Abstract
C18H17N1O3, monoclinic, P21/c (no. 14), a = 14.9752(8) Å, b = 9.0793(5) Å, c = 11.1030(6) Å, β = 106.431(1)°, V = 1447.96(14) Å3, Z = 4, Rgt(F) = 0.0342, wRref(F2) = 0.0903, T = 296(2) K.
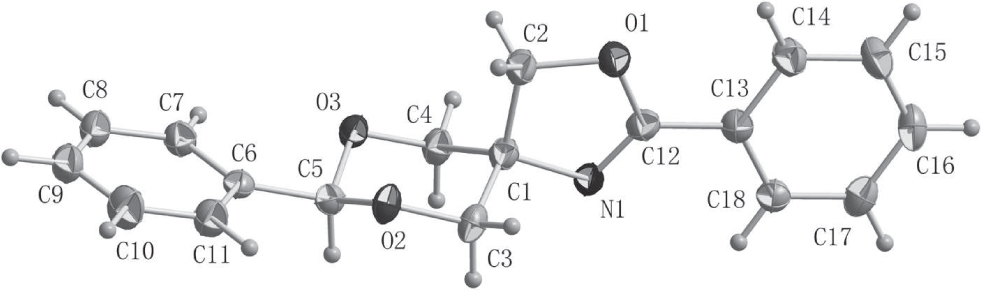
The crystal structure is shown in the figure. Tables 1 and 2 contain details on crystal structure and measurement conditions and a list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Block, colorless |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.09 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω-scans |
| 2θmax, completeness: | 25°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 26710, 2541, 0.018 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 2384 |
| N(param)refined: | 199 |
| Programs: | Bruker programs [1], SHELX [2, 3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| O3 | 0.06061(6) | 0.17715(10) | 0.24804(7) | 0.0270(2) |
| O2 | 0.07766(6) | 0.35823(9) | 0.10810(7) | 0.0275(2) |
| O1 | −0.21461(6) | 0.32501(10) | 0.03002(9) | 0.0349(2) |
| N1 | −0.13695(7) | 0.14691(11) | −0.04115(9) | 0.0265(2) |
| C1 | −0.06661(8) | 0.21939(13) | 0.06214(11) | 0.0256(3) |
| C5 | 0.12246(8) | 0.24476(13) | 0.19006(11) | 0.0233(3) |
| H5 | 0.1456 | 0.1706 | 0.1421 | 0.028* |
| C13 | −0.30284(8) | 0.18096(13) | −0.14404(11) | 0.0261(3) |
| C12 | −0.21330(9) | 0.21354(14) | −0.05101(11) | 0.0276(3) |
| C4 | −0.01397(8) | 0.10583(14) | 0.15649(11) | 0.0278(3) |
| H4A | −0.0559 | 0.0597 | 0.1976 | 0.033* |
| H4B | 0.0109 | 0.0299 | 0.1136 | 0.033* |
| C7 | 0.23114(9) | 0.24366(15) | 0.40891(12) | 0.0295(3) |
| H7 | 0.1974 | 0.1653 | 0.4277 | 0.035* |
| C6 | 0.20345(8) | 0.30765(13) | 0.29031(11) | 0.0247(3) |
| C18 | −0.30567(9) | 0.07887(14) | −0.23862(11) | 0.0282(3) |
| H18 | −0.2510 | 0.0349 | −0.2445 | 0.034* |
| C14 | −0.38491(9) | 0.24658(15) | −0.13635(13) | 0.0337(3) |
| H14 | −0.3835 | 0.3151 | −0.0736 | 0.040* |
| C3 | 0.00384(9) | 0.29752(14) | 0.00848(11) | 0.0273(3) |
| H3A | 0.0293 | 0.2282 | −0.0395 | 0.033* |
| H3B | −0.0269 | 0.3757 | −0.0476 | 0.033* |
| C8 | 0.30866(9) | 0.29594(16) | 0.49914(12) | 0.0338(3) |
| H8 | 0.3265 | 0.2526 | 0.5782 | 0.041* |
| C11 | 0.25427(9) | 0.42562(15) | 0.26460(12) | 0.0340(3) |
| H11 | 0.2363 | 0.4702 | 0.1860 | 0.041* |
| C15 | −0.46869(9) | 0.20986(16) | −0.22206(14) | 0.0404(3) |
| H15 | −0.5236 | 0.2539 | −0.2169 | 0.049* |
| C2 | −0.12262(9) | 0.32762(17) | 0.11823(13) | 0.0373(3) |
| H2A | −0.1245 | 0.2957 | 0.2008 | 0.045* |
| H2B | −0.0960 | 0.4257 | 0.1248 | 0.045* |
| C17 | −0.38951(9) | 0.04279(16) | −0.32366(13) | 0.0353(3) |
| H17 | −0.3912 | −0.0255 | −0.3867 | 0.042* |
| C10 | 0.33164(10) | 0.47703(17) | 0.35560(14) | 0.0412(3) |
| H10 | 0.3651 | 0.5563 | 0.3378 | 0.049* |
| C9 | 0.35948(9) | 0.41159(16) | 0.47264(13) | 0.0374(3) |
| H9 | 0.4121 | 0.4454 | 0.5330 | 0.045* |
| C16 | −0.47099(9) | 0.10798(17) | −0.31533(14) | 0.0410(4) |
| H16 | −0.5274 | 0.0832 | −0.3726 | 0.049* |
Source of material
Tris(hydroxymethyl)aminomethane (TRIS) (2.00 g, 16.5 mmol) and benzaldehyde (5.31 g, 50 mmol) were stirred at 338 K in anhydrous toluene (50 mL) until the reaction mixture becomes clear. The solvent was evaporated to dryness and the residue dissolved in diethylether (50 mL). The etheral solution was washed with a saturated Na2CO3 solution (10 mL). The aqueous phase was extracted with diethylether and the combined organic phase was dried over anhydrous Na2SO4, then evaporated to a white powder. The latter was recrystallized from ethanol to afford colorless crystals (2.73 g, 56%).
Experimental details
All hydrogen atoms were identified in difference Fourier syntheses.
Discussion
Synthetic routes employing oxazolidines as cyclic protection of aminoalcohols have been widely used for the asymmetric synthesis of chiral amines, aminoalcohols and/or aminoacids [4]. Moreover, such derivatives were assumed to have potential as prodrug forms, as they undergo conversion to the parent compound [5]. Indeed, oxazolidine derived from ephedrine has been proved to have sympathomimetic activity in several animal models [6, 7] . In both cases, the success of the approach relies on the cleavage of the 1,3-N,O ring.
Herein, we report on a new oxazolidine derivative, which was synthesized and characterized by single-crystal X-ray diffraction [2, 3] . In the structure, there are two new formed rings, C(1)—C(3)—O(2)—C(5)—O(3)—C(4) and C(1)—N(1)—C(12)—O(1)—C(2). The bond lengths of N(1)—C(1), N(1)—C(12) are 1.4746(15) Å and 1.2706(16) Å respectively. All geometric parameters are in the expected ranges [8, 9] .
Acknowledgement
This work was financially supported by the Scientific Research Fund of Hunan Provincial Education Department (No. 16B104), the Opening Project of Key Laboratory of Comprehensive Utilization of Advantage Plants Resources in Hunan South (No. XNZW16C01) and the Scientific Research Fund of Hunan University of Science and Engineering (No. 16XKY063), Natural Science Foundation of Hunan Province of China (2017JJ3093).
References
Bruker. APEX2, SAINT and SADABS. Brucker AXS Inc., Madison, Wisconsin, USA, (2009).Suche in Google Scholar
Sheldrick, G. M.: SHELXS-97: Program for the Solution of Crystal Structures. University of Göttingen, Germany, (1997).Suche in Google Scholar
Sheldrick, G. M.: A short history of SHELX. Acta Crystallogr. A64 (2008) 112–122.10.1107/S0108767307043930Suche in Google Scholar
Wagner, B.; Gonzalez, G. I.; Tran Hun Dau, M. E.; Zhu, J.: Total synthesis and conformational studies of hapalosin, N-desmethylhapalosin and 8-deoxyhapalosin. Bioorg. Med. Chem. 7 (1999) 737–C747.10.1016/S0968-0896(98)00208-9Suche in Google Scholar
Berrien, J. F.; Royer, J.; Husson, H. P.: A new access to enantiomerically pure (S)-(-)-pipecolic acid and 2-alkylated or 6-alkylated derivatives. J. Org. Chem. 59 (1994) 3769–C3774.10.1021/jo00093a007Suche in Google Scholar
Johansen, M.; Bundgaard, H.: Hydrolysis of oxazolidines – a potential new prodrug type. J. Pharm. Sci. 72 (1983) 1294–C1298.10.1002/jps.2600721115Suche in Google Scholar
Walker, R. B.; Huang, M. J.; Leszczynski, J.: An investigation of isomeric differences in hydrolytic rates of oxazolidines using computational methods. J. Mol. Struct. 549 (2001) 137–C146.10.1016/S0166-1280(01)00489-4Suche in Google Scholar
Jia, G.-K.; Yuan, L.; Yuan, X.-Y.; Li, Z.-Y.: Crystal structure of 5-ethyl-2-(p-tolyl)-1,3-dioxane-5-carboxylic acid, C14H18O4. Z. Kristallogr. NCS 232 (2017) 819–820.10.1515/ncrs-2017-0061Suche in Google Scholar
Jia, G.-K.; Yuan, L.; Zhang, M., Yuan, X.-Y.; Liu, F.: Crystal structure of 2-(2-bromophenyl)-5-methyl-1,3-dioxane-5-carboxylic acid, C12H13BrO4. Z. Kristallogr. NCS 231 (2016) 1207–1208.10.1515/ncrs-2016-0135Suche in Google Scholar
©2017 Min Zhang et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 License.
Artikel in diesem Heft
- Cover and Frontmatter
- Crystal structure of potassium 1-methyl-1H-1,2,3,4-tetrazole-5-thiolate, C2H3N4SK
- Crystal structure of bis(3-(3-ethylureido)-N,N-dimethylpropan-1-aminium) bis (μ3-2-(hydroxymethyl)-2-(oxidomethyl)propane-1,3-bis(olato))-(μ6-oxo)-hexakis(μ2-oxo)-hexaoxo-hexavanadium(V) – dichloromethane (1/1), C27H60Cl2N6O23V6
- Crystal structure of bis(μ3-methanolato-κ3O:O:O)-bis(μ2-methanolato-κ2O:O)-dimethanol-bis{6,6′-(1,3-dihydroxyl-2-acetylpropane-1,3-diyl)bis(2-chloro-4-bromophenolato)}tetramanganese(III) C40H40Br4Cl4Mn4O16
- Synthesis and crystal structure of tetrakis(μ2-methanolato)-dimethanol-bis(μ2-2-acetyl-1,3-bis(3,5-dibromo-2-oxidophenyl)propane-1,3-bis(olato)-κ5O1,O2,O3:O3,O4,O5)tetramanganese(III), C40H40Br8Mn4O16
- Synthesis and crystal structure tetrakis(μ2-methanolato)-dimethanol-bis(μ2-2-acetyl-1,3-bis(3,5-dichloro-2-oxidophenyl)propane-1,3-bis(olato)-κ5O1,O2,O3:O3,O4,O5), tetramanganese(III), C40H40Cl8Mn4O16
- Crystal structure of bis{5-methoxy-2-((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O)}copper(II), C34H34CuN4O6
- Crystal structure of (E)-1-(2-hydroxy-3-{[2-hydroxybenzylidene]amino}phenyl)ethan-1-one, C15H13NO3
- Crystal structure of bis(μ2-acetato-κ2O:O′)-bis{μ2-4-chloro-2-(8-(4-(diethylamino)-2-oxidophenyl)-3,6-dioxa-2,7-diazaocta-1,7-dien-1-yl)phenolato-κ6O:O,N,N′,O′′:O′′}tricobalt(II), C44H49Cl2Co3N6O12
- Crystal structure of bis{μ2-2,4-dichloro-6-(8-(4-(diethylamino)-2-oxidophenyl)-3,6-dioxa-2,7-diazaocta-1,7-dien-1-yl)phenolato-κ5O:O,N,N′,O′}dicobalt(II) acetone solvate, C43H48Br4Co2N6O9
- Synthesis and crystal structure of bis(μ2-acetato-κ2O:O′)-bis{μ2-4-chloro-2-(8-(4-(diethylamino)-2-oxidophenyl)-3,6-dioxa-2,7-diazaocta-1,7-dien-1-yl)phenolato-κ6O:O,N,N′,O′:O′}trizinc(II), C44H49Cl2N6O12Zn3
- Crystal structure of bis{5-(N,N′-diethylamine)-5′-methoxy -2,2′-[ethylenediyldioxybis(nitrilomethylidyne)]diphenolato}-bis(μ2-acetato-κ2O:O′)trizinc(II), C46H56Zn3N6O14
- Crystal structure of tris(cyano-(hydrogen tris(3,5-dimethylpyrazolyl)borate))-iron(III) 4-methoxypyridinium monohydrate, C24H32BN10O2Fe
- Synthesis and crystal structure of bis{1-(((4-(1-(hydroxyimino)ethyl)phenyl)imino)methyl)naphthalen-2-olato-κ2O,N}copper(II), C38H30CuN4O4
- Crystal structure of (E)-1-(4-{[(E)-2-Hydroxy-1-naphthalenylmethylene] amino}phenyl)ethanone oxime, C19H16N2O2
- The pseudosymmetric crystal structure of 2-(2-naphthalenyl)-3-nitro-2H-1-benzopyran, C38H26N2O6
- Crystal structure of 2-benzoyl-3-(4-methoxyphenyl)cyclopropane-1,1-dicarbonitrile, C19H14N2O2
- N′-(5-ethoxycarbonyl-3,4-dimethyl-pyrrol-2-yl-methylidene)-4-hydroxybenzohydrazide monohydrate, C17H21N3O5
- The crystal structure of carbonyl-[4-(2,4-dichlorophenylamino)pent-3-en-2-onato-κ2N,O]-(triphenylphosphine-κP)rhodium(I), RhC30H25Cl2NO2P
- Crystal structure of (E)-5-(diethylamino)-2-(((1,1,2-trihydroxyethyl)iminio)methyl)phenolate, C15H24N2O4
- Crystal structure of poly[(μ2-1,4-bis((2-ethyl-1H-benzo[d]imidazol-1-yl)methyl)benzene-κ2N:N′)(μ2-isophthalato-κ4O,O′:O′′,O′′′)cadmium(II)], C34H30N4O4Cd
- Crystal structure of 5,6-Dihydro-9,10-dimethoxybenzo[g]-1,3-benzodioxolo[5,6-a]chinolizinium 3-(4-hydroxyphenyl)-4-oxo-4H-chromen-7-olate - methanol - water (1/1/1), C36H33NO10
- A single crystal study on 2-(methylcarbamoyl)benzoic acid, C9H9NO3
- Crystal structure of the salt 1,1′-(ethane-1,2-diyl)bis(1,4-diazabicyclo[2.2.2]octan-1-ium) diperchlorate, C14H28N4(ClO4)2
- Crystal structure of 2,5-bis((E)-2-(trifluoromethyl)benzylidene)cyclopentan-1-one, C21H14F6O
- Crystal structure of catena-poly[(μ2-benzene-1,4-dicarboxylato-κ2O:O′)-(1-ethyl-6-fluoro-7-(4-methylpiperazin-1-ium-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylato-κ2O,O′)zinc(II)] 1.25 hydrate, C25H26.5N3O8.25FZn
- Crystal structure of 1-methyl-1,4-diazabicyclo[2.2.2]octan-1-ium poly[aqua-bis(μ2-perchlorato-κ3O,O′:O′′)sodium], C7H17Cl2N2NaO9
- Crystal structure of trimethyammonium 2,6-dicarboxyisonicotinate monohydrate, C11H16N2O7
- Crystal structure of dodecaguanidinium bis(tetrapropylammonium) heptacarbonate, C43H128N38O21
- Crystal structure of poly[tetraaqua-bis(μ6-benzene-1,2,4,5-tetracarboxylato)nickel(II)diyttrium(III)]dihydrate, C20H16NiO22Y2
- Halogen bonds and π–π interactions in the crystal structure of 1,3,5-trifluoro-2,4,6-triiodobenzene–N,N-dimethylformamide (1/1), C9H7F3I3NO
- Crystal structure of guanidinium tetraethylammonium carbonate dihydrate, C10H30N4O5
- The crystal structure of oxonium chlorido-ethylenediaminetetraactetotin(IV) hydrate, C10H17ClN2O10Sn
- Crystal structure of 8-((E)-((4-((E)-1-((benzyloxy)imino)ethyl)phenyl)imino)methyl)-7-hydroxy-4-methyl-2H-chromen-2-one, C26H22N2O4
- Crystal structure of bis{5-(diethylamino)-2-(((2-oxo-2H-chromen-6-yl)imino)methyl)phenolato-κ2O,N}copper(II), C40H38CuN4O6
- Crystal structure of fac-(acetylacetonato-κ2O,O′)tricarbonyl(tri(p-tolyl)phosphine-κP)rhenium(I), C29H28O5PRe
- Crystal structure of fac-(acetylacetonato-κ2O,O′)tricarbonyl(benzyldiphenylphosphine-κP)rhenium(I), C27H24O5PRe
- Crystal structure of triethylammonium bis{3-(((3-oxidonaphthalen-1-yl)methylene)amino)-2-oxo-2H-chromen-4-olato-κ3O,N,O′}manganese(III), C46H38MnN3O8
- Crystal structure of bis(N,N,N-ethyldimethylethanaminium) bis(heptaselenido-κ2Se1,Se7)palladate(II), C12H32N2PdSe14
- Crystal structure of bis(4-(1H-pyrazol-5-yl)pyridine-κ2N:N′)-bis(2-(2-((2,6-difluorophenyl)amino)phenyl)acetate-κO)cadmium(II), C44H34N8CdCl4O4
- Crystal structure of 4,4′-(1,4-phenylene)bis(pyridin-1-ium) catena-poly[diaqua-bis(μ2-3′,5′-dicarboxy-[1,1′-biphenyl]-2,5-dicarboxylato-κ2O:O′] dihydrate, C48H42O22N2Ca
- The crystal structure of (S)-2-benzylsuccinic acid, C11H12O4
- Crystal structure of poly[aqua-(μ3-4-(pyridin-4-yl)isophthalato-κ4O,O′:O′′:O′′′)-(μ2-5-carboxy-2-(pyridin-4-yl)benzoato-κ2O:O′)yttrium(III)], C26H17N2O9Y
- Crystal structure of ethyl 1-(3,4-dimethylphenyl)-5-methyl-1H-1,2,3-triazole-4-carboxylate, C14H17N3O2
- Crystal structure of tetrakis(methanol-κO)-bis{μ2-3-((4-methoxy-2-oxidobenzylidene)amino)-2-oxo-2H-chromen-4-olate-κ4O,N;O′:O′}dizinc(II), C38H38Zn2N2O14
- Crystal structure of bromido(4,4′-dimethoxy-2,2′-bipyridine-κ2N,N′)(isopropyl(diphenyl)phosphane-κP)copper(I), C27H29BrCuN2O2P
- Crystal structure of bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane)-9,9-dioctylfluorene, C41H64B2O4
- Crystal structure of 11-oxo-2,3,6,7-tetrahydro-1H,5H,11H-pyrano[2,3-f]pyrido[3,2,1-ij]quinoline-10-carbaldehyde - a julolidine derivative, C16H15NO3
- Crystal structure of 1,3-dimethyl-5,5-dibenzylbarbituric acid, C20H20N2O3
- Crystal structure of bis(N,N,N-trimethylethanaminium) poly[bis(μ2-heptaselenido-κ2Se1,Se7)palladate(II)], C10H28N2PdSe14
- Crystal structure of 1-{4-[(5-Chloro-2-hydroxy-benzylidene)amino]phenyl} ethanone O-ethyl-oxime, C17H17ClN2O2
- The crystal structure of methyl N-(4-bromophenyl)carbamate, C8H8BrNO2
- Synthesis and crystal structure of 1-{4-[(2-hydroxy-benzylidene)amino]phenyl}ethanone oxime, C15H14N2O2
- Crystal structure of 1,1′-butanebis(3-methyl-1H-imidazol-3-ium)bis(hexafluorophosphate), C12H20F12N4P2
- (E)-N-benzylidene-3-(benzylthio)-5-p-tolyl-4H-1,2,4-triazol-4-amine, C23H20N4S
- Crystal structure of ethyl 5-methyl-1-(pyridin-3-yl)-1H-1,2,3-triazole-4-carboxylate, C11H12N4O2
- Crystal structure of 5,5-difluoro-10-(4-fluorophenyl)-1,3,7,9-tetramethyl-5H-4l4,5l4-dipyrrolo[1,2-c:2′,1′-f][1,3,2]diazaborinine - a Z′ = 3 structure, C19H18B2F3N2
- The crystal structure of the Matrine derivative: 12-(1H-indol-1-yl)dodecahydro-1H,5H,10H-dipyrido[2,1-f:3′,2′,1′-ij][1,6]naphthyridin-10-one hydrate, C23H29N3O
- The crystal structure of tris(μ2-1,3-bis(4,4,4-trifluoro-3-oxido-1-(oxo)but-2-en-1-yl)phenyl-κ4O,O′:O′′,O′′′)-bis(1,2-dimethoxyethane-κ2O,O′)dilutetium(III), C50H38F18Lu2O16
- The crystal structure of (Z)-2-(3-(2-(4-chlorobenzoyl)hydrazono)-2-oxoindolin-1-yl) acetic acid, C17H12ClN3O4
- Synthesis and crystal structure of trans-tetraqua-bis(2-(((7-hydroxy-3-(4-hydroxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)ammonio)acetato-κO)cobalt(II) hexahydrate, C36H48CoN2O28S2
- The crystal structure of N,N-dimethyl-2,6-di-p-tolylpyrimidin-4-amine, C20H21N3
- The crystal structure (E)-4-methyl-N′-(2-nitrobenzylidene)benzenesulfonohydrazide, C14H13N3O4S
- Crystal structure of catena-poly[(μ2-1,3-bis(benzimidazol-1-yl)propane κ2N:N′)-(μ2-5-methoxyisophthalato-κ2O:O′)zinc(II)] hydrate, C26H24ZnN4O6
- The crystal structure of (E)-N′-(quinolin-2-ylmethylene)furan-2-carbohydrazide monohydrate, C15H13N3O3
- Crystal structure of 2,8-diphenyl-3,7,9-trioxa-1-azaspiro[4.5]dec-1-ene, C18H17N1O3
- Crystal structure of diethyl 2-(4-chlorophenyl)-1,3-dioxane-5,5-dicarboxylate, C16H19ClO6
- Crystal structure of 1-(carboxymethyl)-1H-benzo[d][1,2,3]triazole 3-oxide, C8H7N3O3
- Crystal structure of (acetylacetonato-κ2O,O′)-(2-amino-6-chlorobenzoato-κO)-oxido(1,10-phenanthroline-κ2N,N′)vanadium(IV) – trichloromethane (1/1)
- Crystal structure of (1E,4E)-1,5-bis(4-chlorophenyl)penta-1,4-dien-3-one, C17H12Cl2O
- The crystal structure of trans-dibromido-bis(pyridine-κN)platinum(II), C10H10Br2N2Pt
Artikel in diesem Heft
- Cover and Frontmatter
- Crystal structure of potassium 1-methyl-1H-1,2,3,4-tetrazole-5-thiolate, C2H3N4SK
- Crystal structure of bis(3-(3-ethylureido)-N,N-dimethylpropan-1-aminium) bis (μ3-2-(hydroxymethyl)-2-(oxidomethyl)propane-1,3-bis(olato))-(μ6-oxo)-hexakis(μ2-oxo)-hexaoxo-hexavanadium(V) – dichloromethane (1/1), C27H60Cl2N6O23V6
- Crystal structure of bis(μ3-methanolato-κ3O:O:O)-bis(μ2-methanolato-κ2O:O)-dimethanol-bis{6,6′-(1,3-dihydroxyl-2-acetylpropane-1,3-diyl)bis(2-chloro-4-bromophenolato)}tetramanganese(III) C40H40Br4Cl4Mn4O16
- Synthesis and crystal structure of tetrakis(μ2-methanolato)-dimethanol-bis(μ2-2-acetyl-1,3-bis(3,5-dibromo-2-oxidophenyl)propane-1,3-bis(olato)-κ5O1,O2,O3:O3,O4,O5)tetramanganese(III), C40H40Br8Mn4O16
- Synthesis and crystal structure tetrakis(μ2-methanolato)-dimethanol-bis(μ2-2-acetyl-1,3-bis(3,5-dichloro-2-oxidophenyl)propane-1,3-bis(olato)-κ5O1,O2,O3:O3,O4,O5), tetramanganese(III), C40H40Cl8Mn4O16
- Crystal structure of bis{5-methoxy-2-((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O)}copper(II), C34H34CuN4O6
- Crystal structure of (E)-1-(2-hydroxy-3-{[2-hydroxybenzylidene]amino}phenyl)ethan-1-one, C15H13NO3
- Crystal structure of bis(μ2-acetato-κ2O:O′)-bis{μ2-4-chloro-2-(8-(4-(diethylamino)-2-oxidophenyl)-3,6-dioxa-2,7-diazaocta-1,7-dien-1-yl)phenolato-κ6O:O,N,N′,O′′:O′′}tricobalt(II), C44H49Cl2Co3N6O12
- Crystal structure of bis{μ2-2,4-dichloro-6-(8-(4-(diethylamino)-2-oxidophenyl)-3,6-dioxa-2,7-diazaocta-1,7-dien-1-yl)phenolato-κ5O:O,N,N′,O′}dicobalt(II) acetone solvate, C43H48Br4Co2N6O9
- Synthesis and crystal structure of bis(μ2-acetato-κ2O:O′)-bis{μ2-4-chloro-2-(8-(4-(diethylamino)-2-oxidophenyl)-3,6-dioxa-2,7-diazaocta-1,7-dien-1-yl)phenolato-κ6O:O,N,N′,O′:O′}trizinc(II), C44H49Cl2N6O12Zn3
- Crystal structure of bis{5-(N,N′-diethylamine)-5′-methoxy -2,2′-[ethylenediyldioxybis(nitrilomethylidyne)]diphenolato}-bis(μ2-acetato-κ2O:O′)trizinc(II), C46H56Zn3N6O14
- Crystal structure of tris(cyano-(hydrogen tris(3,5-dimethylpyrazolyl)borate))-iron(III) 4-methoxypyridinium monohydrate, C24H32BN10O2Fe
- Synthesis and crystal structure of bis{1-(((4-(1-(hydroxyimino)ethyl)phenyl)imino)methyl)naphthalen-2-olato-κ2O,N}copper(II), C38H30CuN4O4
- Crystal structure of (E)-1-(4-{[(E)-2-Hydroxy-1-naphthalenylmethylene] amino}phenyl)ethanone oxime, C19H16N2O2
- The pseudosymmetric crystal structure of 2-(2-naphthalenyl)-3-nitro-2H-1-benzopyran, C38H26N2O6
- Crystal structure of 2-benzoyl-3-(4-methoxyphenyl)cyclopropane-1,1-dicarbonitrile, C19H14N2O2
- N′-(5-ethoxycarbonyl-3,4-dimethyl-pyrrol-2-yl-methylidene)-4-hydroxybenzohydrazide monohydrate, C17H21N3O5
- The crystal structure of carbonyl-[4-(2,4-dichlorophenylamino)pent-3-en-2-onato-κ2N,O]-(triphenylphosphine-κP)rhodium(I), RhC30H25Cl2NO2P
- Crystal structure of (E)-5-(diethylamino)-2-(((1,1,2-trihydroxyethyl)iminio)methyl)phenolate, C15H24N2O4
- Crystal structure of poly[(μ2-1,4-bis((2-ethyl-1H-benzo[d]imidazol-1-yl)methyl)benzene-κ2N:N′)(μ2-isophthalato-κ4O,O′:O′′,O′′′)cadmium(II)], C34H30N4O4Cd
- Crystal structure of 5,6-Dihydro-9,10-dimethoxybenzo[g]-1,3-benzodioxolo[5,6-a]chinolizinium 3-(4-hydroxyphenyl)-4-oxo-4H-chromen-7-olate - methanol - water (1/1/1), C36H33NO10
- A single crystal study on 2-(methylcarbamoyl)benzoic acid, C9H9NO3
- Crystal structure of the salt 1,1′-(ethane-1,2-diyl)bis(1,4-diazabicyclo[2.2.2]octan-1-ium) diperchlorate, C14H28N4(ClO4)2
- Crystal structure of 2,5-bis((E)-2-(trifluoromethyl)benzylidene)cyclopentan-1-one, C21H14F6O
- Crystal structure of catena-poly[(μ2-benzene-1,4-dicarboxylato-κ2O:O′)-(1-ethyl-6-fluoro-7-(4-methylpiperazin-1-ium-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylato-κ2O,O′)zinc(II)] 1.25 hydrate, C25H26.5N3O8.25FZn
- Crystal structure of 1-methyl-1,4-diazabicyclo[2.2.2]octan-1-ium poly[aqua-bis(μ2-perchlorato-κ3O,O′:O′′)sodium], C7H17Cl2N2NaO9
- Crystal structure of trimethyammonium 2,6-dicarboxyisonicotinate monohydrate, C11H16N2O7
- Crystal structure of dodecaguanidinium bis(tetrapropylammonium) heptacarbonate, C43H128N38O21
- Crystal structure of poly[tetraaqua-bis(μ6-benzene-1,2,4,5-tetracarboxylato)nickel(II)diyttrium(III)]dihydrate, C20H16NiO22Y2
- Halogen bonds and π–π interactions in the crystal structure of 1,3,5-trifluoro-2,4,6-triiodobenzene–N,N-dimethylformamide (1/1), C9H7F3I3NO
- Crystal structure of guanidinium tetraethylammonium carbonate dihydrate, C10H30N4O5
- The crystal structure of oxonium chlorido-ethylenediaminetetraactetotin(IV) hydrate, C10H17ClN2O10Sn
- Crystal structure of 8-((E)-((4-((E)-1-((benzyloxy)imino)ethyl)phenyl)imino)methyl)-7-hydroxy-4-methyl-2H-chromen-2-one, C26H22N2O4
- Crystal structure of bis{5-(diethylamino)-2-(((2-oxo-2H-chromen-6-yl)imino)methyl)phenolato-κ2O,N}copper(II), C40H38CuN4O6
- Crystal structure of fac-(acetylacetonato-κ2O,O′)tricarbonyl(tri(p-tolyl)phosphine-κP)rhenium(I), C29H28O5PRe
- Crystal structure of fac-(acetylacetonato-κ2O,O′)tricarbonyl(benzyldiphenylphosphine-κP)rhenium(I), C27H24O5PRe
- Crystal structure of triethylammonium bis{3-(((3-oxidonaphthalen-1-yl)methylene)amino)-2-oxo-2H-chromen-4-olato-κ3O,N,O′}manganese(III), C46H38MnN3O8
- Crystal structure of bis(N,N,N-ethyldimethylethanaminium) bis(heptaselenido-κ2Se1,Se7)palladate(II), C12H32N2PdSe14
- Crystal structure of bis(4-(1H-pyrazol-5-yl)pyridine-κ2N:N′)-bis(2-(2-((2,6-difluorophenyl)amino)phenyl)acetate-κO)cadmium(II), C44H34N8CdCl4O4
- Crystal structure of 4,4′-(1,4-phenylene)bis(pyridin-1-ium) catena-poly[diaqua-bis(μ2-3′,5′-dicarboxy-[1,1′-biphenyl]-2,5-dicarboxylato-κ2O:O′] dihydrate, C48H42O22N2Ca
- The crystal structure of (S)-2-benzylsuccinic acid, C11H12O4
- Crystal structure of poly[aqua-(μ3-4-(pyridin-4-yl)isophthalato-κ4O,O′:O′′:O′′′)-(μ2-5-carboxy-2-(pyridin-4-yl)benzoato-κ2O:O′)yttrium(III)], C26H17N2O9Y
- Crystal structure of ethyl 1-(3,4-dimethylphenyl)-5-methyl-1H-1,2,3-triazole-4-carboxylate, C14H17N3O2
- Crystal structure of tetrakis(methanol-κO)-bis{μ2-3-((4-methoxy-2-oxidobenzylidene)amino)-2-oxo-2H-chromen-4-olate-κ4O,N;O′:O′}dizinc(II), C38H38Zn2N2O14
- Crystal structure of bromido(4,4′-dimethoxy-2,2′-bipyridine-κ2N,N′)(isopropyl(diphenyl)phosphane-κP)copper(I), C27H29BrCuN2O2P
- Crystal structure of bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane)-9,9-dioctylfluorene, C41H64B2O4
- Crystal structure of 11-oxo-2,3,6,7-tetrahydro-1H,5H,11H-pyrano[2,3-f]pyrido[3,2,1-ij]quinoline-10-carbaldehyde - a julolidine derivative, C16H15NO3
- Crystal structure of 1,3-dimethyl-5,5-dibenzylbarbituric acid, C20H20N2O3
- Crystal structure of bis(N,N,N-trimethylethanaminium) poly[bis(μ2-heptaselenido-κ2Se1,Se7)palladate(II)], C10H28N2PdSe14
- Crystal structure of 1-{4-[(5-Chloro-2-hydroxy-benzylidene)amino]phenyl} ethanone O-ethyl-oxime, C17H17ClN2O2
- The crystal structure of methyl N-(4-bromophenyl)carbamate, C8H8BrNO2
- Synthesis and crystal structure of 1-{4-[(2-hydroxy-benzylidene)amino]phenyl}ethanone oxime, C15H14N2O2
- Crystal structure of 1,1′-butanebis(3-methyl-1H-imidazol-3-ium)bis(hexafluorophosphate), C12H20F12N4P2
- (E)-N-benzylidene-3-(benzylthio)-5-p-tolyl-4H-1,2,4-triazol-4-amine, C23H20N4S
- Crystal structure of ethyl 5-methyl-1-(pyridin-3-yl)-1H-1,2,3-triazole-4-carboxylate, C11H12N4O2
- Crystal structure of 5,5-difluoro-10-(4-fluorophenyl)-1,3,7,9-tetramethyl-5H-4l4,5l4-dipyrrolo[1,2-c:2′,1′-f][1,3,2]diazaborinine - a Z′ = 3 structure, C19H18B2F3N2
- The crystal structure of the Matrine derivative: 12-(1H-indol-1-yl)dodecahydro-1H,5H,10H-dipyrido[2,1-f:3′,2′,1′-ij][1,6]naphthyridin-10-one hydrate, C23H29N3O
- The crystal structure of tris(μ2-1,3-bis(4,4,4-trifluoro-3-oxido-1-(oxo)but-2-en-1-yl)phenyl-κ4O,O′:O′′,O′′′)-bis(1,2-dimethoxyethane-κ2O,O′)dilutetium(III), C50H38F18Lu2O16
- The crystal structure of (Z)-2-(3-(2-(4-chlorobenzoyl)hydrazono)-2-oxoindolin-1-yl) acetic acid, C17H12ClN3O4
- Synthesis and crystal structure of trans-tetraqua-bis(2-(((7-hydroxy-3-(4-hydroxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)ammonio)acetato-κO)cobalt(II) hexahydrate, C36H48CoN2O28S2
- The crystal structure of N,N-dimethyl-2,6-di-p-tolylpyrimidin-4-amine, C20H21N3
- The crystal structure (E)-4-methyl-N′-(2-nitrobenzylidene)benzenesulfonohydrazide, C14H13N3O4S
- Crystal structure of catena-poly[(μ2-1,3-bis(benzimidazol-1-yl)propane κ2N:N′)-(μ2-5-methoxyisophthalato-κ2O:O′)zinc(II)] hydrate, C26H24ZnN4O6
- The crystal structure of (E)-N′-(quinolin-2-ylmethylene)furan-2-carbohydrazide monohydrate, C15H13N3O3
- Crystal structure of 2,8-diphenyl-3,7,9-trioxa-1-azaspiro[4.5]dec-1-ene, C18H17N1O3
- Crystal structure of diethyl 2-(4-chlorophenyl)-1,3-dioxane-5,5-dicarboxylate, C16H19ClO6
- Crystal structure of 1-(carboxymethyl)-1H-benzo[d][1,2,3]triazole 3-oxide, C8H7N3O3
- Crystal structure of (acetylacetonato-κ2O,O′)-(2-amino-6-chlorobenzoato-κO)-oxido(1,10-phenanthroline-κ2N,N′)vanadium(IV) – trichloromethane (1/1)
- Crystal structure of (1E,4E)-1,5-bis(4-chlorophenyl)penta-1,4-dien-3-one, C17H12Cl2O
- The crystal structure of trans-dibromido-bis(pyridine-κN)platinum(II), C10H10Br2N2Pt

