ti6-Core-Based Titanium Oxo Clusters by the Type and Number of Chromophore Ligands. Inorg. Chem. 2019, 58, 16785–16791; https://doi.org/10.1021/acs.inorgchem.9b02951.Suche in Google Scholar PubMed
14. Davidson, M. G.; Johnson, A. L.; Jones, M.; Lunn, M.; Mahon, M. F. Titanium(IV) Complexes of Oximes-Novel Binding Modes. Polyhedron 2007, 26, 975–980; https://doi.org/10.1016/j.poly.2006.09.055.Suche in Google Scholar
15. Wang, C.; Chen, N.; Kong, F.; Wang, S. A Family of Oxime-based titanium-oxo Clusters: Synthesis, Structures, and Photoelectric Responses. CrystEngComm 2022, 24, 3280; https://doi.org/10.1039/D2CE00195K.Suche in Google Scholar
16. Chen, S.; Fang, W.-H.; Zhang, L.; Zhang, J. Synthesis, Structures, and Photocurrent Responses of Polyoxo-Titanium Clusters with Oxime Ligands: from Ti4 to Ti18. Inorg. Chem. 2018, 57, 8850; https://doi.org/10.1021/acs.inorgchem.8b00751.Suche in Google Scholar PubMed
17. Yu, Y.; Wang, H.; Li, L.; Guo, Y.; Fu, Q; Dai, J. Crystal Structure of bis((N-methyl-2-oxyethyl)amine)-bis(μ2-N,N,N-tris(2-oxoethyl)amine)-bis(isopropoxy)-Bis(μ3-oxo)tetratitanium(IV)– Isopropanol (1/2), C34H76N4O16Ti4. Z. Kristallogr. N. Cryst. Struct. 2022, 237, 965–967.10.1515/ncrs-2022-0325Suche in Google Scholar
18. Yu, Y.; Wang, H.; Li, L.; Guo, Y.; Feng, J.; Li, Y. Crystal Structure of bis(μ2-2-oxido-2-phenylacetato-κ3O,O′:O′)-bis(N-oxido-benzamide-κ2O,O′)-bis(propan-2-olato-κ1O)dititanium(IV), C36H38N2O12Ti2. Z. Kristallogr. N. Cryst. Struct. 2022, 237, 957–959.10.1515/ncrs-2022-0326Suche in Google Scholar
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of (S)-N-(10-((2,2-dimethoxyethyl)amino)-1,2,3-trimethoxy-9-oxo-5,6,7,9-tetrahydrobenzo[a]heptalen-7-yl)acetamide, C25H32N2O7
- The crystal structure of 6,6′-difluoro-3,3′-dimethyl-5,5′-di(10H-phenoxazin-10-yl)- [1,1′-biphenyl]-2,2′-dicarbonitrile, C40H24F2N4O2
- Crystal structure of poly[(di-ethylenediamine-κ2N,N′)cadmium(II) tetradedocyloxidohexavanadate] (V4+/V5+ = 2/1), C4H16CdN4O14V6
- The crystal structure of poly[bis(dimethylformamide-κ1N)-(μ4-2′,3,3″,5′-tetrakis(trifluoromethyl)-[1,1′:4′,1″-terphenyl]-4,4″-dicarboxylato-κ4 O,O′: O″,O‴)dicadmium(II)], C27H15CdF12NO5
- Crystal structure of bis(μ2-ferrocenylcarboxylato-O,O′)-(μ3-oxido-κ3O:O:O)-bis(μ2-salicyladoximato-κ2N,O,O′)-(μ2-isopropoxo)-tris(isopropoxy-κ1O trititanium(IV)), C48H55N2O13Fe2Ti3
- Crystal structure of 3-(diethylamino)-7,9,11-trimethyl-8-phenyl-6H,13H-12λ4,13λ4-chromeno[3′,4′:4,5]pyrrolo[1,2-c]pyrrolo[2,1-f][1,3,2]diazaborinin-6-one, C28H26BF2N3O2
- The crystal structure of catena-poly[aqua-μ2-2-nitro-benzene-1,3-dicarboxylato-κ2O,O′)-(1,10-phenanthroline-κ2N,N′)-zinc(II)], C20H13N3O7Zn
- Crystal structure of poly[diaqua-{μ3-1-(3-carboxylatophenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylato-κ4O,O′:O′′:O′′′′}manganese(II)] hydrate
- Crystal structure of N′-((1-hydroxycyclohexyl)(phenyl)methyl)-2-methoxybenzohydrazide methanol solvate, C22H28N2O4
- The cocrystal of caffeic acid — progesterone — water (1/2/1), C51H70O9
- Crystal structure of (((oxido(quinolin-6-yl)methoxy)triphenyl-λ5-stibanyl)oxy)(quinolin-7-yl)methanolate
- Crystal structure of [(E)-6′-(diethylamino)-2-(2-(((E)-pyren-1-ylmethylene)amino)ethyl)-4′-(2-((E)-1,3,3-trimethylindolin-2-ylidene)ethylidene)-1′,2′,3′,4′-tetrahydrospiro[isoindoline-1,9′-xanthen]-3-one]-methanol, solvate C57H56N4O3
- The crystal structure of 1-(acridin-9-yl)pyrrolidine-2,5-dione, C17H22N2O2
- Crystal structure of N-(4-acetylphenyl)-2-(6-methoxynaphthalen-2-yl)propanamide, C22H21NO3
- The crystal structure of 5,10,15,20-tetrakis(4-(1H-1,2,4-triazol-1-yl)phenyl)porphyrin, C52H34N16
- Crystal structure of hexacarbonyl-μ2-[phenylmethanedithiolato-κ4S:S,S′:S′]diiron (Fe–Fe) C13H6Fe2O6S2
- Crystal structure of diiodo-bis(1-((2-propyl-1H-benzo[d]imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κ1N)cadmium(II), C34H34CdI2N10
- Crystal structure of (E)-(3-(3-bromophenyl)acryloyl)ferrocene, C19H15BrFeO
- Crystal structure of catena-poly(μ2-6-chloropyridine-2-carboxylato-κ3N,O:O′)(6-chloropyridine-2-carboxylato-κ2O,N)copper(II), C12H6Cl2N2O4Cu
- Crystal structure of poly[diaqua-μ 3-(5-(3,5-dicarboxy-2,4,6-trimethylbenzyl)-2,4,6-trimethylisophthalato)-κ 6O,O′:O″,O‴:O‴′,O‴″) terbium(III)-monohydrate], C23H28TbO12
- Crystal structure of (E)-2-(((5-chloro-3-methyl-1-phenyl-1H-pyrazol-4-yl)methylene)amino)-3′,6′-dihydroxyspiro[isoindoline-1,9′-xanthen]-3-one – ethanol (1/2), C35H33ClN4O6
- The crystal structure of 3-(5-amino-3-phenylisoxazol-4-yl)-4-chloro-3-hydroxyindolin-2-one, C17H12ClN3O3
- The crystal structure of dimethylammonium 4-[2-(4-fluorophenyl)-4, 5-diphenyl-1H-imidazol-1-yl]benzenesulfonate, C29H26FN3O3S
- Crystal structure of (R)-2-ammonio-3-((5-carboxypentyl)thio)propanoate
- Crystal structure of 4-cyclohexyl-5-(thiophen-2-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione, C12H15N3S2
- The crystal structure of 4,6-bis(dimethylamino)-2-fluoroisophthalonitrile, C12H13FN4
- Hydrogen bonding in the crystal structure of nicotin-1,1′-dium tetrabromidomanganate(II)
- The crystal structure of bis(2-bromobenzyl)(2-((2-oxybenzylidene)amino)-4-methylpentanoato-κ3N, O,O′)tin(IV), C27H27Br2NO3Sn
- Crystal structure of (E)-(3-(p-tolyl)acryloyl)ferrocene, C20H18FeO
- Crystal structure of (E)-7-fluoro-2-((5-(4-methylpiperazin-1-yl)pyridin-2-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C21H22FN3O
- Crystal structure of (E)-7-methoxy-2-((5-(4-methylpiperazin-1-yl)pyridin-2-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C22H25N3O2
- The crystal structure of poly(bis(μ2-1,3,5-tri(1H-imidazol-1-yl)benzene-κ2N:N′)-(μ2-2,3,5,6-tetrafluoroterephthalato-κ2O:O′)-manganese(II), C38H24F4N12O4Mn
- Crystal structure of (3,4-dimethoxybenzyl)triphenylphosphonium bromide ethanol solvate, C29H32BrO3P
- Crystal structure of tetraethylammonium hydrogencarbonate – (diaminomethylene)thiourea – water (2/1/3)
- Crystal structure of N, N-Dimethyl-N′-tosylformimidamide, C10H14N2O2S
- The crystal structure of ethyl 2-methyl-5-oxo-4-(2-methoxyphenyl)-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C20H23N2O4
- Crystal structure of bis(μ2-1,5-bis[(E)-1-(2-hydroxyphenyl)ethylidene] thiocarbonohydrazide)-bis(dimethylformamide)-dizinc(II) dimethylformamide solvate, C40H46N10O6S2Zn2⋅C3H7NO
- Crystal structure of azido-κ1N{hydridotris(3-tert-butyl-5-methylpyrazol-1-yl)borato-κ3N,N′,N″}copper(II), C24H40BCuN9
- The crystal structure of fac-tricarbonyl(1,10-phenanthroline-κ2N,N′)-(azido-κ1N)rhenium(I), C15H8N5O3Re
- Crystal structure of 4-((triphenylphosphonio)methyl)pyridin-1-ium tetrachloridozincate(II), C24H22Cl4NPZn
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of (S)-N-(10-((2,2-dimethoxyethyl)amino)-1,2,3-trimethoxy-9-oxo-5,6,7,9-tetrahydrobenzo[a]heptalen-7-yl)acetamide, C25H32N2O7
- The crystal structure of 6,6′-difluoro-3,3′-dimethyl-5,5′-di(10H-phenoxazin-10-yl)- [1,1′-biphenyl]-2,2′-dicarbonitrile, C40H24F2N4O2
- Crystal structure of poly[(di-ethylenediamine-κ2N,N′)cadmium(II) tetradedocyloxidohexavanadate] (V4+/V5+ = 2/1), C4H16CdN4O14V6
- The crystal structure of poly[bis(dimethylformamide-κ1N)-(μ4-2′,3,3″,5′-tetrakis(trifluoromethyl)-[1,1′:4′,1″-terphenyl]-4,4″-dicarboxylato-κ4 O,O′: O″,O‴)dicadmium(II)], C27H15CdF12NO5
- Crystal structure of bis(μ2-ferrocenylcarboxylato-O,O′)-(μ3-oxido-κ3O:O:O)-bis(μ2-salicyladoximato-κ2N,O,O′)-(μ2-isopropoxo)-tris(isopropoxy-κ1O trititanium(IV)), C48H55N2O13Fe2Ti3
- Crystal structure of 3-(diethylamino)-7,9,11-trimethyl-8-phenyl-6H,13H-12λ4,13λ4-chromeno[3′,4′:4,5]pyrrolo[1,2-c]pyrrolo[2,1-f][1,3,2]diazaborinin-6-one, C28H26BF2N3O2
- The crystal structure of catena-poly[aqua-μ2-2-nitro-benzene-1,3-dicarboxylato-κ2O,O′)-(1,10-phenanthroline-κ2N,N′)-zinc(II)], C20H13N3O7Zn
- Crystal structure of poly[diaqua-{μ3-1-(3-carboxylatophenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylato-κ4O,O′:O′′:O′′′′}manganese(II)] hydrate
- Crystal structure of N′-((1-hydroxycyclohexyl)(phenyl)methyl)-2-methoxybenzohydrazide methanol solvate, C22H28N2O4
- The cocrystal of caffeic acid — progesterone — water (1/2/1), C51H70O9
- Crystal structure of (((oxido(quinolin-6-yl)methoxy)triphenyl-λ5-stibanyl)oxy)(quinolin-7-yl)methanolate
- Crystal structure of [(E)-6′-(diethylamino)-2-(2-(((E)-pyren-1-ylmethylene)amino)ethyl)-4′-(2-((E)-1,3,3-trimethylindolin-2-ylidene)ethylidene)-1′,2′,3′,4′-tetrahydrospiro[isoindoline-1,9′-xanthen]-3-one]-methanol, solvate C57H56N4O3
- The crystal structure of 1-(acridin-9-yl)pyrrolidine-2,5-dione, C17H22N2O2
- Crystal structure of N-(4-acetylphenyl)-2-(6-methoxynaphthalen-2-yl)propanamide, C22H21NO3
- The crystal structure of 5,10,15,20-tetrakis(4-(1H-1,2,4-triazol-1-yl)phenyl)porphyrin, C52H34N16
- Crystal structure of hexacarbonyl-μ2-[phenylmethanedithiolato-κ4S:S,S′:S′]diiron (Fe–Fe) C13H6Fe2O6S2
- Crystal structure of diiodo-bis(1-((2-propyl-1H-benzo[d]imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κ1N)cadmium(II), C34H34CdI2N10
- Crystal structure of (E)-(3-(3-bromophenyl)acryloyl)ferrocene, C19H15BrFeO
- Crystal structure of catena-poly(μ2-6-chloropyridine-2-carboxylato-κ3N,O:O′)(6-chloropyridine-2-carboxylato-κ2O,N)copper(II), C12H6Cl2N2O4Cu
- Crystal structure of poly[diaqua-μ 3-(5-(3,5-dicarboxy-2,4,6-trimethylbenzyl)-2,4,6-trimethylisophthalato)-κ 6O,O′:O″,O‴:O‴′,O‴″) terbium(III)-monohydrate], C23H28TbO12
- Crystal structure of (E)-2-(((5-chloro-3-methyl-1-phenyl-1H-pyrazol-4-yl)methylene)amino)-3′,6′-dihydroxyspiro[isoindoline-1,9′-xanthen]-3-one – ethanol (1/2), C35H33ClN4O6
- The crystal structure of 3-(5-amino-3-phenylisoxazol-4-yl)-4-chloro-3-hydroxyindolin-2-one, C17H12ClN3O3
- The crystal structure of dimethylammonium 4-[2-(4-fluorophenyl)-4, 5-diphenyl-1H-imidazol-1-yl]benzenesulfonate, C29H26FN3O3S
- Crystal structure of (R)-2-ammonio-3-((5-carboxypentyl)thio)propanoate
- Crystal structure of 4-cyclohexyl-5-(thiophen-2-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione, C12H15N3S2
- The crystal structure of 4,6-bis(dimethylamino)-2-fluoroisophthalonitrile, C12H13FN4
- Hydrogen bonding in the crystal structure of nicotin-1,1′-dium tetrabromidomanganate(II)
- The crystal structure of bis(2-bromobenzyl)(2-((2-oxybenzylidene)amino)-4-methylpentanoato-κ3N, O,O′)tin(IV), C27H27Br2NO3Sn
- Crystal structure of (E)-(3-(p-tolyl)acryloyl)ferrocene, C20H18FeO
- Crystal structure of (E)-7-fluoro-2-((5-(4-methylpiperazin-1-yl)pyridin-2-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C21H22FN3O
- Crystal structure of (E)-7-methoxy-2-((5-(4-methylpiperazin-1-yl)pyridin-2-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C22H25N3O2
- The crystal structure of poly(bis(μ2-1,3,5-tri(1H-imidazol-1-yl)benzene-κ2N:N′)-(μ2-2,3,5,6-tetrafluoroterephthalato-κ2O:O′)-manganese(II), C38H24F4N12O4Mn
- Crystal structure of (3,4-dimethoxybenzyl)triphenylphosphonium bromide ethanol solvate, C29H32BrO3P
- Crystal structure of tetraethylammonium hydrogencarbonate – (diaminomethylene)thiourea – water (2/1/3)
- Crystal structure of N, N-Dimethyl-N′-tosylformimidamide, C10H14N2O2S
- The crystal structure of ethyl 2-methyl-5-oxo-4-(2-methoxyphenyl)-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C20H23N2O4
- Crystal structure of bis(μ2-1,5-bis[(E)-1-(2-hydroxyphenyl)ethylidene] thiocarbonohydrazide)-bis(dimethylformamide)-dizinc(II) dimethylformamide solvate, C40H46N10O6S2Zn2⋅C3H7NO
- Crystal structure of azido-κ1N{hydridotris(3-tert-butyl-5-methylpyrazol-1-yl)borato-κ3N,N′,N″}copper(II), C24H40BCuN9
- The crystal structure of fac-tricarbonyl(1,10-phenanthroline-κ2N,N′)-(azido-κ1N)rhenium(I), C15H8N5O3Re
- Crystal structure of 4-((triphenylphosphonio)methyl)pyridin-1-ium tetrachloridozincate(II), C24H22Cl4NPZn

