Abstract
Biochar, an economical adsorbent, has drawn attention for Cu(ii) removal from wastewater. However, its performance is limited by inadequate functional groups and low specific surface area. In this study, coconut husk biochar (CB) was selected as the optimal precursor among four biochars modified with potassium humate. The optimized modified biochar (CMB) exhibited significantly enhanced Cu(ii) adsorption capacity under optimal conditions (pH = 6.0, adsorbent dosage = 10 g·L−1). Energy-dispersive X-ray spectroscopy, Brunauer–Emmett–Teller, Fourier transform infrared spectroscopy, and Boehm titration analyses indicated chemisorption via surface complexation and ion exchange as the dominant adsorption mechanisms. Batch experiments evaluated the influence of initial Cu(ii) concentration, pH, adsorbent dosage, and contact time, while adsorption kinetics and isotherms were analyzed using nonlinear regression. Fixed-bed column experiments further revealed that CMB significantly extended the breakthrough time from 90 min (CB) to 300 min (CMB), improving total Cu(ii) adsorption capacity. Additionally, column desorption tests confirmed excellent regeneration potential. Thus, due to its simple preparation, and superior adsorption efficiency, CMB shows promising applicability in Cu(ii) contaminated wastewater treatment.
1 Introduction
Driven by socioeconomic progress and the rapid pace of industrialization and urbanization, heavy metal pollution has become a growing environmental concern [1]. Copper (Cu(ii)), being a common heavy metal, has a narrow range between its optimal concentration and the point at which it becomes toxic to organisms [2]. Industrial and agricultural activities frequently discharge wastewater containing substantial amounts of Cu(ii) into the environment. Cu(ii) can enter the food chain, causing harmful effects on water, soil, and ecosystems, and posing direct and indirect risks to human health [3]. Therefore, it is crucial to develop efficient methods for the removal of Cu(ii) from wastewater.
Currently, various technologies have been developed to remove Cu(ii) from wastewater, including adsorption [4], membrane separation [5], precipitation/electrode position [6], ion exchange [7], and solvent extraction [8]. Among these methods, adsorption is extensively used due to its wide applicability, high efficiency, and simplicity in operation [9,10].
To enhance the effectiveness of adsorption, various adsorbents have been commonly employed, such as magnetic materials, carbon-based materials, silica-based materials, biomaterials, and other composite materials [11]. These materials, characterized by specific properties such as high pore volume, broad pore size distribution, and large specific surface area, are effective for adsorbing pollutants from wastewater [12]. However, their application is often limited by high costs and complex activation processes required to convert raw materials into functional adsorbents [13]. Therefore, there is a growing need for alternative adsorbents that offer comparable performance but can be produced from low-cost raw materials with minimal processing.
In recent years, biochar has emerged as a preferred material for the adsorption of pollutants from wastewater due to its cost-effectiveness [14]. As an environmentally friendly material, biochar not only has high porosity and a highly functionalized surface area but also demonstrates strong potential as an adsorbent in wastewater treatment [15,16]. However, in most cases, the adsorption performance of biochar is limited and often insufficient for removing heavy metals from wastewater. To improve biochar’s adsorption capacity, it is typically optimized through chemical or physical modification. Wassie and Srivastava [17] demonstrated that chemical modification is an effective means of enhancing the adsorption performance of biochar. Further research has shown that modified biochar significantly increases its specific surface area, porosity, optimizes pore size distribution, and augments the number of surface functional groups, thereby enhancing its capacity for heavy metal ion adsorption [15].
Humic acid is a naturally occurring organic substance widely found in soil, water, and sediments, formed from plant and animal residues through complex chemical transformations mediated by microbial activity [18]. Its structure is complex and rich in oxygen-containing functional groups (e.g., phenolic hydroxyl, carbonyl, and carboxyl groups), which provide humic acid with high chemical reactivity, making it highly applicable, especially in metal ion adsorption and complexation [19]. For example, Alhawas et al. [20] found that the maximum adsorption capacity for Pb was 18.85 mg·g−1 when biochar was modified with humic acid and phosphate rock. Similarly, Yang et al. [21] designed a new adsorbent using magnetic corn stover biochar modified with humic acid, achieving adsorption capacities of 163.9 mg·g−1 for acetamiprid and 123.5 mg·g−1 for thiamethoxam in water.
As a potassium salt derivative of humic acid, potassium humate retains its abundant oxygen-containing functional groups and has enhanced water solubility [22]. This property allows potassium humate to integrate with biochar’s pore structure more effectively, providing more active adsorption sites and thereby enhancing metal ion adsorption capacity. The modified biochar not only retains its original pore structure but also exhibits improved metal ion adsorption efficiency owing to potassium humate. However, direct studies investigating potassium humate’s role in this context remain limited. Although the effectiveness of humic acid analogues in enhancing biochar’s adsorption performance has been demonstrated, such as in Giwa et al. [23] who found that optimizing Fe3O4-modified biochar with sodium humate significantly increased specific surface area and pore structure, improving Hg(ii) removal, the specific mechanisms of potassium humate’s interaction with biochar and its synergistic effects require further in-depth investigation.
In this research, potassium humate was used to modify biochar, a cost-effective and abundant adsorbent, to improve its capacity for removing Cu(ii) from wastewater. Four different types of biochars (coconut husk biochar, sawdust biochar, nutshell biochar, and corn cob biochar) were initially evaluated for their adsorption performance following modification. Preliminary experiments indicated that coconut husk biochar (CB) and potassium humate-modified coconut husk biochar (CMB) exhibited the highest adsorption capacities among all tested biochars. Consequently, the current work focused on investigating the surface chemical properties of CB and CMB and evaluating their performance for Cu(ii) removal. Specifically, the objectives of this study were to: (i) clarify the roles of surface functional groups, specific surface area, and pore structure in Cu(ii) adsorption through surface chemical and structural characterization of CB and CMB using energy-dispersive X-ray spectroscopy (SEM-EDS), Fourier transform infrared spectroscopy (FTIR), Brunauer–Emmett–Teller (BET) analyses, and Boehm titration; (ii) systematically examine the effects of key experimental parameters, including solution pH, adsorbent dosage, initial Cu(ii) concentration and contact time, on adsorption efficiency, kinetics, and isotherm behavior; (iii) evaluate dynamic adsorption and desorption characteristics of CB and CMB using fixed-bed column experiments; and (iv) describe and analyze column adsorption results using the Thomas and Yoon–Nelson models. These findings provide valuable insights into the large-scale application of potassium humate-modified biochar in treating Cu(ii) contaminated wastewater.
2 Materials and methods
2.1 Materials and chemicals
The coconut husk biochar (granular, 1.5 mm), sawdust biochar (columnar, 1.5 mm), and nutshell biochar (granular, 1–1.5 mm) used in the experiment were purchased from Zhiyuan Chemical Reagent Co., Ltd., China. The corn cob biochar (granular, 1–5 mm) used was purchased from Dalian Jiucheng Products Co., Ltd., China. Potassium humate, hydrochloric acid, cuprizone (BCO), CuSO4·5H2O, ethanol, and ammonia, belonging to analytical grades, were purchased from Sinopharm Chemical Reagent Co., Ltd., China. All solutions were prepared using deionized water.
2.2 Preparation of potassium humate modified biochar
The biochar samples of coconut husk, sawdust, nutshell, and corn cob, each weighing 1 g, were separately immersed in 250 mL of potassium humate solutions at concentrations of 1, 2, 3, or 4 g·L−1. The modification treatments ranged from 0 to 480 min, and temperatures were set at 25°C, 45°C, 65°C, or 80°C. After modification, the biochars were thoroughly rinsed with deionized water until the wash pH stabilized. Finally, the biochars were oven-dried at 70°C for 240 min pending use.
2.3 Characterization of biochar
The surface morphology and elemental composition of the biochar were examined using a scanning electron microscope equipped with SEM-EDS (TESCAN MIRA LMS, Czech Republic). The specific surface area and pore volume were measured using the automated area and pore size analyzer (Belsorp-Mini-II, Japan) following the BET method, which relies on nitrogen adsorption and desorption. FTIR (IR PRESTIGE-21, Japan) was used to examine changes in the characteristic functional groups of CB and CMB before and after adsorption, with spectra collected over a wavenumber range of 4,000–400 cm−1 through 30 scans. The surface functional groups on the biochar were quantified using the Boehm titration method [24]. Briefly, biochar samples were separately mixed with NaHCO3, Na2CO3, and NaOH solutions. After thorough shaking for 1,440 min and subsequent filtration, 20 mL of each filtrate was extracted, and phenolphthalein was added as an indicator. The solutions were then titrated with 0.5% HCl to the endpoint. The differences in HCl consumption were used to calculate the contents of various oxygen-containing functional groups.
2.4 Comparison of Cu(ii) adsorption
Each experiment used 1 g of CB or CMB, which was separately added to 100 mL of a 100 mg·L−1 Cu(ii) solution. The mixtures were shaken in a constant-temperature shaker at 25°C for 540 min. The Cu(ii) concentration was measured using the BCO method with a UV–visible spectrophotometer at 600 nm [25].
All experiments were conducted in triplicate, and results were averaged. Unless stated otherwise, the modification of biochar was performed using a potassium humate concentration of 1.0 g·L−1 at 25°C for 360 min in subsequent experiments.
2.5 Batch adsorption experiment
2.5.1 Optimal adsorption conditions
In a batch adsorption experiment, the effects of adsorbent dosage and solution pH on Cu(ii) removal were investigated. To examine the effect of dosage, CB and CMB samples (0.2–4 g) were added separately to 100 mL of a 100 mg·L−1 Cu(ii) solution. The mixtures were shaken at 120 rpm at a constant temperature of 25°C for 540 min. To study the influence of pH, 1 g of CB or CMB was added to 100 mL of a 100 mg·L−1 Cu(ii) solution. The pH of the solution was regulated between 2 and 10 by adding 0.1 mol·L−1 HCl or NaOH. The mixtures were then shaken under identical conditions (25°C, 120 rpm) for 540 min. After the adsorption process, all samples were filtered through a 0.45 µm membrane filter, and the Cu(ii) concentrations were analyzed using a UV–visible spectrophotometer at 600 nm. All experiments were performed in triplicate, and average values were calculated.
In the batch adsorption experiment, the Cu(ii) removal rate R(%) and adsorption capacity q e (mg·g−1) were calculated using the following equations:
where R is the Cu(ii) removal rate, q e is the adsorption capacity (mg·g−1), c 0 and c e are the initial concentration and equilibrium concentration of Cu(ii), respectively (mg·L−1), V is the volume of the solution (L), and M is the mass of the biochar (g).
2.5.2 Adsorption kinetic experiment
The biochar samples of CB and CMB, each weighing 1 g, were added to 100 mL of a 100 mg·L−1 Cu(ii) solution. The mixtures were shaken at a constant temperature of 25°C with an agitation speed of 120 rpm. Samples were collected at intervals of 30, 60, 120, 180, 300, 420, 540, 720, 900, and 1,620 min. Afterward, the solutions were filtered through a 0.45 µm membrane, and the Cu(ii) concentration in the filtrate was measured using a UV–visible spectrophotometer. Each experiment was performed in triplicate.
The experimental data were analyzed using pseudo-first-order kinetic Eq. 3, pseudo-second-order kinetic Eq. 4, Elovich Eq. 5, and Intra-particle diffusion Eq. 6.
where q t is the adsorption capacity at time t (mg·g−1), k 1 and k 2, respectively, are the pseudo-first-order and pseudo-second-order adsorption rate constants (1/min) and (g·(mg−1·min−1)), t is the adsorption time (min), α is the initial adsorption coefficient (mg·(g−1·min−1)), β is the resolution factor (g·mg−1), K Ip is the intra-particle diffusion rate constant (mg·(g−1·min−1/2)) and X Ip is the intercept (mg·g−1).
2.5.3 Isothermal adsorption experiment
The biochar samples of CB and CMB, each weighing 1 g, were added separately to 100 mL of Cu(ii) solutions at concentrations of 10, 20, 40, 60, 80, 100, 150, 200, 250, 300, 400, and 500 mg·L−1. The mixtures were shaken at a constant temperature of 25°C and an agitation speed of 120 rpm. After 540 min, samples were collected, filtered through a 0.45 µm membrane, and analyzed for Cu(ii) concentration using a UV–visible spectrophotometer. Each experiment was conducted in triplicate.
The equilibrium adsorption behavior of both biochars was evaluated through Langmuir (7), Freundlich (8), and Temkin isothermal models (9)
where q m is the maximum adsorption capacity (mg·g−1), K L is the adsorption constant of the Langmuir model (L·g−1), K F is the adsorption capacity (mg(1−n)·L n /g), and N is the Freundlich model adsorption constant. N = 1, N < 1, and N > 1 indicate adsorption processes of linear, chemical, and physical processes, respectively. R = 8.314 (J·(mol−1·K−1)) is the universal gases. T is the absolute temperature, K T is the Temkin constant (L·g−1), and b T is the constant related to the variation in adsorption energy.
2.6 Adsorption in a fixed-bed column
The fixed-bed adsorption apparatus consists of an inlet tank, a constant-flow pump, a chromatographic column, and an outlet tank (Figure S1). The column was packed with 17.5 g of biochar to a bed height of 10 cm. To prevent biochar leakage, 1 cm of cotton was placed at both ends of the column, ensuring uniform solution infiltration. A Cu(ii) solution with a concentration of 100 mg·L−1 was pumped through the column at a constant flow rate of 2 mL·min−1. Effluent samples were frequently collected during the initial stage and subsequently at longer intervals until saturation. The Cu(ii) concentrations in the filtrate were measured using the same method as described for batch experiments. The breakthrough point was defined as the moment when the effluent Cu(ii) concentration reached 10% of the initial concentration, while the saturation point was defined when it reached 90%.
2.6.1 Calculation of adsorption column parameters
Total adsorption in column processes (q) is defined as the adsorbate quantity adsorbed from the start to saturation and calculated using
The unit adsorption amount in dynamic adsorption (q 0) is the saturated adsorption per unit mass of the adsorbent, and computed using
The total removal rate in column adsorption (η) is the ratio of total adsorbed mass to the total mass of adsorbate processed, and determined using
where t total is the total operating time of the fixed bed (min), c t is the Cu(ii) concentration at time t (mg·L−1), Q is the influent flow rate (mL·min−1), and m is the adsorbent mass in the column (g).
2.6.2 Column adsorption model analysis
Column adsorption processes were analyzed using the Thomas (13) and Yoon–Nelson models (14). The Thomas model, based on the Langmuir equation, is used frequently to estimate adsorption capacities and rate constants [26]. The Yoon–Nelson model, a classic for column adsorption, is specifically designed for single-component systems [27]
where K Th is the rate constant of the Thomas model ((mL·min−1)·mg−1), q 0 is the adsorption capacity (mg·g−1), K Y is the rate constant of the Yoon–Nelson model (min), and τ is the time required for C t /C 0 to reach 0.5 (min).
2.6.3 Column desorption
Desorption experiments were conducted using a 4% HCl solution as the eluent on the adsorption-saturated chromatographic column, at a flow rate of 2 mL·min−1. Effluent samples were periodically collected throughout the desorption process. The effluent Cu(ii) concentration was measured similarly to the batch mode, until Cu(ii) was no longer detectable in the effluent.
3 Results and discussion
3.1 Effects of potassium humate modification conditions on Cu(ii) adsorption
Figure 1 illustrates the impacts of potassium humate concentration, modification time, and modification temperature on the Cu(ii) adsorption performance of four biochars (coconut husk, sawdust, nutshell, and corn cob).
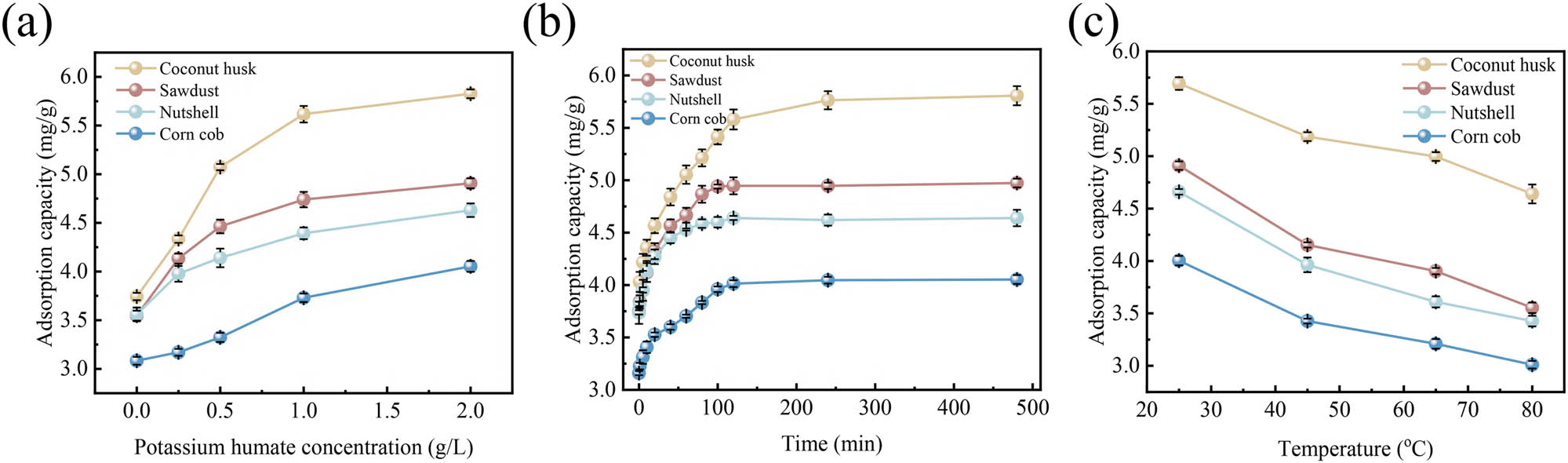
Adsorption capacities of biochars (coconut husk, sawdust, nutshell, and corn cob) for Cu(ii) (mean ± std). (a) Effect of various potassium humate concentrations (modification time 240 min, modification temperature 25°C), (b) effect of different modification times (potassium humate concentration 1.0 g·L−1, modification temperature 25°C), (c) effect of varying modification temperatures (potassium humate concentration 1.0 g·L−1, modification time 240 min) (conditions: Cu(ii) concentration 100 mg·L−1, pH 6, biochar dosage 10 g·L−1, adsorption time 540 min).
The adsorption capacities of biochars generally increased as the concentration of potassium humate increased (Figure 1a). At a concentration of 1.0 g·L−1, the adsorption capacities reached 5.61 mg·g−1 (coconut husk biochar), 4.74 mg·g−1 (sawdust biochar), 4.39 mg·g−1 (nutshell biochar), and 3.73 mg·g−1 (corn cob biochar). This improvement occurred because higher potassium humate concentrations promoted a more thorough interaction with biochars, increasing oxygen-containing functional groups and potassium oxide loading on biochar surfaces, thus enhancing Cu(ii) adsorption [28]. However, increasing potassium humate concentration from 1.0 to 2.0 g·L−1 did not significantly improve adsorption capacities. The increments were less than 0.25 mg·g−1, indicating limited available sites on biochar surfaces for additional functionalization. Therefore, considering both adsorption effectiveness and cost-efficiency, 1.0 g·L−1 was selected as the optimal potassium humate concentration.
Modification time also significantly influenced the Cu(ii) adsorption capacities of the biochars (Figure 1b). As modification time increased, adsorption capacities gradually improved. When modification time extended from 0 to 360 min, the adsorption capacities increased to 5.76 mg·g−1 for coconut husk biochar, 4.94 mg·g−1 for sawdust biochar, 4.62 mg·g−1 for nutshell biochar, and 4.04 mg·g−1 for corn cob biochar. The increase in adsorption capacity was attributed to a more complete reaction between biochar and potassium humate, leading to enhanced surface oxidation, increased oxygen-containing functional groups, and improved microstructure of the biochars, thus promoting the adsorption of Cu(ii). Among these biochars, CMB consistently showed superior adsorption performance, reaching an adsorption capacity of 5.76 mg·g−1 at 360 min, significantly outperforming the other biochars. However, extending the modification time beyond 360–480 min resulted in negligible increases in adsorption capacity (increments less than 0.05 mg·g−1), suggesting that the reaction between potassium humate and biochar had reached equilibrium and that additional modification time did not significantly enhance functional group density or adsorption sites [29]. Therefore, a modification time of 360 min was selected to optimize both adsorption performance and operational efficiency.
Furthermore, modification temperature notably influenced Cu(ii) adsorption capacity (Figure 1c). Increasing the temperature from 25°C to 80°C gradually decreased adsorption capacities from 5.69 to 4.64 mg·g−1 (coconut husk), 4.91 to 3.55 mg·g−1 (sawdust), 4.66 to 3.42 mg·g−1 (nutshell), and 4.00 to 3.01 mg·g−1 (corn cob). Previous studies reported that higher modification temperatures typically enhance adsorption capacities of heavy metals due to improved surface functionalization and porosity [30]. However, the present study found that higher modification temperatures reduced adsorption capacity, likely resulting from diminished active sites, pore structure collapse or blockage, and weakened physical adsorption interactions [31]. Therefore, 25°C was determined as the optimal modification temperature, effectively maximizing Cu(ii) adsorption performance.
3.2 Batch adsorption
Based on the above experiments, optimal conditions for biochar modification with potassium humate were established, and CB was identified as the most effective biochar. Subsequently, the study focused on evaluating the adsorption performance of CB and CMB.
3.2.1 Effects of solution pH and biochar dosage on Cu(ii) adsorption performance
Batch experiments were performed to systematically investigate the influences of solution pH and biochar dosage on the adsorption performance of CB and CMB. As illustrated in Figure 2a, solution pH significantly affected adsorption efficiency. When solution pH increased from 2 to 6, the adsorption capacities and removal efficiencies of Cu(ii) for both CB and CMB progressively increased. At strongly acidic conditions, the high concentration of H+ extensively protonated biochar surfaces, generating a positive charge [32]. This resulted in strong electrostatic repulsion with positively charged Cu(ii), thus inhibiting metal adsorption [33]. Additionally, excess H+ competed directly with Cu(ii) for adsorption sites, further limiting adsorption efficiency [34]. As solution pH rose, decreasing H+ concentrations and increasing OH⁻ reduced biochar surface protonation, mitigating electrostatic repulsion. Consequently, electrostatic attraction between negatively charged biochar surfaces and Cu(ii) was enhanced, improving adsorption efficiency [35]. Nevertheless, once the solution pH exceeded 7, the formation of insoluble Cu(ii) hydroxide precipitates significantly affected the adsorption capacity of the biochars. At pH 7, the residual Cu(ii) concentration in solution after precipitation was approximately 0.57 mg/100 mL, whereas the corresponding adsorption capacities of CB and CMB were only 0.21 and 0.23 mg·g−1, respectively. When the pH increased further to 10, the residual Cu(ii) concentration decreased to approximately 0.18 mg/100 mL due to enhanced precipitation, and the adsorption capacities of CB and CMB were reduced accordingly to 0.11 and 0.18 mg·g−1, respectively. These results clearly indicate that at elevated pH values, copper removal is primarily governed by precipitation.
![Figure 2
Effects of solution pH (a) and biochar dosage (b) on Cu(ii) removal by CB and CMB (mean ± std). ACB, adsorption capacity of coconut husk biochar (mg·g−1); ACMB, adsorption capacity of modified coconut husk biochar (mg·g−1); RCB, removal rate of coconut husk biochar (%); RCMB, removal rate of modified coconut husk biochar (%); CS, amount of Cu(ii) in 100 mL solution (mg) [conditions: Cu(ii) concentration 100 mg·L−1, adsorption time 540 min, biochar dosage in (a) 10 g·L−1, pH in (b) 6].](/document/doi/10.1515/gps-2025-0077/asset/graphic/j_gps-2025-0077_fig_002.jpg)
Effects of solution pH (a) and biochar dosage (b) on Cu(ii) removal by CB and CMB (mean ± std). ACB, adsorption capacity of coconut husk biochar (mg·g−1); ACMB, adsorption capacity of modified coconut husk biochar (mg·g−1); RCB, removal rate of coconut husk biochar (%); RCMB, removal rate of modified coconut husk biochar (%); CS, amount of Cu(ii) in 100 mL solution (mg) [conditions: Cu(ii) concentration 100 mg·L−1, adsorption time 540 min, biochar dosage in (a) 10 g·L−1, pH in (b) 6].
Figure 2b illustrates the influence of biochar dosage on Cu(ii) adsorption performance. Adsorbent dosage significantly impacts the adsorption efficiency by determining the availability of active sites at a given initial concentration [36]. As biochar dosage increased from 5 to 10 g·L−1, Cu(ii) removal efficiencies notably improved from 25.67% to 62.58% for CB and from 26.76% to 70.62% for CMB. However, the adsorption capacities per unit mass decreased substantially from 12.06 to 4.50 mg·g−1 for CB and from 13.37 to 5.62 mg·g−1 for CMB. These results indicated that the enhanced Cu(ii) removal efficiencies at higher biochar dosages were primarily attributed to a greater total number of available adsorption sites, rather than improved intrinsic adsorption properties of the biochar itself. When the biochar dosage further increased beyond 10 g·L−1 (10–40 g·L−1), improvements in Cu(ii) removal efficiency became marginal, reaching only 73.62% for CB and 86.52% for CMB. Concurrently, adsorption capacities experienced substantial reductions, decreasing from 4.50 to 2.37 mg·g−1 for CB and from 5.62 to 3.01 mg·g−1 for CMB. This diminishing return at higher dosages can be explained by increased aggregation of biochar particles, potentially causing blockage of adsorption sites and reduced effective surface areas [37]. Additionally, excessive adsorbent dosage could trigger collisions between particles, resulting in the desorption of weakly adsorbed Cu(ii) and subsequently decreasing overall adsorption efficiency[38]. Considering both adsorption effectiveness and economic feasibility, an optimal biochar dosage of 10 g·L−1 was selected for subsequent experiments.
3.2.2 Adsorption kinetics
To further explore the adsorption behavior of CB and CMB, kinetic experiments were conducted, and the results are shown in Figure 3 and Table 1. As depicted in Figure 3a and b, the adsorption capacities of both biochars increased rapidly during the initial stages, owing to the high Cu(ii) concentration and abundance of unoccupied surface binding sites, which generated a strong mass transfer driving force [32]. As the reaction progressed, active sites were gradually occupied, resulting in a decreased adsorption rate until equilibrium was attained.
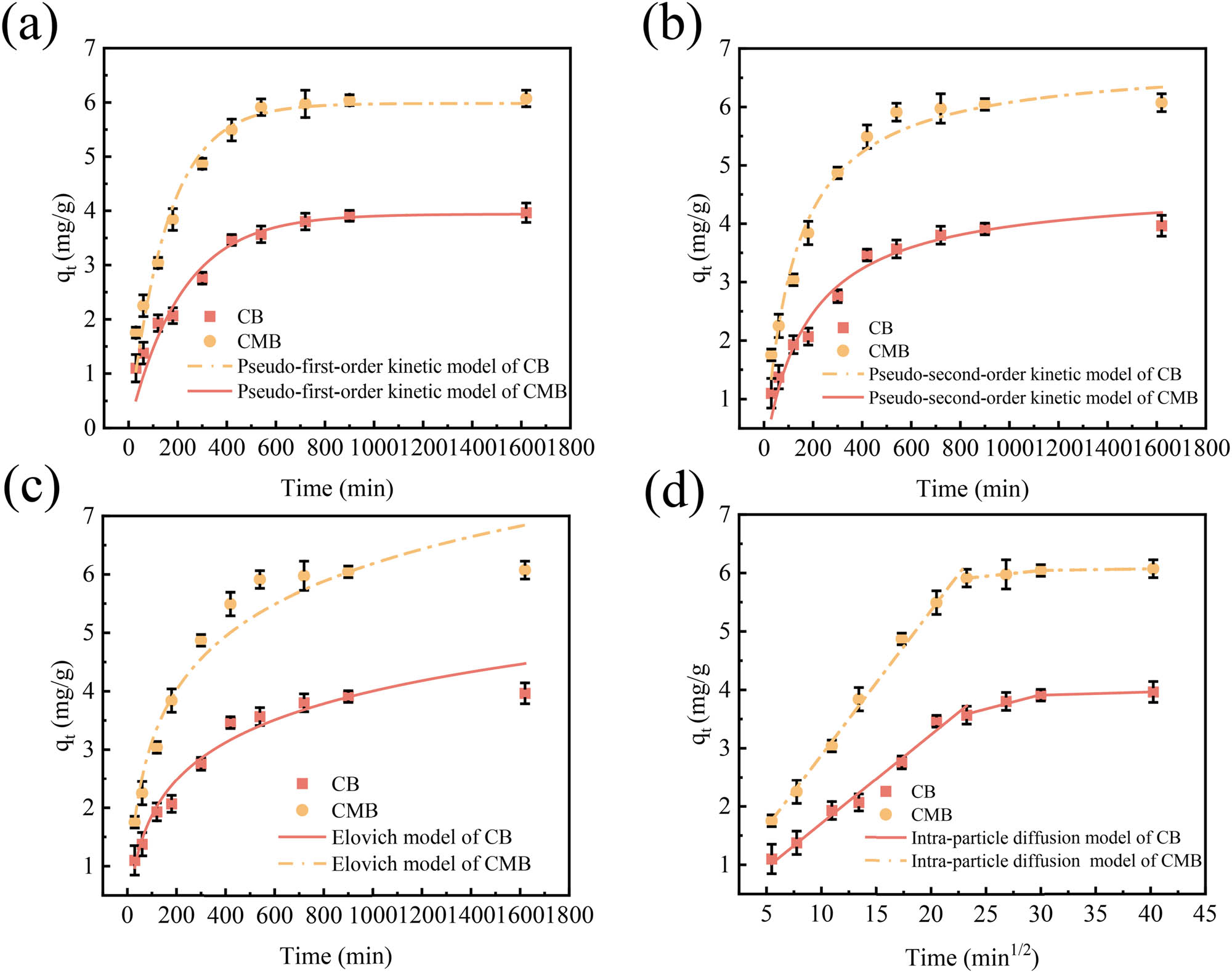
Adsorption kinetics of Cu(ii) onto CB and CMB (mean ± std). (a) Pseudo-first-order kinetic model, (b) pseudo-second-order kinetic model, (c) Elovich model, and (d) intra-particle diffusion model (conditions: Cu(ii) concentration 100 mg·L−1, pH 6, biochar dosage 10 g·L−1, adsorption time 0–1,620 min).
Kinetic parameters for Cu(ii) adsorption by CB and CMB
| Models | Treatments | Parameters | |||
|---|---|---|---|---|---|
| Pseudo-first-order | q e (mg·g−1) | k 1 (1/min) | R 2 | RMSE | |
| CB | 3.9388 | 0.0047 | 0.9477 | 1.3430 | |
| CMB | 5.9815 | 0.0064 | 0.9539 | 2.5450 | |
| Pseudo-second-order | q e (mg·g−1) | k 2 (g·(mg−1·min−1)) | R 2 | RMSE | |
| CB | 4.6461 | 0.0012 | 0.9548 | 1.2500 | |
| CMB | 6.8159 | 0.0012 | 0.9718 | 1.9860 | |
| Elovich | α (mg·(g−1·min−1)) | B (g·mg−1) | R 2 | RMSE | |
| CB | 0.1198 | 0.7238 | 0.9285 | 2.6170 | |
| CMB | 0.0555 | 1.0107 | 0.9512 | 1.5720 | |
| Intra-particle diffusion | K Ip (mg·(g−1·min−1/2)) | X Ip (mg·g−1) | R 2 | RMSE | |
| (First stage) | CB | 0.1525 | 0.1841 | 0.9702 | 0.8820 |
| (Second stage) | CB | 0.0493 | 2.4403 | 0.9474 | 0.1760 |
| (Third stage) | CB | 0.0055 | 3.7446 | 1.0000 | 0.0000 |
| (First stage) | CMB | 0.2478 | 0.4027 | 0.9908 | 0.9880 |
| (Second stage) | CMB | 0.0195 | 5.4570 | 0.9972 | 0.0157 |
| (Third stage) | CMB | 0.0029 | 5.9570 | 1.0000 | 0.0000 |
Nonlinear fitting results revealed that the pseudo-second-order model provided the best correlation with the experimental data, exhibiting higher R 2 values (0.9548 for CB and 0.9718 for CMB) and lower RMSE values (1.2500 and 1.9860, respectively) compared to the pseudo-first-order model (R 2 = 0.9477 and 0.9539, RMSE = 1.3430 and 2.5450). The corresponding theoretical maximum adsorption capacities were 4.64 mg·g−1 for CB and 6.82 mg·g−1 for CMB, which were in good agreement with the actual measured values of 3.75 and 5.83 mg·g−1, respectively. These results suggest that Cu(ii) adsorption onto both CB and CMB primarily occurred through chemisorption [39].
The Elovich and intra-particle diffusion models were also applied to further elucidate the adsorption mechanisms (Figure 3c and d). The Elovich model exhibited good fitting performance with R 2 values of 0.9285 (CB) and 0.9512 (CMB), and RMSE values of 2.6170 (CB) and 1.5720 (CMB), indicating that chemisorption occurred on heterogeneous surfaces [40]. In contrast, the intra-particle diffusion plots displayed multi-linear characteristics and did not pass through the origin, implying that intra-particle diffusion was involved but was not the sole rate-limiting step [35].
As shown in Figure 3d, the Cu(ii) adsorption process could be divided into three distinct phases: an initial rapid phase governed by external (film) diffusion, a slower intermediate phase involving intra-particle diffusion, and a final equilibrium stage. Overall, external diffusion played a significant role in controlling the adsorption rate, while chemisorption remained the dominant adsorption mechanism throughout the process [41].
3.2.3 Isothermal adsorption
Figure 4 and Table 2 present the adsorption isotherm fitting results for Cu(ii) adsorption onto CB and CMB. As shown in Figure 4a and b and Table 2, the Langmuir model provided higher correlation coefficients (R 2 = 0.9896 and 0.9826 for CB and CMB, respectively) and lower RMSE values (1.7110 and 2.2050), compared to the Freundlich model (R 2 = 0.9690 and 0.9365, RMSE = 3.0920 and 3.5260), suggesting that Cu(ii) adsorption by CB and CMB likely occurred via monolayer adsorption on energetically uniform sites. However, it is important to note that Langmuir adsorption can also remain valid under certain conditions involving multilayer adsorption, especially when the first adsorption layer dominates the overall process. For the Freundlich model, the obtained N values were 0.5975 for CB and 0.5612 for CMB, indicating that Cu(ii) adsorption occurred on heterogeneous surfaces with favorable affinity [42,43].
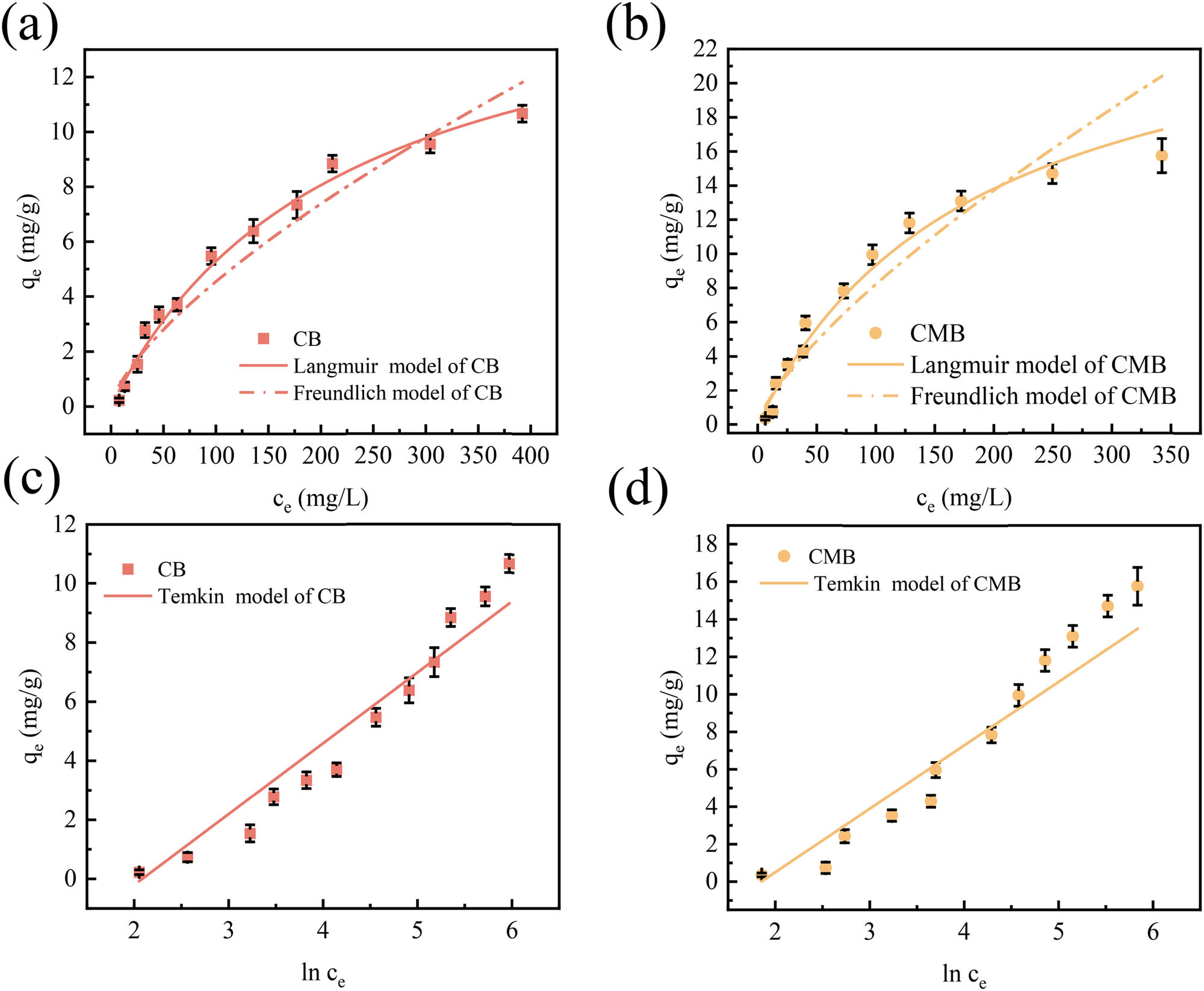
Adsorption isotherms of Cu(ii) onto CB and CMB (mean ± std). (a) Langmuir and Freundlich models for CB, (b) Langmuir and Freundlich models for CMB, (c) Temkin model for CB, and (d) Temkin model for CMB (conditions: initial Cu(ii) concentration 10–600 mg·L−1, pH 6, biochar dosage 10 g·L−1, adsorption time 540 min).
Isotherm parameters of Cu(ii) adsorption by CB and CMB
| Models | Treatments | Parameters | |||
|---|---|---|---|---|---|
| Langmuir | q m (mg·g−1) | K L (L·mg−1) | R 2 | RMSE | |
| CB | 15.1012 | 0.0051 | 0.9896 | 1.7110 | |
| CMB | 21.1305 | 0.0076 | 0.9826 | 2.2050 | |
| Freundlich | K F (mg(1−1/n)·L1/n /g) | N | R 2 | RMSE | |
| CB | 0.3234 | 0.5975 | 0.9690 | 3.0920 | |
| CMB | 0.6675 | 0.5612 | 0.9365 | 3.5260 | |
| Temkin | b T (J·mol−1) | K T (L·g−1) | R 2 | RMSE | |
| CB | 887.4500 | 0.0875 | 0.9536 | 3.1840 | |
| CMB | 574.2700 | 0.1064 | 0.9608 | 3.2940 | |
The fitting results of the Temkin model are shown in Figure 4c and d and Table 2, with R 2 values of 0.9536 for CB and 0.9608 for CMB, and corresponding RMSE values of 3.1840 and 3.2940, respectively. Although the Temkin model exhibited slightly lower R 2 values and higher RMSE values than the Langmuir model, these values were still within an acceptable range. According to the Temkin model, as surface coverage increased, the adsorption heat gradually decreased, suggesting chemisorption likely dominated Cu(ii) removal.
3.3 Fixed-bed column studies
3.3.1 Adsorption and desorption behaviors of Cu(ii) in fixed-bed column
Figure 5a presents the breakthrough and saturation curves for Cu(ii) adsorption on CB and CMB. During the initial phase, the ratio of outlet to inlet concentration (C t /C 0) remained close to zero, indicating a stable removal efficiency above 99%. Over time, the C t /C 0 ratios for both materials increased gradually until reaching their respective breakthrough points: 90 min for CB and 300 min for CMB. This phase represents the optimal period for adsorption [44]. CMB maintained a higher removal efficiency for a longer period, exhibiting its enhanced stability and adsorption capacity. After the breakthrough point, the C t /C 0 ratio increased sharply, indicating the progressive occupation of active adsorption sites and a significant reduction in adsorption capacity [45]. As adsorption progressed, the C t /C 0 ratio for CB and CMB eventually reached their predefined saturation points at 1,080 min and 1,800 min, respectively. Correspondingly, the calculated adsorption capacities were 100.27 mg for CB and 145.20 mg for CMB.
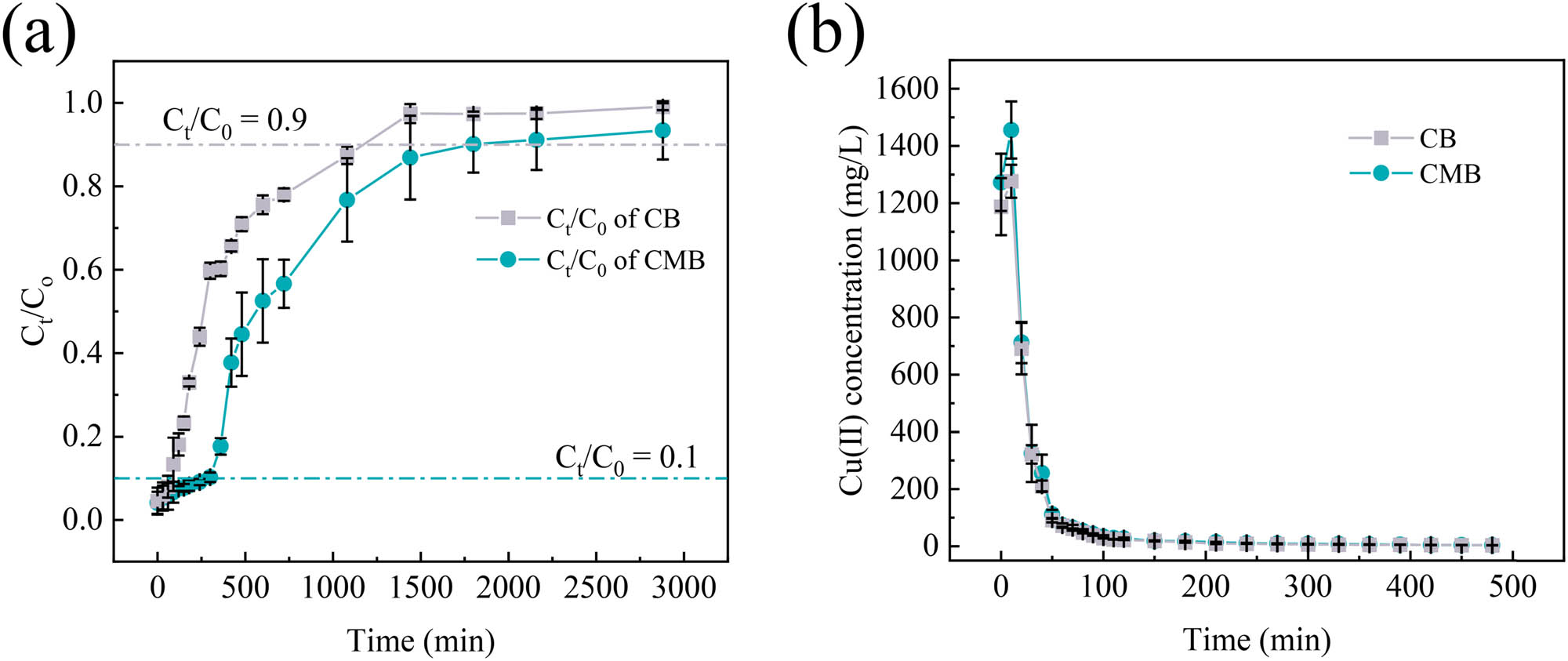
Dynamic adsorption and desorption of Cu(ii) onto CB and CMB (mean ± std): (a) breakthrough and saturation curves for Cu(ii) adsorption onto CB and CMB and (b) dynamic desorption curves of Cu(ii) onto CB and CMB.
Following adsorption, desorption experiments were performed to assess the regeneration potential of CB and CMB, with temporal changes in Cu(ii) concentration in the eluate shown in Figure 5b. Within the initial 10 min, Cu(ii) concentrations rose sharply, peaking at 1,309 mg·L−1 for CB and 1,355 mg·L−1 for CMB, indicating rapid desorption from easily accessible surface adsorption sites. From 10 to 50 min, Cu(ii) concentration declined markedly, signifying the gradual exhaustion of surface-bound Cu(ii) and subsequent desorption from more strongly bound or less accessible sites. Subsequently, from 50 to 420 min, the decline in Cu(ii) concentration slowed notably, reflecting diffusion-limited desorption from deeper internal adsorption sites [44]. After 420 min, the Cu(ii) concentration in the eluates approached zero, signifying completion of the desorption process. It is noteworthy that Cu(ii) concentrations in CMB eluates remained consistently higher compared to CB throughout the desorption process, implying that potassium humate modification not only increased adsorption capacity but also enhanced the subsequent desorption capacity, likely due to additional functional groups and improved accessibility of adsorption sites. The total amounts of Cu(ii) desorbed were 83.27 mg for CB and 91.35 mg for CMB, corresponding to desorption efficiencies of 83% and 63%, respectively. The relatively lower desorption efficiency of CMB is likely due to the stronger chemical binding mechanisms introduced by potassium humate modification, which rendered Cu(ii) more difficult to desorb [33].
In this study, 4% HCl was employed for Cu(ii) desorption due to its high efficiency. However, its potential environmental impact should not be overlooked, and greener, biodegradable eluents will be actively explored in future work. Additionally, further studies will assess CMB’s selectivity, stability, and reusability under realistic wastewater conditions through competitive ion tests and multi-cycle adsorption–desorption experiments.
3.3.2 Fixed-bed kinetic modeling
The experimental data for the column adsorption of Cu(ii) by CB and CMB were analyzed using the Thomas and Yoon–Nelson models (Figure 6 and Table 3). The fitted results showed that the correlation coefficients (R 2) for CB and CMB in the Thomas model were 0.9164 and 0.9352, with corresponding RMSE values of 0.7190 and 0.5560 (Figure 6a). These results indicate a strong fit and reliable prediction of their column adsorption behavior [26].
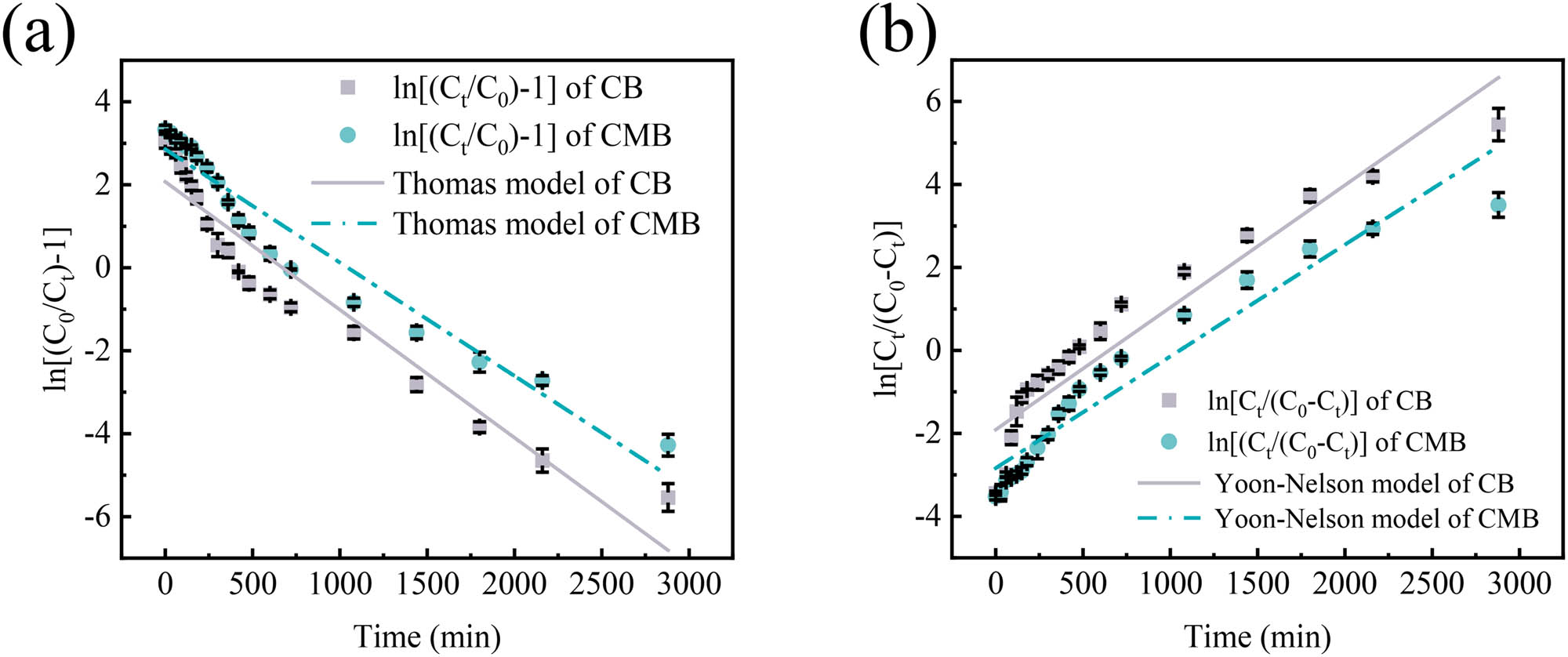
Thomas (a) and Yoon-Nelson (b) model fitting curves for dynamic adsorption of Cu(ii) onto CB and CMB (mean ± std).
Parameters of Thomas and Yoon–Nelson models for dynamic removal of Cu(ii) by CB and CMB
| Models | Treatments | Parameters | |||
|---|---|---|---|---|---|
| Thomas model | q 0 (mg·g−1) | K Th (mL·min−1·mg−1) | R 2 | RMSE | |
| CB | 7.6813 | 0.0031 | 0.9164 | 0.7190 | |
| CMB | 11.9536 | 0.0027 | 0.9352 | 0.5560 | |
| Yoon–Nelson model | K Y (1/min) | τ (min) | R 2 | RMSE | |
| CB | 0.0030 | 649 | 0.9012 | 0.7530 | |
| CMB | 0.0027 | 1054 | 0.9250 | 0.5920 | |
For the Yoon–Nelson model, the R 2 values were 0.9012 for CB and 0.9250 for CMB, with corresponding RMSE values of 0.7530 and 0.5920, demonstrating a reliable representation of the adsorption process under different conditions (Figure 6b). The increased number of adsorption sites in CMB extended the breakthrough time from 649 min (CB) to 1,054 min (CMB), indicating a significant improvement in adsorption capacity as a result of the modification. Moreover, an increase in the Yoon–Nelson rate constant (K Y ) shortened the corresponding adsorption time, resulting in a steeper breakthrough curve and an earlier saturation point [46]. The fitted curves showed that the K Y value for Cu(ii) adsorption on CB was higher than that on CMB, suggesting that CB reached saturation more quickly.
3.4 Adsorption mechanisms
3.4.1 SEM-EDS analysis
The microscopic surface morphologies of the adsorbent materials were observed by SEM [47]. Initially, the surface of CB appeared relatively uniform and smooth, as shown in Figure 7a and b. After modification with potassium humate, the morphology significantly changed, exhibiting an uneven, aggregated, film-like coating, along with numerous small spherical particles on the surface of CMB (Figure 7d and e). These observations clearly confirmed the successful immobilization of potassium humate onto the biochar surface. After Cu(ii) adsorption, SEM images of CMB-Cu (Figure 7h and i) showed that the originally porous structure was partially filled or blocked, indicating that Cu(ii) were successfully adsorbed onto the biochar surface and into its pores.
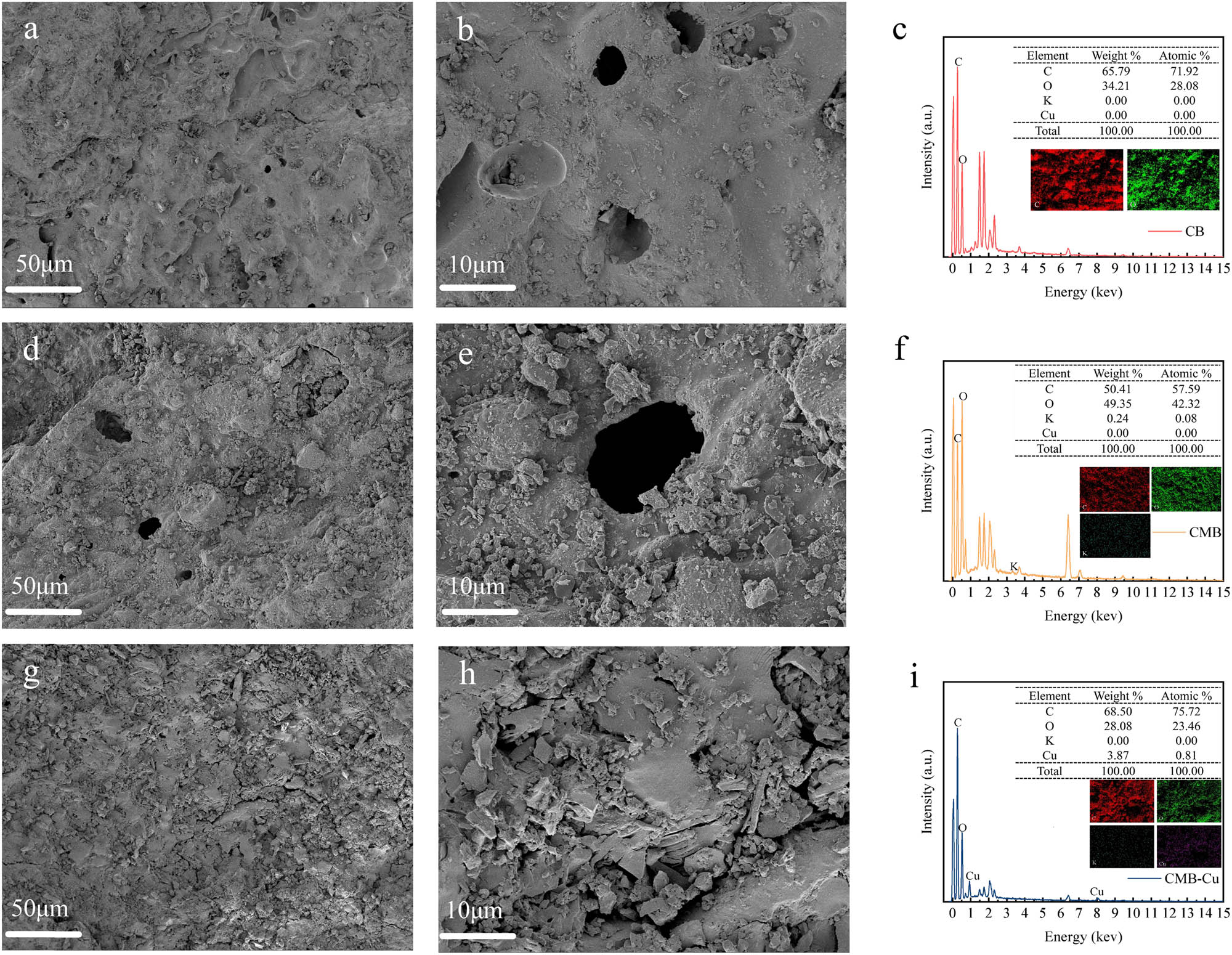
SEM images and EDS elemental mapping of CB, CMB, and CMB-Cu. (a) and (b) SEM images of CB, (c) EDS mapping of CB, (d) and (e) SEM images of CMB, (f) EDS mapping of CMB, (g) and (h) SEM images of CMB-Cu, and (i) EDS mapping of CMB-Cu.
EDS analysis provided further evidence supporting these findings. As shown in Figure 7c, f and j, CB primarily contained C and O elements. After modification, a new potassium (K) peak appeared in the EDS spectrum of CMB, verifying the presence of potassium humate. Following Cu(ii) adsorption, the EDS spectrum of CMB-Cu displayed a distinct Cu peak, and corresponding elemental composition changes were observed: C content increased from 50.41% to 68.50%, O content decreased from 49.35% to 28.08%, K content dropped from 0.24% to 0.00%, while Cu content rose from 0.00% to 3.87%. These compositional changes confirmed the successful adsorption of Cu(ii) onto CMB, suggesting interactions between Cu(ii) and the functional groups introduced by potassium humate. Additionally, the observed decrease in K content indicated that ion exchange played a role during adsorption. EDS elemental mapping further demonstrated uniform distribution of these elements, supporting homogeneous adsorption on the biochar surface, which was consistent with the monolayer adsorption behavior described by the Langmuir isotherm model.
3.4.2 FTIR analysis
FTIR analysis was conducted to investigate changes in surface functional groups of CB, CMB, and their forms after Cu(ii) adsorption (CB-Cu, CMB-Cu). As shown in Figure S2, both CB and CMB exhibited similar characteristic peaks. However, notable differences were observed in peak intensities and positions. Compared to CB, CMB exhibited significantly enhanced peak intensities at approximately 3,448 cm−1 (O–H stretching vibrations) [48], 2,827 cm−1 (C–H stretching vibrations of –CH and –CH2 groups) [49], and 1,608 cm−1 (C═O stretching vibrations from carboxyl groups or aromatic structures) [50]. These changes indicated that potassium humate modification successfully introduced additional oxygen-containing functional groups onto the biochar surface.
After Cu(ii) adsorption, noticeable changes were observed in the FTIR spectra of CB and CMB (denoted as CB-Cu and CMB-Cu, respectively), including reduced peak intensities and slight shifts in key functional group bands. In the case of CMB, the O–H stretching vibration band shifted from 3,448 to 3,435 cm−1 and showed a marked decrease in intensity, indicating that hydroxyl groups were actively involved in coordinating with Cu(ii). Likewise, the absorption band corresponding to C═O stretching in carboxyl groups shifted from 1,608 to 1,614 cm−1, accompanied by a weakening of peak intensity. This suggests that carboxyl groups participated in Cu(ii) binding through deprotonation and complexation processes [51].
Compared with CB, these spectral changes were more prominent in CMB, highlighting the role of additional oxygen-containing functional groups introduced through potassium humate modification. The more substantial peak shifts and reductions in intensity confirmed that the enriched hydroxyl and carboxyl functionalities in CMB played a critical role in enhancing Cu(ii) adsorption, primarily via chemisorption mechanisms.
3.4.3 BET analysis
The textural characteristics of the biochars, determined through BET analysis (Table 4 and Figure S3), showed that CMB possessed a significantly improved surface area and pore structure compared to CB. Specifically, the specific surface area and total pore volume of CMB were 6.3895 m2·g−1 and 0.002364 cm3·g−1, respectively – substantially higher than those of CB (3.5221 m2·g−1 and 0.000795 cm3·g−1). Furthermore, CMB exhibited a more developed micro and mesoporous network, which provided more accessible adsorption sites and promoted faster Cu(ii) diffusion into the internal structure [52]. These improvements were consistent with the results of the Langmuir isotherm analysis, where CMB demonstrated higher maximum adsorption capacity (q m) and equilibrium affinity constant (K L ) than CB. This indicates a greater number of active binding sites and stronger interactions between Cu(ii) and the modified surface.
BET characteristics of CB and CMB
| Thermophysical properties | CB | CMB |
|---|---|---|
| BET surface area (m2·g−1) | 3.5221 | 6.3895 |
| Micropore volume (cm3·g−1) | 0.0008 | 0.0024 |
| Micropore area (m2·g−1) | 1.6863 | 4.8907 |
| Average pore size (nm) | 9.0016 | 4.5747 |
Overall, the increased specific surface area and enhanced pore volume contributed directly to the improved Cu(ii) adsorption performance of CMB. The BET analysis thus supports the conclusion that potassium humate modification effectively improved the structural properties of the biochar, enhancing its adsorption capacity for Cu(ii).
3.4.4 Boehm titration analysis
The surface acidic functional groups (carboxyl, lactonic, and phenolic hydroxyl groups) on CB and CMB were quantitatively determined using the Boehm titration method. As shown in Table 5, CMB exhibited a significantly higher total concentration of acidic functional groups than CB, particularly a marked increase in carboxyl groups (1.50 mmol·g−1 for CMB vs 0.35 mmol·g−1 for CB). Generally, carboxyl groups are among the most acidic oxygen-containing functional groups on biochar surfaces and play a crucial role in the adsorption of heavy metals through ion exchange or complexation mechanisms [53]. Thus, the substantial increase in carboxyl groups on CMB enhanced its capacity to effectively bind Cu(ii), primarily through the formation of surface complexation reactions. Moreover, the increased concentrations of lactonic and phenolic hydroxyl groups in CMB (0.35 and 0.90 mmol·g−1, respectively, compared to 0.12 and 0.45 mmol·g−1 in CB) further enhanced the adsorption capacity by providing additional binding sites for Cu(ii). These conclusions were consistent with the FTIR analysis. Collectively, the results of Boehm titration analyses clearly indicated that the potassium humate modification significantly improved Cu(ii) adsorption through enhanced chemisorption involving carboxyl and hydroxyl functional groups on the biochar surface.
Surface functional group content of CB and CMB
| Sample | Carboxyl (mmol·g−1) (mean ± std) | Lactonic (mmol·g−1) (mean ± std) | Phenolic OH (mmol·g−1) (mean ± std) |
|---|---|---|---|
| CB | 0.35 ± 0.02 | 0.12 ± 0.02 | 0.45 ± 0.05 |
| CMB | 1.50 ± 0.04 | 0.35 ± 0.01 | 0.90 ± 0.07 |
In summary, although the BET surface area of CMB was relatively low (6.4 m2·g−1), the potassium humate immobilized on its surface chemically interacted with Cu(ii) in aqueous solution, thereby enhancing the adsorption performance primarily through surface complexation and ion exchange mechanisms.
3.5 Economic benefit analysis
Although the adsorption capacity of CMB in our study is modest compared with previously reported modified biochars, its enhancement ratio relative to the unmodified biochar is comparatively high [20,54,55]. Importantly, most reported adsorbents exist in powder form, which generally exhibit limitations in practical use, including difficult separation, and poor suitability for fixed-bed systems. In contrast, the granular nature of CMB offers significant practical advantages, such as convenient handling, and excellent suitability for fixed-bed column applications. Additionally, the production cost of CMB is relatively low (approximately $484 per ton, details provided in Text S1). In addition to economic factors, environmental sustainability is crucial for practical implementation. potassium humate, the modifying agent used here, is derived from natural humic substances (e.g., lignite or peat) via a mild alkaline extraction process [56]. This method avoids hazardous solvents, requires minimal energy input, and yields a biodegradable and non-toxic modifier [33].
4 Conclusions
The results demonstrated that potassium humate modification significantly enhanced the Cu(ii) adsorption performance of coconut husk biochar. Compared to CB, CMB exhibited notably higher Cu(ii) adsorption capacity (6.8 mg·g−1), larger specific surface area (6.39 m2·g−1), and increased concentration of acidic functional groups, especially carboxyl groups (1.50 mmol·g−1). Adsorption kinetics and isotherm analyses showed that Cu(ii) adsorption on both CB and CMB fitted well with pseudo-second-order and Langmuir models, respectively, indicating a predominantly chemisorption process and monolayer adsorption behavior. CMB displayed higher kinetic constants and Langmuir parameters (q m and K L ) than CB, confirming its stronger affinity and greater adsorption capacity for Cu(ii). In fixed-bed column experiments, CMB exhibited substantially prolonged breakthrough time (300 min) and higher total Cu(ii) adsorption capacity (145.20 mg) compared to CB (breakthrough time: 90 min, adsorption capacity: 100.27 mg), highlighting enhanced dynamic adsorption performance after modification. Furthermore, column desorption experiments revealed total Cu(ii) desorption amounts of 83.27 mg for CB and 91.35 mg for CMB, corresponding to desorption efficiencies of 83% and 63%, respectively. SEM-EDS, FTIR, BET, and Boehm titration collectively indicated that chemisorption via surface complexation and ion exchange was the primary mechanism for Cu(ii) adsorption onto CMB. Considering its simplicity, low production cost, excellent adsorption performance, and minimal environmental impact owing to the natural and biodegradable properties of potassium humate, CMB demonstrates promising potential for practical applications in copper-containing wastewater treatment and heavy metal pollution remediation.
Acknowledgments
The authors express their gratitude to Hubei University of Arts and Science, Xiangyang, China, for providing the laboratory facilities to conduct this research.
-
Funding information: This work was funded by China Scholarship Council (202308420151) and the Natural Science Foundation of Hubei Province, China (2017CFB608).
-
Author contributions: Ze Zhong: investigation, methodology, formal analysis, writing-original draft. Pengfei Chen: investigation, methodology, formal analysis, validation. Jiachen Chen: methodology, formal analysis. Zhenfeng Cheng: methodology, formal analysis. Jie Zhang: methodology, formal analysis. Kai Luo: methodology, validation, writing – review and editing. Jie Zhu: methodology, validation. Yuqi Li: conceptualization, supervision, funding acquisition, writing – review and editing.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Shahab A, Hui Z, Rad S, Xiao H, Siddique J, Huang LL, et al. A comprehensive review on pollution status and associated health risk assessment of human exposure to selected heavy metals in road dust across different cities of the world. Envrion Geochem Hlth. 2023;45:585–606. 10.1007/s10653-022-01255-3.Search in Google Scholar PubMed
[2] Carić H, Cukrov N, Omanović D. Nautical tourism in marine protected areas (MPAs): Evaluating an impact of copper emission from antifouling coating. Sustainability. 2021;13:11897. 10.3390/su132111897.Search in Google Scholar
[3] Hu Y, Cheng H, Tao S. Environmental and human health challenges of industrial livestock and poultry farming in China and their mitigation. Env Int. 2017;107:111–30. 10.1016/j.envint.2017.07.003.Search in Google Scholar PubMed
[4] Rozyyev V, Gao F, Liu Y, Shevate R, Pathak R, Mane AU, et al. Thiol-functionalized adsorbents through atomic layer deposition and vapor-phase silanization for heavy metal ion removal. ACS Appl Mater Interfaces. 2024;16(26):34030–41. 10.1021/acsami.4c03935.Search in Google Scholar PubMed
[5] Dadari S, Rahimi M, Zinadini S. Novel antibacterial and antifouling PES nanofiltration membrane incorporated with green synthesized nickel-bentonite nanoparticles for heavy metal ions removal. Chem Eng J. 2022;431:134116. 10.1016/j.cej.2021.134116.Search in Google Scholar
[6] Zhang Y, Duan X. Chemical precipitation of heavy metals from wastewater by using the synthetical magnesium hydroxy carbonate. Water Sci Technol. 2020;81:1130–6. 10.2166/wst.2020.208.Search in Google Scholar PubMed
[7] Ulloa L, Martínez-Minchero M, Bringas E, Cobo A, San-Román MF. Split regeneration of chelating resins for the selective recovery of nickel and copper. Sep Purif Technol. 2020;253:117516. 10.1016/j.seppur.2020.117516.Search in Google Scholar
[8] Sorouraddin SM, Farajzadeh MA, Dastoori H. Development of a dispersive liquid-liquid microextraction method based on a ternary deep eutectic solvent as chelating agent and extraction solvent for preconcentration of heavy metals from milk samples. Talanta. 2020;208:120485. 10.1016/j.talanta.2019.120485.Search in Google Scholar PubMed
[9] Jain M, Khan SA, Sharma K, Jadhao PR, Pant KK, Ziora ZM, et al. Current perspective of innovative strategies for bioremediation of organic pollutants from wastewater. Bioresour Technol. 2022;344:126305. 10.1016/j.biortech.2021.126305.Search in Google Scholar PubMed
[10] Salari M, Dehghani MH, Azari A, Motevalli MD, Shabanloo A, Ali I. High performance removal of phenol from aqueous solution by magnetic chitosan based on response surface methodology and genetic algorithm. J Mol J Mol Liq. 2019;285:146–57. 10.1016/j.molliq.2019.04.065.Search in Google Scholar
[11] Verma R, Maji PK, Sarkar S. Removal of hexavalent chromium from impaired water: Polyethylenimine-based sorbents − A review. J Env Chem Eng. 2023;11:109598. 10.1016/j.jece.2023.109598.Search in Google Scholar
[12] Saini K, Sahoo A, Biswas B, Kumar A, Bhaskar T. Preparation and characterization of lignin-derived hard templated carbon(s): Statistical optimization and methyl orange adsorption isotherm studies. Bioresour Technol. 2021;342:125924. 10.1016/j.biortech.2021.125924.Search in Google Scholar PubMed
[13] Li Y, Shao J, Wang X, Deng Y, Yang H, Chen H. Characterization of modified biochars derived from bamboo pyrolysis and their utilization for target component (Furfural) adsorption. Energ Fuel. 2014;28:5119–27. 10.1021/ef500725c.Search in Google Scholar
[14] Jalayeri H, Pepe F. Novel and high-performance biochar derived from pistachio green hull biomass: Production, characterization, and application to Cu(II) removal from aqueous solutions. Ecotox Env Safe. 2019;168:64–71. 10.1016/j.ecoenv.2018.10.058.Search in Google Scholar PubMed
[15] Yin K, Wang J, Zhai S, Xu X, Li T, Sun S, et al. Adsorption mechanisms for cadmium from aqueous solutions by oxidant-modified biochar derived from Platanus orientalis Linn leaves. J Hazard Mater. 2022;428:128261. 10.1016/j.jhazmat.2022.128261.Search in Google Scholar PubMed
[16] Chen H, Li W, Wang J, Xu H, Liu Y, Zhang Z, et al. Adsorption of cadmium and lead ions by phosphoric acid-modified biochar generated from chicken feather: Selective adsorption and influence of dissolved organic matter. Bioresour Technol. 2019;292:121948. 10.1016/j.biortech.2019.121948.Search in Google Scholar PubMed
[17] Wassie AB, Srivastava VC. Chemical treatment of teff straw by sodium hydroxide, phosphoric acid and zinc chloride: adsorptive removal of chromium. Int J Env Sci Te. 2016;13:2415–26. 10.1007/s13762-016-1080-6.Search in Google Scholar
[18] Lebedev V, Miroshnichenko D, Vytrykush N, Pyshyev S, Masikevych A, Filenko O, et al. Novel biodegradable polymers modified by humic acids. Mater Chem Phys. 2024;313:128778. 10.1016/j.matchemphys.2023.128778.Search in Google Scholar
[19] Ding W, Bao S, Zhang Y, Chen B, Wang Z. Antimony(V) Adsorption and Partitioning by Humic Acid-Modified Ferrihydrite: Insights into Environmental Remediation and Transformation Processes. Materials. 2024;17:4172. 10.3390/ma17174172.Search in Google Scholar PubMed PubMed Central
[20] Alhawas MS, Rafique MI, Ahmad M, Al-Wabel MI, Usman ARA, Al-Swadi HA, et al. Ball mill, humic acid, and rock phosphate-modified conocarpus biochar for efficient removal of heavy metals from contaminated water. Sustainability. 2023;15:11474. 10.3390/su151411474.Search in Google Scholar
[21] Yang Y, Ma X, Li Z, Wang Y, Ju C, Cao L, et al. ZIF-8 and humic acid modified magnetic corn stalk biochar: An efficient, magnetically stable, and eco-friendly adsorbent for imidacloprid and thiamethoxam removal. Chem Eng J. 2023;465:142788. 10.1016/j.cej.2023.142788.Search in Google Scholar
[22] Song J, Li Y, Chen L, Zhao D, Yu S, Huang L. Preparation of KHA/SA/MMT composites and their adsorption properties for Rhodamine B. Env Sci Pollut R. 2024;31:24220–34. 10.1007/s11356-024-32652-z.Search in Google Scholar PubMed
[23] Giwa AS, Ndungutse JM, Li Y, Mabi A, Liu X, Vakili M, et al. Modification of biochar with Fe3O4 and humic acid-salt for removal of mercury from aqueous solutions: a review. Env Pollut Bioavail. 2022;34:352–64. 10.1080/26395940.2022.2115402.Search in Google Scholar
[24] Boehm HP. Some aspects of the surface chemistry of carbon blacks and other carbons. Carbon. 1994;32:759–69. 10.1016/0008-6223(94)90031-0.Search in Google Scholar
[25] Gama BMV, Santos JHL, Nascimento GED, Novais MED, Campos NF, Freitas RAD, et al. Adsorption of cadmium and copper using biochar obtained from the residue of the second-generation ethanol production: kinetics and equilibrium study. Chem Eng Commun. 2024;211:961–73. 10.1080/00986445.2024.2316610.Search in Google Scholar
[26] Fu X, Wang P, Wu J, Zheng P, Wang T, Li X, et al. Hydrocotyle vulgaris derived novel biochar beads for phosphorus removal: static and dynamic adsorption assessment. J Env Chem Eng. 2022;10:108177. 10.1016/j.jece.2022.108177.Search in Google Scholar
[27] Meina L, Qiao M, Zhang Q, Xu S, Wang D. Study on the dynamic adsorption and recycling of phosphorus by Fe–Mn oxide/mulberry branch biochar composite adsorbent. Sci Rep. 2024;14:1235. 10.1038/s41598-024-51416-w.Search in Google Scholar PubMed PubMed Central
[28] Huang G, Liu Q, Kang W, Xing B, Chen L, Zhang C. Potassium humate based reduced graphite oxide materials for supercapacitor applications. Electrochim Acta. 2016;196:450–6. 10.1016/j.electacta.2016.02.158.Search in Google Scholar
[29] Han L, Zhang B, Chen L, Feng Y, Yang Y, Sun K. Impact of biochar amendment on soil aggregation varied with incubation duration and biochar pyrolysis temperature. Biochar. 2021;3:339–47. 10.1007/s42773-021-00097-z.Search in Google Scholar
[30] Chen J, Duan Q, Ji C, Liu J, Wang Z, Song J, et al. Modified coconut shell biochars (MCSBCs): Fabrication and their adsorptions for Pb(II). Heliyon. 2024;10:e32422. 10.1016/j.heliyon.2024.e32422.Search in Google Scholar PubMed PubMed Central
[31] Zhang M, Zhu H, Xi B, Tian Y, Sun X, Zhang H, et al. Surface hydrophobic modification of biochar by silane coupling agent KH-570. Processes. 2022;10:301. 10.3390/pr10020301.Search in Google Scholar
[32] Katiyar R, Patel AK, Nguyen T-B, Singhania RR, Chen C-W, Dong C-D. Adsorption of copper (II) in aqueous solution using biochars derived from Ascophyllum nodosum seaweed. Bioresour Technol. 2021;328:124829. 10.1016/j.biortech.2021.124829.Search in Google Scholar PubMed
[33] Babeker TMA, Lv S, Khalil MN, Hao Z, Chen Q. Biochar modified by ammonium pyrrolidine dithiocarbamate for high selective adsorption of copper in wastewater. Sep Purif Technol. 2025;354:129436. 10.1016/j.seppur.2024.129436.Search in Google Scholar
[34] Han L, Qian L, Liu R, Chen M, Yan J, Hu Q. Lead adsorption by biochar under the elevated competition of cadmium and aluminum. Sci Rep. 2017;7:2264. 10.1038/s41598-017-02353-4.Search in Google Scholar PubMed PubMed Central
[35] Sun Y, Li H, Li G, Gao B, Yue Q, Li X. Characterization and ciprofloxacin adsorption properties of activated carbons prepared from biomass wastes by H3PO4 activation. Bioresour Technol. 2016;217:239–44. 10.1016/j.biortech.2016.03.047.Search in Google Scholar PubMed
[36] Abbas Z, Ali S, Rizwan M, Zaheer IE, Malik A, Riaz MA, et al. A critical review of mechanisms involved in the adsorption of organic and inorganic contaminants through biochar. Arab J Geosci. 2018;11:448. 10.1007/s12517-018-3790-1.Search in Google Scholar
[37] Ahmad M, Rajapaksha AU, Lim JE, Zhang M, Bolan N, Mohan D, et al. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere. 2014;99:19–33. 10.1016/j.chemosphere.2013.10.071.Search in Google Scholar PubMed
[38] Annadurai G, Juang RS, Lee DJ. Adsorption of heavy metals from water using banana and orange peels. Water Sci Technol. 2003;47:185–90. 10.2166/wst.2003.0049.Search in Google Scholar
[39] Yuan J, Wang C, Tang Z, Chu T, Zheng C, Han Q, et al. Biochar derived from traditional Chinese medicine residues: An efficient adsorbent for heavy metal Pb(II). Arab J Chem. 2024;17:105606. 10.1016/j.arabjc.2024.105606.Search in Google Scholar
[40] Wang C, Wang H. Pb(II) sorption from aqueous solution by novel biochar loaded with nano-particles. Chemosphere. 2018;192:1–4. 10.1016/j.chemosphere.2017.10.125.Search in Google Scholar PubMed
[41] Guo Y, Zhu Z, Qiu Y, Zhao J. Enhanced adsorption of acid brown 14 dye on calcined Mg/Fe layered double hydroxide with memory effect. Chem Eng J. 2013;219:69–77. 10.1016/j.cej.2012.12.084.Search in Google Scholar
[42] Wijeyawardana P, Nanayakkara N, Gunasekara C, Karunarathna A, Law D, Pramanik BK. Removal of Cu, Pb and Zn from stormwater using an industrially manufactured sawdust and paddy husk derived biochar. Env Technol Inno. 2022;28:102640. 10.1016/j.eti.2022.102640.Search in Google Scholar
[43] Hussain A, Maitra J, Khan KA. Development of biochar and chitosan blend for heavy metals uptake from synthetic and industrial wastewater. Appl Water Sci. 2017;7:4525–37. 10.1007/s13201-017-0604-7.Search in Google Scholar
[44] Dovi E, Aryee AA, Kani AN, Mpatani FM, Li J, Qu L, et al. High-capacity amino-functionalized walnut shell for efficient removal of toxic hexavalent chromium ions in batch and column mode. J Env Chem Eng. 2022;10:107292. 10.1016/j.jece.2022.107292.Search in Google Scholar
[45] Boutaleb Y, Zerdoum R, Bensid N, Abumousa RA, Hattab Z, Bououdina M. Adsorption of Cr(VI) by mesoporous pomegranate peel biowaste from synthetic wastewater under dynamic mode. Water. 2022;14:3885. 10.3390/w14233885.Search in Google Scholar
[46] Syeda HI, Muthukumaran S, Baskaran K. Dynamic adsorption of heavy metals on functionalized and regeneratable biopolymeric aerogels: Fixed-bed column reactor modelling and dual functionality elution technique. Sep Purif Technol. 2025;363:131861. 10.1016/j.seppur.2025.131861.Search in Google Scholar
[47] Plentz Gomes Vasconcelos L, Almeida Albuquerque A, Roberta Cabral Ribeiro K, Beatriz Oliveira Palmeira M, Thalis Vaz Da Costa Capistrano R, Inácio Soletti J, et al. Comparison of adsorption potential of methylene blue and 17β-stradiol on biochar, activated biochar and catalytic biochar from lignocellulosic waste. J Ind Eng Chem. 2025;144:585–95. 10.1016/j.jiec.2024.10.004.Search in Google Scholar
[48] Ahmad S, Sabir A, Khan SM. Synthesis and characterization of pectin/carboxymethyl cellulose-based hybrid hydrogels for heavy metal ions adsorption. Chem Pap. 2023;77:4165–77. 10.1007/s11696-023-02767-7.Search in Google Scholar
[49] Sevilla M, Fuertes AB. Chemical and Structural Properties of Carbonaceous Products Obtained by Hydrothermal Carbonization of Saccharides. Chem-Eur J. 2009;15:4195–203. 10.1002/chem.200802097.Search in Google Scholar PubMed
[50] El-Ghobashy MA, Khamis MM, Elsherbiny AS, Salem IA. Selective removal of ammonia from wastewater using Cu(II)-loaded Amberlite IR-120 resin and its catalytic application for removal of dyes. Env Sci Pollut R. 2023;30:106822–37. 10.1007/s11356-023-25677-3.Search in Google Scholar PubMed PubMed Central
[51] Zhao D, Zhang S, Deng H, Hu L, Li A. Eco‐friendly adsorbent: Insights into the performance and adsorption mechanisms of banana fruit shaft biochar for the removal of Mn(II), Cd(II), Pb(II), and Cu(II). Appl Organomet Chem. 2025;39:e70036. 10.1002/aoc.70036.Search in Google Scholar
[52] Tan L, Nie Y, Chang H, Zhu L, Guo K, Ran X, et al. Adsorption performance of Ni(II) by KOH-modified biochar derived from different microalgae species. Bioresour Technol. 2024;394:130287. 10.1016/j.biortech.2023.130287.Search in Google Scholar PubMed
[53] Xu X, Cao X, Zhao L, Wang H, Yu H, Gao B. Removal of Cu, Zn, and Cd from aqueous solutions by the dairy manure-derived biochar. Env Sci Pollut R. 2013;20:358–68. 10.1007/s11356-012-0873-5.Search in Google Scholar PubMed
[54] Wang Z, Zhao P, Li X, Sun Q, She D. Magnesium chloride-modified potassium humate-based carbon material for efficient removal of phosphate from water. J Taiwan Inst Chem E. 2022;140:104540. 10.1016/j.jtice.2022.104540.Search in Google Scholar
[55] Yang Z, Wu H, Yan X, Bekchanov D, Kong D, Su X. Preparation of Al-doped carbon materials derived from artificial potassium humate prepared from waste cotton cloth and their excellent Cr(VI) adsorption performance. Colloid Surf A. 2024;699:134721. 10.1016/j.colsurfa.2024.134721.Search in Google Scholar
[56] Jin Q, Zhang Y, Wang Q, Li M, Sun H, Liu N, et al. Effects of potassium fulvic acid and potassium humate on microbial biodiversity in bulk soil and rhizosphere soil of Panax ginseng. Microbiol Res. 2022;254:126914. 10.1016/j.micres.2021.126914.Search in Google Scholar PubMed
© 2025 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Optimized green synthesis of silver nanoparticles from guarana seed skin extract with antibacterial potential
- Green adsorbents for water remediation: Removal of Cr(vi) and Ni(ii) using Prosopis glandulosa sawdust and biochar
- Green approach for the synthesis of zinc oxide nanoparticles from methanolic stem extract of Andrographis paniculata and evaluation of antidiabetic activity: In silico GSK-3β analysis
- Development of a green and rapid ethanol-based HPLC assay for aspirin tablets and feasibility evaluation of domestically produced bioethanol in Thailand as a sustainable mobile phase
- A facile biodegradation of polystyrene microplastic by Bacillus subtilis
- Enhanced synthesis of fly ash-derived hydrated sodium silicate adsorbents via low-temperature alkaline hydrothermal treatment for advanced environmental applications
- Impact of metal nanoparticles biosynthesized using camel milk on bacterial growth and copper removal from wastewater
- Preparation of Co/Cr-MOFs for efficient removal of fleroxacin and Rhodamine B
- Applying nanocarbon prepared from coal as an anode in lithium-ion batteries
- Improved electrochemical synthesis of Cu–Fe/brass foil alloy followed by combustion for high-efficiency photoelectrodes and hydrogen production in alkaline solutions
- Precipitation of terephthalic acid from post-consumer polyethylene terephthalate waste fractions
- Biosynthesized zinc oxide nanoparticles: Multifunctional potential applications in anticancer, antibacterial, and B. subtilis DNA gyrase docking
- Anticancer and antimicrobial effects of green-synthesized silver nanoparticles using Teucrium polium leaves extract
- Green synthesis of eco-friendly bioplastics from Chlorella and Lithothamnion algae for safe and sustainable solutions for food packaging
- Optimizing coal water slurry concentration via synergistic coal blending and particle size distribution
- Green synthesis of Ag@Cu and silver nanowire using Pterospermum heterophyllum extracts for surface-enhanced Raman scattering
- Green synthesis of copper oxide nanoparticles from Algerian propolis: Exploring biochemical, structural, antimicrobial, and anti-diabetic properties
- Simultaneous quantification of mefenamic acid and paracetamol in fixed-dose combination tablet dosage forms using the green HPTLC method
- Green synthesis of titanium dioxide nanoparticles using green tea (Camellia sinensis) extract: Characteristics and applications
- Pharmaceutical properties for green fabricated ZnO and Ag nanoparticle-mediated Borago officinalis: In silico predications study
- Synthesis and optimization of gemcitabine-loaded nanoparticles by using Box–Behnken design for treating prostate cancer: In vitro characterization and in vivo pharmacokinetic study
- A comparative analysis of single-step and multi-step methods for producing magnetic activated carbon from palm kernel shells: Adsorption of methyl orange dye
- Sustainable green synthesis of silver nanoparticles using walnut septum waste: Characterization and antibacterial properties
- Efficient electrocatalytic reduction of CO2 to CO over Ni/Y diatomic catalysts
- Greener and magnetic Fe3O4 nanoparticles as a recyclable catalyst for Knoevenagel condensation and degradation of industrial Congo red dye
- Recycling of HDPE-giant reed composites: Processability and performance
- Fabrication of antibacterial chitosan/PVA nanofibers co-loaded with curcumin and cefadroxil for wound healing
- Cost-effective one-pot fabrication of iron(iii) oxychloride–iron(iii) oxide nanomaterials for supercapacitor charge storage
- Novel trimetallic (TiO2–MgO–Au) nanoparticles: Biosynthesis, characterization, antimicrobial, and anticancer activities
- Green-synthesized chromium oxide nanoparticles using pomegranate husk extract: Multifunctional bioactivity in antioxidant potential, lipase and amylase inhibition, and cytotoxicity
- Therapeutic potential of sustainable zinc oxide nanoparticles biosynthesized using Tradescantia spathacea aqueous leaf extract
- Chitosan-coated superparamagnetic iron oxide nanoparticles synthesized using Carica papaya bark extract: Evaluation of antioxidant, antibacterial, and anticancer activity of HeLa cervical cancer cells
- Antioxidant potential of peptide fractions from tuna dark muscle protein isolate: A green enzymatic approach
- Clerodendron phlomoides leaf extract-mediated synthesis of selenium nanoparticles for multi-applications
- Optimization of cellulose yield from oil palm trunks with deep eutectic solvents using response surface methodology
- Nitrogen-doped carbon dots from Brahmi (Bacopa monnieri): Metal-free probe for efficient detection of metal pollutants and methylene blue dye degradation
- High energy density pseudocapacitor based on a nanoporous tungsten(VI) oxide iodide/poly(2-amino-1-mercaptobenzene) composite
- Green synthesized Ag–Cu nanocomposites as an improved strategy to fight multidrug-resistant bacteria by inhibition of biofilm formation: In vitro and in silico assessment study
- In vitro evaluation of antibacterial activity and associated cytotoxicity of biogenic silver nanoparticles using various extracts of Tabernaemontana ventricosa
- Fabrication of novel composite materials by impregnating ZnO particles into bacterial cellulose nanofibers for antimicrobial applications
- Solidification floating organic drop for dispersive liquid–liquid microextraction estimation of copper in different water samples
- Kinetics and synthesis of formation of phosphate composites from low-grade phosphorites in the presence of phosphate–siliceous shales and oil sludge
- Removal of minocycline and terramycin by graphene oxide and Cr/Mn base metal–organic framework composites
- Microfluidic preparation of ceramide E liposomes and properties
- Therapeutic potential of Anamirta cocculus (L.) Wight & Arn. leaf aqueous extract-mediated biogenic gold nanoparticles
- Antioxidant-rich Micromeria imbricata leaf extract as a medium for the eco-friendly preparation of silver-doped zinc oxide nanoparticles with antibacterial properties
- Influence of different colors with light regime on Chlorella sp., biomass, pigments, and lipids quantity and quality
- Experimental vibrational analysis of natural fiber composite reinforced with waste materials for energy absorbing applications
- Green synthesis of sea buckthorn-mediated ZnO nanoparticles: Biological applications and acute nanotoxicity studies
- Production of liquid smoke by consecutive electroporation and microwave-assisted pyrolysis of empty fruit bunches
- Synthesis of MPAA based on polyacrylamide and gossypol resin and applications in the encapsulation of ammophos
- Application of iron-based catalysts in the microwave treatment of environmental pollutants
- Enhanced adsorption of Cu(ii) from wastewater using potassium humate-modified coconut husk biochar
- Adsorption of heavy metal ions from water by Fe3O4 nano-particles
- Green synthesis of parsley-derived silver nanoparticles and their enhanced antimicrobial and antioxidant effects against foodborne resistant bacteria
- Unwrapping the phytofabrication of bimetallic silver–selenium nanoparticles: Antibacterial, Anti-virulence (Targeting magA and toxA genes), anti-diabetic, antioxidant, anti-ovarian, and anti-prostate cancer activities
- Review Article
- Sustainable innovations in garlic extraction: A comprehensive review and bibliometric analysis of green extraction methods
- Rapid Communication
- In situ supported rhodium catalyst on mesoporous silica for chemoselective hydrogenation of nitriles to primary amines
- Special Issue: Valorisation of Biowaste to Nanomaterials for Environmental Applications
- Valorization of coconut husk into biochar for lead (Pb2+) adsorption
- Corrigendum
- Corrigendum to “An updated review on carbon nanomaterials: Types, synthesis, functionalization and applications, degradation and toxicity”
Articles in the same Issue
- Research Articles
- Optimized green synthesis of silver nanoparticles from guarana seed skin extract with antibacterial potential
- Green adsorbents for water remediation: Removal of Cr(vi) and Ni(ii) using Prosopis glandulosa sawdust and biochar
- Green approach for the synthesis of zinc oxide nanoparticles from methanolic stem extract of Andrographis paniculata and evaluation of antidiabetic activity: In silico GSK-3β analysis
- Development of a green and rapid ethanol-based HPLC assay for aspirin tablets and feasibility evaluation of domestically produced bioethanol in Thailand as a sustainable mobile phase
- A facile biodegradation of polystyrene microplastic by Bacillus subtilis
- Enhanced synthesis of fly ash-derived hydrated sodium silicate adsorbents via low-temperature alkaline hydrothermal treatment for advanced environmental applications
- Impact of metal nanoparticles biosynthesized using camel milk on bacterial growth and copper removal from wastewater
- Preparation of Co/Cr-MOFs for efficient removal of fleroxacin and Rhodamine B
- Applying nanocarbon prepared from coal as an anode in lithium-ion batteries
- Improved electrochemical synthesis of Cu–Fe/brass foil alloy followed by combustion for high-efficiency photoelectrodes and hydrogen production in alkaline solutions
- Precipitation of terephthalic acid from post-consumer polyethylene terephthalate waste fractions
- Biosynthesized zinc oxide nanoparticles: Multifunctional potential applications in anticancer, antibacterial, and B. subtilis DNA gyrase docking
- Anticancer and antimicrobial effects of green-synthesized silver nanoparticles using Teucrium polium leaves extract
- Green synthesis of eco-friendly bioplastics from Chlorella and Lithothamnion algae for safe and sustainable solutions for food packaging
- Optimizing coal water slurry concentration via synergistic coal blending and particle size distribution
- Green synthesis of Ag@Cu and silver nanowire using Pterospermum heterophyllum extracts for surface-enhanced Raman scattering
- Green synthesis of copper oxide nanoparticles from Algerian propolis: Exploring biochemical, structural, antimicrobial, and anti-diabetic properties
- Simultaneous quantification of mefenamic acid and paracetamol in fixed-dose combination tablet dosage forms using the green HPTLC method
- Green synthesis of titanium dioxide nanoparticles using green tea (Camellia sinensis) extract: Characteristics and applications
- Pharmaceutical properties for green fabricated ZnO and Ag nanoparticle-mediated Borago officinalis: In silico predications study
- Synthesis and optimization of gemcitabine-loaded nanoparticles by using Box–Behnken design for treating prostate cancer: In vitro characterization and in vivo pharmacokinetic study
- A comparative analysis of single-step and multi-step methods for producing magnetic activated carbon from palm kernel shells: Adsorption of methyl orange dye
- Sustainable green synthesis of silver nanoparticles using walnut septum waste: Characterization and antibacterial properties
- Efficient electrocatalytic reduction of CO2 to CO over Ni/Y diatomic catalysts
- Greener and magnetic Fe3O4 nanoparticles as a recyclable catalyst for Knoevenagel condensation and degradation of industrial Congo red dye
- Recycling of HDPE-giant reed composites: Processability and performance
- Fabrication of antibacterial chitosan/PVA nanofibers co-loaded with curcumin and cefadroxil for wound healing
- Cost-effective one-pot fabrication of iron(iii) oxychloride–iron(iii) oxide nanomaterials for supercapacitor charge storage
- Novel trimetallic (TiO2–MgO–Au) nanoparticles: Biosynthesis, characterization, antimicrobial, and anticancer activities
- Green-synthesized chromium oxide nanoparticles using pomegranate husk extract: Multifunctional bioactivity in antioxidant potential, lipase and amylase inhibition, and cytotoxicity
- Therapeutic potential of sustainable zinc oxide nanoparticles biosynthesized using Tradescantia spathacea aqueous leaf extract
- Chitosan-coated superparamagnetic iron oxide nanoparticles synthesized using Carica papaya bark extract: Evaluation of antioxidant, antibacterial, and anticancer activity of HeLa cervical cancer cells
- Antioxidant potential of peptide fractions from tuna dark muscle protein isolate: A green enzymatic approach
- Clerodendron phlomoides leaf extract-mediated synthesis of selenium nanoparticles for multi-applications
- Optimization of cellulose yield from oil palm trunks with deep eutectic solvents using response surface methodology
- Nitrogen-doped carbon dots from Brahmi (Bacopa monnieri): Metal-free probe for efficient detection of metal pollutants and methylene blue dye degradation
- High energy density pseudocapacitor based on a nanoporous tungsten(VI) oxide iodide/poly(2-amino-1-mercaptobenzene) composite
- Green synthesized Ag–Cu nanocomposites as an improved strategy to fight multidrug-resistant bacteria by inhibition of biofilm formation: In vitro and in silico assessment study
- In vitro evaluation of antibacterial activity and associated cytotoxicity of biogenic silver nanoparticles using various extracts of Tabernaemontana ventricosa
- Fabrication of novel composite materials by impregnating ZnO particles into bacterial cellulose nanofibers for antimicrobial applications
- Solidification floating organic drop for dispersive liquid–liquid microextraction estimation of copper in different water samples
- Kinetics and synthesis of formation of phosphate composites from low-grade phosphorites in the presence of phosphate–siliceous shales and oil sludge
- Removal of minocycline and terramycin by graphene oxide and Cr/Mn base metal–organic framework composites
- Microfluidic preparation of ceramide E liposomes and properties
- Therapeutic potential of Anamirta cocculus (L.) Wight & Arn. leaf aqueous extract-mediated biogenic gold nanoparticles
- Antioxidant-rich Micromeria imbricata leaf extract as a medium for the eco-friendly preparation of silver-doped zinc oxide nanoparticles with antibacterial properties
- Influence of different colors with light regime on Chlorella sp., biomass, pigments, and lipids quantity and quality
- Experimental vibrational analysis of natural fiber composite reinforced with waste materials for energy absorbing applications
- Green synthesis of sea buckthorn-mediated ZnO nanoparticles: Biological applications and acute nanotoxicity studies
- Production of liquid smoke by consecutive electroporation and microwave-assisted pyrolysis of empty fruit bunches
- Synthesis of MPAA based on polyacrylamide and gossypol resin and applications in the encapsulation of ammophos
- Application of iron-based catalysts in the microwave treatment of environmental pollutants
- Enhanced adsorption of Cu(ii) from wastewater using potassium humate-modified coconut husk biochar
- Adsorption of heavy metal ions from water by Fe3O4 nano-particles
- Green synthesis of parsley-derived silver nanoparticles and their enhanced antimicrobial and antioxidant effects against foodborne resistant bacteria
- Unwrapping the phytofabrication of bimetallic silver–selenium nanoparticles: Antibacterial, Anti-virulence (Targeting magA and toxA genes), anti-diabetic, antioxidant, anti-ovarian, and anti-prostate cancer activities
- Review Article
- Sustainable innovations in garlic extraction: A comprehensive review and bibliometric analysis of green extraction methods
- Rapid Communication
- In situ supported rhodium catalyst on mesoporous silica for chemoselective hydrogenation of nitriles to primary amines
- Special Issue: Valorisation of Biowaste to Nanomaterials for Environmental Applications
- Valorization of coconut husk into biochar for lead (Pb2+) adsorption
- Corrigendum
- Corrigendum to “An updated review on carbon nanomaterials: Types, synthesis, functionalization and applications, degradation and toxicity”

