Abstract
As one of the important contaminants of water pollution, the toxic heavy metals have harmful effects on the lives of human beings and the environments. For instance, chromium (Cr), copper (Cu), cadmium (Cd), and nickel (Ni) are listed in the 11 hazardous priority substances of pollutants. Hence, it is of utmost importance to purify water before use. The effective disposal of heavy metals has been arousing worldwide concern in the last few decades. Nano-particles are widely studied as heavy metal adsorbents because of their unique physical and chemical properties. The work purified the contaminated water using the magnetite Fe3O4 nanoparticles with different particle sizes, adsorption time, and pH value. Adsorbents were conveniently separated from the resultant via an external magnetic field due to the magnetic properties of Fe3O4 nanoparticles. Subsequently, a series of characterization methods, including X-ray diffraction, scanning electron microscopy, transmission electron microscopy, and atomic emission spectrophotometer, were used to characterize the structures of nano-particles obtained before and after the purification process and to test heavy metal content. The main results and conclusions in this work are summarized as follows: the removal of heavy metal ions increased with increasing adsorption time and decreasing particle size. The optimal pH of Cr6+, Cu2+, Ni2+, and Cd2+ is 2, 4, and 11, respectively. All the removal rates were above 97%. The experimental results indicate that physical adsorption plays a dominant role in Fe3O4 nanoparticles. It is a promising adsorbent for heavy metal ions.
1 Introduction
Since the twentieth century, damage to the environment has become increasingly serious due to the rapid development of the chemical industry and the continuous improvement of people’s living standards. A prominent problem is the pollution of heavy metals in the natural environment, such as the atmosphere, water, and soil [1]. As early as 2000, Zhang et al. [2] investigated five industrial sites along the Yangtze River estuary. The concentration of Cu2+ and Pb2+ is highest in Shidongkou due to the direct discharge of pollutants nearby. The Yellow River [3], the Pearl River [4], and the Haihe River [5] are polluted by different levels of heavy metals. Weber [6] reported that there are about 15,000 companies engaged in electroplating and metal polishing in the United States. These companies directly or indirectly discharge industrial wastewater, causing heavy metal pollution in the water environment. Thornton and Walsh [7] reported that Swansea, a port city in the south of Wales in the United Kingdom, is the center of the world’s metal smelting industry, causing heavy metal pollution such as Cu, Zn, Pb, and Cd in the water environment. Heavy metals have the characteristics of long existence time, large pollution range, significant environmental toxicity, difficult biodegradation in natural conditions, and concealment [8]. Heavy metals can be bioaccumulated through the food chain, posing a serious threat to biology and human health [2,9–13]. For example, Minamata disease and bone pain disease, which shocked the world, were caused by mercury and cadmium-containing wastewater pollution [14]. Therefore, it is very important to find an effective method to remove heavy metal ions from water.
At present, the commonly used heavy metal wastewater treatment technologies mainly include the chemical precipitation method, oxidation–reduction method, ion exchange, membrane separation method, biological method, etc. [15]. These treatment methods may temporarily transfer heavy metals, but still endanger the environment or have low treatment efficiency [16]. The adsorption method has become one of the key research methods of many relevant scholars because of its simple operation, recyclable heavy metals, low cost, and other advantages [17–21]. The adsorption method can be applied to treat heavy metal ions in water due to various active groups in the adsorbent molecules, such as hydroxyl, thiol, carboxyl, and amino. These active groups can chelate many metal ions by forming ionic bonds or covalent bonds with the adsorbed heavy metal ions to achieve the purpose of adsorbing metal ions, so they can effectively adsorb metal ions in the solution [22,23]. At present, adsorbents with more research include modified zeolite, activated carbon, biochar, lignite, and corncob, but these adsorbents still have defects such as low adsorption efficiency, difficult regeneration, and large interference of coexisting heavy metal ions, which limit their popularization and use [24].
In this article, nano-Fe3O4 particles were used as adsorbents to remove heavy metal ions because nano-Fe3O4 has the characteristics of a large specific surface area and insufficient coordination of surface atoms, which increases the contact with heavy metal ions. Therefore, it is a kind of adsorbent worthy of research, and Fe3O4 has magnetism, which is conducive to recycling. The effects of Fe3O4 particle size, adsorption time, pH, and adsorbent regeneration on the adsorption of Cu2+, Ni2+, Cr6+, Cd2+, and other heavy metal ions were studied. Besides, the adsorption mechanism was discussed.
2 Materials and methods
2.1 Main reagents and instruments
The main reagents include ferrous chloride, ferric chloride, sodium hydroxide, N-methylpyrrolidone, 1,2-propanediol, ammonia, CuSO4·5H2O, NiSO4·6H2O, K2Cr2O6, and CdCl2·2.5H2O; the above drugs are all of analytical grade. The main instruments include 4300DV plasma emission spectrometer, PerkinElmer, Inc., USA; 85-2 digital constant temperature magnetic stirrer, Dadi Automation Instruments Factory, Jintan City, China; CPS-3 ultrasonic grinder, Shenpu Ultrasonic Equipment Factory, Shanghai, China; 600-B electric constant temperature water bath, Xin Kang Medical Instruments Co., Ltd, Jiangyan, China; GL-16A high speed refrigerated centrifuge, Fichal Analytical Instruments Co., Ltd, Shanghai, China; DFZ-1B electric vacuum drying oven, Yongguangming Medical Instruments Factory, Beijing, China; JB90-D elastic electric mixer, Shanghai, China; DHS-25C precision acidity meter, Lida Instruments Factory, Shanghai, China; PW3040/60 X-ray diffractometer Panaco Netherlands; Tecnai G2 20 transmission electron microscope (TEM), Thermo Fisher Scientific, America.
2.2 Preparation of nano-Fe3O4
2.2.1 Polyol method
First, a certain mass of ferrous chloride (FeCl2·4H2O), surfactant N-methylpyrrolidone (PVP), and a certain mass of sodium hydroxide are dissolved in an equal volume of 1,2-propanediol. The 1,2-propanediol solution with dissolved ferrous chloride is placed in a 500 mL Erlenmeyer flask and continuously stirred and heated on the constant temperature magnetic stirrer. When the solution boils, the 1,2-propanediol solution with dissolved sodium hydroxide is slowly added dropwise through a constant-pressure funnel. During the dripping process, black fine particles are constantly produced, and a magnet is employed to check that these particles are magnetic. After the heating switch is turned off, the 1,2-propanediol solution with sodium hydroxide continues to react for 2 h and is cooled to room temperature naturally. Through multiple washings, ultrasonic treatment, water bath treatment, etc., the product is centrifuged and dried in a vacuum drying oven at 60°C for 24 h to obtain black powders.
2.2.2 Co-precipitation method
A certain amount of ferrous chloride (FeCl2·4H2O) and ferric chloride (FeC13·6H2O) mixed solution is added into the Erlenmeyer flask and stirred by a constant temperature magnetic stirrer for 20 min. Then, a certain concentration of ammonia is quickly added into the Erlenmeyer flask under a N2 atmosphere, and stirred vigorously. During the reaction, ammonia helps to adjust the pH of the solution to be greater than 9 (pH > 9), in which the mixed solution gradually changes from orange-red to black. Subsequently, the product is poured into a beaker and placed in a constant temperature water bath at 60°C, then vented with nitrogen and mechanically stirred for 30 min. After the reaction is completed, the product is washed through the pouring method under a magnetic field until it becomes neutral. Next, the product is separated by centrifugation and dried under vacuum for 24 h to obtain nano-magnetic Fe3O4 particles.
2.3 Adsorption experiment
In this experiment, a synthetic wastewater solution was prepared containing four heavy metal ions – nickel (Ni), copper (Cu), cadmium (Cd), and chromium (Cr) – each at a concentration of 40 mg·L−1. To prepare 1,000 mL of this solution, 179.14 mg of NiSO4·6H2O, 157.15 mg of CuSO4·5H2O, 81.27 mg of CdCl2·2.5 H2O, and 113.15 mg of K2Cr2O7 were dissolved. For the adsorption experiment, 200 mL aliquots of the wastewater solution were placed into two beakers labeled No. 1 and No. 2. Then, 5 g of Fe3O4 nanoparticles prepared by the polyol method were added to beaker No. 1, while 5 g of Fe3O4 nanoparticles prepared by the co-precipitation method were added to beaker No. 2. Then, they were treated with magnetic stirring, ultrasound and magnetic stirring, and a certain period of static precipitation under external magnetic field. The metal ion concentration of the supernatant was measured by a plasma emission spectrometer. Under the same conditions, each experiment was repeated five times to get the average value, so as to reduce the error. Relative standard deviation (RSD) was calculated through the formula RSD = σ/μ × 100%, verifying the accuracy of the experiment.
3 Results
3.1 Characterization of nano-Fe3O4
The prepared magnetic nano-Fe3O4 particles were characterized by an X-ray scanner and a TEM, respectively. X-ray diffraction (XRD) testing used Cu target without a monochromator; X-ray tube voltage: 40 kV; X-ray tube current: 40 mA; continuous scanning, scanning range: 20–100°; scanning step size: 0.03342°; and time required for each step: 20.68 s. Figure 1 shows the XRD pattern of Fe3O4 particles synthesized by two methods. Several main diffraction peaks correspond to the crystal planes (220), (311), (400), (422), (511), and (440), respectively. Compared with the PDF card in JCPDS, it was found that the diffraction peak in the standard pattern (00-019-0629) of Fe3O4 corresponds to the face-centered cubic spinel structure (space group: Fd3m 227). The XRD pattern of Fe3O4 with lattice constant (a = 8.396 Å) shows no impurity peaks of other phases, indicating that the sample is pure Fe3O4 particles. The clear and strong diffraction peak indicates that the sample is Fe3O4 particles with good crystallinity. The average particle diameter of the sample can be calculated according to the Scherrer formula: D = kλ/β cos θ (k is the Scherrer constant, k = 0.89, λ is the wavelength of X-rays [for Cu targets, λ = 1.54056 nm], D is the average particle size of crystal particles, β is the full width at half maximum of the diffraction peak, and θ is the Bragg diffraction angle). The average particle size is calculated to be approximately 21 nm for sample A and 6 nm for sample B.
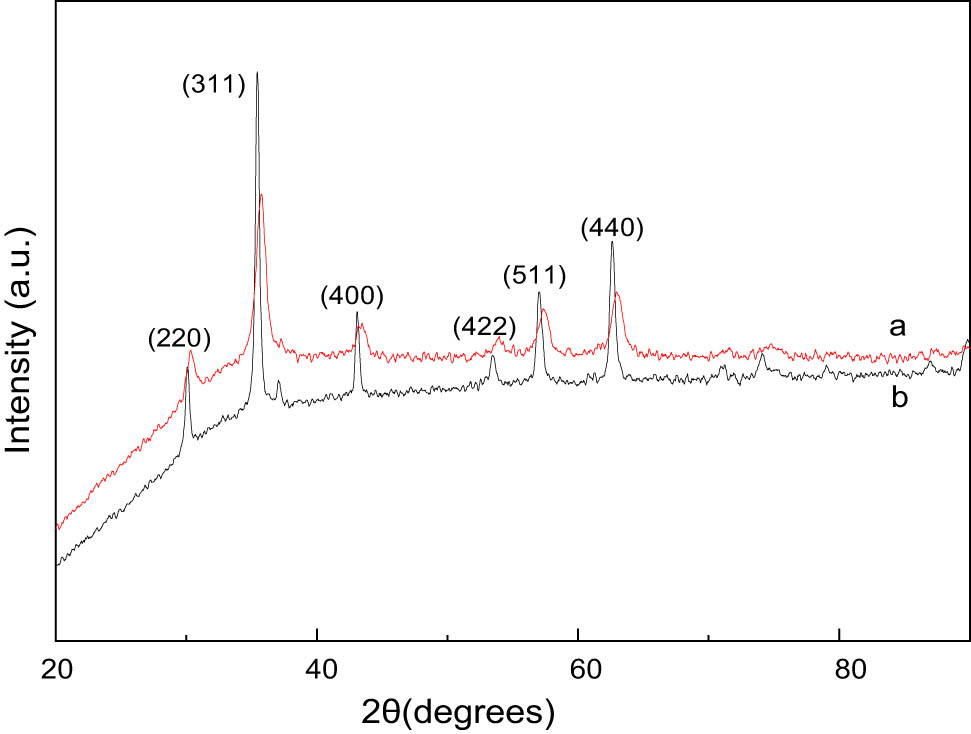
XRD patterns of Fe3O4: (a) polyol process and (b) co-precipitation process.
Figure 2 shows the TEM image, electron diffraction (ED) pattern, and particle size distribution of Fe3O4 nanoparticles prepared by the polyol method and co-precipitation method. The particle size of Fe3O4 prepared by the polyol method is between 25 and 55 nm (Figure 2(a)). The discontinuity of the ED pattern spots also indicates that Fe3O4 grains are larger. The particle size distribution diagram in Figure 2(b) shows that the average particle size is about 33 nm. In Figure 2(c), the particle size of Fe3O4 produced by the co-precipitation method is between 5 and 12 nm, and the continuous pattern of the ED pattern also manifests that the Fe3O4 grains are small. In Figure 2(d), the average particle size is about 8 nm.
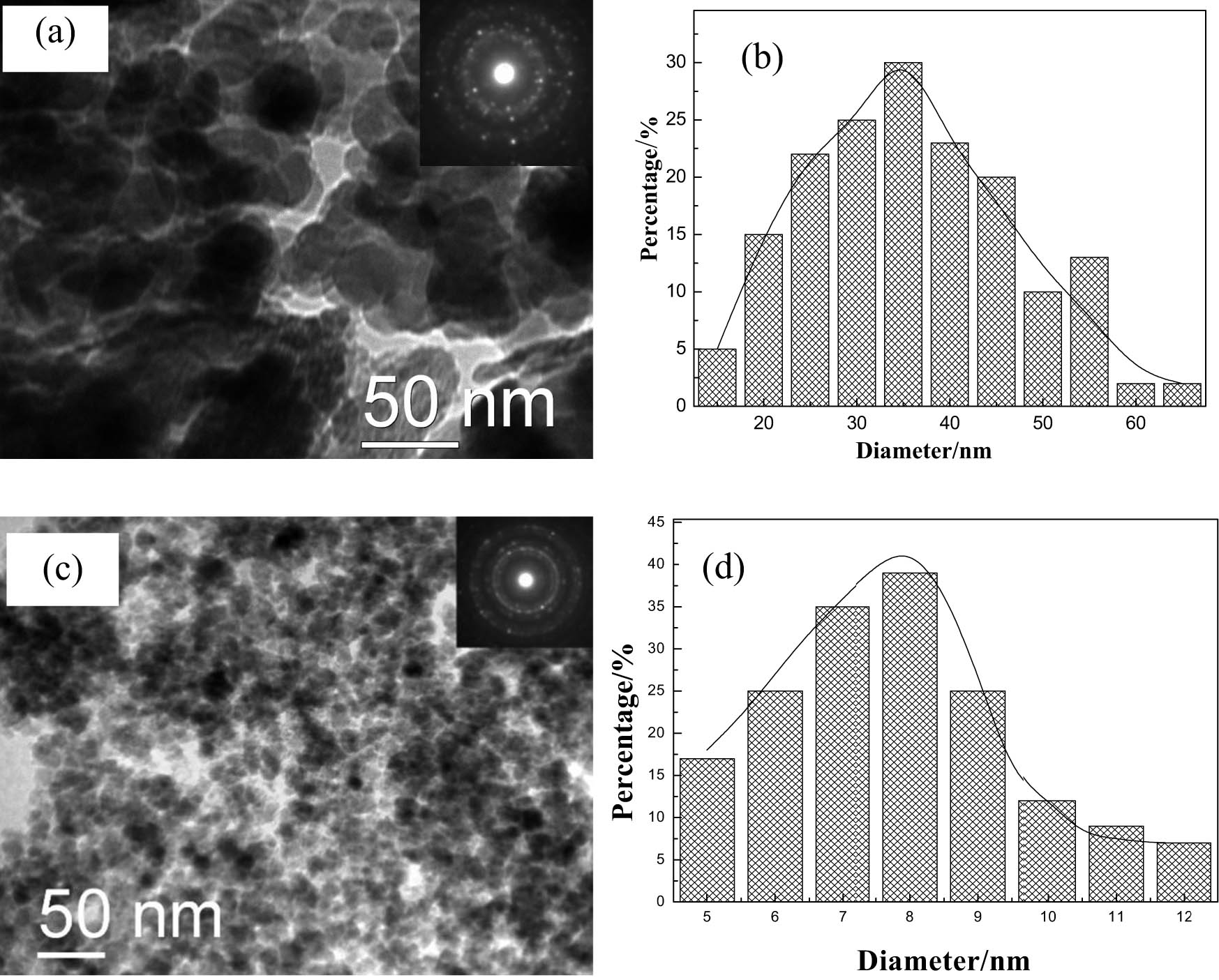
TEM images, ED patterns (a) and (c) and size distributions (b) and (d).
This differs from the results obtained from XRD earlier, partly due to the fast scanning speed of XRD, which ignores some details. On the other hand, each particle may contain several nanoparticles.
3.2 Effect of Fe3O4 particle size on its adsorption of heavy metal ions
Adsorption conditions: pH = 4, temperature was 20°C, adsorbent dosage was 5.00 g·L−1, adsorption time was 16 h. The same treatment was performed on solution Nos. 1 and 2, respectively, with absorption results in Tables 1 and 2.
Results of No. 1 sample (mg·L−1)
| Times | Ni2+ | Cu2+ | Cd2+ | Cr6+ | |
|---|---|---|---|---|---|
| Before treatment | 1 | 34.34 | 41.00 | 76.46 | 50.40 |
| 2 | 34.22 | 41.05 | 76.58 | 50.38 | |
| 3 | 34.18 | 41.21 | 76.53 | 50.36 | |
| 4 | 34.46 | 40.87 | 76.41 | 50.32 | |
| 5 | 34.31 | 41.02 | 76.48 | 50.31 | |
| RSD | — | 0.69% | 0.27% | 0.06% | 0.08% |
| After treatment | 1 | 33.94 | 40.10 | 76.37 | 9.60 |
| 2 | 33.82 | 40.13 | 76.36 | 9.53 | |
| 3 | 33.76 | 40.14 | 76.44 | 9.61 | |
| 4 | 34.02 | 39.98 | 76.34 | 9.56 | |
| 5 | 33.92 | 40.11 | 76.37 | 9.61 | |
| RSD | 0.43% | 0.16% | 0.05% | 0.37% | |
| Absorption | — | 1.19% | 2.27% | 0.12% | 80.97% |
Results of No. 2 sample (mg·L−1)
| Times | Ni2+ | Cu2+ | Cd2+ | Cr6+ | |
|---|---|---|---|---|---|
| Before treatment | 1 | 41.60 | 47.40 | 45.70 | 43.60 |
| 2 | 41.63 | 47.42 | 45.69 | 43.61 | |
| 3 | 41.59 | 47.45 | 45.71 | 43.66 | |
| 4 | 41.62 | 47.38 | 45.73 | 43.61 | |
| 5 | 41.58 | 47.44 | 45.69 | 43.58 | |
| RSD | — | 0.05% | 0.06% | 0.04% | 0.07% |
| After treatment | 1 | 0.880 | 0.129 | 0.886 | 2.220 |
| 2 | 0.883 | 0.125 | 0.885 | 2.222 | |
| 3 | 0.881 | 0.123 | 0.888 | 2.218 | |
| 4 | 0.878 | 0.126 | 0.890 | 2.219 | |
| 5 | 0.881 | 0.130 | 0.887 | 2.223 | |
| RSD | 0.2% | 2.3% | 0.2% | 0.09% | |
| Absorption | — | 97.88% | 99.73% | 98.06% | 94.91% |
In Table 1, the absorption effect of F3O4 prepared by the polyol method on Ni2+, Cu2+, and Cd2+ heavy metal ions is very weak, almost no effect. However, the absorption effect on Cr6+ ions is obvious.
In Table 2, F3O4 produced by the co-precipitation method has a relatively ideal absorption effect on the four heavy metal ions, and the absorption rate is up to 94.91% and over. The RSD calculation results show that their values are all less than 3%, proving that the results are reliable.
The average particle size of Fe3O4 particles prepared by the polyol method and co-precipitation method is 35 and 8 nm, respectively, and the specific surface area measured by the Brunauer–Emmett–Teller method is 4.6 × 101 and 1.9 × 102 m2·g−1, respectively. Therefore, the smaller the size of Fe3O4 nano-particle, the larger the specific surface area, the higher the surface energy, and the more unsaturated bonds on the surface, which improves the contacts and the actions between nano-Fe3O4 and heavy metal ions in water, increasing the adsorption of heavy metal ions. It reveals the application of Fe3O4 nano-particles in adsorbing heavy metal ions in water.
3.3 Effect of adsorption time on adsorption effect
Due to the smaller particle size of Fe3O4 nanoparticles prepared by the co-precipitation method, the adsorption effect of heavy metal ions in water was relatively good, demonstrating superior potential application value. The Fe3O4 prepared by the co-precipitation method was selected for the experiment repeatedly. The supernatant after adsorption for different times was taken and measured to obtain its concentration changes (Figure 3).
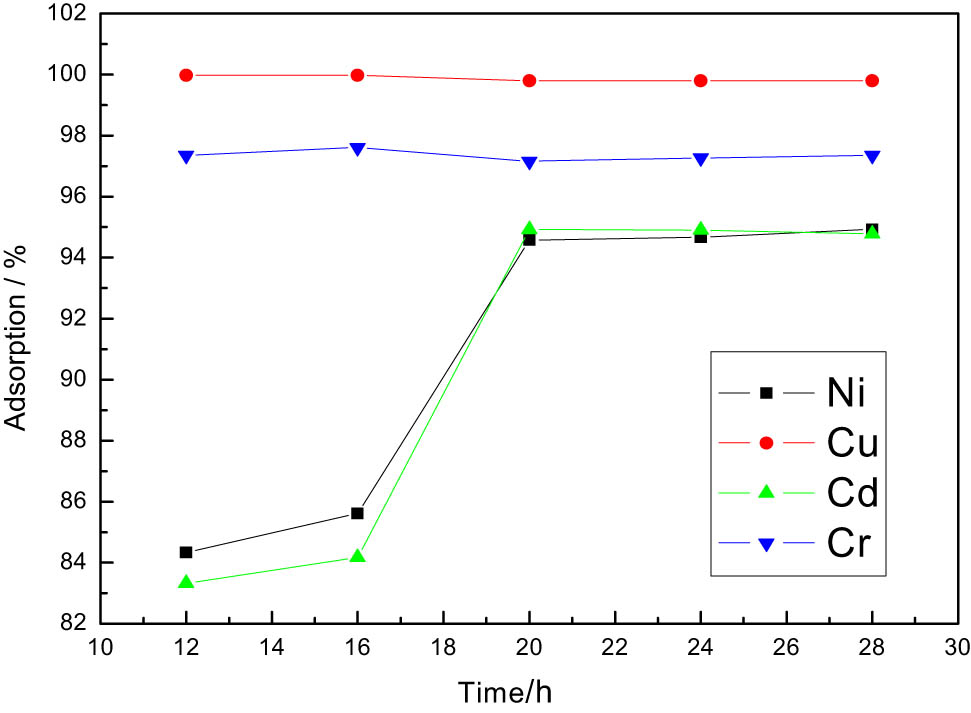
Effects of varying adsorption time.
In Figure 3, when the adsorption time reaches 12 h, Cr6+ and Cu2+ ions reach adsorption equilibrium, and the adsorption rate is the maximum; when the adsorption time is 20 h, Ni2+ and Cd2+ ions reach adsorption equilibrium, and the adsorption rate is the maximum. The increase of adsorption time is conducive to the removal of Ni2+ and Cd2+ ions, but has less impact on Cr6+ and Cu2+ ions.
The adsorption process of adsorbents on adsorbates in solution can be divided into three continuous stages: first, the external diffusion of particles (also known as membrane diffusion) stage, where adsorbates diffuse from the solution to the surface of the adsorbent. The second stage is pore diffusion, during which the adsorbate continues to diffuse toward the adsorption point within the adsorbent pores. Finally, in the adsorption reaction stage, the adsorbate is adsorbed onto the active functional groups within the pores of the adsorbent. Generally, the adsorption rate is mainly controlled by membrane diffusion and pore diffusion. Therefore, extending the adsorption time can promote the active surface point contact between metal ions and Fe3O4 particles, thereby improving the removal rate.
3.4 Effect of pH value on adsorption effect
Similarly, the Fe3O4 obtained by the co-precipitation method is also used, and the pH value of the solution is adjusted from 1 to 12 with 1 M NaOH and H2SO4. The work selected five characteristic points to study their variation patterns. The effect is shown in Figure 4.
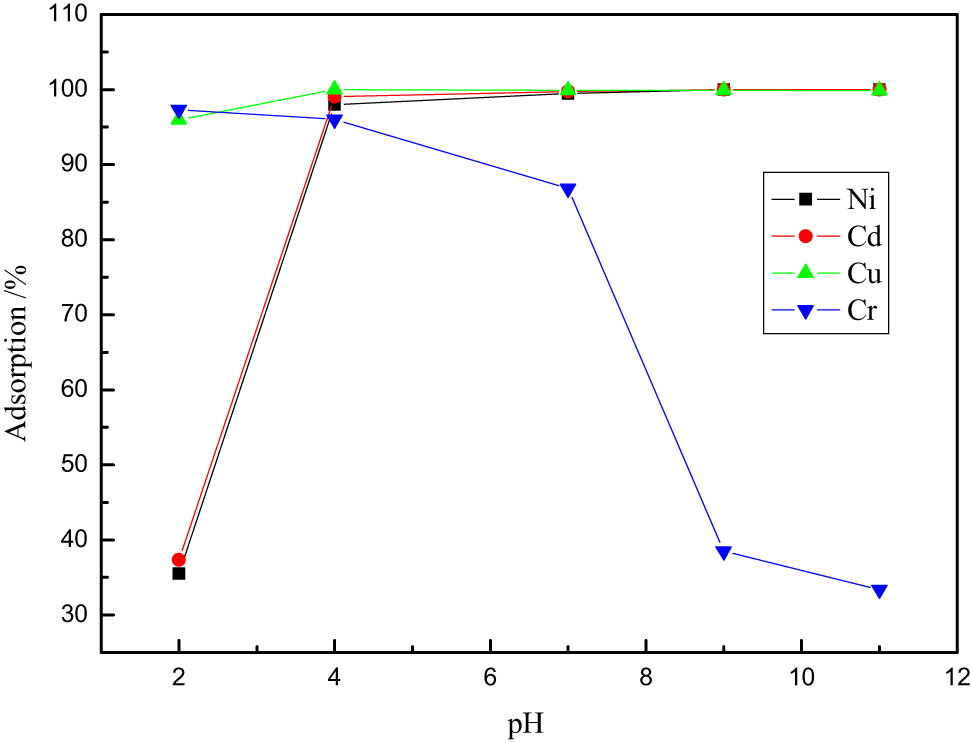
Effects of varying pH.
In Figure 4, when pH = 2, the adsorption rate of Cr6+ reaches the maximum value of 97.30%; when pH = 4, the adsorption rate of Cu2+ reaches the maximum value of 99.97%; and when pH = 11, the adsorption rate of Ni2+ and Cd2+ reaches the maximum value of 99.98%.
It can be seen that pH is one of the most important factors affecting adsorption. When the pH value is low, the heavy metal ions in the solution are in a cationic state. Due to the high concentration of H+ ions, H+ imposes competitive absorption on heavy metal ions, which affects the adsorption effect of heavy metal ions. Therefore, when the pH value is low, the adsorbent has a poor removal effect on heavy metal ions; when the pH value increases, the influence of H+ ions gradually weakens, and when pH = 11, a trace amount of hydroxides is produced, which is to be adsorbed by Fe3O4 particles.
From the above results, it can be seen that each ion has the best adsorption conditions. Under the best adsorption conditions, the adsorption rates of Cr6+, Cu2+, Ni2+, and Cd2+ reach 97.30%, 99.97%, 99.98%, and 99.98%, respectively, which is better than the traditional adsorbents such as modified bio-char, lignite, halloysite, and corncob [25–29]. The common adsorbents described in the references generally have an adsorption rate of less than 95%. Even when a single heavy metal ion is present, the adsorption rate can reach over 99%. However, when there are coexisting ions, the adsorption rate decreases significantly. Fe3O4 nano-particles adsorbent can simultaneously adsorb multiple heavy metal ions, while its adsorption rate can still reach over 97%, which is sufficient to demonstrate its superiority.
4 Discussion
After treating wastewater with Fe3O4 nanoparticles prepared by the co-precipitation method, washed twice with deionized water, added 200 mL of 0.1 mol·L−1 NaOH, diluted to 1,000 mL, stirred magnetically for 1 h, soaked for 4 h, separated Fe3O4 with a magnet, washed twice with deionized water, and then used for adsorption of heavy metal ions. This experiment was repeated five times to study the regenerability of the adsorbent. The results are shown in Figure 5. Through five cycles of regeneration, it was found that the removal rate of the adsorbent for the four ions has not significantly decreased and remains above 97%, indicating that the adsorption is reversible. Therefore, it is inferred that the adsorption mechanism is mainly physical adsorption, which can also be verified from Figure 6.
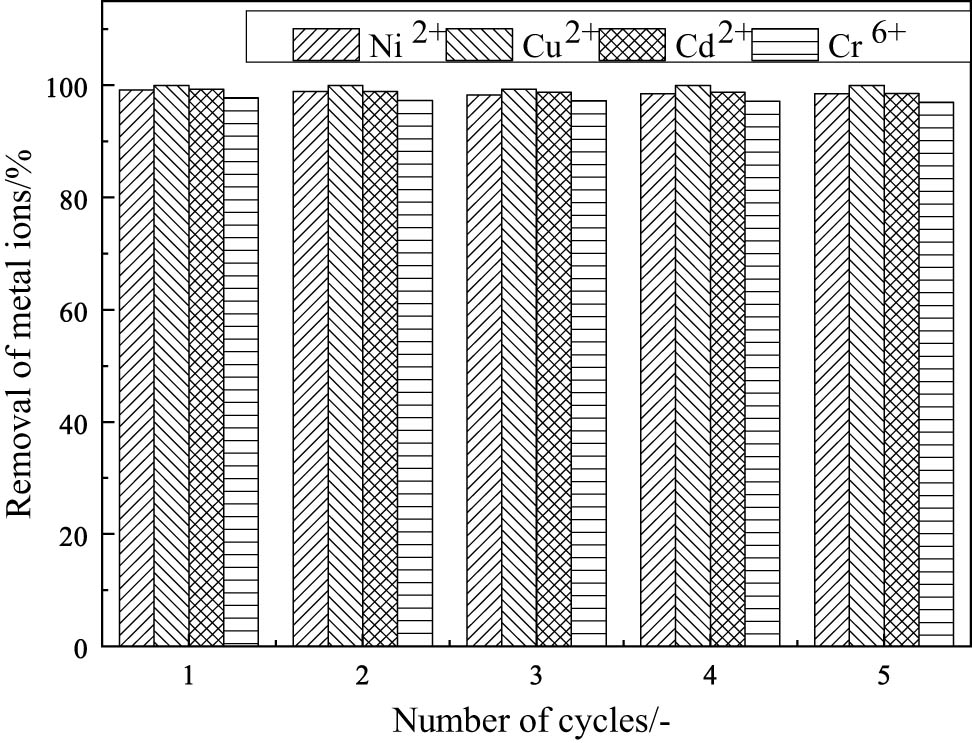
Effect of reusing Fe3O4 on the removal ratio and adsorption capacity of ions.
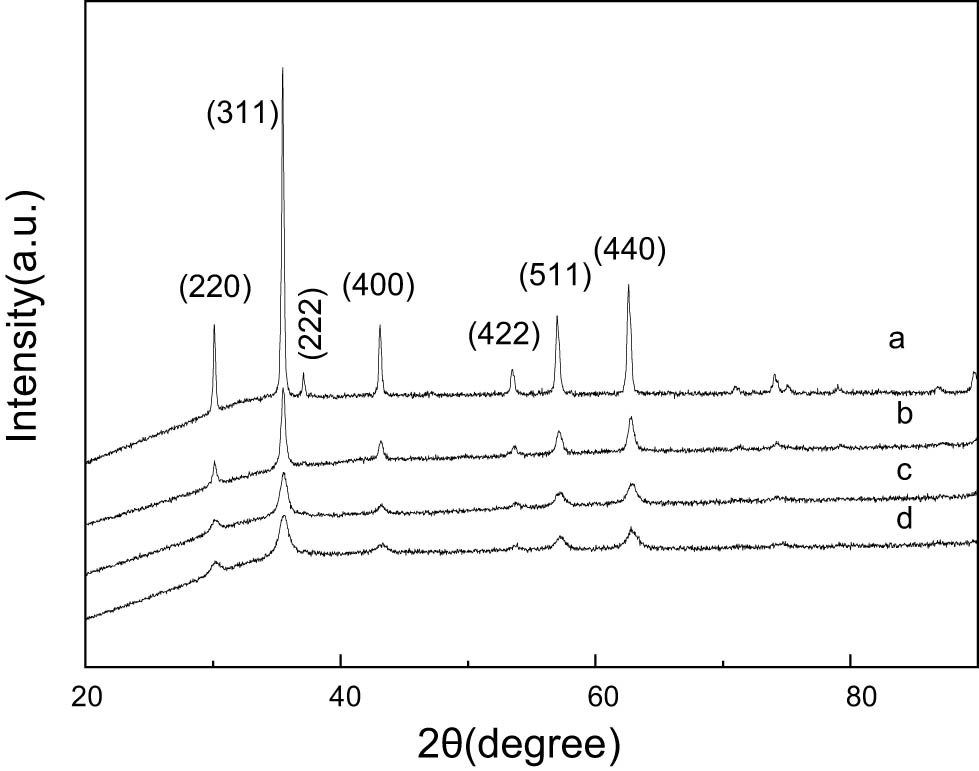
Fe3O4 XRD patterns after purification: (a) treated with Ni2+, (b) treated with Cu2+, (c) treated with Cd2+, and (d) treated with Cr6+.
In Figure 6, the XRD spectrum of the Fe3O4 after wastewater treatment reveals no new impurity peaks. The crystal structure of Fe3O4 remains unchanged during the process, suggesting that the adsorption mechanism is physical rather than chemical.
5 Conclusions
Fe3O4 particles prepared by the co-precipitation method with an average particle size of 8 nm and a specific surface area (4.6 × 101 m2·g−1) showed better adsorption effects on heavy metal ions. When the adsorption time reaches 20 h, the four ions reach adsorption equilibrium. When pH = 2, the Cr6+ ions perform the best adsorption effect; when pH = 4, the adsorption effect of Cu2+ ions is the best; when pH = 11, the adsorption effect of Ni2+ and Cd2+ ions is the best.
Regeneration experiments and XDR patterns showed that magnetic Fe3O4 nanoparticles were utilized as adsorbents whose main adsorption mechanism was physical adsorption. Compared with modified bio-char, lignite, halloysite, and corncob, it had advantages such as a high adsorption rate and reusability. Fe3O4 particle prepared by the co-precipitation method is an ideal adsorbent.
Acknowledgments
The authors are thankful to the Coordination for the Improvement of Northeast Geological S&T Innovation Center of China Geological Survey for financial support.
-
Funding information: This work was supported by the funds from Northeast Geological S&T Innovation Center of China Geological Survey (Grant No. QCJJ2022-25).
-
Author contributions: Li Huiying: writing – original draft, methodology, project administration; Li Xinxin: formal analysis; Wang Yan: writing – review & editing; Zheng Dongmei: visualization; Tang Jian: resources.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Wang H, Fang F, Xie H. Research situation and outlook on heavy metal pollution in water environment of China. Guangdong Trace Elem Sci. 2010;17(1):14–8.Search in Google Scholar
[2] Zhang W, Yu L, Hutchinsan SM, Xu S, Chen Z, Gao X. China’s Yangtze Geomorphic influence on heavy metal accumulation in intertidal sediments. Geomorphology. 2001;41(2–3):195–205.10.1016/S0169-555X(01)00116-7Search in Google Scholar
[3] Niu Y, Cui S. Evaluation of the current status of Yellow River water quality and pollution trends and countermeasures. Environ Forum. 1995;3:37–9.Search in Google Scholar
[4] Ho KC, Hui KCC. Chemical contamination of the east River(Dongjiang)and its implication on sustainable development in the Pearl River Delta. Environ Int. 2001;26(5–6):303–8.10.1016/S0160-4120(01)00004-6Search in Google Scholar
[5] Li H, Huo J, Yu H. Comprehensive assessment of water pollution and water quality for the Haihe River Basin. Water Resour Prot. 2000;4:12–4.Search in Google Scholar
[6] Weber J. Wastewater treatment. Met Finish. 1999;97(1):801–2.10.1016/S0026-0576(99)80075-0Search in Google Scholar
[7] Thornton GJP, Walsh PD. Heavy metals in the waters of the Nanty-Fendrod: change in pollution levels and dynamics associated with the redevelopment of the Lower Swansea Valley, South Waters, UK. Sci Total Environ. 2001;278(1–3):45–55.Search in Google Scholar
[8] Hu N, Li Z, Huang P, Cheng W. The pollution prevention and remediation of heavy metals in infield land in some Suburb Areas China. Bull Mineral Petrol Geochem. 2003;22:251–4.Search in Google Scholar
[9] Bai H, Luo M, Wei S, Jiang Z, He M. The vital function of humic acid with different molecular weight in controlling Cd and Pb bioavailability and toxicity to earthworm (Eisenia fetida) in soil. Environ Pollut. 2020;261:260–4.10.1016/j.envpol.2020.114222Search in Google Scholar PubMed
[10] Thornton GJP, Walsh PD. Heavy metals in the waters of the Nanty-Fendrod: change inpollution levels and dynamics associated with the redevelopment of the Lower Swansea Valley, South Waters, UK. Sci Total Environ. 2001;278(1–3):45–55.10.1016/S0048-9697(00)00887-1Search in Google Scholar PubMed
[11] Li H, Li Z, Liu T, Xiao X, Peng Z, Deng L. A novel technology for biosorption and recovery hexavalent chromium in wastewater by bio-functional magnetic beads. Bioresour Technol. 2008;99:6271–9.10.1016/j.biortech.2007.12.002Search in Google Scholar PubMed
[12] Kokab T, Ashraf HS, Shakoor MB, Jilani A, Ahmad SR, Majid M, et al. Effective removal of Cr (VI) from wastewater using biochar derived from walnut shell. Int J Environ Res Public Health. 2021;18(18):9670.10.3390/ijerph18189670Search in Google Scholar PubMed PubMed Central
[13] Chen S, Zhong M, Wang H, Zhou S, Li W, Wang T, et al. Study on adsorption of Cu2 +, Pb2 +, Cd2 +, and Zn2 + by the KMnO4 modified biochar derived from walnut shell. Int J Environ Sci Technol. 2023;20:1551–68.10.1007/s13762-022-04002-4Search in Google Scholar
[14] Abtahi M, Fakhri Y, Oliveri Conti G, Keramati H, Zandsalimi Y, Bahmani Z, et al. Heavy metals(As, Cr, Pb, Cd and Ni) concentrations in rice (Oryza sativa) from Iran and associated risk assessment: a systematic review. Toxin Rev. 2017;36(1/4):331–41.10.1080/15569543.2017.1354307Search in Google Scholar
[15] Zhang GY, Chen ZN, Tong F, Shen HG, Gao Y, Liu LZ, et al. Effects of different biomass and pyrolysis technique on biochar characterization and immobilization of heavy metal in contaminated soil. J Ecol Rural Environ. 2021;37(1):86–95.Search in Google Scholar
[16] Cao JY, Zhang J, Zhang W, et al. Research progress on remediation technique for hexavalent chromic-contaminated soil. Chin J Soil Sci. 2022;53(5):1220–7.Search in Google Scholar
[17] Yan JY, Yang Y, Wang JH, Ye F, Pan C, Qin Y, et al. Cobalt and nitrogen co-doped biochar enhanced peroxymonosulfate activation for bisphenol A degradation. Acta Mater Compos Sin. 2024;42:1–12.Search in Google Scholar
[18] Shakoor MB, Ali S, Rizwan M, Abbas F, Bibi I, Riaz M, et al. A review of biochar-based sorbents for separation of heavy metals from water. Int J Phytorem. 2020;22(2):111–26.10.1080/15226514.2019.1647405Search in Google Scholar PubMed
[19] Aneta Z, Magdalena S, Ewa S. Sources of soilpollution by heavymetals and the iraccumulation invegetables: a review. Water Air Soil Pollut. 2019;230(7):1–9.10.1007/s11270-019-4221-ySearch in Google Scholar
[20] Kilaruh V, Ponnusamys K, Rames C. Areview on heavy metal pollution, toxicity and remedial measures: current trends and future perspectives. J Mol Liq. 2019;290(9):111197–204.10.1016/j.molliq.2019.111197Search in Google Scholar
[21] Xu J, Liu C, Hsu PC, Zhao J, Wu T, Tang J, et al. Remediation of heavy metal contaminated soil by asymmetrical alternating current electrochemistry. Nat Commun. 2019;10(1):2440–2.10.1038/s41467-019-10472-xSearch in Google Scholar PubMed PubMed Central
[22] Li XM, Xu GJ, Liu Y. Magntic Fe3O4 nanoparticles: synthesis and application in water treatment. Nanosci Nanotechnol-Asia. 2011;1(1):14–24.10.2174/2210681211101010014Search in Google Scholar
[23] You J, Wang L, Zhao Y, Bao W. Areview of amino-functional lizedmagnetic nanoparticles for water treatment: features and prospects. J Clean Prod. 2021;281:124668.10.1016/j.jclepro.2020.124668Search in Google Scholar
[24] Nie Y, Zhao C, Zhou Z, Kong Y, Ma J. Hydrochloric acid-modified fungi-microalgae biochar for adsorption of tetracycline hydrochloride: performance and mechanism. Bioresour Technol. 2023;383:129224.10.1016/j.biortech.2023.129224Search in Google Scholar PubMed
[25] Zhao C, Gong S, Chen Y, Tan W, Wen L, Xuan Y, et al. Comparison of adsorption performance and mechanism analysis of Zn–Fe co-activated magnetic biochar for tetracycline and chromium. J Agro-Environ Sci. 2024;10:22–6.Search in Google Scholar
[26] Xiao X, Yu W, Tan D, Wu X, Qin Z, Wan Q. Preparation of amino-modified halloysite and its adsorption to Cu(Ⅱ) and Cd(Ⅱ). Acta Mineral Sin. 2024;44:1–12.10.3724/j.1000-4734.2024.44.082Search in Google Scholar
[27] Chu H, Gao Y, Zhang W. Study on adsorption of heavy metals lead and cadmium in soil by modified lignite. Multipurp Util Miner Resour (Chinese Journal). 2024;4:4–8.Search in Google Scholar
[28] Hong J, Dai Y, Nie Q, Liao Z, Peng L, Sun D. Research progress in the adsorption of heavy metal ions from wastewater by modified biochar. Chin J Biotechnol. 2024;40(12):4467–79.Search in Google Scholar
[29] Gou L, Sui H, Ren J. Study on the adsorption properties of modified corn cob for heavy metals. Guangzhou Chem Ind. 2024;52(24):141–4.Search in Google Scholar
© 2025 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Optimized green synthesis of silver nanoparticles from guarana seed skin extract with antibacterial potential
- Green adsorbents for water remediation: Removal of Cr(vi) and Ni(ii) using Prosopis glandulosa sawdust and biochar
- Green approach for the synthesis of zinc oxide nanoparticles from methanolic stem extract of Andrographis paniculata and evaluation of antidiabetic activity: In silico GSK-3β analysis
- Development of a green and rapid ethanol-based HPLC assay for aspirin tablets and feasibility evaluation of domestically produced bioethanol in Thailand as a sustainable mobile phase
- A facile biodegradation of polystyrene microplastic by Bacillus subtilis
- Enhanced synthesis of fly ash-derived hydrated sodium silicate adsorbents via low-temperature alkaline hydrothermal treatment for advanced environmental applications
- Impact of metal nanoparticles biosynthesized using camel milk on bacterial growth and copper removal from wastewater
- Preparation of Co/Cr-MOFs for efficient removal of fleroxacin and Rhodamine B
- Applying nanocarbon prepared from coal as an anode in lithium-ion batteries
- Improved electrochemical synthesis of Cu–Fe/brass foil alloy followed by combustion for high-efficiency photoelectrodes and hydrogen production in alkaline solutions
- Precipitation of terephthalic acid from post-consumer polyethylene terephthalate waste fractions
- Biosynthesized zinc oxide nanoparticles: Multifunctional potential applications in anticancer, antibacterial, and B. subtilis DNA gyrase docking
- Anticancer and antimicrobial effects of green-synthesized silver nanoparticles using Teucrium polium leaves extract
- Green synthesis of eco-friendly bioplastics from Chlorella and Lithothamnion algae for safe and sustainable solutions for food packaging
- Optimizing coal water slurry concentration via synergistic coal blending and particle size distribution
- Green synthesis of Ag@Cu and silver nanowire using Pterospermum heterophyllum extracts for surface-enhanced Raman scattering
- Green synthesis of copper oxide nanoparticles from Algerian propolis: Exploring biochemical, structural, antimicrobial, and anti-diabetic properties
- Simultaneous quantification of mefenamic acid and paracetamol in fixed-dose combination tablet dosage forms using the green HPTLC method
- Green synthesis of titanium dioxide nanoparticles using green tea (Camellia sinensis) extract: Characteristics and applications
- Pharmaceutical properties for green fabricated ZnO and Ag nanoparticle-mediated Borago officinalis: In silico predications study
- Synthesis and optimization of gemcitabine-loaded nanoparticles by using Box–Behnken design for treating prostate cancer: In vitro characterization and in vivo pharmacokinetic study
- A comparative analysis of single-step and multi-step methods for producing magnetic activated carbon from palm kernel shells: Adsorption of methyl orange dye
- Sustainable green synthesis of silver nanoparticles using walnut septum waste: Characterization and antibacterial properties
- Efficient electrocatalytic reduction of CO2 to CO over Ni/Y diatomic catalysts
- Greener and magnetic Fe3O4 nanoparticles as a recyclable catalyst for Knoevenagel condensation and degradation of industrial Congo red dye
- Recycling of HDPE-giant reed composites: Processability and performance
- Fabrication of antibacterial chitosan/PVA nanofibers co-loaded with curcumin and cefadroxil for wound healing
- Cost-effective one-pot fabrication of iron(iii) oxychloride–iron(iii) oxide nanomaterials for supercapacitor charge storage
- Novel trimetallic (TiO2–MgO–Au) nanoparticles: Biosynthesis, characterization, antimicrobial, and anticancer activities
- Green-synthesized chromium oxide nanoparticles using pomegranate husk extract: Multifunctional bioactivity in antioxidant potential, lipase and amylase inhibition, and cytotoxicity
- Therapeutic potential of sustainable zinc oxide nanoparticles biosynthesized using Tradescantia spathacea aqueous leaf extract
- Chitosan-coated superparamagnetic iron oxide nanoparticles synthesized using Carica papaya bark extract: Evaluation of antioxidant, antibacterial, and anticancer activity of HeLa cervical cancer cells
- Antioxidant potential of peptide fractions from tuna dark muscle protein isolate: A green enzymatic approach
- Clerodendron phlomoides leaf extract-mediated synthesis of selenium nanoparticles for multi-applications
- Optimization of cellulose yield from oil palm trunks with deep eutectic solvents using response surface methodology
- Nitrogen-doped carbon dots from Brahmi (Bacopa monnieri): Metal-free probe for efficient detection of metal pollutants and methylene blue dye degradation
- High energy density pseudocapacitor based on a nanoporous tungsten(VI) oxide iodide/poly(2-amino-1-mercaptobenzene) composite
- Green synthesized Ag–Cu nanocomposites as an improved strategy to fight multidrug-resistant bacteria by inhibition of biofilm formation: In vitro and in silico assessment study
- In vitro evaluation of antibacterial activity and associated cytotoxicity of biogenic silver nanoparticles using various extracts of Tabernaemontana ventricosa
- Fabrication of novel composite materials by impregnating ZnO particles into bacterial cellulose nanofibers for antimicrobial applications
- Solidification floating organic drop for dispersive liquid–liquid microextraction estimation of copper in different water samples
- Kinetics and synthesis of formation of phosphate composites from low-grade phosphorites in the presence of phosphate–siliceous shales and oil sludge
- Removal of minocycline and terramycin by graphene oxide and Cr/Mn base metal–organic framework composites
- Microfluidic preparation of ceramide E liposomes and properties
- Therapeutic potential of Anamirta cocculus (L.) Wight & Arn. leaf aqueous extract-mediated biogenic gold nanoparticles
- Antioxidant-rich Micromeria imbricata leaf extract as a medium for the eco-friendly preparation of silver-doped zinc oxide nanoparticles with antibacterial properties
- Influence of different colors with light regime on Chlorella sp., biomass, pigments, and lipids quantity and quality
- Experimental vibrational analysis of natural fiber composite reinforced with waste materials for energy absorbing applications
- Green synthesis of sea buckthorn-mediated ZnO nanoparticles: Biological applications and acute nanotoxicity studies
- Production of liquid smoke by consecutive electroporation and microwave-assisted pyrolysis of empty fruit bunches
- Synthesis of MPAA based on polyacrylamide and gossypol resin and applications in the encapsulation of ammophos
- Application of iron-based catalysts in the microwave treatment of environmental pollutants
- Enhanced adsorption of Cu(ii) from wastewater using potassium humate-modified coconut husk biochar
- Adsorption of heavy metal ions from water by Fe3O4 nano-particles
- Green synthesis of parsley-derived silver nanoparticles and their enhanced antimicrobial and antioxidant effects against foodborne resistant bacteria
- Unwrapping the phytofabrication of bimetallic silver–selenium nanoparticles: Antibacterial, Anti-virulence (Targeting magA and toxA genes), anti-diabetic, antioxidant, anti-ovarian, and anti-prostate cancer activities
- Review Article
- Sustainable innovations in garlic extraction: A comprehensive review and bibliometric analysis of green extraction methods
- Rapid Communication
- In situ supported rhodium catalyst on mesoporous silica for chemoselective hydrogenation of nitriles to primary amines
- Special Issue: Valorisation of Biowaste to Nanomaterials for Environmental Applications
- Valorization of coconut husk into biochar for lead (Pb2+) adsorption
- Corrigendum
- Corrigendum to “An updated review on carbon nanomaterials: Types, synthesis, functionalization and applications, degradation and toxicity”
Articles in the same Issue
- Research Articles
- Optimized green synthesis of silver nanoparticles from guarana seed skin extract with antibacterial potential
- Green adsorbents for water remediation: Removal of Cr(vi) and Ni(ii) using Prosopis glandulosa sawdust and biochar
- Green approach for the synthesis of zinc oxide nanoparticles from methanolic stem extract of Andrographis paniculata and evaluation of antidiabetic activity: In silico GSK-3β analysis
- Development of a green and rapid ethanol-based HPLC assay for aspirin tablets and feasibility evaluation of domestically produced bioethanol in Thailand as a sustainable mobile phase
- A facile biodegradation of polystyrene microplastic by Bacillus subtilis
- Enhanced synthesis of fly ash-derived hydrated sodium silicate adsorbents via low-temperature alkaline hydrothermal treatment for advanced environmental applications
- Impact of metal nanoparticles biosynthesized using camel milk on bacterial growth and copper removal from wastewater
- Preparation of Co/Cr-MOFs for efficient removal of fleroxacin and Rhodamine B
- Applying nanocarbon prepared from coal as an anode in lithium-ion batteries
- Improved electrochemical synthesis of Cu–Fe/brass foil alloy followed by combustion for high-efficiency photoelectrodes and hydrogen production in alkaline solutions
- Precipitation of terephthalic acid from post-consumer polyethylene terephthalate waste fractions
- Biosynthesized zinc oxide nanoparticles: Multifunctional potential applications in anticancer, antibacterial, and B. subtilis DNA gyrase docking
- Anticancer and antimicrobial effects of green-synthesized silver nanoparticles using Teucrium polium leaves extract
- Green synthesis of eco-friendly bioplastics from Chlorella and Lithothamnion algae for safe and sustainable solutions for food packaging
- Optimizing coal water slurry concentration via synergistic coal blending and particle size distribution
- Green synthesis of Ag@Cu and silver nanowire using Pterospermum heterophyllum extracts for surface-enhanced Raman scattering
- Green synthesis of copper oxide nanoparticles from Algerian propolis: Exploring biochemical, structural, antimicrobial, and anti-diabetic properties
- Simultaneous quantification of mefenamic acid and paracetamol in fixed-dose combination tablet dosage forms using the green HPTLC method
- Green synthesis of titanium dioxide nanoparticles using green tea (Camellia sinensis) extract: Characteristics and applications
- Pharmaceutical properties for green fabricated ZnO and Ag nanoparticle-mediated Borago officinalis: In silico predications study
- Synthesis and optimization of gemcitabine-loaded nanoparticles by using Box–Behnken design for treating prostate cancer: In vitro characterization and in vivo pharmacokinetic study
- A comparative analysis of single-step and multi-step methods for producing magnetic activated carbon from palm kernel shells: Adsorption of methyl orange dye
- Sustainable green synthesis of silver nanoparticles using walnut septum waste: Characterization and antibacterial properties
- Efficient electrocatalytic reduction of CO2 to CO over Ni/Y diatomic catalysts
- Greener and magnetic Fe3O4 nanoparticles as a recyclable catalyst for Knoevenagel condensation and degradation of industrial Congo red dye
- Recycling of HDPE-giant reed composites: Processability and performance
- Fabrication of antibacterial chitosan/PVA nanofibers co-loaded with curcumin and cefadroxil for wound healing
- Cost-effective one-pot fabrication of iron(iii) oxychloride–iron(iii) oxide nanomaterials for supercapacitor charge storage
- Novel trimetallic (TiO2–MgO–Au) nanoparticles: Biosynthesis, characterization, antimicrobial, and anticancer activities
- Green-synthesized chromium oxide nanoparticles using pomegranate husk extract: Multifunctional bioactivity in antioxidant potential, lipase and amylase inhibition, and cytotoxicity
- Therapeutic potential of sustainable zinc oxide nanoparticles biosynthesized using Tradescantia spathacea aqueous leaf extract
- Chitosan-coated superparamagnetic iron oxide nanoparticles synthesized using Carica papaya bark extract: Evaluation of antioxidant, antibacterial, and anticancer activity of HeLa cervical cancer cells
- Antioxidant potential of peptide fractions from tuna dark muscle protein isolate: A green enzymatic approach
- Clerodendron phlomoides leaf extract-mediated synthesis of selenium nanoparticles for multi-applications
- Optimization of cellulose yield from oil palm trunks with deep eutectic solvents using response surface methodology
- Nitrogen-doped carbon dots from Brahmi (Bacopa monnieri): Metal-free probe for efficient detection of metal pollutants and methylene blue dye degradation
- High energy density pseudocapacitor based on a nanoporous tungsten(VI) oxide iodide/poly(2-amino-1-mercaptobenzene) composite
- Green synthesized Ag–Cu nanocomposites as an improved strategy to fight multidrug-resistant bacteria by inhibition of biofilm formation: In vitro and in silico assessment study
- In vitro evaluation of antibacterial activity and associated cytotoxicity of biogenic silver nanoparticles using various extracts of Tabernaemontana ventricosa
- Fabrication of novel composite materials by impregnating ZnO particles into bacterial cellulose nanofibers for antimicrobial applications
- Solidification floating organic drop for dispersive liquid–liquid microextraction estimation of copper in different water samples
- Kinetics and synthesis of formation of phosphate composites from low-grade phosphorites in the presence of phosphate–siliceous shales and oil sludge
- Removal of minocycline and terramycin by graphene oxide and Cr/Mn base metal–organic framework composites
- Microfluidic preparation of ceramide E liposomes and properties
- Therapeutic potential of Anamirta cocculus (L.) Wight & Arn. leaf aqueous extract-mediated biogenic gold nanoparticles
- Antioxidant-rich Micromeria imbricata leaf extract as a medium for the eco-friendly preparation of silver-doped zinc oxide nanoparticles with antibacterial properties
- Influence of different colors with light regime on Chlorella sp., biomass, pigments, and lipids quantity and quality
- Experimental vibrational analysis of natural fiber composite reinforced with waste materials for energy absorbing applications
- Green synthesis of sea buckthorn-mediated ZnO nanoparticles: Biological applications and acute nanotoxicity studies
- Production of liquid smoke by consecutive electroporation and microwave-assisted pyrolysis of empty fruit bunches
- Synthesis of MPAA based on polyacrylamide and gossypol resin and applications in the encapsulation of ammophos
- Application of iron-based catalysts in the microwave treatment of environmental pollutants
- Enhanced adsorption of Cu(ii) from wastewater using potassium humate-modified coconut husk biochar
- Adsorption of heavy metal ions from water by Fe3O4 nano-particles
- Green synthesis of parsley-derived silver nanoparticles and their enhanced antimicrobial and antioxidant effects against foodborne resistant bacteria
- Unwrapping the phytofabrication of bimetallic silver–selenium nanoparticles: Antibacterial, Anti-virulence (Targeting magA and toxA genes), anti-diabetic, antioxidant, anti-ovarian, and anti-prostate cancer activities
- Review Article
- Sustainable innovations in garlic extraction: A comprehensive review and bibliometric analysis of green extraction methods
- Rapid Communication
- In situ supported rhodium catalyst on mesoporous silica for chemoselective hydrogenation of nitriles to primary amines
- Special Issue: Valorisation of Biowaste to Nanomaterials for Environmental Applications
- Valorization of coconut husk into biochar for lead (Pb2+) adsorption
- Corrigendum
- Corrigendum to “An updated review on carbon nanomaterials: Types, synthesis, functionalization and applications, degradation and toxicity”

