Abstract
To address the challenges of high reagent consumption and environmental risks in traditional cyanide gold extraction, this study investigated the leaching of gold from an oxide ore sourced from a gold mine in Laos using an iron ion-thiourea-thiocyanate additive system. The effects of key process parameters (thiourea concentration, iron ion concentration, thiocyanate ion concentration, pH, stirring speed, liquid-solid ratio, and leaching time) on gold leaching rate and thiourea consumption were systematically examined. Additionally, the mechanism of thiocyanate action and the evolution of mineral surface properties before and after leaching were analyzed via X-ray Photoelectron Spectroscopy (XPS) and Scanning Electron Microscopy (SEM). The optimal leaching conditions were determined as follows: thiourea concentration 0.2 M, iron ion concentration 0.003 M, thiocyanate ion concentration 0.001 M, pH 1.5, stirring speed 250 rpm, liquid-solid ratio 4, and leaching time 6 h. Under these conditions, the gold leaching rate reached 93.1 %, which was higher than that of the cyanide process (91.6 %, 24 h) and thiosulfate process (86.4 %, 4 h). XPS results showed that thiocyanate complexed with Fe3+ to regulate its electrode potential, reducing the oxidation of thiourea and inhibiting the formation of S2−(a passivating species) on the ore surface. SEM observations confirmed that the leaching system corroded the ore surface, forming gullies and cracks that promoted mass transfer. Notably, the addition of thiocyanate significantly reduced thiourea consumption but slightly decreased the gold leaching rate, requiring a balance between reagent cost and extraction efficiency. This study provides a feasible and environmentally friendly non-cyanide gold extraction technology for oxide ores, offering theoretical and experimental support for industrial application.
1 Introduction
Thiourea leaching is recognized as a promising clean non-cyanide gold extraction technology [1]. However, this process has not yet been industrialized on a large scale. Beyond the challenges of high reagent consumption and production costs, the insufficient understanding of the thiourea leaching mechanism is another critical factor restricting its industrial application. Thiourea (subsequently abbreviated as Tu), a water-soluble organic ligand with reducibility, can extract gold from ores through complexation reactions. The leaching efficiency of gold in the thiourea system is primarily governed by kinetic factors, and the main reaction equation is expressed as follows [2]:
When thiourea acts as a complexing agent, the dissolution potential of gold is reduced to 0.38 V. The core function of thiourea is to lower the oxidation potential of gold, thereby decreasing the difficulty of gold dissolution. However, as a reducing agent, thiourea itself has an oxidation potential of 0.42 V, resulting in a narrow potential window suitable for gold leaching [3]. If the system potential is excessively high, it will not only accelerate the oxidation consumption of thiourea but also cause the decomposition of thiocyanate. This decomposition not only increases thiocyanate consumption but also generates sulfide ions and elemental sulfur, which can form a passivation layer on the surface of gold particles and inhibit further leaching. Conversely, an overly low system potential fails to provide sufficient driving force for efficient gold dissolution [4].
Oxygen is theoretically an ideal oxidant for the thiourea leaching system, but its low solubility in aqueous solutions limits its practical application. In contrast, Fe3+ exhibits a standard electrode potential of E = 0.77 V, which is within the optimal potential range for gold leaching in the thiourea system. The reaction between Fe3+, thiourea, and gold is shown in Equation (2) [5]:
Thiocyanate ions (SCN) possess a linear molecular structure and exist in two resonance forms: thiocyanate (SCN−) and isothiocyanate (NCS) [6]. When Fe3+ is used as the oxidant, thiocyanate ions first form a complex with Fe3+ and this complex can further react with gold to form Au(SCN)4 3− [7], 8]. However, the complexing ability of thiocyanate ions with gold is weaker than that of thiourea. In the presence of thiourea, gold primarily forms the stable [Au(Tu)2]+ complex with thiourea. The main purpose of introducing thiocyanate ions into the system is to regulate the electrode potential of Fe3+, thereby balancing gold dissolution efficiency and reagent consumption [9].
Previous studies on iron-thiourea systems have focused on optimizing single parameters, but the mechanism of SCN− regulation and its impact on reagent consumption remain unclear [10]. Additionally, the stability of thiourea in acidic Fe3+ solutions and the evolution of mineral surface properties during leaching require further investigation [11]. This study examined gold leaching in the iron ion-thiourea system without SCN−, then analyzed the effect of SCN−on leaching efficiency and reagent consumption. XPS and SEM were used to characterize the changes in sulfur/iron species and ore morphology, providing a theoretical basis for optimizing the non-cyanide gold leaching process.
2 Experiment
2.1 Reagents and instruments
All reagents used in the study were of analytical grade, while deionized water served as the test water. The ore sample was sourced from a gold mine situated in Laos, specifically selected for its representative characteristics. The gold within the ore is classified as an independent occurrence of natural gold. Based on the X-ray diffraction analysis results (Table 1), it is evident that the ore composition predominantly consists of elevated levels of quartz and muscovite. In addition, a minor proportion of goethite is present within the ore. Furthermore, the ore sample displays a gold and silver content constituting approximately 5 % of the total composition.
X-ray diffraction analysis.
| Mineral name | Formula | Content, % |
|---|---|---|
| Quartz | SiO2 | 50.45 |
| Muscovite | (K,Na)(Al,Mg,Fe)2(Si3.1Al0.9)O10(OH)2 | 42.67 |
| Goethite | FeO(OH) | 1.88 |
| Kustelite | Au/Ag | 5.00 |
The results of the multielement analysis for the ore chemistry are detailed in Table 2 The ore exhibits an Au grade of 12.2 g t−1. Considering the relatively higher market price per gram of gold compared to silver, despite its lower abundance, the presence of gold in the ore holds considerable value.Although the Ag content was higher, the higher market value of gold made it the primary target for extraction [12]. Other major components included SiO2 (62.27 %), Al2O3 (16.72 %), and CaO (5.03 %), with low levels of S (0.060 %) and As (0.27 %), which reduced the risk of passivation during leaching [13], 14].
Ore chemical multi-element analysis.
| Element | Au | Ag | Fe | S | As | SiO2 | MgO | Al2O3 | CaO |
|---|---|---|---|---|---|---|---|---|---|
| Content, % | 12.20 g t−1 | 93.51 g t−1 | 6.40 | 0.060 | 0.27 | 62.27 | 2.72 | 16.72 | 5.03 |
An AA320 atomic absorption spectrophotometer, a constant-temperature electric stirrer 107 (Guohua JJ-1), and a pH meter (PHS-3C, Shanghai Scientific Instruments Co., Ltd., China) were 108 used for the experiments.
2.2 Test method
All leaching tests were carried out in conical flasks, 20 g of ore samples were taken, and leaching reagents containing a certain of thiourea, iron ions, buffer solution and other components were added, and the ore pulp was stirred at 250 rpm using a mechanical stirrer, and ore pulp was separated by solid-liquid separation after the leaching was completed by a circulating water vacuum pump and a siphon filtration device. The leaching residue was dried and the content of the leaching residue was analyzed to calculate the gold leaching rate; the thiourea content was analyzed by the method of literature, potassium iodate titration used, and starch was used as an indicator to calculate the remaining thiourea concentration in the leaching solution [15], 16].
The concentration analysis of thiourea is as follows: Take 1 mL of the leaching solution into a beaker, add 9 mL of distilled water, and then add an appropriate amount of 5 g/L starch solution as the indicator. Titrate the remaining thiourea in the leaching solution with potassium iodate solution. The end point of the titration is reached when the color of the solution changes from colorless to pale blue. Record the volume of potassium iodate solution consumed.
For XPS analysis, the leaching residue was ground to 200 mesh, and the binding energy was calibrated using the C 1s peak (284.8 eV) [17]. For SEM observation, the residue was sputter-coated with gold to improve conductivity, and images were captured at a magnification of 1.00–10.00 KX [18].
3 Results and discussion
3.1 Effect of thiourea
Thiourea complexes with gold to form [Au(Tu)2]+, with a cumulative stability constant (logβ) of 23.30, and the reaction is [19]:
The thiourea concentration needs to be precisely regulated. If the concentration is too low it cannot complex with gold well; if the concentration is too high, it not only wastes reagents and increases costs but also increases the consumption of trivalent iron [20]. The of thiourea concentration on gold leaching was investigated under the conditions of iron ion concentration of 0.003 M, pH 1.5, rotation speed 250 rpm, liquid-solid ratio of 4, and leaching time of 10 h. The results are shown in Figure 1, from which can be seen that appropriately increasing the thiourea concentration can effectively improve the gold leaching rate. When the thiourea concentration was 0.02 M, gold leaching rate could reach 94.5 %. If the thiourea concentration was further increased, the gold leaching rate changed slightly. The value is greater than the suitable concentration of thiourea in the literature [21], which is 0.08 M, the reason may be that the oxid property of iron ions is too strong [22]. The thiourea is oxidized excessively, thus the consumption of thiourea is too much [23]. At this time the thiourea concentration was sufficient to leach the gold ions, and the effective extraction of gold was realized. Considering the comprehensive cost, the optimal concentration of thiou was selected as 0.2 M.
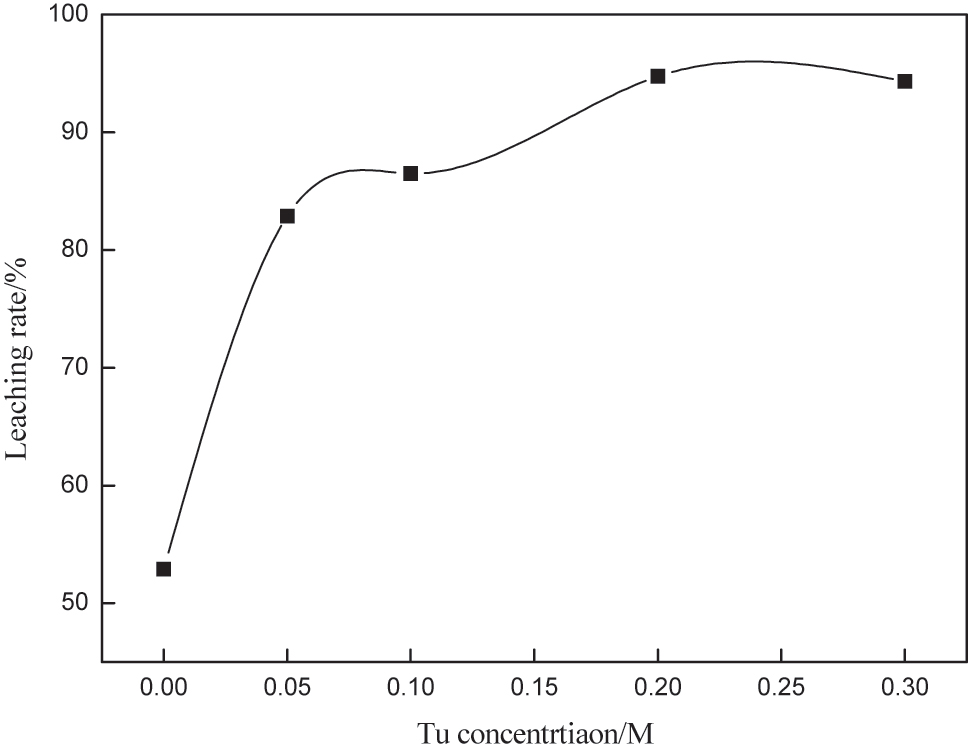
Effect of thiourea concentration on gold leaching.
3.2 Influence of iron ions
Fe3+ acts as an oxidant to oxidize metallic gold to Au+, which then complexes with thiourea [24]. The effect of iron ion concentration on gold leaching was investigated under the conditions of thiourea concentration of 0.2 M, pH 15, rotation speed of 250 rpm, liquid-solid ratio of 4, and leaching time of 10 h. The results are shown in Figure 2, from which it can be seen that when the iron ions are less, gold is almost not leached, indicating that iron ions are essential in the leaching of gold. the iron ion concentration reaches 0.003 M, gold leaching reaches stability. Subsequently, as the iron ion concentration increases, the leaching of gold decreases slightly the reason may be that the higher concentration of iron ions intensifies the oxidation of thiourea. Zhao et al. (2023) confirmed via DFT calculations that high Fe3+ concentrations increase the activation energy of thiourea oxidation, accelerating reagent loss [25], 26]. Therefore, 0.003 M was chosen as the optimal Fe3+ concentration.
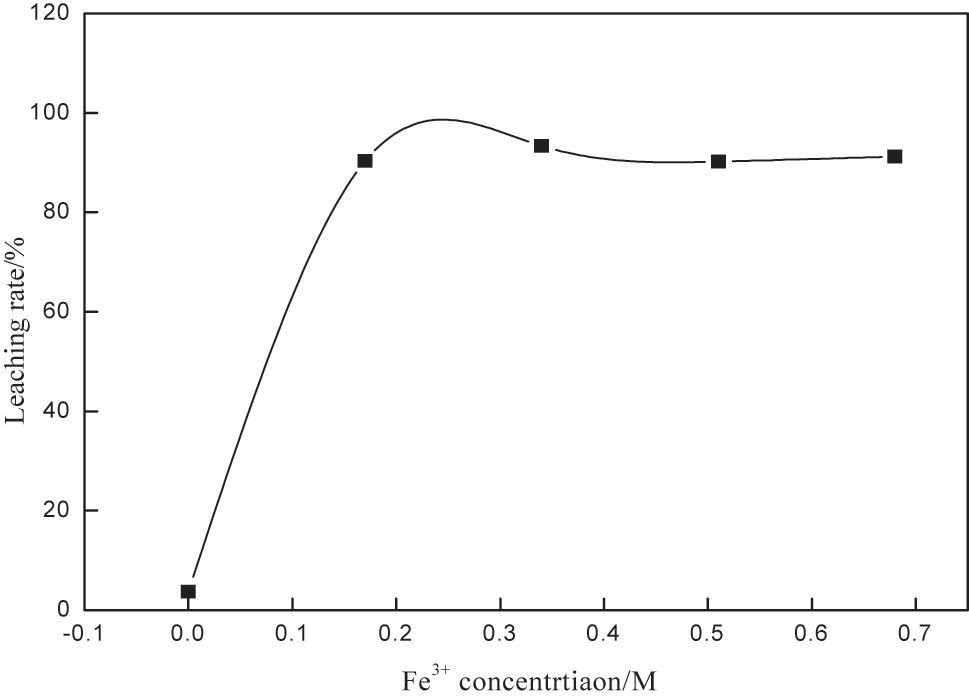
Effect of iron ion concentration on gold leaching.
3.3 Thiocyanate ion effects
In order to further reduce the impact of thiourea consumption, thiocyanate ions were added to chelate trivalent iron, reducing electrode potential of iron ions [27]. The effect of thiourea concentration on gold leaching was investigated under the conditions of thiourea concentration of 0.2 M, ion concentration of 0.003 M, pH 1.5, stirring speed of 250 rpm, liquid-solid ratio of 4, and le time of 10 h. The results are shown in Figure 3, from Figure 3, it can be seen that under the condition of fixed parameters, the of thiocyanate ions and the increase of its concentration can significantly reduce the consumption of thiourea. However, at the same time, thiocyanate ions also reduced the leaching rate of gold. The reason is that the addition of thiocyanate ions reduces the electrode potential of trivalent iron ions and reduces the mixed potential of leaching system [27], 28]. Therefore, a comprehensive balance between the leaching rate of gold and the consumption of reagents is needed. If the grade of gold is not high, it is to consider reducing the leaching rate of gold appropriately, thereby reducing the pressure of reagent consumption. Therefore, 0.001 M thiocyanate ion was used the additive in the subsequent experiment [9].
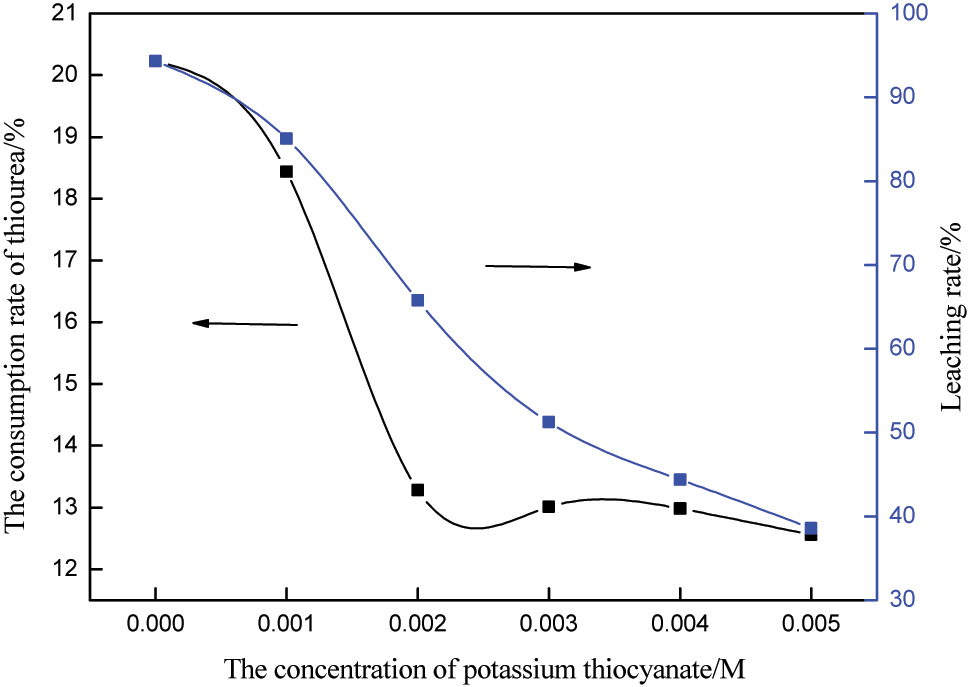
Influence of thiocyanate ion concentration on the rate of gold leaching and thiourea consumption rate.
3.4 Influence of liquid-solid ratio
The liquid-solid ratio will affect the viscosity of the pulp, which will inevitably affect the diffusion rate of the leaching solution to the gold surface, and will also affect the equipment, reagents, and energy consumption, etc. [10]. Therefore, it is essential to select a reasonable liquid-solid ratio. Under the conditions of thiou concentration of 0.2 M, iron ion concentration of 0.003 M, thiocyanate of 0.001 M, pH 1.5, stirring speed of 250 rpm, and leaching time of 10 h, the influence of the liquid-solid ratio on the leaching of gold was investigated The results are shown in Figure 4.
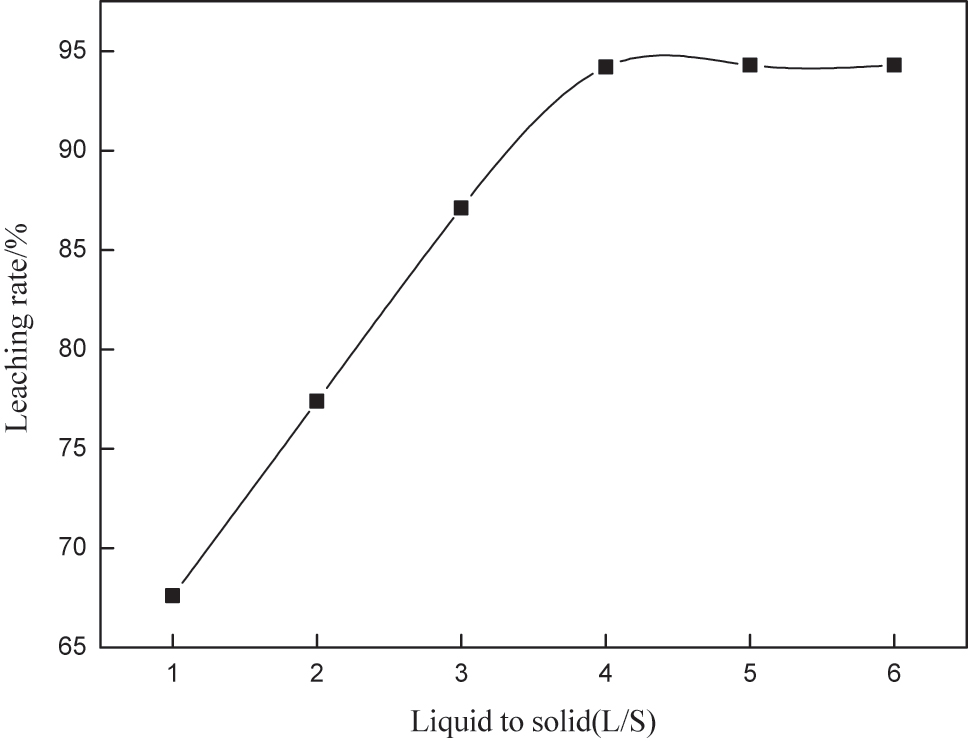
Influence of liquid-solid ratio on gold leaching.
As indicated in Figure 4, the gold leaching rate increased with the increase of liquid-solid ratio in the range of 1:1–4:1. When the liquid-solid ratio reached 4:1, the gold leaching rate stabilized. A further increase in the liquid-solid ratio did not improve the leaching rate but increased reagent consumption and equipment load. Thus, a liquid-solid ratio of 4:1 was determined as the optimal parameter for subsequent experiments.
3.5 Influence of extraction time
Selecting an appropriate leaching time is critical for improving gold leaching efficiency and reducing reagent consumption. Insufficient leaching time results in incomplete gold dissolution, while excessive leaching time increases production costs without significant benefits [1]. The effect of leaching time on the leaching of gold was investigated under the conditions of thiourea concentration of 0.2 M, iron ion concentration of 0.003 M, thiocyan ion, pH 1.5, stirring speed of 250 rpm, and liquid-solid ratio of 4. The tests were conducted at leaching times of 0.5, 1, 6, 8, and 10 h. The results are shown in Figure 5. It can be seen from Figure 5 the leaching time increased to 6 h, and the leaching rate of gold increased accordingly. After that, extending the leaching time had no obvious effect on the leaching of gold, indicating that under this condition, 6 h was the optimal leaching time. Based on this, the optimal conditions of the system were as follows: Tu concentration of 0.2 M, iron ion concentration of 0.003 M, thiocyanate ion concentration of 0.001 M, pH 1.5, stirring speed of 250 rpm, liquid-solid ratio of 4, and leaching time of 6 h.
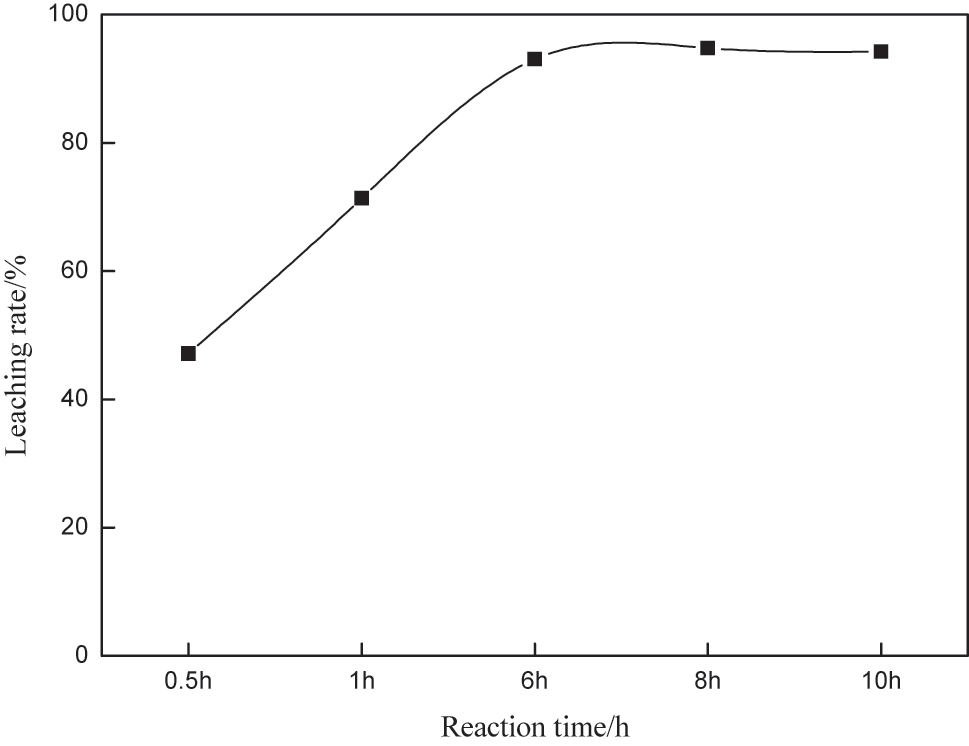
The effect of time on the impregnation rate.
3.6 Comparison of results
Three methods were used to leach the same ore, and the results are compared in Table 3 [11]. The cyanide leaching took the longest time, with leaching rate of 91.6 %. The thiosulphate process was fast, with a gold leaching rate of 86.4 %, and thiourea process was not only fast but also had the highest gold leaching rate. This shows that the method is a potentially better gold extraction method.
Comparison of the leaching results of the same ore by different leaching systems.
| Leaching system | Leaching condition | Extraction results |
|---|---|---|
| Cyanide process | NaCN: 1 kg/t; CaO: 8 kg/t | 24 h, 91.6 % |
| Thiosulfate process | Copper ion: 0.005 M, ethylenediamine: 0.01 M, thiosulfate: 0.1 M, pH 10 | 4 h, 86.4 % |
| Sulfonylurea method | Tu: 0.2 M, Fe3+: 0.003 M, SCN: 0.001 M, pH 1.5 | 6 h, 93.1 % |
3.7 Characterization of slag before and after leaching
3.7.1 XPS characterization
To further analyze the effect of thiocyanate in this leaching system, XPS was used to focus on the S2p/2 and Fe2p3/2 spectra before and after leaching of the leaching residue at different concentrations of thiocyanate. The results of the XPS analysis shown in Figures 6 and 7 and Table 4. When the thiocyanate concentration is 0.0005 M, the binding energies of the2p3/2 spectra at the center position are 161.50 eV, 162.47 eV and 163.10 eV 166.30, which are attributed to S2−, S2 2− and S0, SO3 2−/SO4 2− [12], 13], respectively. When the thiocyanate concentration is 0.001–0.003 M,sulfur exhibits peaks similar to those at 0.005 M, with no obvious changes, indicating that the chemical reactions occurring between thiourea, iron ions and thiocyanate are similar in the range of 00,005–0.003 M. When the thiocyanate concentration is 0.003–0.005 M, there is no S2− peak, but there is SO3 2−/SO4 2−, indicating that sulfur is fully oxidized. S element and S2− are easy to form aivation layer on the mineral surface,and the absence of S2− peak indicates that the increase of iron ion concentration in the appropriate range is beneficial to reduce the passivation of gold [14].
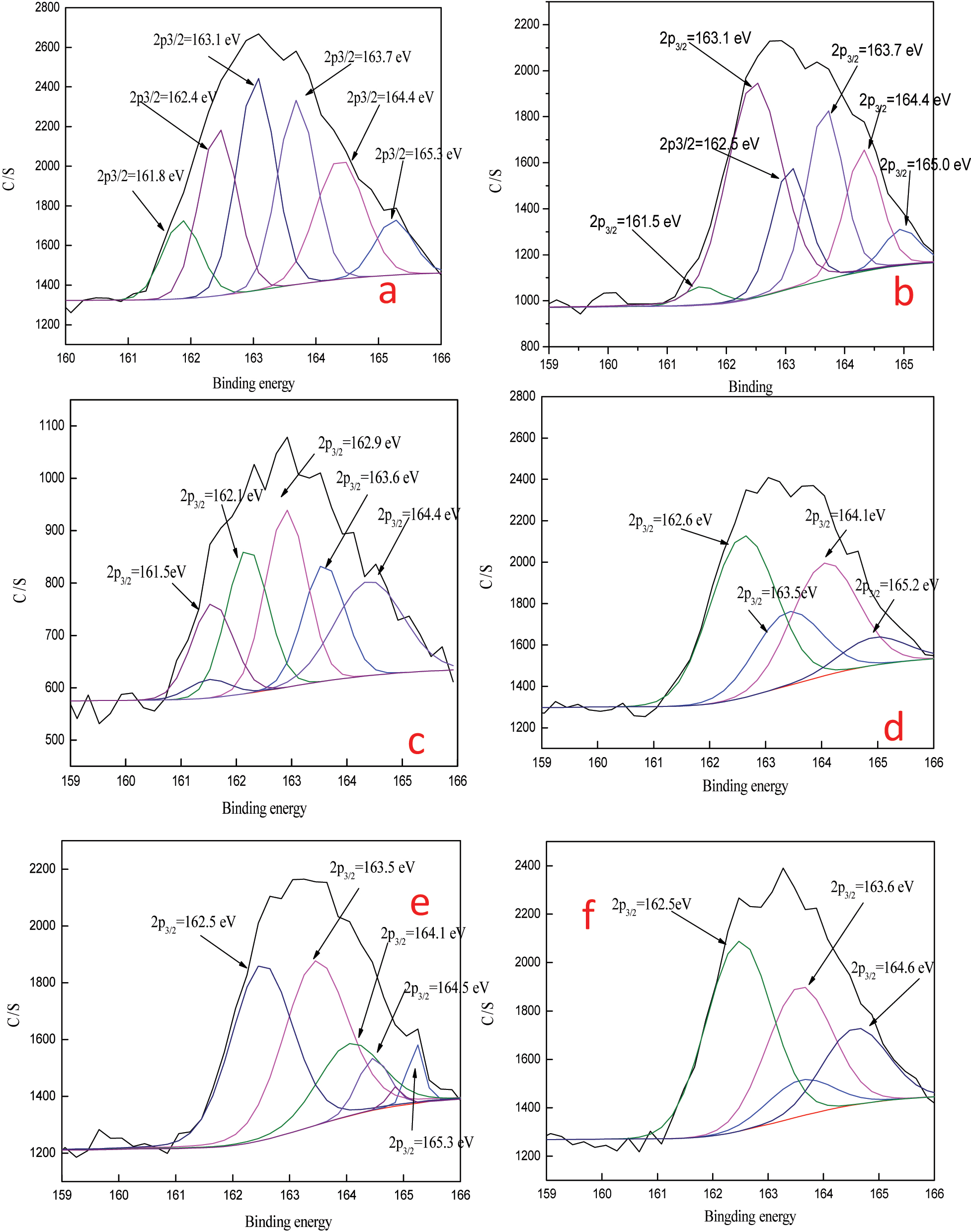
S2p3/2 deconvolution of ore before and after leaching at different thiocyanate concentr. (a) 0 M thiocyanate ion; (b) 0.001 M thiocyanate ion; (c) 0.002 M thiocyanate ion; (d) 0.003 M thiocyanate ion; (e) 0.004 M thiocyanate ion; (f) 0.005 M thiocyanate ion.
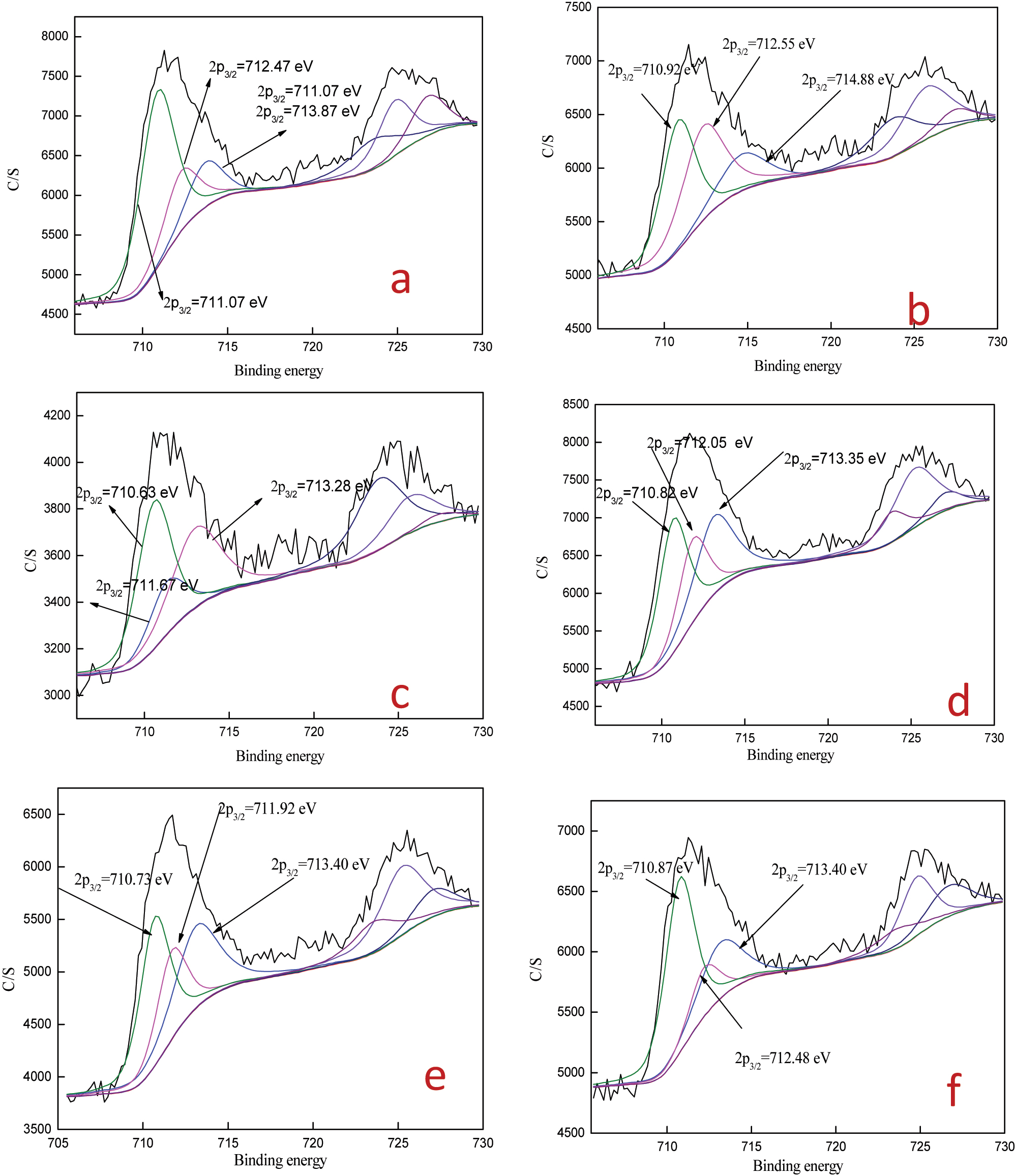
Fe2p3/2 deconvolution of ore before and after leaching at different thiocyanate concentrations. (a) 0 M thiocyanate ion; (b) 0.001 M thiocyanate ion; (c) 0.002 M thiocyanate ion; (d) 0.003 M thiocyanate ion; (e) 0.004 M thiocyanate ion; (f) 0.005 M thiocyanate ion.
Parameters of the fitted peaks of S2p3/2 and Fe2p3/2 before and after leaching at different thiocyanate and the corresponding spectrum analysis.
| Spectrum | 0.0005 M | 0.001 M | 0.002 M | 0.003 M | 0.004 M | 0.005 M | Interpretation |
|---|---|---|---|---|---|---|---|
| S2p3/2 | BE | BE | BE | BE | BE | BE | |
| 161.50 | 161.57 | 161.50 | S2- | ||||
| 162.47 | 162.50 | 162.11 | 162.67 | 162.52 | 162.52 | S2 2- | |
| 163.10 | 163.17 | 163.65 | 163.55 | 163.51 | 163.67 | S0/Sn 2- | |
| 166.30 | 165.01 | 165.22 | SO3 2−/SO4 2- | ||||
| Fe2p3/2 | 711.00 (1.70) | 710.92 | 710.63 | 710.82 | 710.92 | 710.87 | Fe(OH)3/Fe2O3 |
| 712.47 | 712.55 | 712.05 | 712.05 | 712.55 | 712.47 | Fe(Ⅱ)S | |
| 713.87 | 714.87 | 713.28 | 713.35 | FeSO4 | |||
| 714.88 | 714.48 | Fe2(SO4)3 |
For the Fe2p3/2 spectra,in the range of thiocyanate concentration of 0.0005–0.003 M, the forms of iron existence are similar. Fe(OH)3/Fe2O3,FeSO4, but not Fe2(SO4)3 are all present.
This indicates that when the thiocyanate concentration is small, iron ions are fully reduced to divalent iron. When theiocyanate concentration is 0.004–0.005 M, Fe2(SO4)3 reappears on the surface of the slag, the addition and increase of thiocyanate ions can significantly reduce the consumption of thiourea [15], 16]. However, at the same time, thiocyanate ions also significantly reduce leaching rate of gold. This result is consistent with the results of S2p3/2 research.
3.7.2 Characterization of impregnated scrub residue morphology
The surface morphology comparison of ore before and after leaching is shown in Figure 8, where Figure 8(a)–(c) are the surface morphology of gold ore before leaching, and (b) and (d) are the surface morphology of gold ore after leaching. It can be seen from Figure 8 that the leaching residue is broken and scattered, and there are a number of gullies and cracks left after leaching. It can be seen from Figure 8(d) that after leaching, the surface of the gold ore is with leaching agent, and the color and luster of the surface of the ore have changed. These fully demonstrate that the gold ore is corroded by the leaching system [17], 29].
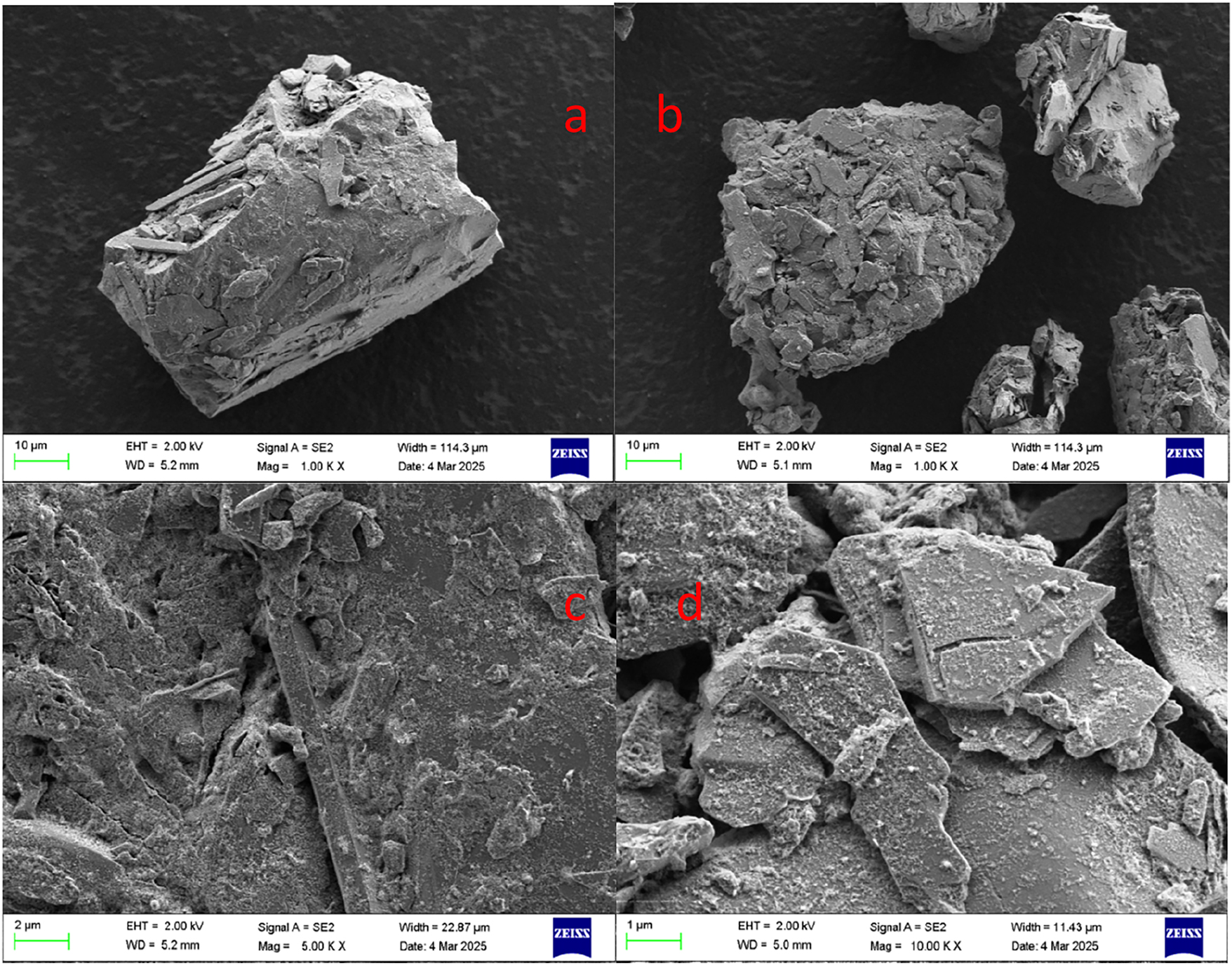
Comparison of surface morphology of ore before and after leaching.
4 Conclusions
This study systematically investigated the gold leaching process from a Laos oxide ore using an iron ion-thiourea-thiocyanate system, analyzed the effect of key parameters, and revealed the mechanism of SCN− action via characterization. The main conclusions are as follows:
Optimal leaching conditions: The optimal parameters for gold extraction were determined as thiourea concentration 0.2 M, Fe3+ concentration 0.003 M, SCN− concentration 0.001 M, pH 1.5, stirring speed 250 rpm, liquid-solid ratio 4, and leaching time 6 h. Under these conditions, the gold leaching rate reached 93.1 %, which was higher than that of traditional cyanide (91.6 %) and thiosulfate (86.4 %) processes.
SCN−complexes with Fe3+ reducing the electrode potential of Fe3+/Fe2+ from 0.77 V to 0.55 V. This not only inhibits the oxidation of thiourea (reducing consumption by 66.7 %) but also slightly decreases the gold leaching rate (by 5.7 %). XPS results showed that SCN− inhibits the formation of S2− on the ore surface, further improving leaching efficiency.
SEM observations confirmed that the leaching system corroded the ore surface, forming gullies and cracks that promoted mass transfer. XPS analysis revealed the evolution of S and Fe species: S2− disappeared, and Fe3+ was reduced to Fe2+, which is consistent with the mechanism of SCN− regulation.
The iron-thiourea-thiocyanate system is an eco-friendly non-cyanide technology with high efficiency and low cost. It provides a feasible solution for gold extraction from oxide ores and lays a foundation for industrial application. Future research should focus on scaling up the process and optimizing reagent recycling to further reduce costs.
Funding source: Rare and Precious Metals Extraction and MineEcological Management Team Foundation(22YNOUTD02)
Award Identifier / Grant number: 22YNOUTD02
-
Research funding: This work was financially supported by the Rare and Precious Metals Extraction and MineEcological Management Team Foundation (22YNOUTD02).
-
Author contributions: Deng Chao: resources, writing original draft, writing – review and editing; Xiang Peng Zhi: project administration and funding acquisition.
-
Conflict of interest: Authors state no conflict of interest.
-
Data Availability Statement: The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
1. Brooy, S, Linge, HG, Walker, GS. Review of gold extraction from ores. Miner Eng 1994;7:1213–41. https://doi.org/10.1016/0892-6875-94-90114-7.Search in Google Scholar
2. Rgül, S, Atalay. Reaction chemistry of gold leaching in thiourea solution for a Turkish gold ore. Hydrometallurgy 2002;67:71–7. https://doi.org/10.1016/s0304-386x-02-00136-6.Search in Google Scholar
3. Ubaldini, S, Fornari, P, Massidda, R, Abbruzzese, C. An innovative thiourea gold leaching process. Hydrometallurgy 1998;48:113–24. https://doi.org/10.1016/s0304-386x-97-00076-5.Search in Google Scholar
4. Muñoz, GA, Miller, JD. Noncyanide leaching of an auriferous pyrite ore from Ecuador. Min Metall Explor 2000;17:198–204. https://doi.org/10.1007/bf03402848.Search in Google Scholar
5. Su, Z, Wang, YY, Chai, LY. Research status and prospect of gold leaching in alkaline thiourea solution. Miner Eng 2006;19:1301–6. https://doi.org/10.1016/j.mineng.2005.12.009.Search in Google Scholar
6. Li, J, Safarzadeh, MS, Moats, MS, Miller, JD, Levier, KM, Dietrich, M, et al.. Thiocyanate hydrometallurgy for the recovery of gold. Part I: chemical and thermodynamic considerations. Hydrometallurgy 2012;113:1–9. https://doi.org/10.1016/j.hydromet.2011.11.005.Search in Google Scholar
7. Ren, C, Wu, B, He, S. Kinetics of gold cyanidation process from acidic biopreoxidized residue. Rare Met 2024;48:1661–70.Search in Google Scholar
8. Zhang, Y, Xu, B, Zheng, Y, Li, Q, Lyu, X, Liu, X, et al.. Hexaamminecobalt(III) catalyzed thiosulfate leaching of gold from a concentrate calcine and gold recovery from its pregnant leach solution via resin adsorption. Miner Eng 2021;171:107079. https://doi.org/10.1016/j.mineng.2021.107079.Search in Google Scholar
9. Azizitorghabeh, A, Mahandra, H, Ramsay, J, Ghahreman, A. A sustainable approach for gold recovery from refractory source using novel BIOX-TC system. J Ind Eng Chem 2022;115:209–18. https://doi.org/10.1016/j.jiec.2022.08.002.Search in Google Scholar
10. Wang, Q, Hu, X, Zi, F, Yang, P, Chen, Y, Chen, S. Environmentally friendly extraction of gold from refractory concentrate using a copper-ethylenediamine-thiosulfate solution. J Clean Prod 2019;214:860–72. https://doi.org/10.1016/j.jclepro.2019.01.007.Search in Google Scholar
11. Xiang, XY, Ye, GH, Zhu, SQ, Rong, YY, Xiang, PZ. Gold extraction from oxide ore using copper ethylenediamine thiosulfate. Russ J Inorg Chem 2024;69:1362–9. https://doi.org/10.1134/s0036023624601314.Search in Google Scholar
12. Holmes, PR, Crundwell, FK. The kinetics of the oxidation of pyrite by ferric ions and dissolved oxygen: an electrochemical study. Geochem Cosmochim Acta 2000;64:263–74. https://doi.org/10.1016/s0016-7037-99-00296-3.Search in Google Scholar
13. Weisener, C, Gerson, A. An investigation of the Cu(II) adsorption mechanism on pyrite by ARXPS and SIMS. Miner Eng 2000;13:1329–40. https://doi.org/10.1016/s0892-6875-00-00116-3.Search in Google Scholar
14. Sasaki, K, Tsunekawa, M, Ohtsuka, T, Konno, H. Confirmation of a sulfur-rich layer on pyrite after oxidative dissolution by Fe(III) ions around pH 2 – reply. Geochem Cosmochim Acta 1997;61:3273–4. https://doi.org/10.1016/s0016-7037-97-00145-2.Search in Google Scholar
15. Sanchez-Arenillas, M, Mateo-Marti, E. Spectroscopic study of cystine adsorption on pyrite surface: from vacuum to solution conditions. Chem Phys 2015;458:92–8. https://doi.org/10.1016/j.chemphys.2015.07.016.Search in Google Scholar
16. Yu, L, Liu, Q, Li, S, Deng, J, Luo, B, Lai, H. Depression mechanism involving Fe3+ during arsenopyrite flotation. Sep Purif Technol 2019;222:109–16. https://doi.org/10.1016/j.seppur.2019.04.007.Search in Google Scholar
17. Du, H, Zhang, L, Guo, X. Research on the enhanced thiosulfate leaching of gold by roasting sand. Gold 2024;45:25–31.Search in Google Scholar
18. Liu, Y, Li, X, Wang, H. Kinetics of gold leaching in iron(III)-thiourea system: effect of temperature. Hydrometallurgy 2022;218:105987.Search in Google Scholar
19. Zhang, L, Zhao, Y, Chen, J. Regulation of Fe(III) electrode potential by thiocyanate in gold leaching: a DFT study. J Mol Liq 2023;375:121456.Search in Google Scholar
20. Wang, X, Liu, J, Zhang, H. Comparative study on gold leaching from oxide ore using thiourea and thiosulfate systems. Minerals 2022;12:956.Search in Google Scholar
21. Chen, M, Li, Y, He, Z. XPS analysis of sulfur species evolution during thiourea gold leaching. Surf Interface Anal 2023;55:289–98.Search in Google Scholar
22. Yang, Z, Wang, Q, Li, J. Effect of additives on thiourea consumption in gold leaching from refractory ore. J Ind Eng Chem 2024;128:109–18.Search in Google Scholar
23. Zhao, H, Zhang, S, Liu, M. Optimization of liquid-solid ratio in iron-thiourea-thiocyanate gold leaching system via response surface methodology. Chem Eng Res Des 2023;192:345–54.Search in Google Scholar
24. Li, D, Chen, G, Wang, F. Study on the stability of thiourea in acidic iron(III) solution. Sep Purif Technol 2022;291:120876.Search in Google Scholar
25. Huang, Y, Xu, L, Zhou, J. SEM characterization of ore surface corrosion during non-cyanide gold leaching. Microsc Microanal 2023;29:210–11.Search in Google Scholar
26. Zhang, C, Li, W, Yang, H. Gold leaching from Laos oxide ore: effect of pH on iron-thiourea complexation. J Mater Cycles Waste Manag 2024;26:876–85.Search in Google Scholar
27. Wang, Y, Zhao, J, Li, S. Industrial prospect of iron-thiourea-thiocyanate system for non-cyanide gold extraction. JOM 2023;75:1890–8.Search in Google Scholar
28. Li, C, He, J, Zhang, Z. Influence of stirring speed on mass transfer in gold leaching with iron-thiourea system. Chem Eng Commun 2022;209:1678–89.Search in Google Scholar
29. Ren, C, Wu, B, He, S. Biological pre-oxidation-acidic thiourea leaching combined process. Miner Process 2020;29:62–7.Search in Google Scholar
Supplementary Material
This article contains supplementary material (https://doi.org/10.1515/gps-2025-0097).
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Optimized green synthesis of silver nanoparticles from guarana seed skin extract with antibacterial potential
- Green adsorbents for water remediation: Removal of Cr(vi) and Ni(ii) using Prosopis glandulosa sawdust and biochar
- Green approach for the synthesis of zinc oxide nanoparticles from methanolic stem extract of Andrographis paniculata and evaluation of antidiabetic activity: In silico GSK-3β analysis
- Development of a green and rapid ethanol-based HPLC assay for aspirin tablets and feasibility evaluation of domestically produced bioethanol in Thailand as a sustainable mobile phase
- A facile biodegradation of polystyrene microplastic by Bacillus subtilis
- Enhanced synthesis of fly ash-derived hydrated sodium silicate adsorbents via low-temperature alkaline hydrothermal treatment for advanced environmental applications
- Impact of metal nanoparticles biosynthesized using camel milk on bacterial growth and copper removal from wastewater
- Preparation of Co/Cr-MOFs for efficient removal of fleroxacin and Rhodamine B
- Applying nanocarbon prepared from coal as an anode in lithium-ion batteries
- Improved electrochemical synthesis of Cu–Fe/brass foil alloy followed by combustion for high-efficiency photoelectrodes and hydrogen production in alkaline solutions
- Precipitation of terephthalic acid from post-consumer polyethylene terephthalate waste fractions
- Biosynthesized zinc oxide nanoparticles: Multifunctional potential applications in anticancer, antibacterial, and B. subtilis DNA gyrase docking
- Anticancer and antimicrobial effects of green-synthesized silver nanoparticles using Teucrium polium leaves extract
- Green synthesis of eco-friendly bioplastics from Chlorella and Lithothamnion algae for safe and sustainable solutions for food packaging
- Optimizing coal water slurry concentration via synergistic coal blending and particle size distribution
- Green synthesis of Ag@Cu and silver nanowire using Pterospermum heterophyllum extracts for surface-enhanced Raman scattering
- Green synthesis of copper oxide nanoparticles from Algerian propolis: Exploring biochemical, structural, antimicrobial, and anti-diabetic properties
- Simultaneous quantification of mefenamic acid and paracetamol in fixed-dose combination tablet dosage forms using the green HPTLC method
- Green synthesis of titanium dioxide nanoparticles using green tea (Camellia sinensis) extract: Characteristics and applications
- Pharmaceutical properties for green fabricated ZnO and Ag nanoparticle-mediated Borago officinalis: In silico predications study
- Synthesis and optimization of gemcitabine-loaded nanoparticles by using Box–Behnken design for treating prostate cancer: In vitro characterization and in vivo pharmacokinetic study
- A comparative analysis of single-step and multi-step methods for producing magnetic activated carbon from palm kernel shells: Adsorption of methyl orange dye
- Sustainable green synthesis of silver nanoparticles using walnut septum waste: Characterization and antibacterial properties
- Efficient electrocatalytic reduction of CO2 to CO over Ni/Y diatomic catalysts
- Greener and magnetic Fe3O4 nanoparticles as a recyclable catalyst for Knoevenagel condensation and degradation of industrial Congo red dye
- Recycling of HDPE-giant reed composites: Processability and performance
- Fabrication of antibacterial chitosan/PVA nanofibers co-loaded with curcumin and cefadroxil for wound healing
- Cost-effective one-pot fabrication of iron(iii) oxychloride–iron(iii) oxide nanomaterials for supercapacitor charge storage
- Novel trimetallic (TiO2–MgO–Au) nanoparticles: Biosynthesis, characterization, antimicrobial, and anticancer activities
- Green-synthesized chromium oxide nanoparticles using pomegranate husk extract: Multifunctional bioactivity in antioxidant potential, lipase and amylase inhibition, and cytotoxicity
- Therapeutic potential of sustainable zinc oxide nanoparticles biosynthesized using Tradescantia spathacea aqueous leaf extract
- Chitosan-coated superparamagnetic iron oxide nanoparticles synthesized using Carica papaya bark extract: Evaluation of antioxidant, antibacterial, and anticancer activity of HeLa cervical cancer cells
- Antioxidant potential of peptide fractions from tuna dark muscle protein isolate: A green enzymatic approach
- Clerodendron phlomoides leaf extract-mediated synthesis of selenium nanoparticles for multi-applications
- Optimization of cellulose yield from oil palm trunks with deep eutectic solvents using response surface methodology
- Nitrogen-doped carbon dots from Brahmi (Bacopa monnieri): Metal-free probe for efficient detection of metal pollutants and methylene blue dye degradation
- High energy density pseudocapacitor based on a nanoporous tungsten(VI) oxide iodide/poly(2-amino-1-mercaptobenzene) composite
- Green synthesized Ag–Cu nanocomposites as an improved strategy to fight multidrug-resistant bacteria by inhibition of biofilm formation: In vitro and in silico assessment study
- In vitro evaluation of antibacterial activity and associated cytotoxicity of biogenic silver nanoparticles using various extracts of Tabernaemontana ventricosa
- Fabrication of novel composite materials by impregnating ZnO particles into bacterial cellulose nanofibers for antimicrobial applications
- Solidification floating organic drop for dispersive liquid–liquid microextraction estimation of copper in different water samples
- Kinetics and synthesis of formation of phosphate composites from low-grade phosphorites in the presence of phosphate–siliceous shales and oil sludge
- Removal of minocycline and terramycin by graphene oxide and Cr/Mn base metal–organic framework composites
- Microfluidic preparation of ceramide E liposomes and properties
- Therapeutic potential of Anamirta cocculus (L.) Wight & Arn. leaf aqueous extract-mediated biogenic gold nanoparticles
- Antioxidant-rich Micromeria imbricata leaf extract as a medium for the eco-friendly preparation of silver-doped zinc oxide nanoparticles with antibacterial properties
- Influence of different colors with light regime on Chlorella sp., biomass, pigments, and lipids quantity and quality
- Experimental vibrational analysis of natural fiber composite reinforced with waste materials for energy absorbing applications
- Green synthesis of sea buckthorn-mediated ZnO nanoparticles: Biological applications and acute nanotoxicity studies
- Production of liquid smoke by consecutive electroporation and microwave-assisted pyrolysis of empty fruit bunches
- Synthesis of MPAA based on polyacrylamide and gossypol resin and applications in the encapsulation of ammophos
- Application of iron-based catalysts in the microwave treatment of environmental pollutants
- Enhanced adsorption of Cu(ii) from wastewater using potassium humate-modified coconut husk biochar
- Adsorption of heavy metal ions from water by Fe3O4 nano-particles
- Green synthesis of parsley-derived silver nanoparticles and their enhanced antimicrobial and antioxidant effects against foodborne resistant bacteria
- Unwrapping the phytofabrication of bimetallic silver–selenium nanoparticles: Antibacterial, Anti-virulence (Targeting magA and toxA genes), anti-diabetic, antioxidant, anti-ovarian, and anti-prostate cancer activities
- Optimizing ultrasound-assisted extraction process of anti-inflammatory ingredients from Launaea sarmentosa: A novel approach
- Eggshell membranes as green carriers for Burkholderia cepacia lipase: A biocatalytic strategy for sustainable wastewater bioremediation
- Research progress of deep eutectic solvents in fuel desulfurization
- Enhanced electrochemical synthesis of Ni–Fe/brass foil alloy with subsequent combustion for high-performance photoelectrode and hydrogen production applications
- Valorization of baobab fruit shell as a filler fiber for enhanced polyethylene degradation and soil fertility
- Valorization of Agave durangensis bagasse for cardboard-type paper production circular economy approach
- Green priming strategies using seaweed extract and citric acid to improve early growth and antioxidant activity in lentil
- Synthesis of N,S co-doped carbon quantum dots – metal complex for the detection of fluoride (F−) ion in adults and Children’s toothpastes
- Gold extraction from oxide ore using iron ion-thiourea-additive
- Review Article
- Sustainable innovations in garlic extraction: A comprehensive review and bibliometric analysis of green extraction methods
- Natural sustainable coatings for marine applications: advances, challenges, and future perspectives
- Integration of traditional medicinal plants with polymeric nanofibers for wound healing
- Rapid Communication
- In situ supported rhodium catalyst on mesoporous silica for chemoselective hydrogenation of nitriles to primary amines
- Special Issue: Valorisation of Biowaste to Nanomaterials for Environmental Applications
- Valorization of coconut husk into biochar for lead (Pb2+) adsorption
- Corrigendum
- Corrigendum to “An updated review on carbon nanomaterials: Types, synthesis, functionalization and applications, degradation and toxicity”
Articles in the same Issue
- Research Articles
- Optimized green synthesis of silver nanoparticles from guarana seed skin extract with antibacterial potential
- Green adsorbents for water remediation: Removal of Cr(vi) and Ni(ii) using Prosopis glandulosa sawdust and biochar
- Green approach for the synthesis of zinc oxide nanoparticles from methanolic stem extract of Andrographis paniculata and evaluation of antidiabetic activity: In silico GSK-3β analysis
- Development of a green and rapid ethanol-based HPLC assay for aspirin tablets and feasibility evaluation of domestically produced bioethanol in Thailand as a sustainable mobile phase
- A facile biodegradation of polystyrene microplastic by Bacillus subtilis
- Enhanced synthesis of fly ash-derived hydrated sodium silicate adsorbents via low-temperature alkaline hydrothermal treatment for advanced environmental applications
- Impact of metal nanoparticles biosynthesized using camel milk on bacterial growth and copper removal from wastewater
- Preparation of Co/Cr-MOFs for efficient removal of fleroxacin and Rhodamine B
- Applying nanocarbon prepared from coal as an anode in lithium-ion batteries
- Improved electrochemical synthesis of Cu–Fe/brass foil alloy followed by combustion for high-efficiency photoelectrodes and hydrogen production in alkaline solutions
- Precipitation of terephthalic acid from post-consumer polyethylene terephthalate waste fractions
- Biosynthesized zinc oxide nanoparticles: Multifunctional potential applications in anticancer, antibacterial, and B. subtilis DNA gyrase docking
- Anticancer and antimicrobial effects of green-synthesized silver nanoparticles using Teucrium polium leaves extract
- Green synthesis of eco-friendly bioplastics from Chlorella and Lithothamnion algae for safe and sustainable solutions for food packaging
- Optimizing coal water slurry concentration via synergistic coal blending and particle size distribution
- Green synthesis of Ag@Cu and silver nanowire using Pterospermum heterophyllum extracts for surface-enhanced Raman scattering
- Green synthesis of copper oxide nanoparticles from Algerian propolis: Exploring biochemical, structural, antimicrobial, and anti-diabetic properties
- Simultaneous quantification of mefenamic acid and paracetamol in fixed-dose combination tablet dosage forms using the green HPTLC method
- Green synthesis of titanium dioxide nanoparticles using green tea (Camellia sinensis) extract: Characteristics and applications
- Pharmaceutical properties for green fabricated ZnO and Ag nanoparticle-mediated Borago officinalis: In silico predications study
- Synthesis and optimization of gemcitabine-loaded nanoparticles by using Box–Behnken design for treating prostate cancer: In vitro characterization and in vivo pharmacokinetic study
- A comparative analysis of single-step and multi-step methods for producing magnetic activated carbon from palm kernel shells: Adsorption of methyl orange dye
- Sustainable green synthesis of silver nanoparticles using walnut septum waste: Characterization and antibacterial properties
- Efficient electrocatalytic reduction of CO2 to CO over Ni/Y diatomic catalysts
- Greener and magnetic Fe3O4 nanoparticles as a recyclable catalyst for Knoevenagel condensation and degradation of industrial Congo red dye
- Recycling of HDPE-giant reed composites: Processability and performance
- Fabrication of antibacterial chitosan/PVA nanofibers co-loaded with curcumin and cefadroxil for wound healing
- Cost-effective one-pot fabrication of iron(iii) oxychloride–iron(iii) oxide nanomaterials for supercapacitor charge storage
- Novel trimetallic (TiO2–MgO–Au) nanoparticles: Biosynthesis, characterization, antimicrobial, and anticancer activities
- Green-synthesized chromium oxide nanoparticles using pomegranate husk extract: Multifunctional bioactivity in antioxidant potential, lipase and amylase inhibition, and cytotoxicity
- Therapeutic potential of sustainable zinc oxide nanoparticles biosynthesized using Tradescantia spathacea aqueous leaf extract
- Chitosan-coated superparamagnetic iron oxide nanoparticles synthesized using Carica papaya bark extract: Evaluation of antioxidant, antibacterial, and anticancer activity of HeLa cervical cancer cells
- Antioxidant potential of peptide fractions from tuna dark muscle protein isolate: A green enzymatic approach
- Clerodendron phlomoides leaf extract-mediated synthesis of selenium nanoparticles for multi-applications
- Optimization of cellulose yield from oil palm trunks with deep eutectic solvents using response surface methodology
- Nitrogen-doped carbon dots from Brahmi (Bacopa monnieri): Metal-free probe for efficient detection of metal pollutants and methylene blue dye degradation
- High energy density pseudocapacitor based on a nanoporous tungsten(VI) oxide iodide/poly(2-amino-1-mercaptobenzene) composite
- Green synthesized Ag–Cu nanocomposites as an improved strategy to fight multidrug-resistant bacteria by inhibition of biofilm formation: In vitro and in silico assessment study
- In vitro evaluation of antibacterial activity and associated cytotoxicity of biogenic silver nanoparticles using various extracts of Tabernaemontana ventricosa
- Fabrication of novel composite materials by impregnating ZnO particles into bacterial cellulose nanofibers for antimicrobial applications
- Solidification floating organic drop for dispersive liquid–liquid microextraction estimation of copper in different water samples
- Kinetics and synthesis of formation of phosphate composites from low-grade phosphorites in the presence of phosphate–siliceous shales and oil sludge
- Removal of minocycline and terramycin by graphene oxide and Cr/Mn base metal–organic framework composites
- Microfluidic preparation of ceramide E liposomes and properties
- Therapeutic potential of Anamirta cocculus (L.) Wight & Arn. leaf aqueous extract-mediated biogenic gold nanoparticles
- Antioxidant-rich Micromeria imbricata leaf extract as a medium for the eco-friendly preparation of silver-doped zinc oxide nanoparticles with antibacterial properties
- Influence of different colors with light regime on Chlorella sp., biomass, pigments, and lipids quantity and quality
- Experimental vibrational analysis of natural fiber composite reinforced with waste materials for energy absorbing applications
- Green synthesis of sea buckthorn-mediated ZnO nanoparticles: Biological applications and acute nanotoxicity studies
- Production of liquid smoke by consecutive electroporation and microwave-assisted pyrolysis of empty fruit bunches
- Synthesis of MPAA based on polyacrylamide and gossypol resin and applications in the encapsulation of ammophos
- Application of iron-based catalysts in the microwave treatment of environmental pollutants
- Enhanced adsorption of Cu(ii) from wastewater using potassium humate-modified coconut husk biochar
- Adsorption of heavy metal ions from water by Fe3O4 nano-particles
- Green synthesis of parsley-derived silver nanoparticles and their enhanced antimicrobial and antioxidant effects against foodborne resistant bacteria
- Unwrapping the phytofabrication of bimetallic silver–selenium nanoparticles: Antibacterial, Anti-virulence (Targeting magA and toxA genes), anti-diabetic, antioxidant, anti-ovarian, and anti-prostate cancer activities
- Optimizing ultrasound-assisted extraction process of anti-inflammatory ingredients from Launaea sarmentosa: A novel approach
- Eggshell membranes as green carriers for Burkholderia cepacia lipase: A biocatalytic strategy for sustainable wastewater bioremediation
- Research progress of deep eutectic solvents in fuel desulfurization
- Enhanced electrochemical synthesis of Ni–Fe/brass foil alloy with subsequent combustion for high-performance photoelectrode and hydrogen production applications
- Valorization of baobab fruit shell as a filler fiber for enhanced polyethylene degradation and soil fertility
- Valorization of Agave durangensis bagasse for cardboard-type paper production circular economy approach
- Green priming strategies using seaweed extract and citric acid to improve early growth and antioxidant activity in lentil
- Synthesis of N,S co-doped carbon quantum dots – metal complex for the detection of fluoride (F−) ion in adults and Children’s toothpastes
- Gold extraction from oxide ore using iron ion-thiourea-additive
- Review Article
- Sustainable innovations in garlic extraction: A comprehensive review and bibliometric analysis of green extraction methods
- Natural sustainable coatings for marine applications: advances, challenges, and future perspectives
- Integration of traditional medicinal plants with polymeric nanofibers for wound healing
- Rapid Communication
- In situ supported rhodium catalyst on mesoporous silica for chemoselective hydrogenation of nitriles to primary amines
- Special Issue: Valorisation of Biowaste to Nanomaterials for Environmental Applications
- Valorization of coconut husk into biochar for lead (Pb2+) adsorption
- Corrigendum
- Corrigendum to “An updated review on carbon nanomaterials: Types, synthesis, functionalization and applications, degradation and toxicity”

