Abstract
C21H14F6O, triclinic, P1̅ (no. 2), a = 7.8470(16) Å, b = 7.9390(16) Å, c = 14.993(3) Å, α = 83.25(3)°, β = 89.18(3)°, γ = 73.79(3)°, V = 890.5(3) Å, Z = 2, Rgt(F) = 0.0658, wRref(F2) = 0.1261, T = 293 K.
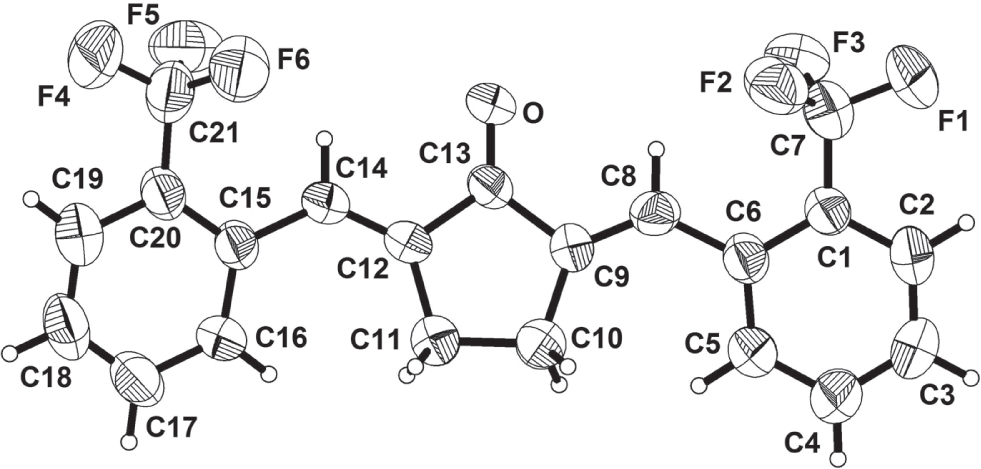
The crystal structure is shown in the figure. Tables 1 and 2 contain details on crystal structure and measurement conditions and a list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colorless block |
| Size: | 0.30 × 0.22 × 0.20 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.13 mm−1 |
| Diffractometer, scan mode: | Nonius CAD4, ω/2θ |
| θmax: | 25.0° |
| N(hkl)measured, N(hkl)unique: | 3150, 3150 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 1298 |
| N(param)refined: | 253 |
| Programs: | SHELX [13] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| O | 0.3518(3) | 0.1788(3) | 0.12515(15) | 0.0671(8) |
| F1 | 0.2333(4) | −0.1261(4) | 0.51161(16) | 0.1268(12) |
| C1 | 0.2977(5) | −0.2847(5) | 0.3855(3) | 0.0588(11) |
| F2 | 0.1382(4) | 0.0185(3) | 0.38446(16) | 0.0995(9) |
| C2 | 0.2981(6) | −0.4343(6) | 0.4439(3) | 0.0852(14) |
| H2A | 0.2831 | −0.4267 | 0.5051 | 0.102* |
| F3 | 0.4119(4) | −0.0540(3) | 0.41798(16) | 0.0978(9) |
| C3 | 0.3207(6) | −0.5934(6) | 0.4112(3) | 0.0956(16) |
| H3A | 0.3198 | −0.6932 | 0.4503 | 0.115* |
| F4 | 0.0923(5) | 0.7135(4) | −0.21608(18) | 0.1480(14) |
| C4 | 0.3442(6) | −0.6054(6) | 0.3227(3) | 0.0887(15) |
| H4A | 0.3614 | −0.7137 | 0.3008 | 0.106* |
| F5 | 0.3074(4) | 0.5680(4) | −0.12766(19) | 0.1099(10) |
| C5 | 0.3425(6) | −0.4567(6) | 0.2647(3) | 0.0771(13) |
| H5A | 0.3568 | −0.4666 | 0.2037 | 0.093* |
| F6 | 0.0446(4) | 0.5745(3) | −0.09335(18) | 0.1072(9) |
| C6 | 0.3202(5) | −0.2930(5) | 0.2941(3) | 0.0612(11) |
| C7 | 0.2711(7) | −0.1148(7) | 0.4241(3) | 0.0746(13) |
| C8 | 0.3224(5) | −0.1373(5) | 0.2300(3) | 0.0609(11) |
| H8A | 0.3457 | −0.0451 | 0.2560 | 0.073* |
| C9 | 0.2966(5) | −0.1071(5) | 0.1416(3) | 0.0573(11) |
| C10 | 0.2523(5) | −0.2174(5) | 0.0759(2) | 0.0708(12) |
| H10A | 0.1384 | −0.2389 | 0.0893 | 0.085* |
| H10B | 0.3421 | −0.3302 | 0.0784 | 0.085* |
| C11 | 0.2460(5) | −0.1119(5) | −0.0179(2) | 0.0659(11) |
| H11A | 0.3397 | −0.1740 | −0.0549 | 0.079* |
| H11B | 0.1327 | −0.0962 | −0.0477 | 0.079* |
| C12 | 0.2704(5) | 0.0626(5) | −0.0040(2) | 0.0596(11) |
| C13 | 0.3126(5) | 0.0609(5) | 0.0919(2) | 0.0542(10) |
| C14 | 0.2613(5) | 0.2052(5) | −0.0631(2) | 0.0576(11) |
| H14A | 0.2824 | 0.3005 | −0.0392 | 0.069* |
| C15 | 0.2229(5) | 0.2331(5) | −0.1600(2) | 0.0591(11) |
| C16 | 0.2400(5) | 0.0915(5) | −0.2090(3) | 0.0710(12) |
| H16A | 0.2774 | −0.0230 | −0.1798 | 0.085* |
| C17 | 0.2023(6) | 0.1174(7) | −0.3013(3) | 0.0861(14) |
| H17A | 0.2120 | 0.0207 | −0.3325 | 0.103* |
| C18 | 0.1514(6) | 0.2838(7) | −0.3455(3) | 0.0898(16) |
| H18A | 0.1281 | 0.3011 | −0.4070 | 0.108* |
| C19 | 0.1347(6) | 0.4255(6) | −0.2989(3) | 0.0817(14) |
| H19A | 0.0989 | 0.5393 | −0.3290 | 0.098* |
| C20 | 0.1705(5) | 0.4016(6) | −0.2071(3) | 0.0654(12) |
| C21 | 0.1536(8) | 0.5632(7) | −0.1621(4) | 0.0916(15) |
Source of materials
The title compound was synthesized by Aldol condensation between two molecules of 2-(trifluoromethyl)benzaldehyde and cyclopentanone, which was synthesized according our earlier published method [1, 2] . In detail: 5.0 mmol cyclopentanone was added to a solution of 10 mmol 2-(trifluoromethyl)benzaldehyde in MeOH (10 mL). The solution was stirred at room temperature for 30 min, followed by dropwise addition of NaOCH3/CH3OH (1.0 mL, 5.0 mmol). The mixture was stirred at room temperature and monitored with TLC. When the reaction was finished, the residue was poured into saturated NH4Cl solution and filtered. The precipitate was washed with water and cold ethanol, and dried in vacuum. The solid was further purified by silica gel chromatography (CH2Cl2/CH3OH). All chemicals used for the synthesis were commercially available and were used without further purification. The yellow crystals of the title compound were obtained by slow evaporation of a CH3OH solution at room temperature.
Experimental details
Position of the H atoms were calculated based on geometric criteria (C—H = 0.97 and 0.93 for methyl and aromatic atoms, respectively) and placed in their calculated position and refined, using a riding model with Uiso(H) = 1.5 Ueq (C) for methyl and Uiso(H) = 1.2 Ueq(C) for all others.
Discussion
Curcumin has been demonstrated to possess a wide range of pharmaceutical activities, such as anti-tumor [3, 4] , anti-inflammation [5], anti-oxidation [6], anti-bacterial [7], and cardiovascular protection [8, 9] . However, its high metabolic instability and low bioavailability have dramatically limited its practical application [10]. Recently, our research group has synthesized a series of mono-carbonyl analogs of curcumin (MACs) by deleting β-diketone moiety [11], [12]. The title compound is an Curcumin analogon with one central keto group only.
The symmetrical unit of this complex is constructed by two 2-(trifluoromethyl)benzylidene groups and a cyclopentanone linker. The C—C bond lengths of the cyclopentanone moiety are with 1.477(4) to 1.543(4) Å quite normal. The C8—C9 and C12—C14 bond lengths of 1.326(4) and 1.340(4) Å conform to a double bond. The C6—C8 and C14—C15 bond lengths of 1.477(5) and 1.465(5) Å are between double and single bonds. The shortening of the C—C bonds showing the partial double-bond character of the C—C bonds, which are influenced by adjacent double bonds.
Funding source: Wenzhou Medical University
Award Identifier / Grant number: wyx2016101001
Award Identifier / Grant number: wyx2016101026
Award Identifier / Grant number: wyx2016101107
Funding statement: We are grateful to the project of students academic research of Wenzhou Medical University (wyx2016101001, wyx2016101026, wyx2016101107) for financial support.
References
Liang, G.; Shao, L.; Wang, Y.; Zhao, C.; Chu, Y.; Xiao, J.; Zhao, Y.; Li, X.; Yang, S.: Exploration and synthesis of curcumin analogues with improved structural stability both in vitro and in vivo as cytotoxic agents. Bioorg. Med. Chem. 17 (2009) 2623–2631.10.1016/j.bmc.2008.10.044Search in Google Scholar PubMed
Zhao, C.; Yang, J.; Huang, Y.; Liang, G.; Li, X.: Crystal structure of ortho-(2E,5E)-2,5-bis(2-methoxybenzylidene) cyclopentanone, C21H20O3. Z. Kristallogr. NCS. 224 (2009) 337–338.10.1524/ncrs.2009.0150Search in Google Scholar
Gao, X.; Wang, B. L.; Wu, Q. J.; Wei, X. W.; Zheng, F. J.; Men, K.; Shi, H. S.; Huang, N.; Wei, Y. Q.; Gong, C. Y.: Combined delivery and anti-cancer activity of paclitaxel and curcumin using polymeric micelles. J. Biomed. Nanotech. 11 (2015) 578–589.10.1166/jbn.2015.1964Search in Google Scholar PubMed
Kumar, D.; Kumar, M.; Saravanan, C.; Singh, S. K.: Curcumin: a potential candidate for matrix metalloproteinase inhibitors. Expert. Opin. Ther. Targets 16 (2012) 959–972.10.1517/14728222.2012.710603Search in Google Scholar PubMed
Zhang, Y. L.; Zhao, L. P.; Wu, J. Z.; Jiang, X.; Dong, L. L.; Xu, F. L.; Zou, P.; Dai, Y. R.; Shan, X. O.; Yang, S. L.: Synthesis and evaluation of a series of novel asymmetrical curcumin Analogs for the treatment of inflammation. Molecules. 19 (2014) 7287–7307.10.3390/molecules19067287Search in Google Scholar PubMed PubMed Central
Yu, C.; Wei, C.; Liao, V.: Curcumin-mediated oxidative stress resistance in Caenorhabditis elegans is modulated by age-1, akt-1, pdk-1, osr-1, unc-43, sek-1, skn-1, sir-2.1, and mev-1. Free Radical Res. 48 (2014) 371–379.10.3109/10715762.2013.872779Search in Google Scholar PubMed
Negi, P. S.; Jayaprakasha, G. K.; Jagan, M.; Sakariah, K. K.: Antibacterial activity of turmeric oil: a byproduct from curcumin manufacture. J. Agric. Food. Chem. 47 (1999) 4297–4300.10.1021/jf990308dSearch in Google Scholar PubMed
Kapakos, G.; Youreva, V.; Srivastava, A. K.: Cardiovascular protection by curcumin: molecular aspects. Indian J. Biochem. Biophys. 49 (2012) 306–315.Search in Google Scholar
Zeng, C.; Zhong, P.; Zhao, Y.; Kanchana, K.; Zhang, Y.; Khan, Z. A.; Chakrabarti, S.; Wu, L.; Wang, J.; Liang, G.: Curcumin protects hearts from FFA-induced injury by activating Nrf2 and inactivating NF-κB both in vitro and in vivo. J. Mol. Cell. Cardiol. 79 (2014) 1–12.10.1016/j.yjmcc.2014.10.002Search in Google Scholar PubMed
Mythri, R. B.; Bharath, M. M.: Curcumin: a potential neuroprotective agent in Parkinson′s disease. Curr. Pharm. Des. 18 (2012) 91–9.10.2174/138161212798918995Search in Google Scholar PubMed
Liu, Z.; Tang, L.; Zou, P.; Zhang, Y.; Wang, Z.; Fang, Q.; Jiang, L.; Chen, G.; Xu, Z.; Zhang, H.; Liang, G.: Synthesis and biological evaluation of allylated and prenylated mono-carbonyl analogues of curcumin as anti-inflammatory agents. Eur. J. Med. Chem. 74 (2014) 671–682.10.1016/j.ejmech.2013.10.061Search in Google Scholar PubMed
Zhang, Y.; Jiang, X.; Peng, K.; Chen, C.; Fu, L.; Wang, Z.; Feng, J.; Liu, Z.; Zhang, H.; Liang, G.; Pan, Z.: Discovery and evaluation of novel anti-inflammatory derivatives of natural bioactive curcumin. Drug. Des. Devel. Ther. 8 (2014) 2161–2171.10.2147/DDDT.S69914Search in Google Scholar PubMed PubMed Central
Sheldrick G. M.: A short history of SHELX. Acta Crystallogr. A64 (2008) 112–122.10.1107/S0108767307043930Search in Google Scholar PubMed
©2017 Wenxin Zhang et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 License.
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of potassium 1-methyl-1H-1,2,3,4-tetrazole-5-thiolate, C2H3N4SK
- Crystal structure of bis(3-(3-ethylureido)-N,N-dimethylpropan-1-aminium) bis (μ3-2-(hydroxymethyl)-2-(oxidomethyl)propane-1,3-bis(olato))-(μ6-oxo)-hexakis(μ2-oxo)-hexaoxo-hexavanadium(V) – dichloromethane (1/1), C27H60Cl2N6O23V6
- Crystal structure of bis(μ3-methanolato-κ3O:O:O)-bis(μ2-methanolato-κ2O:O)-dimethanol-bis{6,6′-(1,3-dihydroxyl-2-acetylpropane-1,3-diyl)bis(2-chloro-4-bromophenolato)}tetramanganese(III) C40H40Br4Cl4Mn4O16
- Synthesis and crystal structure of tetrakis(μ2-methanolato)-dimethanol-bis(μ2-2-acetyl-1,3-bis(3,5-dibromo-2-oxidophenyl)propane-1,3-bis(olato)-κ5O1,O2,O3:O3,O4,O5)tetramanganese(III), C40H40Br8Mn4O16
- Synthesis and crystal structure tetrakis(μ2-methanolato)-dimethanol-bis(μ2-2-acetyl-1,3-bis(3,5-dichloro-2-oxidophenyl)propane-1,3-bis(olato)-κ5O1,O2,O3:O3,O4,O5), tetramanganese(III), C40H40Cl8Mn4O16
- Crystal structure of bis{5-methoxy-2-((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O)}copper(II), C34H34CuN4O6
- Crystal structure of (E)-1-(2-hydroxy-3-{[2-hydroxybenzylidene]amino}phenyl)ethan-1-one, C15H13NO3
- Crystal structure of bis(μ2-acetato-κ2O:O′)-bis{μ2-4-chloro-2-(8-(4-(diethylamino)-2-oxidophenyl)-3,6-dioxa-2,7-diazaocta-1,7-dien-1-yl)phenolato-κ6O:O,N,N′,O′′:O′′}tricobalt(II), C44H49Cl2Co3N6O12
- Crystal structure of bis{μ2-2,4-dichloro-6-(8-(4-(diethylamino)-2-oxidophenyl)-3,6-dioxa-2,7-diazaocta-1,7-dien-1-yl)phenolato-κ5O:O,N,N′,O′}dicobalt(II) acetone solvate, C43H48Br4Co2N6O9
- Synthesis and crystal structure of bis(μ2-acetato-κ2O:O′)-bis{μ2-4-chloro-2-(8-(4-(diethylamino)-2-oxidophenyl)-3,6-dioxa-2,7-diazaocta-1,7-dien-1-yl)phenolato-κ6O:O,N,N′,O′:O′}trizinc(II), C44H49Cl2N6O12Zn3
- Crystal structure of bis{5-(N,N′-diethylamine)-5′-methoxy -2,2′-[ethylenediyldioxybis(nitrilomethylidyne)]diphenolato}-bis(μ2-acetato-κ2O:O′)trizinc(II), C46H56Zn3N6O14
- Crystal structure of tris(cyano-(hydrogen tris(3,5-dimethylpyrazolyl)borate))-iron(III) 4-methoxypyridinium monohydrate, C24H32BN10O2Fe
- Synthesis and crystal structure of bis{1-(((4-(1-(hydroxyimino)ethyl)phenyl)imino)methyl)naphthalen-2-olato-κ2O,N}copper(II), C38H30CuN4O4
- Crystal structure of (E)-1-(4-{[(E)-2-Hydroxy-1-naphthalenylmethylene] amino}phenyl)ethanone oxime, C19H16N2O2
- The pseudosymmetric crystal structure of 2-(2-naphthalenyl)-3-nitro-2H-1-benzopyran, C38H26N2O6
- Crystal structure of 2-benzoyl-3-(4-methoxyphenyl)cyclopropane-1,1-dicarbonitrile, C19H14N2O2
- N′-(5-ethoxycarbonyl-3,4-dimethyl-pyrrol-2-yl-methylidene)-4-hydroxybenzohydrazide monohydrate, C17H21N3O5
- The crystal structure of carbonyl-[4-(2,4-dichlorophenylamino)pent-3-en-2-onato-κ2N,O]-(triphenylphosphine-κP)rhodium(I), RhC30H25Cl2NO2P
- Crystal structure of (E)-5-(diethylamino)-2-(((1,1,2-trihydroxyethyl)iminio)methyl)phenolate, C15H24N2O4
- Crystal structure of poly[(μ2-1,4-bis((2-ethyl-1H-benzo[d]imidazol-1-yl)methyl)benzene-κ2N:N′)(μ2-isophthalato-κ4O,O′:O′′,O′′′)cadmium(II)], C34H30N4O4Cd
- Crystal structure of 5,6-Dihydro-9,10-dimethoxybenzo[g]-1,3-benzodioxolo[5,6-a]chinolizinium 3-(4-hydroxyphenyl)-4-oxo-4H-chromen-7-olate - methanol - water (1/1/1), C36H33NO10
- A single crystal study on 2-(methylcarbamoyl)benzoic acid, C9H9NO3
- Crystal structure of the salt 1,1′-(ethane-1,2-diyl)bis(1,4-diazabicyclo[2.2.2]octan-1-ium) diperchlorate, C14H28N4(ClO4)2
- Crystal structure of 2,5-bis((E)-2-(trifluoromethyl)benzylidene)cyclopentan-1-one, C21H14F6O
- Crystal structure of catena-poly[(μ2-benzene-1,4-dicarboxylato-κ2O:O′)-(1-ethyl-6-fluoro-7-(4-methylpiperazin-1-ium-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylato-κ2O,O′)zinc(II)] 1.25 hydrate, C25H26.5N3O8.25FZn
- Crystal structure of 1-methyl-1,4-diazabicyclo[2.2.2]octan-1-ium poly[aqua-bis(μ2-perchlorato-κ3O,O′:O′′)sodium], C7H17Cl2N2NaO9
- Crystal structure of trimethyammonium 2,6-dicarboxyisonicotinate monohydrate, C11H16N2O7
- Crystal structure of dodecaguanidinium bis(tetrapropylammonium) heptacarbonate, C43H128N38O21
- Crystal structure of poly[tetraaqua-bis(μ6-benzene-1,2,4,5-tetracarboxylato)nickel(II)diyttrium(III)]dihydrate, C20H16NiO22Y2
- Halogen bonds and π–π interactions in the crystal structure of 1,3,5-trifluoro-2,4,6-triiodobenzene–N,N-dimethylformamide (1/1), C9H7F3I3NO
- Crystal structure of guanidinium tetraethylammonium carbonate dihydrate, C10H30N4O5
- The crystal structure of oxonium chlorido-ethylenediaminetetraactetotin(IV) hydrate, C10H17ClN2O10Sn
- Crystal structure of 8-((E)-((4-((E)-1-((benzyloxy)imino)ethyl)phenyl)imino)methyl)-7-hydroxy-4-methyl-2H-chromen-2-one, C26H22N2O4
- Crystal structure of bis{5-(diethylamino)-2-(((2-oxo-2H-chromen-6-yl)imino)methyl)phenolato-κ2O,N}copper(II), C40H38CuN4O6
- Crystal structure of fac-(acetylacetonato-κ2O,O′)tricarbonyl(tri(p-tolyl)phosphine-κP)rhenium(I), C29H28O5PRe
- Crystal structure of fac-(acetylacetonato-κ2O,O′)tricarbonyl(benzyldiphenylphosphine-κP)rhenium(I), C27H24O5PRe
- Crystal structure of triethylammonium bis{3-(((3-oxidonaphthalen-1-yl)methylene)amino)-2-oxo-2H-chromen-4-olato-κ3O,N,O′}manganese(III), C46H38MnN3O8
- Crystal structure of bis(N,N,N-ethyldimethylethanaminium) bis(heptaselenido-κ2Se1,Se7)palladate(II), C12H32N2PdSe14
- Crystal structure of bis(4-(1H-pyrazol-5-yl)pyridine-κ2N:N′)-bis(2-(2-((2,6-difluorophenyl)amino)phenyl)acetate-κO)cadmium(II), C44H34N8CdCl4O4
- Crystal structure of 4,4′-(1,4-phenylene)bis(pyridin-1-ium) catena-poly[diaqua-bis(μ2-3′,5′-dicarboxy-[1,1′-biphenyl]-2,5-dicarboxylato-κ2O:O′] dihydrate, C48H42O22N2Ca
- The crystal structure of (S)-2-benzylsuccinic acid, C11H12O4
- Crystal structure of poly[aqua-(μ3-4-(pyridin-4-yl)isophthalato-κ4O,O′:O′′:O′′′)-(μ2-5-carboxy-2-(pyridin-4-yl)benzoato-κ2O:O′)yttrium(III)], C26H17N2O9Y
- Crystal structure of ethyl 1-(3,4-dimethylphenyl)-5-methyl-1H-1,2,3-triazole-4-carboxylate, C14H17N3O2
- Crystal structure of tetrakis(methanol-κO)-bis{μ2-3-((4-methoxy-2-oxidobenzylidene)amino)-2-oxo-2H-chromen-4-olate-κ4O,N;O′:O′}dizinc(II), C38H38Zn2N2O14
- Crystal structure of bromido(4,4′-dimethoxy-2,2′-bipyridine-κ2N,N′)(isopropyl(diphenyl)phosphane-κP)copper(I), C27H29BrCuN2O2P
- Crystal structure of bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane)-9,9-dioctylfluorene, C41H64B2O4
- Crystal structure of 11-oxo-2,3,6,7-tetrahydro-1H,5H,11H-pyrano[2,3-f]pyrido[3,2,1-ij]quinoline-10-carbaldehyde - a julolidine derivative, C16H15NO3
- Crystal structure of 1,3-dimethyl-5,5-dibenzylbarbituric acid, C20H20N2O3
- Crystal structure of bis(N,N,N-trimethylethanaminium) poly[bis(μ2-heptaselenido-κ2Se1,Se7)palladate(II)], C10H28N2PdSe14
- Crystal structure of 1-{4-[(5-Chloro-2-hydroxy-benzylidene)amino]phenyl} ethanone O-ethyl-oxime, C17H17ClN2O2
- The crystal structure of methyl N-(4-bromophenyl)carbamate, C8H8BrNO2
- Synthesis and crystal structure of 1-{4-[(2-hydroxy-benzylidene)amino]phenyl}ethanone oxime, C15H14N2O2
- Crystal structure of 1,1′-butanebis(3-methyl-1H-imidazol-3-ium)bis(hexafluorophosphate), C12H20F12N4P2
- (E)-N-benzylidene-3-(benzylthio)-5-p-tolyl-4H-1,2,4-triazol-4-amine, C23H20N4S
- Crystal structure of ethyl 5-methyl-1-(pyridin-3-yl)-1H-1,2,3-triazole-4-carboxylate, C11H12N4O2
- Crystal structure of 5,5-difluoro-10-(4-fluorophenyl)-1,3,7,9-tetramethyl-5H-4l4,5l4-dipyrrolo[1,2-c:2′,1′-f][1,3,2]diazaborinine - a Z′ = 3 structure, C19H18B2F3N2
- The crystal structure of the Matrine derivative: 12-(1H-indol-1-yl)dodecahydro-1H,5H,10H-dipyrido[2,1-f:3′,2′,1′-ij][1,6]naphthyridin-10-one hydrate, C23H29N3O
- The crystal structure of tris(μ2-1,3-bis(4,4,4-trifluoro-3-oxido-1-(oxo)but-2-en-1-yl)phenyl-κ4O,O′:O′′,O′′′)-bis(1,2-dimethoxyethane-κ2O,O′)dilutetium(III), C50H38F18Lu2O16
- The crystal structure of (Z)-2-(3-(2-(4-chlorobenzoyl)hydrazono)-2-oxoindolin-1-yl) acetic acid, C17H12ClN3O4
- Synthesis and crystal structure of trans-tetraqua-bis(2-(((7-hydroxy-3-(4-hydroxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)ammonio)acetato-κO)cobalt(II) hexahydrate, C36H48CoN2O28S2
- The crystal structure of N,N-dimethyl-2,6-di-p-tolylpyrimidin-4-amine, C20H21N3
- The crystal structure (E)-4-methyl-N′-(2-nitrobenzylidene)benzenesulfonohydrazide, C14H13N3O4S
- Crystal structure of catena-poly[(μ2-1,3-bis(benzimidazol-1-yl)propane κ2N:N′)-(μ2-5-methoxyisophthalato-κ2O:O′)zinc(II)] hydrate, C26H24ZnN4O6
- The crystal structure of (E)-N′-(quinolin-2-ylmethylene)furan-2-carbohydrazide monohydrate, C15H13N3O3
- Crystal structure of 2,8-diphenyl-3,7,9-trioxa-1-azaspiro[4.5]dec-1-ene, C18H17N1O3
- Crystal structure of diethyl 2-(4-chlorophenyl)-1,3-dioxane-5,5-dicarboxylate, C16H19ClO6
- Crystal structure of 1-(carboxymethyl)-1H-benzo[d][1,2,3]triazole 3-oxide, C8H7N3O3
- Crystal structure of (acetylacetonato-κ2O,O′)-(2-amino-6-chlorobenzoato-κO)-oxido(1,10-phenanthroline-κ2N,N′)vanadium(IV) – trichloromethane (1/1)
- Crystal structure of (1E,4E)-1,5-bis(4-chlorophenyl)penta-1,4-dien-3-one, C17H12Cl2O
- The crystal structure of trans-dibromido-bis(pyridine-κN)platinum(II), C10H10Br2N2Pt
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of potassium 1-methyl-1H-1,2,3,4-tetrazole-5-thiolate, C2H3N4SK
- Crystal structure of bis(3-(3-ethylureido)-N,N-dimethylpropan-1-aminium) bis (μ3-2-(hydroxymethyl)-2-(oxidomethyl)propane-1,3-bis(olato))-(μ6-oxo)-hexakis(μ2-oxo)-hexaoxo-hexavanadium(V) – dichloromethane (1/1), C27H60Cl2N6O23V6
- Crystal structure of bis(μ3-methanolato-κ3O:O:O)-bis(μ2-methanolato-κ2O:O)-dimethanol-bis{6,6′-(1,3-dihydroxyl-2-acetylpropane-1,3-diyl)bis(2-chloro-4-bromophenolato)}tetramanganese(III) C40H40Br4Cl4Mn4O16
- Synthesis and crystal structure of tetrakis(μ2-methanolato)-dimethanol-bis(μ2-2-acetyl-1,3-bis(3,5-dibromo-2-oxidophenyl)propane-1,3-bis(olato)-κ5O1,O2,O3:O3,O4,O5)tetramanganese(III), C40H40Br8Mn4O16
- Synthesis and crystal structure tetrakis(μ2-methanolato)-dimethanol-bis(μ2-2-acetyl-1,3-bis(3,5-dichloro-2-oxidophenyl)propane-1,3-bis(olato)-κ5O1,O2,O3:O3,O4,O5), tetramanganese(III), C40H40Cl8Mn4O16
- Crystal structure of bis{5-methoxy-2-((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O)}copper(II), C34H34CuN4O6
- Crystal structure of (E)-1-(2-hydroxy-3-{[2-hydroxybenzylidene]amino}phenyl)ethan-1-one, C15H13NO3
- Crystal structure of bis(μ2-acetato-κ2O:O′)-bis{μ2-4-chloro-2-(8-(4-(diethylamino)-2-oxidophenyl)-3,6-dioxa-2,7-diazaocta-1,7-dien-1-yl)phenolato-κ6O:O,N,N′,O′′:O′′}tricobalt(II), C44H49Cl2Co3N6O12
- Crystal structure of bis{μ2-2,4-dichloro-6-(8-(4-(diethylamino)-2-oxidophenyl)-3,6-dioxa-2,7-diazaocta-1,7-dien-1-yl)phenolato-κ5O:O,N,N′,O′}dicobalt(II) acetone solvate, C43H48Br4Co2N6O9
- Synthesis and crystal structure of bis(μ2-acetato-κ2O:O′)-bis{μ2-4-chloro-2-(8-(4-(diethylamino)-2-oxidophenyl)-3,6-dioxa-2,7-diazaocta-1,7-dien-1-yl)phenolato-κ6O:O,N,N′,O′:O′}trizinc(II), C44H49Cl2N6O12Zn3
- Crystal structure of bis{5-(N,N′-diethylamine)-5′-methoxy -2,2′-[ethylenediyldioxybis(nitrilomethylidyne)]diphenolato}-bis(μ2-acetato-κ2O:O′)trizinc(II), C46H56Zn3N6O14
- Crystal structure of tris(cyano-(hydrogen tris(3,5-dimethylpyrazolyl)borate))-iron(III) 4-methoxypyridinium monohydrate, C24H32BN10O2Fe
- Synthesis and crystal structure of bis{1-(((4-(1-(hydroxyimino)ethyl)phenyl)imino)methyl)naphthalen-2-olato-κ2O,N}copper(II), C38H30CuN4O4
- Crystal structure of (E)-1-(4-{[(E)-2-Hydroxy-1-naphthalenylmethylene] amino}phenyl)ethanone oxime, C19H16N2O2
- The pseudosymmetric crystal structure of 2-(2-naphthalenyl)-3-nitro-2H-1-benzopyran, C38H26N2O6
- Crystal structure of 2-benzoyl-3-(4-methoxyphenyl)cyclopropane-1,1-dicarbonitrile, C19H14N2O2
- N′-(5-ethoxycarbonyl-3,4-dimethyl-pyrrol-2-yl-methylidene)-4-hydroxybenzohydrazide monohydrate, C17H21N3O5
- The crystal structure of carbonyl-[4-(2,4-dichlorophenylamino)pent-3-en-2-onato-κ2N,O]-(triphenylphosphine-κP)rhodium(I), RhC30H25Cl2NO2P
- Crystal structure of (E)-5-(diethylamino)-2-(((1,1,2-trihydroxyethyl)iminio)methyl)phenolate, C15H24N2O4
- Crystal structure of poly[(μ2-1,4-bis((2-ethyl-1H-benzo[d]imidazol-1-yl)methyl)benzene-κ2N:N′)(μ2-isophthalato-κ4O,O′:O′′,O′′′)cadmium(II)], C34H30N4O4Cd
- Crystal structure of 5,6-Dihydro-9,10-dimethoxybenzo[g]-1,3-benzodioxolo[5,6-a]chinolizinium 3-(4-hydroxyphenyl)-4-oxo-4H-chromen-7-olate - methanol - water (1/1/1), C36H33NO10
- A single crystal study on 2-(methylcarbamoyl)benzoic acid, C9H9NO3
- Crystal structure of the salt 1,1′-(ethane-1,2-diyl)bis(1,4-diazabicyclo[2.2.2]octan-1-ium) diperchlorate, C14H28N4(ClO4)2
- Crystal structure of 2,5-bis((E)-2-(trifluoromethyl)benzylidene)cyclopentan-1-one, C21H14F6O
- Crystal structure of catena-poly[(μ2-benzene-1,4-dicarboxylato-κ2O:O′)-(1-ethyl-6-fluoro-7-(4-methylpiperazin-1-ium-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylato-κ2O,O′)zinc(II)] 1.25 hydrate, C25H26.5N3O8.25FZn
- Crystal structure of 1-methyl-1,4-diazabicyclo[2.2.2]octan-1-ium poly[aqua-bis(μ2-perchlorato-κ3O,O′:O′′)sodium], C7H17Cl2N2NaO9
- Crystal structure of trimethyammonium 2,6-dicarboxyisonicotinate monohydrate, C11H16N2O7
- Crystal structure of dodecaguanidinium bis(tetrapropylammonium) heptacarbonate, C43H128N38O21
- Crystal structure of poly[tetraaqua-bis(μ6-benzene-1,2,4,5-tetracarboxylato)nickel(II)diyttrium(III)]dihydrate, C20H16NiO22Y2
- Halogen bonds and π–π interactions in the crystal structure of 1,3,5-trifluoro-2,4,6-triiodobenzene–N,N-dimethylformamide (1/1), C9H7F3I3NO
- Crystal structure of guanidinium tetraethylammonium carbonate dihydrate, C10H30N4O5
- The crystal structure of oxonium chlorido-ethylenediaminetetraactetotin(IV) hydrate, C10H17ClN2O10Sn
- Crystal structure of 8-((E)-((4-((E)-1-((benzyloxy)imino)ethyl)phenyl)imino)methyl)-7-hydroxy-4-methyl-2H-chromen-2-one, C26H22N2O4
- Crystal structure of bis{5-(diethylamino)-2-(((2-oxo-2H-chromen-6-yl)imino)methyl)phenolato-κ2O,N}copper(II), C40H38CuN4O6
- Crystal structure of fac-(acetylacetonato-κ2O,O′)tricarbonyl(tri(p-tolyl)phosphine-κP)rhenium(I), C29H28O5PRe
- Crystal structure of fac-(acetylacetonato-κ2O,O′)tricarbonyl(benzyldiphenylphosphine-κP)rhenium(I), C27H24O5PRe
- Crystal structure of triethylammonium bis{3-(((3-oxidonaphthalen-1-yl)methylene)amino)-2-oxo-2H-chromen-4-olato-κ3O,N,O′}manganese(III), C46H38MnN3O8
- Crystal structure of bis(N,N,N-ethyldimethylethanaminium) bis(heptaselenido-κ2Se1,Se7)palladate(II), C12H32N2PdSe14
- Crystal structure of bis(4-(1H-pyrazol-5-yl)pyridine-κ2N:N′)-bis(2-(2-((2,6-difluorophenyl)amino)phenyl)acetate-κO)cadmium(II), C44H34N8CdCl4O4
- Crystal structure of 4,4′-(1,4-phenylene)bis(pyridin-1-ium) catena-poly[diaqua-bis(μ2-3′,5′-dicarboxy-[1,1′-biphenyl]-2,5-dicarboxylato-κ2O:O′] dihydrate, C48H42O22N2Ca
- The crystal structure of (S)-2-benzylsuccinic acid, C11H12O4
- Crystal structure of poly[aqua-(μ3-4-(pyridin-4-yl)isophthalato-κ4O,O′:O′′:O′′′)-(μ2-5-carboxy-2-(pyridin-4-yl)benzoato-κ2O:O′)yttrium(III)], C26H17N2O9Y
- Crystal structure of ethyl 1-(3,4-dimethylphenyl)-5-methyl-1H-1,2,3-triazole-4-carboxylate, C14H17N3O2
- Crystal structure of tetrakis(methanol-κO)-bis{μ2-3-((4-methoxy-2-oxidobenzylidene)amino)-2-oxo-2H-chromen-4-olate-κ4O,N;O′:O′}dizinc(II), C38H38Zn2N2O14
- Crystal structure of bromido(4,4′-dimethoxy-2,2′-bipyridine-κ2N,N′)(isopropyl(diphenyl)phosphane-κP)copper(I), C27H29BrCuN2O2P
- Crystal structure of bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane)-9,9-dioctylfluorene, C41H64B2O4
- Crystal structure of 11-oxo-2,3,6,7-tetrahydro-1H,5H,11H-pyrano[2,3-f]pyrido[3,2,1-ij]quinoline-10-carbaldehyde - a julolidine derivative, C16H15NO3
- Crystal structure of 1,3-dimethyl-5,5-dibenzylbarbituric acid, C20H20N2O3
- Crystal structure of bis(N,N,N-trimethylethanaminium) poly[bis(μ2-heptaselenido-κ2Se1,Se7)palladate(II)], C10H28N2PdSe14
- Crystal structure of 1-{4-[(5-Chloro-2-hydroxy-benzylidene)amino]phenyl} ethanone O-ethyl-oxime, C17H17ClN2O2
- The crystal structure of methyl N-(4-bromophenyl)carbamate, C8H8BrNO2
- Synthesis and crystal structure of 1-{4-[(2-hydroxy-benzylidene)amino]phenyl}ethanone oxime, C15H14N2O2
- Crystal structure of 1,1′-butanebis(3-methyl-1H-imidazol-3-ium)bis(hexafluorophosphate), C12H20F12N4P2
- (E)-N-benzylidene-3-(benzylthio)-5-p-tolyl-4H-1,2,4-triazol-4-amine, C23H20N4S
- Crystal structure of ethyl 5-methyl-1-(pyridin-3-yl)-1H-1,2,3-triazole-4-carboxylate, C11H12N4O2
- Crystal structure of 5,5-difluoro-10-(4-fluorophenyl)-1,3,7,9-tetramethyl-5H-4l4,5l4-dipyrrolo[1,2-c:2′,1′-f][1,3,2]diazaborinine - a Z′ = 3 structure, C19H18B2F3N2
- The crystal structure of the Matrine derivative: 12-(1H-indol-1-yl)dodecahydro-1H,5H,10H-dipyrido[2,1-f:3′,2′,1′-ij][1,6]naphthyridin-10-one hydrate, C23H29N3O
- The crystal structure of tris(μ2-1,3-bis(4,4,4-trifluoro-3-oxido-1-(oxo)but-2-en-1-yl)phenyl-κ4O,O′:O′′,O′′′)-bis(1,2-dimethoxyethane-κ2O,O′)dilutetium(III), C50H38F18Lu2O16
- The crystal structure of (Z)-2-(3-(2-(4-chlorobenzoyl)hydrazono)-2-oxoindolin-1-yl) acetic acid, C17H12ClN3O4
- Synthesis and crystal structure of trans-tetraqua-bis(2-(((7-hydroxy-3-(4-hydroxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)ammonio)acetato-κO)cobalt(II) hexahydrate, C36H48CoN2O28S2
- The crystal structure of N,N-dimethyl-2,6-di-p-tolylpyrimidin-4-amine, C20H21N3
- The crystal structure (E)-4-methyl-N′-(2-nitrobenzylidene)benzenesulfonohydrazide, C14H13N3O4S
- Crystal structure of catena-poly[(μ2-1,3-bis(benzimidazol-1-yl)propane κ2N:N′)-(μ2-5-methoxyisophthalato-κ2O:O′)zinc(II)] hydrate, C26H24ZnN4O6
- The crystal structure of (E)-N′-(quinolin-2-ylmethylene)furan-2-carbohydrazide monohydrate, C15H13N3O3
- Crystal structure of 2,8-diphenyl-3,7,9-trioxa-1-azaspiro[4.5]dec-1-ene, C18H17N1O3
- Crystal structure of diethyl 2-(4-chlorophenyl)-1,3-dioxane-5,5-dicarboxylate, C16H19ClO6
- Crystal structure of 1-(carboxymethyl)-1H-benzo[d][1,2,3]triazole 3-oxide, C8H7N3O3
- Crystal structure of (acetylacetonato-κ2O,O′)-(2-amino-6-chlorobenzoato-κO)-oxido(1,10-phenanthroline-κ2N,N′)vanadium(IV) – trichloromethane (1/1)
- Crystal structure of (1E,4E)-1,5-bis(4-chlorophenyl)penta-1,4-dien-3-one, C17H12Cl2O
- The crystal structure of trans-dibromido-bis(pyridine-κN)platinum(II), C10H10Br2N2Pt

