Abstract
C17H16F3NO2, monoclinic, P21/n, a = 5.7291(4) Å, b = 37.994(3) Å, c = 6.9806(5) Å, β = 100.575(7)°, V = 1493.7(2) Å3, Z = 4, R gt (F) = 0.0598, wR ref (F2) = 0.1310, T = 100 K.
1 Source of materials
The title compound was prepared according to the following synthetic protocol. A p-aminobenzoate (165 mg, 1.0 mmol), p-toluenesulfonic acid monohydrate 100 mg and 4-(trifluoromethyl)benzaldehyde (226 mg, 1.3 mmol) in dry toluene (20 mL) were heated at reflux temperature under nitrogen atmosphere for 48 h. The mixture was cooled to room temperature and filtered. The filtrate was washed with 5 % aqueous sodium hydroxide and water, and dried over anhydrous magnesium sulfate. The crude product was purifed on a silica-gel column using a mixture of petroleum ether and ethyl acetate, yielding light yellow solid (yield: 50 %). The yellow crystals of the title compound were obtained by slow evaporation of dichloromethane/hexane at room temperature and the suitable single crystal was studied by X-ray diffraction method (Table 1).
Data collection and handling.
| Crystal: | colourless block |
| Size: | 0.15 × 0.12 × 0.11 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.12 mm−1 |
| Diffractometer, scan mode: | Rigaku SuperNova, ω scans |
| θmax, completeness: | 29.7°, 100 % |
| N(hkl)measured, N(hkl)unique, Rint: | 7665, 3549, 0.041 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 2,374 |
| N(param)refined: | 213 |
| Programs: | Rigaku, 1 SHELX 2 , 3 |
2 Experimental details
The hydrogen atoms were refined with constraints or restraints.
3 Comment
Diarylamines are important building blocks in organic synthesis and are present as privileged structures in numerous pharmaceuticals and biologically active compounds. Due to the importance to medicinal chemistry, many methods exist for diarylamine synthesis, with transition metal-catalyzed C–N bond formation being especially prominent in recent years. 4 , 5 , 6 , 7 Up to date, there are only a few reports on diarylamine compounds especially their crystal structures. 8 , 9 , 10
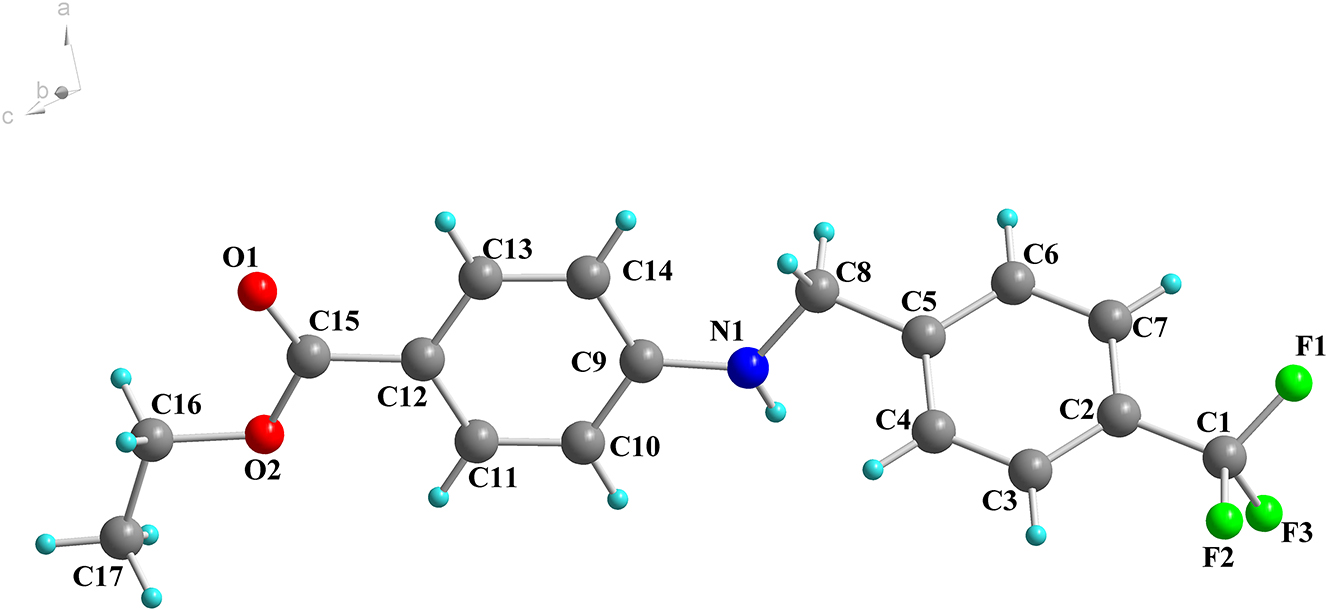
There is one molecule in the asymmetric unit of the title structure which is shown in the figure. The p-methyltrifluorophenyl group is attached to the 4-position of the ethyl benzoate. The CCN bond distance is 1.442(3) Å for C8⋯N1, which is normal for arylamine. The three bond angles around the amino group are 124.44(18)∘ for C8–N1–C9, 111.07(17)∘ and 123.0(2)∘ for N1–C9–C14, respectively. The dihedral angel between the benzene rings is 57.56∘. Molecules are linked by NH⋯O hydrogen bonds to form one-dimensional supramolecular structure. These chains are linked by CHC interactions with 2.967 Å for nitrogen (N3) atom to the nearest oxygen (O1) atom.
Acknowledgements
We gratefully acknowledge support the Fundamental Research Funds for Henan Provincial Department of Science and Technology(No: 242102320275).
References
1. Oxford Diffraction Ltd CrysAlisPRO: Abingdon, Oxfordshire, England, 2006.Search in Google Scholar
2. Sheldrick, G. M. SHELXT – Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Search in Google Scholar PubMed PubMed Central
3. Sheldrick, G. M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar PubMed PubMed Central
4. Ruiz–Castillo, P.; Buchwald, S. L. Applications of Palladium-Catalyzed C–N Cross-Coupling Reactions. Chem. Rev. 2016, 116, 12564–12649; https://doi.org/10.1021/acs.chemrev.6b00512.Search in Google Scholar PubMed PubMed Central
5. Chen, J. Q.; Li, J. H.; Dong, Z. B. A Review on the Latest Progress of Chan-Lam Coupling Reaction. Adv. Synth. Catal. 2020, 362, 3311–3331; https://doi.org/10.1002/adsc.202000495.Search in Google Scholar
6. Sambiagio, C.; Marsden, S. P.; Blacker, A. J.; McGowan, P. C. Copper Catalysed Ullmann Type Chemistry: from Mechanistic Aspects to Modern Development. Chem. Soc. Rev. 2014, 43, 3525–3550; https://doi.org/10.1039/c3cs60289c.Search in Google Scholar PubMed
7. Thomas, S.; Jonathan, M. L.; Sam, B.; Michael, F. G. Diarylamine Synthesis via Desulfifinylative Smiles Rearrangement. Org. Lett. 2022, 24, 1132–1135; https://doi.org/10.1021/acs.orglett.1c04122.Search in Google Scholar PubMed PubMed Central
8. Wang, Z. S.; Chen, Y. B.; Zhang, H. W.; Sun, Z.; Zhu, C.; Ye, L. W. Ynamide Smiles Rearrangement Triggered by Visible-Light-Mediated Regioselective Ketyl-Ynamide Coupling: Rapid Access to Functionalized Indoles and Isoquinolines. J. Am. Chem. Soc. 2020, 142, 3636–3644; https://doi.org/10.1021/jacs.9b13975.Search in Google Scholar PubMed
9. Yan, J.; Cheo, H. W.; Teo, W. K.; Shi, X.; Wu, H.; Idres, S. B.; Deng, L. W. J.; Wu, J. A Radical Smiles Rearrangement Promoted by Neutral Eosin Y as a Direct Hydrog Transfer Photocatalyst. J. Am. Chem. Soc. 2020, 142, 11357–11362; https://doi.org/10.1021/jacs.0c02052.Search in Google Scholar PubMed
10. Alpers, D.; Cole, K. P.; Stephenson, C. R. J. Visible Light Mediated Aryl Migration by Homolytic C–N Cleavage of Aryl Amines. Angew. Chem. Int. Ed. 2018, 57, 12167–12170; https://doi.org/10.1002/anie.201806659.Search in Google Scholar PubMed
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of 5,5′-bis(2,4,6-trinitrophenyl)-2,2′-bi(1,3,4-oxadiazole), C16H4N10O14
- Crystal structure of catena-poly[(μ3-4,4′-oxydibenzoato- κ5 O,O′: O″,O‴:O‴)-bis(2,4,6-tri(3-pyridine)-1,3,5-triazine-κ1 N)cadmium(II)], C50H32CdN12O5
- The crystal structure of 1,4-diazepane-1,4-diium potassium trinitrate, C5H14KN5O9
- The crystal structure of benzyl 2,2,5,5-tetramethylthiazolidine-4-carboxylate, C15H21NO2S
- Crystal structure of 2-hydroxyethyl-triphenylphosphonium tetracyanidoborate, C24H20BN4OP
- The crystal structure of 1-methyl-3-(N-methylnitrous amide–N-methylene) imidazolidine-2,4,5-trione
- Crystal structure of N-((3-cyano-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-(2,2,2-trifluoroacetyl)-1H-pyrazol-5-yl)carbamoyl)-2,6-difluorobenzamide, C20H7Cl2F8N5O3S
- Crystal structure of 5-(2,2-difluoropropyl)-5-methylbenzo[4,5]imidazo[2,1-a] isoquinolin-6(5H)-one, C20H18F2N2O
- The crystal structure of N′,N″-[1,2-bis(4-chlorophenyl)ethane-1,2-diylidene]bis(furan-2- carbohydrazide), C24H16Cl2N4O4
- Crystal structure of [(4-bromobenzyl)triphenylphosphonium] tetrabromoantimony(III), [C25H21BrP]+[SbBr4]−
- Crystal structure of [(4-bromobenzyl)triphenylphosphonium] tetrabromidoindium(III), [C25H21BrP]+[InBr4]−
- The crystal structure of 4-carboxy-2-oxobutan-1-aminium chloride, C5H10ClNO3
- Crystal structure of (4-(4-chlorophenyl)-1H-pyrrole-3-carbonyl)ferrocene, C21H16ClFeNO
- The crystal structure of dichlorido(η6-p-cymene)(triphenylarsine)ruthenium(II), C28H29AsCl2Ru
- Crystal structure of (Z)-2-hydroxy-N′-(1-(o-tolyl)ethylidene)benzohydrazide, C16H16N2O2
- The crystal structure of 10-(1-bromoethyl)-14-(bromomethyl)dibenzo[a, c]acridine, C24H17NBr2
- Synthesis and crystal structure of 6-methoxy-7-[(4-methoxyphenyl)methoxy]-2H-1-benzopyran-2-one, C18H16O5
- Synthesis and crystal structure of ethyl 4-((4-trifluoromethylbenzyl)amino)benzo, C17H16F3NO2
- The crystal structure of (Z)-2-(tert-butyl)-6-(7-(tert-butyl)-5-methylbenzo[d][1,3]oxathiol-2-ylidene)-4-methylcyclohexa-2,4-dien-1-one, C23H28O2S
- The crystal structure of (R)-2-aminobutanamide hydrochloride, C4H11ClN2O
- Crystal structure of bromido[hydridotris(3-tert-butyl-5-isopropylpyrazolyl)borato-κ3 N,N′,N″]copper(II), C30H52BBrCuN6
- Crystal structure of chlorido{hydridotris[3-mesityl-5-methyl-1H-pyrazol-1-yl-κN3]borato}-copper(II) dichloromethane monosolvate
- Crystal structure of 4-[3,5-bis(propan-2-yl)-1H-pyrazol-4-yl]pyridine, C14H19N3
- Crystal structure of ((4-(4-bromophenyl)-1H-pyrrol-3-yl)methyl)ferrocene, C21H16BrFeNO
- Crystal structure of [(4-chlorobenzyl)triphenylphosphonium] dichloridocopper(I), {[C25H21ClP]+[CuCl2]−}n
- The crystal structure of {Cu(2,9-diisopropyl-4,7-diphenyl-1,10-phenanthroline)[4,5-bis(diphenylphosphino)-9,9-dimethylxanthene]}+ PF6−·1.5(EtOAC)
- Crystal structure of 3,5-bis(t-butyl)-1H-pyrazol-4-amine, C11H21N3
- Crystal structure of [(2,4-dichlorobenzyl)triphenylphosphonium] trichloridocopper(II), [C25H20Cl2P]+[CuCl3]−
- The crystal structure of dipotassium sulfide, K2S
- Crystal structure of (4-(4-methoxyphenyl)-1H-pyrrole-3-carbonyl)ferrocene, C22H19FeNO2
- Crystal structure of (E)-6-(4-methylpiperazin-1-yl)-2-(4-(trifluoromethyl)benzylidene)-3, 4-dihydronaphthalen-1(2H)-one, C23H23F3N2O
- Crystal structure of (E)-6-morpholino-2-(4-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C22H20F3NO2
- Crystal structure of Ce9Ir37Ge25
- The crystal structure of ethyl 6-(2-nitrophenyl)imidazo[2,1-b]thiazole-3-carboxylate, C14H11N3O4S
- Crystal structure of (4-(4-isopropylphenyl)-1H-pyrrol-3-yl)(ferrocenyl)methanone, C24H23FeNO
- Crystal structure of bis(methylammonium) tetrathiotungstate(VI), (CH3NH3)2[WS4]
- Crystal structure of 6,11-dihydro-12H-benzo[e]indeno[1,2-b]oxepin-12-one, C17H12O2
- Crystal structure of 3-[(4-phenylpiperidin-1-yl)methyl]-5-(thiophen-2-yl)-2,3-dihydro-1,3,4- oxadiazole-2-thione, C18H19N3OS2
- Crystal structure of N-isopropyl-1,8-naphthalimide C15H13NO2
- TiNiSi-type EuPdBi
- Crystal structure of 1-(p-tolylphenyl)-4-(2-thienoyl)-3-methyl-1H-pyrazol-5-ol, C16H14N2O2S
- The crystal structure of 3-(3-carboxypropyl)-2-nitro-1H-pyrrole 1-oxide, C7H9N3O5
- The crystal structure of tetraaqua-bis(2-(2-methyl-5-nitro-1H-imidazol-1-yl)acetato-k2O:N)-tetrakis(2-(2-methyl-5-nitro-1H-imidazol-1-yl)acetato-k1N)trizinc(II) hexahydrate C36H52N18O32Zn3
- The crystal structure of 4-(3-carboxy-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinolin-7-yl)piperazin-1-ium 4-hydroxy-3,5-dimethoxybenzoate monohydrate, C25H30FN3O9
- Crystal structure of bis(DL-1-carboxy-2-(1H-indol-3-yl)ethan-1-aminium) oxalate — acetic acid (1/2)
- Crystal structure of methyl (E)-4-((4-methylphenyl)sulfonamido)but-2-enoate, C12H15NO4S
- The crystal structure of actarit, C10H11NO3
- The crystal structure of bicyclol, C19H18O9
- The crystal structure of topiroxostat, C13H8N6
- Crystal structure of 2,2-dichloro-N-methyl-N-(4-p-tolylthiazol-2-yl)acetamide, C13H12Cl2N2OS
- Crystal structure of 4-(trifluoromethyl)-7-coumarinyl trifluoromethanesulfonate C11H4F6O5S
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ6O6)-((Z)-N,N′-bis(2-(dimethylamino)phenyl)carbamimidato-κ1N)potassium(I)
- Crystal structure of (Z)-2-(5-((4-(dimethylamino)naphthalen-1-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid, C18H16N2O3S2
- Crystal structure of (4-fluorobenzyl)triphenylphosphonium bromide, C25H21BrFP
- The crystal structure of dichlorido-[6-(pyridin-2-yl)phenanthridine-κ2N, N′]zinc(II)-chloroform (1/1), C19H13N2ZnCl5
- Crystal structure of (E)-(3-(2,4-dichlorophenyl)acryloyl)ferrocene, C19H14Cl2FeO
- The crystal structure of (E)-7-chloro-1-cyclopropyl-6-fluoro-3-((2-hydroxybenzylidene)amino)quinolin-4(1H)-one, C19H14ClFN2O2
- Crystal structure of 2-bromo-11-(((fluoromethyl)sulfonyl)methyl)-6-methyl-6,11-dihydrodibenzo[c,f][1,2]thiazepine 5,5-dioxide, C16H13BrFNO4S2
- Crystal structure of 2-chloro-11-(((fluoromethyl)sulfonyl)methyl)-6-methyl-6,11-dihydrodibenzo[c,f][1,2]thiazepine 5,5-dioxide, C16H13ClFNO4S2
- Crystal structure of 5-(2,2-difluoropropyl)-5-methyl-6-oxo-5,6-dihydrobenzo[4,5]imidazo[2,1-a]isoquinoline-3-carbonitrile, C20H15F2N3O
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of 5,5′-bis(2,4,6-trinitrophenyl)-2,2′-bi(1,3,4-oxadiazole), C16H4N10O14
- Crystal structure of catena-poly[(μ3-4,4′-oxydibenzoato- κ5 O,O′: O″,O‴:O‴)-bis(2,4,6-tri(3-pyridine)-1,3,5-triazine-κ1 N)cadmium(II)], C50H32CdN12O5
- The crystal structure of 1,4-diazepane-1,4-diium potassium trinitrate, C5H14KN5O9
- The crystal structure of benzyl 2,2,5,5-tetramethylthiazolidine-4-carboxylate, C15H21NO2S
- Crystal structure of 2-hydroxyethyl-triphenylphosphonium tetracyanidoborate, C24H20BN4OP
- The crystal structure of 1-methyl-3-(N-methylnitrous amide–N-methylene) imidazolidine-2,4,5-trione
- Crystal structure of N-((3-cyano-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-(2,2,2-trifluoroacetyl)-1H-pyrazol-5-yl)carbamoyl)-2,6-difluorobenzamide, C20H7Cl2F8N5O3S
- Crystal structure of 5-(2,2-difluoropropyl)-5-methylbenzo[4,5]imidazo[2,1-a] isoquinolin-6(5H)-one, C20H18F2N2O
- The crystal structure of N′,N″-[1,2-bis(4-chlorophenyl)ethane-1,2-diylidene]bis(furan-2- carbohydrazide), C24H16Cl2N4O4
- Crystal structure of [(4-bromobenzyl)triphenylphosphonium] tetrabromoantimony(III), [C25H21BrP]+[SbBr4]−
- Crystal structure of [(4-bromobenzyl)triphenylphosphonium] tetrabromidoindium(III), [C25H21BrP]+[InBr4]−
- The crystal structure of 4-carboxy-2-oxobutan-1-aminium chloride, C5H10ClNO3
- Crystal structure of (4-(4-chlorophenyl)-1H-pyrrole-3-carbonyl)ferrocene, C21H16ClFeNO
- The crystal structure of dichlorido(η6-p-cymene)(triphenylarsine)ruthenium(II), C28H29AsCl2Ru
- Crystal structure of (Z)-2-hydroxy-N′-(1-(o-tolyl)ethylidene)benzohydrazide, C16H16N2O2
- The crystal structure of 10-(1-bromoethyl)-14-(bromomethyl)dibenzo[a, c]acridine, C24H17NBr2
- Synthesis and crystal structure of 6-methoxy-7-[(4-methoxyphenyl)methoxy]-2H-1-benzopyran-2-one, C18H16O5
- Synthesis and crystal structure of ethyl 4-((4-trifluoromethylbenzyl)amino)benzo, C17H16F3NO2
- The crystal structure of (Z)-2-(tert-butyl)-6-(7-(tert-butyl)-5-methylbenzo[d][1,3]oxathiol-2-ylidene)-4-methylcyclohexa-2,4-dien-1-one, C23H28O2S
- The crystal structure of (R)-2-aminobutanamide hydrochloride, C4H11ClN2O
- Crystal structure of bromido[hydridotris(3-tert-butyl-5-isopropylpyrazolyl)borato-κ3 N,N′,N″]copper(II), C30H52BBrCuN6
- Crystal structure of chlorido{hydridotris[3-mesityl-5-methyl-1H-pyrazol-1-yl-κN3]borato}-copper(II) dichloromethane monosolvate
- Crystal structure of 4-[3,5-bis(propan-2-yl)-1H-pyrazol-4-yl]pyridine, C14H19N3
- Crystal structure of ((4-(4-bromophenyl)-1H-pyrrol-3-yl)methyl)ferrocene, C21H16BrFeNO
- Crystal structure of [(4-chlorobenzyl)triphenylphosphonium] dichloridocopper(I), {[C25H21ClP]+[CuCl2]−}n
- The crystal structure of {Cu(2,9-diisopropyl-4,7-diphenyl-1,10-phenanthroline)[4,5-bis(diphenylphosphino)-9,9-dimethylxanthene]}+ PF6−·1.5(EtOAC)
- Crystal structure of 3,5-bis(t-butyl)-1H-pyrazol-4-amine, C11H21N3
- Crystal structure of [(2,4-dichlorobenzyl)triphenylphosphonium] trichloridocopper(II), [C25H20Cl2P]+[CuCl3]−
- The crystal structure of dipotassium sulfide, K2S
- Crystal structure of (4-(4-methoxyphenyl)-1H-pyrrole-3-carbonyl)ferrocene, C22H19FeNO2
- Crystal structure of (E)-6-(4-methylpiperazin-1-yl)-2-(4-(trifluoromethyl)benzylidene)-3, 4-dihydronaphthalen-1(2H)-one, C23H23F3N2O
- Crystal structure of (E)-6-morpholino-2-(4-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C22H20F3NO2
- Crystal structure of Ce9Ir37Ge25
- The crystal structure of ethyl 6-(2-nitrophenyl)imidazo[2,1-b]thiazole-3-carboxylate, C14H11N3O4S
- Crystal structure of (4-(4-isopropylphenyl)-1H-pyrrol-3-yl)(ferrocenyl)methanone, C24H23FeNO
- Crystal structure of bis(methylammonium) tetrathiotungstate(VI), (CH3NH3)2[WS4]
- Crystal structure of 6,11-dihydro-12H-benzo[e]indeno[1,2-b]oxepin-12-one, C17H12O2
- Crystal structure of 3-[(4-phenylpiperidin-1-yl)methyl]-5-(thiophen-2-yl)-2,3-dihydro-1,3,4- oxadiazole-2-thione, C18H19N3OS2
- Crystal structure of N-isopropyl-1,8-naphthalimide C15H13NO2
- TiNiSi-type EuPdBi
- Crystal structure of 1-(p-tolylphenyl)-4-(2-thienoyl)-3-methyl-1H-pyrazol-5-ol, C16H14N2O2S
- The crystal structure of 3-(3-carboxypropyl)-2-nitro-1H-pyrrole 1-oxide, C7H9N3O5
- The crystal structure of tetraaqua-bis(2-(2-methyl-5-nitro-1H-imidazol-1-yl)acetato-k2O:N)-tetrakis(2-(2-methyl-5-nitro-1H-imidazol-1-yl)acetato-k1N)trizinc(II) hexahydrate C36H52N18O32Zn3
- The crystal structure of 4-(3-carboxy-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinolin-7-yl)piperazin-1-ium 4-hydroxy-3,5-dimethoxybenzoate monohydrate, C25H30FN3O9
- Crystal structure of bis(DL-1-carboxy-2-(1H-indol-3-yl)ethan-1-aminium) oxalate — acetic acid (1/2)
- Crystal structure of methyl (E)-4-((4-methylphenyl)sulfonamido)but-2-enoate, C12H15NO4S
- The crystal structure of actarit, C10H11NO3
- The crystal structure of bicyclol, C19H18O9
- The crystal structure of topiroxostat, C13H8N6
- Crystal structure of 2,2-dichloro-N-methyl-N-(4-p-tolylthiazol-2-yl)acetamide, C13H12Cl2N2OS
- Crystal structure of 4-(trifluoromethyl)-7-coumarinyl trifluoromethanesulfonate C11H4F6O5S
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ6O6)-((Z)-N,N′-bis(2-(dimethylamino)phenyl)carbamimidato-κ1N)potassium(I)
- Crystal structure of (Z)-2-(5-((4-(dimethylamino)naphthalen-1-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid, C18H16N2O3S2
- Crystal structure of (4-fluorobenzyl)triphenylphosphonium bromide, C25H21BrFP
- The crystal structure of dichlorido-[6-(pyridin-2-yl)phenanthridine-κ2N, N′]zinc(II)-chloroform (1/1), C19H13N2ZnCl5
- Crystal structure of (E)-(3-(2,4-dichlorophenyl)acryloyl)ferrocene, C19H14Cl2FeO
- The crystal structure of (E)-7-chloro-1-cyclopropyl-6-fluoro-3-((2-hydroxybenzylidene)amino)quinolin-4(1H)-one, C19H14ClFN2O2
- Crystal structure of 2-bromo-11-(((fluoromethyl)sulfonyl)methyl)-6-methyl-6,11-dihydrodibenzo[c,f][1,2]thiazepine 5,5-dioxide, C16H13BrFNO4S2
- Crystal structure of 2-chloro-11-(((fluoromethyl)sulfonyl)methyl)-6-methyl-6,11-dihydrodibenzo[c,f][1,2]thiazepine 5,5-dioxide, C16H13ClFNO4S2
- Crystal structure of 5-(2,2-difluoropropyl)-5-methyl-6-oxo-5,6-dihydrobenzo[4,5]imidazo[2,1-a]isoquinoline-3-carbonitrile, C20H15F2N3O

