Abstract
C18H21N3O2, triclinic, P1̅ (no. 2), a = 8.5155(4) Å, b = 10.6415(4) Å, c = 19.0732(10) Å, α = 80.918(4)°, β = 89.689(4)°, γ = 80.666(4)°, V = 1683.74(14) Å3, Z = 4, Rgt(F) = 0.055, wRref(F2) = 0.133, T = 100 K.
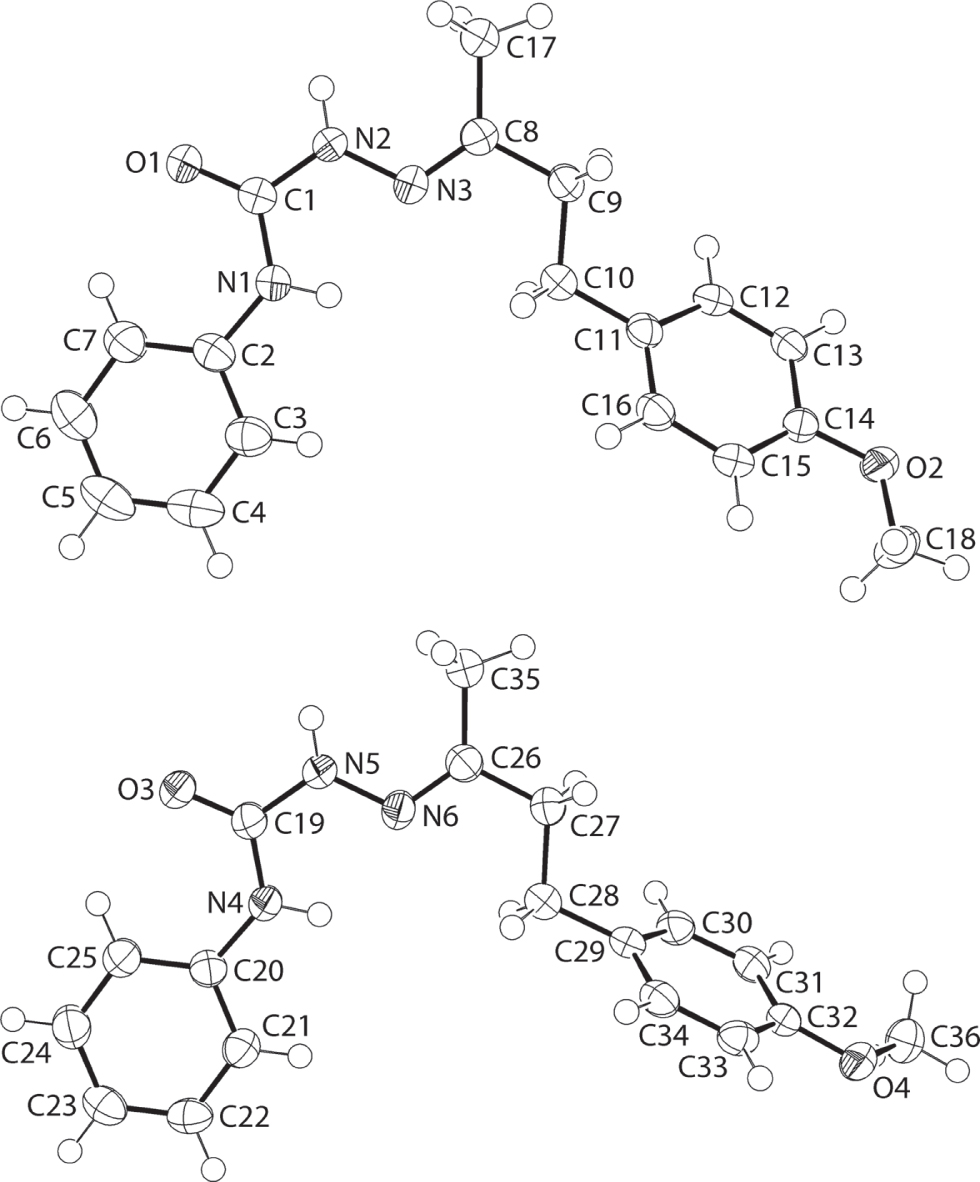
The asymmetric unit of the title crystal structure is shown in the figure. Tables 1 and 2 contain details of the measurement method and a list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless prism |
| Size: | 0.44 × 0.29 × 0.10 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.8 cm−1 |
| Diffractometer, scan mode: | SuperNova Dual, ω scans |
| 2θmax, completeness: | 55°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 15637, 7710, 0.040 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 4964 |
| N(param)refined: | 431 |
| Programs: | Agilent [1], SHELX [2, 3], ORTEP [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| O1 | 0.62938(16) | 0.17977(12) | 0.45757(7) | 0.0325(4) |
| O2 | 0.20571(15) | 0.95078(12) | −0.03809(6) | 0.0274(3) |
| N1 | 0.65711(19) | 0.26364(15) | 0.34056(8) | 0.0265(4) |
| H1N | 0.621(2) | 0.3328(14) | 0.3101(9) | 0.032* |
| N2 | 0.4832(2) | 0.37184(15) | 0.41117(8) | 0.0278(4) |
| H2N | 0.437(2) | 0.3733(18) | 0.4526(7) | 0.033* |
| N3 | 0.44148(18) | 0.46161(14) | 0.35062(8) | 0.0240(4) |
| C1 | 0.5933(2) | 0.26540(17) | 0.40598(10) | 0.0252(4) |
| C2 | 0.7657(2) | 0.16579(17) | 0.31674(10) | 0.0250(4) |
| C3 | 0.8087(2) | 0.18784(19) | 0.24596(10) | 0.0288(5) |
| H3 | 0.7658 | 0.2666 | 0.2167 | 0.035* |
| C4 | 0.9134(2) | 0.0962(2) | 0.21768(12) | 0.0353(5) |
| H4 | 0.9415 | 0.1122 | 0.1692 | 0.042* |
| C5 | 0.9768(3) | −0.0181(2) | 0.25965(12) | 0.0380(5) |
| H5 | 1.0492 | −0.0810 | 0.2405 | 0.046* |
| C6 | 0.9343(3) | −0.04031(19) | 0.32977(12) | 0.0378(5) |
| H6 | 0.9779 | −0.1194 | 0.3586 | 0.045* |
| C7 | 0.8291(2) | 0.05033(18) | 0.35935(11) | 0.0315(5) |
| H7 | 0.8010 | 0.0336 | 0.4078 | 0.038* |
| C8 | 0.3416(2) | 0.56302(17) | 0.35577(10) | 0.0239(4) |
| C9 | 0.2972(2) | 0.65680(17) | 0.28843(9) | 0.0239(4) |
| H9A | 0.3307 | 0.7399 | 0.2933 | 0.029* |
| H9B | 0.1799 | 0.6727 | 0.2823 | 0.029* |
| C10 | 0.3715(2) | 0.61082(17) | 0.22189(9) | 0.0238(4) |
| H10A | 0.4886 | 0.5933 | 0.2288 | 0.029* |
| H10B | 0.3366 | 0.5282 | 0.2170 | 0.029* |
| C11 | 0.3316(2) | 0.70338(16) | 0.15329(9) | 0.0211(4) |
| C12 | 0.1768(2) | 0.76840(16) | 0.13697(9) | 0.0213(4) |
| H12 | 0.0956 | 0.7563 | 0.1705 | 0.026* |
| C13 | 0.1385(2) | 0.84978(16) | 0.07345(9) | 0.0215(4) |
| H13 | 0.0326 | 0.8937 | 0.0641 | 0.026* |
| C14 | 0.2555(2) | 0.86751(16) | 0.02301(9) | 0.0211(4) |
| C15 | 0.4095(2) | 0.80246(16) | 0.03690(10) | 0.0231(4) |
| H15 | 0.4896 | 0.8123 | 0.0026 | 0.028* |
| C16 | 0.4454(2) | 0.72220(17) | 0.10202(10) | 0.0235(4) |
| H16 | 0.5516 | 0.6789 | 0.1116 | 0.028* |
| C17 | 0.2642(2) | 0.59499(18) | 0.42328(10) | 0.0302(5) |
| H17A | 0.1897 | 0.5354 | 0.4386 | 0.045* |
| H17B | 0.2064 | 0.6836 | 0.4149 | 0.045* |
| H17C | 0.3461 | 0.5868 | 0.4604 | 0.045* |
| C18 | 0.3235(2) | 0.9762(2) | −0.08930(10) | 0.0324(5) |
| H18A | 0.4076 | 1.0111 | −0.0676 | 0.049* |
| H18B | 0.2746 | 1.0390 | −0.1295 | 0.049* |
| H18C | 0.3694 | 0.8959 | −0.1060 | 0.049* |
| O3 | 0.33971(16) | 0.34631(12) | 0.54839(7) | 0.0327(4) |
| O4 | 0.82627(15) | −0.44318(12) | 1.03135(7) | 0.0284(3) |
| N4 | 0.32024(18) | 0.26414(14) | 0.66636(8) | 0.0252(4) |
| H4N | 0.361(2) | 0.1956(14) | 0.6972(9) | 0.030* |
| N5 | 0.48823(19) | 0.15496(15) | 0.59335(8) | 0.0268(4) |
| H5N | 0.537(2) | 0.1558(18) | 0.5519(7) | 0.032* |
| N6 | 0.53493(18) | 0.06471(14) | 0.65343(8) | 0.0245(4) |
| C19 | 0.3792(2) | 0.26156(17) | 0.59999(10) | 0.0251(4) |
| C20 | 0.2120(2) | 0.36143(17) | 0.69096(10) | 0.0231(4) |
| C21 | 0.1890(2) | 0.34831(18) | 0.76388(10) | 0.0259(4) |
| H21 | 0.2450 | 0.2756 | 0.7942 | 0.031* |
| C22 | 0.0855(2) | 0.44006(18) | 0.79290(10) | 0.0283(4) |
| H22 | 0.0713 | 0.4302 | 0.8428 | 0.034* |
| C23 | 0.0029(2) | 0.54582(18) | 0.74927(11) | 0.0295(5) |
| H23 | −0.0687 | 0.6087 | 0.7688 | 0.035* |
| C24 | 0.0255(2) | 0.55916(19) | 0.67699(11) | 0.0323(5) |
| H24 | −0.0312 | 0.6319 | 0.6470 | 0.039* |
| C25 | 0.1297(2) | 0.46828(18) | 0.64711(10) | 0.0289(5) |
| H25 | 0.1443 | 0.4792 | 0.5972 | 0.035* |
| C26 | 0.6373(2) | −0.03487(17) | 0.64658(10) | 0.0243(4) |
| C27 | 0.6851(2) | −0.13092(17) | 0.71260(9) | 0.0246(4) |
| H27A | 0.8021 | −0.1428 | 0.7188 | 0.030* |
| H27B | 0.6570 | −0.2149 | 0.7057 | 0.030* |
| C28 | 0.6084(2) | −0.09378(17) | 0.78052(10) | 0.0266(4) |
| H28A | 0.6317 | −0.0079 | 0.7865 | 0.032* |
| H28B | 0.4915 | −0.0868 | 0.7757 | 0.032* |
| C29 | 0.6656(2) | −0.18903(17) | 0.84620(9) | 0.0224(4) |
| C30 | 0.6270(2) | −0.31287(17) | 0.85763(10) | 0.0252(4) |
| H30 | 0.5632 | −0.3376 | 0.8232 | 0.030* |
| C31 | 0.6792(2) | −0.40153(17) | 0.91802(10) | 0.0249(4) |
| H31 | 0.6523 | −0.4858 | 0.9242 | 0.030* |
| C32 | 0.7710(2) | −0.36547(17) | 0.96925(10) | 0.0219(4) |
| C33 | 0.8103(2) | −0.24187(17) | 0.95914(10) | 0.0241(4) |
| H33 | 0.8729 | −0.2165 | 0.9938 | 0.029* |
| C34 | 0.7577(2) | −0.15610(17) | 0.89839(10) | 0.0250(4) |
| H34 | 0.7853 | −0.0721 | 0.8921 | 0.030* |
| C35 | 0.7140(3) | −0.06337(19) | 0.57839(10) | 0.0321(5) |
| H35A | 0.6314 | −0.0586 | 0.5421 | 0.048* |
| H35B | 0.7772 | −0.1501 | 0.5863 | 0.048* |
| H35C | 0.7835 | 0.0000 | 0.5624 | 0.048* |
| C36 | 0.7948(3) | −0.57233(18) | 1.04164(11) | 0.0332(5) |
| H36A | 0.6798 | −0.5715 | 1.0446 | 0.050* |
| H36B | 0.8472 | −0.6199 | 1.0858 | 0.050* |
| H36C | 0.8360 | −0.6144 | 1.0016 | 0.050* |
Source of materials
To a solution of 4-phenylsemicarbazide (0.151 g, 1 mmol) in heated absolute ethanol (20 mL) was added slowly a heated ethanol solution (20 mL) of 4-methoxy-2-butanone (0.102 g, 1 mmol) while stirring for 20 min. The white precipitate was filtered, washed with cold ethanol and dried in vacuo. Single crystals were grown at room temperature from slow evaporation of a mixture of ethanol and acetonitrile (1:1 v/v). IR (cm−1) 3338 (N—H), 1665 (C=O), 1597 (C=N), 1239 (C—N), 1025 (C=S). MS: m/z 311.25 [M]+.
Experimental details
The C-bound H atoms were geometrically placed (C—H = 0.95−0.99 Å) and refined as riding with Uiso(H) = 1.2–1.5 Ueq(C). The N-bound H-atoms were located in a difference Fourier map but were refined with a distance restraint of N—H = 0.88 ± 0.01 Å, and with Uiso(H) set to 1.2 Ueq(N).
Comment
It is well known that the condensation of semicarbazides with aldehydes/ketones gives rise to a class of potential Schiff base ligands. These molecules attract interest in terms of potential biological activity, most notably in the context of their anti-convulsant properties with the 4-(4-fluorophenoxy)benzaldehyde semicarbazone being the subject of considerable investigations in this regard [5]. In connection with on-going studies of the biological activity of transition metal thiosemicarbazone complexes [6], recently the synthesis and characterization of a new Schiff base ligand, derived from the reaction of aryl semicarbazide and vanillylacetone, was described [7]. In continuation of these studies, the title Schiff base molecule was characterized.
Two independent molecules comprise the asymmetric unit of the title compound. As seen from the Figure (70% displacement ellipsoids), the molecules present many similarities but, with the obvious difference related to the relative disposition of the methoxyphenyl residues. The molecule comprises a di-substituted urea residue. At one end, there is a phenyl ring while at the other, an imine (Z-configuration) group connects the urea residue to the 4-methoxyphenyl ring via an ethane link. The four atoms of the urea core are strictly planar with a r.m.s. deviation of 0.0004 Å for the fitted atoms [0.0014 Å for the O3-molecule]. The amine-N—H and imine-N atoms are syn, a disposition that enables the formation of intramolecular amine-N–H⋯N(imine) hydrogen bonds [N1—H1n⋯N3: 2.111(16) Å and 113.8(14)°; N4—H4n⋯N6: 2.125(16) Å and 113.7(13)°]. The dihedral angle between the CN2O plane and the adjacent phenyl ring is 3.85(13)° [7.54(12)°], consistent with a co-planar relationship. The dihedral angles between the outer phenyl rings are 37.56(8) and 66.56(5)° for the O1- and O3-molecules, respectively.
The most prominent feature of the molecular packing is the formation of an eight-membered {⋯HNCO}2 amide synthon formed between the two independent molecules comprising the asymmetric unit [N2—H2n⋯O3: 2.001(14) Å and 171.0(18)°; N5—H5n⋯O1: 1.956(14) Å and 172.8(17)°].
There is a sole literature precedent for molecules of this type as discussed recently [7]. The structure of this molecule, derived from the reaction of semicarbazide and vanillylacetone, presents very similar features to that described above.
Acknowledgements
We thank the staff of the University of Malaya’s X-ray diffraction laboratory for the data collection. The Universiti Putra Malaysia, under the research University Grant Scheme (RUGS Nos. 9199834 and 9174000), and the Malaysian Ministry of Science, Technology and Innovation (Grant No. 09-02-04-0752-EA001) are thanked for support.
References
Agilent: Agilent Technologies, Yarnton, England (2011).Search in Google Scholar
Sheldrick, G. M.: A short history of SHELX. Acta Crystallogr. A71 (2015) 3–8.10.1107/S2053273314026370Search in Google Scholar PubMed PubMed Central
Sheldrick, G. M.: Crystal structure refinement with SHELXL. Acta Crystallogr. C71 (2015) 3–8.10.1107/S2053229614024218Search in Google Scholar PubMed PubMed Central
Farrugia, L. J.: WinGX and ORTEP for Windows: an update. J. Appl. Cryst. 45 (2012) 849–854.10.1107/S0021889812029111Search in Google Scholar
Pandeya, S. N.: Semicarbazone – a versatile therapeutic pharmacophore for fragment based anticonvulsant drug design. Acta Pharm. 62 (2012) 263–286.10.2478/v10007-012-0030-1Search in Google Scholar PubMed
Yusof, E. N. M.; Ravoof, T. B. S. A.; Tiekink, E. R. T.; Veerakumarasivam, A.; Crouse, K. A.; Tahir, M. I. M.; Ahmad, H.: Synthesis, characterization and biological evaluation of transition metal complexes derived from N, S bidentate ligands. Int. J. Mol. Sci. 16 (2015) 11034–11054.10.3390/ijms160511034Search in Google Scholar PubMed PubMed Central
Tan, M. Y.; Crouse, K. A.; Ravoof, T. B. S. A.; Jotani, M. M.; Tiekink, E. R. T.: 3-{(E)-[4-(4-Hydroxy-3-methoxyphenyl)butan-2-ylidene]amino}-1-phenylurea: crystal structure and Hirshfeld surface analysis. Acta Crystallogr. E74 (2018) 21–27.10.1107/S2056989017017273Search in Google Scholar PubMed PubMed Central
©2018 Ming Yueh Tan et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 License.
Articles in the same Issue
- Cover and Frontmatter
- Bis(tetraethylammonium) carbonate – boric acid – water (1/2/5), C17H56B2N2O14
- Crystal structure of 4-methoxy-6-phenyl-2H-pyran-2-one, C12H10O3
- Crystal structure of tris{(3-((E)-(((E)-2-oxidobenzylidene)hydrazono)methyl)-2-oxo-2H-chromen-4-olato-κ3O,N:N′)}dicobalt(III)tris(dimethylformamide), C60H50Co2N9O15
- Crystal structure of poly[bis(1-methyl-[4,4′-bipyridin]-1-ium-κN)-tetrakis(μ3-sulfato-κ3O:O′:O′′)trizinc(II)], C22H22Zn3N4O16S4
- Crystal structure of (5Z,10Z)-3,13-dichloro-17,18-dioxo-5,11-diphenyl-8,9,17,18-tetrahydro-7H-dibenzo[e,n][1,4,8,12]tetraazacyclopentadecine-16,19-diido-κ4N,N′,N′′,N′′′)copper(II), C31H22N4O2Cl2Cu
- Crystal structure of bis{5-methoxy-2-(((2-oxo-2H-chromen-6-yl)imino)methyl)phenolato-κ2N,O}zinc(II), C34H24N2O8Zn
- Crystal structure of 2,2′-((((ethane-1,2-diylbis(oxy))bis(2,1-phenylene))bis(azanylylidene))bis(methanylylidene))diphenolato-κ2N;κ4O)nickel(II), C28H22N2O4Ni
- Crystal structure of 1,1′-((1E,1′E)-(((ethane-1,2-diylbis(oxy))bis(2,1-phenylene))bis(azanylylidene))bis(methanylylidene))bis(naphthalen-2-olato)cobalt(II), C36H26N2O4Co
- Crystal structure of camptothecin, C20H16N2O4
- Crystal structure of (2-(chlorophenyl)-5-methyl-1,3-dioxane-5-carboxylato–κ2O,O′)(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)nickel(II) perchlorate monohydrate, Ni(C16H36N4)(C12H12O4Cl)ClO4⋅H2O
- Crystal structure of 3-(2-chloro-6-methoxyquinolin-3-yl)-5-phenylisoxazole (C19H13ClN2O2)
- Crystal structure of tetrakis(μ2-acetato-κ2O:O′)-bis{[(E)-2,6-diisopropyl-N-(pyridin-3-ylmethylene)aniline]copper (II)}, C44H56Cu2N4O8
- Crystal structure of diethyl 2-(2-chlorophenyl)-1,3-dioxane-5,5-dicarboxylate, C16H19Cl1O6
- Crystal structure of (μ2-2,2′-bipyridine-3,3′-dicarboxylato)-bis(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane)-di-nickel(II) perchlorate N,N′-dimethylformamide solvate, C50H92Cl2N12Ni2O14
- Crystal structure of catena-poly[triaqua(μ2-1,2-bis(4-pyridyl)ethane-κ2N:N′)-(1,2-bis(4-pyridyl)ethane-κN)nickel(II)] 2-aminonicotinate nitrate – 1,2-bis(4-pyridyl)ethane – water (2/1/8), C36H44N8NiO12
- Hydrothermal synthesis and crystal structure of poly[bis(μ2-3-(3,5-dicarboxyphenoxy)phthalato-κ3O,O′:O′′)-(μ2-1,2-di(pyridin-4-yl)ethane-κ2N:N′)copper(II)], C22H14CuNO9
- Crystal structure of catena-poly[aqua-(methanol-κO)-bis(μ2-4-(pyridin-4-yl)benzoato-κ2N:O)-bis(triphenylphospine-κP)disilver(I)], C61H52Ag2N2O6P2
- The crystal structure of 6-(4-bromobenzyl)-1,3,5-trimethyl-7-phenyl-1,5-dihydro-2H-pyrrolo[3,2-d]pyrimidine-2,4(3H)-dione, C22H20BrN3O2
- Synthesis and crystal structure of catena-poly[bis(2-(2-((2,6-dichlorophenyl)amino)phenyl)acetato-κO;κ2O′,O′′)-(μ2-1,4-di(pyridin-4-yl)benzene-κ2N:N′)zinc(II)], C44H32N4ZnCl4O4
- Crystal structure of diaqua-bis[N-phenyl-2-(quinolin-8-yloxy)acetamide-κ3-N,O,O′]-nitrato(κ2O,O′)-cerium(III) dinitrate - acetone (1/2), C40H44N7O17Ce
- Crystal structure of the 2D coordination polymer poly[aqua(μ2-2,2′-(1,2-phenylene)diacetato-κ3O,O′:O′)-(μ2-4,4′-bis((1H-1,2,4-triazol-1-yl)methyl)-1,1′-biphenyl-κ2N:N′)cobalt(II)], C28H26CoN6O5
- Crystal structure of (dimethylformamide-κO)(perchlorato-κ2O,O′){μ2-6,6′-((1,2-phenylenebis(azanylylidene))bis(methanylylidene))bis(4-bromo-2-methoxyphenolate)-κ8N,N′,O:O,O′:O′,O′′,O′′′}sodium(I)nickel(II), C25H23Br2ClN3NaNiO9
- The crystal structure of catena-poly[bis((4-aminophenyl)sulfonyl)(pyrimidin-2-yl)amido-κ2N,N′)-bis(μ2-4,4′-bipyridine-N,N′-κ2N:N′)zinc(II) – methanol (1/2), C32H34N10O6S2Zn
- Synthesis and crystal structure poly[aqua(μ3-2-(((7-hydroxy-3-(4-hydroxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)ammonio)acetate-κ4O,O′:O′′:O′′′) sodium] monohydrate, C18H18NNaO11S
- Crystal structure of methyl 4′-amino-3′,5′-diisopropyl-[1,1′-biphenyl]-4-carboxylate, C20H25NO2
- Crystal structure of (η6-1-isopropyl-4-methyl benzene)-(N-(2,5-dichlorophenyl)-1-(pyridin-2-yl)methanimine-κ2N,N′)ruthenium(II) perchlorate, C22H22Cl4N2O4Ru
- Crystal structure of 2-(2-(1-Chlorocyclopropyl)-3-(2-chlorophenyl)-2-hydroxypropyl)-1H-1,2,4-triazole-3(2H)-thione, C14H15Cl2N3OS
- Crystal structure of methyl 4′-amino-3′,5′-dimethyl-[1,1′-biphenyl]-4-carboxylate, C16H17NO2
- The crystal structure of 1-(5-ferrocenyl-3-(trifluoromethyl)-1H-pyrazol-1-yl)pentan-1-on, C19H19F3FeN2O
- The crystal structure of (3S,12R,20R,24S)-3,12-diacetyl-20,24-epoxy-dammarane-3,12,25-triol acetone solvate, C34H56O6
- Crystal structure of methyl 10-(pyridin-4-yl)-anthracene-9-carboxylate, C21H15NO2
- Crystal structure of catena-poly[diaqua-bis(di(N2,N6-dihydroxypyridine-2,6-dicarboxamide))potassium(I)]tetrahydrate, C14H25N6O14K
- Crystal structure of poly{[μ2-(E)-1,4-bis(1H-benzo[d]imidazol-1-yl)but-2-ene-κ2N:N′][μ3–cyclohexane-1,4-dicarboxylato-κ4O,O′:O′′:O′′′]cadmium(II)}, C26H26CdN4O4
- Crystal structure of poly[aqua(μ3-[2,2′-bipyridine]-3,3′-dicarboxylato-κ4N,N′:O:O′)zinc(II)] – dimethylformamide (1/1), C15H15N3O6Zn
- The crystal structure of poly[tetraaqua-tris(μ2-2,6-di(1H-imidazol-1-yl)naphthalene-κ2N:N′)-bis(thiophene-2,5-dicarboxylato-κ1O)]dicobalt(II), C30H24CoN6O6S
- Crystal structure of (S)-1-(5-(anthracen-9-yl)-3-phenyl-4,5-dihydro-1H-pyrazol-1-yl)propan-1-one, C26H22N2O
- Crystal structure of 5-methyl-3,3-diphenyl-1-tosyl-1,2,3,4-tetrahydropyridine, C25H25NO2S
- Synthesis and crystal structure of μ-[1,1′-di(mesitylphosphanido)ferrocene]bis[η5-cyclopentadienylnickel(II)] tetrahydrofurane solvate, C42H48FeNi2OP2
- Synthesis and crystal structure of (E)-1-(4-(((E)-5-chloro-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C16H15ClN2O2
- Crystal structure of bis(1-(phenylsulfonyl)-2-(1-(pyrazin-2-yl)ethylidene)hydrazin-1-ido-κ3N,N′,O)nickel(II), C24H22N8O4S2Ni
- Crystal Structure of bis(1-(phenylsulfonyl)-2-(1-(pyrazin-2-yl)ethylidene)hydrazin-1-ido-κ3N,N′,O)copper(II), C24H22N8O4S2Cu
- Synthesis and crystal structure of poly[aqua{μ3-(1S,2S)-1-((7-hydroxy-3-(4-hydroxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)pyrrolidin-1-ium-2-carboxylato-κ4O,O′:O′′:O′′′}sodium(I)] monohydrate, C21H22NNaO11S
- Halogen bonds in the crystal structure of 1,4-diiodotetrafluorobenzene–1,2-bis(4-pyridyl)propane (1/1), C19H14F4I2N2
- Crystal structure of bis(μ-N-i-propyl-N-n-propyldithiocarbamato-κ2S:S′) bis(N-i-propyl-N-n-propyldithiocarbamato-κ2S,S′)dizinc(II), C28H56N4S8Zn2
- Crystal structure of bis(μ-N-i-propyl-N-n-propyldithiocarbamato-κ3S,S′:S)bis(N-i-propyl-N-n-propyldithiocarbamato-κ2S,S′)dicadmium(II), C28H56Cd2N4S8
- Crystal structure of bis(μ2-di-n-butyldithiocarbamato-κ3S,S′:S;κ3S:S:S′)-hexacarbonyl-di-rhenium(I), C24H36N2O6Re2
- Crystal structure of 7-(4-methylphenyl)imidazo[1,2-a][1,3,5]triazin-4-amine, C12H11N5
- Crystal structure of the co-crystal O-isopropyl phenylcarbamothioate – 4,4′-bipyridine (2/1), C15H17N2OS
- Crystal structure of the coordination polymer catena-poly[chlorido-{μ2-2-(((3,5-dimethyl-1H-pyrazol-1-yl)methyl)amino)-3-hydroxybutanoato-κ4N,N,O:O′}copper(II)], C11H16ClCuN2O3
- Synthesis and crystal structure of bis(μ2-acetato-κ2O:O′)-di(ethanol)-bis{μ2-5-(N,N′-diethylamine)-5′-methoxyl-2,2′-[ethylenediyldioxybis(nitrilomethylidyne)]diphenolato-κ6O:O,N,N,O′:O′}trinickel(II) – ethanol – acetonitrile (1/2/2), C58H86Ni3N8O18
- Crystal structure of the bis((E)-O-ethyl-N-phenylthiocarbamate) – 4,4′-bipyridine co-crystal (2/1), C28H30N4O2S2
- Crystal structure of the (E)-O-methyl-N-phenyl-thiocarbamate – 4,4′-bipyridine (1/1), C18H17N3OS
- Crystal structure of bis(μ2-diethyldithiocarbamato-κ3S,S′:S′)-bis(tricyclohexylphosphane-κP)dicopper(I), C46H86Cu2N2P2S4
- Crystal structure of N-(3-chlorophenyl)ethoxycarbothioamide, C9H10ClNOS
- Crystal structure of bis(μ2-pyrrolidine-1-carbodithioato-κ3S,S′:S;κ3S:S:S′)-bis(tricyclohexylphosphane-P)-di-copper(I), C46H82Cu2N2P2S4
- Crystal structure of N-(2-chlorophenyl)methoxycarbothioamide, C8H8ClNOS
- Crystal structure of chlorido-methanol-(N-(2-(oxy)-3-methoxybenzylidene)pyridine-4-carbohydrazonato-κ3O,N,O′)-(4-methylphenyl)methyl-tin(IV), C23H24ClN3O4Sn
- Crystal structure of N-(3-chlorophenyl)(propan-2-yloxy)carbothioamide, C10H12ClNOS
- Crystal structure of 1-[(Z)-[4-(4-methoxyphenyl)butan-2-ylidene]amino]-3-phenylurea, C18H21N3O2
- A triclinic polymorph of bis(μ-N,N-bis(2-hydroxyethyl)dithiocarbamato-κ3S,S′:S′) bis(N,N-bis(2-hydroxyethyl)dithiocarbamato-κ2S:S′)zinc(II), C20H40N4O8S8Zn2
Articles in the same Issue
- Cover and Frontmatter
- Bis(tetraethylammonium) carbonate – boric acid – water (1/2/5), C17H56B2N2O14
- Crystal structure of 4-methoxy-6-phenyl-2H-pyran-2-one, C12H10O3
- Crystal structure of tris{(3-((E)-(((E)-2-oxidobenzylidene)hydrazono)methyl)-2-oxo-2H-chromen-4-olato-κ3O,N:N′)}dicobalt(III)tris(dimethylformamide), C60H50Co2N9O15
- Crystal structure of poly[bis(1-methyl-[4,4′-bipyridin]-1-ium-κN)-tetrakis(μ3-sulfato-κ3O:O′:O′′)trizinc(II)], C22H22Zn3N4O16S4
- Crystal structure of (5Z,10Z)-3,13-dichloro-17,18-dioxo-5,11-diphenyl-8,9,17,18-tetrahydro-7H-dibenzo[e,n][1,4,8,12]tetraazacyclopentadecine-16,19-diido-κ4N,N′,N′′,N′′′)copper(II), C31H22N4O2Cl2Cu
- Crystal structure of bis{5-methoxy-2-(((2-oxo-2H-chromen-6-yl)imino)methyl)phenolato-κ2N,O}zinc(II), C34H24N2O8Zn
- Crystal structure of 2,2′-((((ethane-1,2-diylbis(oxy))bis(2,1-phenylene))bis(azanylylidene))bis(methanylylidene))diphenolato-κ2N;κ4O)nickel(II), C28H22N2O4Ni
- Crystal structure of 1,1′-((1E,1′E)-(((ethane-1,2-diylbis(oxy))bis(2,1-phenylene))bis(azanylylidene))bis(methanylylidene))bis(naphthalen-2-olato)cobalt(II), C36H26N2O4Co
- Crystal structure of camptothecin, C20H16N2O4
- Crystal structure of (2-(chlorophenyl)-5-methyl-1,3-dioxane-5-carboxylato–κ2O,O′)(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)nickel(II) perchlorate monohydrate, Ni(C16H36N4)(C12H12O4Cl)ClO4⋅H2O
- Crystal structure of 3-(2-chloro-6-methoxyquinolin-3-yl)-5-phenylisoxazole (C19H13ClN2O2)
- Crystal structure of tetrakis(μ2-acetato-κ2O:O′)-bis{[(E)-2,6-diisopropyl-N-(pyridin-3-ylmethylene)aniline]copper (II)}, C44H56Cu2N4O8
- Crystal structure of diethyl 2-(2-chlorophenyl)-1,3-dioxane-5,5-dicarboxylate, C16H19Cl1O6
- Crystal structure of (μ2-2,2′-bipyridine-3,3′-dicarboxylato)-bis(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane)-di-nickel(II) perchlorate N,N′-dimethylformamide solvate, C50H92Cl2N12Ni2O14
- Crystal structure of catena-poly[triaqua(μ2-1,2-bis(4-pyridyl)ethane-κ2N:N′)-(1,2-bis(4-pyridyl)ethane-κN)nickel(II)] 2-aminonicotinate nitrate – 1,2-bis(4-pyridyl)ethane – water (2/1/8), C36H44N8NiO12
- Hydrothermal synthesis and crystal structure of poly[bis(μ2-3-(3,5-dicarboxyphenoxy)phthalato-κ3O,O′:O′′)-(μ2-1,2-di(pyridin-4-yl)ethane-κ2N:N′)copper(II)], C22H14CuNO9
- Crystal structure of catena-poly[aqua-(methanol-κO)-bis(μ2-4-(pyridin-4-yl)benzoato-κ2N:O)-bis(triphenylphospine-κP)disilver(I)], C61H52Ag2N2O6P2
- The crystal structure of 6-(4-bromobenzyl)-1,3,5-trimethyl-7-phenyl-1,5-dihydro-2H-pyrrolo[3,2-d]pyrimidine-2,4(3H)-dione, C22H20BrN3O2
- Synthesis and crystal structure of catena-poly[bis(2-(2-((2,6-dichlorophenyl)amino)phenyl)acetato-κO;κ2O′,O′′)-(μ2-1,4-di(pyridin-4-yl)benzene-κ2N:N′)zinc(II)], C44H32N4ZnCl4O4
- Crystal structure of diaqua-bis[N-phenyl-2-(quinolin-8-yloxy)acetamide-κ3-N,O,O′]-nitrato(κ2O,O′)-cerium(III) dinitrate - acetone (1/2), C40H44N7O17Ce
- Crystal structure of the 2D coordination polymer poly[aqua(μ2-2,2′-(1,2-phenylene)diacetato-κ3O,O′:O′)-(μ2-4,4′-bis((1H-1,2,4-triazol-1-yl)methyl)-1,1′-biphenyl-κ2N:N′)cobalt(II)], C28H26CoN6O5
- Crystal structure of (dimethylformamide-κO)(perchlorato-κ2O,O′){μ2-6,6′-((1,2-phenylenebis(azanylylidene))bis(methanylylidene))bis(4-bromo-2-methoxyphenolate)-κ8N,N′,O:O,O′:O′,O′′,O′′′}sodium(I)nickel(II), C25H23Br2ClN3NaNiO9
- The crystal structure of catena-poly[bis((4-aminophenyl)sulfonyl)(pyrimidin-2-yl)amido-κ2N,N′)-bis(μ2-4,4′-bipyridine-N,N′-κ2N:N′)zinc(II) – methanol (1/2), C32H34N10O6S2Zn
- Synthesis and crystal structure poly[aqua(μ3-2-(((7-hydroxy-3-(4-hydroxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)ammonio)acetate-κ4O,O′:O′′:O′′′) sodium] monohydrate, C18H18NNaO11S
- Crystal structure of methyl 4′-amino-3′,5′-diisopropyl-[1,1′-biphenyl]-4-carboxylate, C20H25NO2
- Crystal structure of (η6-1-isopropyl-4-methyl benzene)-(N-(2,5-dichlorophenyl)-1-(pyridin-2-yl)methanimine-κ2N,N′)ruthenium(II) perchlorate, C22H22Cl4N2O4Ru
- Crystal structure of 2-(2-(1-Chlorocyclopropyl)-3-(2-chlorophenyl)-2-hydroxypropyl)-1H-1,2,4-triazole-3(2H)-thione, C14H15Cl2N3OS
- Crystal structure of methyl 4′-amino-3′,5′-dimethyl-[1,1′-biphenyl]-4-carboxylate, C16H17NO2
- The crystal structure of 1-(5-ferrocenyl-3-(trifluoromethyl)-1H-pyrazol-1-yl)pentan-1-on, C19H19F3FeN2O
- The crystal structure of (3S,12R,20R,24S)-3,12-diacetyl-20,24-epoxy-dammarane-3,12,25-triol acetone solvate, C34H56O6
- Crystal structure of methyl 10-(pyridin-4-yl)-anthracene-9-carboxylate, C21H15NO2
- Crystal structure of catena-poly[diaqua-bis(di(N2,N6-dihydroxypyridine-2,6-dicarboxamide))potassium(I)]tetrahydrate, C14H25N6O14K
- Crystal structure of poly{[μ2-(E)-1,4-bis(1H-benzo[d]imidazol-1-yl)but-2-ene-κ2N:N′][μ3–cyclohexane-1,4-dicarboxylato-κ4O,O′:O′′:O′′′]cadmium(II)}, C26H26CdN4O4
- Crystal structure of poly[aqua(μ3-[2,2′-bipyridine]-3,3′-dicarboxylato-κ4N,N′:O:O′)zinc(II)] – dimethylformamide (1/1), C15H15N3O6Zn
- The crystal structure of poly[tetraaqua-tris(μ2-2,6-di(1H-imidazol-1-yl)naphthalene-κ2N:N′)-bis(thiophene-2,5-dicarboxylato-κ1O)]dicobalt(II), C30H24CoN6O6S
- Crystal structure of (S)-1-(5-(anthracen-9-yl)-3-phenyl-4,5-dihydro-1H-pyrazol-1-yl)propan-1-one, C26H22N2O
- Crystal structure of 5-methyl-3,3-diphenyl-1-tosyl-1,2,3,4-tetrahydropyridine, C25H25NO2S
- Synthesis and crystal structure of μ-[1,1′-di(mesitylphosphanido)ferrocene]bis[η5-cyclopentadienylnickel(II)] tetrahydrofurane solvate, C42H48FeNi2OP2
- Synthesis and crystal structure of (E)-1-(4-(((E)-5-chloro-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C16H15ClN2O2
- Crystal structure of bis(1-(phenylsulfonyl)-2-(1-(pyrazin-2-yl)ethylidene)hydrazin-1-ido-κ3N,N′,O)nickel(II), C24H22N8O4S2Ni
- Crystal Structure of bis(1-(phenylsulfonyl)-2-(1-(pyrazin-2-yl)ethylidene)hydrazin-1-ido-κ3N,N′,O)copper(II), C24H22N8O4S2Cu
- Synthesis and crystal structure of poly[aqua{μ3-(1S,2S)-1-((7-hydroxy-3-(4-hydroxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)pyrrolidin-1-ium-2-carboxylato-κ4O,O′:O′′:O′′′}sodium(I)] monohydrate, C21H22NNaO11S
- Halogen bonds in the crystal structure of 1,4-diiodotetrafluorobenzene–1,2-bis(4-pyridyl)propane (1/1), C19H14F4I2N2
- Crystal structure of bis(μ-N-i-propyl-N-n-propyldithiocarbamato-κ2S:S′) bis(N-i-propyl-N-n-propyldithiocarbamato-κ2S,S′)dizinc(II), C28H56N4S8Zn2
- Crystal structure of bis(μ-N-i-propyl-N-n-propyldithiocarbamato-κ3S,S′:S)bis(N-i-propyl-N-n-propyldithiocarbamato-κ2S,S′)dicadmium(II), C28H56Cd2N4S8
- Crystal structure of bis(μ2-di-n-butyldithiocarbamato-κ3S,S′:S;κ3S:S:S′)-hexacarbonyl-di-rhenium(I), C24H36N2O6Re2
- Crystal structure of 7-(4-methylphenyl)imidazo[1,2-a][1,3,5]triazin-4-amine, C12H11N5
- Crystal structure of the co-crystal O-isopropyl phenylcarbamothioate – 4,4′-bipyridine (2/1), C15H17N2OS
- Crystal structure of the coordination polymer catena-poly[chlorido-{μ2-2-(((3,5-dimethyl-1H-pyrazol-1-yl)methyl)amino)-3-hydroxybutanoato-κ4N,N,O:O′}copper(II)], C11H16ClCuN2O3
- Synthesis and crystal structure of bis(μ2-acetato-κ2O:O′)-di(ethanol)-bis{μ2-5-(N,N′-diethylamine)-5′-methoxyl-2,2′-[ethylenediyldioxybis(nitrilomethylidyne)]diphenolato-κ6O:O,N,N,O′:O′}trinickel(II) – ethanol – acetonitrile (1/2/2), C58H86Ni3N8O18
- Crystal structure of the bis((E)-O-ethyl-N-phenylthiocarbamate) – 4,4′-bipyridine co-crystal (2/1), C28H30N4O2S2
- Crystal structure of the (E)-O-methyl-N-phenyl-thiocarbamate – 4,4′-bipyridine (1/1), C18H17N3OS
- Crystal structure of bis(μ2-diethyldithiocarbamato-κ3S,S′:S′)-bis(tricyclohexylphosphane-κP)dicopper(I), C46H86Cu2N2P2S4
- Crystal structure of N-(3-chlorophenyl)ethoxycarbothioamide, C9H10ClNOS
- Crystal structure of bis(μ2-pyrrolidine-1-carbodithioato-κ3S,S′:S;κ3S:S:S′)-bis(tricyclohexylphosphane-P)-di-copper(I), C46H82Cu2N2P2S4
- Crystal structure of N-(2-chlorophenyl)methoxycarbothioamide, C8H8ClNOS
- Crystal structure of chlorido-methanol-(N-(2-(oxy)-3-methoxybenzylidene)pyridine-4-carbohydrazonato-κ3O,N,O′)-(4-methylphenyl)methyl-tin(IV), C23H24ClN3O4Sn
- Crystal structure of N-(3-chlorophenyl)(propan-2-yloxy)carbothioamide, C10H12ClNOS
- Crystal structure of 1-[(Z)-[4-(4-methoxyphenyl)butan-2-ylidene]amino]-3-phenylurea, C18H21N3O2
- A triclinic polymorph of bis(μ-N,N-bis(2-hydroxyethyl)dithiocarbamato-κ3S,S′:S′) bis(N,N-bis(2-hydroxyethyl)dithiocarbamato-κ2S:S′)zinc(II), C20H40N4O8S8Zn2

