Abstract
C21H21BrO2, monoclinic, P21/c (no. 14), a = 22.175(2) Å, b = 5.8375(6) Å, c = 14.8729(14) Å, β = 100.936(1)°, V = 1890.3(3) Å3, Z = 4, R gt (F) = 0.0343, wRref(F2) = 0.0839, T = 296(2) K.
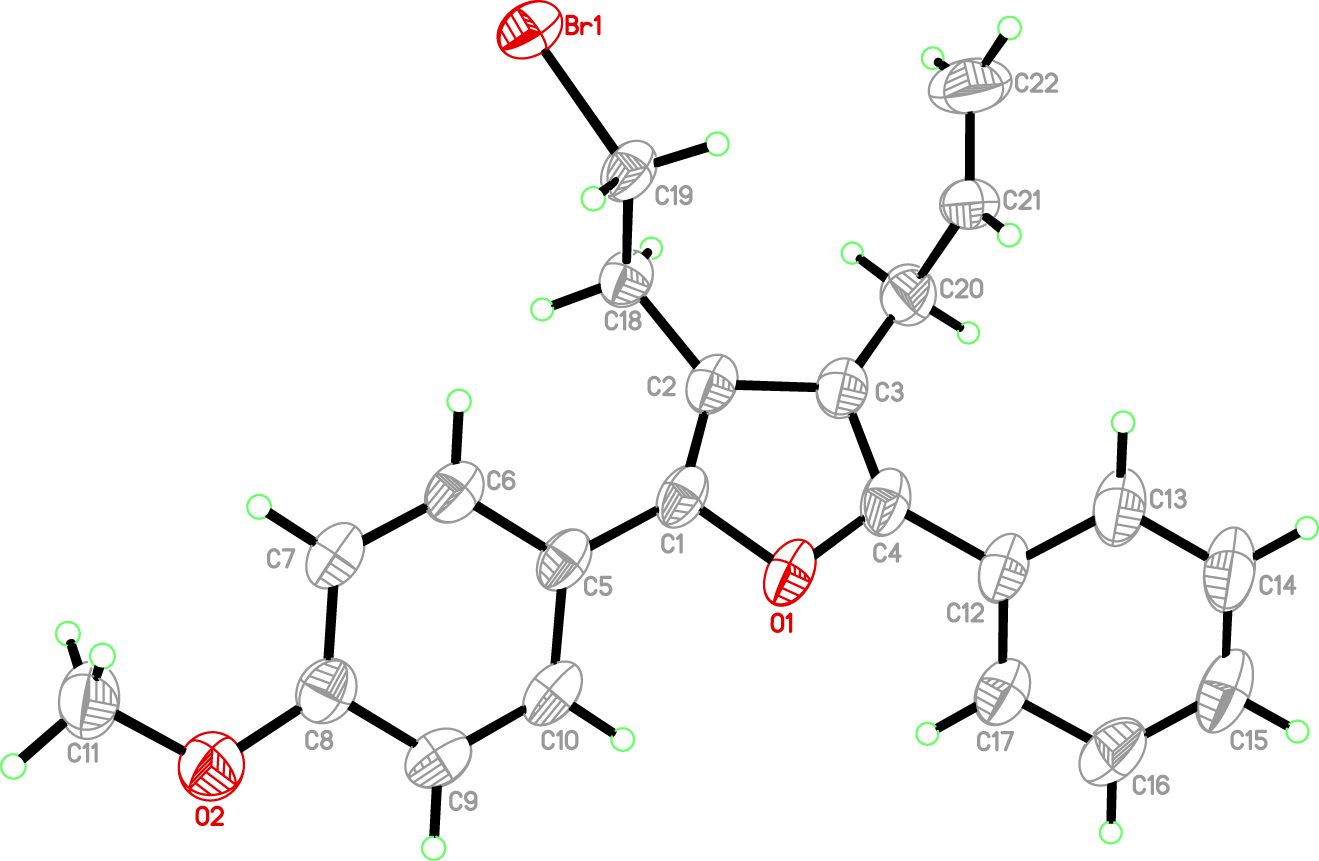
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colorless block |
| Size: | 0.20 × 0.18 × 0.16 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 2.19 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| θmax, completeness: | 26.4°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 13,804, 3862, 0.026 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 2606 |
| N(param)refined: | 246 |
| Programs: | Bruker [1], SHELX [2] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| Br1 | 0.19913 (2) | 0.11721 (5) | 0.58231 (2) | 0.07259 (13) |
| O1 | 0.25684 (8) | 0.8688 (3) | 0.29775 (13) | 0.0648 (5) |
| O2 | −0.01240 (10) | 0.6470 (4) | 0.09392 (14) | 0.0846 (6) |
| C1 | 0.21593 (11) | 0.7301 (4) | 0.33181 (19) | 0.0576 (7) |
| C2 | 0.24030 (12) | 0.6690 (4) | 0.4188 (2) | 0.0583 (7) |
| C3 | 0.29994 (12) | 0.7735 (4) | 0.4413 (2) | 0.0642 (7) |
| C4 | 0.30784 (12) | 0.8922 (4) | 0.3659 (2) | 0.0670 (7) |
| C5 | 0.15738 (12) | 0.6980 (4) | 0.27012 (18) | 0.0560 (6) |
| C6 | 0.11978 (12) | 0.5091 (5) | 0.27505 (19) | 0.0647 (7) |
| H6 | 0.133117 | 0.395143 | 0.317935 | 0.078* |
| C7 | 0.06316 (13) | 0.4864 (5) | 0.21787 (19) | 0.0676 (7) |
| H7 | 0.038800 | 0.359235 | 0.223124 | 0.081* |
| C8 | 0.04278 (13) | 0.6513 (5) | 0.15330 (19) | 0.0655 (7) |
| C9 | 0.08002 (14) | 0.8386 (5) | 0.1461 (2) | 0.0708 (8) |
| H9 | 0.066937 | 0.950232 | 0.102090 | 0.085* |
| C10 | 0.13588 (13) | 0.8607 (4) | 0.20323 (19) | 0.0655 (7) |
| H10 | 0.160118 | 0.987725 | 0.197201 | 0.079* |
| C11 | −0.05025 (14) | 0.4501 (6) | 0.0955 (2) | 0.0943 (10) |
| H11A | −0.027821 | 0.315260 | 0.084901 | 0.142* |
| H11B | −0.086290 | 0.463583 | 0.048487 | 0.142* |
| H11C | −0.062121 | 0.438954 | 0.154141 | 0.142* |
| C12 | 0.35502 (13) | 1.0473 (5) | 0.3453 (2) | 0.0705 (8) |
| C13 | 0.41696 (13) | 1.0052 (5) | 0.3779 (3) | 0.0924 (10) |
| H13 | 0.428885 | 0.873088 | 0.411497 | 0.111* |
| C14 | 0.46111 (16) | 1.1584 (7) | 0.3608 (3) | 0.1053 (12) |
| H14 | 0.502426 | 1.129507 | 0.383561 | 0.126* |
| C15 | 0.44432 (18) | 1.3508 (7) | 0.3108 (3) | 0.1068 (12) |
| H15 | 0.474182 | 1.452939 | 0.299420 | 0.128* |
| C16 | 0.38348 (18) | 1.3948 (6) | 0.2770 (3) | 0.0995 (11) |
| H16 | 0.372265 | 1.526487 | 0.242665 | 0.119* |
| C17 | 0.33849 (14) | 1.2433 (5) | 0.2937 (2) | 0.0797 (8) |
| H17 | 0.297312 | 1.273365 | 0.270412 | 0.096* |
| C18 | 0.21078 (11) | 0.5314 (4) | 0.48488 (18) | 0.0589 (6) |
| H18A | 0.221628 | 0.597685 | 0.545610 | 0.071* |
| H18B | 0.166466 | 0.538227 | 0.466192 | 0.071* |
| C19 | 0.23132 (12) | 0.2853 (4) | 0.4880 (2) | 0.0658 (7) |
| H19A | 0.275841 | 0.278759 | 0.500898 | 0.079* |
| H19B | 0.216926 | 0.214340 | 0.428813 | 0.079* |
| C20 | 0.34128 (14) | 0.7729 (5) | 0.5333 (3) | 0.0919 (10) |
| H20Aa | 0.317788 | 0.756748 | 0.581689 | 0.110* |
| H20Ba | 0.365073 | 0.913174 | 0.543041 | 0.110* |
| H20Cb | 0.314614 | 0.815673 | 0.575217 | 0.110* |
| H20Db | 0.368077 | 0.903453 | 0.531420 | 0.110* |
| C21a | 0.3836 (3) | 0.5629 (12) | 0.5304 (5) | 0.075 (3) |
| H21Aa | 0.398778 | 0.533492 | 0.477423 | 0.090* |
| C22a | 0.3982 (4) | 0.4288 (15) | 0.5994 (6) | 0.112 (3) |
| H22Aa | 0.383296 | 0.456453 | 0.652781 | 0.134* |
| H22Ba | 0.423678 | 0.303759 | 0.596059 | 0.134* |
| C21Bb | 0.3832 (4) | 0.5905 (12) | 0.5848 (6) | 0.072 (3) |
| H21Bb | 0.394940 | 0.588107 | 0.648282 | 0.087* |
| C22Bb | 0.4008 (4) | 0.4407 (16) | 0.5325 (7) | 0.081 (3) |
| H22Cb | 0.387837 | 0.450450 | 0.469410 | 0.097* |
| H22Eb | 0.426607 | 0.322024 | 0.557629 | 0.097* |
-
aOccupancy: 0.539(11), bOccupancy: 0.461(11).
Source of material
The title compound was synthesized according to our previous reported method, only the (4-methoxyphenyl) (1-(p-tolylethynyl) cyclopropyl)methanone was replaced by (4-methoxyphenyl) (1-(phenylethynyl)cyclopropyl) methanone [3]. The crystal suitable for single crystal structure determination was obtained by recrystallization from tetrahydrofuran at room temperature.
Experimental details
All H atoms were included in calculated positions and refined as riding atoms with C–H = 0.93–0.97 Å, with Uiso = 1.5 Ueq(C) for methyl H atoms and 1.2 Ueq(C) for the remaining H atoms. Some atoms are disordered over two positions. The two parts overlapped each other with site occupation factors of 0.539 and 0.461. The disorder is omitted in the figure for clarity.
Comment
Furan derivatives have attracted more and more attention due to their wide variety of biological and pharmacological activities [3], [4], [5], [6], [7], [8], [9], [10], [11], [12]. Recently we have reported the structure of the structurally related 3-allyl-4-(2-bromoethyl)-5-(4-methoxyphenyl)-2-(p-tolyl)furan [3]. In continuation of our research on furan derivatives, the molecular and crystal structure of the title compound were determined.
In the crystal structure, the asymmetric unit of the title compound consists of one formula unit (cf. Figure). The bond lengths are comparable with those found in our previous work [3] and that of others [12, 13]. The dihedral angle formed by the two aromatic rings is 22.319(1) Å. The furan ring and the two aromatic rings form dihedral angles of 26.389(1)° and 37.263(1)°, respectively. In the crystal, weak intermolecular C–H…Br hydrogen bonds link the molecules into one dimensional chains along the b axis.
Funding source: Nanyang Institute of Technology
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This work was financial supported by Nanyang Institute of Technology.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Bruker. Apex2, Saint and Sadabs; Bruker AXS Inc.: Madison, Wisconsin, USA, 2008.Suche in Google Scholar
2. Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Suche in Google Scholar
3. Xu, X.-L., Li, J. Crystal structure of 3-allyl-4-(2-bromoethyl)-5-(4-methoxyphenyl)-2-(p-tolyl)furan, C23H23BrO2. Z. Kristallogr. NCS 2021, 236, 1161–1163; https://doi.org/10.1515/ncrs-2021-0258.Suche in Google Scholar
4. Aziz, M. A., Serya, R. A. T., Lasheen, D. S., Abouzid, K. A. M. Furo[2,3-d]pyrimidine based derivatives as kinase inhibitors and anticancer agents. J. Future Pharm. Sci. 2016, 2, 1–8; https://doi.org/10.1016/j.fjps.2015.12.001.Suche in Google Scholar
5. Zhu, M., Fu, W.-J., Xu, C., Zou, G.-L., Wang, Z.-Q., Ji, B.-M. Highly efficient synthesis of multisubstituted furans through cupric halide-mediated intramolecular halocyclization of 1-(1-alkynyl)cyclopropyl ketones. Eur. J. Org Chem. 2012, 2012, 4609–4615; https://doi.org/10.1002/ejoc.201200601.Suche in Google Scholar
6. Komogortsev, A. N., Melekhina, V. G., Lichitsky, B. V., Minyaev, M. E. Novel one-pot approach to 2-aminofuran derivatives via multicomponent reaction of 3-hydroxy-4H-pyran-4-ones, α-ketoaldehydes and methylene active nitriles. Tetrahedron Lett. 2020, 61, 152384; https://doi.org/10.1016/j.tetlet.2020.152384.Suche in Google Scholar
7. Su, Z., Xie, Z., Wang, S., Luo, N., Wang, C. Direct synthesis of highly functionalized furans from donor-acceptor cyclopropanes via DBU-mediated ring expansion reactions. Org. Biomol. Chem. 2019, 17, 7342–7351; https://doi.org/10.1039/c9ob01308c.Suche in Google Scholar
8. Nie, Y.-L., Wu, Y.-D., Wang, C.-X., Lin, R., Xie, Y., Fang, D.-S., Jiang, H., Lian, Y.-Y. Compounds from marine-derived Verrucosispora sp. FIM06054 and their potential antitumour activities. Nat. Prod. Res. 2014, 28, 2134–2139.10.1080/14786419.2014.926350Suche in Google Scholar PubMed
9. Tsuji, H., Nakamura, E. Design and functions of semiconducting fused polycyclic furans for optoelectronic applications. Acc. Chem. Res. 2017, 50, 396–406; https://doi.org/10.1021/acs.accounts.6b00595.Suche in Google Scholar
10. Mishra, A., Ulaganathan, M., Edison, E., Borah, P., Mishra, A., Sreejith, S., Madhavi, S., Stuparu, M. C. Polymeric nanomaterials based on the buckybowl motif: synthesis through ring-opening metathesis polymerization and energy storage applications. ACS Macro Lett. 2017, 6, 1212–1216; https://doi.org/10.1021/acsmacrolett.7b00746.Suche in Google Scholar
11. Hu, S.-L., Xu, C.-H., Zhang, S.-S., Sun, W.-B., Gao, Q. Synthesis, crystal structure and binding properties of a new fluorescent molecular clip based on 2,5-diphenylfuran. J. Chem. Res. 2013, 37, 210–212.10.3184/174751913X13626742215922Suche in Google Scholar
12. Wang, R.-L., Shi, J.-X., Zheng, X.-F., Zhou, Y. 2-(3-Bromo-4-butoxyphenyl)-3,4-dimethyl-5-(3,4,5-trimethoxyphenyl)furan. Acta Crystallogr. 2005, E61, o2619–o2620; https://doi.org/10.1107/s1600536805022543.Suche in Google Scholar
13. Zang, W., Wei, Y., Shi, M. Gold(I)-catalyzed cascade cyclization of O-tethered 1,7-enynes bearing a cyclopropane moiety: construction of multi-substituted furans. Chem. Commun. 2019, 55, 8126–8129; https://doi.org/10.1039/c9cc03735g.Suche in Google Scholar
© 2021 Haiyan Yu and Jihong Li, published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of (E)-7-hydroxy-2-((6-methoxypyridin-3-yl)methylene)-3, 4-dihydronaphthalen-1(2H)-one, C17H15NO3
- Crystal structure of (E)-7-methoxy-2-((2-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1 (2H)-one, C18H17NO3
- The crystal structure of N 6,N 6′-di(pyridin-2-yl)-[2,2′-bipyridine]-6,6′-diamine, C20H16N6
- The crystal structure of {N 1,N 2-bis[2,4-dimethyl-6-(4-(tert-butyl)phenyl)(phenyl)methyl]acenaphthylene-1,2-diimino-κ2 N, N′}-dibromido-nickel(II) – dichloromethane(1/2), C64H64Br2Cl4N2Ni
- Synthesis and crystal structure of nonacarbonyltris[(2-thia-1,3,5-triaza-7-phosphatricylco[3.3.1.1]decane-κ1 P)-2,2-dioxide]triruthenium(0) – acetonitrile (7/6), C25.71H32.57N9.86O15P3S3Ru3
- A new polymorph of 1-(4-nitrophenyl)-1H-benzimidazole (C13H9N3O2)
- The crystal structure of 2,2′-((1E,1′E)-(naphthalene-2,3 diylbis(azanylylidene)) bis(methanylylidene))bis(4-methylphenol), C26H22N2O2
- The crystal structure of bis(μ2-iodido)-bis(η6-benzene)-bis(iodido)-diosmium(II), C12H12I4Os2
- Redetermination of the crystal structure of bis{hydridotris(3,5-dimethylpyrazol-1-yl-κN 3)borato}copper(II), C30H44B2CuN12
- Crystal structure of (E)-3-((4-(tert-butyl)phenyl)thio)-4-hydroxypent-3-en-2-one, C15H20O2S
- Crystal structure of 2,2′-(p-tolylazanediyl)bis(1-phenylethan-1-one), C23H21NO2
- Redetermination of the crystal structure of the crystal sponge the poly[tetrakis(μ3-2,4,6-tris(pyridin-4-yl)-1,3,5-triazine)-dodecaiodidohexazinc(II) nitrobenzene solvate], C72H48I12N24Zn6⋅10(C6H5NO2)
- Crystal structure of (4′E)-6′-(diethylamino)-2-[(E)-[(6-methylpyridin-2-yl)methylidene]amino]-4′-{2-[(2E)-1,3,3-trimethyl-2,3-dihydro-1H-indol-2-ylidene]ethylidene}-1′,2,2′,3,3′,4′-hexahydrospiro[isoindole-1,9′-xanthene]-3-one, C44H45N5O2
- Crystal structure of (E)-7-fluoro-2-(3-fluorobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C17H12F2O1
- Crystal structure of tetrabutylammonium sulfanilate – 1-(diaminomethylene)thiourea (1/2)
- Crystal structure of [2,2′-{azanediyl)bis[(propane-3,1-diyl)(azanylylidene)methylylidene]} bis(3,5-dichlorophenolato)-κ2O,O′]-isothiocyanato-κN-iron(III), C21H19Cl4FeN4O2S
- Crystal structure of (4-chlorophenyl)(4-hydroxyphenyl)methanone, C13H9ClO2
- Crystal structure of 6,6′-((pentane-1,3-diylbis(azaneylylidene))bis(methaneylylidene))bis(2,4-dibromolphenolato-κ4 N,N′,O,O′)copper(II),) C19H16Br4CuN2O2
- Chlorido-(2,2′-(ethane-bis(5-methoxyphenolato))-κ4 N,N′,O,O′)manganese(III) monohydrate, C19H18Cl2CuN2O2
- Crystal structure of 2,6-di-tert-butyl-4-(4-methoxybenzylidene)cyclohexa-2,5-dien-1-one, C22H28O2
- Crystal structure of [6,6′-(((2,2-dimethylpropane-1,3-diyl)bis(azanylylidene))bis(methanylylidene))bis(2-chlorophenolato)-κ4N,N′,O,O′]copper(II)
- Crystal structure of 2-chloro-3-((thiophen-2-ylmethyl)amino)naphthalene-1,4-dione, C30H20O4N2Cl2S2
- Crystal structure of bis{hydridotris(3-trifluoromethyl-5-methylpyrazolyl-1-yl)borato-κN 3}manganese(II), C30H26B2F18MnN12
- Crystal structure of 1-(2-methylphenyl)-2-(2-methylbenzo[b]thienyl)-3,3,4,4,5,5-hexafluorocyclopent-ene, C21H14F6S
- Crystal structure of 2-(3-((carbamimidoylthio)methyl)benzyl)isothiouronium hexafluorophosphate monohydrate, C10H17F6N4OPS2
- Crystal structure of 4,5-diiodo-1,3-dimesityl-1H-1,2,3-triazol-3-ium chloride – chloroform (1/1), C21H23Cl4I2N3
- Crystal structure of azido-k1 N-{6,6′-((((methylazanediyl)bis(propane-3,1-diyl))bis(azanylylidene))bis(methanylylidene))bis(2,4-dibromophenolato)k5 N,N′,N″,O,O′}cobalt(III)-methanol (1/1)), C21H23Br4CoN6O3
- The crystal structure of 2-(4-((carbamimidoylthio)methyl)benzyl)isothiouronium hexafluorophosphate monohydrate, C10H17F6N4OPS2
- Crystal structure of 1,1′-(methane-1,1-diyl)bis(3-methyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C9H14F12N4P2
- Crystal structure of (4′E)-6′-(diethylamino)-2-[(E)-[(pyren-1-yl)methylidene]amino]-4′-{2-[(2E)-1,3,3-trimethyl-2,3-dihydro-1H-indol-2-ylidene]ethylidene}-1′,2,2′,3,3′,4′-hexahydrospiro[isoindole-1,9′-xanthene]-3-one, C54H48N4O2
- Crystal structure of poly[bis(μ2-2,6-bis(1-imidazoly)pyridine-κ2 N,N′)-bis(thiocyanato-κ1 N)copper(II)] dithiocyanate, C24H18CuN12S2
- Cones with a three-fold symmetry constructed from three hydrogen bonded theophyllinium cations that coat [FeCl4]− anions in the crystal structure of tris(theophyllinium) bis(tetrachloridoferrate(III)) chloride trihydrate, C21H33Cl9Fe2N12O9
- Crystal structure of 14-O-[(4-(4-hydroxypiperidine-1-yl)-6-methylpyrimidine-2-yl)thioacetyl]-mutilin monohydrate, C32H49N3O6S
- The crystal structure of (E)-3-chloro-2-(2-(4-methylbenzylidene)hydrazinyl)pyridine, C13H12ClN3
- The crystal structure of 4-phenyl-4-[2-(pyridine-4-carbonyl)hydrazinylidene]butanoic acid, C16H15N3O3
- The crystal structure of 6-amino-5-carboxypyridin-1-ium pentaiodide monohydrate C6H9I5N2O3
- Crystal structure of bis(μ3-oxido)-bis(μ2-2-formylbenzoato-k2O:O′)-bis(2-(dimethoxymethyl)-benzoato-κO)-oktakismethyl-tetratin(IV)
- Crystal structure of 2-((E)-(((E)-2-hydroxy-4-methylbenzylidene) hydrazineylidene)methyl)-4-methylphenol, C16H16N2O2
- Crystal structure of (E)-amino(2-((5-methylfuran-2-yl)methylene)hydrazinyl) methaniminium nitrate monohydrate, C14H26N10O10
- The crystal structure of N′-(2-chloro-6-hydroxybenzylidene)thiophene-2-carbohydrazide monohydrate, C12H11ClN2O3S
- Crystal structure of catena-poly[(μ2-1,1′-(biphenyl-4,4′-diyl)bis(1H-imidazol)-κ2N:N′)-bis(4-bromobenzoate-κ1O)zinc(II)], C64H44Br4N8O8Zn2
- The crystal structure of catena-poly[(1-(4-carboxybenzyl)pyridin-1-ium-4-carboxylato-κ1O)-(μ2-oxalato-κ4 O:O′:O″:O‴)dioxidouranium(VI)], C16H11NO10U
- Crystal structure of 3-allyl-4-(2-bromoethyl)-5-(4-methoxyphenyl)-2-phenylfuran, C22H21BrO2
- Halogen bonds in the crystal structure of 4,3′:5′,4″-terpyridine — 1,3-diiodotetrafluorobenzene (1/1), C21H11F4I2N3
- Crystal structure of 2-(1H-indol-3-yl)ethan-1-aminium 2-(4-acetylphenoxy)acetate, C20H22N2O4
- Chalcogen bonds in the crystal structure of 4,7-dibromo-2,1,3-benzoselenadiazole, C6H2Br2N2Se
- The crystal structure of 1,4-bis((1H-benzimidazol-2-yl)methyl)-piperazine-2,5-dione dihydrate, C20H22N6O4
- The crystal structure of C19H20O8
- The crystal structure of KNa3Te8O18·5H2O exhibiting a ∞2[Te4O9]2− layer
- Erratum
- Erratum to: Crystal structure of (Z)-3-(6-bromo-1H-indol-3-yl)-1,3-diphenylprop-2-en-1-one, C23H16BrNO
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of (E)-7-hydroxy-2-((6-methoxypyridin-3-yl)methylene)-3, 4-dihydronaphthalen-1(2H)-one, C17H15NO3
- Crystal structure of (E)-7-methoxy-2-((2-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1 (2H)-one, C18H17NO3
- The crystal structure of N 6,N 6′-di(pyridin-2-yl)-[2,2′-bipyridine]-6,6′-diamine, C20H16N6
- The crystal structure of {N 1,N 2-bis[2,4-dimethyl-6-(4-(tert-butyl)phenyl)(phenyl)methyl]acenaphthylene-1,2-diimino-κ2 N, N′}-dibromido-nickel(II) – dichloromethane(1/2), C64H64Br2Cl4N2Ni
- Synthesis and crystal structure of nonacarbonyltris[(2-thia-1,3,5-triaza-7-phosphatricylco[3.3.1.1]decane-κ1 P)-2,2-dioxide]triruthenium(0) – acetonitrile (7/6), C25.71H32.57N9.86O15P3S3Ru3
- A new polymorph of 1-(4-nitrophenyl)-1H-benzimidazole (C13H9N3O2)
- The crystal structure of 2,2′-((1E,1′E)-(naphthalene-2,3 diylbis(azanylylidene)) bis(methanylylidene))bis(4-methylphenol), C26H22N2O2
- The crystal structure of bis(μ2-iodido)-bis(η6-benzene)-bis(iodido)-diosmium(II), C12H12I4Os2
- Redetermination of the crystal structure of bis{hydridotris(3,5-dimethylpyrazol-1-yl-κN 3)borato}copper(II), C30H44B2CuN12
- Crystal structure of (E)-3-((4-(tert-butyl)phenyl)thio)-4-hydroxypent-3-en-2-one, C15H20O2S
- Crystal structure of 2,2′-(p-tolylazanediyl)bis(1-phenylethan-1-one), C23H21NO2
- Redetermination of the crystal structure of the crystal sponge the poly[tetrakis(μ3-2,4,6-tris(pyridin-4-yl)-1,3,5-triazine)-dodecaiodidohexazinc(II) nitrobenzene solvate], C72H48I12N24Zn6⋅10(C6H5NO2)
- Crystal structure of (4′E)-6′-(diethylamino)-2-[(E)-[(6-methylpyridin-2-yl)methylidene]amino]-4′-{2-[(2E)-1,3,3-trimethyl-2,3-dihydro-1H-indol-2-ylidene]ethylidene}-1′,2,2′,3,3′,4′-hexahydrospiro[isoindole-1,9′-xanthene]-3-one, C44H45N5O2
- Crystal structure of (E)-7-fluoro-2-(3-fluorobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C17H12F2O1
- Crystal structure of tetrabutylammonium sulfanilate – 1-(diaminomethylene)thiourea (1/2)
- Crystal structure of [2,2′-{azanediyl)bis[(propane-3,1-diyl)(azanylylidene)methylylidene]} bis(3,5-dichlorophenolato)-κ2O,O′]-isothiocyanato-κN-iron(III), C21H19Cl4FeN4O2S
- Crystal structure of (4-chlorophenyl)(4-hydroxyphenyl)methanone, C13H9ClO2
- Crystal structure of 6,6′-((pentane-1,3-diylbis(azaneylylidene))bis(methaneylylidene))bis(2,4-dibromolphenolato-κ4 N,N′,O,O′)copper(II),) C19H16Br4CuN2O2
- Chlorido-(2,2′-(ethane-bis(5-methoxyphenolato))-κ4 N,N′,O,O′)manganese(III) monohydrate, C19H18Cl2CuN2O2
- Crystal structure of 2,6-di-tert-butyl-4-(4-methoxybenzylidene)cyclohexa-2,5-dien-1-one, C22H28O2
- Crystal structure of [6,6′-(((2,2-dimethylpropane-1,3-diyl)bis(azanylylidene))bis(methanylylidene))bis(2-chlorophenolato)-κ4N,N′,O,O′]copper(II)
- Crystal structure of 2-chloro-3-((thiophen-2-ylmethyl)amino)naphthalene-1,4-dione, C30H20O4N2Cl2S2
- Crystal structure of bis{hydridotris(3-trifluoromethyl-5-methylpyrazolyl-1-yl)borato-κN 3}manganese(II), C30H26B2F18MnN12
- Crystal structure of 1-(2-methylphenyl)-2-(2-methylbenzo[b]thienyl)-3,3,4,4,5,5-hexafluorocyclopent-ene, C21H14F6S
- Crystal structure of 2-(3-((carbamimidoylthio)methyl)benzyl)isothiouronium hexafluorophosphate monohydrate, C10H17F6N4OPS2
- Crystal structure of 4,5-diiodo-1,3-dimesityl-1H-1,2,3-triazol-3-ium chloride – chloroform (1/1), C21H23Cl4I2N3
- Crystal structure of azido-k1 N-{6,6′-((((methylazanediyl)bis(propane-3,1-diyl))bis(azanylylidene))bis(methanylylidene))bis(2,4-dibromophenolato)k5 N,N′,N″,O,O′}cobalt(III)-methanol (1/1)), C21H23Br4CoN6O3
- The crystal structure of 2-(4-((carbamimidoylthio)methyl)benzyl)isothiouronium hexafluorophosphate monohydrate, C10H17F6N4OPS2
- Crystal structure of 1,1′-(methane-1,1-diyl)bis(3-methyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C9H14F12N4P2
- Crystal structure of (4′E)-6′-(diethylamino)-2-[(E)-[(pyren-1-yl)methylidene]amino]-4′-{2-[(2E)-1,3,3-trimethyl-2,3-dihydro-1H-indol-2-ylidene]ethylidene}-1′,2,2′,3,3′,4′-hexahydrospiro[isoindole-1,9′-xanthene]-3-one, C54H48N4O2
- Crystal structure of poly[bis(μ2-2,6-bis(1-imidazoly)pyridine-κ2 N,N′)-bis(thiocyanato-κ1 N)copper(II)] dithiocyanate, C24H18CuN12S2
- Cones with a three-fold symmetry constructed from three hydrogen bonded theophyllinium cations that coat [FeCl4]− anions in the crystal structure of tris(theophyllinium) bis(tetrachloridoferrate(III)) chloride trihydrate, C21H33Cl9Fe2N12O9
- Crystal structure of 14-O-[(4-(4-hydroxypiperidine-1-yl)-6-methylpyrimidine-2-yl)thioacetyl]-mutilin monohydrate, C32H49N3O6S
- The crystal structure of (E)-3-chloro-2-(2-(4-methylbenzylidene)hydrazinyl)pyridine, C13H12ClN3
- The crystal structure of 4-phenyl-4-[2-(pyridine-4-carbonyl)hydrazinylidene]butanoic acid, C16H15N3O3
- The crystal structure of 6-amino-5-carboxypyridin-1-ium pentaiodide monohydrate C6H9I5N2O3
- Crystal structure of bis(μ3-oxido)-bis(μ2-2-formylbenzoato-k2O:O′)-bis(2-(dimethoxymethyl)-benzoato-κO)-oktakismethyl-tetratin(IV)
- Crystal structure of 2-((E)-(((E)-2-hydroxy-4-methylbenzylidene) hydrazineylidene)methyl)-4-methylphenol, C16H16N2O2
- Crystal structure of (E)-amino(2-((5-methylfuran-2-yl)methylene)hydrazinyl) methaniminium nitrate monohydrate, C14H26N10O10
- The crystal structure of N′-(2-chloro-6-hydroxybenzylidene)thiophene-2-carbohydrazide monohydrate, C12H11ClN2O3S
- Crystal structure of catena-poly[(μ2-1,1′-(biphenyl-4,4′-diyl)bis(1H-imidazol)-κ2N:N′)-bis(4-bromobenzoate-κ1O)zinc(II)], C64H44Br4N8O8Zn2
- The crystal structure of catena-poly[(1-(4-carboxybenzyl)pyridin-1-ium-4-carboxylato-κ1O)-(μ2-oxalato-κ4 O:O′:O″:O‴)dioxidouranium(VI)], C16H11NO10U
- Crystal structure of 3-allyl-4-(2-bromoethyl)-5-(4-methoxyphenyl)-2-phenylfuran, C22H21BrO2
- Halogen bonds in the crystal structure of 4,3′:5′,4″-terpyridine — 1,3-diiodotetrafluorobenzene (1/1), C21H11F4I2N3
- Crystal structure of 2-(1H-indol-3-yl)ethan-1-aminium 2-(4-acetylphenoxy)acetate, C20H22N2O4
- Chalcogen bonds in the crystal structure of 4,7-dibromo-2,1,3-benzoselenadiazole, C6H2Br2N2Se
- The crystal structure of 1,4-bis((1H-benzimidazol-2-yl)methyl)-piperazine-2,5-dione dihydrate, C20H22N6O4
- The crystal structure of C19H20O8
- The crystal structure of KNa3Te8O18·5H2O exhibiting a ∞2[Te4O9]2− layer
- Erratum
- Erratum to: Crystal structure of (Z)-3-(6-bromo-1H-indol-3-yl)-1,3-diphenylprop-2-en-1-one, C23H16BrNO

