Abstract
In this study, Ag@Cu alloy nanoparticles and silver nanowires (AgNWs) were synthesized by a green method using the Pterospermum heterophyllum extract. To study the influence of the precursor ratio on the synthesis of Ag@Cu, the molar ratio of Ag Cu was changed to 10:7, 10:6, 10:5, and 10:4. To study the influence of the precursor concentration on the formation of AgNWs, the AgNO3 concentration was varied with values such as 3, 4, 5, 6, 7, and 8 mM. The results showed that spherical Ag@Cu were formed uniformly when the Ag:Cu molar ratio was high. The branched structures appeared when the Ag:Cu molar ratio was 10:6 and 10:7. The formation of AgNWs strongly depended on the precursor concentration, similar to the polyol method. 5 mM of AgNO3 was the most suitable concentration for the synthesis of AgNWs. Ag@Cu and AgNWs have been studied for surface-enhanced Raman scattering effects on MB dye. The results showed that both types of particles could enhance Raman scattering with enhancement factors up to 108 and 109. This proved that the green method synthesized Ag@Cu and AgNWs for products with equivalent applications to the chemical methods.
Graphical abstract

1 Introduction
Metal nanoparticles are increasingly asserting their position and important role in nanotechnology, especially gold and silver nanoparticles [1,2,3,4,5]. Recently, alloy-like multilayer nanostructures have attracted the attention of many research groups because their unique properties provide applications in many different fields, such as storage information, photonics, sensing, antibacterial, catalysis, and surface-enhanced Raman scattering (SERs) [6,7,8,9,10]. Among metal nanostructures, silver nanostructures are one of the materials with the most applications because silver nanostructures have the plasmon resonance frequency in the visible light region, and they also can be antibacterial, virus and cancer cell killing [11,12,13,14,15,16]. Therefore, the synthesis and exploitation of applications of silver nanostructures, as well as their alloys, have become the topic of most attention.
There are many methods to synthesize silver nanostructures and their alloys with other metals such as Cu, Fe, Ni, Pt, and Pd, such as electrochemical, wet chemical, ionic, sputtering, or green method [17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32]. Among these methods, the green method, using plant extracts, fungi, or bacteria as reducing agents for the synthesis process, produces eco-friendly nanomaterials. Ag@Cu bimetallic nanostructures and silver nanowires (AgNWs) have been synthesized by some similar methods, such as the polyol method using polyvinylpyrrolidone (PVP) and ethylene glycol (EG) with AgNO3 as a precursor [33,34] or the green method using ascorbic acid [35], palm leaves extract [36], or citrus fruit (lemon) peel extract [37]. However, silver nanoparticles synthesized by the green method often have a spherical or near-spherical shape. It is very difficult to synthesize AgNWs.
In this work, we used Pterospermum heterophyllum leaf extract as a surfactant as well as a reducing agent for the synthesis of Ag@Cu alloy nanoparticles and AgNWs. The influence of the Ag:Cu ratio on the optical properties and the structure of Ag@Cu was discussed. During the synthesis of AgNWs, the influence of the concentration of AgNO3 on the formation and growth of AgNWs was also investigated. At the same time, the SERs effects of the two synthesized materials were researched and compared.
2 Experimental section
2.1 Chemicals and materials
Silver nitrate (AgNO3, ≥99.0%), PVP ((C6H9NO) n , 360.000 MW), and copper(ii) nitrate (Cu(NO3)2·3H2O, 99.0%) were purchased from Merck. Sodium chloride (NaCl, 99.8%) and methylene blue (MB) dye were purchased from Sigma Aldrich. Deionized water was produced from a Mini-Q machine in the laboratory. Pterospermum heterophyllum leaves were obtained from the experimental garden at the University.
2.2 Preparation of Pterospermum heterophyllum leaf extract
Leaves were harvested from the garden. After washing several times with double distilled water, they were dried at 50°C for approximately 24 h. Dry leaves (10 g) were crushed and boiled in a water bath containing 100 ml of deionized water at 100°C for 30 min. The extract was filtered through a 0.4 μm filter to remove large leaf debris. The resulting solution was stored at 4°C.
2.3 Green synthesis of Ag@Cu alloy nanoparticles
An amount of 0.1 mM AgNO3 was added to the flask containing a 0.1 mM Cu(NO3)2 ·3H2O solution. The solution mixture was stirred at 250 rpm at room temperature. Pterospermum heterophyllum extract (0.5 mL) was then added to the reaction vessel. The solution was magnetically stirred at 250 rpm for 6 h at room temperature. To investigate the effect of the ratio of precursors on the formation of Ag@Cu alloy nanoparticles, 5.88, 6.25, 6.67, and 7.14 mL of 0.1 mM AgNO3 were placed in containers 4.12, 3.75, 3.33, and 2.86 mL of 0.1 mM Cu(NO3)2·3H2O. Therefore, the volumes of AgNO3 and Cu(NO3)2·3H2O solution were adjusted so that the molar ratios of Ag:Cu were 10:7, 10:6, 10:5, and 10:4, respectively. The total volume of the two precursor solutions remained constant at 10 mL to ensure that the extract concentration was the same in the reaction profiles.
2.4 Green synthesis of AgNWs
AgNWs were synthesized by the polyol method using Pterospermum heterophyllum extract as a surfactant and a reducing agent. This method was improved on the polyol method using EG. In the synthesis, AgCl was freshly synthesized to increase synthesis efficiency. The experiments were performed in a three-necked flask at 160°C. A total of 18 g of PVP dispersed in 50 mL of Pterospermum heterophyllum extract was stirred at 500 rpm, and after 10 min, 40 mg of AgCl was added to the solution. To investigate the influence of AgNO3 concentrations on the formation and growth of AgNWs, 1.5, 2.0, 2.5, 3.0, 3.5, and 4.0 mL of 0.1 M AgNO3 was added to the solution after 5 min, and the reaction was maintained for 2 h. The concentration of AgNO3 in the solution was varied by 3, 4, 5, 6, 7, and 8 mM.
2.5 SERs effect of Ag@Cu and AgNWs
Ag@Cu and AgNWs were centrifuged three times with deionized water at appropriate centrifugation speeds. The obtained precipitate was spread on a glass slide with a spot about 1 mm in diameter to make the SERs substrates. Then, 10 μL of MB dye with different concentrations such as 10−6, 10−7, 10−8, 10−9, 10−10, and 10−11 M was slowly loaded onto the SERs substrates. These samples were measured for Raman scattering to investigate their surface enhancement ability.
2.6 Material characterization
Scanning electron microscope (SEM) images and energy-dispersive X-ray spectroscopy (EDS) were carried out by using a Hitachi S4800 scanning electron microscope operating at 10 kV. The optical properties of Ag@Cu and AgNWs were obtained through the UV–Vis absorption spectra obtained using a Jasco V-770 UV–Vis spectrophotometer. The structure and physical properties of the synthesized materials were studied by the X-ray diffraction (XRD) spectra using an X-ray diffractometer (Bruker D8 Advance, Germany). The functional groups on Ag@Cu and leaf extract were determined by Fourier transform infrared (FTIR) spectroscopy (FTIR 1S – Mitshumitshi, wavenumber range: 400–4,000 cm−1). The SERs effect of AgNWs and Ag@Cu were collected from a Raman spectrometer (Raman Horiba XploRa plus Raman Microprobe) using a laser beam with an excitation wavelength of 532.0 nm, a diameter of 4.0 mm, a laser power of 3.2 mW, and a reception time of 8.0 s.
3 Results and discussion
3.1 Ag@Cu alloy nanoparticles
SEM images of Ag@Cu alloy nanoparticles with various molar ratios of Ag:Cu (10:4, 10:5, 10:6, and 10:7) are shown in Figure 1a–d, respectively. All images are magnified 100k times and have the same 100 nm scale. The results show that as the molar ratio of Ag:Cu increases, the size of the particles becomes larger. This means that for the same volume of AgNO3 and the Cu(NO3)2·3H2O precursor mixture when the volume of AgNO3 increases, the volume of Cu(NO3)2·3H2O decreases, and the Ag@Cu alloy nanoparticles are larger. With samples of molar ratio of Ag:Cu of 10:4 (Figure 1a) and 10:5 (Figure 1b), the particles are relatively uniform and have a spherical shape. When the Cu concentration increases, the molar ratios of Ag:Cu are 10:6 (Figure 1c) and 10:7 (Figure 1d); the particles in the solution tend to aggregate together, lengthen, and form branching structures. This is explained as follows: when the concentration increases, only a small part of Cu atoms is the core component of the Ag@Cu core@shell structure, and the majority of Cu atoms agglomerate and disperse outside the Ag@Cu structure [38,39]. On the other hand, due to the high concentration of Cu, it leads to an incomplete reduction reaction. Cu atoms are easily oxidized, and the particles aggregate together to form branched structures [39,40]. It is also difficult to identify the individual particles. The stability of these particles in solution is much poorer than samples with low Cu concentrations.

SEM images of Ag@Cu alloy nanoparticles when the Ag:Cu molar ratios are 10:4 (a), 10:5 (b), 10:6 (c), and 10:7 (d), respectively. The size scale is 100 nm.
The presence of distinct peaks in the EDS spectrum and elemental composition originating from alloy affirmed the successful formation of Ag@Cu alloy nanoparticles (Figure 2). Figure 2 shows the SEM image and EDS spectrum along with the composition table of elements in the measured samples Ag@Cu 10:4 (Figure 2a and b) and Ag@Cu 10:7 (Figure 2c and d). The energy peak at 3 keV is typical for silver nanocrystals, and the energy peaks at 1, 8, and 9 keV are typical for Cu crystals. It can be seen that when changing the molar ratio of Ag and Cu, the percentage of the corresponding elements in the sample also changes. The percentage of atoms in the Ag@Cu 10:4 sample is almost unchanged compared to the ratio of the original precursors. However, when the Cu concentration in the precursor increases, the percentage of Cu atoms in the Ag@Cu 10:7 sample decreases compared to the original precursors. This proves that as the Cu(NO3)2 concentration increases, the Cu atoms tend to disperse outside the Ag@Cu core structure. This result is also consistent with previous publications on the synthesis of Ag@Cu alloy nanoparticles [35,41]. The movement of Cu particles outside the core@shell structure leads to uneven distribution of Ag and Cu elements. As a result, the particles grow unevenly. Larger and longer particles usually contain more Cu elements. The appearance of O and Si elements in the EDS spectrum of the samples is due to the high energy electron beam interacting with the glass (SiO2). However, the oxygen in the EDS could be from the metal oxides as well. Several other elements such as carbon, sulfur, and aluminum are components of the microscope slide, where the Ag@Cu sample is spread during the EDS measurement [42,43].

EDS spectrum and elemental composition in Ag@Cu samples with Ag:Cu molar ratios of 10:4 (a) and (b) and 10:7 (c) and (d).
Figure 3 presents the UV-Vis absorption spectra (Figure 3a) and normalized absorption spectra (Figure 3b) of Ag@Cu alloy nanoparticles with different molar ratios of Ag:Cu 10:7, 10:6, 10:5, and 10:4. It can be seen that when the molar ratio of Ag:Cu increases from 10:7 to 10:4, the absorption spectra appear some absorption shoulders at longer wavelengths. This is explained by the fact that when the Cu concentration in the solution is high, the particles are not completely spherical but have additional branched structures, and Cu particles form outside the core@shell structure. Therefore, the absorption spectra have additional resonance modes oscillating at low energy. The absorption spectrum of the Ag@Cu 10:5 sample has a plasmon resonance peak at 370 nm. This is the characteristic spectrum of spherical nanoparticles. This result is also reflected through the SEM images of the particle samples in Figure 1. When the concentration of Cu2+ ions is low, and the concentration of Ag+ is high, the Cu atoms are located almost in the core of the Ag shell. The particles grow isotropically, forming a spherical core@shell structure. As the concentration of Cu in the solution increases, the plasmon resonance peak shifts toward longer waves (from 390 to 370 nm). This is mainly due to the contribution of electrons on Cu nanoparticles.

UV-Vis absorption (a) and normalized absorption (b) spectra of Ag@Cu alloy nanoparticles with vary molar ratios of Ag and Cu of 10:7, 10:6, 10:5, and 10:4.
The FTIR spectrum was used to identify functional groups and organic compounds in the extract and Ag@Cu alloy nanoparticles, thereby confirming the role of the groups of the extract in the biological reduction process to form nanoparticles. Figure 4 presents the FTIR of the Pterospermum heterophyllum extract and Ag@Cu alloy nanoparticles (Ag@Cu 10:6). The concentration of the extract was diluted 20 times to match the concentration of the extract used for particle synthesis. It can be seen that most of the peaks of the extract and the nanoparticles coincide with each other. This suggests that Ag@Cu alloy nanoparticles are coated with biomolecules from the Pterospermum heterophyllum extract, creating a stable environment for the particles in the solution. The strong peak at wave number 1,050 cm−1 corresponds to the C–O–C stretch bond [44]. The weak absorption band at 1,380 cm−1 is characteristic of the aromatic amine group [44].

FTIR spectra of the Pterospermum heterophyllum extract and of Ag@Cu alloy nanoparticles synthesized from the extract (a); X-ray diffraction patterns of the synthesized Ag@Cu structures with Ag:Cu ratios of 10:7, 10:6, 10:5, and 10:4 (b).
Vibrations of the carboxylic acid stretch group are shown in the absorption bands at 2,890 and 2,975 cm−1 [44]. The significant decrease of the absorption peak at wave number 3,330 cm−1 (corresponding to the bond of the alcohol hydroxyl –OH stretch group) and the peak at 1,050 cm−1 on the FTIR spectra of Ag@Cu alloy nanoparticles shows that these functional groups play an important role in reducing Ag+ and Cu2+ precursors to form nanoparticles. These bonds exist mainly in compounds in the extract, such as flavonoids, alkaloids, phenylpropanoids, and steroids [45].
X-ray diffraction patterns of Ag@Cu alloy nanoparticles synthesized with molar ratios of different Ag:Cu precursors of 10:7, 10:6, 10:5, and 10:4 are shown in Figure 4b. The 2θ angles are 38.3°, 44.4°, 64.7°, and 77.4°, corresponding to the (111), (200), (220), and (311) planes of the Ag crystal. These diffraction peaks are shown on all three samples with different intensity ratios. The Ag@Cu 10:4 and 10:5 samples have the highest diffraction intensity typical of silver crystals, and the half-broadness of the spectrum is the smallest. This can lead to a hypothesis that Cu exists mainly as single atoms dispersed in the silver lattice and is oxidized to a very low degree. On the contrary, when the concentration of Cu is high, the diffraction pattern appears with additional diffraction peaks characteristic of Cu (111) at 42.5° and diffraction peaks characteristic of copper oxide (Cu2O) at 36.57 and 63.5° [39]. The appearance of the peaks of Cu2O and Cu in the XRD pattern of the Ag@Cu 10:6 and 10:7 samples once again confirms that Cu exists as an amorphous phase or it can be partially dispersed under a single-atom form in the silver lattice structure. The average crystal size was determined based on the width of the diffraction peaks using the Debye-Scherrer formula: D = (k λ)/(β cos θ), where D is the average crystal size of the powder sample, k is the geometric factor or Scherrer constant, β is the angular full-width at half-maximum (FWHM) of the XRD peak (rad), λ is the wavelength of Cu kα, and θ is the Bragg diffraction angle of the corresponding peak. The crystalline domain size of the prepared Ag NPs and Cu NPs was found from the Scherer equation to be around 9.5 and 5.5 nm, respectively.
3.2 AgNWs
SEM images of AgNWs were obtained when changing the AgNO3 concentration in the solution from 3 to 8 mM. It can be seen that AgNWs tend to become shorter and wider as the concentration of AgNO3 increases. This result is similar to that of the polyol method [46–48]. When the AgNO3 concentration is low (3 mM), the AgNWs have a small aspect ratio (AR) and heterogeneous size (Figure 5a and b). When the AgNO3 concentration gradually increases to 5 mM, the AgNWs become uniform; the AR reaches 150 with widths of 20 nm ± 2 nm and lengths of 30 μm ± 5 nm (Figure 5c). The AgNWs are not uniform in size, and the AR decreases significantly when AgNO3 concentration continues to increase. At the same time, silver nanoparticles appear in several other shapes, the most numerous being spherical and spherical nanoparticles (Figure 5d–f). The higher the AgNO3 concentration, the higher the proportion of spherical silver nanoparticles and the fewer AgNWs. This is explained as follows: when the concentration of Ag+ in the solution is high, the possibility of random collisions between Ag+ ions with each other and with particles in the solution increases. Therefore, the chemical reaction rate increases, leading to larger Ag nanoparticles. The PVP capping agents then cover the Ag nanoparticles, making the particles stable in solution. The fast reaction rate causes the formation of silver nanospheres and a few AgNWs with small ARs. Thus, according to our survey, 5 mM of AgNO3 is the most suitable concentration to synthesize AgNWs uniformly and with the fewest by-products.

SEM images of AgNWs atthe AgNO3 precursor concentration of 3 mM (a), 4 mM (b), 5 mM (c), 6 mM (d), 7 mM (e), and 8 mM (f).
The optical properties of silver nanoparticles using Pterospermum heterophyllum extract obtained with various AgNO3 concentrations are shown in Figure 6a. It can be seen that the absorption spectra, as well as the shape of the SEM images, depend strongly on the concentration of the AgNO3 precursor. When the concentration of AgNO3 in the solution changes from 3 to 5 mM, the absorption spectra of the solution obtained have the same characteristic shape of AgNWs with a high-intensity resonance peak at wavelength 380–390 nm and an absorption shoulder at 347 nm. The resonance peak has the highest intensity, and the wavelength shifts toward the longest wave of 390 nm when the concentration of AgNO3 is 5 mM. This is consistent with the structure obtained from SEM images when the AR of AgNWs is the largest. When the concentration of AgNO3 is high, from 6 to 8 mM, the absorption spectra are attributed to the superposition of the spectra caused by nanoparticles with different shapes.

UV-Vis absorption spectra of silver nanoparticles when AgNO3 concentration changes from 3 to 8 mM (a) and X-ray diffraction pattern of AgNWs synthesized with 5 mM of AgNO3 (b).
Figure 6b is the XRD pattern of AgNWs synthesized using 5 mM AgNO3. The crystal structure of AgNWs is observed through diffraction peaks at positions 2θ = 32.1°, 38.2°, 44.4°, 64.4°, and 75.2°, corresponding to the characteristic lattice planes of the face-centered cubic structure such as (122), (200), (220), and (311) [JCPDS no 04–0783]. The detection of no other peaks from any other phase evidenced that single-phase Ag with cubic structure nanoparticles has been obtained directly.
3.3 SERs of Ag@Cu alloy nanoparticles and AgNWs
The Ag@Cu 10:5 sample and the AgNW sample synthesized using 5 mM AgNO3 were used as substrates to investigate the SERs effect on MB dye. The SERs spectra of MB, when enhanced by Ag@Cu alloy nanoparticles and AgNWs are shown in Figure 7. The characteristic scattering peaks for MB are observed at different wavenumbers such as 1,614, 1,440, and 450 cm−1, present the bond patterns ν(C–C) ring stretches, ν(C N) symmetric and asymmetric stretches, and δ(C–N–C) skeletal deformation mode, respectively. It can be seen that when using the Ag@Cu alloy nanoparticles and AgNWs as SERs substrates, the characteristic scattering peaks of MB dye still have high intensity even when the concentration of MB is as low as 10−11 M. This shows the ability to increase surface Raman scattering of silver nanostructures. The peaks at wave numbers 241, 672, 1,440, and 1,614 cm−1 have the strongest scattering enhancement. The scattering peak at 1,514 cm−1 of MB dye was shifted to 1,570 cm−1 when enhanced by AgNWs. This may be due to the effect of bond formation between AgNWs and MB molecule leading to relaxation of the ring bond ν(C–C). To determine the Raman scattering enhancement factor (EF) of Ag@Cu alloy nanoparticles and AgNWs, we calculated the EF for some characteristic scattering peaks with an initial MB concentration of 10−2 M for Ag@Cu/MB 10−10 M and AgNWs/MB 10−10 M according to the following formula:
where I SERs is the Raman signal intensity at 1,614 cm−1 peak in case the MB molecules adsorbed on AgNWs and Ag@Cu alloy nanoparticle substrate; I NOR is the Raman signal intensity at 1,614 cm−1 on a glass; C NOR is the concentration of 10−2 M initial MB and C SERs is the concentration of MB when absorbed on the SERs substrate. The EF values are determined in Table 1. It can be seen that at the characteristic peaks of MB dye, the EF of AgNWs is higher than that of Ag@Cu alloy nanoparticles.
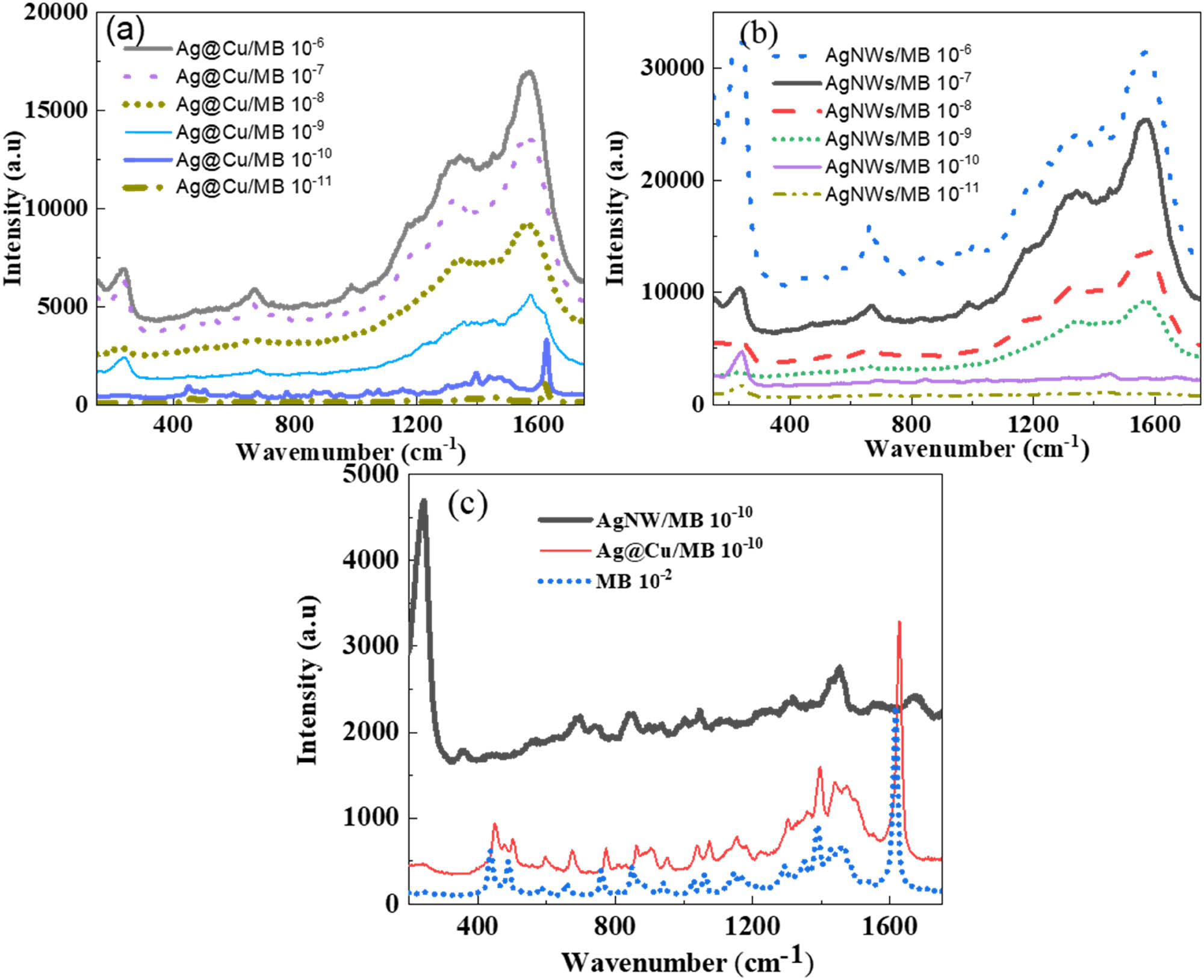
SERs spectra of MB with different concentrations from 10−11 M to 10−6 M on the Ag@Cu alloy nanoparticles substrate (a), on AgNW substrate (b), and Raman scattering spectrum of 10−2 M MB dye on the glass substrate and SERs spectra of 10−10 M MB on Ag@Cu and AgNW substrate (c).
Raman scattering intensity of MB dye at different wavenumbers on the substrate of Ag@Cu or AgNWs and EF at peaks for 10−10 M of MB
| Wavenumber (cm−1) | Intensity | ||||
|---|---|---|---|---|---|
| MB 10−2 M | Ag@Cu/MB 10−10 M | AgNWs/MB 10−10 M | EF Ag@Cu/MB 10−10 M | EF AgNWs/MB 10−10 M | |
| 241 | 145 | 500 | 4,718 | 3.45 × 108 | 3.25 × 109 |
| 672 | 250 | 684 | 2,208 | 2.74 × 108 | 8.83 × 108 |
| 1,440 | 677 | 1,462 | 2,825 | 2.15 × 108 | 4.17 × 108 |
| 1,614 | 2,231 | 3,367 | 2,441 | 1.51 × 108 | 1.09 × 108 |
The EFs are up to 8–9 orders of magnitude compared to without metal nanoparticles as the SERs substrate. This shows that AgNWs and Ag@Cu alloy nanoparticles can enhance surface Raman scattering well for MB dye. The SERs technique works through the electromagnetic effect in which molecules come close to gold or silver particles. When the incident laser light hits the nanoparticle surface, local surface plasmons can be excited, which significantly enhances the Raman signal. The mechanism contributed to surface-enhanced Raman spectroscopy (SERs) is the electromagnetic enhancement of SERs-active silver nanoparticles. SERs “hot-spot” is generated in the gap between two close nanoparticles. The rougher the surface structure of silver nanoparticles and the more “hot-spot” they have, the better their ability to enhance Raman signals [49]. The analysis results of Raman signals enhanced by Ag@Cu and AgNWs show that AgNWs (width 20 nm, length 30 μm) have an enhancement efficiency one order of magnitude higher than Ag@Cu 10:5. The Ag@Cu spherical structure has higher symmetry than the AgNW structure and the presence of the Cu shell also reduces the ability of the excited electromagnetic field to interact with electrons on the Ag surface.
4 Conclusions
Ag@Cu alloy nanoparticles and AgNWs were synthesized by the green method using Pterospermum heterophyllum extract. The –OH and C–O–C groups of the extract play an important role in reducing the precursors to form nanoparticles in the solution. The results show that the formation and growth of Ag@Cu alloy nanoparticles and AgNWs depend greatly on the concentration of precursors. For the synthesis of Ag@Cu alloy nanoparticles, when the Ag:Cu ratios are 10:4 and 10:5, the synthesized particles have a relatively uniform spherical shape. The particles become long and branched when the Ag:Cu ratio is small (10:6 and 10:7). The uniform AgNWs with an AR of 150 and no by-products are prepared using 5 mM of AgNO3. The synthesized materials are used as used as SERs substrates that can detect 10−10 M MB dye with EF up to 108 (for Ag@Cu alloy nanoparticles) and 109 (for AgNWs). These results show that organic reducing agents can be used to replace inorganic reducing agents in the synthesis of nanostructures to diversify applications, especially biomedical applications.
Acknowledgments
The authors would like to express our gratitude to Thai Nguyen University of Education for proving conditions and supporting to this study.
-
Author contributions: Khoa Tien Cao: writing – original draft, writing – review and editing, methodology, formal analysis; Hue Thi Do: writing – original draft, formal analysis, visualization, project administration.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Pasparakis G. Recent developments in the use of gold and silver nanoparticles in biomedicine. WIREs Nanomed Nanobiotechnol. 2022;14:e1817.10.1002/wnan.1817Search in Google Scholar PubMed PubMed Central
[2] Sakthi Devi R, Girigoswami A, Siddharth M, Girigoswami K. Applications of gold and silver nanoparticles in theranostics. Appl Biochem Biotechnol. 2022;194:4187–219.10.1007/s12010-022-03963-zSearch in Google Scholar PubMed PubMed Central
[3] Slepička P, Kasálková NS, Siegel J, Kolská Z, Švorčík V. Methods of gold and silver nanoparticles preparation. Materials. 2020;13:1–22.10.3390/ma13010001Search in Google Scholar PubMed PubMed Central
[4] Alaqad K, Saleh TA. Gold and silver nanoparticles: Synthesis methods, characterization routes and applications towards drugs. J Environ Anal Toxicol. 2016;6(4):1000384.10.4172/2161-0525.1000384Search in Google Scholar
[5] Yaqoob SB, Adnan R, Rameez Khan RM, Rashid M. Gold, silver, and palladium nanoparticles: A chemical tool for biomedical applications. Front Chem. 2020;8:376.10.3389/fchem.2020.00376Search in Google Scholar PubMed PubMed Central
[6] Porrati F, Sachser R, Gazzadi GC, Frabboni S, Terfort A, Huth M. Alloy multilayers and ternary nanostructures by direct-write approach. Nanotechnology. 14 de setembro de 2017;28(41):451302.10.1088/1361-6528/aa8619Search in Google Scholar PubMed
[7] Avila PRT, da Silva EP, Rodrigues AM, Aristizabal K, Pineda F, Coelho RS, et al. On manufacturing multilayer-like nanostructures using misorientation gradients in PVD films. Sci Rep. 1o de dezembro de 2019;9(1):15898.10.1038/s41598-019-52226-1Search in Google Scholar PubMed PubMed Central
[8] Migranov MS, Shehtman SR, Sukhova NA, Mitrofanov AP, Gusev AS, Migranov AM, et al. Study of tribotechnical properties of multilayer nanostructured coatings and contact processes during milling of titanium alloys. Coatings. 1o de janeiro de 2023;13(1):171.10.3390/coatings13010171Search in Google Scholar
[9] Kalska-Szostko B, Klekotka U, Olszewski W, Satuła D. Multilayered and alloyed Fe-Co and Fe-Ni nanowires physicochemical studies. J Magn Magn Mater. 15 de agosto de 2019;484:67–73.10.1016/j.jmmm.2019.03.016Search in Google Scholar
[10] Boillat R, Isanaka SP, Liou F. The effect of nanostructures in aluminum alloys processed using additive manufacturing on microstructural evolution and mechanical performance behavior. Crystals. 2021;11:524.10.3390/cryst11050524Search in Google Scholar
[11] Xu L, Wang YY, Huang J, Chen CY, Wang ZX, Xie H. Silver nanoparticles: Synthesis, medical applications and biosafety. Theranostics. 2020;10:8996–9031. Ivyspring International Publisher.10.7150/thno.45413Search in Google Scholar PubMed PubMed Central
[12] Nie P, Zhao Y, Xu H. Synthesis, applications, toxicity and toxicity mechanisms of silver nanoparticles: A review. Ecotoxicol Environ Saf. 2023;253:114636.10.1016/j.ecoenv.2023.114636Search in Google Scholar PubMed
[13] Husain S, Nandi A, Simnani FZ, Saha U, Ghosh A, Sinha A, et al. Emerging trends in advanced translational applications of silver nanoparticles: A progressing dawn of nanotechnology. J Funct Biomater. 2023;14:47–76.10.3390/jfb14010047Search in Google Scholar PubMed PubMed Central
[14] Kale SK, Parishwad GV, Husainy ASN, Patil AS. Emerging agriculture applications of silver nanoparticles. ES Food Agrofor. 1o de março de 2021;3:17–22.Search in Google Scholar
[15] Bruna T, Maldonado-Bravo F, Jara P, Caro N. Silver nanoparticles and their antibacterial applications. Int J Mol Sci. 2021;22:7202.10.3390/ijms22137202Search in Google Scholar PubMed PubMed Central
[16] Urnukhsaikhan E, Bold BE, Gunbileg A, Sukhbaatar N, Mishig-Ochir T. Antibacterial activity and characteristics of silver nanoparticles biosynthesized from Carduus crispus. Sci Rep. 1o de dezembro de 2021;11(1):21047.10.1038/s41598-021-00520-2Search in Google Scholar PubMed PubMed Central
[17] Shafiq A, Deshmukh AR, AbouAitah K, Kim BS. Green synthesis of controlled shape silver nanostructures and their peroxidase, catalytic degradation, and antibacterial activity. J Funct Biomater. 1o de junho de 2023;14(6):325.10.3390/jfb14060325Search in Google Scholar PubMed PubMed Central
[18] Zhang Z, Shen W, Xue J, Liu Y, Liu Y, Yan P, et al. Recent advances in synthetic methods and applications of silver nanostructures. Nanoscale Res Lett. 2018;13:54–72.10.1186/s11671-018-2450-4Search in Google Scholar PubMed PubMed Central
[19] Al Tamimi S, Ashraf S, Abdulrehman T, Parray A, Mansour SA, Haik Y, et al. Synthesis and analysis of silver–copper alloy nanoparticles of different ratios manifest anticancer activity in breast cancer cells. Cancer Nanotechnol. 2020;11(1):13–29.10.1186/s12645-020-00069-1Search in Google Scholar
[20] Ścigała A, Szczęsny R, Kamedulski P, Trzcinski M, Szłyk E. Copper nitride/silver nanostructures synthesized via wet chemical reduction method for the oxygen reduction reaction. J Nanopart Res. 1o de fevereiro de 2023;25(2):28–43.10.1007/s11051-023-05671-zSearch in Google Scholar
[21] Zhang W, Hu G, Zhang W, Zhang Y, He J, Yuan Y, et al. A facile strategy for the synthesis of silver nanostructures with different morphologies. Mater Chem Phys. 1o de setembro de 2019;235:121629.10.1016/j.matchemphys.2019.05.017Search in Google Scholar
[22] Borah R, Verbruggen SW. Silver-gold bimetallic alloy versus core-shell nanoparticles: Implications for plasmonic enhancement and photothermal applications. J Phys Chem C. 2020;124(22):12081–94.10.1021/acs.jpcc.0c02630Search in Google Scholar
[23] Ha Pham TT, Dien ND, Vu XH. Facile synthesis of silver/gold alloy nanoparticles for ultra-sensitive rhodamine B detection. RSC Adv. 17 de junho de 2021;11(35):21475–88.10.1039/D1RA02576GSearch in Google Scholar PubMed PubMed Central
[24] Firdhouse MJ, Lalitha P. Biosynthesis of silver nanoparticles and its applications. J Nanotechnol. 2015;2015:829526–44.10.1155/2015/829526Search in Google Scholar
[25] Abdul Kareem EA, Sultan AE, Oraibi HM. Synthesis and characterization of silver nanoparticles: A review. Ibn AL-Haitham J Pure Appl Sci. 20 de julho de 2023;36(3):177–200.10.30526/36.3.3050Search in Google Scholar
[26] Rheima AM, Mohammed MA, Jaber SH, Hameed SA. Synthesis of silver nanoparticles using the UV-irradiation technique in an antibacterial application. J Southwest Jiaotong Univ. 2019;54(5):388–99.10.35741/issn.0258-2724.54.5.34Search in Google Scholar
[27] Hoang NH, Nguyen VN, Nguyen TT, Nguyen TMD. Impact of microwave synthesis time on the shape of silver nanostructures and their antibacterial activity. J Met Mater Miner. 2023;33(1):101–6.10.55713/jmmm.v33i1.1631Search in Google Scholar
[28] Vala AK, Trivedi H, Gosai H, Panseriya H, Dave B. Biosynthesized silver nanoparticles and their therapeutic applications. Em: Comprehensive Analytical Chemistry. Elsevier B.V.; 2021. p. 547–84.10.1016/bs.coac.2020.12.010Search in Google Scholar
[29] Adebayo AE, Oke AM, Lateef A, Oyatokun AA, Abisoye OD, Adiji IP, et al. Biosynthesis of silver, gold and silver–gold alloy nanoparticles using Persea americana fruit peel aqueous extract for their biomedical properties. Nanotechnol Environ Eng. 1o de dezembro de 2019;4:13.10.1007/s41204-019-0060-8Search in Google Scholar
[30] Shumi G, Demissie TB, Eswaramoorthy R, Bogale RF, Kenasa G, Desalegn T. Biosynthesis of silver nanoparticles functionalized with histidine and phenylalanine amino acids for potential antioxidant and antibacterial activities. ACS Omega. 11 de julho de 2023;8(27):24371–86.10.1021/acsomega.3c01910Search in Google Scholar PubMed PubMed Central
[31] Ajaykumar AP, Sabira O, Sebastian M, Varma SR, Roy KB, Binitha VS, et al. A novel approach for the biosynthesis of silver nanoparticles using the defensive gland extracts of the beetle, Luprops tristis Fabricius. Sci Rep. 2023;13(1):10186.10.1038/s41598-023-37175-0Search in Google Scholar PubMed PubMed Central
[32] Ojo SA, Lateef A, Azeez MA, Oladejo SM, Akinwale AS, Asafa TB, et al. Biomedical and catalytic applications of gold and silver-gold alloy nanoparticles biosynthesized using cell-free extract of bacillus safensis LAU 13: Antifungal, dye degradation, anti-coagulant and thrombolytic activities. IEEE Trans Nanobiosci. 1o de julho de 2016;15(5):433–42.10.1109/TNB.2016.2559161Search in Google Scholar PubMed
[33] Rahman LU, Qureshi R, Yasinzai MM, Shah A. Synthesis and spectroscopic characterization of Ag-Cu alloy nanoparticles prepared in various ratios. C R Chim. junho de 2012;15(6):533–8.10.1016/j.crci.2012.03.012Search in Google Scholar
[34] Hikmah N, Idrus NF, Jai J, Hadi A. Synthesis and characterization of silver-copper core-shell nanoparticles using polyol method for antimicrobial agent. Em: IOP Conference Series: Earth and Environmental Science. Institute of Physics Publishing; 2016.10.1088/1755-1315/36/1/012050Search in Google Scholar
[35] Valodkar M, Modi S, Pal A, Thakore S. Synthesis and anti-bacterial activity of Cu, Ag and Cu-Ag alloy nanoparticles: A green approach. Mater Res Bull. março de 2011;46(3):384–9.10.1016/j.materresbull.2010.12.001Search in Google Scholar
[36] Mohamad NAN, Arham NA, Junaidah J, Hadi A, Idris SA. Green synthesis of Ag, Cu and AgCu nanoparticles using palm leaves extract as the reducing and stabilizing agents. Em: IOP Conference Series: Materials Science and Engineering. Institute of Physics Publishing; 2018.10.1088/1757-899X/358/1/012063Search in Google Scholar
[37] Shareef SN, Bhavani KS, Anusha T, Priyanka P, Rao MS. Eco-friendly green synthesis of Ag@Cu bimetallic nanoparticles: Evaluation of their structural, morphological and electrochemical characterizations. Vietnam J Chem. 1o de abril de 2023;61(2):220–6.10.1002/vjch.202200126Search in Google Scholar
[38] He L, Liu C, Tang J, Zhou Y, Yang H, Liu R, et al. Self-catalytic stabilized Ag-Cu nanoparticles with tailored SERS response for plasmonic photocatalysis. Appl Surf Sci. 15 de março de 2018;434:265–72.10.1016/j.apsusc.2017.10.155Search in Google Scholar
[39] Yang G, Zou Q, Wang P, Lai H, Lai T, Zeng X, et al. Towards understanding the facile synthesis of well-covered Cu-Ag core-shell nanoparticles from a complexing model. J Alloy Compd. 2021;874:159900.10.1016/j.jallcom.2021.159900Search in Google Scholar
[40] Liu X, Zheng Z, Wang C, Liu W, Kong L. Effects of temperature and dispersants on the phases and morphology of Ag–Cu nanoparticles. J Mater Sci: Mater Electron. 1o de outubro de 2016;27(10):10065–9.10.1007/s10854-016-5079-zSearch in Google Scholar
[41] Köroğlu M, Ebin B, Stopic S, Gürmen S, Friedrich B. One step production of silver-copper (Agcu) nanoparticles. Metals (Basel). 2021;11(9):1466.10.3390/met11091466Search in Google Scholar
[42] Khodabandehloo A, Rahbar-Kelishami A, Shayesteh H. Methylene blue removal using Salix babylonica (Weeping willow) leaves powder as a low-cost biosorbent in batch mode: Kinetic, equilibrium, and thermodynamic studies. J Mol Liq. 1o de outubro de 2017;244:540–8.10.1016/j.molliq.2017.08.108Search in Google Scholar
[43] Han R, Zhang L, Song C, Zhang M, Zhu H, Zhang LJ. Characterization of modified wheat straw, kinetic and equilibrium study about copper ion and methylene blue adsorption in batch mode. Carbohydr Polym. 17 de março de 2010;79(4):1140–9.10.1016/j.carbpol.2009.10.054Search in Google Scholar
[44] Dai F, Zhuang Q, Huang G, Deng H, Zhang X. Infrared spectrum characteristics and quantification of OH groups in coal. ACS Omega. 16 de maio de 2023;8(19):17064–76.10.1021/acsomega.3c01336Search in Google Scholar PubMed PubMed Central
[45] Yang L, Liu R, Fan A, Zhao J, Zhang Y, He J. Chemical composition of Pterospermum heterophyllum root and its anti-arthritis effect on adjuvant-induced arthritis in rats via modulation of inflammatory responses. Front Pharmacol. 2020;11:584849. 10.3389/fphar.2020.584849.Search in Google Scholar PubMed PubMed Central
[46] Dzido G, Smolska A, Farooq MO. Rapid synthesis of silver nanowires in the polyol process with conventional and microwave heating. Appl Sci (Switz). 1o de abril de 2023;13(8):4963–77.10.3390/app13084963Search in Google Scholar
[47] Hemmati S, Harris MT, Barkey DP. Polyol silver nanowire synthesis and the outlook for a green process. J Nanomater. 2020;2020:9341983–2008.10.1155/2020/9341983Search in Google Scholar
[48] Inose T, Toyouchi S, Lu G, Umemoto K, Tezuka Y, Lyu B, et al. Water-mediated polyol synthesis of pencil-like sharp silver nanowires suitable for nonlinear plasmonics. Chem Commun. 2019;55(77):11630–3.10.1039/C9CC04743CSearch in Google Scholar PubMed
[49] Tim B, Błaszkiewicz P, Kotkowiak M. Recent advances in metallic nanoparticle assemblies for surface-enhanced spectroscopy. Int J Mol Sci. 2021 Dec 28;23(1):291. 10.3390/ijms23010291.Search in Google Scholar PubMed PubMed Central
© 2025 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Synthesis of N,S co-doped carbon quantum dots – metal complex for the detection of fluoride (F−) ion in adults and Children’s toothpastes
- Research Articles
- Optimized green synthesis of silver nanoparticles from guarana seed skin extract with antibacterial potential
- Green adsorbents for water remediation: Removal of Cr(vi) and Ni(ii) using Prosopis glandulosa sawdust and biochar
- Green approach for the synthesis of zinc oxide nanoparticles from methanolic stem extract of Andrographis paniculata and evaluation of antidiabetic activity: In silico GSK-3β analysis
- Development of a green and rapid ethanol-based HPLC assay for aspirin tablets and feasibility evaluation of domestically produced bioethanol in Thailand as a sustainable mobile phase
- A facile biodegradation of polystyrene microplastic by Bacillus subtilis
- Enhanced synthesis of fly ash-derived hydrated sodium silicate adsorbents via low-temperature alkaline hydrothermal treatment for advanced environmental applications
- Impact of metal nanoparticles biosynthesized using camel milk on bacterial growth and copper removal from wastewater
- Preparation of Co/Cr-MOFs for efficient removal of fleroxacin and Rhodamine B
- Applying nanocarbon prepared from coal as an anode in lithium-ion batteries
- Improved electrochemical synthesis of Cu–Fe/brass foil alloy followed by combustion for high-efficiency photoelectrodes and hydrogen production in alkaline solutions
- Precipitation of terephthalic acid from post-consumer polyethylene terephthalate waste fractions
- Biosynthesized zinc oxide nanoparticles: Multifunctional potential applications in anticancer, antibacterial, and B. subtilis DNA gyrase docking
- Anticancer and antimicrobial effects of green-synthesized silver nanoparticles using Teucrium polium leaves extract
- Green synthesis of eco-friendly bioplastics from Chlorella and Lithothamnion algae for safe and sustainable solutions for food packaging
- Optimizing coal water slurry concentration via synergistic coal blending and particle size distribution
- Green synthesis of Ag@Cu and silver nanowire using Pterospermum heterophyllum extracts for surface-enhanced Raman scattering
- Green synthesis of copper oxide nanoparticles from Algerian propolis: Exploring biochemical, structural, antimicrobial, and anti-diabetic properties
- Simultaneous quantification of mefenamic acid and paracetamol in fixed-dose combination tablet dosage forms using the green HPTLC method
- Green synthesis of titanium dioxide nanoparticles using green tea (Camellia sinensis) extract: Characteristics and applications
- Pharmaceutical properties for green fabricated ZnO and Ag nanoparticle-mediated Borago officinalis: In silico predications study
- Synthesis and optimization of gemcitabine-loaded nanoparticles by using Box–Behnken design for treating prostate cancer: In vitro characterization and in vivo pharmacokinetic study
- A comparative analysis of single-step and multi-step methods for producing magnetic activated carbon from palm kernel shells: Adsorption of methyl orange dye
- Sustainable green synthesis of silver nanoparticles using walnut septum waste: Characterization and antibacterial properties
- Efficient electrocatalytic reduction of CO2 to CO over Ni/Y diatomic catalysts
- Greener and magnetic Fe3O4 nanoparticles as a recyclable catalyst for Knoevenagel condensation and degradation of industrial Congo red dye
- Recycling of HDPE-giant reed composites: Processability and performance
- Fabrication of antibacterial chitosan/PVA nanofibers co-loaded with curcumin and cefadroxil for wound healing
- Cost-effective one-pot fabrication of iron(iii) oxychloride–iron(iii) oxide nanomaterials for supercapacitor charge storage
- Novel trimetallic (TiO2–MgO–Au) nanoparticles: Biosynthesis, characterization, antimicrobial, and anticancer activities
- Green-synthesized chromium oxide nanoparticles using pomegranate husk extract: Multifunctional bioactivity in antioxidant potential, lipase and amylase inhibition, and cytotoxicity
- Therapeutic potential of sustainable zinc oxide nanoparticles biosynthesized using Tradescantia spathacea aqueous leaf extract
- Chitosan-coated superparamagnetic iron oxide nanoparticles synthesized using Carica papaya bark extract: Evaluation of antioxidant, antibacterial, and anticancer activity of HeLa cervical cancer cells
- Antioxidant potential of peptide fractions from tuna dark muscle protein isolate: A green enzymatic approach
- Clerodendron phlomoides leaf extract-mediated synthesis of selenium nanoparticles for multi-applications
- Optimization of cellulose yield from oil palm trunks with deep eutectic solvents using response surface methodology
- Nitrogen-doped carbon dots from Brahmi (Bacopa monnieri): Metal-free probe for efficient detection of metal pollutants and methylene blue dye degradation
- High energy density pseudocapacitor based on a nanoporous tungsten(VI) oxide iodide/poly(2-amino-1-mercaptobenzene) composite
- Green synthesized Ag–Cu nanocomposites as an improved strategy to fight multidrug-resistant bacteria by inhibition of biofilm formation: In vitro and in silico assessment study
- In vitro evaluation of antibacterial activity and associated cytotoxicity of biogenic silver nanoparticles using various extracts of Tabernaemontana ventricosa
- Fabrication of novel composite materials by impregnating ZnO particles into bacterial cellulose nanofibers for antimicrobial applications
- Solidification floating organic drop for dispersive liquid–liquid microextraction estimation of copper in different water samples
- Kinetics and synthesis of formation of phosphate composites from low-grade phosphorites in the presence of phosphate–siliceous shales and oil sludge
- Removal of minocycline and terramycin by graphene oxide and Cr/Mn base metal–organic framework composites
- Microfluidic preparation of ceramide E liposomes and properties
- Therapeutic potential of Anamirta cocculus (L.) Wight & Arn. leaf aqueous extract-mediated biogenic gold nanoparticles
- Antioxidant-rich Micromeria imbricata leaf extract as a medium for the eco-friendly preparation of silver-doped zinc oxide nanoparticles with antibacterial properties
- Influence of different colors with light regime on Chlorella sp., biomass, pigments, and lipids quantity and quality
- Experimental vibrational analysis of natural fiber composite reinforced with waste materials for energy absorbing applications
- Green synthesis of sea buckthorn-mediated ZnO nanoparticles: Biological applications and acute nanotoxicity studies
- Production of liquid smoke by consecutive electroporation and microwave-assisted pyrolysis of empty fruit bunches
- Synthesis of MPAA based on polyacrylamide and gossypol resin and applications in the encapsulation of ammophos
- Application of iron-based catalysts in the microwave treatment of environmental pollutants
- Enhanced adsorption of Cu(ii) from wastewater using potassium humate-modified coconut husk biochar
- Adsorption of heavy metal ions from water by Fe3O4 nano-particles
- Green synthesis of parsley-derived silver nanoparticles and their enhanced antimicrobial and antioxidant effects against foodborne resistant bacteria
- Unwrapping the phytofabrication of bimetallic silver–selenium nanoparticles: Antibacterial, Anti-virulence (Targeting magA and toxA genes), anti-diabetic, antioxidant, anti-ovarian, and anti-prostate cancer activities
- Optimizing ultrasound-assisted extraction process of anti-inflammatory ingredients from Launaea sarmentosa: A novel approach
- Eggshell membranes as green carriers for Burkholderia cepacia lipase: A biocatalytic strategy for sustainable wastewater bioremediation
- Research progress of deep eutectic solvents in fuel desulfurization
- Enhanced electrochemical synthesis of Ni–Fe/brass foil alloy with subsequent combustion for high-performance photoelectrode and hydrogen production applications
- Valorization of baobab fruit shell as a filler fiber for enhanced polyethylene degradation and soil fertility
- Valorization of Agave durangensis bagasse for cardboard-type paper production circular economy approach
- Green priming strategies using seaweed extract and citric acid to improve early growth and antioxidant activity in lentil
- Gold extraction from oxide ore using iron ion-thiourea-additive
- Development of loess-derived P-type molecular sieve as a sustainable antibacterial agent
- Review Article
- Sustainable innovations in garlic extraction: A comprehensive review and bibliometric analysis of green extraction methods
- Natural sustainable coatings for marine applications: advances, challenges, and future perspectives
- Integration of traditional medicinal plants with polymeric nanofibers for wound healing
- Rapid Communication
- In situ supported rhodium catalyst on mesoporous silica for chemoselective hydrogenation of nitriles to primary amines
- Special Issue: Valorisation of Biowaste to Nanomaterials for Environmental Applications
- Valorization of coconut husk into biochar for lead (Pb2+) adsorption
- Corrigendum
- Corrigendum to “An updated review on carbon nanomaterials: Types, synthesis, functionalization and applications, degradation and toxicity”
Articles in the same Issue
- Synthesis of N,S co-doped carbon quantum dots – metal complex for the detection of fluoride (F−) ion in adults and Children’s toothpastes
- Research Articles
- Optimized green synthesis of silver nanoparticles from guarana seed skin extract with antibacterial potential
- Green adsorbents for water remediation: Removal of Cr(vi) and Ni(ii) using Prosopis glandulosa sawdust and biochar
- Green approach for the synthesis of zinc oxide nanoparticles from methanolic stem extract of Andrographis paniculata and evaluation of antidiabetic activity: In silico GSK-3β analysis
- Development of a green and rapid ethanol-based HPLC assay for aspirin tablets and feasibility evaluation of domestically produced bioethanol in Thailand as a sustainable mobile phase
- A facile biodegradation of polystyrene microplastic by Bacillus subtilis
- Enhanced synthesis of fly ash-derived hydrated sodium silicate adsorbents via low-temperature alkaline hydrothermal treatment for advanced environmental applications
- Impact of metal nanoparticles biosynthesized using camel milk on bacterial growth and copper removal from wastewater
- Preparation of Co/Cr-MOFs for efficient removal of fleroxacin and Rhodamine B
- Applying nanocarbon prepared from coal as an anode in lithium-ion batteries
- Improved electrochemical synthesis of Cu–Fe/brass foil alloy followed by combustion for high-efficiency photoelectrodes and hydrogen production in alkaline solutions
- Precipitation of terephthalic acid from post-consumer polyethylene terephthalate waste fractions
- Biosynthesized zinc oxide nanoparticles: Multifunctional potential applications in anticancer, antibacterial, and B. subtilis DNA gyrase docking
- Anticancer and antimicrobial effects of green-synthesized silver nanoparticles using Teucrium polium leaves extract
- Green synthesis of eco-friendly bioplastics from Chlorella and Lithothamnion algae for safe and sustainable solutions for food packaging
- Optimizing coal water slurry concentration via synergistic coal blending and particle size distribution
- Green synthesis of Ag@Cu and silver nanowire using Pterospermum heterophyllum extracts for surface-enhanced Raman scattering
- Green synthesis of copper oxide nanoparticles from Algerian propolis: Exploring biochemical, structural, antimicrobial, and anti-diabetic properties
- Simultaneous quantification of mefenamic acid and paracetamol in fixed-dose combination tablet dosage forms using the green HPTLC method
- Green synthesis of titanium dioxide nanoparticles using green tea (Camellia sinensis) extract: Characteristics and applications
- Pharmaceutical properties for green fabricated ZnO and Ag nanoparticle-mediated Borago officinalis: In silico predications study
- Synthesis and optimization of gemcitabine-loaded nanoparticles by using Box–Behnken design for treating prostate cancer: In vitro characterization and in vivo pharmacokinetic study
- A comparative analysis of single-step and multi-step methods for producing magnetic activated carbon from palm kernel shells: Adsorption of methyl orange dye
- Sustainable green synthesis of silver nanoparticles using walnut septum waste: Characterization and antibacterial properties
- Efficient electrocatalytic reduction of CO2 to CO over Ni/Y diatomic catalysts
- Greener and magnetic Fe3O4 nanoparticles as a recyclable catalyst for Knoevenagel condensation and degradation of industrial Congo red dye
- Recycling of HDPE-giant reed composites: Processability and performance
- Fabrication of antibacterial chitosan/PVA nanofibers co-loaded with curcumin and cefadroxil for wound healing
- Cost-effective one-pot fabrication of iron(iii) oxychloride–iron(iii) oxide nanomaterials for supercapacitor charge storage
- Novel trimetallic (TiO2–MgO–Au) nanoparticles: Biosynthesis, characterization, antimicrobial, and anticancer activities
- Green-synthesized chromium oxide nanoparticles using pomegranate husk extract: Multifunctional bioactivity in antioxidant potential, lipase and amylase inhibition, and cytotoxicity
- Therapeutic potential of sustainable zinc oxide nanoparticles biosynthesized using Tradescantia spathacea aqueous leaf extract
- Chitosan-coated superparamagnetic iron oxide nanoparticles synthesized using Carica papaya bark extract: Evaluation of antioxidant, antibacterial, and anticancer activity of HeLa cervical cancer cells
- Antioxidant potential of peptide fractions from tuna dark muscle protein isolate: A green enzymatic approach
- Clerodendron phlomoides leaf extract-mediated synthesis of selenium nanoparticles for multi-applications
- Optimization of cellulose yield from oil palm trunks with deep eutectic solvents using response surface methodology
- Nitrogen-doped carbon dots from Brahmi (Bacopa monnieri): Metal-free probe for efficient detection of metal pollutants and methylene blue dye degradation
- High energy density pseudocapacitor based on a nanoporous tungsten(VI) oxide iodide/poly(2-amino-1-mercaptobenzene) composite
- Green synthesized Ag–Cu nanocomposites as an improved strategy to fight multidrug-resistant bacteria by inhibition of biofilm formation: In vitro and in silico assessment study
- In vitro evaluation of antibacterial activity and associated cytotoxicity of biogenic silver nanoparticles using various extracts of Tabernaemontana ventricosa
- Fabrication of novel composite materials by impregnating ZnO particles into bacterial cellulose nanofibers for antimicrobial applications
- Solidification floating organic drop for dispersive liquid–liquid microextraction estimation of copper in different water samples
- Kinetics and synthesis of formation of phosphate composites from low-grade phosphorites in the presence of phosphate–siliceous shales and oil sludge
- Removal of minocycline and terramycin by graphene oxide and Cr/Mn base metal–organic framework composites
- Microfluidic preparation of ceramide E liposomes and properties
- Therapeutic potential of Anamirta cocculus (L.) Wight & Arn. leaf aqueous extract-mediated biogenic gold nanoparticles
- Antioxidant-rich Micromeria imbricata leaf extract as a medium for the eco-friendly preparation of silver-doped zinc oxide nanoparticles with antibacterial properties
- Influence of different colors with light regime on Chlorella sp., biomass, pigments, and lipids quantity and quality
- Experimental vibrational analysis of natural fiber composite reinforced with waste materials for energy absorbing applications
- Green synthesis of sea buckthorn-mediated ZnO nanoparticles: Biological applications and acute nanotoxicity studies
- Production of liquid smoke by consecutive electroporation and microwave-assisted pyrolysis of empty fruit bunches
- Synthesis of MPAA based on polyacrylamide and gossypol resin and applications in the encapsulation of ammophos
- Application of iron-based catalysts in the microwave treatment of environmental pollutants
- Enhanced adsorption of Cu(ii) from wastewater using potassium humate-modified coconut husk biochar
- Adsorption of heavy metal ions from water by Fe3O4 nano-particles
- Green synthesis of parsley-derived silver nanoparticles and their enhanced antimicrobial and antioxidant effects against foodborne resistant bacteria
- Unwrapping the phytofabrication of bimetallic silver–selenium nanoparticles: Antibacterial, Anti-virulence (Targeting magA and toxA genes), anti-diabetic, antioxidant, anti-ovarian, and anti-prostate cancer activities
- Optimizing ultrasound-assisted extraction process of anti-inflammatory ingredients from Launaea sarmentosa: A novel approach
- Eggshell membranes as green carriers for Burkholderia cepacia lipase: A biocatalytic strategy for sustainable wastewater bioremediation
- Research progress of deep eutectic solvents in fuel desulfurization
- Enhanced electrochemical synthesis of Ni–Fe/brass foil alloy with subsequent combustion for high-performance photoelectrode and hydrogen production applications
- Valorization of baobab fruit shell as a filler fiber for enhanced polyethylene degradation and soil fertility
- Valorization of Agave durangensis bagasse for cardboard-type paper production circular economy approach
- Green priming strategies using seaweed extract and citric acid to improve early growth and antioxidant activity in lentil
- Gold extraction from oxide ore using iron ion-thiourea-additive
- Development of loess-derived P-type molecular sieve as a sustainable antibacterial agent
- Review Article
- Sustainable innovations in garlic extraction: A comprehensive review and bibliometric analysis of green extraction methods
- Natural sustainable coatings for marine applications: advances, challenges, and future perspectives
- Integration of traditional medicinal plants with polymeric nanofibers for wound healing
- Rapid Communication
- In situ supported rhodium catalyst on mesoporous silica for chemoselective hydrogenation of nitriles to primary amines
- Special Issue: Valorisation of Biowaste to Nanomaterials for Environmental Applications
- Valorization of coconut husk into biochar for lead (Pb2+) adsorption
- Corrigendum
- Corrigendum to “An updated review on carbon nanomaterials: Types, synthesis, functionalization and applications, degradation and toxicity”

