Abstract
C14H26N10O10, monoclinic, P21 (no. 4), a = 12.3337(3) Å, b = 3.87010(10) Å, c = 24.2368(6) Å, β = 97.644(2)°, V = 1146.61(5) Å3, Z = 2, R gt (F) = 0.0400, wRref(F2) = 0.1092, T = 170 K.
Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Yellow plate |
| Size: | 0.42 × 0.11 × 0.01 mm |
| Wavelength: | Cu Kα radiation (1.54184 Å) |
| μ: | 1.06 mm−1 |
| Diffractometer, scan mode: | XtaLAB Synergy R, ω |
| θmax, completeness: | 75.1°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 10,934, 4198, 0.041 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 3982 |
| N(param)refined: | 312 |
| Programs: | CrysAlisPRO [1], SHELX [2], Olex2 [3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| O1 | 0.26216 (12) | 0.6229 (5) | 0.55776 (6) | 0.0427 (4) |
| N1 | 0.38488 (16) | 0.9592 (6) | 0.64397 (8) | 0.0411 (4) |
| N2 | 0.43925 (15) | 1.1364 (6) | 0.68867 (8) | 0.0419 (4) |
| H2 | 0.407808 | 1.179062 | 0.717537 | 0.050* |
| N3 | 0.59227 (16) | 1.4148 (7) | 0.72964 (9) | 0.0495 (5) |
| H3A | 0.658960 | 1.480600 | 0.730010 | 0.059* |
| H3B | 0.557878 | 1.461439 | 0.757354 | 0.059* |
| N4 | 0.59248 (17) | 1.1657 (7) | 0.64332 (9) | 0.0502 (5) |
| H4A | 0.659176 | 1.229094 | 0.642849 | 0.060* |
| H4B | 0.558661 | 1.052957 | 0.615647 | 0.060* |
| C1 | 0.1824 (2) | 0.4463 (7) | 0.52321 (10) | 0.0462 (6) |
| C2 | 0.0960 (2) | 0.3859 (8) | 0.55111 (11) | 0.0487 (6) |
| H2A | 0.031852 | 0.271008 | 0.537299 | 0.058* |
| C3 | 0.1210 (2) | 0.5293 (7) | 0.60506 (11) | 0.0464 (6) |
| H3 | 0.076973 | 0.526809 | 0.633356 | 0.056* |
| C4 | 0.22208 (18) | 0.6711 (7) | 0.60727 (9) | 0.0406 (5) |
| C5 | 0.2069 (3) | 0.3684 (9) | 0.46697 (12) | 0.0582 (7) |
| H5A | 0.214299 | 0.580204 | 0.447200 | 0.087* |
| H5B | 0.148404 | 0.234001 | 0.447550 | 0.087* |
| H5C | 0.273905 | 0.239891 | 0.469318 | 0.087* |
| C6 | 0.28879 (18) | 0.8536 (7) | 0.65051 (10) | 0.0414 (5) |
| H6 | 0.262060 | 0.896467 | 0.683965 | 0.050* |
| C7 | 0.54233 (18) | 1.2409 (7) | 0.68622 (10) | 0.0413 (5) |
| O1A | 0.76376 (11) | 1.3943 (5) | 0.94291 (6) | 0.0396 (4) |
| N1A | 0.87729 (15) | 1.2428 (6) | 0.85690 (8) | 0.0423 (5) |
| N2A | 0.93133 (15) | 1.1463 (7) | 0.81334 (8) | 0.0465 (5) |
| H2AA | 0.898660 | 1.028638 | 0.785936 | 0.056* |
| N3A | 1.08727 (17) | 1.1520 (8) | 0.77224 (9) | 0.0554 (6) |
| H3AA | 1.154823 | 1.206798 | 0.772184 | 0.066* |
| H3AB | 1.052959 | 1.039691 | 0.744658 | 0.066* |
| N4A | 1.08741 (16) | 1.4126 (7) | 0.85745 (9) | 0.0503 (5) |
| H4AA | 1.154966 | 1.469234 | 0.857971 | 0.060* |
| H4AB | 1.053087 | 1.468296 | 0.884792 | 0.060* |
| C1A | 0.68667 (18) | 1.4417 (7) | 0.97817 (9) | 0.0410 (5) |
| C2A | 0.58899 (19) | 1.3233 (7) | 0.95376 (11) | 0.0448 (5) |
| H2AB | 0.523651 | 1.327295 | 0.968980 | 0.054* |
| C3A | 0.60408 (18) | 1.1913 (7) | 0.90055 (10) | 0.0436 (5) |
| H3AC | 0.551014 | 1.091883 | 0.874508 | 0.052* |
| C4A | 0.71098 (18) | 1.2390 (6) | 0.89532 (10) | 0.0395 (5) |
| C5A | 0.7272 (2) | 1.5969 (8) | 1.03287 (10) | 0.0499 (6) |
| H5AA | 0.791151 | 1.474605 | 1.049336 | 0.075* |
| H5AB | 0.671395 | 1.581509 | 1.056854 | 0.075* |
| H5AC | 0.745324 | 1.835055 | 1.027883 | 0.075* |
| C6A | 0.77561 (18) | 1.1574 (7) | 0.85241 (9) | 0.0418 (5) |
| H6A | 0.743827 | 1.040961 | 0.820770 | 0.050* |
| C7A | 1.03632 (19) | 1.2400 (7) | 0.81460 (10) | 0.0441 (6) |
| O2 | 0.30805 (13) | 1.3944 (7) | 0.76875 (7) | 0.0540 (5) |
| O3 | 0.31049 (15) | 1.6358 (8) | 0.84949 (8) | 0.0645 (6) |
| O4 | 0.45007 (14) | 1.6807 (7) | 0.80558 (8) | 0.0565 (5) |
| N5 | 0.35664 (15) | 1.5716 (6) | 0.80870 (8) | 0.0447 (5) |
| O2A | 0.80373 (14) | 0.7363 (6) | 0.73062 (8) | 0.0582 (6) |
| O3A | 0.80719 (17) | 0.4827 (7) | 0.65154 (8) | 0.0649 (6) |
| O4A | 0.94760 (16) | 0.7633 (9) | 0.68933 (10) | 0.0806 (9) |
| N5A | 0.85311 (16) | 0.6597 (8) | 0.69018 (8) | 0.0492 (5) |
| O5 | 0.48651 (18) | 0.8605 (12) | 0.53592 (10) | 0.0981 (11) |
| H5D | 0.480166 | 0.689310 | 0.513761 | 0.147* |
| H5E | 0.434336 | 0.807510 | 0.553971 | 0.147* |
| O6 | 0.99548 (15) | 1.6258 (8) | 0.95986 (8) | 0.0637 (6) |
| H6B | 0.992218 | 1.796659 | 0.981628 | 0.096* |
| H6C | 0.929169 | 1.582980 | 0.947335 | 0.096* |
Source of material
5-Methylfurfural (1.10 g, 0.01 mol) was placed in 15 ml ethanol at room temperature, added into aminoguanidine nitrate (1.37 g, 0.01 mol) solution containing 10 ml water and 8 ml ethanol, heated and stirred for 8 h cooled to room temperature, then the precipitate was removed, and the filtrate was left standing to precipitate light yellow crystal.
Experimental details
Hydrogen atoms were placed in their geometrically idealized positions and constrained to ride on their parent atoms.
Comment
Guanidine groups easy form hydrogen bonds, have good stability and strong physiological activity, and are used as intermediates in the synthesis of drugs [4, 5]. Schiff base compounds and their metal complexes are used in medicine [6], catalysis [7], analytical chemistry [8], and an important application in the field of photochromism [9]. According to previous studies [10, 11] we synthesized the title compound by the method of amine carbonyl condensation.
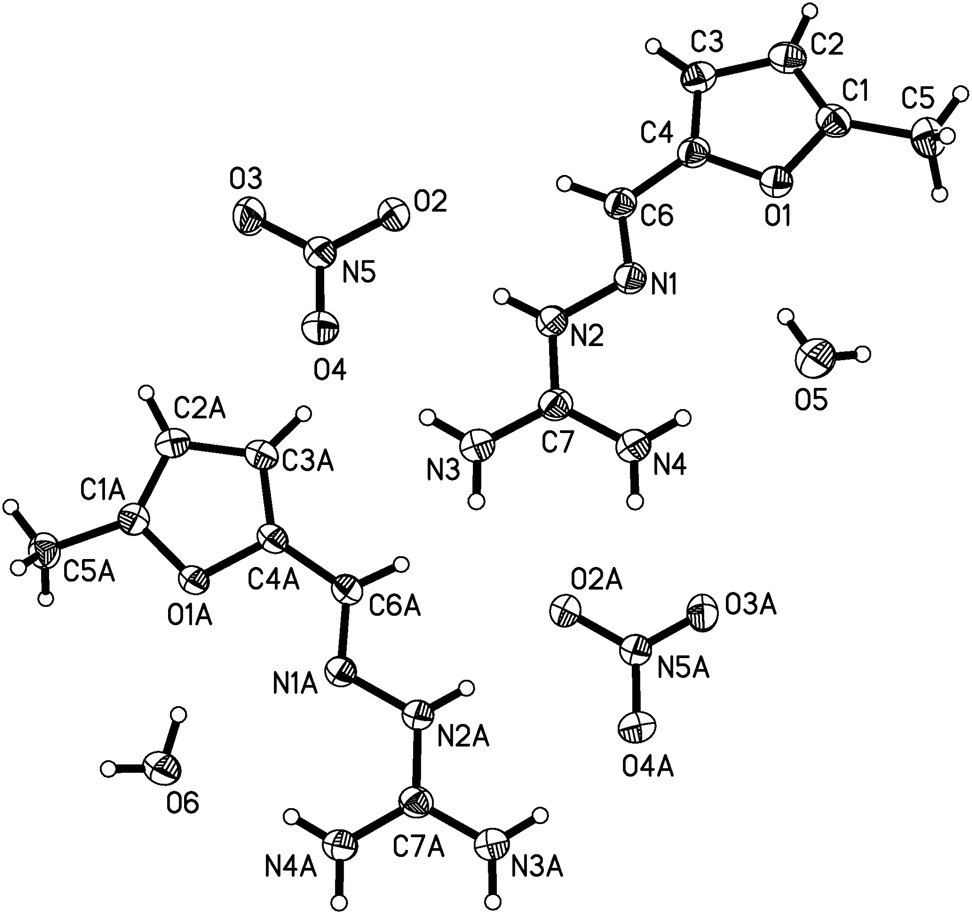
The structure of the title compound contains pairs of crystallographically independent cations, anions and water molecules (see the figure). The N–N and C=N bond distances in the two molecules are almost the same, N(1)–N(2) is 1.378(3) Å, N(1A)–N(2A) is 1.373(3) Å, N1=C6 is 1.283(3) Å, N(1A) = C(6A) is 1.288(3) Å, and all other bond lengths and bond angles are within the normal range [11].
Funding source: Henan University of Science and Technology
Award Identifier / Grant number: 135100001
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This study was financially supported by Henan University of Science and Technology Distinguished Professor Open Fund (Grant No. 135100001).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Agilent Technologies. CRYSALISPRO; Agilent Technologies: Santa Clara, CA, USA, 2017.Suche in Google Scholar
2. Sheldrick, G. M. SHELXTL – integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Suche in Google Scholar
3. Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Suche in Google Scholar
4. Thomas, S. J., Balónová, B., Cinatl, J.Jr., Wass, M. N., Serpell, C. J., Blight, B. A., Michaelis, M. Thiourea and guanidine compounds and their iridium complexes in drug-resistant cancer cell lines: structure-activity relationships and direct luminescent imaging. ChemMedChem 2020, 15, 349–353; https://doi.org/10.1002/cmdc.201900591.Suche in Google Scholar
5. Bhat, M., Madhu, N., Sagar, B. K., Vijay Sekhar, E. Sulfisoxazole guanidinyl derivatives: synthesis, characterization and docking studies for potential anti-TB agents. Res. J. Pharm. Technol. 2019, 12, 1726–1730; https://doi.org/10.5958/0974-360x.2019.00288.9.Suche in Google Scholar
6. Baumeister, J. E., Reinig, K. M., Barnes, C. L., Kelley, S. P., Jurisson, S. S. Technetium and rhenium Schiff base compounds for nuclear medicine: syntheses of rhenium analogues to 99mTc-Furifosmin. Inorg. Chem. 2018, 57, 12920–12933; https://doi.org/10.1021/acs.inorgchem.8b02156.Suche in Google Scholar
7. Rahaman, G. F., Aziz, A. A., Debashree, B., Saptarshi, R., Supriya, S., Lakshi, S., Lochan, G. R., Biswajit, S. Correction: a ferrocene functionalized Schiff base containing Cu(II) complex: synthesis, characterization and parts-per-million level catalysis for azide alkyne cycloaddition. Dalton Trans. 2021, 50, 6735.10.1039/D1DT90072BSuche in Google Scholar
8. Ye, N., Wang, X., Liu, Q., Hu, X. Covalent bonding of Schiff base network-1 as a stationary phase for capillary electrochromatography. Anal. Chim. Acta 2018, 1128, 113–120; https://doi.org/10.1016/j.aca.2018.04.037.Suche in Google Scholar
9. Zhuge, Y., Zheng, C., Liao, G., Pu, S. Structure and photochromic properties of a novel diarylethene containing a 9-fluorenone hydrazone Schiff base moiety. J. Chem. Res. 2020, 44, 137–141; https://doi.org/10.1177/1747519819891029.Suche in Google Scholar
10. Radanović, M. M., Rodić, M. V., Armaković, S., Armaković, S. J., Vojinović-Ješić, L. S., Leovac, V. M. Pyridoxylidene aminoguanidine and its copper(II) complexes - syntheses, structure, and DFT calculations. J. Coord. Chem. 2017, 70, 2870–2887.10.1080/00958972.2017.1367388Suche in Google Scholar
11. Liu, X.-J., Liu, E., Jin, Z.-S., Li, Z.-Y., Jian, F.-F., Liang, T. Crystal structure of (E)-amino(2-(4-(dimethylamino)benzylidene) hydrazineyl)methaniminium nitrate, C10H16N6O3. Z. Kristallogr. N. Cryst. Struct. 2021, 236, 795–796; https://doi.org/10.1515/ncrs-2021-0092.Suche in Google Scholar
© 2021 Ze-Sen Jin et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of (E)-7-hydroxy-2-((6-methoxypyridin-3-yl)methylene)-3, 4-dihydronaphthalen-1(2H)-one, C17H15NO3
- Crystal structure of (E)-7-methoxy-2-((2-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1 (2H)-one, C18H17NO3
- The crystal structure of N 6,N 6′-di(pyridin-2-yl)-[2,2′-bipyridine]-6,6′-diamine, C20H16N6
- The crystal structure of {N 1,N 2-bis[2,4-dimethyl-6-(4-(tert-butyl)phenyl)(phenyl)methyl]acenaphthylene-1,2-diimino-κ2 N, N′}-dibromido-nickel(II) – dichloromethane(1/2), C64H64Br2Cl4N2Ni
- Synthesis and crystal structure of nonacarbonyltris[(2-thia-1,3,5-triaza-7-phosphatricylco[3.3.1.1]decane-κ1 P)-2,2-dioxide]triruthenium(0) – acetonitrile (7/6), C25.71H32.57N9.86O15P3S3Ru3
- A new polymorph of 1-(4-nitrophenyl)-1H-benzimidazole (C13H9N3O2)
- The crystal structure of 2,2′-((1E,1′E)-(naphthalene-2,3 diylbis(azanylylidene)) bis(methanylylidene))bis(4-methylphenol), C26H22N2O2
- The crystal structure of bis(μ2-iodido)-bis(η6-benzene)-bis(iodido)-diosmium(II), C12H12I4Os2
- Redetermination of the crystal structure of bis{hydridotris(3,5-dimethylpyrazol-1-yl-κN 3)borato}copper(II), C30H44B2CuN12
- Crystal structure of (E)-3-((4-(tert-butyl)phenyl)thio)-4-hydroxypent-3-en-2-one, C15H20O2S
- Crystal structure of 2,2′-(p-tolylazanediyl)bis(1-phenylethan-1-one), C23H21NO2
- Redetermination of the crystal structure of the crystal sponge the poly[tetrakis(μ3-2,4,6-tris(pyridin-4-yl)-1,3,5-triazine)-dodecaiodidohexazinc(II) nitrobenzene solvate], C72H48I12N24Zn6⋅10(C6H5NO2)
- Crystal structure of (4′E)-6′-(diethylamino)-2-[(E)-[(6-methylpyridin-2-yl)methylidene]amino]-4′-{2-[(2E)-1,3,3-trimethyl-2,3-dihydro-1H-indol-2-ylidene]ethylidene}-1′,2,2′,3,3′,4′-hexahydrospiro[isoindole-1,9′-xanthene]-3-one, C44H45N5O2
- Crystal structure of (E)-7-fluoro-2-(3-fluorobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C17H12F2O1
- Crystal structure of tetrabutylammonium sulfanilate – 1-(diaminomethylene)thiourea (1/2)
- Crystal structure of [2,2′-{azanediyl)bis[(propane-3,1-diyl)(azanylylidene)methylylidene]} bis(3,5-dichlorophenolato)-κ2O,O′]-isothiocyanato-κN-iron(III), C21H19Cl4FeN4O2S
- Crystal structure of (4-chlorophenyl)(4-hydroxyphenyl)methanone, C13H9ClO2
- Crystal structure of 6,6′-((pentane-1,3-diylbis(azaneylylidene))bis(methaneylylidene))bis(2,4-dibromolphenolato-κ4 N,N′,O,O′)copper(II),) C19H16Br4CuN2O2
- Chlorido-(2,2′-(ethane-bis(5-methoxyphenolato))-κ4 N,N′,O,O′)manganese(III) monohydrate, C19H18Cl2CuN2O2
- Crystal structure of 2,6-di-tert-butyl-4-(4-methoxybenzylidene)cyclohexa-2,5-dien-1-one, C22H28O2
- Crystal structure of [6,6′-(((2,2-dimethylpropane-1,3-diyl)bis(azanylylidene))bis(methanylylidene))bis(2-chlorophenolato)-κ4N,N′,O,O′]copper(II)
- Crystal structure of 2-chloro-3-((thiophen-2-ylmethyl)amino)naphthalene-1,4-dione, C30H20O4N2Cl2S2
- Crystal structure of bis{hydridotris(3-trifluoromethyl-5-methylpyrazolyl-1-yl)borato-κN 3}manganese(II), C30H26B2F18MnN12
- Crystal structure of 1-(2-methylphenyl)-2-(2-methylbenzo[b]thienyl)-3,3,4,4,5,5-hexafluorocyclopent-ene, C21H14F6S
- Crystal structure of 2-(3-((carbamimidoylthio)methyl)benzyl)isothiouronium hexafluorophosphate monohydrate, C10H17F6N4OPS2
- Crystal structure of 4,5-diiodo-1,3-dimesityl-1H-1,2,3-triazol-3-ium chloride – chloroform (1/1), C21H23Cl4I2N3
- Crystal structure of azido-k1 N-{6,6′-((((methylazanediyl)bis(propane-3,1-diyl))bis(azanylylidene))bis(methanylylidene))bis(2,4-dibromophenolato)k5 N,N′,N″,O,O′}cobalt(III)-methanol (1/1)), C21H23Br4CoN6O3
- The crystal structure of 2-(4-((carbamimidoylthio)methyl)benzyl)isothiouronium hexafluorophosphate monohydrate, C10H17F6N4OPS2
- Crystal structure of 1,1′-(methane-1,1-diyl)bis(3-methyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C9H14F12N4P2
- Crystal structure of (4′E)-6′-(diethylamino)-2-[(E)-[(pyren-1-yl)methylidene]amino]-4′-{2-[(2E)-1,3,3-trimethyl-2,3-dihydro-1H-indol-2-ylidene]ethylidene}-1′,2,2′,3,3′,4′-hexahydrospiro[isoindole-1,9′-xanthene]-3-one, C54H48N4O2
- Crystal structure of poly[bis(μ2-2,6-bis(1-imidazoly)pyridine-κ2 N,N′)-bis(thiocyanato-κ1 N)copper(II)] dithiocyanate, C24H18CuN12S2
- Cones with a three-fold symmetry constructed from three hydrogen bonded theophyllinium cations that coat [FeCl4]− anions in the crystal structure of tris(theophyllinium) bis(tetrachloridoferrate(III)) chloride trihydrate, C21H33Cl9Fe2N12O9
- Crystal structure of 14-O-[(4-(4-hydroxypiperidine-1-yl)-6-methylpyrimidine-2-yl)thioacetyl]-mutilin monohydrate, C32H49N3O6S
- The crystal structure of (E)-3-chloro-2-(2-(4-methylbenzylidene)hydrazinyl)pyridine, C13H12ClN3
- The crystal structure of 4-phenyl-4-[2-(pyridine-4-carbonyl)hydrazinylidene]butanoic acid, C16H15N3O3
- The crystal structure of 6-amino-5-carboxypyridin-1-ium pentaiodide monohydrate C6H9I5N2O3
- Crystal structure of bis(μ3-oxido)-bis(μ2-2-formylbenzoato-k2O:O′)-bis(2-(dimethoxymethyl)-benzoato-κO)-oktakismethyl-tetratin(IV)
- Crystal structure of 2-((E)-(((E)-2-hydroxy-4-methylbenzylidene) hydrazineylidene)methyl)-4-methylphenol, C16H16N2O2
- Crystal structure of (E)-amino(2-((5-methylfuran-2-yl)methylene)hydrazinyl) methaniminium nitrate monohydrate, C14H26N10O10
- The crystal structure of N′-(2-chloro-6-hydroxybenzylidene)thiophene-2-carbohydrazide monohydrate, C12H11ClN2O3S
- Crystal structure of catena-poly[(μ2-1,1′-(biphenyl-4,4′-diyl)bis(1H-imidazol)-κ2N:N′)-bis(4-bromobenzoate-κ1O)zinc(II)], C64H44Br4N8O8Zn2
- The crystal structure of catena-poly[(1-(4-carboxybenzyl)pyridin-1-ium-4-carboxylato-κ1O)-(μ2-oxalato-κ4 O:O′:O″:O‴)dioxidouranium(VI)], C16H11NO10U
- Crystal structure of 3-allyl-4-(2-bromoethyl)-5-(4-methoxyphenyl)-2-phenylfuran, C22H21BrO2
- Halogen bonds in the crystal structure of 4,3′:5′,4″-terpyridine — 1,3-diiodotetrafluorobenzene (1/1), C21H11F4I2N3
- Crystal structure of 2-(1H-indol-3-yl)ethan-1-aminium 2-(4-acetylphenoxy)acetate, C20H22N2O4
- Chalcogen bonds in the crystal structure of 4,7-dibromo-2,1,3-benzoselenadiazole, C6H2Br2N2Se
- The crystal structure of 1,4-bis((1H-benzimidazol-2-yl)methyl)-piperazine-2,5-dione dihydrate, C20H22N6O4
- The crystal structure of C19H20O8
- The crystal structure of KNa3Te8O18·5H2O exhibiting a ∞2[Te4O9]2− layer
- Erratum
- Erratum to: Crystal structure of (Z)-3-(6-bromo-1H-indol-3-yl)-1,3-diphenylprop-2-en-1-one, C23H16BrNO
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of (E)-7-hydroxy-2-((6-methoxypyridin-3-yl)methylene)-3, 4-dihydronaphthalen-1(2H)-one, C17H15NO3
- Crystal structure of (E)-7-methoxy-2-((2-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1 (2H)-one, C18H17NO3
- The crystal structure of N 6,N 6′-di(pyridin-2-yl)-[2,2′-bipyridine]-6,6′-diamine, C20H16N6
- The crystal structure of {N 1,N 2-bis[2,4-dimethyl-6-(4-(tert-butyl)phenyl)(phenyl)methyl]acenaphthylene-1,2-diimino-κ2 N, N′}-dibromido-nickel(II) – dichloromethane(1/2), C64H64Br2Cl4N2Ni
- Synthesis and crystal structure of nonacarbonyltris[(2-thia-1,3,5-triaza-7-phosphatricylco[3.3.1.1]decane-κ1 P)-2,2-dioxide]triruthenium(0) – acetonitrile (7/6), C25.71H32.57N9.86O15P3S3Ru3
- A new polymorph of 1-(4-nitrophenyl)-1H-benzimidazole (C13H9N3O2)
- The crystal structure of 2,2′-((1E,1′E)-(naphthalene-2,3 diylbis(azanylylidene)) bis(methanylylidene))bis(4-methylphenol), C26H22N2O2
- The crystal structure of bis(μ2-iodido)-bis(η6-benzene)-bis(iodido)-diosmium(II), C12H12I4Os2
- Redetermination of the crystal structure of bis{hydridotris(3,5-dimethylpyrazol-1-yl-κN 3)borato}copper(II), C30H44B2CuN12
- Crystal structure of (E)-3-((4-(tert-butyl)phenyl)thio)-4-hydroxypent-3-en-2-one, C15H20O2S
- Crystal structure of 2,2′-(p-tolylazanediyl)bis(1-phenylethan-1-one), C23H21NO2
- Redetermination of the crystal structure of the crystal sponge the poly[tetrakis(μ3-2,4,6-tris(pyridin-4-yl)-1,3,5-triazine)-dodecaiodidohexazinc(II) nitrobenzene solvate], C72H48I12N24Zn6⋅10(C6H5NO2)
- Crystal structure of (4′E)-6′-(diethylamino)-2-[(E)-[(6-methylpyridin-2-yl)methylidene]amino]-4′-{2-[(2E)-1,3,3-trimethyl-2,3-dihydro-1H-indol-2-ylidene]ethylidene}-1′,2,2′,3,3′,4′-hexahydrospiro[isoindole-1,9′-xanthene]-3-one, C44H45N5O2
- Crystal structure of (E)-7-fluoro-2-(3-fluorobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C17H12F2O1
- Crystal structure of tetrabutylammonium sulfanilate – 1-(diaminomethylene)thiourea (1/2)
- Crystal structure of [2,2′-{azanediyl)bis[(propane-3,1-diyl)(azanylylidene)methylylidene]} bis(3,5-dichlorophenolato)-κ2O,O′]-isothiocyanato-κN-iron(III), C21H19Cl4FeN4O2S
- Crystal structure of (4-chlorophenyl)(4-hydroxyphenyl)methanone, C13H9ClO2
- Crystal structure of 6,6′-((pentane-1,3-diylbis(azaneylylidene))bis(methaneylylidene))bis(2,4-dibromolphenolato-κ4 N,N′,O,O′)copper(II),) C19H16Br4CuN2O2
- Chlorido-(2,2′-(ethane-bis(5-methoxyphenolato))-κ4 N,N′,O,O′)manganese(III) monohydrate, C19H18Cl2CuN2O2
- Crystal structure of 2,6-di-tert-butyl-4-(4-methoxybenzylidene)cyclohexa-2,5-dien-1-one, C22H28O2
- Crystal structure of [6,6′-(((2,2-dimethylpropane-1,3-diyl)bis(azanylylidene))bis(methanylylidene))bis(2-chlorophenolato)-κ4N,N′,O,O′]copper(II)
- Crystal structure of 2-chloro-3-((thiophen-2-ylmethyl)amino)naphthalene-1,4-dione, C30H20O4N2Cl2S2
- Crystal structure of bis{hydridotris(3-trifluoromethyl-5-methylpyrazolyl-1-yl)borato-κN 3}manganese(II), C30H26B2F18MnN12
- Crystal structure of 1-(2-methylphenyl)-2-(2-methylbenzo[b]thienyl)-3,3,4,4,5,5-hexafluorocyclopent-ene, C21H14F6S
- Crystal structure of 2-(3-((carbamimidoylthio)methyl)benzyl)isothiouronium hexafluorophosphate monohydrate, C10H17F6N4OPS2
- Crystal structure of 4,5-diiodo-1,3-dimesityl-1H-1,2,3-triazol-3-ium chloride – chloroform (1/1), C21H23Cl4I2N3
- Crystal structure of azido-k1 N-{6,6′-((((methylazanediyl)bis(propane-3,1-diyl))bis(azanylylidene))bis(methanylylidene))bis(2,4-dibromophenolato)k5 N,N′,N″,O,O′}cobalt(III)-methanol (1/1)), C21H23Br4CoN6O3
- The crystal structure of 2-(4-((carbamimidoylthio)methyl)benzyl)isothiouronium hexafluorophosphate monohydrate, C10H17F6N4OPS2
- Crystal structure of 1,1′-(methane-1,1-diyl)bis(3-methyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C9H14F12N4P2
- Crystal structure of (4′E)-6′-(diethylamino)-2-[(E)-[(pyren-1-yl)methylidene]amino]-4′-{2-[(2E)-1,3,3-trimethyl-2,3-dihydro-1H-indol-2-ylidene]ethylidene}-1′,2,2′,3,3′,4′-hexahydrospiro[isoindole-1,9′-xanthene]-3-one, C54H48N4O2
- Crystal structure of poly[bis(μ2-2,6-bis(1-imidazoly)pyridine-κ2 N,N′)-bis(thiocyanato-κ1 N)copper(II)] dithiocyanate, C24H18CuN12S2
- Cones with a three-fold symmetry constructed from three hydrogen bonded theophyllinium cations that coat [FeCl4]− anions in the crystal structure of tris(theophyllinium) bis(tetrachloridoferrate(III)) chloride trihydrate, C21H33Cl9Fe2N12O9
- Crystal structure of 14-O-[(4-(4-hydroxypiperidine-1-yl)-6-methylpyrimidine-2-yl)thioacetyl]-mutilin monohydrate, C32H49N3O6S
- The crystal structure of (E)-3-chloro-2-(2-(4-methylbenzylidene)hydrazinyl)pyridine, C13H12ClN3
- The crystal structure of 4-phenyl-4-[2-(pyridine-4-carbonyl)hydrazinylidene]butanoic acid, C16H15N3O3
- The crystal structure of 6-amino-5-carboxypyridin-1-ium pentaiodide monohydrate C6H9I5N2O3
- Crystal structure of bis(μ3-oxido)-bis(μ2-2-formylbenzoato-k2O:O′)-bis(2-(dimethoxymethyl)-benzoato-κO)-oktakismethyl-tetratin(IV)
- Crystal structure of 2-((E)-(((E)-2-hydroxy-4-methylbenzylidene) hydrazineylidene)methyl)-4-methylphenol, C16H16N2O2
- Crystal structure of (E)-amino(2-((5-methylfuran-2-yl)methylene)hydrazinyl) methaniminium nitrate monohydrate, C14H26N10O10
- The crystal structure of N′-(2-chloro-6-hydroxybenzylidene)thiophene-2-carbohydrazide monohydrate, C12H11ClN2O3S
- Crystal structure of catena-poly[(μ2-1,1′-(biphenyl-4,4′-diyl)bis(1H-imidazol)-κ2N:N′)-bis(4-bromobenzoate-κ1O)zinc(II)], C64H44Br4N8O8Zn2
- The crystal structure of catena-poly[(1-(4-carboxybenzyl)pyridin-1-ium-4-carboxylato-κ1O)-(μ2-oxalato-κ4 O:O′:O″:O‴)dioxidouranium(VI)], C16H11NO10U
- Crystal structure of 3-allyl-4-(2-bromoethyl)-5-(4-methoxyphenyl)-2-phenylfuran, C22H21BrO2
- Halogen bonds in the crystal structure of 4,3′:5′,4″-terpyridine — 1,3-diiodotetrafluorobenzene (1/1), C21H11F4I2N3
- Crystal structure of 2-(1H-indol-3-yl)ethan-1-aminium 2-(4-acetylphenoxy)acetate, C20H22N2O4
- Chalcogen bonds in the crystal structure of 4,7-dibromo-2,1,3-benzoselenadiazole, C6H2Br2N2Se
- The crystal structure of 1,4-bis((1H-benzimidazol-2-yl)methyl)-piperazine-2,5-dione dihydrate, C20H22N6O4
- The crystal structure of C19H20O8
- The crystal structure of KNa3Te8O18·5H2O exhibiting a ∞2[Te4O9]2− layer
- Erratum
- Erratum to: Crystal structure of (Z)-3-(6-bromo-1H-indol-3-yl)-1,3-diphenylprop-2-en-1-one, C23H16BrNO

