Abstract
C13H14Cl1N3, orthorhombic, Pma2 (no. 28), a = 6.7623(2) Å, b = 21.1431(6) Å, c = 8.8708(2) Å, Z = 4, V = 1268.31(6) Å3, Rgt(F) = 0.0362, wRref(F2) = 0.0969, T = 200(2) K.
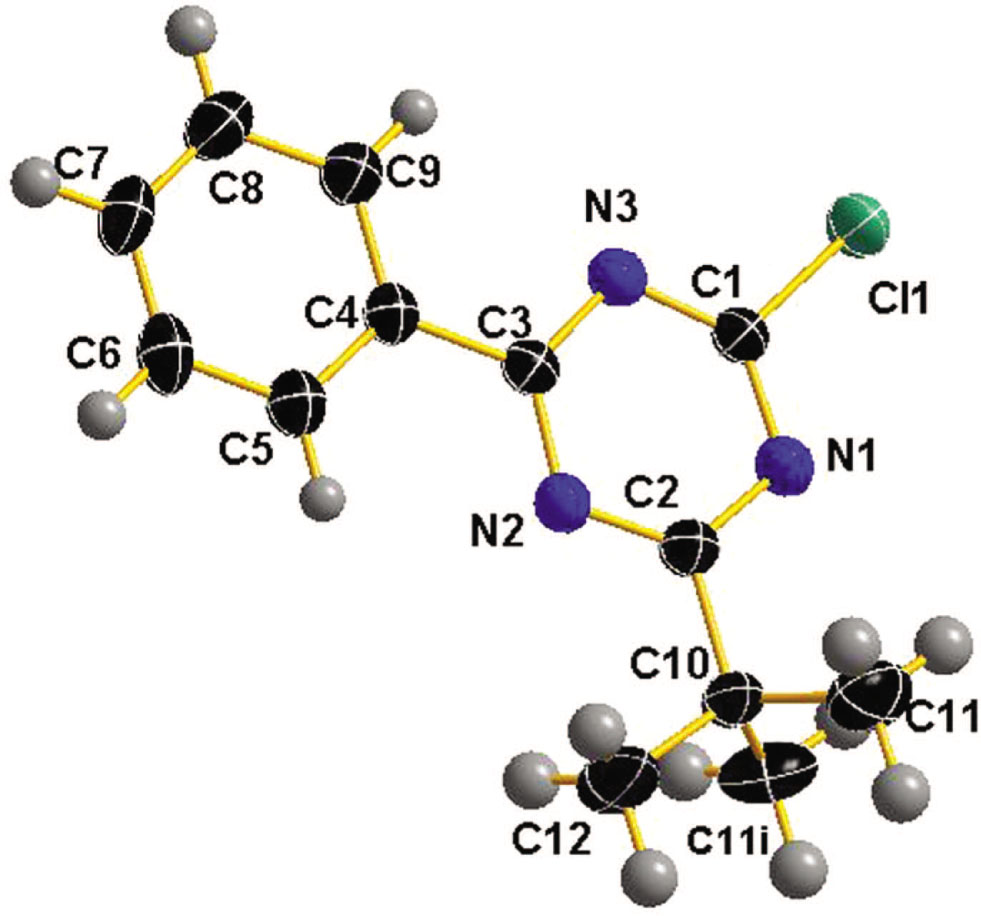
One of two crystallographically independent molecules of the asymmetric unit of the title structure is shown in the figure. Tables 1 and 2 contain details on crystal structure and measurement conditions and a list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colorless block |
| Size: | 0.15 × 0.12 × 0.10 mm |
| Wavelength: | Cu Kα radiation (1.54178Å) |
| μ: | 2.50 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω-scans |
| θmax, completeness: | 68.2°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 14980, 2537, 0.068 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 2324 |
| N(param)refined: | 215 |
| Programs: | Bruker programs [1], SHELX [2] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| Cl1 | −0.250000 | 0.71123(5) | 1.41697(10) | 0.0471(3) |
| N1 | −0.250000 | 0.66798(16) | 1.1436(4) | 0.0369(8) |
| N2 | −0.250000 | 0.55879(16) | 1.0871(4) | 0.0342(8) |
| N3 | −0.250000 | 0.59247(17) | 1.3425(4) | 0.0337(8) |
| C1 | −0.250000 | 0.65043(18) | 1.2865(5) | 0.0330(8) |
| C2 | −0.250000 | 0.61896(19) | 1.0459(5) | 0.0355(9) |
| C3 | −0.250000 | 0.54733(18) | 1.2360(4) | 0.0307(8) |
| C4 | −0.250000 | 0.48098(18) | 1.2860(5) | 0.0322(8) |
| C5 | −0.250000 | 0.4322(2) | 1.1796(5) | 0.0392(10) |
| H5 | −0.250000 | 0.441954 | 1.075116 | 0.047* |
| C6 | −0.250000 | 0.3698(2) | 1.2257(6) | 0.0463(11) |
| H6 | −0.250000 | 0.336897 | 1.152811 | 0.056* |
| C7 | −0.250000 | 0.3551(2) | 1.3770(6) | 0.0468(12) |
| H7 | −0.250000 | 0.312133 | 1.408149 | 0.056* |
| C8 | −0.250000 | 0.4029(2) | 1.4836(6) | 0.0490(12) |
| H8 | −0.250000 | 0.392480 | 1.587801 | 0.059* |
| C9 | −0.250000 | 0.4659(2) | 1.4393(5) | 0.0402(10) |
| H9 | −0.250000 | 0.498505 | 1.512914 | 0.048* |
| C10 | −0.250000 | 0.6356(2) | 0.8796(5) | 0.0410(10) |
| C11 | −0.4336(6) | 0.6765(2) | 0.8475(4) | 0.0662(10) |
| H11A | −0.429089 | 0.714704 | 0.909757 | 0.099* |
| H11B | −0.553170 | 0.652291 | 0.871417 | 0.099* |
| H11C | −0.435345 | 0.688360 | 0.740698 | 0.099* |
| C12 | −0.250000 | 0.5773(3) | 0.7819(6) | 0.084(2) |
| Cl2 | −0.250000 | 0.20354(5) | 1.14771(11) | 0.0488(4) |
| N4 | −0.250000 | 0.08493(16) | 1.0723(4) | 0.0332(8) |
| N5 | −0.250000 | 0.05119(16) | 0.8158(4) | 0.0330(7) |
| N6 | −0.250000 | 0.16102(16) | 0.8735(4) | 0.0354(7) |
| C13 | −0.250000 | 0.14331(19) | 1.0165(4) | 0.0330(8) |
| C14 | −0.250000 | 0.11263(18) | 0.7758(5) | 0.0329(8) |
| C15 | −0.250000 | 0.04038(18) | 0.9650(4) | 0.0307(8) |
| C16 | −0.250000 | −0.02651(18) | 1.0143(5) | 0.0313(8) |
| C17 | −0.250000 | −0.07472(19) | 0.9077(5) | 0.0372(9) |
| H17 | −0.250000 | −0.064532 | 0.803370 | 0.045* |
| C18 | −0.250000 | −0.1374(2) | 0.9523(6) | 0.0432(10) |
| H18 | −0.250000 | −0.170019 | 0.878681 | 0.052* |
| C19 | −0.250000 | −0.1527(2) | 1.1049(6) | 0.0447(11) |
| H19 | −0.250000 | −0.195758 | 1.135843 | 0.054* |
| C20 | −0.250000 | −0.1054(2) | 1.2097(5) | 0.0465(11) |
| H20 | −0.250000 | −0.115859 | 1.313849 | 0.056* |
| C21 | −0.250000 | −0.0420(2) | 1.1667(5) | 0.0394(9) |
| H21 | −0.250000 | −0.009616 | 1.240954 | 0.047* |
| C22 | −0.250000 | 0.12908(19) | 0.6090(5) | 0.0368(9) |
| C23 | −0.4284(9) | 0.1696(4) | 0.5768(5) | 0.115(3) |
| H23A | −0.548881 | 0.144632 | 0.591296 | 0.172* |
| H23B | −0.422628 | 0.184785 | 0.472452 | 0.172* |
| H23C | −0.429579 | 0.205900 | 0.645614 | 0.172* |
| C24 | −0.250000 | 0.0713(4) | 0.5126(8) | 0.144(6) |
| H12A | −0.368(5) | 0.5518(16) | 0.805(3) | 0.215* |
| H12Ba | −0.253(5) | 0.5897(9) | 0.6756(13) | 0.215* |
| H24A | −0.367(5) | 0.0460(16) | 0.535(3) | 0.215* |
| H24Ba | −0.250(5) | 0.0840(9) | 0.4066(14) | 0.215* |
aOccupancy: 0.5.
Source of material
Under the atmosphere of N2, to the solution of 1,3,5-triazine (18.44 g, 0.1 mol) in tetrahydrofuran (100 mL) was added the solution of PhMgBr in Et2O (1 mol/L, 110 mL) dropwise at 258 K. The mixture was stirred for 2 h then quenched with 5 mL of water. The solvent was evaporated to get a yellow solid which was purified by silica gel chromatograph to afford 2-phenyl-4,6-dichloro-1,3,5-triazine (17.41 g, yield 77%). Under the atmosphere of N2, to the solution of 2-phenyl-4,6-dichloro-1,3,5-triazine (11.30 g, 0.05 mol) in tetrahydrofuran (100 mL) was added the solution of t-butMgBr in Et2O (1 mol/L, 55 mL) dropwise at 273 K. Then CuI (95.25 mg, 0.5 mmol) was added to the reaction system. The mixture was stirred and warmed to room temperature. After half an hour, the reaction was quenched with 5 mL of water. The solvent was evaporated to get a yellow solid which was purified by silica gel chromatograph to afford the title compound (8.05 g, yield 65%). 1HNMR: 8.56 ppm (2H, d, J =8 Hz), 7.62 ppm (1H, t, J =8 Hz), 7.54 ppm (2H, t, J =8 Hz), 1.48 ppm (9H,s). Crystals were obtained from an petroleumether/ethyl acetate mixture (1:1, v:v) by slow evaporation at room temperature.
Experimental details
The hydrogen atoms were placed at calculated positions and refined as riding atoms with isotropic displacement parameters.
Discussion
1,3,5-Triazine derivatives are important intermediates which were widely used in drug development, pesticide research, chemical materials and catalytic application, due to its especial pharmacological activities, biological activities [3], [4], photoelectric properties [5] and catalytic properties. Because of these advantages, the synthesis of new triazine derivatives have drawn more attentions in recent years [6]. As an important unit, tertiary butylgroup was widely used in constructing efficient chiral ligands or chiral catalysts, due to its large steric stabilisation. So, 2-(tert-butyl)-4-chloro-6-phenyl-1,3,5-triazine was firstly synthesized using 1,3,5-triazine as starting material. This derivative can be used to synthesize many important chiral ligands by substitution reactions with chiral sulfamide, chiral sulfenamide, chiral amine, chiralphosphine and so on.
This title crystal structure consists of the C13H14ClN3 molecules, in which all bond lengths are in normal ranges. There are two cystallographically independent molecules in the asymmetric unit. In one of independent molecule, the bond length of C1—Cl1 and C10—C11 are 1.730(4) Å and 1.538(5) Å respectively. The bond length of C1—N1 is 1.320(5) Å, which is shorter than C7—C8 1.383(7) Å. The angle of C1—N1—C2 = 113.6(3)° is smaller than that of C4—C5—C6 = 120.3(4)°. There are π-π stacking interactions between the adjacent molecules in different layer. The distance between the adjacent aromatic rings in different layers is less than 3.5 Å, which is within normal range [7]. No classical hydrogen bonds were observed as following: C5—H5⋯N2 (d(H5⋯N2) = 2.47 Å), C6—H6⋯Cl2 (d(H6⋯Cl2) = 2.82 Å), C17—H17⋯N5 (d(H17⋯N5) = 2.45 Å) and C23—H23C⋯N6 (d(H23C⋯N6) = 2.54 Å).
Funding source: China Postdoctoral Science Foundation
Award Identifier / Grant number: 2016M602994
Funding statement: We acknowledge the supports by Key project of Shaanxi Provincial Education Department (17JS029), Natural Science Basic Research Program of Shaanxi(2017JM8070). China Postdoctoral Science Foundation funded project(2016M602994).
References
Bruker. APEX2, SAINT and SADABS. Bruker AXS Inc., Madison, WI, USA (2012).Search in Google Scholar
Sheldrick, G. M.: Crystal structure refinement with SHELXL. Acta Crystallogr. Sect.C: 27 (2015) 3–8.10.1107/S2053229614024218Search in Google Scholar PubMed PubMed Central
Sharma, A.; Ghabbour, H.; Khan, S. T.; Torre, B. G.; Albericio, F.; El-Faham A.: Novelpyrazolyl-s-triazine derivatives, molecular structure and antimicrobial activity. J. Mol. Struct. 1145 (2017) 244–253.10.1016/j.molstruc.2017.05.040Search in Google Scholar
Xiao, Y.; Jin, B.; Peng, R.; Zhao, J.; Liu, Q.; Chu, S.; Synthesis, characterization and properties of a new energetic salt 2,4-diamino-6-methyl-1,3,5-triazine dinitramide. J. Mol. Struct. 1146 (2017) 417–423.10.1016/j.molstruc.2017.06.030Search in Google Scholar
Wang, T.; Sun, H.; Lu, T.; Bridgmohan, C. N.; Li,F.; Liu, D.; Hu , W.; Li, W.; Zhou, X.; Wang, L.: Dissociation exists in s-triazine based donor-accepter organic systemsby photo-induced electron transfer. Dyes. Pigments. 139 (2017) 264–273.10.1016/j.dyepig.2016.12.030Search in Google Scholar
Wang, K.; Tang , Y.; Jiang, Q.; Lan,Y.; Huang, H.; Liu , D.; Zhong, C.: A thiophene-containing covalent triazine-based framework with ultramicropore for CO2 capture. J. Energy Chem. 26 (2017) 902–908.10.1016/j.jechem.2017.07.007Search in Google Scholar
Deng, L.; Liu, H.; Li, S.: Crystal structure of 4-(chloromethyl)-3-nitrobenzoic acid, C8H6ClNO4. Z. Kristallogr. NCS 232 (2017) 647–648.10.1515/ncrs-2016-0389Search in Google Scholar
©2018 Xiao Song et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 License.
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of 2-amino-4-(2,4-dinitrophenyl)-3-cyano-7,7-dimethyl-5-oxo-4H-5,6,7,8-tetrahydrobenzo-pyran, C18H16N4O6
- Crystal structure of catena-poly[(μ3-5-carboxy-2-(pyridin-4-yl)benzoato-κ5O,O′:O′′,O′′′:O′′′)(1,10-phenanthroline-κ2N,N′)cadmium(II)], C100H60N12O16Cd4
- Crystal structure of 2-amino-4-(3,4,5-trimethoxy-phenyl)-7-methyl-5-oxo-4H,5H-pyrano[4,3-b]pyran-3-carbonitrile, C19H18N2O6
- Crystal structure of 1-{4-[(2-hydroxy-5-methyl benzylidene)amino]phenyl}ethanone O-ethyl-oxime, C18H20N2O2
- Crystal structure of bis{4-methyl-2-((E)-((4-((E)-1-(ethoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O)}copper(II), C36H38CuN4O4
- Crystal structure of bis{5-methoxy-2-((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}nickel(II), C34H34N4NiO6
- Crystal structure of poly[μ8-3-carboxyphthalat-κ8-O:O1,O1,O1:O2:O3,O3:O4)silver(I)], C9H4Ag2O6
- Crystal structure of 2-((E)-((4-((E)-1-(hydroxyimino)ethyl)phenyl)iminio)methyl)-5-methoxyphenolate, C16H16N2O3
- Crystal structure of (E)-1-(4-(((E)-2-hydroxy-3-methoxybenzylidene)amino)phenyl)ethan-1-one oxime, C16H16N2O3
- The crystal structure of 2-(tert-butyl)-4-chloro-6-phenyl-1,3,5-triazine, C13H14Cl1N3
- Crystal structure of (6,6′-(((((2-aminoethyl)azanediyl)bis(ethane-2,1-diyl))bis(azanylylidene))bis(methanylylidene))bis(2,4-dichlorophenolato)-κ6N,N′,N′′,N′′′,O,O′)cadmium(II) – ethanol – water (1/1/1), C22H28CdCl4N4O4
- Crystal structure of 6-chloro-N-methylpyrimidin-4-amine, C5H6ClN3
- Synthesis and crystal structure of bis{((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)-2-naphtholato-κ2N,O}nickel(II), C40H34N4NiO4
- Crystal structure of (E)-2-(4-bromophenyl)ethenesulfonyl fluoride (C8H6BrFO2S)
- Synthesis and crystal structure of 2,2′-ethylenedioxybis(benzimide)-2,2′-bis[O-(1-propyloxyamide)]oxime-4,4′,6,6′-tetrachlorodiphenol, C36H34Cl4N4O8
- Crystal structure of bis(N-(1-(pyrazin-2-yl)ethylidene)nicotinohydrazonato-κ3N,N′,O)cadmium(II) – methanol (1/1), C26H28N10O4Zn
- Crystal structure of diaqua-(N-(1-(pyrazin-2-yl)ethylidene)nicotinohydrazonato-κ3N,N′,O)-bis(nitrato-κ2O,O′)samarium(III), C12H14N7O9Sm
- Crystal structure of hexaaqua-{(E)-N′-(1-(pyrazin-2-yl)ethylidene)isonicotinohydrazide-κ3N,N′,O}praseodym(III) trichloride monohydrate, C12H25Cl3N5O8Pr
- Crystal structure of methyl 4-(3-cyanophenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydro-quinoline-3-carboxylate, C21H22N2O3
- Crystal structure of ethyl 2-amino-4-(2-methoxyphenyl)-5-oxo-4H,5H-pyrano[3,2-c]chromene-3-carboxylate, C22H19NO6
- Crystal structure of 2,12-dibromo-5,15-dihexyl-5,15-dihydrobenzo [1,2-b:5,6-c′]dicarbazole, C38H38Br2N2
- Crystal structure of ethyl 2-amino-4-(3-hydroxy-4-methoxyphenyl)-5-oxo-4H,5H-pyrano[3,2-c]chromene-3-carboxylate, C22H19NO7
- Crystal structure of ethyl 2-amino-4-(3,4-dimethylphenyl)-5-oxo-4H,5H-pyrano[3,2-c] chromene-3-carboxylate, C23H21NO5
- Crystal structure of 2-hydroxy-N′-(pyrimidin-2-yl)benzohydrazide, C11H10N4O2
- Crystal structure of 2-(3,4-dimethylphenyl)-1,8-naphthyridine, C16H14N2
- Crystal structure of ethyl 4-(3,4-dimethylphenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C23H29NO3
- Crystal structure of ethyl 4-(3-cyanophenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C22H24N2O3
- Crystal structure of 7β,9β-dihydroxy-15-oxo-ent-kauran-16-en-19,6β-olide, C20H26O5
- Crystal structure of Ent-7β,20-epoxy-kaur-16-en-1β,6α,7α,14α,15α-pentaol-20-one, C20H30O8
- Crystal structure of 1,4-bis(benzo[d][1,3]dioxol-5-ylmethyl)dihydro-1H,3H-furo[3,4-c]furan-3a(4H)-yl acetate, C22H20O8
- Crystal structure of methyl 2-((4-((2-nitrophenoxy)methyl)-1H-1,2,3-triazol-1-yl)methyl) benzoate, C18H16N4O5
- Crystal structure of diaqua-bis(5-carboxy-1-methyl-1H-imidazole-4-carboxylato-κ2N,O)zinc(II), C12H14N4O10Zn
- Hydrothemal synthesis and crystal structure of triaqua-bis(5-carboxy-1-methyl-1H-imidazole-4-carboxylato-κ2N,O;κ1O)manganese(II), C12H16N4O11Mn
- Redetermination of methyl 4-(4-chlorophenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydro-quinoline-3-carboxylate, C20H22ClNO3
- Crystal structure of bis{1-[(benzotriazol-1-yl)methyl]-1-H-1,3-(2-methyl-imdazol)-κN}-dithiocyano-κN-zinc(II) C24H22N12S2Zn
- Crystal structure of (5-fluoro-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)methyl furan-2-carboxylate, C10H7FN2O5
- Crystal structure of bis(η6-cymene)-tri-μ2-chlorido-ruthenium(II) tetrafluoroborate, C20H28BCl3F4Ru2
- Crystal structure of 3,6-diphenyl-7H-[1,2,4]-triazolo[3,4-b][1,3,4]thiadiazine, C16H12N4S
- Synthesis and crystal structure of 1,3-bis[(3,4-dicyano)phenoxy]-4,6-dinitro-benzene, C22H8N6O6
- Crystal structure of ethyl 2-amino-4-(2,6-dichlorophenyl)-7-methyl-5-oxo-4H,5H-pyrano [4,3-b]pyran-3-carboxylate, C18H15Cl2NO5
- Crystal structure of poly[μ3-hydroxy-(μ5-(5-(2-carboxylatophenoxy)isophthalato-κ6O1:O2:O3:O4:O5,O6)-(μ2-1,4-di(1H-imidazol-1-yl)butane-κ2N:N′)dicobalt(II)] hemihydrate, C25H22Co2N4O8.5
- Crystal structure of methyl 4-(4-bromophenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C20H22BrNO3
- Crystal structure of 9,10-dimethoxy-5,6-dihydro-[1,3]dioxolo[4,5-g]isoquinolino[3,2-a]isoquinolin-7-ium 5-hydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-chromen-7-olate trihydrate, C35H33NO12
- Crystal structure of 2,5-bis(4-(10H-phenothiazin-10-yl)phenyl)-1,3,4-oxadiazole, C38H24N4OS2
- The crystal structure of (E)-2-(4-hydroxyphenyl)-5-(prop-1-en-1-yl)benzofuran-3-carbaldehyde, C18H14O3
- Crystal structure of bis{5H-dibenzo[c,f][1,5]oxabismocin-12(7H)-yl} carbonate, C29H24O5Bi2
- Crystal structure of ethyl-5-formyl-3,4-dimethylpyrrole-2-carboxylate — N-(5-ethoxycarbonyl-3,4-dimethylpyrrole)-2-methylene-5-nitrobenzene-1,2-diamine (1:1), C26H31N5O7
- Crystal structure of (E)-2-(1-((2-aminophenyl)imino)ethyl)-6-bromo-4-chlorophenol, C14H12BrClN2O
- Crystal structure of (Z)-2-(4-chlorophenyl)-4-(furan-2-yl(phenylamino)methylene)-5-methyl-2,4-dihydro-3H-pyrazol-3-one, C21H16ClN3O2
- Crystal structure of N2,N4-dibutyl-6-chloro-N2,N4-bis(1,2,2,6,6-pentamethylpiperidin-4-yl)-1,3,5-triazine-2,4-diamine, C31H58ClN7 – Important intermediate of Chimassorb 119 synthesis
- Crystal structure of 1-(5-bromo-2-(4-methoxyphenyl)-1H-indol-7-yl)ethanone oxime, C17H15BrN2O2
- Crystal structure of poly-{diaqua-bis[(μ2-3-nitrobenzenesulfonylglycine-κ3N:O:O′)(4,4′-bipyridine)manganese(II)]}-dimethylformamide (1/1), C39H35Mn2N9O15S2
- Crystal structure of 4-(dimethylamino)-1-(prop-2-yn-1-yl)pyridin-1-ium perchlorate, C10H13ClN2O4
- Crystal structure of 2-amino-5-oxo-4-(4-chloro-phenyl)-4,5,6,7-tetrahydro-cyclopenta[b]pyran-3-carbonitrile, C15H11ClN2O2
- Crystal structure of triaqua-(pyridine-2,6-dicarboxylato-κ3O,N,O′)cobalt(II) – 6-phenyl-1,3,5-triazine-2,4-diamine (1/1), C16H18CoN6O7
- Crystal structure of ethyl 2-amino-4-(3-cyanophenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C21H22N2O4
- Crystal structure of 2-(4-chloro-2,6-dinitrophenyl)-1-(4-chloro-3-nitrophenyl)diazene 1-oxide, C12H5Cl2N5O7
- Crystal structure of 3,4-dimethyl-2,6-dinitrophenol, C8H8N2O5
- Crystal structure of 1,2-dimethyl-3,5-dinitrobenzene, C8H8N2O4
- Crystal structure of ethyl 4-(3-chlorophenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C21H24ClNO3
- Crystal structure of N-(4-chlorophenyl)-2-(2,6-dichlorophenyl)acetamide, C14H10Cl3NO
- Crystal structure of 2-amino-4-(4-fluorophenyl)-3-cyano-5-oxo-4H,5H-pyrano[3,2c] chromene, C19H11FN2O3
- The crystal structure of (4-nitrophenyl) (5-ferrocenyl-3-(trifluoromethyl)-1H-pyrazol-1-yl) methanone, C21H12F3FeN3O3
- Crystal structure of 5,5′-((3-hydroxy-4-methoxyphenyl)methylene)bis(1,3-diethyl-6-hydroxy-2-thioxo-2,3-dihydropyrimidin-4(1H)-one), C24H30N4O6S2
- Crystal structure of (3aR,4R,5R,7R,8S,9R,9aS,12R)-7-ethyl-5-(1-hydroxy-2-((R)-3-hydroxypyrrolidin-1-yl)ethoxy)-4,7,9,12-tetramethyldecahydro-4,9a-propanocyclopenta[8]annulene-3,8-diol – a pleuromutilin derivative, C26H41NO5
- Crystal structure of bis(μ2-3-formyl-5-methoxy-2-oxidobenzoato-κ3O,O′:O′)-hexapyridine-dicadmium(II) – pyridine (1/1), C53H47Cd2N7O10
- Crystal structure of ethyl 2-amino-4-(3-methoxyphenyl)-5-oxo-4H,5H-pyrano[3,2-c]chromene-3-carboxylate, C22H19NO6
- Crystal structure of methyl 4-(3,5-ditrifluoromethylphenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate — water (2/1), C22H21F6NO3
- Crystal structure of 2-(4-bromophenyl)-2,3-dihydro-1H-perimidine, C17H13BrN2
- Crystal structure of (5-ethyl-2-(4-methoxyphenyl)-1,3-dioxan-5-yl)methanol, C14H20O4
- Crystal structure of (E)-N-(4-bromo-2-(1-(hydroxyimino)ethyl)phenyl)benzamide, C15H13BrN2O2
- Crystal structure of caffeinium triiodide – caffeine (1/1), C16H21I3N8O4
- Crystal structure of methyl 2-methyl-4-(3-methoxyphenyl)-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C19H21NO4
- Crystal structure of (E)-3-(3,5-di-tert-butyl-4-hydroxyphenyl)-1-phenylprop-2-en-1-one, C23H28O2
- Crystal structure of 1,4-bis(2-azidoethyl)piperazine-1,4-diium dichloride, C8H18N8Cl2
- The crystal structure of dichlorido-(1,3-bis(2,6-dimethylphenyl)-1H-imidazol-2(3H)-ylidene)-(morpholine-κ1N)palladium(II), C23H29Cl2N3OPd(II)
- The crystal structure of 1-((5-chloro-3-methyl-1-phenyl-1H-pyrazole-4-yl)methyl)-1,3-diphenylurea, C24H21ClN4O
- Crystal structure of 6-(2-bromoacetamido)tetrahydro-2H-pyran-2,3,4,5-Tetrayl tetraacetate, C16H22BrNO10
- Crystal structure of 5-methylpyrazine-2-carbohydrazide, C6H8N4O
- Crystal structure of catena-poly[(μ2-5-(tert-butyl)isophthalato-κ4O,O′:O′′,O′′′)(-4′-(pyridin-4-yl)-2,2′:6′,2′′-terpyridine-κ3N,N′,N′′)manganese(II)], C32H28N4O5Mn
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of 2-amino-4-(2,4-dinitrophenyl)-3-cyano-7,7-dimethyl-5-oxo-4H-5,6,7,8-tetrahydrobenzo-pyran, C18H16N4O6
- Crystal structure of catena-poly[(μ3-5-carboxy-2-(pyridin-4-yl)benzoato-κ5O,O′:O′′,O′′′:O′′′)(1,10-phenanthroline-κ2N,N′)cadmium(II)], C100H60N12O16Cd4
- Crystal structure of 2-amino-4-(3,4,5-trimethoxy-phenyl)-7-methyl-5-oxo-4H,5H-pyrano[4,3-b]pyran-3-carbonitrile, C19H18N2O6
- Crystal structure of 1-{4-[(2-hydroxy-5-methyl benzylidene)amino]phenyl}ethanone O-ethyl-oxime, C18H20N2O2
- Crystal structure of bis{4-methyl-2-((E)-((4-((E)-1-(ethoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O)}copper(II), C36H38CuN4O4
- Crystal structure of bis{5-methoxy-2-((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}nickel(II), C34H34N4NiO6
- Crystal structure of poly[μ8-3-carboxyphthalat-κ8-O:O1,O1,O1:O2:O3,O3:O4)silver(I)], C9H4Ag2O6
- Crystal structure of 2-((E)-((4-((E)-1-(hydroxyimino)ethyl)phenyl)iminio)methyl)-5-methoxyphenolate, C16H16N2O3
- Crystal structure of (E)-1-(4-(((E)-2-hydroxy-3-methoxybenzylidene)amino)phenyl)ethan-1-one oxime, C16H16N2O3
- The crystal structure of 2-(tert-butyl)-4-chloro-6-phenyl-1,3,5-triazine, C13H14Cl1N3
- Crystal structure of (6,6′-(((((2-aminoethyl)azanediyl)bis(ethane-2,1-diyl))bis(azanylylidene))bis(methanylylidene))bis(2,4-dichlorophenolato)-κ6N,N′,N′′,N′′′,O,O′)cadmium(II) – ethanol – water (1/1/1), C22H28CdCl4N4O4
- Crystal structure of 6-chloro-N-methylpyrimidin-4-amine, C5H6ClN3
- Synthesis and crystal structure of bis{((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)-2-naphtholato-κ2N,O}nickel(II), C40H34N4NiO4
- Crystal structure of (E)-2-(4-bromophenyl)ethenesulfonyl fluoride (C8H6BrFO2S)
- Synthesis and crystal structure of 2,2′-ethylenedioxybis(benzimide)-2,2′-bis[O-(1-propyloxyamide)]oxime-4,4′,6,6′-tetrachlorodiphenol, C36H34Cl4N4O8
- Crystal structure of bis(N-(1-(pyrazin-2-yl)ethylidene)nicotinohydrazonato-κ3N,N′,O)cadmium(II) – methanol (1/1), C26H28N10O4Zn
- Crystal structure of diaqua-(N-(1-(pyrazin-2-yl)ethylidene)nicotinohydrazonato-κ3N,N′,O)-bis(nitrato-κ2O,O′)samarium(III), C12H14N7O9Sm
- Crystal structure of hexaaqua-{(E)-N′-(1-(pyrazin-2-yl)ethylidene)isonicotinohydrazide-κ3N,N′,O}praseodym(III) trichloride monohydrate, C12H25Cl3N5O8Pr
- Crystal structure of methyl 4-(3-cyanophenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydro-quinoline-3-carboxylate, C21H22N2O3
- Crystal structure of ethyl 2-amino-4-(2-methoxyphenyl)-5-oxo-4H,5H-pyrano[3,2-c]chromene-3-carboxylate, C22H19NO6
- Crystal structure of 2,12-dibromo-5,15-dihexyl-5,15-dihydrobenzo [1,2-b:5,6-c′]dicarbazole, C38H38Br2N2
- Crystal structure of ethyl 2-amino-4-(3-hydroxy-4-methoxyphenyl)-5-oxo-4H,5H-pyrano[3,2-c]chromene-3-carboxylate, C22H19NO7
- Crystal structure of ethyl 2-amino-4-(3,4-dimethylphenyl)-5-oxo-4H,5H-pyrano[3,2-c] chromene-3-carboxylate, C23H21NO5
- Crystal structure of 2-hydroxy-N′-(pyrimidin-2-yl)benzohydrazide, C11H10N4O2
- Crystal structure of 2-(3,4-dimethylphenyl)-1,8-naphthyridine, C16H14N2
- Crystal structure of ethyl 4-(3,4-dimethylphenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C23H29NO3
- Crystal structure of ethyl 4-(3-cyanophenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C22H24N2O3
- Crystal structure of 7β,9β-dihydroxy-15-oxo-ent-kauran-16-en-19,6β-olide, C20H26O5
- Crystal structure of Ent-7β,20-epoxy-kaur-16-en-1β,6α,7α,14α,15α-pentaol-20-one, C20H30O8
- Crystal structure of 1,4-bis(benzo[d][1,3]dioxol-5-ylmethyl)dihydro-1H,3H-furo[3,4-c]furan-3a(4H)-yl acetate, C22H20O8
- Crystal structure of methyl 2-((4-((2-nitrophenoxy)methyl)-1H-1,2,3-triazol-1-yl)methyl) benzoate, C18H16N4O5
- Crystal structure of diaqua-bis(5-carboxy-1-methyl-1H-imidazole-4-carboxylato-κ2N,O)zinc(II), C12H14N4O10Zn
- Hydrothemal synthesis and crystal structure of triaqua-bis(5-carboxy-1-methyl-1H-imidazole-4-carboxylato-κ2N,O;κ1O)manganese(II), C12H16N4O11Mn
- Redetermination of methyl 4-(4-chlorophenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydro-quinoline-3-carboxylate, C20H22ClNO3
- Crystal structure of bis{1-[(benzotriazol-1-yl)methyl]-1-H-1,3-(2-methyl-imdazol)-κN}-dithiocyano-κN-zinc(II) C24H22N12S2Zn
- Crystal structure of (5-fluoro-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)methyl furan-2-carboxylate, C10H7FN2O5
- Crystal structure of bis(η6-cymene)-tri-μ2-chlorido-ruthenium(II) tetrafluoroborate, C20H28BCl3F4Ru2
- Crystal structure of 3,6-diphenyl-7H-[1,2,4]-triazolo[3,4-b][1,3,4]thiadiazine, C16H12N4S
- Synthesis and crystal structure of 1,3-bis[(3,4-dicyano)phenoxy]-4,6-dinitro-benzene, C22H8N6O6
- Crystal structure of ethyl 2-amino-4-(2,6-dichlorophenyl)-7-methyl-5-oxo-4H,5H-pyrano [4,3-b]pyran-3-carboxylate, C18H15Cl2NO5
- Crystal structure of poly[μ3-hydroxy-(μ5-(5-(2-carboxylatophenoxy)isophthalato-κ6O1:O2:O3:O4:O5,O6)-(μ2-1,4-di(1H-imidazol-1-yl)butane-κ2N:N′)dicobalt(II)] hemihydrate, C25H22Co2N4O8.5
- Crystal structure of methyl 4-(4-bromophenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C20H22BrNO3
- Crystal structure of 9,10-dimethoxy-5,6-dihydro-[1,3]dioxolo[4,5-g]isoquinolino[3,2-a]isoquinolin-7-ium 5-hydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-chromen-7-olate trihydrate, C35H33NO12
- Crystal structure of 2,5-bis(4-(10H-phenothiazin-10-yl)phenyl)-1,3,4-oxadiazole, C38H24N4OS2
- The crystal structure of (E)-2-(4-hydroxyphenyl)-5-(prop-1-en-1-yl)benzofuran-3-carbaldehyde, C18H14O3
- Crystal structure of bis{5H-dibenzo[c,f][1,5]oxabismocin-12(7H)-yl} carbonate, C29H24O5Bi2
- Crystal structure of ethyl-5-formyl-3,4-dimethylpyrrole-2-carboxylate — N-(5-ethoxycarbonyl-3,4-dimethylpyrrole)-2-methylene-5-nitrobenzene-1,2-diamine (1:1), C26H31N5O7
- Crystal structure of (E)-2-(1-((2-aminophenyl)imino)ethyl)-6-bromo-4-chlorophenol, C14H12BrClN2O
- Crystal structure of (Z)-2-(4-chlorophenyl)-4-(furan-2-yl(phenylamino)methylene)-5-methyl-2,4-dihydro-3H-pyrazol-3-one, C21H16ClN3O2
- Crystal structure of N2,N4-dibutyl-6-chloro-N2,N4-bis(1,2,2,6,6-pentamethylpiperidin-4-yl)-1,3,5-triazine-2,4-diamine, C31H58ClN7 – Important intermediate of Chimassorb 119 synthesis
- Crystal structure of 1-(5-bromo-2-(4-methoxyphenyl)-1H-indol-7-yl)ethanone oxime, C17H15BrN2O2
- Crystal structure of poly-{diaqua-bis[(μ2-3-nitrobenzenesulfonylglycine-κ3N:O:O′)(4,4′-bipyridine)manganese(II)]}-dimethylformamide (1/1), C39H35Mn2N9O15S2
- Crystal structure of 4-(dimethylamino)-1-(prop-2-yn-1-yl)pyridin-1-ium perchlorate, C10H13ClN2O4
- Crystal structure of 2-amino-5-oxo-4-(4-chloro-phenyl)-4,5,6,7-tetrahydro-cyclopenta[b]pyran-3-carbonitrile, C15H11ClN2O2
- Crystal structure of triaqua-(pyridine-2,6-dicarboxylato-κ3O,N,O′)cobalt(II) – 6-phenyl-1,3,5-triazine-2,4-diamine (1/1), C16H18CoN6O7
- Crystal structure of ethyl 2-amino-4-(3-cyanophenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C21H22N2O4
- Crystal structure of 2-(4-chloro-2,6-dinitrophenyl)-1-(4-chloro-3-nitrophenyl)diazene 1-oxide, C12H5Cl2N5O7
- Crystal structure of 3,4-dimethyl-2,6-dinitrophenol, C8H8N2O5
- Crystal structure of 1,2-dimethyl-3,5-dinitrobenzene, C8H8N2O4
- Crystal structure of ethyl 4-(3-chlorophenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C21H24ClNO3
- Crystal structure of N-(4-chlorophenyl)-2-(2,6-dichlorophenyl)acetamide, C14H10Cl3NO
- Crystal structure of 2-amino-4-(4-fluorophenyl)-3-cyano-5-oxo-4H,5H-pyrano[3,2c] chromene, C19H11FN2O3
- The crystal structure of (4-nitrophenyl) (5-ferrocenyl-3-(trifluoromethyl)-1H-pyrazol-1-yl) methanone, C21H12F3FeN3O3
- Crystal structure of 5,5′-((3-hydroxy-4-methoxyphenyl)methylene)bis(1,3-diethyl-6-hydroxy-2-thioxo-2,3-dihydropyrimidin-4(1H)-one), C24H30N4O6S2
- Crystal structure of (3aR,4R,5R,7R,8S,9R,9aS,12R)-7-ethyl-5-(1-hydroxy-2-((R)-3-hydroxypyrrolidin-1-yl)ethoxy)-4,7,9,12-tetramethyldecahydro-4,9a-propanocyclopenta[8]annulene-3,8-diol – a pleuromutilin derivative, C26H41NO5
- Crystal structure of bis(μ2-3-formyl-5-methoxy-2-oxidobenzoato-κ3O,O′:O′)-hexapyridine-dicadmium(II) – pyridine (1/1), C53H47Cd2N7O10
- Crystal structure of ethyl 2-amino-4-(3-methoxyphenyl)-5-oxo-4H,5H-pyrano[3,2-c]chromene-3-carboxylate, C22H19NO6
- Crystal structure of methyl 4-(3,5-ditrifluoromethylphenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate — water (2/1), C22H21F6NO3
- Crystal structure of 2-(4-bromophenyl)-2,3-dihydro-1H-perimidine, C17H13BrN2
- Crystal structure of (5-ethyl-2-(4-methoxyphenyl)-1,3-dioxan-5-yl)methanol, C14H20O4
- Crystal structure of (E)-N-(4-bromo-2-(1-(hydroxyimino)ethyl)phenyl)benzamide, C15H13BrN2O2
- Crystal structure of caffeinium triiodide – caffeine (1/1), C16H21I3N8O4
- Crystal structure of methyl 2-methyl-4-(3-methoxyphenyl)-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C19H21NO4
- Crystal structure of (E)-3-(3,5-di-tert-butyl-4-hydroxyphenyl)-1-phenylprop-2-en-1-one, C23H28O2
- Crystal structure of 1,4-bis(2-azidoethyl)piperazine-1,4-diium dichloride, C8H18N8Cl2
- The crystal structure of dichlorido-(1,3-bis(2,6-dimethylphenyl)-1H-imidazol-2(3H)-ylidene)-(morpholine-κ1N)palladium(II), C23H29Cl2N3OPd(II)
- The crystal structure of 1-((5-chloro-3-methyl-1-phenyl-1H-pyrazole-4-yl)methyl)-1,3-diphenylurea, C24H21ClN4O
- Crystal structure of 6-(2-bromoacetamido)tetrahydro-2H-pyran-2,3,4,5-Tetrayl tetraacetate, C16H22BrNO10
- Crystal structure of 5-methylpyrazine-2-carbohydrazide, C6H8N4O
- Crystal structure of catena-poly[(μ2-5-(tert-butyl)isophthalato-κ4O,O′:O′′,O′′′)(-4′-(pyridin-4-yl)-2,2′:6′,2′′-terpyridine-κ3N,N′,N′′)manganese(II)], C32H28N4O5Mn

