Novel trimetallic (TiO2–MgO–Au) nanoparticles: Biosynthesis, characterization, antimicrobial, and anticancer activities
-
Mohamed Khalil Yousef Soliman
, Mostafa Abdel-Maksoud
Abstract
Trimetallic nanoparticles have garnered significant attention due to their promising biological activities. In this study, the aqueous extract of banana peel was utilized as a reducing and stabilizing agent for the green synthesis of novel trimetallic titanium dioxide–magnesium oxide–gold nanoparticles (TiO2–MgO–Au NPs). The biosynthesized nanoparticles were spherical, with an average size of 55 nm as observed by transmission electron microscopy and 70 nm based on dynamic light scattering measurements. Antimicrobial tests revealed that the nanoparticles exhibited minimum inhibitory concentration values of 500 µg·mL−1 against Bacillus subtilis and Escherichia coli, 250 µg·mL−1 against Pseudomonas aeruginosa and Candida albicans, and 125 µg·mL−1 against Staphylococcus aureus. The nanoparticles demonstrated significant antibiofilm activity, inhibiting methicillin-resistant S. aureus biofilm formation by 86.8% at 250 µg·mL−1 and by 25.1% at 15.62 µg·mL−1. The MTT assay showed strong cytotoxic effects on MCF-7 and HepG2 cancer cells, with the lowest IC50 value of 11.09 ± 1.02 µg·mL−1 observed in MCF-7 cells. Additionally, ELISA results confirmed that TiO2–MgO–Au NPs enhanced the activation of caspase-8 while reducing the levels of VEGFR-2. In conclusion, the biosynthesized TiO2–MgO–Au NPs showed significant antimicrobial, antibiofilm, and anticancer potential, particularly against breast cancer cells, indicating their potential as a novel therapeutic agent.
1 Introduction
Antibiotics are highly effective in protecting against a wide range of bacterial infections. However, one strategy bacteria use to enhance their resistance to antibiotics is mutation, leading to the emergence of multidrug-resistant (MDR) species and significantly diminishing the therapeutic efficacy of antibiotics. Concerns about the rise of antibiotic resistance are growing as bacteria actively develop and adapt their defense mechanisms against conventional antibiotics [1]. Furthermore, bacteria have evolved defense mechanisms against several inhibitors, such as the production of biofilms. Biofilms, composed of abiotic or biotic materials, are formed by live microorganisms that establish a strong attachment to the surface they colonize [2,3]. The substances released by the bacteria in the biofilm mediate this interaction [4,5]. Moreover, bacteria’s resistance to well-known antimicrobial drugs is caused by the biofilm matrix surrounding their cells. Therefore, many researchers are increasingly focusing on developing non-traditional antibiotics, including innovative nano-antibiotic compounds, to combat microorganism resistance [6,7]. Undoubtedly, creating new compounds capable of eliminating and preventing bacterial proliferation through innovative mechanisms is the solution to these challenges [8].
Chemotherapy, radiation therapy, and surgery improve cancer outcomes but have significant side effects. Nausea, exhaustion, hair loss, infertility, and secondary malignancies can result after chemotherapy. Radiation therapy can lead to skin irritation, fatigue, organ damage, and an increased risk of developing secondary malignancies. Surgical procedures can cause bleeding, infection, deformity, and psychological discomfort. These treatments are beneficial, but they emphasize the need for balanced approaches that regard the patient’s well-being and continued research toward safer treatments [9]. Therefore, the primary goal of research in this field is to develop new methods or anti-cancer drugs that offer superior efficacy, minimal toxicity, excellent biocompatibility, and the ability to degrade naturally.
Nanotechnology is rapidly emerging as a crucial field, driving significant advancements in pharmaceutical delivery, bioavailability, imaging, and treatment while considering all relevant factors. Additionally, it effectively reduces the negative consequences associated with these procedures [10]. The use of nanoparticles as anticancer drugs offers multiple advantages. It can enhance the stability and longevity of the distribution of medications [11]. Nanoparticles can be precisely delivered to selectively target cancer cells, minimizing damage to healthy tissues. Nanoparticles can overcome the problem of multiple drug resistance by avoiding the P-glycoprotein efflux pump. Furthermore, they can deliver multiple therapeutic drugs simultaneously, facilitating the use of combination therapy. Specific stimuli can activate nanoparticles with advanced sensing capabilities to precisely release their payloads at the targeted site. This precise distribution strategy enhances the efficacy of treatment and reduces the negative side effects. Furthermore, nanoparticles can be designed to overcome drug resistance and improve drug solubility and loading efficiency. Nanoparticles possess unique attributes that make them an ideal platform for cancer therapy, including enhanced efficacy and improved safety [12,13].
Among different nanosized materials, MgO and TiO2 nanoparticles have recently been thoroughly explored for their powerful anticancer activities. Various studies have demonstrated that minuscule nanoparticles composed of metal oxides could selectively target and eradicate cancer cells, even in very minute concentrations [14]. MgO and TiO2 nanoparticles exhibit anticancer properties due to their capability to produce reactive oxygen species (ROS), which can trigger oxidative stress and apoptosis in tumor cells. The nanoparticles exhibited the capacity to disrupt the mitochondrial membrane. In addition, the nanoparticles can specifically gather in cancer cells, reducing harm to healthy cells [15].
Many inorganic NPs have antibacterial characteristics, including Ag, Au, Cu, CuO, Se, TiO2, and ZnO [16,17,18,19,20,21]. Along with the other inorganic NPs, zinc oxide nanoparticles (ZnONPs) have received a lot of attention due to their affordable, easy-to-prepare nature that is safe for both humans and animals [22,23]. TiO2NPs, biofabricated using Staphylococcus aureus, exhibit remarkable antibacterial and antibiofilm properties against various bacterial species, including Bacillus subtilis and Escherichia coli [24]. These nanoparticles are also extensively used in the production of medical supplies [25]. According to researchers, gold nanoparticles may prevent S. aureus and Pseudomonas aeruginosa from forming biofilms [26].
Multimetallic nanoparticles, composed of two or more different metallic elements, have emerged as promising multifunctional agents with both antimicrobial and anticancer properties [27,28,29,30]. These nanoparticles exploit the synergistic effects of the constituent metals to disrupt bacterial cell membranes, inhibit microbial enzymes and metabolic processes, and generate ROS that trigger oxidative stress [28]. This multi-pronged antimicrobial action allows trimetallic nanoparticles to be effective against a broad spectrum of pathogens, including drug-resistant strains [31]. Simultaneously, trimetallic nanoparticles can selectively target cancer cells by exploiting differences in cellular uptake, disrupting mitochondrial function, and triggering apoptotic pathways. The versatile design of trimetallic nanoparticles enables the tuning of their physicochemical properties to maximize antimicrobial efficacy while preserving their ability to target and destroy cancer cells [28]. This dual functionality makes trimetallic nanoparticles attractive candidates for applications in antimicrobial coatings, wound dressings, and targeted cancer therapeutics, where their multifunctional nature can be leveraged to address pressing clinical challenges. This study aimed to utilize banana peel extract for the green biosynthesis of novel trimetallic (TiO2–MgO–Au) nanoparticles for the first time and to evaluate their antimicrobial and anticancer activities.
2 Materials and methods
2.1 Materials
Banana peels that were collected were supplied from a local store in Giza, Egypt. Most of the chemicals and reagents needed for the experiment were obtained from Sigma Aldrich, including Ti(NO3)4·4H2O (titanium(iv) nitrate tetrahydrate) as a titanium source, magnesium(ii) nitrate hexahydrate (Mg(NO3)2·6H2O), HAuCl4·3H2O (hydrogen tetrachloroaurate(iii) hydrate), and sodium hydroxide as a precipitating agent. MTT (3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium-bromide), Taxol (paclitaxel), and DMSO (dimethyl sulfoxide) were purchased from Sigma Chemical Co. (Missouri, USA). Fetal bovine serum (FBS), phosphate buffer saline (PBS), trypsin-EDTA, and Dulbecco’s modified Eagle’s medium (DMEM), penicillin/streptomycin (Pen/Strep) were procured from Gibco (Gibco, TFS Inc., USA).
2.2 Banana peel extract preparation
Fresh bananas were sourced from local merchants, and their peels were sliced into small pieces and washed three times with distilled water to remove dirt and impurities. The cleaned peel pieces were dried using paper towels and boiled for 20 min at 100°C in a beaker containing 250 mL of double-distilled water and 100 g of peels. The resulting mixture was filtered three times using Whatman No. 1 filter paper, yielding a pale yellow extract, which was then refrigerated at 4°C for further use [32].
2.3 Biosynthesis of trimetallic TiO2–MgO–Au NPs using banana peel extract
Ten milliliters of 0.01 M solutions of Ti(NO3)4·4H2O, Mg(NO3)2·6H2O, and HAuCl4·3H2O were precisely combined and stirred at room temperature for approximately 1 h. After that, the extracted banana peel (30 mL) was added following Kamli et al.’s [33] procedure with a few adjustments. Subsequently, 20 mL of the banana peel extract was added, adjusting the pH to 9.0. The optimal synthesis of trimetallic TiO2–MgO–Au nanoparticles was achieved by maintaining the reaction conditions at an incubation temperature of 35°C with continuous agitation (250 rpm) in a shaking incubator for approximately 24 h [34]. At the end of the incubation period, the solution changed to a pale brown color, signifying the successful formation of trimetallic TiO2–MgO–Au nanoparticles. To get rid of peel biomolecules that were weakly attached, the produced trimetallic TiO2–MgO–Au NPs needed to be washed five times using distilled water. Afterward, the nanoparticles were separated by centrifugation at 15,000 rpm for 5 min, collected, and dried in an oven at 200°C overnight [35].
2.4 Characterization of trimetallic TiO2–MgO–Au NPs
The color shift of the banana peel extract from pale yellow to pale brown upon mixing with the metal precursors is the first clue that the synthesis of trimetallic TiO2–MgO–Au NPs has occurred. Then, UV-visible spectroscopy (JENWAY 6305, Staffordshire, UK) was used to determine the absorbance of the faint brown hue at wavelengths between 200 and 800 nm. The synthesized solution (2 mL) was put in a quartz cuvette, and its absorbance was determined at regular intervals at various wavelengths to find the maximum absorbance [36]. Moreover, the chemical functional groups included in the produced trimetallic TiO2–MgO–Au NPs were determined by FTIR analysis (Cary-660 model, KBr pellet technique, wavenumber range: 400–4,000 cm⁻¹). Moreover, the surface morphology of trimetallic TiO2–MgO–Au NPs was evaluated using SEM (ZEISS, EVO-MA10, Germany). Using EDX (Bruker, Germany), the elemental composition, purity, and distribution of the constituents in the produced trimetallic TiO2–MgO–Au NPs were examined. Additionally, we used transmission electron microscopy (TEM) (JEM-2100 Plus, Jeol, Japan) to determine the morphologies, average, and exact sizes of the produced trimetallic TiO2–MgO–Au NPs. The average particle size distribution and zeta potential analysis of the trimetallic TiO2–MgO–Au NPs was determined using dynamic light scattering (DLS) (Nano ZS, Malvern, UK) [37]. The crystal size and crystallinity were evaluated by using XRD-6000 (Shimadzu Scientific Instruments, Japan).
2.5 Antibacterial activity
Green synthesized trimetallic TiO2–MgO–Au NPs were tested against five different microbial strains: B. subtilis (ATCC 6633), S. aureus (ATCC 6538), P. aeruginosa (ATCC 9027), E. coli (ATCC 25922), and Candida albicans (ATCC 10231). Each microbial strain was evenly spread on sterile Petri plates containing Muller–Hinton agar using the agar diffusion well method, following incubation of pure strains grown in Muller–Hinton broth. Using a clean cork borer, four 7 mm circular wells were created in the plates. To assess antimicrobial efficacy, 0.1 mL of TiO2 salt, MgO salt, HAuCl4, and TiO2–MgO–Au NPs were added to each well. The Petri dishes were then incubated at 37°C overnight, and the inhibition zones were measured [38,39].
2.6 Determination of MIC
Using the broth-based microdilution technique, the minimum inhibitory concentrations (MIC) of TiO2–MgO–Au NPs were found for B. subtilis (ATCC 6633), S. aureus (ATCC 6538), P. aeruginosa (ATCC 9027), E. coli (ATCC 25922), and C. albicans (ATCC 10231). TiO2–MgO–Au NPs were already synthesized in various amounts (from 1,000 to 15.62 μg·mL−1). Different amounts of TiO2–MgO–Au NPs were introduced to sterilized microtiter plate wells after 100 μL of double-strength Mueller–Hinton broth. Microbial cell suspension (20 μL) matching (OD of 0.5 McFarland standard) was added to all wells except the negative control well. Positive control wells were populated with microbial solutions to determine if MH broth would sustain microbial growth. These plates were incubated for 24 h at 37°C. The wells were then filled with 30 µL of HiMedia’s resazurin solution (0.02% wt/v), and then the plate was incubated for a further 6 h to check for microbial growth. In cases when the strains had been propagated properly, the color of the developing control wells changed from blue to red, while the color of the control or negative control wells remained constant in the absence of contamination. Three runs of the examination were conducted [7].
2.7 Assay for biofilm inhibition
Using methicillin-resistant S. aureus (MRSA), clinically relevant isolation with a powerful biofilm-forming agent, the MTP technique was utilized to evaluate the ability of TiO2–MgO–Au NPs to prevent or diminish the development of bacterial biofilms. We made some changes to the biofilm research to make it better than the previous one [5,40]. MTP-containing TSB media were added to gradient doses of TiO2–MgO–Au NPs, supplemented with 1% glucose. The organisms investigated were cultivated on MH broth for 24 h at 37°C after being diluted 1:100 in TSB. The growth of the cell intensity (OD620 nm) was monitored during the incubation period, and then the planktonic cells were removed from the plates. The resultant biofilm was fixed for 10 min using 200 μL of 95% methanol as the solvent and washed three times using PBS at pH 7.4. This was done after removing all the components of the well contents so as not to disturb the biofilms that had grown. Once the 200 μL wells were filled with 0.3% w/v crystal violet, they were left at room temperature for around 15 min. Subsequently, the plates were washed using distilled water, and then the wells were filled with a 30% acetic acid reagent for the quantitative evaluation of biofilm formation. The absorbance was measured at OD 540 nm using the STATFAX-USA microplate reader. The results were confirmed by comparing the comparative wells that were treated with the untreated wells [7].
2.8 Anticancer activity
2.8.1 Cell viability assay
The study used two cell lines from ATCC (USA): HepG2, derived from hepatic cancer, and MCF-7, derived from breast cancer. The cells were cultured in DMEM supplemented with 10% FBS and 1% penicillin/streptomycin solution (TFS Inc., USA) at 37°C with 5% carbon dioxide. The MTT method was used to measure the cytotoxic capability [41]. The cells were distributed in 96-well plates with a mean density of 1.2 × 104 cells/well and incubated for 24 h to allow for development. Then, the medium containing different concentrations of nanoparticles was replenished. The MTT assay was performed after 48 h by introducing 100 μL of a solution comprising 5 mg·mL−1 MTT in PBS. The wells were then placed in an incubator set at body temperature for 4 h. DMSO (100 μL) was introduced to every well to form the crystals of formazan. The plates were incubated at body temperature for 10 min. Optical densities were measured at 570 nm and obtained using a microplate reader (Epoc-2 C, Bio Tek, USA).
2.8.2 Assessment of caspase-8 activity
ELISA kits from DRG International Inc. (USA) were used to determine caspase-8 activity (human, EIA-4863).
2.8.3 In vitro cell-based VEGFR-2 TK inhibitory assay
The inhibitory effect of TiO2–MgO–Au NPs towards VEGFR-2 was assessed in vitro by applying ten-fold serial dilution procedures (1.0, 0.1, 0.01, 0.001 µM) employing the VEGFR-2 (KDR) Kinase Assay Kit (Catalog no. # 40325) following the manufacturer’s instructions. Concisely, 25 µL per well of the mix was made and poured into every well. Inhibitor solution (5 µL) was added to every well, designated as a “Test Inhibitor.” Then, 5 µL of an equal liquid without an inhibitor was added to the positive control and blank. Subsequently, 600 µL of kinase buffer and 2.4 mL of water were mixed to create a 3 mL kinase buffer. Twenty microliters of the kinase buffer were added to the “Blank” wells. The amount of VEGFR-2 required for the experiment was calculated, and kinase buffer was used to dilute the enzyme to a concentration of 1 ng·µL−1. Test Inhibitor Control and Positive Control wells were filled with 20 µL of diluted VEGFR-2 enzyme to initiate the reaction. The mixtures were incubated for 45 min in an incubator with a temperature setting of 30°C. Each well received a volume of 50 µL of Kinase-Glo Max reagent. For 15 min, the plate was left at room temperature. Luminescence was measured using a microplate reader.
2.9 Statistical analysis
The GraphPad Prism 8.0 (CA, USA) was employed to conduct data assessment and graphical demonstrations. All findings are expressed as mean ± SD, and all investigations were done three times (n = 3). The statistical analysis was performed using a one-way analysis of variance and Tukey’s multiple comparison tests, where P < 0.05 was considered significant.
3 Results and discussion
3.1 Biosynthesis of trimetallic TiO2–MgO–Au NPs using banana peel extract
There are several advantages of using plant extracts for nanoparticle production as opposed to other biological synthesis methods that include microorganisms. Numerous aspects, including the minimal lab needs for nanoparticle synthesis, the appropriateness for commercial production, the avoidance of impurities, the speedier approach, the single-step process, handling safety, and the greater stability of the nanoparticles owing to significant metabolite secretion, all contribute to this conclusion [42]. The primary function of plant secondary metabolites, including phenols, carbohydrates, proteins, flavonoids, steroids, alkaloids, tannins, sugars, and terpenoids, is to reduce and stabilize nanoparticles [43]. Indeed, variability in biochemical composition poses challenges for reproducibility across batches; however, ensuring consistent processing conditions, such as using standardized ripeness levels or controlled extraction methods, is essential to mitigate these issues. In the present study, trimetallic TiO2–MgO–Au NPs were prepared using an extract from banana peels. The high polyphenolic content in banana peel extract substantially enhances its reducing capacity, facilitating effective nanoparticle synthesis and improving its stability [44]. Banana peel extract contains functional groups such as carboxyl, amine, and hydroxyl groups, which act as reducing agents and stabilize nanoparticles by capping their surface [45]. Nanoparticle stability is influenced by several environmental factors, particularly pH and temperature. The stability of nanoparticles is highly sensitive to the pH of the medium in which they are stored. In highly acidic or alkaline environments, nanoparticles may undergo dissolution, aggregation, or surface modifications that affect their stability. For example, nanoparticles synthesized under more neutral conditions tend to exhibit better long-term stability. Under extreme pH conditions, the surface charge can change, leading to aggregation or destabilization. Studies have shown that nanoparticles with a neutral surface charge are more stable in a wide range of pH levels [46].
Temperature is another crucial factor influencing the long-term stability of nanoparticles. Higher temperatures can accelerate degradation processes such as oxidation, agglomeration, or changes in surface charge. The stability of nanoparticles is generally improved when they are stored at lower temperatures, as this slows down the rate of degradation reactions. However, long-term storage at high temperatures may lead to the transformation or sintering of nanoparticles, potentially resulting in a decrease in their functional properties. For instance, nanoparticles that are stored at room temperature or under refrigeration often exhibit greater stability and retain their biological activity for extended periods [47]. Trimetallic nanostructures, which are subsequently stabilized and capped, are produced as a result of metabolites in the banana peel extract decreasing the metal precursors. A simple and eco-friendly process was developed by Dlugaszewska and Dobrucka [48] to generate Au/Pt/Ag trimetallic nanoparticles using an aqueous Lamii albi flos extract. Furthermore, Meliloti officinalis extract was used to bio-prepare Au–ZnO–Ag trimetallic nanoparticles (∼20 nm) [49]. Aqueous leaf extracts of Froriepia subpinnata and Eryngium campestre were used to biosynthesize Cu/Cr/Ni trimetallic oxide nanoparticles reliably at moderate temperatures [50]. These NPs demonstrated exceptional antibacterial activities against E. coli and S. aureus. Rao and Paria [51], by modifying the mix of phytochemicals in extracts from Aegle marmelos (leaf extract) and Syzygium aromaticum buds, alloy-like Ag–Au–Pd trimetallic NPs (∼8–11 nm) were green-fabricated in 10 min under ambient conditions. In another study, Kaur et al. [52] synthesized TiO2–Al2O3–ZnFe2O4 nanocomposites using hydrothermally prepared Hibiscus rosa sinesis flower extract. Furthermore, Aspergillus niger was successfully used in the entire mycosynthesis of trimetallic copper, selenium, and zinc oxide nanoparticles (Tri-CSZ NPs) by Hashem et al. [27].
3.2 Characterization
3.2.1 UV–vis spectroscopy
The first indication of trimetallic TiO2–MgO–Au NPs production is the plant’s aqueous extract changing from pale yellow to pale brown after being mixed with Ti(NO3)4·4H2O, Mg(NO3)2·6H2O, and HAuCl4·3H2O. This change validated the ability of metabolites to reduce metal ions and build nanoscale structures. The new hue’s absorbance was measured between 200 and 800 nm to determine the maximum SPR. There were two maximums of SPR at 270 and 550 nm, which corresponded to (TiO2, MgO) and Au absorbance, respectively (Figure 1). An absorption band associated with phytobiomolecules may be seen at 280 nm in the UV-visible spectra of banana peel extract. Hassan et al. [53] verified the excitation of green-synthesized TiO2 NPs using a UV–Vis spectrophotometer at 270 and 290 nm. Furthermore, the specific absorption peak was observed at 267 nm for magnesium oxide nanoparticles synthesized by using Trigonella foenum-graecum leaf extract by Vergheese and Vishal [54]. According to Hassanisaadi et al. [55], the UV-Vis spectra of biosynthesized AuNPs were observed at 545 nm.
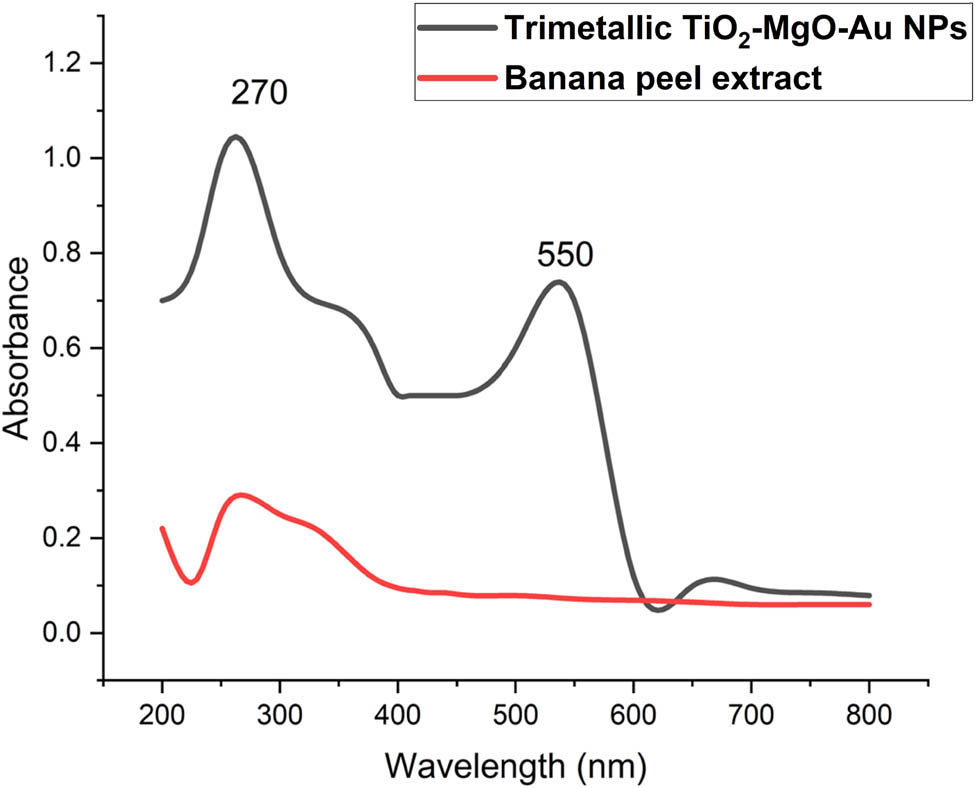
UV-Vis spectroscopy of banana peel extract and biosynthesized trimetallic TiO2–MgO–Au NPs.
According to many studies conducted by Alshehri and Malik, the biosynthesis of trimetallic Cu–Co–Ni NPs utilizing an extract of Origanum vulgar L. shows absorption peaks in its UV–visible spectra at 270 and 320 nm [34]. The green-produced NPs from the combined leaf extracts of F. subpinnata and E. campestre had the highest absorbance at about 220 nm, according to Vaseghi et al. [50]. It is crucial to keep in mind that the interaction of numerous chemicals in the reaction mixture may prevent separate peaks from forming for each of the individual metals that make up the trimetallic structure. As shown by Kannaiyan et al. [56], the UV–Vis absorbance of the C. sativum extract was compared with the collected tri-metallic oxide NPs. The three distinctive peaks of tri-metallic oxide NPs, which exhibited wavelengths of 261 nm, 426 nm, and 564 nm, correspond to the tri-metallic oxide Ni/Cr/Cu NPs, as previously reported. Furthermore, Vaseghi et al. [50] observed that the nanocomposite’s absorption spectra showed the appearance of the largest absorption peak at 265 nm, which suggested the existence of a distinct surface plasmon resonance.
3.2.2 FT-IR spectroscopy
FT-IR spectroscopy was used to identify the trimetallic TiO2–MgO–Au NPs that were biosynthesized. The FT-IR spectra of the synthetic composite are in the 400–4,000 cm−1 spectral region. Ten distinct peaks at wavenumbers 3,200, 2,668, 2,340, 2,076, 1,830, 1,643, 1,405, 1,095, 570, and 439 cm−1 were found in the FT-IR spectra of trimetallic TiO2–MgO–Au NPs (Figure 2). Several groups found in banana peel extract belonged to a variety of substances, including proteins, amino acids, amines, and polysaccharides. The peaks at 3,200, 2,668, 2,340, 2,076, 1,830, and 1,095 cm−1 are caused by the stretching bonds of O–H, C–H, C═O, C═C, and C–O, respectively, that are found in bioorganic molecules such as phenolics, amino acids, and carboxylic acid compounds [57]. Conversely, the nanocomposite’s spectrum displayed all of these vibrations at a greater intensity because of the weak van der Waals contacts between the metallic and biological NPs [58]. According to Botteon et al. [59], metal–metal interactions inside the nanocomposite are responsible for the new, strong bands in the 400–600 cm−1 range. Both the new bands at 1,643 and 1,405 cm−1, which are often associated with the stretching and bending vibrations of the carboxylate anion [60], most likely resulted from the oxidation of –C–OH in phytochemicals during the reduction of metal ions [61]. The results are consistent with previous studies [34,50,57] on the manufacture of trimetallic nanoparticles using plant aqueous extract.

FTIR analysis of biosynthesized trimetallic TiO2–MgO–Au NPs using banana peel extract.
3.3 Morphological and elemental analysis
TEM and SEM were used to examine the surface morphology and particle size of the biosynthesized TiO2–MgO–Au NPs (Figure 3). In this case, an extract from banana peels was used to create trimetallic TiO2–MgO–Au NPs; while many plant extracts share similar properties, the composition of banana peel extract, especially its polysaccharides and high antioxidant levels, offers distinct benefits in creating smaller, more uniform nanoparticles with robust stability [62]. TEM was used to examine their distribution, size, and shape. The produced trimetallic TiO2–MgO–Au NPs, in this instance, had spherical forms and ranged in size from 50 to 70 nm, with an average size of 55 nm (Figure 3a). Therefore, smaller nanoparticles are more reactive in biological environments [63]. Similarly, Hussein et al. [57] claim that TEM images display the spherical form of nanoparticles with a particle size of 50–90 nm. Vaseghi et al. [50] revealed that the TEM images of Cu/Cr/Ni trimetallic oxide NPs biosynthesized by aqueous leaf extracts of E. campestre and F. subpinnata show a variety of sizes and shapes.
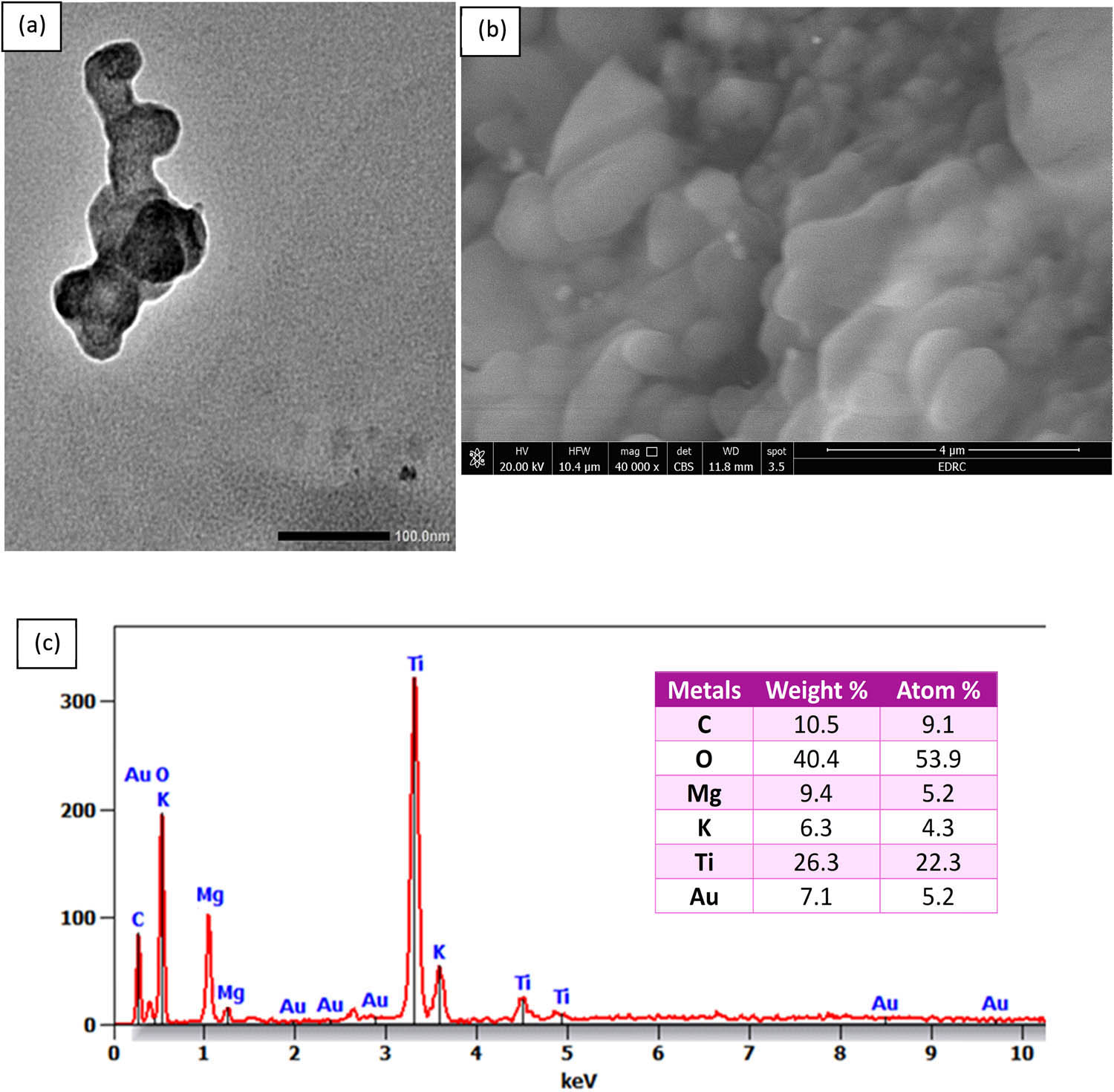
(a) TEM image, (b) SEM image, and (c) EDX analysis of biosynthesized trimetallic TiO2–MgO–Au NPs by using banana peel extract.
The nanocomposite’s morphology was examined using SEM. Figure 3b displays the recorded SEM images. Because of the high surface energy-imposed strong interparticle contact, the trimetallic composite structure displayed an oval and irregular crystalline porous structure, which is characteristic of metallic nanocomposites. When smaller nanostructures merge to form larger ones, the result is a mixed nanostructure. This is evident in the SEM images and indicates the formation of a nanocomposite [57]. Additional research by Alshehri and Malik [34] demonstrates through SEM micrographs of biosynthesized Cu–Co–Ni trimetallic nanoparticles that structural porosity is caused by the agglomeration of nanoflakes.
Verifying the presence and distribution of the required metals inside the trimetallic structure can be facilitated by EDX analysis, which offers information on the elemental composition of a nanostructure. Knowing the elemental composition of NPs is essential to creating materials with appropriate qualities and their use for certain applications. Using EDX analysis, the trimetallic TiO2–MgO–Au NPs’ elementary mapping was determined. According to the acquired data, the as-formed sample mostly consists of Ti, Mg, O, and Au ions, as shown by their respective weight percentages (26.3%, 9.4%, 40.4%, and 7.1%) and atomic percentages (22.3%, 5.2%, 53.9%, and 5.2%) (Figure 3c). The trimetallic synthesis that was previously studied using chemical techniques and plant or microbial extracts was consistent with our findings [34,50]. Plant extract capping agents that coat nanoparticles for stability may be the cause for the C and K peaks [64]. Alshehri and Malik [34], using EDX analysis, determined the weight percentages of 17.34% Co, 10.37% Ni, and 34.01% Cu, resulting in a 2:1:3 ratio for the Cu–Co–Ni trimetallic nanoparticles made with the extract from Origanum vulgare L. in a different study. It appears that surface biomolecules act as capping agents when weak C and O signals are present, and in the elemental mapping, Ni is shown in white, Co in green, and Cu in red.
3.4 XRD, DLS, and zeta potential analysis
The trimetallic TiO2–MgO–Au NPs were examined using XRD. The trimetallic TiO2–MgO–Au NPs XRD spectra, as displayed, revealed ten powerful reflection peaks at 2θ of 25.4°, 36.8°, 38.0°, 42,7°, 48.1°, 54.5°, 62.2°, 64.5°, 74.5°, and 77.6°, respectively. These represent the Bragg diffraction of the (101), (111), (200), (211), (220), and (311) planes, as shown in Figure 4a. Upon analyzing the produced nanoparticles using XRD, they found unique diffraction peaks at four different angles: 25.4°, 38.0°, 48.1°, and 54.5°. These angles correspond to the (101), (111), (200), and (211) planes, respectively, and are in line with a typical TiO2-NP phase pattern (JCPDS No. 21-1272) [65]. MgO-NPs exhibited characteristic peaks at 2θ values of 36.8°, 42,7°, 62.2°, and 74.5°, corresponding to the (111), (200), (220), and (311) planes (JCPDS: 9000493) [66]. Five distinct diffraction peaks were discovered for the hexagonal structure of Au-NP, and they were indexed with the planes (111), (220), and (311) at 38.0°, 64.5°, and 77.6°, respectively (JCPDS: 00-407-84) [67]. These peaks lined up with certain crystallographic planes, such as (220), (211), (211), (111), (200), and (311). Notably, the crystalline structure of the trimetallic TiO2–MgO–Au NPs was verified by these peaks. Using Scherrer’s equation and the XRD pattern, the average crystallite size of the trimetallic TiO2–MgO–Au NPs was found to be 60 nm. The discrepancy between DLS and TEM’s recorded size values of vesicles is expected. This is because the sample preparation for TEM measurement involves dehydration and staining, which can affect the size. Moreover, DLS provides an average size for particles moving in dispersion and is highly sensitive to particle agglomeration or clustering. Even minor aggregation can significantly increase the measured hydrodynamic diameter, as DLS calculates an average size weighted by the intensity of scattered light, but TEM captures a specific field. A similar discrepancy was noticed in previous work and was discussed similarly [68]. The trimetallic nanocomposite (Ru/Ag/Pd) XRD patterns were shown by Hussein et al. [57], and the (111), (200), and (220) planes of Pd-NP were matched with the high-intensity peaks at 2θ = 40.11, 47.75, and 68.31, which match JCPDS 87-0641. At 2θ values of 38.45°, 44.85°, 67.55°, and 77.5°, Ag-NPs showed peaks that corresponded to the (111), (200), (220), and (311) planes, respectively (JCPDS: 04-0783). Ru-NPs revealed peaks for the (100), (002), (101), (102) planes (69.42°, 43.82°, 46.12°, 58.32°, and 69.42°) (JCPDS: 06–0663). Pure crystalline trimetallic nanoparticles were present since no impurity peaks were seen. By using Scherrer’s equation, the crystal size was determined to be 15.67 nm. The same result was obtained by Alshehri and Malik [34] for biogenic Cu–Co–Ni trimetallic nanoparticles.

XRD (a) and DLS (b) of the biosynthesized trimetallic TiO2–MgO–Au NPs.
The size distribution of the particles in a suspension or solution may be measured using the DLS method. It offers details on how different particle sizes are distributed within a sample; this information is commonly shown as a histogram or intensity-weighted size distribution curve. Particle populations that are polydisperse (varying in size) or monodisperse (uniform in size) can be identified by the analysis. DLS examination of the trimetallic TiO2–MgO–Au NPs revealed an average particle size distribution of about 70 nm (Figure 4b). They came to the conclusion that the greater size might have been caused by trace quantities of larger particles formed by contamination or agglomeration, which could add uncertainty to particle size measurements [69]. Similarly, the particle size was roughly 190 nm for the Cu–Fe–Ag NPs that were subjected to DLS analysis by Roy et al. [70]. Monodisperse models are more consistent with the polydispersity index (PDI) values of less than 0.05. Conversely, values higher than 0.7 are expected to indicate the dispersion of polydispersity particles [71]. In this study, the PDI value was 0.472 for the approved PDI levels. The present data show that the trimetallic TiO2–MgO–Au NPs biosynthesized have a moderately polydisperse size distribution.
The zeta potential is a key parameter in assessing the colloidal stability of nanoparticle suspensions, influenced by factors such as surface chemistry, particle roughness, and adsorbed biomolecules [101]. A high absolute zeta potential value (greater than ±30 mV) typically indicates strong electrostatic repulsion, preventing aggregation and ensuring stability [102]. Conversely, values approaching 0 mV suggest reduced repulsive forces, leading to particle agglomeration. Some studies suggest that particles with zeta potential values exceeding ±20 mV can still exhibit moderate stability, depending on other stabilizing interactions [103,104].
In this study, the biosynthesized trimetallic TiO2–MgO–Au NPs exhibited a bimodal zeta potential distribution, with peaks at approximately −20 and +25 mV, indicating a heterogeneous surface charge, as shown in Figure 5. This variation may result from differences in the particle size, surface composition, or functional groups adsorbed from the biological extract used in the synthesis [102]. In this study, the observed zeta potential values indicate a moderate level of colloidal stability, which aligns with the PDI value (0.472), confirming the polydisperse nature of the synthesized nanoparticles. El-Sawaf et al. [105] reported a zeta potential of 21.5 mV for the trimetallic CuO/Ag/ZnO nanocomposite synthesized using Ziziphus spina-christi plant extract.

Zeta potential analysis of the biosynthesized trimetallic TiO2–MgO–Au NPs.
3.5 Antimicrobial activity of trimetallic TiO2–MgO–Au NPs
In the present investigation, the agar well-diffusion technique was used to evaluate the antimicrobial performance of biologically generated TiO2–MgO–Au NPs against a variety of five microbial pathogens, including Gram-positive (S. aureus ATCC 6538, B. subtilis ATCC 6633), Gram-negative (E. coli ATCC 25922, P. aeruginosa ATCC 9027), and C. albicans ATCC 10231. TiO2–MgO–Au NPs strongly inhibited C. albicans, S. aureus, B. subtilis, E. coli, and P. aeruginosa by 33.8 ± 0.25 mm, 36.6 ± 0.75 mm, 24.7 ± 0.55 mm, 23.16 ± 0.4 mm, and 25.9 ± 0.32 mm, respectively, when compared to each microbe’s area of inhibition (Figure 6).

Antimicrobial activity of trimetallic TiO2–MgO–Au NPs using the agar well technique.
The inhibitory impact of various concentrations of TiO2–MgO–Au NPs (15.62–1,000 µg·mL−1) was examined to determine the MICs of the NPs against the indicated microbial pathogens. The lowest MIC for S. aureus was 125 µg·mL−1, whereas the MIC for P. aeruginosa and C. albicans was 250 µg·mL−1, and that for B. subtilis and E. coli was 500 µg·mL−1 (Figure 7). The obtained result was consistent with studies concerning the antibacterial efficacy of trimetallic nanoalloys [72,73]. Multiple studies indicated that the efficacy of nanoparticles versus diverse pathogenic bacteria was ranked (from highest to lowest) as follows: tri, bi, and monometallic. The trimetallic Au/Pt/Ag exhibited superior antibacterial efficacy against E. faecalis, S. aureus, E. coli, and C. albicans in comparison to monometallic variants [48]. One possible explanation for this action is the additive nature of trimetallic compounds as opposed to bi- or monometallic ones [74].

MIC of trimetallic TiO2–MgO–Au NPs toward the tested microbial strains.
The FDA has also approved MgO NPs for use as harmless substances [75]; they received research attention for their potential use in biological fields. Magnesium oxide nanoparticles (MgO NPs) are non-toxic and easily obtainable and have antibacterial characteristics against both Gram-positive and Gram-negative bacteria, fungi, and viruses, along with traits that limit biofilm formation [76,77]. MgO NPs have shown their ability to inhibit S. aureus, P. aeruginosa, along with E. coli [75,78]. They prevented K. pneumoniae and S. aureus from forming biofilms [77]. TiO2 NPs were demonstrated to have antimicrobial capabilities against a variety of bacteria, including E. coli, S. aureus, P. aeruginosa, and P. expansum [79]. Enhanced antimicrobial effectiveness of AuNPs towards Gram-negative bacteria has been found; this may be because of thinner cell walls and more sustained electrostatic contacts [80]. It has been noted that the type of microbe and strain, as well as the size, functionalization, and quantity of AuNPs, directly affect the antibacterial action [81]. Here, NPs serve as antimicrobial substances that can be employed to administer traditional antimicrobials or to fight resistance to antimicrobial treatments directly. The potential of NPs to enter and damage microbial cell membranes through membrane-damaging hardness, reduce cellular permeability, or generate antimicrobial properties (e.g., the generation of ROS, interactions between proteins and nucleic acids, deactivation of enzymes, excessive expression regarding efflux pumps and expulsion of metal ions), and prevent the development of biofilms [82–84]. The trimetallic (TiO2–MgO–Au) NPs can inhibit pathogenic microbes via impact on the cell wall, cell membrane, and protein synthesis that leads to suppressing the activity of the microbes as mentioned in previous studies [28,85,86].
3.6 Anti-biofilm activity of trimetallic TiO2–MgO–Au NPs
The antibiofilm efficacy of TiO2–Mg–ZnO) NPs against S. aureus MRSA in the current investigation showed significant results. Thus, when used at quantities below the MIC amount, TiO2–MgO–Au NPs displayed the greatest efficacy against the development of biofilms caused by MRSA: 250, 125, 62.5, 31.25, and 15.62 μg·mL−1 decreased the generation of biofilm by 86.8%, 77.1%, 63.3%, 53.4%, 37.6%, and 25.1%, respectively. (Figure 8). According to their respective quantitative and qualitative assessments, TiO2–MgO–Au NPs prevented the very initial stages of MRSA biofilm creation. The biofilm inhibition results using crystal violet were in agreement with those of Khan et al. [87].
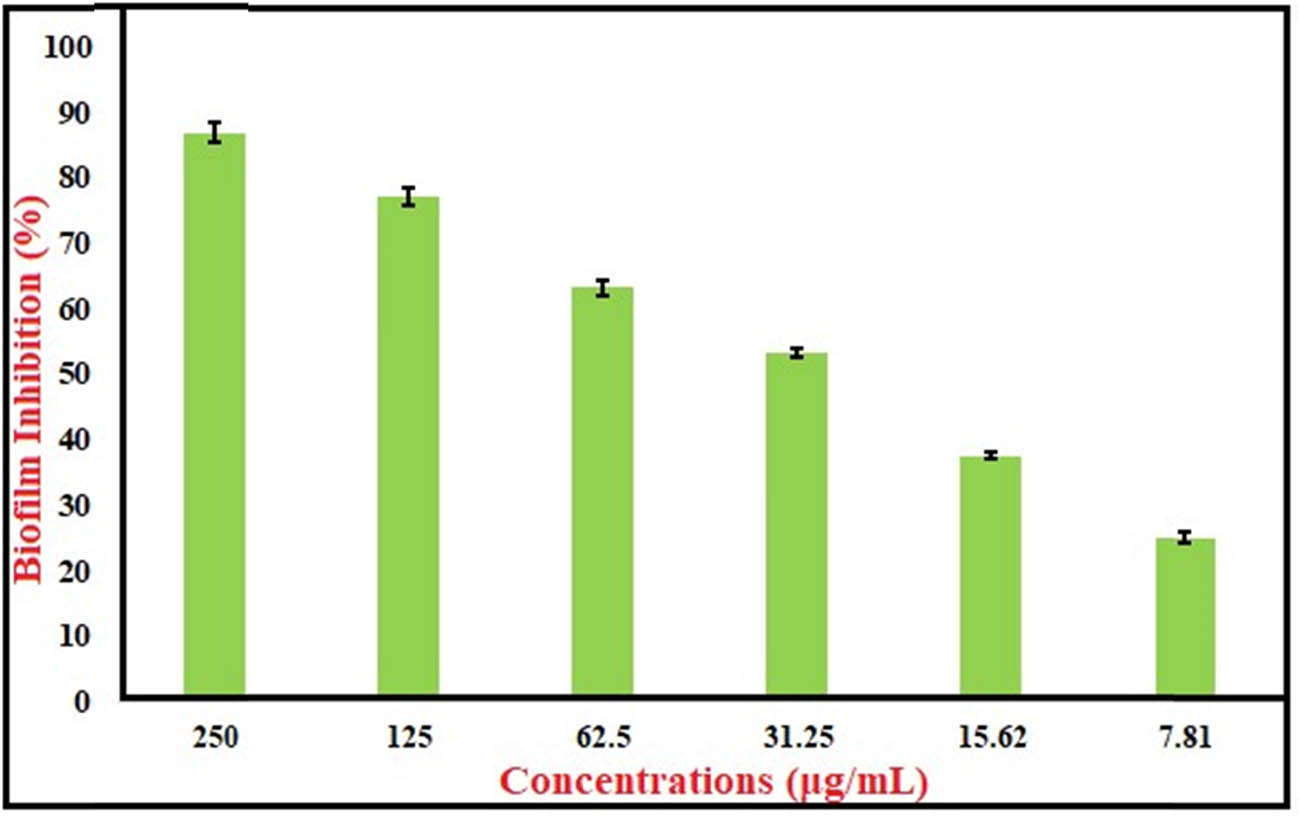
Antibiofilm activity of trimetallic TiO2–MgO–Au NPs.
It was found that P. aeruginosa biofilms were inhibited by intracellularly produced gold nanoparticles employing Laccaria fraternal. The nanoparticles’ gold content was about 15%, and they had a 93% reduction in biofilms [88]. AuNPs showed notable biofilm inhibition against P. aeruginosa and E. coli with sub-MICs, according to Anwar et al. [89]. At concentrations that ranged from 0.25 to 0.5× MICs, the nanocomposite, including AuNPs along with reduced graphene oxide (Au-RGO), showed comparable results, dependent on concentration, removal of established mature biofilms of MRSA and P. aeruginosa [90]. Moreover, the MIC of TiO2 NPs demonstrated strong antibiofilm action towards P. aeruginosa by successfully obstructing the planktonic cells’ adhesion to the substratum [91]. According to Achudhan et al., G-TiO2 NPs were evaluated against fungus (C. albicans) as well as bacteria (Citrobacter freundii and S. mutans) to determine their antimicrobial and antibiofilm properties. At 100 μg·mL−1, each of the fungus and bacterial biofilms were significantly suppressed [92]. Mg-NPs inhibited the biofilms of S. pyogenes, S. epidermis, and P. aeruginosa by 87.15%, 76.35%, and 49.14%, respectively, at an average concentration of 0.98 µg·mL−1. The bacteria in concern had the same minimal biofilm inhibitory concentrations (MBICs) of 1.95 µg·mL−1 for the first two strains and 7.81 µg·mL−1 for P. aeruginosa [93]. The effectiveness of TMNC in inhibiting biofilm generation by S. aureus as well as E. coli was assessed by employing crystal violet staining, as noted in prior research, at different inhibition percentages. Elevated TMNC doses inhibited biofilm creation by S. aureus and E. coli by 85% and 83%, respectively [94]. The biofilm inhibition proportion of the nanocomposite for both bacterial strains increased with a higher TMNC ratio. This is due to the antibiofilm capability of TMNCs. The quantity of TMNC enhances biofilm inhibition and also increases for both bacterial strains. This followed a prior publication by Garza-Cervantes et al. about the progressive reduction in biofilm development as the amount of composite nanoparticles increased [95].
3.7 Cytotoxic effect of TiO2–MgO–Au NPs
The cytotoxic activities of TiO2–MgO–Au NPs were assessed on HepG2 and MCF-7. The MCF-7 cells exhibited the most significant cytotoxic impact, as evidenced by their minimal IC50 values (Figure 9). TiO2–MgO–Au NPs resulted in a minimal IC50 value of 11.09 ± 1.02 µg·mL−1, while the IC50 for taxol was 8.96 ± 0.98 µg·mL−1. The MCF-7 cells are more susceptible to the cytotoxic effects of TiO2–MgO–Au NPs due to their increased expression of receptors and cell surface markers in breast cancer cells, which increase susceptibility. Conversely, HepG2 cells exhibit a greater antioxidant capacity than MCF-7 cells. This antioxidant defense can potentially reduce the cytotoxic activity of TiO2–MgO–Au nanoparticles toward HepG2 cells, thereby mitigating their cytotoxic effects [96,97].

In vitro cytotoxic effects of TiO2–MgO–Au towards MCF-7 and HepG2 cell lines. The data are displayed as the mean ± SD obtained from three separate and independent trials. *Statistically significant within the Taxol group at p < 0.001.
3.8 Effect of TiO2–MgO–Au NPs on caspase-8 activity
TiO2–MgO–Au NPs influence the apoptotic marker caspase-8 (Table 1). Exposure of MCF-7 cells to TiO2–MgO–Au NPs significantly increased caspase-8 activity (1.095 ± 0.04 ng·mL−1) in comparison to the control (0.341 ± 0.02 ng·mL−1). Furthermore, when exposed to TiO2–MgO–Au NPs, caspase-8 activity was significantly higher compared with Taxol treatments (0.986 ± 0.03 ng·mL−1). Apoptosis triggers the activation of DNA fragmentation enzymes by activating caspase-8 [98]. These compounds induced apoptosis in MCF-7 cells by activating caspase-8.
Effect of TiO2–MgO–Au on caspase-8 (ng·mL−1) in MCF-7 cells in comparison to taxol; data are displayed as mean ± SD
| Comp. ID | Caspase-8 (ng·mL−1) |
|---|---|
| MCF-7 | |
| Control | 0.341 ± 0.02 |
| Taxol | 0.986 ± 0.03 |
| TiO2–MgO–Au | 1.095 ± 0.04 |
3.9 Effect of TiO2–MgO–Au NPs on VEGFR-2 activity
Using the inhibition concentration–response curve, the 50% inhibition concentration value (IC50) was found. The positive control used in this experiment was sorafenib. The comparison of IC50 values for VEGFR-2 inhibition between TiO2–MgO–Au and the established inhibitors like sorafenib reveals significant differences in their potential anti-angiogenic activities. The close IC50 values of TiO2–MgO–Au to sorafenib suggest that these differences may not be substantial. An IC50 value of 0.305 ± 0.018 µg·mL−1 for the TiO2–MgO–Au NPs indicated a strong inhibitory effect (Table 2).
Effect of TiO2–MgO–Au on VEGFR-2 in MCF-7 cells compared to Sorafenib
| Comp. ID | VEGFR-2 IC50 (µg·mL−1) |
|---|---|
| MCF-7 | |
| Sorafenib | 0.141 ± 0.005 |
| TiO2–MgO–Au | 0.305 ± 0.018* |
*Statistically significant from the sorafenib group at p < 0.001.
Breast cancer cells exhibit elevated VEGF expression in comparison to normal tissues. The anti-angiogenic effect is suggested by the decreased expression of the VEGF receptor (VEGFR-2). VEGF is a powerful angiogenic agent that stimulates the formation of blood vessels, angiogenesis, and the development of tumors in breast cancer. However, a reduction in VEGFR-2 expression hampers the capacity of VEGF to promote angiogenesis. The simultaneous decrease in VEGFR expression might result in a decline in the formation of new blood vessels, which can be advantageous in cancer therapy, where excessive blood vessel formation can promote tumor development and spread [99,100].
4 Conclusion
The current study successfully demonstrated the green biosynthesis of novel trimetallic TiO2–MgO–Au NPs using banana peel extract for the first time. Detailed characterization revealed that the TiO2–MgO–Au NPs were spherical with an average size of 55 nm. The biosynthesized TiO2–MgO–Au NPs exhibited potent antimicrobial and antibiofilm activities, with the lowest MIC of 125 μg·mL−1 against S. aureus. Moreover, the TiO2–MgO–Au NPs demonstrated significant cytotoxic effects on breast cancer cells (MCF-7), with an IC50 value of 11.09 ± 1.02 μg·mL−1, which was achieved through the activation of caspase-8 and the minimization of VEGFR-2 levels. These findings highlight the promising potential of the green-synthesized trimetallic TiO2–MgO–Au NPs as a multifunctional nanomaterial with antimicrobial, antibiofilm, and anticancer properties, which can have valuable applications in the biomedical field.
Acknowledgments
The authors extend their appreciation to the ongoing Research Funding Program (ORF-2025-552) King Saud University, Riyadh, Saud Arabia.
-
Funding information: The authors extend their appreciation to the ongoing Research Funding Program (ORF-2025-552) King Saud University, Riyadh, Saud Arabia.
-
Author contributions: Mohamed Khalil Yousef Soliman: formal analysis, investigation, methodology, resources, software; and writing – original draft; Ahmed Soliman Doghish: conceptualization, investigation, project administration, supervision, visualization, and validation; Amr Hosny Hashem: conceptualization, data curing, formal analysis, investigation, methodology, project administration, resources, software, validation, visualization, writing-original draft, and writing – review and editing; Mostafa Abdel-Maksoud: data curing, formal analysis, and investigation; Walaa Ahmed El-Dakroury: conceptualization, data curing, formal analysis, investigation, and methodology; Abdulaziz Alamri: software, validation, and visualization; hossam ebaid: formal analysis, investigation, and methodology; Mohamed Sayed Hasanin: investigation, methodology, resources, and software; Ebrahim Saied: conceptualization, data curing, formal analysis, investigation, methodology, project administration, resources, software, validation, visualization, writing-original draft, and writing – review and editing.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Vimbela GV, Ngo SM, Fraze C, Yang L, Stout DA. Antibacterial properties and toxicity from metallic nanomaterials. Int J Nanomed. 2017;24:3941–65. 10.2147/IJN.S134526. PMID: 28579779.Search in Google Scholar PubMed PubMed Central
[2] Roy R, Tiwari M, Donelli G, Tiwari V. Strategies for combating bacterial biofilms: A focus on anti-biofilm agents and their mechanisms of action. Virulence. 2018;9(1):522–54. 10.1080/21505594.2017.1313372. PMID: 28362216.Search in Google Scholar PubMed PubMed Central
[3] Ibrahim SA, Fayed EA, Rizk HF, Desouky SE, Ragab A. Hydrazonoyl bromide precursors as DHFR inhibitors for the synthesis of bis-thiazolyl pyrazole derivatives; antimicrobial activities, antibiofilm, and drug combination studies against MRSA. Bioorg Chem. 2021;116:105339. 10.1016/j.bioorg.2021.105339. PMID: 34530234.Search in Google Scholar PubMed
[4] Nassar O, Desouky SE, El-Sherbiny GM, Abu-Elghait M. Correlation between phenotypic virulence traits and antibiotic resistance in Pseudomonas aeruginosa clinical isolates. Microb Pathogenesis. 2022;162:105339. 10.1016/j.micpath.2021.105339. PMID: 34861345.Search in Google Scholar PubMed
[5] Soliman MK, Salem SS, Abu-Elghait M, Azab MS. Biosynthesis of silver and gold nanoparticles and their efficacy towards antibacterial, antibiofilm, cytotoxicity, and antioxidant activities. Appl Biochem Biotechnol. 2023;195(2):1158–83. 10.1007/s12010-022-04199-7. PMID: 36342621.Search in Google Scholar PubMed PubMed Central
[6] Waters EM, Rowe SE, O’Gara JP, Conlon BP. Convergence of Staphylococcus aureus persister and biofilm research: can biofilms be defined as communities of adherent persister cells? PLoS Pathog. 2016;12(12):e1006012. 10.1371/journal.ppat.1006012. PMID: 28033390.Search in Google Scholar PubMed PubMed Central
[7] Soliman MK, Abu-Elghait M, Salem SS, Azab MS. Multifunctional properties of silver and gold nanoparticles synthesis by Fusarium pseudonygamai. Biomass Convers Biorefin. 2024;14(22):28253–70.10.1007/s13399-022-03507-9Search in Google Scholar
[8] Huh AJ, Kwon YJ. “Nanoantibiotics”: a new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J Control Release. 2011;156(2):128–45. 10.1016/j.jconrel.2011.07.002. PMID: 21763369.Search in Google Scholar PubMed
[9] Brianna, Lee SH. Chemotherapy: how to reduce its adverse effects while maintaining the potency? Med Oncol. 2023;40(3):88. 10.1007/s12032-023-01954-6. PMID: 36735206.Search in Google Scholar PubMed
[10] Shehabeldine AM, Doghish AS, El-Dakroury WA, Hassanin MM, Al-Askar AA, AbdElgawad H, et al. Antimicrobial, antibiofilm, and anticancer activities of syzygium aromaticum essential oil nanoemulsion. Molecules. 2023;28(15):5812. 10.3390/molecules28155812. PMID: 37570781.Search in Google Scholar PubMed PubMed Central
[11] El-Sayyad GS, Elfadil D, Mosleh MA, Hasanien YA, Mostafa A, Abdelkader RS, et al. Eco-friendly strategies for biological synthesis of green nanoparticles with promising applications. BioNanoScience. 2024;2:1–43.10.1007/s12668-024-01494-xSearch in Google Scholar
[12] Wang C, Zhang S. Advantages of nanomedicine in cancer therapy: A review. ACS Appl Nano Mater. 2023;6(24):22594–610.10.1021/acsanm.3c04487Search in Google Scholar
[13] Saied E, Abdel-Maksoud MA, Alfuraydi AA, Kiani BH, Bassyouni M, Al-Qabandi OA, et al. Endophytic Aspergillus hiratsukae mediated biosynthesis of silver nanoparticles and their antimicrobial and photocatalytic activities. Front Microbiol. 2024;15:1345423. 10.3389/fmicb.2024.1345423. PMID: 38533339.Search in Google Scholar PubMed PubMed Central
[14] Annu, Bhat ZI, Imtiyaz K, Rizvi MM, Ikram S, Shin DK. Comparative study of ZnO-and-TiO2-nanoparticles-functionalized polyvinyl alcohol/chitosan bionanocomposites for multifunctional biomedical applications. Polymers. 2023;15(16):3477. 10.3390/polym15163477. PMID: 37631534.Search in Google Scholar PubMed PubMed Central
[15] Tas A, Cakmak NK, Agbektas T, Silig Y. Cytotoxic activity of zinc oxide/titanium dioxide nanoparticles on prostate cancer cells. Int J Chem Technol. 2019;3(2):113–20.10.32571/ijct.613536Search in Google Scholar
[16] Saied E, Hashem AH, Ali OM, Selim S, Almuhayawi MS, Elbahnasawy MA. Photocatalytic and antimicrobial activities of biosynthesized silver nanoparticles using Cytobacillus firmus. Life. 2022;12(9):1331. 10.3390/life12091331. PMID: 36143368.Search in Google Scholar PubMed PubMed Central
[17] Hashem AH, El-Sayyad GS. Antimicrobial and anticancer activities of biosynthesized bimetallic silver-zinc oxide nanoparticles (Ag-ZnO NPs) using pomegranate peel extract. Biomass Convers Biorefin. 2024;14(17):20345–57.10.1007/s13399-023-04126-8Search in Google Scholar
[18] El-Khawaga AM, Elsayed MA, Gobara M, Suliman AA, Hashem AH, Zaher AA, et al. Green synthesized ZnO nanoparticles by Saccharomyces cerevisiae and their antibacterial activity and photocatalytic degradation. Biomass Convers Biorefin. 2023;15:1–2.10.1007/s13399-023-04827-0Search in Google Scholar
[19] Hashem AH, Saied E, Ali OM, Selim S, Al Jaouni SK, Elkady FM, et al. Pomegranate peel extract stabilized selenium nanoparticles synthesis: promising antimicrobial potential, antioxidant activity, biocompatibility, and hemocompatibility. Appl Biochem Biotechnol. 2023;195(10):5753–76. 10.1007/s12010-023-04326-y. PMID: 36705842.Search in Google Scholar PubMed
[20] Saied M, Hasanin M, Abdelghany TM, Amin BH, Hashem AH. Anticandidal activity of nanocomposite based on nanochitosan, nanostarch and mycosynthesized copper oxide nanoparticles against multidrug-resistant Candida. Int J Biol Macromol. 2023;242:124709. 10.1016/j.ijbiomac.2023.124709. PMID: 37141971.Search in Google Scholar PubMed
[21] Saied E, Mekky AE, Al-Askar AA, Hagag AF, El-bana AA, Ashraf M, et al. Aspergillus terreus-mediated selenium nanoparticles and their antimicrobial and photocatalytic activities. Crystals. 2023;13(3):450.10.3390/cryst13030450Search in Google Scholar
[22] Hashem AH, El-Naggar ME, Abdelaziz AM, Abdelbary S, Hassan YR, Hasanin MS. Bio-based antimicrobial food packaging films based on hydroxypropyl starch/polyvinyl alcohol loaded with the biosynthesized zinc oxide nanoparticles. Int J Biol Macromol. 2023;249:126011. 10.1016/j.ijbiomac.2023.126011. PMID: 37517763.Search in Google Scholar PubMed
[23] Al-Askar AA, Hashem AH, Elhussieny NI, Saied E. Green biosynthesis of zinc oxide nanoparticles using Pluchea indica leaf extract: antimicrobial and photocatalytic activities. Molecules. 2023;28(12):4679. 10.3390/molecules28124679. PMID: 37375234.Search in Google Scholar PubMed PubMed Central
[24] Landage KS, Arbade GK, Khanna P, Bhongale CJ. Biological approach to synthesize TiO2 nanoparticles using Staphylococcus aureus for antibacterial and anti-biofilm applications. J Microbiol Exp. 2020;8(1):36–43.10.15406/jmen.2020.08.00283Search in Google Scholar
[25] Manna J, Begum G, Kumar KP, Misra S, Rana RK. Enabling antibacterial coating via bioinspired mineralization of nanostructured ZnO on fabrics under mild conditions. ACS Appl Mater Interfaces. 2013;5(10):4457–63. 10.1021/am400933n. PMID: 23607588.Search in Google Scholar PubMed
[26] Sathyanarayanan MB, Balachandranath R, Genji Srinivasulu Y, Kannaiyan SK, Subbiahdoss G. The effect of gold and iron‐oxide nanoparticles on biofilm‐forming pathogens. Int Sch Res Not. 2013;2013(1):272086.10.1155/2013/272086Search in Google Scholar PubMed PubMed Central
[27] Hashem AH, Al-Askar AA, Haponiuk J, Abd-Elsalam KA, Hasanin MS. Biosynthesis, characterization, and antifungal activity of novel trimetallic copper oxide–selenium–zinc oxide nanoparticles against some mucorales fungi. Microorganisms. 2023;11(6):1380. 10.3390/microorganisms11061380. PMID: 37374882.Search in Google Scholar PubMed PubMed Central
[28] Basavegowda N, Baek KH. Multimetallic nanoparticles as alternative antimicrobial agents: challenges and perspectives. Molecules. 2021;26(4):912. 10.3390/molecules26040912. PMID: 33572219.Search in Google Scholar PubMed PubMed Central
[29] Gaber SE, Hashem AH, El-Sayyad GS, Attia MS. Antifungal activity of myco-synthesized bimetallic ZnO-CuO nanoparticles against fungal plant pathogen Fusarium oxysporum. Biomass Convers Biorefin. 2024;14(20):25395–409.10.1007/s13399-023-04550-wSearch in Google Scholar
[30] Hasanin MS, Hashem AH, Al-Askar AA, Haponiuk J, Saied E. A novel nanocomposite based on mycosynthesized bimetallic zinc-copperoxide nanoparticles, nanocellulose and chitosan: characterization, antimicrobial and photocatalytic activities. Electron J Biotechnol. 2023;65:45–55.10.1016/j.ejbt.2023.05.001Search in Google Scholar
[31] Skłodowski K, Chmielewska-Deptuła SJ, Piktel E, Wolak P, Wollny T, Bucki R. Metallic nanosystems in the development of antimicrobial strategies with high antimicrobial activity and high biocompatibility. Int J Mol Sci. 2023;24(3):2104. 10.3390/ijms24032104. PMID: 36768426.Search in Google Scholar PubMed PubMed Central
[32] Hameed RS, Fayyad RJ, Nuaman RS, Hamdan NT, Maliki SA. Synthesis and characterization of a novel titanium nanoparticals using banana peel extract and investigate its antibacterial and insecticidal activity. J Pure Appl Microbiol. 2019;13(4):2241–9.10.22207/JPAM.13.4.38Search in Google Scholar
[33] Kamli MR, Srivastava V, Hajrah NH, Sabir JS, Hakeem KR, Ahmad A, et al. Facile bio-fabrication of Ag-Cu-Co trimetallic nanoparticles and its fungicidal activity against Candida auris. J Fungi. 2021;7(1):62. 10.3390/jof7010062. PMID: 33477480.Search in Google Scholar PubMed PubMed Central
[34] Alshehri AA, Malik MA. Facile one-pot biogenic synthesis of Cu-Co-Ni trimetallic nanoparticles for enhanced photocatalytic dye degradation. Catalysts. 2020;10(10):1138.10.3390/catal10101138Search in Google Scholar
[35] Nazir MA, Hasan M, Mustafa G, Tariq T, Ahmed MM, Golzari Dehno R, et al. Zinc oxide nano-fertilizer differentially effect on morphological and physiological identity of redox-enzymes and biochemical attributes in wheat (Triticum aestivum L.). Sci Rep. 2024;14(1):13091. 10.1038/s41598-024-63987-9. PMID: 38849601.Search in Google Scholar PubMed PubMed Central
[36] Sangwan S, Seth R. Synthesis, characterization and stability of gold nanoparticles (AuNPs) in different buffer systems. J Clust Sci. 2022;33:749–64. 10.1007/s10876-020-01956-8.Search in Google Scholar
[37] Shukla V, Niveria K, Shashidhar P, Verma AK. Dynamic light scattering (DLS) particle size analysis for biomedical nanotechnology. In Analytical Techniques for Biomedical Nanotechnology. Bristol, UK: IOP Publishing; 2023. p. 16.10.1088/978-0-7503-3379-5ch16Search in Google Scholar
[38] Perez C. Antibiotic assay by agar-well diffusion method. Acta Biol Med Exp. 1990;15:113–5.Search in Google Scholar
[39] Humphries RM, Ambler J, Mitchell SL, Castanheira M, Dingle T, Hindler JA, et al. CLSI methods development and standardization working group best practices for evaluation of antimicrobial susceptibility tests. J Clin Microbiol. 2018;56(4):10–128. 10.1128/JCM.01934-17. PMID: 29367292.Search in Google Scholar PubMed PubMed Central
[40] Khattab AM, Abo-Taleb HA, Abdelaziz AM, El-Tabakh MA, El-Feky MM, Abu-Elghait M. Daphnia magna and Gammarus pulex, novel promising agents for biomedical and agricultural applications. Sci Rep. 2022;12(1):13690. 10.1038/s41598-022-17790-z. PMID: 35953507.Search in Google Scholar PubMed PubMed Central
[41] Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1–2):55–63. 10.1016/0022-1759(83)90303-4. PMID: 6606682.Search in Google Scholar PubMed
[42] Adeyemi JO, Oriola AO, Onwudiwe DC, Oyedeji AO. Plant extracts mediated metal-based nanoparticles: synthesis and biological applications. Biomolecules. 2022;12(5):627. 10.3390/biom12050627. PMID: 35625555.Search in Google Scholar PubMed PubMed Central
[43] Godeto YG, Ayele A, Ahmed IN, Husen A, Bachheti RK. Medicinal plant-based metabolites in nanoparticles synthesis and their cutting-edge applications: an overview. Secondary metabolites from medicinal plants. Vol. 2. Cham, Switzerland: Springer Nature; 2023. p. 1–34.10.1201/9781003213727-1Search in Google Scholar
[44] Rangga WD, Setiawan D, Khosiatun K. Biosynthesis and kinetics of silver nanoparticles formation by reduction using banana kepok (Musa balbisiana) peel extract. ASEAN J Chem Eng. 2017;17(2):77–85.10.22146/ajche.49557Search in Google Scholar
[45] Bankar A, Joshi B, Kumar AR, Zinjarde S. Banana peel extract mediated novel route for the synthesis of palladium nanoparticles. Mater Lett. 2010;64(18):1951–3.10.1016/j.matlet.2010.06.021Search in Google Scholar
[46] Khan AI, Arasu AV. A review of influence of nanoparticle synthesis and geometrical parameters on thermophysical properties and stability of nanofluids. Therm Sci Eng Prog. 2019;11:334–64.10.1016/j.tsep.2019.04.010Search in Google Scholar
[47] Patil S, Chandrasekaran R. Biogenic nanoparticles: a comprehensive perspective in synthesis, characterization, application and its challenges. J Genet Eng Biotechnol. 2020;18(1):67. 10.1186/s43141-020-00081-3. PMID: 33104931.Search in Google Scholar PubMed PubMed Central
[48] Dlugaszewska J, Dobrucka R. Effectiveness of biosynthesized trimetallic Au/Pt/Ag nanoparticles on planktonic and biofilm Enterococcus faecalis and Enterococcus faecium forms. J Clust Sci. 2019;30:1091–101.10.1007/s10876-019-01570-3Search in Google Scholar
[49] Dobrucka R. Biogenic synthesis of trimetallic nanoparticles Au/ZnO/Ag using Meliloti officinalis extract. Int J Environ Anal Chem. 2020;100(9):981–91.10.1080/03067319.2019.1646736Search in Google Scholar
[50] Vaseghi Z, Tavakoli O, Nematollahzadeh A. Rapid biosynthesis of novel Cu/Cr/Ni trimetallic oxide nanoparticles with antimicrobial activity. J Environ Chem Eng. 2018;6(2):1898–911.10.1016/j.jece.2018.02.038Search in Google Scholar
[51] Rao KJ, Paria S. Mixed phytochemicals mediated synthesis of multifunctional Ag–Au–Pd nanoparticles for glucose oxidation and antimicrobial applications. ACS Appl Mater Interfaces. 2015;7(25):14018–25. 10.1021/acsami.5b03089. PMID: 26043395.Search in Google Scholar PubMed
[52] Kaur M, Singh J, Chauhan M, Kumar V, Singh K. Green synthesis of TiO2-Al2O3-ZnFe2O4 nanocomposite using the Hibiscus rosa sinesis and evaluation of its photocatalytic applications. Open Ceram. 2024;18:100571.10.1016/j.oceram.2024.100571Search in Google Scholar
[53] Hassan H, Omoniyi KI, Okibe FG, Nuhu AA, Echioba EG. Evaluation of antibacterial potential of biosynthesized plant leave extract mediated titanium oxide nanoparticles using Hypheae thiebeace and anannos seneglensis. J Appl Sci Environ Manag. 2019;23(10):1795–804.10.4314/jasem.v23i10.5Search in Google Scholar
[54] Vergheese M, Vishal SK. Green synthesis of magnesium oxide nanoparticles using Trigonella foenum-graecum leaf extract and its antibacterial activity. J Pharmacogn Phytochem. 2018;7(3):1193–200.Search in Google Scholar
[55] Hassanisaadi M, Bonjar GH, Rahdar A, Pandey S, Hosseinipour A, Abdolshahi R. Environmentally safe biosynthesis of gold nanoparticles using plant water extracts. Nanomaterials. 2021;11(8):2033. PMID: 34443864 PMCID: PMC8400837 10.3390/nano11082033.Search in Google Scholar PubMed PubMed Central
[56] Kannaiyan SK, Rengaraj R, Gayathri PK, Lavanya G, Hemapriya D. Antimicrobial activity of green synthesized tri-metallic oxide Ni/Cr/Cu nanoparticles. J Nig Soc Phys Sci. 2021;3:144–7. 10.46481/jnsps.2021.237.Search in Google Scholar
[57] Hussein S, Mahmoud AM, Elgebaly HA, Hendawy OM, Hassanein EH, Moustafa SM, et al. Green synthesis of trimetallic nanocomposite (Ru/Ag/Pd)‐Np and its in vitro antimicrobial and anticancer activities. J Chem. 2022;2022(1):4593086.10.1155/2022/4593086Search in Google Scholar
[58] Khan AU, Arooj A, Tahir K, Ibrahim MM, Jevtovic V, AL-Abdulkarim HA, et al. Facile fabrication of novel Ag2S-ZnO/GO nanocomposite with its enhanced photocatalytic and biological applications. J Mol Struct. 2022;1251:131991.10.1016/j.molstruc.2021.131991Search in Google Scholar
[59] Botteon CE, Silva LB, Ccana-Ccapatinta GV, Silva TS, Ambrosio SR, Veneziani RC, et al. Biosynthesis and characterization of gold nanoparticles using Brazilian red propolis and evaluation of its antimicrobial and anticancer activities. Sci Rep. 2021;11(1):1974. PMID: 33479338 PMCID: PMC7820602. 10.1038/s41598-021-81281-w.Search in Google Scholar PubMed PubMed Central
[60] Henzie J, Grünwald M, Widmer-Cooper A, Geissler PL, Yang P. Self-assembly of uniform polyhedral silver nanocrystals into densest packings and exotic superlattices. Nat Mater. 2012;11(2):131–7. 10.1038/nmat3178. PMID: 22101811.Search in Google Scholar PubMed
[61] Nassar AM, Elseman AM, Alsohaimi IH, Alotaibi NF, Khan A. Diaqua oxalato strontium (II) complex as a precursor for facile fabrication of Ag-NPs@ SrCO3, characterization, optical properties, morphological studies and adsorption efficiency. J Coord Chem. 2019;72(5–7):771–85.10.1080/00958972.2019.1588964Search in Google Scholar
[62] Hussien NA. Antimicrobial potential of biosynthesized zinc oxide nanoparticles using banana peel and date seeds extracts. Sustainability. 2023;15(11):9048.10.3390/su15119048Search in Google Scholar
[63] Canaparo R, Foglietta F, Limongi T, Serpe L. Biomedical applications of reactive oxygen species generation by metal nanoparticles. Materials. 2020;14(1):53. 10.3390/ma14010053. PMID: 33374476.Search in Google Scholar PubMed PubMed Central
[64] Sidhu AK, Verma N, Kaushal P. Role of biogenic capping agents in the synthesis of metallic nanoparticles and evaluation of their therapeutic potential. Front Nanotechnol. 2022;3:801620.10.3389/fnano.2021.801620Search in Google Scholar
[65] Rathi VH, Jeice AR. Green fabrication of titanium dioxide nanoparticles and their applications in photocatalytic dye degradation and microbial activities. Chem Phys Impact. 2023;6:100197.10.1016/j.chphi.2023.100197Search in Google Scholar
[66] Saidi NS, Ying KJ, Yusoff HM, Badar N. Synthesis and characterization of magnesium oxide nanoparticles by using banana peel (Musa Acuminata Cavendish) extract. Malay J Anal Sci. 2023;27(5):1017–34.Search in Google Scholar
[67] Deokar GK, Ingale AG. Green synthesis of gold nanoparticles (Elixir of Life) from banana fruit waste extract–an efficient multifunctional agent. RSC Adv. 2016;6(78):74620–9.10.1039/C6RA14567ASearch in Google Scholar
[68] Said AR, Arafa MF, El-Dakroury WA, Alshehri S, El Maghraby GM. Bilosomes and niosomes for enhanced intestinal absorption and in vivo efficacy of cytarabine in treatment of Acute Myeloid Leukemia. Pharmaceuticals. 2024;17(12):1572. 10.3390/ph17121572. PMID: 39770414.Search in Google Scholar PubMed PubMed Central
[69] Caputo F, Vogel R, Savage J, Vella G, Law A, Della Camera G, et al. Measuring particle size distribution and mass concentration of nanoplastics and microplastics: addressing some analytical challenges in the sub-micron size range. J Colloid Interface Sci. 2021;588:401–17. 10.1016/j.jcis.2020.12.039. PMID: 33422789.Search in Google Scholar PubMed
[70] Roy A, Kunwar S, Bhusal U, Idris DS, Alghamdi S, Chidambaram K, et al. Dye degradation activity of biogenically synthesized Cu/Fe/Ag trimetallic nanoparticles. Green Process Synth. 2024;13(1):20230267.10.1515/gps-2023-0267Search in Google Scholar
[71] Xu F. Review of analytical studies on TiO2 nanoparticles and particle aggregation, coagulation, flocculation, sedimentation, stabilization. Chemosphere. 2018;212:662–77. 10.1016/j.chemosphere.2018.08.108. PMID: 30173113.Search in Google Scholar PubMed
[72] Orshiso TA, Zereffa EA, Murthy HA, Demissie TB, Ghotekar S, Pagar K, et al. One-pot biopreparation of trimetallic ZnO–MgO–CuO nanoparticles: enhanced cytotoxicity, antibacterial activities and molecular docking studies. Chem Afr. 2024;7(4):1963–80. 10.1007/s42250-023-00830-0.Search in Google Scholar
[73] Nguyen TT, Nguyen YN, Tran XT, Nguyen TT, Van Tran T. Green synthesis of CuO, ZnO and CuO/ZnO nanoparticles using Annona glabra leaf extract for antioxidant, antibacterial and photocatalytic activities. J Environ Chem Eng. 2023;11(5):111003.10.1016/j.jece.2023.111003Search in Google Scholar
[74] Haque B, Gupta A, Roy A, Malik A, Khan AA. Green fabrication of Ag–Ni–Mn-Zn nanoparticles from watermelon peels and its antioxidant, dye degradation and molecular docking studies. Clean Technol Environ Policy. 2024;7:1–28.10.1007/s10098-024-02906-ySearch in Google Scholar
[75] Cai L, Chen J, Liu Z, Wang H, Yang H, Ding W. Magnesium oxide nanoparticles: effective agricultural antibacterial agent against Ralstonia solanacearum. Front Microbiology. 2018;9:790. 10.3389/fmicb.2018.00790. PMID: 29922237.Search in Google Scholar PubMed PubMed Central
[76] Noori AJ, Kareem FA. The effect of magnesium oxide nanoparticles on the antibacterial and antibiofilm properties of glass-ionomer cement. Heliyon. 2019;5(10):e02568. 10.1016/j.heliyon.2019.e02568. PMID: 31667407.Search in Google Scholar PubMed PubMed Central
[77] Shkodenko L, Kassirov I, Koshel E. Metal oxide nanoparticles against bacterial biofilms: Perspectives and limitations. Microorganisms. 2020;8(10):1545. 10.3390/microorganisms8101545. PMID: 33036373.Search in Google Scholar PubMed PubMed Central
[78] Sharma G, Soni R, Jasuja ND. Phytoassisted synthesis of magnesium oxide nanoparticles with Swertia chirayaita. J Taibah Univ Sci. 2017;11(3):471–7.10.1016/j.jtusci.2016.09.004Search in Google Scholar
[79] Ekielski A. Interactions between food ingredients and nanocomponents used for composite packaging. In: Grumezescu AM, Holban AM, editors. Food packaging and preservation. London, UK: Academic Press; 2019. p. 669–74.10.1016/B978-0-08-100596-5.21850-7Search in Google Scholar
[80] Behzad F, Naghib SM, Tabatabaei SN, Zare Y, Rhee KY. An overview of the plant-mediated green synthesis of noble metal nanoparticles for antibacterial applications. J Ind Eng Chem. 2021;94:92–104.10.1016/j.jiec.2020.12.005Search in Google Scholar
[81] Ortiz-Benítez EA, Velázquez-Guadarrama N, Durán Figueroa NV, Quezada H, Olivares-Trejo JD. Antibacterial mechanism of gold nanoparticles on Streptococcus pneumoniae. Metallomics. 2019;11(7):1265–76. 10.1039/c9mt00084d. PMID: 31173034.Search in Google Scholar PubMed
[82] Lee NY, Ko WC, Hsueh PR. Nanoparticles in the treatment of infections caused by multidrug-resistant organisms. Front Pharmacol. 2019;10:1153. 10.3389/fphar.2019.01153. PMID: 31636564.Search in Google Scholar PubMed PubMed Central
[83] Varier KM, Gudeppu M, Chinnasamy A, Thangarajan S, Balasubramanian J, Li Y, et al. Nanoparticles: antimicrobial applications and its prospects. Advanced nanostructured materials for environmental remediation. Amsterdam, Netherlands: Elsevier; 2019. p. 321–55. 10.1007/978-3-030-04477-0_12.Search in Google Scholar
[84] Spirescu VA, Chircov C, Grumezescu AM, Andronescu E. Polymeric nanoparticles for antimicrobial therapies: An up-to-date overview. Polymers. 2021;13(5):724. 10.3390/polym13050724. PMID: 33673451.Search in Google Scholar PubMed PubMed Central
[85] Baig U, Ansari MA, Gondal MA, Akhtar S, Khan FA, Falath WS. Single step production of high-purity copper oxide-titanium dioxide nanocomposites and their effective antibacterial and anti-biofilm activity against drug-resistant bacteria. Mater Sci Eng: C. 2020;113:110992. 10.1016/j.msec.2020.110992. PMID: 32487404.Search in Google Scholar PubMed
[86] N Oktar F, Yetmez M, Ficai D, Ficai A, Dumitru F, Pica A. Molecular mechanism and targets of the antimicrobial activity of metal nanoparticles. Curr Top Med Chem. 2015;15(16):1583–8. 10.2174/1568026615666150414141601. PMID: 25877090.Search in Google Scholar PubMed
[87] Khan F, Manivasagan P, Lee JW, Pham DT, Oh J, Kim YM. Fucoidan-stabilized gold nanoparticle-mediated biofilm inhibition, attenuation of virulence and motility properties in Pseudomonas aeruginosa PAO1. Mar Drugs. 2019;17(4):208. 10.3390/md17040208. PMID: 30987163.Search in Google Scholar PubMed PubMed Central
[88] Samanta S, Singh BR, Adholeya A. Intracellular synthesis of gold nanoparticles using an ectomycorrhizal strain EM-1083 of Laccaria fraterna and its nanoanti-quorum sensing potential against Pseudomonas aeruginosa. Indian J Microbiol. 2017;57:448–60. 10.1007/s12088-017-0662-4. PMID: 29151646.Search in Google Scholar PubMed PubMed Central
[89] Anwar A, Perveen S, Ahmed S, Siddiqui R, Shah MR, Khan NA. Silver nanoparticle conjugation with thiopyridine exhibited potent antibacterial activity against Escherichia coli and further enhanced by copper capping. Jundishapur J Microbiol. 2019;12(3):e74455. 10.5812/jjm.74455.Search in Google Scholar
[90] Aljaafari A, Ahmed F, Husain FM. Bio-inspired facile synthesis of graphene-based nanocomposites: elucidation of antimicrobial and biofilm inhibitory potential against foodborne pathogenic bacteria. Coatings. 2020;10(12):1171.10.3390/coatings10121171Search in Google Scholar
[91] Rajkumari J, Magdalane CM, Siddhardha B, Madhavan J, Ramalingam G, Al-Dhabi NA, et al. Synthesis of titanium oxide nanoparticles using Aloe barbadensis mill and evaluation of its antibiofilm potential against Pseudomonas aeruginosa PAO1. J Photochem Photobiol B: Biol. 2019;201:111667. 10.1016/j.jphotobiol.2019.111667. PMID: 31683167.Search in Google Scholar PubMed
[92] Achudhan D, Vijayakumar S, Malaikozhundan B, Divya M, Jothirajan M, Subbian K, et al. The antibacterial, antibiofilm, antifogging and mosquitocidal activities of titanium dioxide (TiO2) nanoparticles green-synthesized using multiple plants extracts. J Environ Chem Eng. 2020;8(6):104521.10.1016/j.jece.2020.104521Search in Google Scholar
[93] Younis IY, El-Hawary SS, Eldahshan OA, Abdel-Aziz MM, Ali ZY. Green synthesis of magnesium nanoparticles mediated from Rosa floribunda charisma extract and its antioxidant, antiaging and antibiofilm activities. Sci Rep. 2021;11(1):16868. 10.1038/s41598-021-96377-6. PMID: 34413416.Search in Google Scholar PubMed PubMed Central
[94] Sivasubramanian K, Tamilselvi Y, Velmurugan P, Al-Otibi FO, Alharbi RI, Mohanavel V, et al. Enhanced applications in dentistry through autoclave-assisted sonochemical synthesis of Pb/Ag/Cu trimetallic nanocomposites. Ultrason Sonochem. 2024;22:106966. 10.1016/j.ultsonch.2024.106966. PMID: 38924854.Search in Google Scholar PubMed PubMed Central
[95] Garza-Cervantes JA, Escárcega-González CE, Barriga Castro ED, Mendiola-Garza G, Marichal-Cancino BA, López-Vázquez MA, et al. Antimicrobial and antibiofilm activity of biopolymer-Ni, Zn nanoparticle biocomposites synthesized using R. mucilaginosa UANL-001L exopolysaccharide as a capping agent. Int J Nanomed. 2019;10:2557–71. 10.2147/IJN.S196470. PMID: 31118605.Search in Google Scholar PubMed PubMed Central
[96] Lotfian H, Nemati F. Cytotoxic effect of TiO2 nanoparticles on breast cancer cell line (MCF-7). IIOAB J. 2016;7:219–24.Search in Google Scholar
[97] Guo M, Sun Y, Zhang XD. Enhanced radiation therapy of gold nanoparticles in liver cancer. Appl Sci. 2017;7(3):232.10.3390/app7030232Search in Google Scholar
[98] Gong L, Tang Y, An R, Lin M, Chen L, Du J. RTN1-C mediates cerebral ischemia/reperfusion injury via ER stress and mitochondria-associated apoptosis pathways. Cell Death Dis. 2017;8(10):e3080. 10.1038/cddis.2017.465. PMID: 28981095.Search in Google Scholar PubMed PubMed Central
[99] Onodera R, Jimma Y, Suzuki A, Habano W, Ozawa S, Terashima J. The regulation pathway of VEGF gene expression is different between 2D cells and 3D spheroids in human lung cancer cells. Biol Pharm Bull. 2023;46(4):608–13. 10.1248/bpb.b22-00772. PMID: 37005305.Search in Google Scholar PubMed
[100] Amirchaghmaghi E, Rezaei A, Moini A, Roghaei MA, Hafezi M, Aflatoonian R. Gene expression analysis of VEGF and its receptors and assessment of its serum level in unexplained recurrent spontaneous abortion. Cell J (Yakhteh). 2015;16(4):538. 10.22074/cellj.2015.498. PMID: 25685744.Search in Google Scholar PubMed PubMed Central
[101] Pochapski DJ, Carvalho dos Santos C, Leite GW, Pulcinelli SH, Santilli CV. Zeta potential and colloidal stability predictions for inorganic nanoparticle dispersions: Effects of experimental conditions and electrokinetic models on the interpretation of results. Langmuir. 2021;37(45):13379–89. 10.1021/acs.langmuir.1c02056.Search in Google Scholar PubMed
[102] Sharma D, Kanchi S, Bisetty K. Biogenic synthesis of nanoparticles: a review. Arab J Chem. 2019;12(8):3576–600. 10.1016/j.arabjc.2015.11.002.Search in Google Scholar
[103] Mahmood K, Amara U, Siddique S, Usman M, Peng Q, Khalid M, et al. Green synthesis of Ag@ CdO nanocomposite and their application towards brilliant green dye degradation from wastewater. J Nanostruct Chem. 2022;12:329–41. 10.1007/s40097-021-00418-5.Search in Google Scholar
[104] Karvekar OS, Sarvalkar PD, Vadanagekar AS, Singhan RD, Jadhav SM, Nimbalkar MS, et al. Biogenic synthesis of silver anchored ZnO nanorods as nano catalyst for organic transformation reactions and dye degradation. Appl Nanosci. 2022;12(7):2207–26. 10.1007/s13204-022-02470-1.Search in Google Scholar PubMed PubMed Central
[105] El-Sawaf AK, El-Moslamy SH, Kamoun EA, Hossain K. Green synthesis of trimetallic CuO/Ag/ZnO nanocomposite using Ziziphus spina-christi plant extract: characterization, statistically experimental designs, and antimicrobial assessment. Sci Rep. 2024;14(1):19718. 10.1038/s41598-024-67579-5.Search in Google Scholar PubMed PubMed Central
© 2025 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Optimized green synthesis of silver nanoparticles from guarana seed skin extract with antibacterial potential
- Green adsorbents for water remediation: Removal of Cr(vi) and Ni(ii) using Prosopis glandulosa sawdust and biochar
- Green approach for the synthesis of zinc oxide nanoparticles from methanolic stem extract of Andrographis paniculata and evaluation of antidiabetic activity: In silico GSK-3β analysis
- Development of a green and rapid ethanol-based HPLC assay for aspirin tablets and feasibility evaluation of domestically produced bioethanol in Thailand as a sustainable mobile phase
- A facile biodegradation of polystyrene microplastic by Bacillus subtilis
- Enhanced synthesis of fly ash-derived hydrated sodium silicate adsorbents via low-temperature alkaline hydrothermal treatment for advanced environmental applications
- Impact of metal nanoparticles biosynthesized using camel milk on bacterial growth and copper removal from wastewater
- Preparation of Co/Cr-MOFs for efficient removal of fleroxacin and Rhodamine B
- Applying nanocarbon prepared from coal as an anode in lithium-ion batteries
- Improved electrochemical synthesis of Cu–Fe/brass foil alloy followed by combustion for high-efficiency photoelectrodes and hydrogen production in alkaline solutions
- Precipitation of terephthalic acid from post-consumer polyethylene terephthalate waste fractions
- Biosynthesized zinc oxide nanoparticles: Multifunctional potential applications in anticancer, antibacterial, and B. subtilis DNA gyrase docking
- Anticancer and antimicrobial effects of green-synthesized silver nanoparticles using Teucrium polium leaves extract
- Green synthesis of eco-friendly bioplastics from Chlorella and Lithothamnion algae for safe and sustainable solutions for food packaging
- Optimizing coal water slurry concentration via synergistic coal blending and particle size distribution
- Green synthesis of Ag@Cu and silver nanowire using Pterospermum heterophyllum extracts for surface-enhanced Raman scattering
- Green synthesis of copper oxide nanoparticles from Algerian propolis: Exploring biochemical, structural, antimicrobial, and anti-diabetic properties
- Simultaneous quantification of mefenamic acid and paracetamol in fixed-dose combination tablet dosage forms using the green HPTLC method
- Green synthesis of titanium dioxide nanoparticles using green tea (Camellia sinensis) extract: Characteristics and applications
- Pharmaceutical properties for green fabricated ZnO and Ag nanoparticle-mediated Borago officinalis: In silico predications study
- Synthesis and optimization of gemcitabine-loaded nanoparticles by using Box–Behnken design for treating prostate cancer: In vitro characterization and in vivo pharmacokinetic study
- A comparative analysis of single-step and multi-step methods for producing magnetic activated carbon from palm kernel shells: Adsorption of methyl orange dye
- Sustainable green synthesis of silver nanoparticles using walnut septum waste: Characterization and antibacterial properties
- Efficient electrocatalytic reduction of CO2 to CO over Ni/Y diatomic catalysts
- Greener and magnetic Fe3O4 nanoparticles as a recyclable catalyst for Knoevenagel condensation and degradation of industrial Congo red dye
- Recycling of HDPE-giant reed composites: Processability and performance
- Fabrication of antibacterial chitosan/PVA nanofibers co-loaded with curcumin and cefadroxil for wound healing
- Cost-effective one-pot fabrication of iron(iii) oxychloride–iron(iii) oxide nanomaterials for supercapacitor charge storage
- Novel trimetallic (TiO2–MgO–Au) nanoparticles: Biosynthesis, characterization, antimicrobial, and anticancer activities
- Green-synthesized chromium oxide nanoparticles using pomegranate husk extract: Multifunctional bioactivity in antioxidant potential, lipase and amylase inhibition, and cytotoxicity
- Therapeutic potential of sustainable zinc oxide nanoparticles biosynthesized using Tradescantia spathacea aqueous leaf extract
- Chitosan-coated superparamagnetic iron oxide nanoparticles synthesized using Carica papaya bark extract: Evaluation of antioxidant, antibacterial, and anticancer activity of HeLa cervical cancer cells
- Antioxidant potential of peptide fractions from tuna dark muscle protein isolate: A green enzymatic approach
- Clerodendron phlomoides leaf extract-mediated synthesis of selenium nanoparticles for multi-applications
- Optimization of cellulose yield from oil palm trunks with deep eutectic solvents using response surface methodology
- Nitrogen-doped carbon dots from Brahmi (Bacopa monnieri): Metal-free probe for efficient detection of metal pollutants and methylene blue dye degradation
- High energy density pseudocapacitor based on a nanoporous tungsten(VI) oxide iodide/poly(2-amino-1-mercaptobenzene) composite
- Green synthesized Ag–Cu nanocomposites as an improved strategy to fight multidrug-resistant bacteria by inhibition of biofilm formation: In vitro and in silico assessment study
- In vitro evaluation of antibacterial activity and associated cytotoxicity of biogenic silver nanoparticles using various extracts of Tabernaemontana ventricosa
- Fabrication of novel composite materials by impregnating ZnO particles into bacterial cellulose nanofibers for antimicrobial applications
- Solidification floating organic drop for dispersive liquid–liquid microextraction estimation of copper in different water samples
- Kinetics and synthesis of formation of phosphate composites from low-grade phosphorites in the presence of phosphate–siliceous shales and oil sludge
- Removal of minocycline and terramycin by graphene oxide and Cr/Mn base metal–organic framework composites
- Microfluidic preparation of ceramide E liposomes and properties
- Therapeutic potential of Anamirta cocculus (L.) Wight & Arn. leaf aqueous extract-mediated biogenic gold nanoparticles
- Antioxidant-rich Micromeria imbricata leaf extract as a medium for the eco-friendly preparation of silver-doped zinc oxide nanoparticles with antibacterial properties
- Influence of different colors with light regime on Chlorella sp., biomass, pigments, and lipids quantity and quality
- Experimental vibrational analysis of natural fiber composite reinforced with waste materials for energy absorbing applications
- Green synthesis of sea buckthorn-mediated ZnO nanoparticles: Biological applications and acute nanotoxicity studies
- Production of liquid smoke by consecutive electroporation and microwave-assisted pyrolysis of empty fruit bunches
- Synthesis of MPAA based on polyacrylamide and gossypol resin and applications in the encapsulation of ammophos
- Application of iron-based catalysts in the microwave treatment of environmental pollutants
- Enhanced adsorption of Cu(ii) from wastewater using potassium humate-modified coconut husk biochar
- Adsorption of heavy metal ions from water by Fe3O4 nano-particles
- Green synthesis of parsley-derived silver nanoparticles and their enhanced antimicrobial and antioxidant effects against foodborne resistant bacteria
- Unwrapping the phytofabrication of bimetallic silver–selenium nanoparticles: Antibacterial, Anti-virulence (Targeting magA and toxA genes), anti-diabetic, antioxidant, anti-ovarian, and anti-prostate cancer activities
- Optimizing ultrasound-assisted extraction process of anti-inflammatory ingredients from Launaea sarmentosa: A novel approach
- Eggshell membranes as green carriers for Burkholderia cepacia lipase: A biocatalytic strategy for sustainable wastewater bioremediation
- Research progress of deep eutectic solvents in fuel desulfurization
- Enhanced electrochemical synthesis of Ni–Fe/brass foil alloy with subsequent combustion for high-performance photoelectrode and hydrogen production applications
- Valorization of baobab fruit shell as a filler fiber for enhanced polyethylene degradation and soil fertility
- Valorization of Agave durangensis bagasse for cardboard-type paper production circular economy approach
- Green priming strategies using seaweed extract and citric acid to improve early growth and antioxidant activity in lentil
- Review Article
- Sustainable innovations in garlic extraction: A comprehensive review and bibliometric analysis of green extraction methods
- Natural sustainable coatings for marine applications: advances, challenges, and future perspectives
- Integration of traditional medicinal plants with polymeric nanofibers for wound healing
- Rapid Communication
- In situ supported rhodium catalyst on mesoporous silica for chemoselective hydrogenation of nitriles to primary amines
- Special Issue: Valorisation of Biowaste to Nanomaterials for Environmental Applications
- Valorization of coconut husk into biochar for lead (Pb2+) adsorption
- Corrigendum
- Corrigendum to “An updated review on carbon nanomaterials: Types, synthesis, functionalization and applications, degradation and toxicity”
Articles in the same Issue
- Research Articles
- Optimized green synthesis of silver nanoparticles from guarana seed skin extract with antibacterial potential
- Green adsorbents for water remediation: Removal of Cr(vi) and Ni(ii) using Prosopis glandulosa sawdust and biochar
- Green approach for the synthesis of zinc oxide nanoparticles from methanolic stem extract of Andrographis paniculata and evaluation of antidiabetic activity: In silico GSK-3β analysis
- Development of a green and rapid ethanol-based HPLC assay for aspirin tablets and feasibility evaluation of domestically produced bioethanol in Thailand as a sustainable mobile phase
- A facile biodegradation of polystyrene microplastic by Bacillus subtilis
- Enhanced synthesis of fly ash-derived hydrated sodium silicate adsorbents via low-temperature alkaline hydrothermal treatment for advanced environmental applications
- Impact of metal nanoparticles biosynthesized using camel milk on bacterial growth and copper removal from wastewater
- Preparation of Co/Cr-MOFs for efficient removal of fleroxacin and Rhodamine B
- Applying nanocarbon prepared from coal as an anode in lithium-ion batteries
- Improved electrochemical synthesis of Cu–Fe/brass foil alloy followed by combustion for high-efficiency photoelectrodes and hydrogen production in alkaline solutions
- Precipitation of terephthalic acid from post-consumer polyethylene terephthalate waste fractions
- Biosynthesized zinc oxide nanoparticles: Multifunctional potential applications in anticancer, antibacterial, and B. subtilis DNA gyrase docking
- Anticancer and antimicrobial effects of green-synthesized silver nanoparticles using Teucrium polium leaves extract
- Green synthesis of eco-friendly bioplastics from Chlorella and Lithothamnion algae for safe and sustainable solutions for food packaging
- Optimizing coal water slurry concentration via synergistic coal blending and particle size distribution
- Green synthesis of Ag@Cu and silver nanowire using Pterospermum heterophyllum extracts for surface-enhanced Raman scattering
- Green synthesis of copper oxide nanoparticles from Algerian propolis: Exploring biochemical, structural, antimicrobial, and anti-diabetic properties
- Simultaneous quantification of mefenamic acid and paracetamol in fixed-dose combination tablet dosage forms using the green HPTLC method
- Green synthesis of titanium dioxide nanoparticles using green tea (Camellia sinensis) extract: Characteristics and applications
- Pharmaceutical properties for green fabricated ZnO and Ag nanoparticle-mediated Borago officinalis: In silico predications study
- Synthesis and optimization of gemcitabine-loaded nanoparticles by using Box–Behnken design for treating prostate cancer: In vitro characterization and in vivo pharmacokinetic study
- A comparative analysis of single-step and multi-step methods for producing magnetic activated carbon from palm kernel shells: Adsorption of methyl orange dye
- Sustainable green synthesis of silver nanoparticles using walnut septum waste: Characterization and antibacterial properties
- Efficient electrocatalytic reduction of CO2 to CO over Ni/Y diatomic catalysts
- Greener and magnetic Fe3O4 nanoparticles as a recyclable catalyst for Knoevenagel condensation and degradation of industrial Congo red dye
- Recycling of HDPE-giant reed composites: Processability and performance
- Fabrication of antibacterial chitosan/PVA nanofibers co-loaded with curcumin and cefadroxil for wound healing
- Cost-effective one-pot fabrication of iron(iii) oxychloride–iron(iii) oxide nanomaterials for supercapacitor charge storage
- Novel trimetallic (TiO2–MgO–Au) nanoparticles: Biosynthesis, characterization, antimicrobial, and anticancer activities
- Green-synthesized chromium oxide nanoparticles using pomegranate husk extract: Multifunctional bioactivity in antioxidant potential, lipase and amylase inhibition, and cytotoxicity
- Therapeutic potential of sustainable zinc oxide nanoparticles biosynthesized using Tradescantia spathacea aqueous leaf extract
- Chitosan-coated superparamagnetic iron oxide nanoparticles synthesized using Carica papaya bark extract: Evaluation of antioxidant, antibacterial, and anticancer activity of HeLa cervical cancer cells
- Antioxidant potential of peptide fractions from tuna dark muscle protein isolate: A green enzymatic approach
- Clerodendron phlomoides leaf extract-mediated synthesis of selenium nanoparticles for multi-applications
- Optimization of cellulose yield from oil palm trunks with deep eutectic solvents using response surface methodology
- Nitrogen-doped carbon dots from Brahmi (Bacopa monnieri): Metal-free probe for efficient detection of metal pollutants and methylene blue dye degradation
- High energy density pseudocapacitor based on a nanoporous tungsten(VI) oxide iodide/poly(2-amino-1-mercaptobenzene) composite
- Green synthesized Ag–Cu nanocomposites as an improved strategy to fight multidrug-resistant bacteria by inhibition of biofilm formation: In vitro and in silico assessment study
- In vitro evaluation of antibacterial activity and associated cytotoxicity of biogenic silver nanoparticles using various extracts of Tabernaemontana ventricosa
- Fabrication of novel composite materials by impregnating ZnO particles into bacterial cellulose nanofibers for antimicrobial applications
- Solidification floating organic drop for dispersive liquid–liquid microextraction estimation of copper in different water samples
- Kinetics and synthesis of formation of phosphate composites from low-grade phosphorites in the presence of phosphate–siliceous shales and oil sludge
- Removal of minocycline and terramycin by graphene oxide and Cr/Mn base metal–organic framework composites
- Microfluidic preparation of ceramide E liposomes and properties
- Therapeutic potential of Anamirta cocculus (L.) Wight & Arn. leaf aqueous extract-mediated biogenic gold nanoparticles
- Antioxidant-rich Micromeria imbricata leaf extract as a medium for the eco-friendly preparation of silver-doped zinc oxide nanoparticles with antibacterial properties
- Influence of different colors with light regime on Chlorella sp., biomass, pigments, and lipids quantity and quality
- Experimental vibrational analysis of natural fiber composite reinforced with waste materials for energy absorbing applications
- Green synthesis of sea buckthorn-mediated ZnO nanoparticles: Biological applications and acute nanotoxicity studies
- Production of liquid smoke by consecutive electroporation and microwave-assisted pyrolysis of empty fruit bunches
- Synthesis of MPAA based on polyacrylamide and gossypol resin and applications in the encapsulation of ammophos
- Application of iron-based catalysts in the microwave treatment of environmental pollutants
- Enhanced adsorption of Cu(ii) from wastewater using potassium humate-modified coconut husk biochar
- Adsorption of heavy metal ions from water by Fe3O4 nano-particles
- Green synthesis of parsley-derived silver nanoparticles and their enhanced antimicrobial and antioxidant effects against foodborne resistant bacteria
- Unwrapping the phytofabrication of bimetallic silver–selenium nanoparticles: Antibacterial, Anti-virulence (Targeting magA and toxA genes), anti-diabetic, antioxidant, anti-ovarian, and anti-prostate cancer activities
- Optimizing ultrasound-assisted extraction process of anti-inflammatory ingredients from Launaea sarmentosa: A novel approach
- Eggshell membranes as green carriers for Burkholderia cepacia lipase: A biocatalytic strategy for sustainable wastewater bioremediation
- Research progress of deep eutectic solvents in fuel desulfurization
- Enhanced electrochemical synthesis of Ni–Fe/brass foil alloy with subsequent combustion for high-performance photoelectrode and hydrogen production applications
- Valorization of baobab fruit shell as a filler fiber for enhanced polyethylene degradation and soil fertility
- Valorization of Agave durangensis bagasse for cardboard-type paper production circular economy approach
- Green priming strategies using seaweed extract and citric acid to improve early growth and antioxidant activity in lentil
- Review Article
- Sustainable innovations in garlic extraction: A comprehensive review and bibliometric analysis of green extraction methods
- Natural sustainable coatings for marine applications: advances, challenges, and future perspectives
- Integration of traditional medicinal plants with polymeric nanofibers for wound healing
- Rapid Communication
- In situ supported rhodium catalyst on mesoporous silica for chemoselective hydrogenation of nitriles to primary amines
- Special Issue: Valorisation of Biowaste to Nanomaterials for Environmental Applications
- Valorization of coconut husk into biochar for lead (Pb2+) adsorption
- Corrigendum
- Corrigendum to “An updated review on carbon nanomaterials: Types, synthesis, functionalization and applications, degradation and toxicity”

