Abstract
C16H15NO3, monoclinic, P21/c (no. 14), a = 9.802(1) Å, b = 14.492(1) Å, c = 9.667(1) Å, β = 112.027(1)°, V = 1272.9 Å3, Z = 4, Rgt(F) = 0.0422, wRref(F2) = 0.1249, T = 296 K.
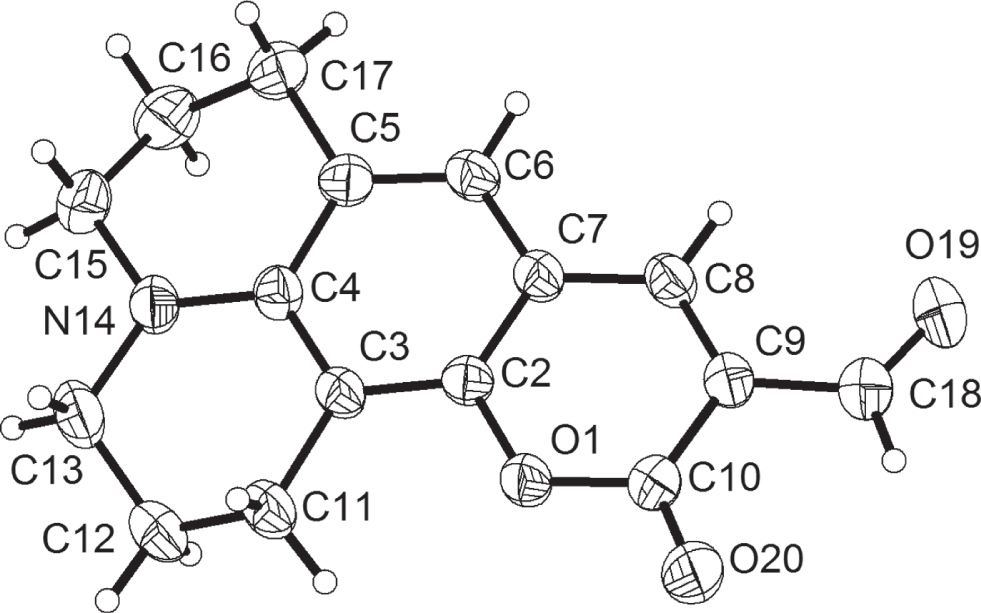
The crystal structure is shown in the figure. Displacement ellipsoids are drawn at the 50% probability level and H atoms are shown as small spheres of arbitrary radii. Tables 1 and 2 contain details on crystal structure and measurement conditions and a list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | purple block |
| Size: | 0.22 × 0.19 × 0.18 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.10 mm−1 |
| Diffractometer, scan mode: | Bruker SMART, φ and ω-scans |
| 2θmax, completeness: | 28.3°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 34108, 3173, 0.062 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 2240 |
| N(param)refined: | 186 |
| Programs: | Bruker [1], SHELX [2], Diamond [3], ORTEP, WinGX [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| O1 | 0.47668(10) | 0.11771(5) | 0.18064(10) | 0.0376(2) |
| C2 | 0.55368(13) | 0.18839(8) | 0.14740(13) | 0.0311(3) |
| C3 | 0.66213(13) | 0.16271(8) | 0.09744(13) | 0.0325(3) |
| C4 | 0.74475(13) | 0.23394(8) | 0.06400(12) | 0.0320(3) |
| C5 | 0.70775(14) | 0.32889(9) | 0.07403(13) | 0.0351(3) |
| C6 | 0.60049(14) | 0.34942(8) | 0.12724(13) | 0.0359(3) |
| H6 | 0.5789 | 0.4110 | 0.1367 | 0.043* |
| C7 | 0.52149(13) | 0.28073(8) | 0.16821(13) | 0.0326(3) |
| C8 | 0.41771(13) | 0.29746(8) | 0.23347(14) | 0.0348(3) |
| H8 | 0.3985 | 0.3580 | 0.2525 | 0.042* |
| C9 | 0.34402(13) | 0.22727(9) | 0.26989(13) | 0.0346(3) |
| C10 | 0.36769(14) | 0.13314(9) | 0.23612(14) | 0.0383(3) |
| C11 | 0.69726(15) | 0.06282(9) | 0.08249(17) | 0.0450(3) |
| H11A | 0.6368 | 0.0407 | −0.0162 | 0.054* |
| H11B | 0.6749 | 0.0261 | 0.1552 | 0.054* |
| C12 | 0.85793(16) | 0.05188(10) | 0.10690(17) | 0.0496(4) |
| H12A | 0.9181 | 0.0650 | 0.2103 | 0.060* |
| H12B | 0.8768 | −0.0113 | 0.0859 | 0.060* |
| C13 | 0.89856(17) | 0.11638(9) | 0.00698(17) | 0.0484(4) |
| H13A | 1.0034 | 0.1119 | 0.0291 | 0.058* |
| H13B | 0.8471 | 0.0982 | −0.0962 | 0.058* |
| N14 | 0.86116(12) | 0.21214(7) | 0.02647(12) | 0.0389(3) |
| C15 | 0.94382(16) | 0.28225(10) | −0.01761(16) | 0.0454(3) |
| H15A | 0.9008 | 0.2901 | −0.1250 | 0.054* |
| H15B | 1.0444 | 0.2613 | 0.0085 | 0.054* |
| C16 | 0.94457(15) | 0.37357(10) | 0.05624(17) | 0.0506(4) |
| H16A | 0.9925 | 0.4195 | 0.0170 | 0.061* |
| H16B | 0.9999 | 0.3682 | 0.1627 | 0.061* |
| C17 | 0.78955(15) | 0.40408(9) | 0.02897(17) | 0.0455(3) |
| H17A | 0.7386 | 0.4184 | −0.0759 | 0.055* |
| H17B | 0.7920 | 0.4595 | 0.0861 | 0.055* |
| C18 | 0.24370(16) | 0.24397(11) | 0.34589(15) | 0.0435(3) |
| H18 | 0.2000(19) | 0.1914(12) | 0.3647(18) | 0.069(5)* |
| O19 | 0.21677(11) | 0.31875(7) | 0.38520(11) | 0.0536(3) |
| O20 | 0.30346(12) | 0.06559(7) | 0.25281(13) | 0.0588(3) |
Source of material
The title compound was synthesized according to standard Vilsmeier-Haack conditions, under which formylation at 3-position of julolidine [2,3]quinolone occurred efficiently [5, 6] . All chemicals used were commercially available of AR grade, and were used as received without further purification. The purple crystals of the title compound were obtained by slow evaporation of an ethanol solution at room temperature.
Experimental details
The hydrogen atoms were placed geometrically and refined using a riding model with d(C—H) = 0.93 Å (aromatic), 0.97 Å (-CH2-), 0.925 Å (-COH). Uiso(H) = 1.2 Ueq(C) for COH and CH2 groups.
Discussion
Fluorescent molecules fascinate the physicists and chemists owing to the application of fluorescence signal in smart material artificial intelligence. Intensive effort and development have been made and engineered according to requirement of device fabrication and material research [7], [8], [9], [10].
The coumarin moiety (O1/C2/C3/C4/C5/C6/C7/C8/C9/C10) is planar with the mean deviation 0.043 Å. However, the adjacent aliphatic moiety is twisted out of the molecular plane due to the envelope conformation. Two carbonyl oxygen atoms are present in molecule, which are polarized and are gathered by more electron density. Chances are that oxygen atoms may involve H⋯O interactions. Actually, C—H⋯O, C–H⋯π, and π⋯π interactions are predominant weak forces that joint the molecule into a crystal. All the oxygen atoms including O1, O19, and O20 are involved in C—H⋯O interactions: C11–H11A⋯O1i1 (i1: x, y, 1 + z), C12—H12B⋯O19i2 (i2: x, 1/2 − y, 1/2 + z), C15–H15B⋯O19i2, C16—H16B⋯O19i2, C6–H6⋯O20i3 (i3: x, y, 1 + z), and C17—H17B⋯O20i3. The distance of C⋯O varied from 3.53 to 3.75 Å, which falls inside the normal C⋯O interval of C–H⋯O interactions [11]. The H⋯O distances range from 2.50 to 2.78 Å and the C—H⋯O angles vary from 138° to 153°, indicating very weak H⋯O interactions. It is worthy to note that the H⋯O interactions with O19 and O20 involve in the crystal are more complicated than the interaction with O1, in which one oxygen atom involve two or more hydrogen bonds. The carbonyl C18 = O19, hovering above the carbon ring (O1/C2/C7/C8/C9C10), configure the π⋯π interactions.
Apart from the interactions mentioned above, more weaker interactions that cannot be neglected are C—H⋯π interactions, to which hydrogen atoms of methylene contribute and interact with the coumarin ring.
Acknowledgement
This work was supported by the National Natural Science Foundation of China (21272060). This work was supported by the industrial Fundamental Technology Development Program (10076350) funded by the Ministry of Trade, Industry and Energy (MOTIE) of Korea.
References
Bruker. APEX2 (Version 2.1.4), SAINT (Version 7.34A) and SADABS (Version 2004/1). Bruker AXS Inc., Madison, Wisconsin, USA (2005).Suche in Google Scholar
Sheldrick, G. M.: A short history of SHELX. Acta Crystallogr. A64 (2008) 112–122.10.1107/S0108767307043930Suche in Google Scholar PubMed
Brandenburg, K.; Putz, H.: Diamond-crystal and molecular structure visualization. Crystal impact GbR, Rathausgasse 30, D-53111 Bonn, Germany (2005).Suche in Google Scholar
Farrugia, L. J.: WinGX and ORTEP for windows: an update. Appl. Cryst. 45 (2012) 849–854.10.1107/S0021889812029111Suche in Google Scholar
Lee, B.; Chen, S.; Heinis, C.; Scopelliti, R.; Severin, K.: Pattern-based sensing of peptides and aminoglycosides with a single molecular probe. Org. Lett. 15 (2013) 3456–3459.10.1021/ol401495cSuche in Google Scholar PubMed
Yuan, L.; Lin, W.; Song, J.; Yang, Y.: Development of an ICT-based ratiometric fluorescent hypochlorite probe suitable for living cell imaging. Chemm. Commun. 47 (2011) 12691–12693.10.1039/c1cc15762kSuche in Google Scholar PubMed
Li, X.; Ji, G., Son, Y.-A.: Tunable emission of hydrazine-containing bipyrrole fluorine-boron complexes by linear extension. Dye Pigments 124 (2016) 232–240.10.1016/j.dyepig.2015.09.022Suche in Google Scholar
Qu, D.-H.; Wang, Q.-C.; Zhang, Q.-W.; Ma, X.; Tian, H.: Photoresponsive host-guest functional systems. Chem. Rev. 115 (2015) 7543–7588.10.1021/cr5006342Suche in Google Scholar PubMed
Li, H.; Qu, D.-H.: Recent advances in new-type molecular switches. Sci. China Chem. 58 (2015) 916–921.10.1007/s11426-015-5417-7Suche in Google Scholar
Li, X.; Son, Y.-A.: Efficient luminescence from easily prepared fluorine-boron core complexes based on benzothiazole and benzoxazole. Dye Pigments 107 (2014) 182–187.10.1016/j.dyepig.2014.04.001Suche in Google Scholar
Desiraju, G. R.: The C–H⋯O hydrogen bond in crystals: What is it? Acc. Chem. Res. 24 (1991) 290–296.10.1021/ar00010a002Suche in Google Scholar
©2017 Yingfan Liu et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 License.
Artikel in diesem Heft
- Cover and Frontmatter
- Crystal structure of potassium 1-methyl-1H-1,2,3,4-tetrazole-5-thiolate, C2H3N4SK
- Crystal structure of bis(3-(3-ethylureido)-N,N-dimethylpropan-1-aminium) bis (μ3-2-(hydroxymethyl)-2-(oxidomethyl)propane-1,3-bis(olato))-(μ6-oxo)-hexakis(μ2-oxo)-hexaoxo-hexavanadium(V) – dichloromethane (1/1), C27H60Cl2N6O23V6
- Crystal structure of bis(μ3-methanolato-κ3O:O:O)-bis(μ2-methanolato-κ2O:O)-dimethanol-bis{6,6′-(1,3-dihydroxyl-2-acetylpropane-1,3-diyl)bis(2-chloro-4-bromophenolato)}tetramanganese(III) C40H40Br4Cl4Mn4O16
- Synthesis and crystal structure of tetrakis(μ2-methanolato)-dimethanol-bis(μ2-2-acetyl-1,3-bis(3,5-dibromo-2-oxidophenyl)propane-1,3-bis(olato)-κ5O1,O2,O3:O3,O4,O5)tetramanganese(III), C40H40Br8Mn4O16
- Synthesis and crystal structure tetrakis(μ2-methanolato)-dimethanol-bis(μ2-2-acetyl-1,3-bis(3,5-dichloro-2-oxidophenyl)propane-1,3-bis(olato)-κ5O1,O2,O3:O3,O4,O5), tetramanganese(III), C40H40Cl8Mn4O16
- Crystal structure of bis{5-methoxy-2-((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O)}copper(II), C34H34CuN4O6
- Crystal structure of (E)-1-(2-hydroxy-3-{[2-hydroxybenzylidene]amino}phenyl)ethan-1-one, C15H13NO3
- Crystal structure of bis(μ2-acetato-κ2O:O′)-bis{μ2-4-chloro-2-(8-(4-(diethylamino)-2-oxidophenyl)-3,6-dioxa-2,7-diazaocta-1,7-dien-1-yl)phenolato-κ6O:O,N,N′,O′′:O′′}tricobalt(II), C44H49Cl2Co3N6O12
- Crystal structure of bis{μ2-2,4-dichloro-6-(8-(4-(diethylamino)-2-oxidophenyl)-3,6-dioxa-2,7-diazaocta-1,7-dien-1-yl)phenolato-κ5O:O,N,N′,O′}dicobalt(II) acetone solvate, C43H48Br4Co2N6O9
- Synthesis and crystal structure of bis(μ2-acetato-κ2O:O′)-bis{μ2-4-chloro-2-(8-(4-(diethylamino)-2-oxidophenyl)-3,6-dioxa-2,7-diazaocta-1,7-dien-1-yl)phenolato-κ6O:O,N,N′,O′:O′}trizinc(II), C44H49Cl2N6O12Zn3
- Crystal structure of bis{5-(N,N′-diethylamine)-5′-methoxy -2,2′-[ethylenediyldioxybis(nitrilomethylidyne)]diphenolato}-bis(μ2-acetato-κ2O:O′)trizinc(II), C46H56Zn3N6O14
- Crystal structure of tris(cyano-(hydrogen tris(3,5-dimethylpyrazolyl)borate))-iron(III) 4-methoxypyridinium monohydrate, C24H32BN10O2Fe
- Synthesis and crystal structure of bis{1-(((4-(1-(hydroxyimino)ethyl)phenyl)imino)methyl)naphthalen-2-olato-κ2O,N}copper(II), C38H30CuN4O4
- Crystal structure of (E)-1-(4-{[(E)-2-Hydroxy-1-naphthalenylmethylene] amino}phenyl)ethanone oxime, C19H16N2O2
- The pseudosymmetric crystal structure of 2-(2-naphthalenyl)-3-nitro-2H-1-benzopyran, C38H26N2O6
- Crystal structure of 2-benzoyl-3-(4-methoxyphenyl)cyclopropane-1,1-dicarbonitrile, C19H14N2O2
- N′-(5-ethoxycarbonyl-3,4-dimethyl-pyrrol-2-yl-methylidene)-4-hydroxybenzohydrazide monohydrate, C17H21N3O5
- The crystal structure of carbonyl-[4-(2,4-dichlorophenylamino)pent-3-en-2-onato-κ2N,O]-(triphenylphosphine-κP)rhodium(I), RhC30H25Cl2NO2P
- Crystal structure of (E)-5-(diethylamino)-2-(((1,1,2-trihydroxyethyl)iminio)methyl)phenolate, C15H24N2O4
- Crystal structure of poly[(μ2-1,4-bis((2-ethyl-1H-benzo[d]imidazol-1-yl)methyl)benzene-κ2N:N′)(μ2-isophthalato-κ4O,O′:O′′,O′′′)cadmium(II)], C34H30N4O4Cd
- Crystal structure of 5,6-Dihydro-9,10-dimethoxybenzo[g]-1,3-benzodioxolo[5,6-a]chinolizinium 3-(4-hydroxyphenyl)-4-oxo-4H-chromen-7-olate - methanol - water (1/1/1), C36H33NO10
- A single crystal study on 2-(methylcarbamoyl)benzoic acid, C9H9NO3
- Crystal structure of the salt 1,1′-(ethane-1,2-diyl)bis(1,4-diazabicyclo[2.2.2]octan-1-ium) diperchlorate, C14H28N4(ClO4)2
- Crystal structure of 2,5-bis((E)-2-(trifluoromethyl)benzylidene)cyclopentan-1-one, C21H14F6O
- Crystal structure of catena-poly[(μ2-benzene-1,4-dicarboxylato-κ2O:O′)-(1-ethyl-6-fluoro-7-(4-methylpiperazin-1-ium-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylato-κ2O,O′)zinc(II)] 1.25 hydrate, C25H26.5N3O8.25FZn
- Crystal structure of 1-methyl-1,4-diazabicyclo[2.2.2]octan-1-ium poly[aqua-bis(μ2-perchlorato-κ3O,O′:O′′)sodium], C7H17Cl2N2NaO9
- Crystal structure of trimethyammonium 2,6-dicarboxyisonicotinate monohydrate, C11H16N2O7
- Crystal structure of dodecaguanidinium bis(tetrapropylammonium) heptacarbonate, C43H128N38O21
- Crystal structure of poly[tetraaqua-bis(μ6-benzene-1,2,4,5-tetracarboxylato)nickel(II)diyttrium(III)]dihydrate, C20H16NiO22Y2
- Halogen bonds and π–π interactions in the crystal structure of 1,3,5-trifluoro-2,4,6-triiodobenzene–N,N-dimethylformamide (1/1), C9H7F3I3NO
- Crystal structure of guanidinium tetraethylammonium carbonate dihydrate, C10H30N4O5
- The crystal structure of oxonium chlorido-ethylenediaminetetraactetotin(IV) hydrate, C10H17ClN2O10Sn
- Crystal structure of 8-((E)-((4-((E)-1-((benzyloxy)imino)ethyl)phenyl)imino)methyl)-7-hydroxy-4-methyl-2H-chromen-2-one, C26H22N2O4
- Crystal structure of bis{5-(diethylamino)-2-(((2-oxo-2H-chromen-6-yl)imino)methyl)phenolato-κ2O,N}copper(II), C40H38CuN4O6
- Crystal structure of fac-(acetylacetonato-κ2O,O′)tricarbonyl(tri(p-tolyl)phosphine-κP)rhenium(I), C29H28O5PRe
- Crystal structure of fac-(acetylacetonato-κ2O,O′)tricarbonyl(benzyldiphenylphosphine-κP)rhenium(I), C27H24O5PRe
- Crystal structure of triethylammonium bis{3-(((3-oxidonaphthalen-1-yl)methylene)amino)-2-oxo-2H-chromen-4-olato-κ3O,N,O′}manganese(III), C46H38MnN3O8
- Crystal structure of bis(N,N,N-ethyldimethylethanaminium) bis(heptaselenido-κ2Se1,Se7)palladate(II), C12H32N2PdSe14
- Crystal structure of bis(4-(1H-pyrazol-5-yl)pyridine-κ2N:N′)-bis(2-(2-((2,6-difluorophenyl)amino)phenyl)acetate-κO)cadmium(II), C44H34N8CdCl4O4
- Crystal structure of 4,4′-(1,4-phenylene)bis(pyridin-1-ium) catena-poly[diaqua-bis(μ2-3′,5′-dicarboxy-[1,1′-biphenyl]-2,5-dicarboxylato-κ2O:O′] dihydrate, C48H42O22N2Ca
- The crystal structure of (S)-2-benzylsuccinic acid, C11H12O4
- Crystal structure of poly[aqua-(μ3-4-(pyridin-4-yl)isophthalato-κ4O,O′:O′′:O′′′)-(μ2-5-carboxy-2-(pyridin-4-yl)benzoato-κ2O:O′)yttrium(III)], C26H17N2O9Y
- Crystal structure of ethyl 1-(3,4-dimethylphenyl)-5-methyl-1H-1,2,3-triazole-4-carboxylate, C14H17N3O2
- Crystal structure of tetrakis(methanol-κO)-bis{μ2-3-((4-methoxy-2-oxidobenzylidene)amino)-2-oxo-2H-chromen-4-olate-κ4O,N;O′:O′}dizinc(II), C38H38Zn2N2O14
- Crystal structure of bromido(4,4′-dimethoxy-2,2′-bipyridine-κ2N,N′)(isopropyl(diphenyl)phosphane-κP)copper(I), C27H29BrCuN2O2P
- Crystal structure of bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane)-9,9-dioctylfluorene, C41H64B2O4
- Crystal structure of 11-oxo-2,3,6,7-tetrahydro-1H,5H,11H-pyrano[2,3-f]pyrido[3,2,1-ij]quinoline-10-carbaldehyde - a julolidine derivative, C16H15NO3
- Crystal structure of 1,3-dimethyl-5,5-dibenzylbarbituric acid, C20H20N2O3
- Crystal structure of bis(N,N,N-trimethylethanaminium) poly[bis(μ2-heptaselenido-κ2Se1,Se7)palladate(II)], C10H28N2PdSe14
- Crystal structure of 1-{4-[(5-Chloro-2-hydroxy-benzylidene)amino]phenyl} ethanone O-ethyl-oxime, C17H17ClN2O2
- The crystal structure of methyl N-(4-bromophenyl)carbamate, C8H8BrNO2
- Synthesis and crystal structure of 1-{4-[(2-hydroxy-benzylidene)amino]phenyl}ethanone oxime, C15H14N2O2
- Crystal structure of 1,1′-butanebis(3-methyl-1H-imidazol-3-ium)bis(hexafluorophosphate), C12H20F12N4P2
- (E)-N-benzylidene-3-(benzylthio)-5-p-tolyl-4H-1,2,4-triazol-4-amine, C23H20N4S
- Crystal structure of ethyl 5-methyl-1-(pyridin-3-yl)-1H-1,2,3-triazole-4-carboxylate, C11H12N4O2
- Crystal structure of 5,5-difluoro-10-(4-fluorophenyl)-1,3,7,9-tetramethyl-5H-4l4,5l4-dipyrrolo[1,2-c:2′,1′-f][1,3,2]diazaborinine - a Z′ = 3 structure, C19H18B2F3N2
- The crystal structure of the Matrine derivative: 12-(1H-indol-1-yl)dodecahydro-1H,5H,10H-dipyrido[2,1-f:3′,2′,1′-ij][1,6]naphthyridin-10-one hydrate, C23H29N3O
- The crystal structure of tris(μ2-1,3-bis(4,4,4-trifluoro-3-oxido-1-(oxo)but-2-en-1-yl)phenyl-κ4O,O′:O′′,O′′′)-bis(1,2-dimethoxyethane-κ2O,O′)dilutetium(III), C50H38F18Lu2O16
- The crystal structure of (Z)-2-(3-(2-(4-chlorobenzoyl)hydrazono)-2-oxoindolin-1-yl) acetic acid, C17H12ClN3O4
- Synthesis and crystal structure of trans-tetraqua-bis(2-(((7-hydroxy-3-(4-hydroxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)ammonio)acetato-κO)cobalt(II) hexahydrate, C36H48CoN2O28S2
- The crystal structure of N,N-dimethyl-2,6-di-p-tolylpyrimidin-4-amine, C20H21N3
- The crystal structure (E)-4-methyl-N′-(2-nitrobenzylidene)benzenesulfonohydrazide, C14H13N3O4S
- Crystal structure of catena-poly[(μ2-1,3-bis(benzimidazol-1-yl)propane κ2N:N′)-(μ2-5-methoxyisophthalato-κ2O:O′)zinc(II)] hydrate, C26H24ZnN4O6
- The crystal structure of (E)-N′-(quinolin-2-ylmethylene)furan-2-carbohydrazide monohydrate, C15H13N3O3
- Crystal structure of 2,8-diphenyl-3,7,9-trioxa-1-azaspiro[4.5]dec-1-ene, C18H17N1O3
- Crystal structure of diethyl 2-(4-chlorophenyl)-1,3-dioxane-5,5-dicarboxylate, C16H19ClO6
- Crystal structure of 1-(carboxymethyl)-1H-benzo[d][1,2,3]triazole 3-oxide, C8H7N3O3
- Crystal structure of (acetylacetonato-κ2O,O′)-(2-amino-6-chlorobenzoato-κO)-oxido(1,10-phenanthroline-κ2N,N′)vanadium(IV) – trichloromethane (1/1)
- Crystal structure of (1E,4E)-1,5-bis(4-chlorophenyl)penta-1,4-dien-3-one, C17H12Cl2O
- The crystal structure of trans-dibromido-bis(pyridine-κN)platinum(II), C10H10Br2N2Pt
Artikel in diesem Heft
- Cover and Frontmatter
- Crystal structure of potassium 1-methyl-1H-1,2,3,4-tetrazole-5-thiolate, C2H3N4SK
- Crystal structure of bis(3-(3-ethylureido)-N,N-dimethylpropan-1-aminium) bis (μ3-2-(hydroxymethyl)-2-(oxidomethyl)propane-1,3-bis(olato))-(μ6-oxo)-hexakis(μ2-oxo)-hexaoxo-hexavanadium(V) – dichloromethane (1/1), C27H60Cl2N6O23V6
- Crystal structure of bis(μ3-methanolato-κ3O:O:O)-bis(μ2-methanolato-κ2O:O)-dimethanol-bis{6,6′-(1,3-dihydroxyl-2-acetylpropane-1,3-diyl)bis(2-chloro-4-bromophenolato)}tetramanganese(III) C40H40Br4Cl4Mn4O16
- Synthesis and crystal structure of tetrakis(μ2-methanolato)-dimethanol-bis(μ2-2-acetyl-1,3-bis(3,5-dibromo-2-oxidophenyl)propane-1,3-bis(olato)-κ5O1,O2,O3:O3,O4,O5)tetramanganese(III), C40H40Br8Mn4O16
- Synthesis and crystal structure tetrakis(μ2-methanolato)-dimethanol-bis(μ2-2-acetyl-1,3-bis(3,5-dichloro-2-oxidophenyl)propane-1,3-bis(olato)-κ5O1,O2,O3:O3,O4,O5), tetramanganese(III), C40H40Cl8Mn4O16
- Crystal structure of bis{5-methoxy-2-((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O)}copper(II), C34H34CuN4O6
- Crystal structure of (E)-1-(2-hydroxy-3-{[2-hydroxybenzylidene]amino}phenyl)ethan-1-one, C15H13NO3
- Crystal structure of bis(μ2-acetato-κ2O:O′)-bis{μ2-4-chloro-2-(8-(4-(diethylamino)-2-oxidophenyl)-3,6-dioxa-2,7-diazaocta-1,7-dien-1-yl)phenolato-κ6O:O,N,N′,O′′:O′′}tricobalt(II), C44H49Cl2Co3N6O12
- Crystal structure of bis{μ2-2,4-dichloro-6-(8-(4-(diethylamino)-2-oxidophenyl)-3,6-dioxa-2,7-diazaocta-1,7-dien-1-yl)phenolato-κ5O:O,N,N′,O′}dicobalt(II) acetone solvate, C43H48Br4Co2N6O9
- Synthesis and crystal structure of bis(μ2-acetato-κ2O:O′)-bis{μ2-4-chloro-2-(8-(4-(diethylamino)-2-oxidophenyl)-3,6-dioxa-2,7-diazaocta-1,7-dien-1-yl)phenolato-κ6O:O,N,N′,O′:O′}trizinc(II), C44H49Cl2N6O12Zn3
- Crystal structure of bis{5-(N,N′-diethylamine)-5′-methoxy -2,2′-[ethylenediyldioxybis(nitrilomethylidyne)]diphenolato}-bis(μ2-acetato-κ2O:O′)trizinc(II), C46H56Zn3N6O14
- Crystal structure of tris(cyano-(hydrogen tris(3,5-dimethylpyrazolyl)borate))-iron(III) 4-methoxypyridinium monohydrate, C24H32BN10O2Fe
- Synthesis and crystal structure of bis{1-(((4-(1-(hydroxyimino)ethyl)phenyl)imino)methyl)naphthalen-2-olato-κ2O,N}copper(II), C38H30CuN4O4
- Crystal structure of (E)-1-(4-{[(E)-2-Hydroxy-1-naphthalenylmethylene] amino}phenyl)ethanone oxime, C19H16N2O2
- The pseudosymmetric crystal structure of 2-(2-naphthalenyl)-3-nitro-2H-1-benzopyran, C38H26N2O6
- Crystal structure of 2-benzoyl-3-(4-methoxyphenyl)cyclopropane-1,1-dicarbonitrile, C19H14N2O2
- N′-(5-ethoxycarbonyl-3,4-dimethyl-pyrrol-2-yl-methylidene)-4-hydroxybenzohydrazide monohydrate, C17H21N3O5
- The crystal structure of carbonyl-[4-(2,4-dichlorophenylamino)pent-3-en-2-onato-κ2N,O]-(triphenylphosphine-κP)rhodium(I), RhC30H25Cl2NO2P
- Crystal structure of (E)-5-(diethylamino)-2-(((1,1,2-trihydroxyethyl)iminio)methyl)phenolate, C15H24N2O4
- Crystal structure of poly[(μ2-1,4-bis((2-ethyl-1H-benzo[d]imidazol-1-yl)methyl)benzene-κ2N:N′)(μ2-isophthalato-κ4O,O′:O′′,O′′′)cadmium(II)], C34H30N4O4Cd
- Crystal structure of 5,6-Dihydro-9,10-dimethoxybenzo[g]-1,3-benzodioxolo[5,6-a]chinolizinium 3-(4-hydroxyphenyl)-4-oxo-4H-chromen-7-olate - methanol - water (1/1/1), C36H33NO10
- A single crystal study on 2-(methylcarbamoyl)benzoic acid, C9H9NO3
- Crystal structure of the salt 1,1′-(ethane-1,2-diyl)bis(1,4-diazabicyclo[2.2.2]octan-1-ium) diperchlorate, C14H28N4(ClO4)2
- Crystal structure of 2,5-bis((E)-2-(trifluoromethyl)benzylidene)cyclopentan-1-one, C21H14F6O
- Crystal structure of catena-poly[(μ2-benzene-1,4-dicarboxylato-κ2O:O′)-(1-ethyl-6-fluoro-7-(4-methylpiperazin-1-ium-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylato-κ2O,O′)zinc(II)] 1.25 hydrate, C25H26.5N3O8.25FZn
- Crystal structure of 1-methyl-1,4-diazabicyclo[2.2.2]octan-1-ium poly[aqua-bis(μ2-perchlorato-κ3O,O′:O′′)sodium], C7H17Cl2N2NaO9
- Crystal structure of trimethyammonium 2,6-dicarboxyisonicotinate monohydrate, C11H16N2O7
- Crystal structure of dodecaguanidinium bis(tetrapropylammonium) heptacarbonate, C43H128N38O21
- Crystal structure of poly[tetraaqua-bis(μ6-benzene-1,2,4,5-tetracarboxylato)nickel(II)diyttrium(III)]dihydrate, C20H16NiO22Y2
- Halogen bonds and π–π interactions in the crystal structure of 1,3,5-trifluoro-2,4,6-triiodobenzene–N,N-dimethylformamide (1/1), C9H7F3I3NO
- Crystal structure of guanidinium tetraethylammonium carbonate dihydrate, C10H30N4O5
- The crystal structure of oxonium chlorido-ethylenediaminetetraactetotin(IV) hydrate, C10H17ClN2O10Sn
- Crystal structure of 8-((E)-((4-((E)-1-((benzyloxy)imino)ethyl)phenyl)imino)methyl)-7-hydroxy-4-methyl-2H-chromen-2-one, C26H22N2O4
- Crystal structure of bis{5-(diethylamino)-2-(((2-oxo-2H-chromen-6-yl)imino)methyl)phenolato-κ2O,N}copper(II), C40H38CuN4O6
- Crystal structure of fac-(acetylacetonato-κ2O,O′)tricarbonyl(tri(p-tolyl)phosphine-κP)rhenium(I), C29H28O5PRe
- Crystal structure of fac-(acetylacetonato-κ2O,O′)tricarbonyl(benzyldiphenylphosphine-κP)rhenium(I), C27H24O5PRe
- Crystal structure of triethylammonium bis{3-(((3-oxidonaphthalen-1-yl)methylene)amino)-2-oxo-2H-chromen-4-olato-κ3O,N,O′}manganese(III), C46H38MnN3O8
- Crystal structure of bis(N,N,N-ethyldimethylethanaminium) bis(heptaselenido-κ2Se1,Se7)palladate(II), C12H32N2PdSe14
- Crystal structure of bis(4-(1H-pyrazol-5-yl)pyridine-κ2N:N′)-bis(2-(2-((2,6-difluorophenyl)amino)phenyl)acetate-κO)cadmium(II), C44H34N8CdCl4O4
- Crystal structure of 4,4′-(1,4-phenylene)bis(pyridin-1-ium) catena-poly[diaqua-bis(μ2-3′,5′-dicarboxy-[1,1′-biphenyl]-2,5-dicarboxylato-κ2O:O′] dihydrate, C48H42O22N2Ca
- The crystal structure of (S)-2-benzylsuccinic acid, C11H12O4
- Crystal structure of poly[aqua-(μ3-4-(pyridin-4-yl)isophthalato-κ4O,O′:O′′:O′′′)-(μ2-5-carboxy-2-(pyridin-4-yl)benzoato-κ2O:O′)yttrium(III)], C26H17N2O9Y
- Crystal structure of ethyl 1-(3,4-dimethylphenyl)-5-methyl-1H-1,2,3-triazole-4-carboxylate, C14H17N3O2
- Crystal structure of tetrakis(methanol-κO)-bis{μ2-3-((4-methoxy-2-oxidobenzylidene)amino)-2-oxo-2H-chromen-4-olate-κ4O,N;O′:O′}dizinc(II), C38H38Zn2N2O14
- Crystal structure of bromido(4,4′-dimethoxy-2,2′-bipyridine-κ2N,N′)(isopropyl(diphenyl)phosphane-κP)copper(I), C27H29BrCuN2O2P
- Crystal structure of bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane)-9,9-dioctylfluorene, C41H64B2O4
- Crystal structure of 11-oxo-2,3,6,7-tetrahydro-1H,5H,11H-pyrano[2,3-f]pyrido[3,2,1-ij]quinoline-10-carbaldehyde - a julolidine derivative, C16H15NO3
- Crystal structure of 1,3-dimethyl-5,5-dibenzylbarbituric acid, C20H20N2O3
- Crystal structure of bis(N,N,N-trimethylethanaminium) poly[bis(μ2-heptaselenido-κ2Se1,Se7)palladate(II)], C10H28N2PdSe14
- Crystal structure of 1-{4-[(5-Chloro-2-hydroxy-benzylidene)amino]phenyl} ethanone O-ethyl-oxime, C17H17ClN2O2
- The crystal structure of methyl N-(4-bromophenyl)carbamate, C8H8BrNO2
- Synthesis and crystal structure of 1-{4-[(2-hydroxy-benzylidene)amino]phenyl}ethanone oxime, C15H14N2O2
- Crystal structure of 1,1′-butanebis(3-methyl-1H-imidazol-3-ium)bis(hexafluorophosphate), C12H20F12N4P2
- (E)-N-benzylidene-3-(benzylthio)-5-p-tolyl-4H-1,2,4-triazol-4-amine, C23H20N4S
- Crystal structure of ethyl 5-methyl-1-(pyridin-3-yl)-1H-1,2,3-triazole-4-carboxylate, C11H12N4O2
- Crystal structure of 5,5-difluoro-10-(4-fluorophenyl)-1,3,7,9-tetramethyl-5H-4l4,5l4-dipyrrolo[1,2-c:2′,1′-f][1,3,2]diazaborinine - a Z′ = 3 structure, C19H18B2F3N2
- The crystal structure of the Matrine derivative: 12-(1H-indol-1-yl)dodecahydro-1H,5H,10H-dipyrido[2,1-f:3′,2′,1′-ij][1,6]naphthyridin-10-one hydrate, C23H29N3O
- The crystal structure of tris(μ2-1,3-bis(4,4,4-trifluoro-3-oxido-1-(oxo)but-2-en-1-yl)phenyl-κ4O,O′:O′′,O′′′)-bis(1,2-dimethoxyethane-κ2O,O′)dilutetium(III), C50H38F18Lu2O16
- The crystal structure of (Z)-2-(3-(2-(4-chlorobenzoyl)hydrazono)-2-oxoindolin-1-yl) acetic acid, C17H12ClN3O4
- Synthesis and crystal structure of trans-tetraqua-bis(2-(((7-hydroxy-3-(4-hydroxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)ammonio)acetato-κO)cobalt(II) hexahydrate, C36H48CoN2O28S2
- The crystal structure of N,N-dimethyl-2,6-di-p-tolylpyrimidin-4-amine, C20H21N3
- The crystal structure (E)-4-methyl-N′-(2-nitrobenzylidene)benzenesulfonohydrazide, C14H13N3O4S
- Crystal structure of catena-poly[(μ2-1,3-bis(benzimidazol-1-yl)propane κ2N:N′)-(μ2-5-methoxyisophthalato-κ2O:O′)zinc(II)] hydrate, C26H24ZnN4O6
- The crystal structure of (E)-N′-(quinolin-2-ylmethylene)furan-2-carbohydrazide monohydrate, C15H13N3O3
- Crystal structure of 2,8-diphenyl-3,7,9-trioxa-1-azaspiro[4.5]dec-1-ene, C18H17N1O3
- Crystal structure of diethyl 2-(4-chlorophenyl)-1,3-dioxane-5,5-dicarboxylate, C16H19ClO6
- Crystal structure of 1-(carboxymethyl)-1H-benzo[d][1,2,3]triazole 3-oxide, C8H7N3O3
- Crystal structure of (acetylacetonato-κ2O,O′)-(2-amino-6-chlorobenzoato-κO)-oxido(1,10-phenanthroline-κ2N,N′)vanadium(IV) – trichloromethane (1/1)
- Crystal structure of (1E,4E)-1,5-bis(4-chlorophenyl)penta-1,4-dien-3-one, C17H12Cl2O
- The crystal structure of trans-dibromido-bis(pyridine-κN)platinum(II), C10H10Br2N2Pt

