Abstract
This study investigates the potential of zinc oxide (ZnO) and nickel oxide (NiO) nanoparticles (NPs), biosynthesized from camel milk, to combat bacterial resistance and enhance heavy metal removal from water. The antimicrobial efficacy against various pathogens, including Bacillus subtilis, Staphylococcus aureus, Escherichia coli, Methicillin-resistant Staphylococcus aureus, Pseudomonas aeruginosa, and Candida tropicalis were studied. Characterization of the NPs was conducted using UV-vis, Fourier transform infrared, X-ray diffraction, transmission electron microscope, and atomic force microscopy techniques. Results showed that ZnO NPs exhibited the highest antimicrobial activity, with an inhibition zone of 16 mm against Pseudomonas aeruginosa and 13 mm against Candida tropicalis, while NiO NPs displayed reduced activity against all selected microorganisms. Additionally, ZnO NPs demonstrated an impressive Cu(ii) ion removal rate of 96.76% at pH 8.4, with a contact time of 90 min, using 0.5 g·L−1 of adsorbent at an initial concentration of 200 mg·L−1. Adsorption kinetics followed the pseudo-second-order model, with isotherm data fitting the Langmuir model (Q max = 100.0 mg·g−1, R 2 = 0.9905). Thermodynamic analysis indicated an exothermic process (∆H° = −4,127.4 J·mol−1) and spontaneous physical adsorption. Future research should focus on scaling up the biosynthesis of ZnO NPs for practical antimicrobial therapies and wastewater treatment technologies, alongside exploring their long-term environmental impact.
1 Introduction
Antimicrobial resistance and untreated heavy metal effluents are major public health and environmental problems worldwide. The extensive use of antimicrobials has inevitably led to increasing antimicrobial resistance. The growing population has also dramatically exacerbated water pollution by heavy metals from batteries, mining, stabilizers, tanneries, paper industry, fertilizer industry, and galvanizing plants for thermoplastics [1]. These heavy metals accumulate in soil and plant roots; they can enter the human body through the consumption of leafy vegetables and cause health hazards, carcinogenesis [2], immunotoxicity [3], and neurotoxicity [4]. Nevertheless, heavy metals have favored the emergence and spread of antibiotic resistance [5]. Therefore, it is important to develop safer alternatives from natural products and/or nanomaterials to replace the synthetically used antimicrobial agents and the harsh physicochemical methods of wastewater treatment.
Nanotechnology has been recognized for many years as a promising technology for biomedical applications [6] and as an alternative therapeutic agent for the treatment of antimicrobial resistance, which is known to be a major threat to human health, as well as for the removal of heavy metals from wastewater. Among the wide variety of nanoparticles (NPs), metal NPs are most commonly used as a specific target drug due to their exceptional and unique properties and bioavailability [7].
A wide range of metal NPs has been extensively used, namely, iron oxide, zinc oxide (ZnO), silver, gold, and nickel (Ni) [8]. However, ZnO NPs have many advantages over the other types of metallic NPs. First, they are not expensive, environmentally friendly, easy to produce, and non-toxic [9] and have shown strong biomedical properties due to their biocompatibility [10]. However, one still encounters limitations in the use of nanomedicines, mainly related to their mechanism of action. Nevertheless, green synthesized ZnO NPs have shown efficient antimicrobial activity rivaling the previously used conventional chemical and physical methods.
Nanoparticles have also proven to be a good choice for the removal of heavy metals from water, as they are easy to produce, inexpensive, and environmentally friendly compared to the chemical and physical methods previously used [11]. The presence of heavy metals in wastewater is a major concern for human health and the aquatic environment due to their carcinogenic properties, so their removal from the contaminated sources is a must. Many previous studies focused on the adsorption and penetration ability of the adsorbents, which has been further improved by the use of nanoadsorbents [12]. With an increased surface area/volume ratio, NPs exhibit better adsorption behavior and provide good long-term performance. For example, ZnO NPs have been reported to provide better adsorption capacity than previously used ultrafiltration membranes and external chemicals through surface complexation [13,14].
Although copper is essential in trace amounts for various biological functions, it poses significant environmental and health risks when present in elevated concentrations in wastewater [15]. Its toxicity can lead to adverse effects on aquatic life, including fish and invertebrates, disrupting ecosystems and reducing biodiversity [16]. In addition, exposure to high concentrations of copper can affect human health, potentially causing gastrointestinal distress, liver damage, and neurological problems [17]. As industrial processes, mining, and urban runoff contribute to copper pollution, effective remediation strategies are crucial [18]. These strategies aim not only to mitigate environmental impacts, but also to protect public health and ensure the safety of water resources. Implementing sustainable and efficient methods to remove copper from wastewater is essential to address these challenges and promote ecological balance [15].
Camel milk has attracted attention as a sustainable and environmentally friendly resource for the biosynthesis of NPs due to its unique biochemical composition containing proteins, vitamins, and minerals [19]. These components act as natural stabilizers and reducing agents during nanoparticle synthesis and facilitate the formation of NPs with desired size and shape [20]. The use of camel milk not only increases the efficiency of nanoparticle production but also ensures biocompatibility and minimizes toxicity, making these NPs suitable for various applications in medicine, agriculture, and environmental remediation [21].
In addition, the rich nutritional profile of camel milk contributes to the enhanced antimicrobial and antioxidant properties of the synthesized NPs, further increasing their potential for use in biomedical and therapeutic fields [22]. This approach is in line with green chemistry principles and promotes sustainable practices while harnessing the therapeutic benefits of camel milk in nanotechnology.
Camel milk was specifically used for the green synthesis of nanomaterials for the effective removal of copper from water. Camel milk with its high protein content provides natural stabilizers and reducing agents during nanoparticle synthesis, thus improving the efficiency and biocompatibility of the resulting nanomaterials. The synthesized NPs have unique properties that facilitate the adsorption and removal of copper ions, thus reducing toxicity in contaminated water. This approach minimizes the introduction of additional organic material into aquatic ecosystems. The use of camel milk therefore aims to develop environmentally friendly nanomaterials that effectively combat copper pollution while maintaining the ecological balance.
This study highlights the diverse biological activities of the synthesized nanomaterials by performing in vitro antimicrobial assays using the well diffusion technique. This approach enabled the determination of the minimum inhibitory concentration (MIC) and plate count, which provides information on the efficacy of the NPs against various pathogens. In addition, a time-killing assay was performed to evaluate the antimicrobial effect of the NPs over time. Furthermore, this study fills a critical research gap in the field of wastewater treatment and nanotechnology, especially with regard to the use of sustainable materials for the biosynthesis of effective nanomaterials for the remediation of heavy metals [23].
Previous methods of copper removal often rely on chemical processes or synthetic adsorbents [24], which can be costly, environmentally harmful, and potentially generate secondary pollutants. In contrast, this research uses camel milk as an environmentally friendly biosynthetic agent for NPs, which significantly improves the environmental friendliness of the process [25]. By demonstrating the efficacy of ZnO NPs and NiO NPs synthesized from camel milk to remove copper ions, this study demonstrates a novel approach that combines the benefits of nanotechnology with sustainable practices. The use of camel milk not only increases the efficiency of copper adsorption, but also ensures that the resulting NPs are biocompatible and non-toxic, addressing both environmental and health concerns. This study fills a gap by providing a cost-effective, environmentally friendly alternative for the remediation of heavy metals and paves the way for future research and applications in wastewater treatment.
2 Materials and methods
2.1 Microorganism
The microorganisms used in the present study were Bacillus subtilis ATCC 6633, MRSA ATCC, Staphylococcus aureus ATCC 29213 as the Gram-positive bacteria, the Gram-negative bacteria were Pseudomonas aeruginosa ATCC 27853, Escherichia coli ATCC 25966, and Candida tropicalis ATCC 66019 as the yeast isolate, all were provided from the microbiology department of the College of Science, King Saud University.
2.2 Chemicals and reagents
Pure materials and solvents from Sigma Aldrich (Hamburg, Germany) were used in this study and were not further purified: Zinc sulfate monohydrate (≥99.0%), nickel nitrate hexahydrate (99.9%), hydrated copper sulfate (CuSO4.5H2O, 98.0%), sodium hydroxide (99.0%), hydrochloric acid (37.5%), and ethanol (≥99.8%).
2.3 Instruments
Ulrospec-7500 UV-vis spectrophotometer (Ottawa, Canada), Fourier transform infrared (FTIR, PerkinElmer, Llantrisant, United Kingdom), X-ray diffractometer (Thermo Electron, Ecublens, Switzerland), transmission electron microscope (TEM, JEOL-1400, Tokyo, Japan), JEOL scanning electron microscope in combination with energy dispersive X-rays (SEM, JEOL, Tokyo, Japan) and Zetasizer Advance Range analyzer (Malvern, United Kingdom).
2.4 Nanoparticles preparation from camel milk sample
The camel milk samples were collected from healthy camels from a Saudi farm in Riyadh, Saudi Arabia. The milk was filled into sterile bottles and stored on ice until transportation to the laboratory.
2.5 Camel milk-mediated synthesis of NPs
The synthesis of ZnO NPs or NiO NPs was carried out by mixing 200 mL of a 2.0 mM aqueous zinc sulfate solution or nickel nitrate hexahydrate separately with 50 mL of camel milk and heating the mixed solution to 80°C. Then, a few drops of 0.2 M sodium hydroxide were added for 30 min, with constant stirring to reach a pH of 8. The resulting precipitate was centrifuged at 4,500 rpm for 15 min and decanted, and the formed ZnO NPs or NiO NPs precipitate were rinsed with deionized water (DI) and then with ethanol to remove organic or excess sodium hydroxide [26].
The resulting NPs were collected and subjected to calcination at 100°C for 12 h before being stored in an airtight container for further studies (Scheme 1).

Biosynthesis illustration of metal oxide NPs using camel milk.
2.6 Characterization
The formation of ZnO NPs and NiO NPs was confirmed by various spectroscopic and microscopic analyzes. These methods include UV-Vis spectroscopy for behavioral investigation, FTIR spectroscopy for functional groups, dynamic light scattering and zeta potential (ZP) for particle size distribution and surface charge, X-ray diffraction (XRD) analysis for grain size, EDX and mapping analysis, and surface morphology, size and aggregation of NPs studied by J-Image software in TEM, and 3D images of SEM/atomic force microscopy (AFM).
2.7 In vitro antimicrobial assay
The standard well-diffusion agar technique was performed to determine the antibacterial potential of ZnO NPs and NiO NPs synthesized in camel milk media. Pure cultures of all microbial isolates, cultured overnight, were smeared evenly onto Muller-Hinton (MH) plates (Oxoid, USA) using sterile cotton swabs. 6 mm wells were made on the surface of the agar plates using a sterile gel puncture. 100 µL of each camel milk NPs was added to the corresponding well and then incubated at 37°C for 18–24 h. The assay was repeated three times and the average of inhibition zones was measured and expressed in mm. Standard tetracycline (30 µg) was used as a positive control. The camel milk metal NP showing the highest antimicrobial activity was selected for further experiments.
2.8 MIC determination
The MIC of the metal NPs was determined using the broth microdilution method according to the Clinical and Laboratory Standards Institute guidelines. In brief, two-fold serial dilutions of the NPs in the concentration range of 1.0–0.01 mg·L−1 were prepared in 2.0 mL MH broth tubes. 5 µL of the microbial strains were inoculated into each tube. After incubation at 37℃ for 18–24 h, 100 µL of the observed MICs were spread on the surface of MH agar plates for plate count.
2.9 Time killing assay
Susceptible strains were inoculated in sterile tubes containing 1.0 mL of the metal NPs and incubated at 0, 2, 4, 18, and 24 h time intervals. Following incubation, 100 µL of each time interval were loaded and spread on MH agar plates for direct bacterial plate count.
2.10 Elimination of Cu(ii) ions from wastewater
2.10.1 Batch adsorption experiments
ZnO NPs were tested in a batch process for the adsorption of Cu(ii) ions from water samples. Water samples of Cu(ii) in volumes of 50 mL, initially concentrated to 100–500 mg·L−1, were transferred to a 250 mL flask. About 0.5 g·L−1 ZnO NPs were added with continuous shaking at 150 rpm, varying the dosage and the amount of the adsorbent. The pH of the solution was optimized (from 2 to 8). The solutions were decanted and the Cu(ii) concentrations were measured after the adsorption time (10–120 min) by complexometric dosing. Eq. 1 was used to estimate the equilibrium metal adsorptive quantity based on data from batch studies:
where q
e,
The removal efficiency % of Cu(ii) ions was determined using the following relationship:
3 Results and discussion
3.1 Mechanism of formation of NPs
The biosynthesis of ZnO NPs and NiO NPs using camel milk involves a series of biochemical processes that leverage the natural components of the milk, including proteins, lipids, and lactose, which act as reducing and stabilizing agents. The process begins with the collection of fresh camel milk, which contains a rich array of bioactive compounds, including enzymes and metabolites. To initiate nanoparticle synthesis, the milk is often subjected to mild heating or pH adjustment to create an optimal environment for nanoparticle formation. Zinc and nickel salts are used. Upon the introduction of these metal salts, the bioactive compounds in the milk facilitate the reduction of metal ions to their respective metal oxides. For ZnO NPs, the carboxyl and hydroxyl groups present in the milk proteins interact with the zinc ions, leading to nucleation and subsequent growth of NPs. Similarly, for NiO NPs, the proteins and other organic molecules in camel milk help in reducing nickel ions, promoting the formation of NiO through controlled aggregation. Following synthesis, the NPs are usually purified by centrifugation, and the residual milk components can be removed to yield a cleaner product. Characterization techniques are employed to analyze the size, shape, and crystalline structure of the NPs, confirming their formation and properties (Scheme 2).

Mechanism of biosynthesis of ZnO NPs and NiO NPs using camel milk constituents as reducing, stabilizing, and capping agents.
3.2 Characterization of the prepared nanosorbents
UV-Vis spectroscopy was used to evaluate the optical properties of the individual synthesized metal oxide NPs. The UV-Vis absorption spectra of zinc sulfate together with the camel milk and the metal NPs were shown (Supporting information S1a). Three peaks at 258, 277, and 374 nm were observed for zinc sulfate, camel milk, and ZnO NPs, respectively. These results are consistent with those reported in the current literature [27]. The absorption spectra (Supporting information S1b) again revealed three distinct peaks at 250, 277, and 350 nm, which are assigned to Ni(NO3)2·6H2O, camel milk, and NiO NPs, respectively. The results obtained are in agreement with those reported in the literature [28].
The optical band gaps (Figure 1a–d) of the synthesized ZnO NPs and NiO NPs can be determined by the Tauc relationship (3):
where A is the constant of transition possibility, hυ is the energy of the incident photon, and q is the optical absorption index, respectively. The calculated band gaps of ZnO NPs and NiO NPs were found to be 3.32 and 3.54 eV, respectively.

Optical spectra and Tauc plot of (a) and (b) ZnO NPs and (c) and (d) NiO NPs synthesized using camel milk, respectively.
FTIR analysis is utilized to investigate the active functional groups of present compounds in pre-synthesized metal oxide NPs using camel milk. The FTIR spectra were indicated spanning between 4,000 and 500 cm⁻¹. The camel milk FTIR spectrum (Figure 2a) revealed one wide peak positioned at 4,343 cm⁻¹ (sharp O–H stretching of free alcohol), 3,402 cm−1 (broad O–H stretching intermolecular bonded of alcohol), 2,924 and 2,854 cm−1 (weak C–H and –CH2 stretching of fats), 2,368 cm−1 (strong stretching O═C═O of carbon dioxide), 1,743 cm−1 (strong C═O stretching of aldehyde), 1,658 and 1,550 cm−1 (strong stretching C═O of amide I and amide II in the protein), 1,458 cm−1 (medium –CH3 bending), 1,141 cm−1 (strong stretching of C–OH), and 609–331 cm−1 (strong stretching C-halide compounds) [29].

FTIR spectra of (a) camel milk, (b) ZnO NPs, and (c) NiO NPs measured at 4,000–500 cm−1.
The synthesized ZnO NPs FTIR analysis indicated different functional groups (Figure 2b) with a broad peak at 3,741 cm−1 (sharp O–H stretching of free alcohol), 3,425 cm−1 (broad O–H stretching of the intermolecular bond of alcohol), 2,924 and 2,854 cm−1 (weak C–H and –CH2 stretching of fats), 2,368 cm−1 (strong O═C═O stretching of carbon dioxide), 1,743 cm−1 (strong C═O stretching of aldhydes), 1,651 cm−1 (strong C═O stretching of amide I in the proton), 1,381 cm−1 (average −CH3 bending), 1,118 cm−1 (strong C–OH stretching), and 609 cm−1 (strong C-halide stretching), the 432 cm−1 band indicated Zn–O bonding [30].
Similarly, NiO NPs FTIR spectrum (Figure 2c) exhibited various absorption bands positioned at 3,749 cm−1 (sharp O–H stretching of free alcohol), 3,441 cm−1 (broad O–H stretching of the intermolecular bond of alcohol), 2,924 cm−1 (weak C–H and –CH2 stretching of fats), 2,376 cm−1 (strong O═C═O stretching of carbon dioxide), 1,743 cm−1 (strong C═O stretching of aldehydes), 1,651 cm−1 (strong C═O stretching of amide I in the proton), 1,465 cm−1 (average –CH3 bending), 1,111 cm−1 (strong C–OH stretching), and 624–393 cm−1 attributed to Ni–O bonding [31].
Both metal nanoparticles’ sizes were determined using a zetasizer. In the DI water, the synthesized ZnO and NiO NPs’ particle size distribution revealed hydrodynamic average diameter sizes of 60.25 and 35.5 nm, with polydispersity index values of 0.368 and 0.298, respectively (Supporting information, S2). On the other hand, the ZPs of ZnO and NiO NPs suspended in a DI water stock solution were found to be −10.0 and −10.8 mV, respectively, indicating greater hydrodynamic sizes than their actual sizes in aqueous media. In addition, the particle exhibited an aggregating tendency, caused by a rise in ionic concentration, and resulted in weakened repulsion forces between the NPs (Supporting information, S3).
Through XRD profiles in the 2θ range of 20–80° with a scan speed of 0.03°/min, the crystallinity and crystalline phase of synthesized samples were ascertained. The XRD peak was used to calculate the crystallite size of the NPs. Scherrer’s Eq. 4 was used to calculate the average grain size of the NPs.
where the CuKα radiation wavelength is represented by λ (0.15406 Å), the full-width at half-maximum (FWHM) is indicated by β, the shape factor is represented by k (k = 0.9), and the Bragg’s angle of the peak is indicated by θ. The XRD pattern of ZnO NPs (Figure 3a) shows various diffraction peaks at 2θ values of 31.64°, 34.43°, 36.29°, 47.75°, 56.51°, 62.90°, 67.98°, and 69.23° related to (1 0 0), (0 0 2), (1 0 1), (1 0 2), (1 1 0), (1 0 3), (1 1 2), and (2 0 1), respectively. The results revealed the formation of hexagonal ZnO NPs with an average crystallite size of 18.39 ± 1.05 nm. It was found that the ZnO-NPs diffractogram matched the standard ZnO (JCPDS card No-36-1451) [32].

XRD profiles of (a) ZnO NPs and (b) NiO NPs synthesized using camel milk.
Through the XRD pattern of NiO NPs (Figure 3b), the crystallinity and crystalline phase of the synthesized sample showed NiO with spherical NPs with a face-centered cubic structure at 2θ values of 37.46°, 43.51°, 63.16°, 75.65°, and 79.71°, which corresponded to (1 1 1), (2 0 0), (2 0 2), (3 1 1), and (2 2 2), respectively. This diagram indicated that no more peaks were observed for the presence of pure NPs. The collected data (JCPDS # 96-101-0096) matched the standard pattern [33]. The average crystallite size of NiO NPs was found to be 19.92 ± 1.6 nm.
ZnO NPs and NiO NPs synthesized with camel milk were further detected using TEM analysis revealed the size, shape, and agglomeration state of the synthesized NPs (Figure 4a–d). The dislocation density of the synthesized ZnO NPs and NiO NPs was calculated as follows:
where
where ε, ß, θ is the micro strain of NPs, FWHM of the peak, and Bragg angle, respectively. The micro strain is able to give information about the defects present in the lattice [34]. The calculated micro strains at 100°C for ZnO NPs and NiO NPs were found to be 5.04 × 10−3 and 4.17 × 10−3 for the abovementioned NPs.

TEM images of (a) and (b) ZnO NPs and (c) and (d) NiO NPs measured at different magnifications 100,000–200,000×.
Both ZnO and NiO-NP samples were analyzed for surface morphology and particle size using Image-J software. The NPs were observed as polydisperse hexagonal and spherical particles, with a particle size distribution of 62.63 ± 8 and 29.15 ± 5 nm for the aforementioned nanomaterials, respectively (Figure 5a–d).

TEM and particles size using image-J software of (a) and (b) ZnO NPs and (c) and (d) NiO NPs.
The field emission scanning electron microscope (FESEM) is the study of material surface phenomena, where numerous qualitative details of a material, including composition, morphology, and crystallography can be obtained. Put differently, FESEM offers details about the sample’s surface characteristics and texture, as well as the size, form, and particles distribution [35]. The primary elements that have a significant impact on an adsorbent’s capacity to absorb biomolecules are its surface area, shape, charge, and aggregation state.
The FESEM, in the present study, was utilized to verify the size and the 3D profile as well as to assess the surface characteristics of the synthesized nanomaterials. Results revealed well-defined hexagonal and spherical shapes with a solid, dense structure, and an average size of 80 and 45 nm for ZnO NPs and NiO NPs, respectively (Figure 6a and b).

(a) and (b) FESEM images and (c) and (d) AFM 3D images of ZnO NPs and NiO NPs synthesized using camel milk.
The elemental configuration of ZnO NPs and NiO NPs synthesized using camel milk and biogenically synthesized using SEM/EDX spectrometer were confirmed (Figure 7a and b). In the ZnO NPs sample, the weight percentages of Zn and O were 73.31% and 26.31%, respectively, while the atomic percentages were 40.67% and 59.33%. NiO NPs showed different weight percentages, 54.88% for Ni and 45.12% for O, with atomic percentages of 24.89% and 75.11%, respectively (Figure 7c and d).

(a) and (b) SEM images and (c) and (d) EDX analysis of ZnO NPs and NiO NPs synthesized using camel milk.
Moreover, the X-rays are restricted to the surface area and excited by the tiny electron beam, enabling the detection of particles or specific area spectra. As a result, by scanning the beam, spectral data for the full-view field can be acquired, producing an element map. The ZnO NPs and NiO NPs EDX mapping is displayed in (Supporting information, S4).
3.3 Surface area and pore diameter
ZnO and NiO NPs’ Barret–Joyner–Halenda (BJH) pore size distribution diagrams and N2 adsorption/desorption isotherms were examined. At a relative pressure (P/P o) in the range of 0.1–1.0, both samples exhibited a typical IV pattern of the IUPAC isotherm type with a prominent hysteresis loop in the low-pressure region of the open structured mesopores. ZnO NPs and NiO NPs, respectively, had surface areas of 134.2 ± 0.1 and 187 ± 0.4 m2·g−1 and pore volumes of 0.47 and 0.51 cm3·g−1, according to surface area analysis using the Brunauer–Emmett–Teller (BET) method (Figure 8a and b).

Pore diameter and surface area using BET/BJH for (a) ZnO NPs and (b) NiO NPs.
3.4 Thermal stability of the synthesized nano sorbents
The thermogravimetric stability (TGA) of the formed ZnO NPs and NiO NPs was examined (Figure 9) with 25–600°C as the temperature range of thermal stability. The ZnO NPs sample’s TGA curve demonstrated the highest stability. A 10% weight reduction was noted at 380°C. On the other hand, the NiO NP sample’s curve displayed various weight loss regions. Thermal degradation caused by moisture and solvent was observed at temperatures between 160°C and 250°C, resulting in weight losses of approximately 2.5% and 7%, respectively. At 380°C, a 25% decrease in weight was observed as a result of the camel milk polysaccharides’ carbon chains used in the synthesis breaking down. The last areas of weight loss were discovered to be 50%, 68%, and 69% of body weight, respectively, at a temperature range of 420–600°C.

TGA analysis of ZnO NPs and NiO NPs synthesized using camel milk.
3.5 Greenness of the synthesis process using complex green analytical procedure index (GAPI) and analytical greenness metric (AGREE) score software
The greenness profile was used to evaluate the analytical environmentally friendly method used relative to other documented methods. Four requirements were evaluated: corrosiveness, toxicity, hazardousness, or bioaccumulative property (with a pH range between 2 and 12), and the waste following consumption should weigh less than 50 g/sample [36].
Ultrapure water and camel milk are known to be non-corrosive and non-hazardous, and for this, they were used in this analysis. Furthermore, not a lot of waste was generated. Consequently, the suggested method conforms fairly well to the five greenness profile requirements (Figure 10a) and is considered more environmentally friendly in terms of time and reagents with a green score greater than 0.6 (Figure 10b) when compared to other chemical methods previously used in the synthesis of nanomaterials [37]. The new co-precipitation approach for the preparation of ZnO and NiO NPs using camel milk revealed a final AGREE score of 0.87 for its greenness features. The parameter 2 with orange color of sample amount means that the sample treatment was not performed online. The parameter 8 with yellow color indicates partial compliance with the greenness criteria.

(a) GAPI pictogram and (b) AGREE score for assessment of the greenness of synthesis method of metal oxide nanoparticle.
Greenness index using the spider diagram metric: The spider diagram greenness index was used to evaluate the reagents used in the proposed synthesis procedure. The Safety Data Sheets (SDS), which detail the numerous solvent properties and their environmental, health, and safety impacts at each stage of the process, provide the data on which this tool is based. By combining five categories of assessment criteria (health effects, general properties, odor, fire safety, and stability), a hierarchical spider diagram was created that provides a visual representation of the overall level of sustainability of the chemicals used. Following a predetermined methodology, each cluster is assigned a score from −5 (least green) to +5 (greenest). For each of the five key criteria, the results are presented visually in the form of a primary spider diagram, which provides an overview of the environmental friendliness of the solvents. The spider diagrams allow a more detailed analysis of the specific characteristics of each criterion [38]. The SDS sections of several solvents are not complete. Each attribute with missing data is scored zero. The “greenness index table” contains a list of all criteria and the percentage of available data used to generate the greenness index results. The percentage indicates the level of confidence in the assessment. It is noteworthy that this study is one of the first to use the greenness index in the analytical domain. The primary spider diagram shows that the overall greenness index of DI water and ethanol is on the safe side (Figure 11). The supporting data for the other values are included in Supporting information, S5. In addition, the eco-friendliness index provides the average values and the percentage of valuable data currently available for the solvents used are summarized in Table 1.

Primary spider diagram for DI water and ethanol in the green synthesis process of nanomaterials.
Greenness index data for DI water and ethanol in the synthesis process of metal oxide NPs
| Criteria | DI water score | Available information (%) | Ethanol score | Available information (%) |
|---|---|---|---|---|
| Health hazard | 5 | 100 | 2.63 | 100 |
| General properties | 4.29 | 100 | 0.70 | 93.50 |
| Stability | 1.94 | 87.50 | 2.70 | 87.71 |
| Fire safely | 5 | 100 | 0.61 | 100 |
| Odor | 5 | 100 | 0.00 | 100 |
| Average | 4.25 | 97.50 | 1.328 | 96.24 |
3.6 Antimicrobial activity assay
Nanobodies in camels were investigated and found to be efficient for the treatment of microbial infections as well as targeting cancer cells, adding as such more unique features to camel milk [39]. The current work established the potential activity of ZnO NPs and NiO NPs, both synthesized from camel milk against some pathogenic bacteria (Figure 12). Higher antibacterial activity was mainly noted with ZnO NPs against Gram-negative bacteria (P. aeruginosa and E. coli) (16 and 11 mm) and the yeast isolate C. tropicalis (13 mm) than against Gram-positive bacteria (S. aureus, B. subtilis, and MRSA) (Table 2). Similar observation was noted with previous report [40], where S. aureus showed no significant effect to ZnO NPs activity.

Well diffusion method showing the inhibition zone of both metal oxide NPs against the selected strains.
Average inhibition zone (mm) of camel milk mediated synthesis metal oxide NPs
| Microorganisms | Variable NPs extract average inhibition zone (mm) | |||
|---|---|---|---|---|
| ZnO NP | NiO NP | TE (30 µg) | Previous data (ZnO NP) (10 mg) | |
| Gram-positive | ||||
| S. aureus ATCC 29213 | 10 | 12 | 11.5 | 9.3 |
| B. subtilis ATCC 6633 | — | — | — | 15 |
| MRSA | — | — | — | |
| Gram-negative | ||||
| E.coli ATCC 25966 | 13 | 11 | 11 | |
| P. aeruginosa ATCC 27853 | 17 | 13.5 | 16 | 12 |
| Yeast | ||||
| C. tropicalis ATCC 66019 | 13 | 13 | ||
While the Gram-positive bacteria were more resistant to the nano extracts, the Gram-negative bacteria and the yeast isolate showed greater sensitivity to ZnO NPs when compared to nickel NPs. These findings led to the selection of ZnO NPs for additional examination.
The MIC for the two selected microorganism strains: P. aeruginosa and C. tropicalis were observed at MIC 50 of (0.5 mg·mL−1) with a lower bacterial count (200 and 180 colonies/plate) compared to MICs 25 and 12 with a bacterial count of >300 (Figure 13a and b).
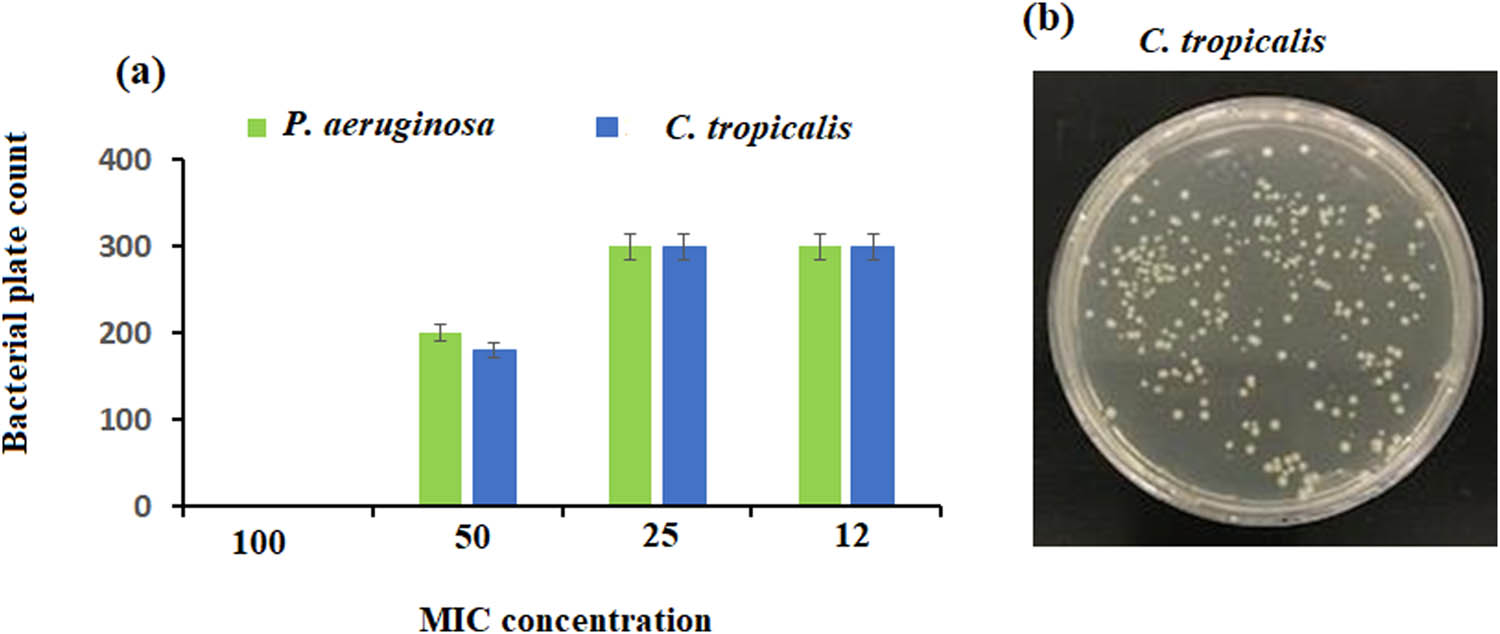
(a) MIC in vitro assay of P. aeruginosa and C. tropicalis showed similar MIC 50 of 0.5 mg·mL−1 concentration against ZnO NPs indicated by (b) a lower count on MH agar plates.
The best time for killing the bacterial strains was observed after 2 and 24 h of incubation, as shown in Table 3. No microbial growth was observed on MH agar plates after 2 and 24 h of incubation, while only a few bacterial colonies were observed after 4 h of incubation.
Time killing assay
| Microorganisms | Time intervals (hours)/microbial plate count | |||
|---|---|---|---|---|
| 0 (control) | 2 | 4 | 24 | |
| P. aeruginosa | >300 | — | 7 | |
| C. tropicalis | >300 | — | 2 | |
This could be due to the thicker cell wall structure, which makes it more difficult for ZnO NPs to enter the cell [41]. The exact mechanism by which ZnO-NPs influence bacterial growth is not yet fully understood. In general, it appears that these NPs release metal ions that act directly on the bacterial cell wall and disrupt its permeability [8], thereby damaging the first line of defense [42]. Once the metal NPs penetrated the bacterial cells, they impair the biochemical processes of the bacterium and cause DNA damage and protein denaturation, which leads to cell death, as the bacteria can no longer multiply at this point [43] as indicated in Scheme 3.
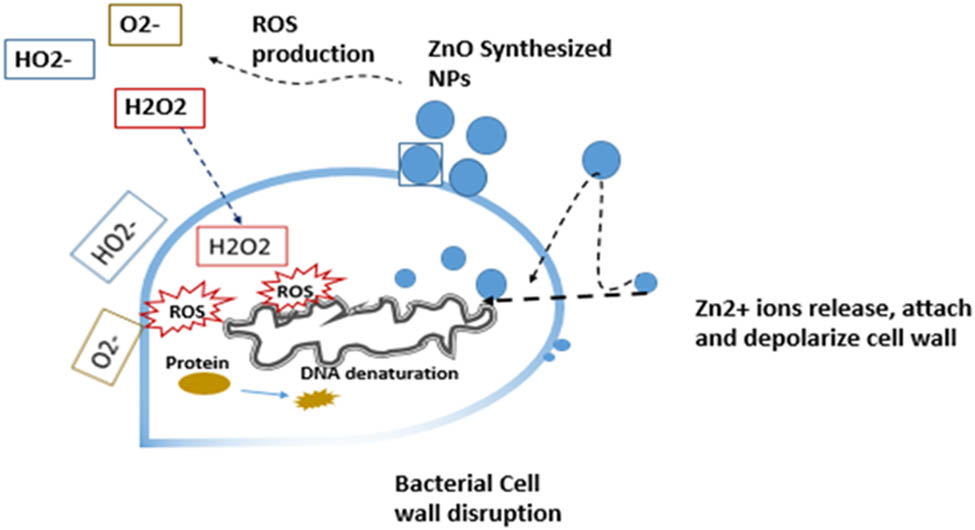
Possible mechanism of metal NPs (Zinc oxide as an example) synthesized from camel milk against bacterial cells. NPs attach to the bacterial cell, they start to release metal ions that act directly on the bacterial cell wall and disrupt its permeability. Once inside the bacterial cell, they impair the biochemical processes of the bacterium by oxidative stress (reactive oxygen species, ROS), cause DNA damage, and protein denaturation leading to cell death.
Several studies investigating the membrane interactions of metal NPs with the bacterial cell have shown that this binding based mainly on the ionic charges of both the NPs and the bacterial cell membrane. The cationic properties of ZnO NPs allow them to attach to the negatively charged bacterial cell surface through electrostatic interaction, which leads to their accumulation and thus to cell damage. This in turn is based on the different cell wall structures of Gram-positive and Gram-negative bacteria, which represent a natural barrier for the uptake of many antimicrobial agents [42]. Another earlier study too stated that binding of titanium oxide NPs to Gram-negative E. coli depolarized the membrane potential, which increased cell permeability and led to leakage of intracellular proteins, DNA, and ions, which in turn resulted in cell death, but this was not the case with Gram-positive Staphylococcus aureus [44]. This is consistent with the available data, in which Gram-negative bacteria showed a higher sensitivity to the zinc oxide NPs than Gram-positive bacteria. Another antibacterial activity of metallic NPs could be attributed to oxidative stress (ROS), which having high reactivity and oxidizing potential, is considered hazardous to microorganisms [45]. NPs similarly, once are in contact with the bacterial surface, trigger a free radicals accumulation inducing as such oxidative stress similar to the effect of high energy electron production when NPs are exposed to UV light induction [46].
In addition, both the shape and size of the metallic oxide NPs play an important role in determining their efficiency as antimicrobial agents. Another study [47] stated that hexagonal ZnO NPs caused lethal damage to various bacteria. Moreover, a single-pot biogenic synthesis of NiO NPs with spherical morphology for antimicrobial activities was developed [48]. Thus, the strong antimicrobial activity shown in this study is also due to the hexagonal and spherical shape of the metal oxide NPs, which were investigated as ZnO and NiO NPs, respectively. Hence, due to their surface charge, size, and shape, or by combining all three, NPs might exhibit their antibacterial activity [49]. The buildup and toxicity of metal NPs at high concentrations in human tissues restricts their broad application, even though bactericidal activity is sought [50].
3.7 Adsorption removal of Cu(ii) from wastewater
3.7.1 Factors influencing adsorption potential
3.7.1.1 Effect of sorbent dose
The influence of biogenic synthesized ZnO NPs using camel milk on the efficient removal of Cu(ii) ions was investigated with adsorbent dosages of 0.1–1.0 g·L−1 using 50 mL of 200 mg·L−1 Cu(ii) ions at 25°C, agitation speed of 150 rpm, 90 min contact time, and pH of 6.2. The % removal of Cu(ii) ions dramatically increased with adsorbent doses from 0.1 to 0.5 g, referred to an increase in the active sites number [51]. It was noticed that at adsorbent doses higher than 0.5 g, adsorption was uniform. As a result, for all upcoming experiments, an adsorbent dose of 0.5 g was determined to be the ideal dose for a generally acceptable Cu(ii) ions removal efficiency (Figure 14a).

Optimal experimental conditions (a) ZnO NPs dosage (g·L−1), (b) contact time (min), (c) initial Cu(ii) concentration (mg·L−1), and (d) pH of test solution from 2 to 8.
3.7.1.2 Effect of contact time
The contact time greatly affected the sorbate kinetics at a specific initial concentration [52]. The effect of contact time on the removal efficiency of 200 mg·L−1 was tested by changing the time period from 0 to 180 min, and using an equilibrium dose of ZnO NPs (0.5 g·L−1) under agitation speed of 150 rpm, pH = 6.2 at room temperature (25°C). The quickest adsorption of Cu ions was observed at 30 min (Figure 14b). Then, the process slowed down and reached equilibrium at 60 min. Increased contact time up to 90 min showed unsignificant increase in the adsorption (less than 1%), suggesting that adsorption might be completed in the contact time less than 90 min. As a result, further studies were performed during contact time 90 min.
3.7.1.3 Effect of initial Cu(ii) concentration
Under the abovementioned conditions, the removal % and uptake values of ZnO NPs were investigated by altering the initial Cu(ii) concentration from 100 to 500 mg·L−1. The ZnO NPs were separately dispersed for 90 min to ascertain the impact of initial Cu(ii) concentration on adsorption quantity. Figure 14c presents the results which state that when Cu(ii) concentration rises, the adsorption rate slows down after initially increasing. The adsorption process slows down as Cu(ii) concentration increases due to competition for active adsorption sites [53]. According to the data, the removal percentage using ZnO NPs sorbent decreased slightly from 99.9% at 100 ppm to 99.7% at 200 mg·L−1, and it significantly decreased to 50.47% at 500 mg·L−1. The initial Cu(ii) concentration in subsequent experiments was 200 mg·L−1.
3.7.1.4 Effect of initial pH values and sorption mechanism
An essential component of the adsorption process is the pH of the solution which influences the adsorbent’s surface charge, the ionization level, and the speciation of the adsorbent. In this study, the effect of pH on the copper adsorption under the above determined parameters, the adsorption of heavy metals Cu(ii) ions onto 0.5 g·L−1 of adsorbent at room temperature, an initial concentration of 200 mg·L−1 Cu(ii) ions, 150 rpm stirring speed, and 90 min contact time, was investigated. The pH of the solution was adjusted to 2–8 with either a 0.1 M HCl or NaOH solution. It was found that with increased pH values from 2 to 6, the removal efficiency increased to reach 96.8%. The surface charge of the adsorbent can be used to explain the fluctuations in the removal of the heavy metals Cu(ii) in function of the pH value. At a strong acidic solution (pH 2), no copper ions are held in solution. This can be explained by the high concentration of H+ cations in the solution, causing competition between the H+ cations and the Cu2+ cations for the negatively charged space. Thus, pH 6.2 was selected for further studies (Figure 14d).
Eq. 7 can be used to calculate the distribution coefficient Kd to assess the adsorbent surface’s affinity.
where C s and C w stand for the Cu(ii) concentration in the adsorbent (mg·g−1) and the concentration of Cu(ii) that remains after removal (mg·L−1), respectively. The obtained K d values as a function of pH are displayed in Figure 15a. It is important to keep in mind that the target pollutant’s binding capability increases with its K d value [54].

(a) Initial pH of 50 mg·L−1 Cu(ii) solution vs log K d and (b) initial pH of Cu(ii) solution (50 mg·L−1) vs final pH of Cu(ii) solution using ZnO NPs.
The findings demonstrated the efficacy of ZnO NPs in removing Cu(ii) ions from water samples. The zero-point charge of the adsorbent accounted for the variation in absorbance with respect to the starting pH of the sample. Figure 15b shows the plot of the pH of the Cu(ii) working solution at the beginning vs the end to determine the zero point of ZnO NPs. The findings showed that the zero-point charges of ZnO NPs at pH = 8.4. Moreover, the maximum sorption capacities of the adsorbent at 25°C was found to be 3.5 mg·g−1.
3.7.1.5 Effect of competing ions
It was investigated how co-ions in contaminated water, including Ca2+, Mg2+, Mn2+, Ag+, Na+, and K+, affect the ability to remove Cu(ii) ions. At room temperature, the Cu(ii) content was stable at 200 mg·L−1, while the ion concentration was 500 mg·L−1. The study was done under the following conditions: a Cu(ii) concentration of 200 mg·L−1, a contact time of 90 min, an adsorbent dose of 0.5 g·L−1, a pH of 6.2 and a temperature of 25°C. In the presence of Ca2+, Mg2+, Mn2+, Ag+, Na+, and K+ ions, the adsorption capacity is drastically reduced (Figure 16a). This indicated that the competition of these ions with the active sites on the adsorbent surface delayed Cu(ii) removal [55].
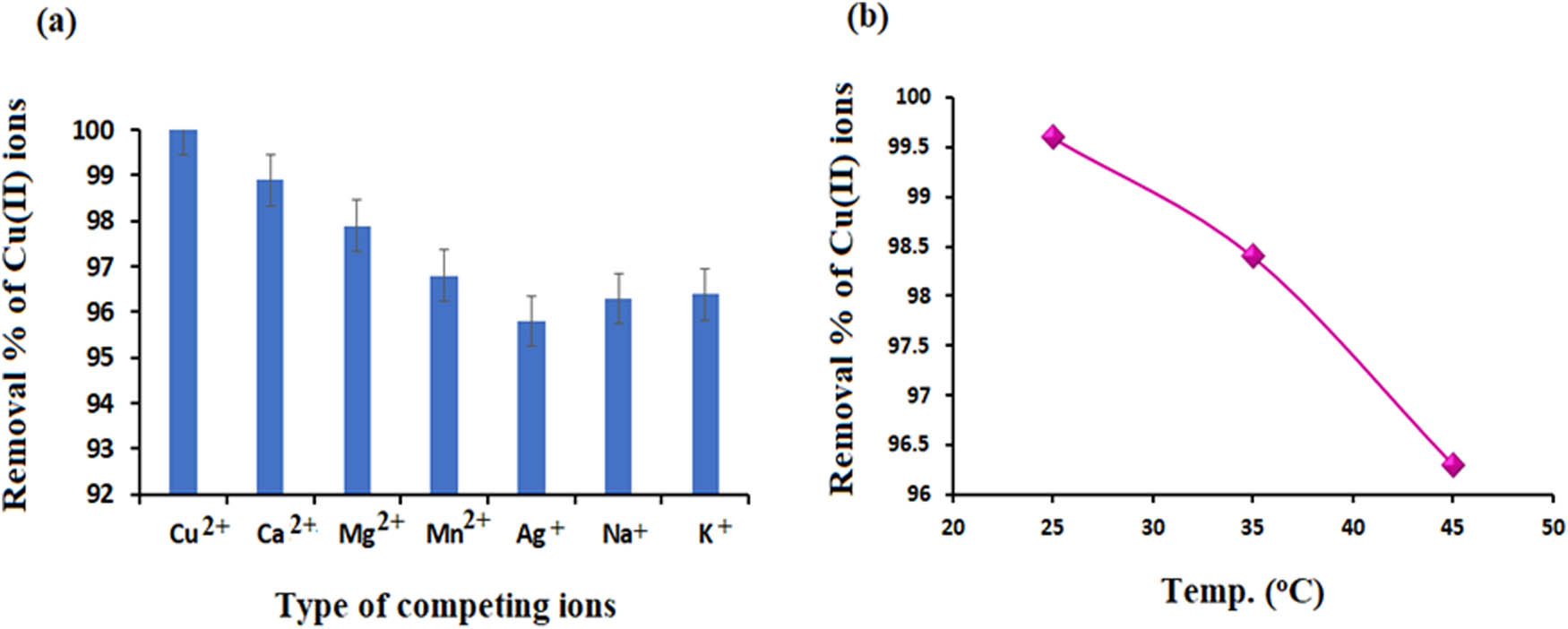
(a) Removal % of Cu(ii) ions in the presence of various cations (b) The effect of temperature in the range from 25°C to 45°C on the removal % of Cu(ii) ions.
3.7.1.6 Effect of different temperatures
There are two ways that temperature affects the adsorption process. As the viscosity of the solution reduces, it is known that a rise in temperature speeds up the dispersion of the adsorbed molecules between the inner pores and the outer layer of the ZnO NPs. Furthermore, for a given adsorbate, the temperature alters the adsorbent’s equilibrium capacity [56]. 0.5 g of the researched sorbent was used to remove 200 mg·L−1 Cu(ii) ions from aqueous solutions. The temperature effect was evaluated at various temperatures between 25°C and 45°C, with a pH of 6.2, a stirring speed of 150 rpm, and a contact period of 90 min.
The data showed that the percentage of dissolved Cu(ii) ions decreased slightly with increasing temperature (Figure 16b). The Cu(ii) adsorption capacity dropped from 99.9% to 98.75% when the temperature was increased from 25°C to 45°C. This trend indicated that the sorption of Cu(ii) from aqueous solutions is an exothermic process. The Le-Chatelier principle [57] states that an increase in the mobility of metal ions can facilitate the reverse desorption process, which in turn can explain the decrease in copper(ii) absorption at high temperatures.
3.8 Adsorption kinetics
To understand the correlation between adsorption time, concentration, and equilibrium adsorption capacity, an understanding of adsorption kinetic models is required. The control steps of the adsorption rate of ZnO NPs can be understood using the pseudo first-order Eq. 8.
The kinetics of Cu(ii) ions adsorption on the surface of ZnO NPs was also investigated utilizing Eq. 9 of pseudo-second order.
where the values q
e (mg·g−1) and q
t
(mg·g−1) represent the adsorption capacity of ZnO NPs on Cu(ii) ions at adsorption equilibrium and at a specific time point. However, the K
1 (h−1) and K
2 (g·mg−1·h−1) correspond to the first- and second-pseudo-order rate constants [58]. Figure 17 shows the linear plots of pseudo-first-order ln

(a) Pseudo-first-order and (b) pseudo-second-order Cu(ii) ions removal using ZnO NPs.
The intercepts and slopes were utilized to estimate the kinetic values of the Cu(ii) ion adsorption on the surface of ZnO NPs. The kinetics of Cu(ii) ion adsorption onto nanomaterials were investigated using two models: pseudo-first order and pseudo-second order. Table 4 provides an overview of the calculated parameters.
Adsorption values of kinetic models
| Initial Conc. of Cu(ii) solution (mg·L−1) | Pseudo-first order | ||
|---|---|---|---|
| 200 | q e (mg·g−1) | K 1 (min−1) | R 2 |
| 65.19 | −0.00028 | 0.9262 | |
| Pseudo-second order | |||
| q e (mg·g−1) | K 2 (g·mg−1·h−1) | R 2 | |
| 4.861 | 0.9818 | 0.9958 | |
The most appropriate model was selected based on the correlation coefficient (R 2) and the numerical matching between the tested and estimated qe values. The pseudo-second-order kinetic model for the above sorbent provides a better explanation for the experimental results. The adsorption capacity was 77.39 mg·g−1 for ZnO NPs which was confirmed by the correlation coefficient R 2 which has a value of 0.9958.
3.9 Adsorption isotherms
To evaluate and understand the adsorption system between both adsorbent and adsorbate, adsorption isotherms were studied [59].
Langmuir adsorption isotherm model (10):
Freundlich adsorption isotherm model (11):
where C e is the equilibrium concentration of Cu(ii) ions (mg·L−1), q e and Q m are the adsorption capacity and saturation adsorption capacity (mg·g−1), respectively, and K L is the Langmuir constant (L·mg−1). A Freundlich constant (K F, L·mg−1) is a constant that might represent the saturated adsorption quantity and 1/n is the adsorption intensity.
The adsorption mechanism was studied in detail during the sorption process using the adsorption isotherm model. The adsorption effect was significantly influenced by the temperature of the adsorption environment. Compared to a diagram generated using the Freundlich adsorption isotherm model, a diagram generated using the Langmuir model is more similar to the experimental diagram.
The recorded results are summarized in Table 5. The slope and intercept of the linear graphs were calculated (Figure 18a). The values for R L (0.0002 L·mg−1), the constant K L (169.7 L·mg−1), and Q max (100.0 mg·g−1) were determined. The regression coefficient for the correlation of the adsorbent was 0.9943. The Langmuir isotherm model described better removal of Cu(ii) ions when using ZnO NPs, with a higher Langmuir correlation coefficient (r 2). For the Freundlich adsorption isotherm model, the K F values (1.403), 1/n (1.1897), and correlation coefficient (0.9905) were estimated (Figure 18b).
Adsorption isotherm parameters determination using Langmuir and Freundlich isotherms for Cu(ii) ions adsorption on ZnO NPs surface
| Adsorbents | Langmuir isotherm | Freundlich isotherm | |||||
|---|---|---|---|---|---|---|---|
| Q max (mg·g−1) | K L (L·mg−1) | R L (L·mg−1) | R 2 | K F (mg·g−1) | 1/n | R 2 | |
| ZnO NPs | 100.0 | 169.7 | 0.0002 | 0.9943 | 1.403 | 1.1897 | 0.9905 |

(a) Langmuir and (b) Freundlich adsorption isotherms for copper removal using ZnO NPs.
3.10 Thermodynamic studies
Standard enthalpy (∆H°, kJ·mol−1), standard entropy (∆S°, kJ·mol−1), and change in Gibbs free energy (∆G°, kJ·mol−1) are the thermodynamic parameters that are taken into account to ascertain the spontaneity and other aspects of a given adsorption process. Five different temperatures 298, 308, 318, 328, and 333 K were used in this study. The adsorption thermodynamics were estimated using the following formulas:
where R is the molar gas constant (8.314 JK·mol−1), T is the temperature (K), and K c is the thermodynamic constant.
Figure 19 shows the thermodynamic plot of Cu(ii) ions adsorption on the surface of ZnO NPs. The slope and intercept of the given graph were used to calculate the values of ∆H° and ∆S°. The chemisorption method or physical adsorption was examined using the parameter ∆G°. When the ∆G° value is negative, adsorption happens on its own. The positive ∆H° readings indicate endothermic process. During adsorption, an increase in disorder at the solid–liquid interface is shown by positive ∆S° values [60]. The obtained results were found to be ∆H° value (−4,127.4 J·mol−1), ∆S° (−88.5774 kJ·mol−1), and ∆G° values (−9,910.29, −11,523.2, −12,928.4, −14,998.5, and −16,556 J·mol−1), for the abovementioned temperature degrees, respectively. The negative ∆H° value indicated the exothermic adsorption process. The affinity of adsorbent materials for retaining Cu(ii) ions on their surfaces consistently with limited mobilities of copper ions was revealed by a negative entropy of adsorption. The negative ∆G° values indicated that the elimination process of Cu(ii) ions on the sorbent is a physical process, spontaneous, with electrostatic interaction, and does not require a time of induction (Table 6).

Thermodynamic graph of copper removal using ZnO NPs as sorbent.
Thermodynamic data of copper adsorption on ZnO NPs
| Adsorbents | C 0 (mg·L−1) | T (K) | ∆G° (J·mol−1) | ∆H° (J·mol−1) | ∆S° JK−1·mol−1 | R 2 |
|---|---|---|---|---|---|---|
| ZnO NPs | 200 | 298 | −9,910.29 | −4,127.4 | −88.5774 | 0.9949 |
| 308 | −11,523.2 | |||||
| 318 | −12,928.5 | |||||
| 328 | −14,998.5 | |||||
| 333 | −16,556.0 |
3.11 Elimination of copper from wastewater samples and reusability of adsorbents
In this study, five wastewater samples containing a Cu(ii) ion concentration of 200 mg·L−1 were subjected to treatment with ZnO NPs (0.5 g·L−1). The main objective was to evaluate the effectiveness of ZnO NPs in removing Cu(ii) ions from wastewater. After treatment, the results showed a significant reduction in Cu(ii) concentration, indicating that the ZnO NPs are very effective in adsorbing and precipitating these ions. Various factors may have contributed to this effectiveness, including the high surface area of the NPs, which enhances interaction with the Cu(ii) ions, and their potential catalytic properties that could facilitate the removal process.
ZnO NPs show significant antibacterial efficacy in wastewater treatment due to several key properties as stated earlier [42–48]. Their antimicrobial mechanism primarily involves the generation of ROS which can damage bacterial cell membranes, DNA, and proteins, ultimately leading to cell death. The high surface-to-volume ratio of ZnO NPs improves their interaction with bacteria, increases adsorption, and facilitates more effective antimicrobial action [42]. Factors such as the size and shape of the NPs play a crucial role, with smaller particles often exhibiting greater efficacy due to their increased surface area and ability to penetrate bacterial cells more effectively [42–48]. Additionally, the concentration of ZnO NPs in wastewater is critical; higher concentrations generally yield greater bacterial inhibition, although there exists a threshold beyond which further increases may not enhance effectiveness and could potentially be toxic to beneficial microorganisms. However, the emergence of bacterial resistance is a concern, as excessive or continuous use of ZnO NPs may lead to the development of resistant strains, necessitating careful management.
Additionally, the regeneration of the synthesized ZnO NPs was also studied. The regeneration study was conducted by sonicating the filtrated sorbent with 3 mL of ethanol followed by 3 mL of DI water, filtered, and then oven dried at 100°C for 1 h. The starting elimination effectiveness was assumed to be 100%, and the consequent performance was approximated. The average efficiency of ZnO NPs was 96.75% with relative standard deviation values of 0.52%. However, the lowest efficiency was 76.32% for the above sorbent. These results indicated that the penetration of copper ions into the internal sorbent sites was easy and hindered the recovery (Figure 20).

Regeneration % of sorbent after five removal cycles.
3.12 Potential limitations for industrial applications
This study has some limitations that should be considered for future applications. First, the scalability of the biogenic synthesis process for industrial use is a crucial factor. While the current method effectively produces ZnO and NiO NPs at laboratory scale, scaling up to larger quantities suitable for commercial applications poses challenges related to cost, production time, and consistent quality. The efficiency of camel milk as a stabilizing agent on a larger scale needs to be evaluated to ensure that the synthesis process remains economically viable. Second, the long-term stability of the synthesized NPs in real wastewater environments is another important aspect. The effectiveness of ZnO and NiO NPs can be influenced by factors such as pH fluctuations, temperature changes, and the presence of organic matter or competing ions in actual wastewater. Research is necessary to determine how these variables impact the structural integrity, reactivity, and overall performance of the NPs over time. Moreover, assessing potential leaching of the NPs into the environment is vital, as this could pose environmental risks. Evaluating the performance of these NPs in diverse wastewater conditions will provide insight into their practical applicability and durability, ensuring that they can deliver sustained results in real-world scenarios.
4 Conclusion
In this study, the biogenic synthesis of ZnO and NiO NPs using camel milk was demonstrated, emphasizing their potential against bacterial resistance and heavy metal removal from aqueous solutions in an accessible and environmentally friendly method. ZnO NPs showed significant antimicrobial activity with inhibition zones of 16 mm against Pseudomonas aeruginosa and 13 mm against Candida tropicalis, while NiO NPs showed lower efficacy against the selected bacteria in the study. Additionally, ZnO NPs removed 96.76% of Cu(ii) ions at a pH of 8.4 in 90 min at a concentration of 0.5 g·L−1 and an initial Cu(ii) concentration of 200 mg·L−1. The adsorption kinetics followed a pseudo-second-order model, and the isothermal data were consistent with the Langmuir model, with a maximum capacity (Q max) of 100.0 mg·g−1 and R 2 = 0.9905. Thermodynamic analysis revealed an exothermic process (∆H° = −4,127.4 J·mol−1) and spontaneous physical adsorption. These results indicate that ZnO- and NiO-NPs are promising candidates for future environmental and clinical applications.
Acknowledgements
The authors provide great appreciation for Researchers Supporting Project in King Saud University for supporting this study.
-
Funding information: This research was funded by Researchers Supporting Project in King Saud University and the code number is (RSP2024R247).
-
Author contributions: Amal Mohamed Al-Mohaimeed: conceptualization, supervision, and funding acquisition; Maha Farouk El-Tohamy: method of analysis, investigations, and writing – original draft; Nadine Mohamad Safouh Moubayed: method of analysis, investigations, data curation, validation, writing, and editing the manuscript. All authors agreed on the last version of the manuscript.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: All data generated or analyzed during this study are included in this published article (and its supplementary information files).
References
[1] Espinoza-Guillen JA, Alderete-Malpartida MB, Gallegos-Huamán RI, Paz-Rosales YM, Domínguez-Vivar RM, Bujaico-Leon C. Ecological risk assessment and identification of sources of heavy metals contamination in sewage sludge from municipal wastewater treatment plants in the metropolitan area of Lima-Callao, Peru. Env Dev Sustain. 2022;26:1559. 10.1007/s10668-022-02774-w.Search in Google Scholar
[2] Marufi N, Oliveri Conti G, Ahmadinejad P, Ferrante M, Mohammadi AA. Carcinogenic and non-carcinogenic human health risk assessments of heavy metals contamination in drinking water supplies in Iran: a systematic review. Rev Env Health. 2024;39:91. 10.1515/reveh-2022-0060.Search in Google Scholar PubMed
[3] Yang F, Liao J, Yu W, Pei R, Qiao N, Han Q, et al. Copper induces oxidative stress with triggered NF-κB pathway leading to inflammatory responses in immune organs of chicken. Ecotoxicol Env Saf. 2020;200:110715. 10.1016/j.ecoenv.2020.110715.Search in Google Scholar PubMed
[4] Vellingiri B, Suriyanarayanan A, Selvaraj P, Abraham KS, Pasha MY, Winster H, et al. Role of heavy metals (copper (Cu), arsenic (As), cadmium (Cd), iron (Fe) and lithium (Li)) induced neurotoxicity. Chemosphere. 2022;301:134625. 10.1016/j.chemosphere.2022.134625.Search in Google Scholar PubMed
[5] Komijani M, Shamabadi NS, Shahin K, Eghbalpour F, Tahsili MR, Bahram M. Heavy metal pollution promotes antibiotic resistance potential in the aquatic environment. Env Poll. 2021;274:116569. 10.1016/j.envpol.2021.116569.Search in Google Scholar PubMed
[6] Rajendran R, Mani AJ. Photocatalytic, antibacterial and anticancer activity of silver-doped zinc oxide nanoparticles. Saud Chem Soc. 2020;24:1010. 10.1016/j.jscs.2020.10.008.Search in Google Scholar
[7] Salem SS. A mini review on green nanotechnology and its development in biological effects. Arch Microbiol. 2023;205:128. 10.1007/s00203-023-03467-2.Search in Google Scholar PubMed PubMed Central
[8] Sukri SN, Shameli K, Wong MM, Teow SY, Chew J, Ismail NA. Cytotoxicity and antibacterial activities of plant-mediated synthesized zinc oxide (ZnO) nanoparticles using Punica granatum (pomegranate) fruit peels extract. J Mol Struct. 2019;1189:57. 10.1016/j.molstruc.2019.04.026.Search in Google Scholar
[9] Abdussalam-Mohammed W. Comparison of chemical and biological properties of metal nanoparticles (Au, Ag), with metal oxide nanoparticles (ZnO-NPs) and their applications. Adv J Chem Sect A. 2020;3:111. 10.33945/SAMI/AJCA.2020.2.8.Search in Google Scholar
[10] Sadhasivam S, Shanmugam M, Umamaheswaran PD, Venkattappan AA. Zinc oxide nanoparticles: green synthesis and biomedical applications. J Clust Sci. 2021;32:1441. 10.1007/s10876-020-01918-0.Search in Google Scholar
[11] Lu J, Wang Y, Jin M, Yuan Z, Bond P, Guo J. Both silver ions and silver nanoparticles facilitate the horizontal transfer of plasmid-mediated antibiotic resistance genes. Water Res. 2020;169:115229. 10.1016/j.watres.2019.115229.Search in Google Scholar PubMed
[12] Younas M, Rezakazemi M, Daud M, Wazir MB, Ahmad S, Ullah N, et al. Recent progress and remaining challenges in post-combustion CO2 capture using metal–organic frameworks (MOFs). Prog Energy Comb Sci. 2020;80:100849. 10.1016/j.pecs.2020.100849.Search in Google Scholar
[13] Akpomie KG, Conradie J, Adegoke KA, Oyedotun KO, Ighalo JO, Amaku JF, et al. Adsorption mechanism and modeling of radionuclides and heavy metals onto ZnO nanoparticles: a review. Appl Water Sci. 2023;13:20. 10.1007/s13201-022-01827-9.Search in Google Scholar
[14] Yang X, Ma X, Yuan J, Feng X, Zhao Y, Chen L. Enhanced the antifouling and antibacterial performance of PVC/ZnO‐CMC nanoparticles ultrafiltration membrane. J Appl Polym Sci. 2023;140:e53412. 10.1002/app.53412.Search in Google Scholar
[15] Alkhanjaf AA, Sharma S, Sharma M, Kumar R, Arora NK, Kumar B, et al. Microbial strategies for copper pollution remediation: Mechanistic insights and recent advances. Env Poll. 2024;346:123588. 10.1016/j.envpol.2024.123588.Search in Google Scholar PubMed
[16] Pesce S, Mamy L, Sanchez W, Artigas J, Bérard A, Betoulle S, et al. The use of copper as plant protection product contributes to environmental contamination and resulting impacts on terrestrial and aquatic biodiversity and ecosystem functions. Env Sci Poll Res. 2024;32:1–7. 10.1007/s11356-024-32145-z.Search in Google Scholar PubMed
[17] Rafati Rahimzadeh M, Rafati Rahimzadeh M, Kazemi S, Moghadamnia AA. Copper poisoning with emphasis on its clinical manifestations and treatment of intoxication. Adv Public Health. 2024;2024(1):6001014. 10.1155/2024/6001014.Search in Google Scholar
[18] Dehkordi MM, Nodeh ZP, Dehkordi KS, Khorjestan RR, Ghaffarzadeh M. Soil, air, and water pollution from mining and industrial activities: sources of pollution, environmental impacts, and prevention and control methods. Results Eng. 2024;23:102729. 10.1016/j.rineng.2024.102729.Search in Google Scholar
[19] Rachwał K, Gustaw K. Lactic acid bacteria in sustainable food production. Sustainability. 2024;16(8):3362. 10.3390/su16083362.Search in Google Scholar
[20] Mittal A, Mahala N, Dhanawade NH, Dubey SK, Dubey US. Evaluation of the cytotoxic activity of sorafenib‐loaded camel milk casein nanoparticles against hepatocarcinoma cells. Biotechnol J. 2024;19(3):2300449. 10.1002/biot.202300449.Search in Google Scholar PubMed
[21] ElMosbah DE, Ibrahim M, Khalil H, El-Asssal M, El Miniawy H. The improvement potential of camel milk whey as a natural remedy in comparison with Rivastigmine chitosan-loaded nanoparticles in aluminum chloride induced Alzheimer-like disease in rats. Egypt J Vet Sci. 2024;55(5):1435–46. 10.21608/ejvs.2024.260943.1768.Search in Google Scholar
[22] Tarbiah N, Alkhattabi N, Baz LA, Al Mokhashab M, Afsa HA, Alhusayni N, et al. Comparing between Camel’s Milk and Bovine’s Milk antioxidant activity using DPPH method. Mod Phytomorphol. 2024;19:70–7. 10.5281/zenodo.200121.Search in Google Scholar
[23] Feisal NA, Kamaludin NH, Ahmad MA, Ibrahim TN. A comprehensive review of nanomaterials for efficient heavy metal ions removal in water treatment. J Water Process Eng. 2024;64:105566. 10.1016/j.jwpe.2024.105566.Search in Google Scholar
[24] Omdehghiasi H, Korayem AH, Yeganeh-Bakhtiary A. Highly efficient green-synthesized sucrose-derived graphene silica and carbonate sand composites for copper(ii) ions removal. Int J Env Sci Technol. 2024;22:1–8. 10.1007/s13762-024-06078-6.Search in Google Scholar
[25] Hegazi A, Elshazly EH, Abdou AM, Abd Allah F, Abdel-Rahman EH. Potential antibacterial properties of silver nanoparticles conjugated with cow and camel milks. Glob Veterinaria. 2014;12:745. 10.5829/idosi.gv.2014.12.06.83198.Search in Google Scholar
[26] Flis Z, Szatkowski P, Pielichowska K, Molik E. The potential of sheep or camel milk constituents to contribute to novel dressings for diabetic wounds. Int J Mol Sci. 2023;24:17551. 10.3390/ijms242417551.Search in Google Scholar PubMed PubMed Central
[27] Meng F, Duan M, Wu W, Shao S, Qin Y, Zhang M. Enzymatic construction Au NPs-rGO based MIP electrochemical sensor for adulteration detection of bovine-derived allergen in camel milk. Food Chem. 2024;436:137638. 10.1016/j.foodchem.2023.137638.Search in Google Scholar PubMed
[28] Jagan KS, Surendhiran S, Savitha S, Balu KS, Karthik A. Distinct nickel-precursor synthesized NiO NPs on the degradation of biological staining dyes: a green route. J Mater Sci Mater Electron. 2024;35:169. 10.1007/s10854-023-11846-0.Search in Google Scholar
[29] PD DA, Plashintania DR, Putri RM, Wibowo I, Ramli Y, Herdianto S, et al. Synthesis of zinc oxide nanoparticles using methanol propolis extract (Pro-ZnO NPs) as antidiabetic and antioxidant. PLoS One. 2023;18:e0289125. 10.1371/journal.pone.0289125.Search in Google Scholar PubMed PubMed Central
[30] Chandekar KV, Palanivel B, Alkallas FH, Trabelsi AB, Khan A, Ashraf IM, et al. Photocatalytic activities of Mg doped NiO NPs for degradation of Methylene blue dye for harmful contaminants: A kinetics, mechanism and recyclability. J Phys Chem Solids. 2023;178:111345. 10.1016/j.jpcs.2023.111345.Search in Google Scholar
[31] Yao Z, Zhang X, Nie P, Lv H, Yang Y, Zou W, et al. Identification of milk adulteration in camel milk using FT-Mid-Infrared spectroscopy and machine learning models. Foods. 2023;12:4517, https://www.mdpi.com/2304-8158/12/24/4517#.10.3390/foods12244517Search in Google Scholar PubMed PubMed Central
[32] Krishnamoorthy N, Sivasankarapillai VS, Natarajan VK, Eldesoky GE, Wabaidur SM, Eswaran M, et al. Biocidal activity of ZnO NPs against pathogens and antioxidant activity-a greener approach by Citrus hystrix leaf extract as bio-reductant. Biochem Eng J. 2023;192:108818. 10.1016/j.bej.2023.108818.Search in Google Scholar
[33] Fang Z, Fan H, Zhao X, Lin G, Li B, Wang J, et al. Unveiling the nature of glucose hydrogenation over Raney Ni: DFT and AIMD simulations. Appl Catal A: Gen. 2023;667:119462. 10.1016/j.apcata.2023.119462.Search in Google Scholar
[34] Khan M, Alam MS, Ahmed SF. Effect of nickel incorporation on structural and optical properties of zinc oxide thin films deposited by RF/DC sputtering technique. Mater Phys Mech. 2023;51:19. 10.18149/MPM.5112023_3.Search in Google Scholar
[35] Adeyemi JO. Kei-apple-mediated NiO nanoparticles and biological studies: anti-inflammatory and cytotoxicity study against HeLa and HEK 293 cell lines. Mater Res Exp. 2023;10:075401. 10.1088/2053-1591/ace29e.Search in Google Scholar
[36] Chan YB, Selvanathan V, Tey LH, Akhtaruzzaman M, Anur FH, Djearamane S, et al. Effect of calcination temperature on structural, morphological and optical properties of copper oxide nanostructures derived from Garcinia mangostana L. leaf extract. Nanomaterials. 2022;12(20):3589. 10.3390/nano12203589.Search in Google Scholar PubMed PubMed Central
[37] Tzu FM, Hsu HS, Chen JS. Non-contact optical detection of foreign materials adhered to color filter and thin-film transistor. Micromachines. 2022;13:101. 10.3390/mi13010101.Search in Google Scholar PubMed PubMed Central
[38] Semysim FA, Hussain BK, Hussien MA, Azooz EA, Snigur D. Assessing the greenness and environmental friendliness of analytical methods: Modern approaches and recent computational programs. Crit Rev Anal Chem. 2024;12:1. 10.1080/10408347.2024.2304552.Search in Google Scholar PubMed
[39] Khan MZ, Xiao J, Ma Y, Ma J, Liu S, Khan A, et al. Research development on anti-microbial and antioxidant properties of camel milk and its role as an anti-cancer and anti-hepatitis agent. Antioxidants. 2021;10:788. 10.3390/antiox10050788.Search in Google Scholar PubMed PubMed Central
[40] Selvanathan V, Aminuzzaman M, Tan LX, Eddy YFW, Cheah SG, Heng MH, et al. Synthesis, characterization, and preliminary in vitro antibacterial evaluation of ZnO nanoparticles derived from soursop (Annona muricata L.) leaf extract as a green reducing agent. J Mater Res Technol. 2022;20:2931–41.10.1016/j.jmrt.2022.08.028Search in Google Scholar
[41] Mthana MS, Mthiyane MN, Ekennia AC, Singh M, Onwudiwe D. Cytotoxicity and antibacterial effects of silver doped zinc oxide nanoparticles prepared using fruit extract of Capsicum chinense. Sci Afr. 2022;17:e01365. 10.1016/j.sciaf.2022.e01365.Search in Google Scholar
[42] Chan YB, Aminuzzaman M, Rahman Md K, Win YF, Sultana S, Cheah S-Y, et al. Green synthesis of ZnO nanoparticles using the mangosteen (Garcinia mangostana L.) leaf extract: Comparative preliminary in vitro antibacterial study. Green Process Synth. 2024;13:20230251. 10.1515/gps-2023-0251.Search in Google Scholar
[43] Khan MU, Pirzadeh M, Förster CY, Shityakov S, Shariati MA. Role of milk-derived antibacterial peptides in modern food biotechnology: Their synthesis, applications and future perspectives. Biomolecules. 2018;8:110. 10.3390/biom8040110.Search in Google Scholar PubMed PubMed Central
[44] Khater MS, Kulkarni GR, Khater SS, Gholap H, Patil R. Study to elucidate effect of titanium dioxide nanoparticles on bacterial membrane potential and membrane permeability. Mater Res Exp. 2020;7:035005. 10.1088/2053-1591/ab731a.Search in Google Scholar
[45] Mammari N, Lamouroux E, Boudier A, Duval RE. Current knowledge on the oxidative-stress-mediated antimicrobial properties of metal-based nanoparticles. Microorganisms. 2022;10:5437. 10.3390/microorganisms10020437.Search in Google Scholar PubMed PubMed Central
[46] Akhtar S, Shahzad K, Mushtaq S, Ali I, Rafe MH, Fazal-ul-Karim SM. Antibacterial and antiviral potential of colloidal Titanium dioxide (TiO2) nanoparticles suitable for biological applications. Mater Res Exp. 2019;6:105409. 10.1088/2053-1591/ab3b27.Search in Google Scholar
[47] Habeeb SA, Hammadi AH, Abed D, Al-Jibouri LF. Green synthesis of metronidazole or clindamycin-loaded hexagonal zinc oxide nanoparticles from Ziziphus extracts and its antibacterial activity. Pharmacia. 2022;69:855. 10.3897/pharmacia.69.e91057.Search in Google Scholar
[48] Singh A, Goyal V, Singh J, Kaur H, Kumar S, Batoo KM, et al. Structurally and morphologically engineered single-pot biogenic synthesis of NiO nanoparticles with enhanced photocatalytic and antimicrobial activities. J Clean Prod. 2022;343:131026. 10.1016/j.jclepro.2022.131026.Search in Google Scholar
[49] Makabenta JM, Nabawy A, Li CH, Schmidt-Malan S, Patel R, Rotello VM. Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections. Nat Rev Microbiol. 2021;19:23. 10.1038/s41579-020-0420-1.Search in Google Scholar PubMed PubMed Central
[50] Duval RE, Gouyau J, Lamouroux E. Limitations of recent studies dealing with the antibacterial properties of silver nanoparticles: Fact and opinion. Nanomaterials. 2019;9:1775. 10.3390/nano9121775.Search in Google Scholar PubMed PubMed Central
[51] Alterary SS, Al-Alshaikh MA, Elhadi AM, Cao W. Design, synthesis, and evaluation of novel magnetic nanoparticles combined with thiophene derivatives for the removal of Cr (VI) from an aqueous solution. ACS Omega. 2024;9:7835, https://pubs.acs.org/toc/acsodf/9/7.10.1021/acsomega.3c07517Search in Google Scholar PubMed PubMed Central
[52] Pavithra S, Thandapani G, Sugashini S, Sudha PN, Alkhamis HH, Alrefaei AF, et al. Batch adsorption studies on surface tailored chitosan/orange peel hydrogel composite for the removal of Cr(VI) and Cu(II) ions from synthetic wastewater. Chemosphere. 2021;271:129415. 10.1016/j.chemosphere.2020.129415.Search in Google Scholar PubMed
[53] Kaya N, Erdem F. Modeling of copper removal from aqueous solutions by using carbon-based adsorbents derived from hazelnut and walnut shells by artificial neural network. Sigma J Eng Nat Sci. 2022;40:695. 10.14744/sigma.2022.00085.Search in Google Scholar
[54] Balciunaitiene A, Liaudanskas M, Puzeryte V, Viskelis J, Janulis V, Viskelis P, et al. Eucalyptus globulus and Salvia officinalis extracts mediated green synthesis of silver nanoparticles and their application as an antioxidant and antimicrobial agent. Plants. 2022;11:1085. 10.3390/plants11081085.Search in Google Scholar PubMed PubMed Central
[55] Adeeyo RO, Edokpayi JN, Bello OS, Adeeyo AO, Odiyo JO. Influence of selective conditions on various composite sorbents for enhanced removal of copper (II) ions from aqueous environments. Int J Env Res Public Health. 2019;16:4596. 10.3390/ijerph16234596.Search in Google Scholar PubMed PubMed Central
[56] Whitehead B, Brennessel WW, Michtavy SS, Silva HA, Kim J, Milner PJ, et al. Selective adsorption of fluorinated super greenhouse gases within a metal–organic framework with dynamic corrugated ultramicropores. Chem Sci. 2024;15:5964. 10.1039/D3SC07007G.Search in Google Scholar
[57] Phey ML, Abdullah TA, Ali UF, Mohamud MY, Ikram M, Nabgan W. Reverse water gas shift reaction over a Cu/ZnO catalyst supported on regenerated spent bleaching earth (RSBE) in a slurry reactor: the effect of the Cu/Zn ratio on the catalytic activity. RSC Adv. 2023;13:3039. 10.1039/D2RA07617A.Search in Google Scholar PubMed PubMed Central
[58] Onursal N, Dal MC. Investigation of isotherm and thermodynamic parameters of adsorption of copper (II) ions in aqueous solution with natural mixed type Siirt clay (NMTSC-2) and new (second) linear equation derived from Harkins–Jura isotherm. Chem Pap. 2024;78:749. 10.1007/s11696-023-03116-4.Search in Google Scholar
[59] Alterary S, Amina M, El-Tohamy M. Impact of silver-doped alumina nanocomposite on water decontamination by remodeling of biogenic waste. Env Sci Poll Res. 2023;30(31):77044–62. 10.1007/s11356-023-27941-y.Search in Google Scholar PubMed
[60] Alarfaj N, Al Musayeib N, Amina M, El-Tohamy M. Synthesis and characterization of polysiphonia/cerium oxide/nickel oxide nanocomposites for the removal of toxins from contaminated water and antibacterial potential. Env Sci Poll Res. 2024;31(11):17064–96. 10.1007/s11356-024-32199-z.Search in Google Scholar PubMed
© 2025 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Synthesis of N,S co-doped carbon quantum dots – metal complex for the detection of fluoride (F−) ion in adults and Children’s toothpastes
- Research Articles
- Optimized green synthesis of silver nanoparticles from guarana seed skin extract with antibacterial potential
- Green adsorbents for water remediation: Removal of Cr(vi) and Ni(ii) using Prosopis glandulosa sawdust and biochar
- Green approach for the synthesis of zinc oxide nanoparticles from methanolic stem extract of Andrographis paniculata and evaluation of antidiabetic activity: In silico GSK-3β analysis
- Development of a green and rapid ethanol-based HPLC assay for aspirin tablets and feasibility evaluation of domestically produced bioethanol in Thailand as a sustainable mobile phase
- A facile biodegradation of polystyrene microplastic by Bacillus subtilis
- Enhanced synthesis of fly ash-derived hydrated sodium silicate adsorbents via low-temperature alkaline hydrothermal treatment for advanced environmental applications
- Impact of metal nanoparticles biosynthesized using camel milk on bacterial growth and copper removal from wastewater
- Preparation of Co/Cr-MOFs for efficient removal of fleroxacin and Rhodamine B
- Applying nanocarbon prepared from coal as an anode in lithium-ion batteries
- Improved electrochemical synthesis of Cu–Fe/brass foil alloy followed by combustion for high-efficiency photoelectrodes and hydrogen production in alkaline solutions
- Precipitation of terephthalic acid from post-consumer polyethylene terephthalate waste fractions
- Biosynthesized zinc oxide nanoparticles: Multifunctional potential applications in anticancer, antibacterial, and B. subtilis DNA gyrase docking
- Anticancer and antimicrobial effects of green-synthesized silver nanoparticles using Teucrium polium leaves extract
- Green synthesis of eco-friendly bioplastics from Chlorella and Lithothamnion algae for safe and sustainable solutions for food packaging
- Optimizing coal water slurry concentration via synergistic coal blending and particle size distribution
- Green synthesis of Ag@Cu and silver nanowire using Pterospermum heterophyllum extracts for surface-enhanced Raman scattering
- Green synthesis of copper oxide nanoparticles from Algerian propolis: Exploring biochemical, structural, antimicrobial, and anti-diabetic properties
- Simultaneous quantification of mefenamic acid and paracetamol in fixed-dose combination tablet dosage forms using the green HPTLC method
- Green synthesis of titanium dioxide nanoparticles using green tea (Camellia sinensis) extract: Characteristics and applications
- Pharmaceutical properties for green fabricated ZnO and Ag nanoparticle-mediated Borago officinalis: In silico predications study
- Synthesis and optimization of gemcitabine-loaded nanoparticles by using Box–Behnken design for treating prostate cancer: In vitro characterization and in vivo pharmacokinetic study
- A comparative analysis of single-step and multi-step methods for producing magnetic activated carbon from palm kernel shells: Adsorption of methyl orange dye
- Sustainable green synthesis of silver nanoparticles using walnut septum waste: Characterization and antibacterial properties
- Efficient electrocatalytic reduction of CO2 to CO over Ni/Y diatomic catalysts
- Greener and magnetic Fe3O4 nanoparticles as a recyclable catalyst for Knoevenagel condensation and degradation of industrial Congo red dye
- Recycling of HDPE-giant reed composites: Processability and performance
- Fabrication of antibacterial chitosan/PVA nanofibers co-loaded with curcumin and cefadroxil for wound healing
- Cost-effective one-pot fabrication of iron(iii) oxychloride–iron(iii) oxide nanomaterials for supercapacitor charge storage
- Novel trimetallic (TiO2–MgO–Au) nanoparticles: Biosynthesis, characterization, antimicrobial, and anticancer activities
- Green-synthesized chromium oxide nanoparticles using pomegranate husk extract: Multifunctional bioactivity in antioxidant potential, lipase and amylase inhibition, and cytotoxicity
- Therapeutic potential of sustainable zinc oxide nanoparticles biosynthesized using Tradescantia spathacea aqueous leaf extract
- Chitosan-coated superparamagnetic iron oxide nanoparticles synthesized using Carica papaya bark extract: Evaluation of antioxidant, antibacterial, and anticancer activity of HeLa cervical cancer cells
- Antioxidant potential of peptide fractions from tuna dark muscle protein isolate: A green enzymatic approach
- Clerodendron phlomoides leaf extract-mediated synthesis of selenium nanoparticles for multi-applications
- Optimization of cellulose yield from oil palm trunks with deep eutectic solvents using response surface methodology
- Nitrogen-doped carbon dots from Brahmi (Bacopa monnieri): Metal-free probe for efficient detection of metal pollutants and methylene blue dye degradation
- High energy density pseudocapacitor based on a nanoporous tungsten(VI) oxide iodide/poly(2-amino-1-mercaptobenzene) composite
- Green synthesized Ag–Cu nanocomposites as an improved strategy to fight multidrug-resistant bacteria by inhibition of biofilm formation: In vitro and in silico assessment study
- In vitro evaluation of antibacterial activity and associated cytotoxicity of biogenic silver nanoparticles using various extracts of Tabernaemontana ventricosa
- Fabrication of novel composite materials by impregnating ZnO particles into bacterial cellulose nanofibers for antimicrobial applications
- Solidification floating organic drop for dispersive liquid–liquid microextraction estimation of copper in different water samples
- Kinetics and synthesis of formation of phosphate composites from low-grade phosphorites in the presence of phosphate–siliceous shales and oil sludge
- Removal of minocycline and terramycin by graphene oxide and Cr/Mn base metal–organic framework composites
- Microfluidic preparation of ceramide E liposomes and properties
- Therapeutic potential of Anamirta cocculus (L.) Wight & Arn. leaf aqueous extract-mediated biogenic gold nanoparticles
- Antioxidant-rich Micromeria imbricata leaf extract as a medium for the eco-friendly preparation of silver-doped zinc oxide nanoparticles with antibacterial properties
- Influence of different colors with light regime on Chlorella sp., biomass, pigments, and lipids quantity and quality
- Experimental vibrational analysis of natural fiber composite reinforced with waste materials for energy absorbing applications
- Green synthesis of sea buckthorn-mediated ZnO nanoparticles: Biological applications and acute nanotoxicity studies
- Production of liquid smoke by consecutive electroporation and microwave-assisted pyrolysis of empty fruit bunches
- Synthesis of MPAA based on polyacrylamide and gossypol resin and applications in the encapsulation of ammophos
- Application of iron-based catalysts in the microwave treatment of environmental pollutants
- Enhanced adsorption of Cu(ii) from wastewater using potassium humate-modified coconut husk biochar
- Adsorption of heavy metal ions from water by Fe3O4 nano-particles
- Green synthesis of parsley-derived silver nanoparticles and their enhanced antimicrobial and antioxidant effects against foodborne resistant bacteria
- Unwrapping the phytofabrication of bimetallic silver–selenium nanoparticles: Antibacterial, Anti-virulence (Targeting magA and toxA genes), anti-diabetic, antioxidant, anti-ovarian, and anti-prostate cancer activities
- Optimizing ultrasound-assisted extraction process of anti-inflammatory ingredients from Launaea sarmentosa: A novel approach
- Eggshell membranes as green carriers for Burkholderia cepacia lipase: A biocatalytic strategy for sustainable wastewater bioremediation
- Research progress of deep eutectic solvents in fuel desulfurization
- Enhanced electrochemical synthesis of Ni–Fe/brass foil alloy with subsequent combustion for high-performance photoelectrode and hydrogen production applications
- Valorization of baobab fruit shell as a filler fiber for enhanced polyethylene degradation and soil fertility
- Valorization of Agave durangensis bagasse for cardboard-type paper production circular economy approach
- Green priming strategies using seaweed extract and citric acid to improve early growth and antioxidant activity in lentil
- Gold extraction from oxide ore using iron ion-thiourea-additive
- Development of loess-derived P-type molecular sieve as a sustainable antibacterial agent
- Review Article
- Sustainable innovations in garlic extraction: A comprehensive review and bibliometric analysis of green extraction methods
- Natural sustainable coatings for marine applications: advances, challenges, and future perspectives
- Integration of traditional medicinal plants with polymeric nanofibers for wound healing
- Rapid Communication
- In situ supported rhodium catalyst on mesoporous silica for chemoselective hydrogenation of nitriles to primary amines
- Special Issue: Valorisation of Biowaste to Nanomaterials for Environmental Applications
- Valorization of coconut husk into biochar for lead (Pb2+) adsorption
- Corrigendum
- Corrigendum to “An updated review on carbon nanomaterials: Types, synthesis, functionalization and applications, degradation and toxicity”
Articles in the same Issue
- Synthesis of N,S co-doped carbon quantum dots – metal complex for the detection of fluoride (F−) ion in adults and Children’s toothpastes
- Research Articles
- Optimized green synthesis of silver nanoparticles from guarana seed skin extract with antibacterial potential
- Green adsorbents for water remediation: Removal of Cr(vi) and Ni(ii) using Prosopis glandulosa sawdust and biochar
- Green approach for the synthesis of zinc oxide nanoparticles from methanolic stem extract of Andrographis paniculata and evaluation of antidiabetic activity: In silico GSK-3β analysis
- Development of a green and rapid ethanol-based HPLC assay for aspirin tablets and feasibility evaluation of domestically produced bioethanol in Thailand as a sustainable mobile phase
- A facile biodegradation of polystyrene microplastic by Bacillus subtilis
- Enhanced synthesis of fly ash-derived hydrated sodium silicate adsorbents via low-temperature alkaline hydrothermal treatment for advanced environmental applications
- Impact of metal nanoparticles biosynthesized using camel milk on bacterial growth and copper removal from wastewater
- Preparation of Co/Cr-MOFs for efficient removal of fleroxacin and Rhodamine B
- Applying nanocarbon prepared from coal as an anode in lithium-ion batteries
- Improved electrochemical synthesis of Cu–Fe/brass foil alloy followed by combustion for high-efficiency photoelectrodes and hydrogen production in alkaline solutions
- Precipitation of terephthalic acid from post-consumer polyethylene terephthalate waste fractions
- Biosynthesized zinc oxide nanoparticles: Multifunctional potential applications in anticancer, antibacterial, and B. subtilis DNA gyrase docking
- Anticancer and antimicrobial effects of green-synthesized silver nanoparticles using Teucrium polium leaves extract
- Green synthesis of eco-friendly bioplastics from Chlorella and Lithothamnion algae for safe and sustainable solutions for food packaging
- Optimizing coal water slurry concentration via synergistic coal blending and particle size distribution
- Green synthesis of Ag@Cu and silver nanowire using Pterospermum heterophyllum extracts for surface-enhanced Raman scattering
- Green synthesis of copper oxide nanoparticles from Algerian propolis: Exploring biochemical, structural, antimicrobial, and anti-diabetic properties
- Simultaneous quantification of mefenamic acid and paracetamol in fixed-dose combination tablet dosage forms using the green HPTLC method
- Green synthesis of titanium dioxide nanoparticles using green tea (Camellia sinensis) extract: Characteristics and applications
- Pharmaceutical properties for green fabricated ZnO and Ag nanoparticle-mediated Borago officinalis: In silico predications study
- Synthesis and optimization of gemcitabine-loaded nanoparticles by using Box–Behnken design for treating prostate cancer: In vitro characterization and in vivo pharmacokinetic study
- A comparative analysis of single-step and multi-step methods for producing magnetic activated carbon from palm kernel shells: Adsorption of methyl orange dye
- Sustainable green synthesis of silver nanoparticles using walnut septum waste: Characterization and antibacterial properties
- Efficient electrocatalytic reduction of CO2 to CO over Ni/Y diatomic catalysts
- Greener and magnetic Fe3O4 nanoparticles as a recyclable catalyst for Knoevenagel condensation and degradation of industrial Congo red dye
- Recycling of HDPE-giant reed composites: Processability and performance
- Fabrication of antibacterial chitosan/PVA nanofibers co-loaded with curcumin and cefadroxil for wound healing
- Cost-effective one-pot fabrication of iron(iii) oxychloride–iron(iii) oxide nanomaterials for supercapacitor charge storage
- Novel trimetallic (TiO2–MgO–Au) nanoparticles: Biosynthesis, characterization, antimicrobial, and anticancer activities
- Green-synthesized chromium oxide nanoparticles using pomegranate husk extract: Multifunctional bioactivity in antioxidant potential, lipase and amylase inhibition, and cytotoxicity
- Therapeutic potential of sustainable zinc oxide nanoparticles biosynthesized using Tradescantia spathacea aqueous leaf extract
- Chitosan-coated superparamagnetic iron oxide nanoparticles synthesized using Carica papaya bark extract: Evaluation of antioxidant, antibacterial, and anticancer activity of HeLa cervical cancer cells
- Antioxidant potential of peptide fractions from tuna dark muscle protein isolate: A green enzymatic approach
- Clerodendron phlomoides leaf extract-mediated synthesis of selenium nanoparticles for multi-applications
- Optimization of cellulose yield from oil palm trunks with deep eutectic solvents using response surface methodology
- Nitrogen-doped carbon dots from Brahmi (Bacopa monnieri): Metal-free probe for efficient detection of metal pollutants and methylene blue dye degradation
- High energy density pseudocapacitor based on a nanoporous tungsten(VI) oxide iodide/poly(2-amino-1-mercaptobenzene) composite
- Green synthesized Ag–Cu nanocomposites as an improved strategy to fight multidrug-resistant bacteria by inhibition of biofilm formation: In vitro and in silico assessment study
- In vitro evaluation of antibacterial activity and associated cytotoxicity of biogenic silver nanoparticles using various extracts of Tabernaemontana ventricosa
- Fabrication of novel composite materials by impregnating ZnO particles into bacterial cellulose nanofibers for antimicrobial applications
- Solidification floating organic drop for dispersive liquid–liquid microextraction estimation of copper in different water samples
- Kinetics and synthesis of formation of phosphate composites from low-grade phosphorites in the presence of phosphate–siliceous shales and oil sludge
- Removal of minocycline and terramycin by graphene oxide and Cr/Mn base metal–organic framework composites
- Microfluidic preparation of ceramide E liposomes and properties
- Therapeutic potential of Anamirta cocculus (L.) Wight & Arn. leaf aqueous extract-mediated biogenic gold nanoparticles
- Antioxidant-rich Micromeria imbricata leaf extract as a medium for the eco-friendly preparation of silver-doped zinc oxide nanoparticles with antibacterial properties
- Influence of different colors with light regime on Chlorella sp., biomass, pigments, and lipids quantity and quality
- Experimental vibrational analysis of natural fiber composite reinforced with waste materials for energy absorbing applications
- Green synthesis of sea buckthorn-mediated ZnO nanoparticles: Biological applications and acute nanotoxicity studies
- Production of liquid smoke by consecutive electroporation and microwave-assisted pyrolysis of empty fruit bunches
- Synthesis of MPAA based on polyacrylamide and gossypol resin and applications in the encapsulation of ammophos
- Application of iron-based catalysts in the microwave treatment of environmental pollutants
- Enhanced adsorption of Cu(ii) from wastewater using potassium humate-modified coconut husk biochar
- Adsorption of heavy metal ions from water by Fe3O4 nano-particles
- Green synthesis of parsley-derived silver nanoparticles and their enhanced antimicrobial and antioxidant effects against foodborne resistant bacteria
- Unwrapping the phytofabrication of bimetallic silver–selenium nanoparticles: Antibacterial, Anti-virulence (Targeting magA and toxA genes), anti-diabetic, antioxidant, anti-ovarian, and anti-prostate cancer activities
- Optimizing ultrasound-assisted extraction process of anti-inflammatory ingredients from Launaea sarmentosa: A novel approach
- Eggshell membranes as green carriers for Burkholderia cepacia lipase: A biocatalytic strategy for sustainable wastewater bioremediation
- Research progress of deep eutectic solvents in fuel desulfurization
- Enhanced electrochemical synthesis of Ni–Fe/brass foil alloy with subsequent combustion for high-performance photoelectrode and hydrogen production applications
- Valorization of baobab fruit shell as a filler fiber for enhanced polyethylene degradation and soil fertility
- Valorization of Agave durangensis bagasse for cardboard-type paper production circular economy approach
- Green priming strategies using seaweed extract and citric acid to improve early growth and antioxidant activity in lentil
- Gold extraction from oxide ore using iron ion-thiourea-additive
- Development of loess-derived P-type molecular sieve as a sustainable antibacterial agent
- Review Article
- Sustainable innovations in garlic extraction: A comprehensive review and bibliometric analysis of green extraction methods
- Natural sustainable coatings for marine applications: advances, challenges, and future perspectives
- Integration of traditional medicinal plants with polymeric nanofibers for wound healing
- Rapid Communication
- In situ supported rhodium catalyst on mesoporous silica for chemoselective hydrogenation of nitriles to primary amines
- Special Issue: Valorisation of Biowaste to Nanomaterials for Environmental Applications
- Valorization of coconut husk into biochar for lead (Pb2+) adsorption
- Corrigendum
- Corrigendum to “An updated review on carbon nanomaterials: Types, synthesis, functionalization and applications, degradation and toxicity”

