Green priming strategies using seaweed extract and citric acid to improve early growth and antioxidant activity in lentil
-
Kartik Bhardwaj
, Ravish Choudhary
and Amine Assouguem
Abstract
Achieving strong early seedling vigour is a crucial determinant of crop success, particularly in adversity-prone and rainfed areas. This study investigates the influence of seed priming with seaweed extract and citric acid on the early seedling vigour (EVS) and physio-biochemical properties of lentil (Lens culinaris Medik.). Seaweed extract and citric acid rich in naturally plant regulators, phytoelicitor activity, evoke phytohormonal responses, phytostimulatory properties micronutrients, and antioxidants, was used in concentrations of 1 %, 2 %, 3 %, and 4 %, while citric acid was applied at 50, 100, and 150 ppm. Seeds were soaked in the priming solutions for 18 h, followed by shade drying before sowing. The impact on seed germination, root and shoot length, seedling dry weight, vigour index, and antioxidant activity was assessed in laboratory conditions. The results demonstrated that both priming agents positively influenced early germination and growth. Seaweed extracts enhanced root and shoot elongation (7.16 and 9.04 cm), while citric acid improved membrane stability and reactive oxygen species scavenging. The most effective treatment was of 4 % seaweed extract and 50 ppm citric acid, which showed a significant increase in germination percentage (93.33 % and 63.33 %), seedling vigour index SVI-I &II (34.53, 3.73 and 962.133.67), and early biomass accumulation. Enhanced mobilization of seed reserves and higher antioxidant enzyme activities Chlorophyll (5.51 and 1.92 mg/g/FW) and H2O2 were also recorded in primed seeds. These physiological enhancements are indicative of better resilience and robust establishment potential laboratory conditions. This research highlights that seaweed extract and citric acid priming offers a practical, eco-friendly approach for improving early seedling performance and may be integrated into sustainable lentil production systems.
1 Introduction
Lentil (Lens culinaris Medik.) is a vital pulse crop grown extensively across arid and semi-arid regions of the world. As a rich source of plant-based protein, essential amino acids, dietary fibre, and micronutrients like iron and zinc, lentils play a crucial role in human nutrition and food security, particularly in vegetarian and low-income populations [1]. Beyond its nutritional profile, lentils contribute to soil health through nitrogen fixation, thus supporting sustainable agricultural practices. Despite its global significance, lentil production often suffers from suboptimal yields due to poor seed vigor, nutrient deficiencies, and inadequate agronomic inputs, which collectively hinder its potential to meet rising global food and nutritional demands. India contributes more than 30 % of the world’s production of lentils, making it the top producer and consumer [2]. Major lentil-growing states include Madhya Pradesh, Uttar Pradesh, Bihar, and West Bengal, where it is cultivated primarily under rainfed conditions during the rabi season. However, average yields remain below potential due to erratic rainfall patterns, marginal soils, and limited use of improved seed technologies. In contrast, countries like Canada, Turkey, and Australia have achieved significantly higher productivity through the adoption of modern agronomic practices, including micronutrient management and seed enhancement techniques [3]. This contrast highlights the critical need for sustainable interventions to bridge the yield gap in Indian lentil cultivation.
Seed priming is a simple yet highly effective pre-sowing technique that enhances seed germination, seedling vigor, and stress tolerance in crops, particularly legumes [4]. It works by initiating early metabolic processes without actual germination, thus preparing seeds for rapid growth once sown. Biostimulants like citric acid and seaweed extract have shown promising results by improving enzymatic activity, antioxidant defence, and nutrient uptake [4], [5], [6]. This eco-friendly strategy supports sustainable agriculture by improving plant performance under suboptimal conditions and reducing dependence on chemical inputs [7]. Seed germination is a foundational process that determines crop establishment, stand density, and final yield. In lentils, uneven and delayed germination often results in poor plant population, which compromises productivity. Seed priming has emerged as a cost-effective and practical technique to improve germination rates, seedling vigor, and early plant growth under both optimal and stress-prone environments [8]. Among various priming agents, citric acid has attracted attention for its dual role in enhancing seed physiology and modifying the biochemical environment of seeds during early growth stages.
Citric acid, a naturally occurring tricarboxylic acid and an intermediate of the Krebs cycle, plays multiple roles in plant systems. It not only regulates cellular pH and ion balance but also acts as a chelating agent for micronutrients, thus improving their bioavailability in the rhizosphere [9]. Recent studies have shown that citric acid priming can improve seed imbibition, accelerate germination, and activate antioxidant enzyme systems, leading to increased tolerance against oxidative stress and better seedling establishment [10]. In lentils, the use of citric acid during seed priming has demonstrated promise in enhancing root growth, shoot length, and biomass accumulation during early stages of development. Citric acid also has a good interaction with growing methods that are enhanced by micronutrients. Micronutrients such as zinc, iron, and boron are essential for key metabolic processes in lentils, but their availability is often limited in Indian soils due to high pH and low organic matter. The chelating nature of citric acid helps solubilize these micronutrients, improving their uptake efficiency by the plant [11]. When applied together with micronutrient fertilizers or through seed treatments, citric acid can thus facilitate balanced nutrition and promote physiological resilience under suboptimal growing conditions.
Another promising technique for enhancing seed quality is the application of seed water extract (SWE), which is a natural bio-stimulant derived from plants and enriched with phytohormones, amino acids, organic acids and contributes to identifying eco-friendly, low-cost, and sustainable strategies for enhancing lentil productivity under adverse conditions. SWE treatments have been shown to improve seed germination and seedling vigor by promoting hormonal balance and improving cellular hydration [12]. When SWE is used alongside citric acid, it can further stimulate metabolic activation during the critical lag phase of germination, offering a synergistic effect that enhances seed performance [13]. These seed management strategies are particularly vital in climate-resilient agriculture, where abiotic stressors such as drought, salinity and nutrient imbalances present significant challenges to lentil cultivation [14], 15]. In recent years, lentil research has increasingly focused on sustainable intensification, prioritizing not only yield improvements but also enhanced nutritional quality and resilience [16]. Within this framework, citric acid-mediated seed priming has emerged as a simple yet highly effective strategy. Its multifaceted role stimulating physiological processes, enhancing nutrient uptake, and boosting antioxidant defines systems make it an invaluable tool for improving lentil growth under micronutrient-enriched conditions [17].
This study aims to fill that gap by systematically investigating the role of citric acid and seaweed extract (SWE) in enhancing germination parameters and physio-biochemical traits, through seed priming. By optimizing seed priming with citric acid and SWE application, this research hopes to develop a scalable, eco-friendly intervention that can significantly contribute to lentil productivity and nutritional quality, especially in nutrient-depleted soils of South Asia. The outcomes of this study are expected to offer practical implications for farmers, researchers, and policymakers working towards sustainable pulse production under climate-sensitive agricultural systems. Hence, the present study was undertaken to evaluate the influence of citric acid and seaweed extract on seed quality and physio-biochemical attributes of lentil. In this crop, research on the role of these priming agents is still scarce, with little to no published information on their effects on germination, vigor, chlorophyll content, and antioxidant activity. While seaweed extracts and citric acid have been extensively examined as plant growth enhancers in several other crops, their application in lentil seed priming has not been thoroughly investigated. This study, therefore, fills an important gap by systematically assessing the impact of citric acid and seaweed extract on key physiological and biochemical traits in lentil, offering new insights into their potential as eco-friendly and sustainable seed-priming agents.
2 Materials and methods
2.1 Experimental design and treatments
The experiment was conducted at laboratory at Amity Institute of Organic Agriculture, Amity University, Noida. IPL - 316 variety of lentil was taken from ICAR – IARI, New Delhi and this variety of lentil was used in the experiment. At the time of standardization (Table 1) different combinations of sea weed extract (SWE) and citric acid (CA) at various durations and concentrations for seed priming were used. We carried out the final seed priming with four different treatments viz., T1 = Control, T2 = Hydro priming (18 h), T3 = Citric acid 50 ppm, T4 = Sea weed extract 4 % and these were used for estimation of yield attributes and yield traits.
Treatment details of seaweed extract and citric acid seed priming concentrations, including standardized priming protocols used in the experiment.
| Sea weed extract | Citric acid | After standardization |
|---|---|---|
| T1 = control | T1 = control | |
| T2 = S.W.E 1 % | T2 = C.A 50 ppm | T1 = control |
| T3 = S.W.E 2 % | T3 = C.A 100 ppm | T2 = Hydro priming (18 hr) |
| T4 = S.W.E 3 % | T4 = C.A 200 ppm | T3 = C.A. 50 ppm |
| T5 = S.W.E 4 % | T5 = C.A 300 ppm | T4 = S.W.E 4 % |
The standardization of Citric acid (CA) and Sea weed extract (SWE), five different concentrations of citric acid Control, 50 ppm, 100 ppm, 200 ppm, and 300 ppm were studied in order to standardize the treatment of lentil seeds. The goal was to ascertain the ideal concentration for encouraging early germination and seedling development. Important metrics were measured to assess the effects of each treatment, including germination percentage, root length, and shoot length. Four concentrations of seaweed extract Control, 1 %, 2 %, 3 %, and 4 % were made and used in order to find the ideal concentration for treating lentil seeds. In order to standardize the most advantageous concentration for encouraging early seedling growth and development under controlled settings, the efficacy of each treatment was evaluated by measuring the germination percentage, root length, and shoot length.
The effects of hydropriming, seaweed extract, citric acid, and an untreated control on the growth of lentil seeds were compared in this study. Following treatment, the seeds were incubated at 25 ± 2 °C in sterile Petri dishes lined with moist filter paper. After 10 days, measurements were made of the root length, shoot length, and germination percentage. Comparing seaweed extract and citric acid to hydropriming and the control, the results showed that both considerably enhanced seedling performance. These treatments produced the best seedling growth, demonstrating their potential to improve the development of lentil seeds in regulated environments.
2.2 Seed quality parameter
2.2.1 Germination percentage [18]
Citric acid and seaweed extract were applied in different concentrations to test the germination percentage of lentil seeds. After 2 min of surface sterilization with 0.1 % sodium hypochlorite, healthy seeds were thoroughly rinsed with distilled water. After that, they were immersed in solutions of seaweed extract (1 %, 2 %, 3 %, and 4 %) and citric acid (50, 100, 200, and 300 ppm) for 18 h in the ratio of 1:1 w/v. Distilled water was used to treat the control group. The treated seeds were incubated at 25 ± 2 °C in sterile Petri dishes lined with damp filter paper. Seeds were considered to have germinated when the radicle expanded at least 2 mm, and germination was tracked every day for 10 days till final count. After three replications of each treatment, the germination percentage was determined, and the mean data were used for additional analysis.
Germination Percentage = (Number of seeds germinated/Total number of seeds sown) × 100.
2.2.2 Seed vigour index-I&II [18]
Using the same setup as the germination test, 50 lentil seeds from each treatment were put on a double layer of moist filter paper and incubated at 25 °C to assess seedling vigor. Using the number of normal seedlings, the standard germination percentage was calculated. The length of the roots, shoots, and complete seedlings in centimetres were measured on the final day after 10 normal seedlings were randomly selected from each replication. To determine the dry weight of the seedlings, they were then dried in a hot air oven for 48 h at 70 ± 1 °C. The germination % was multiplied by the entire length of the seedling to determine the Seed Vigour Index-I (SVI–I), and the germination percentage and seedling dry weight were used to determine the Seed Vigour Index-II (SVI-II). The toughness and physiological potential of lentil seedlings under different treatments were evaluated with the aid of these parameters.
The vigour indices were calculated using the method.
Vigour index I = Germination (%) × Total seedling length
Vigour index II = Germination (%) × Seedling dry weight
2.2.3 Seedling dry weight [18]
Ten normal seedlings chosen at random and used to measure seedling length were dried for 72 h at 42 °C in a hot air oven. Their dry weight, which was measured and represented in milligrams per seedling after cooling, served as a crucial indicator of biomass accumulation and seedling vigor under various treatments.
2.2.4 Root and shoot length [18]
Using a ruler, the root and shoot lengths of best five seedlings per treatment were measured in centimetres following the germination phase. To assure accuracy, the process was repeated three times, and average data were noted to provide consistency and dependability for statistical analysis across all treatment groups.
2.3 Physio biochemical activity
2.3.1 Chlorophyll content
The pigments were extracted by carefully weighing around 1 g of fresh seedling tissue and homogenizing it with 3 ml of 80 % acetone. The supernatant was then separated by carefully transferring the resultant mixture into an Eppendorf tube and centrifuging it for 10 min at 10,000 rpm. A UV-Visible spectrophotometer was used to detect the absorbance of the clear extract at four distinct wavelengths (470 nm, 645 nm, 652 nm, and 663 nm) after centrifugation. Following [19] method, these data were used to calculate the concentration of photosynthetic pigments, such as carotenoids and chlorophyll.
Total Chl = Chl a + Chl b
2.3.2 Histochemical examination of H2O2 in seedlings using DAB
In lentil seedlings, reactive oxygen species (ROS) including hydrogen peroxide and superoxide anion were found using the 3,3′-diaminobenzidine (DAB) staining method, as detailed by [20]. A brown formazan precipitate, which is a sign of ROS buildup, is created when the DAB and hydrogen peroxide combine in the presence of peroxidase. In addition, lipid peroxides were histochemical detected in both leaves and roots following the method of [21], allowing for the visualization of oxidative damage. The samples were kept in darkness to allow proper staining, and chlorophyll was removed with ethanol for better visibility. Image analysis tools were used to quantify ROS accumulation, offering valuable insights into plant responses to oxidative stress.
DAB staining solution (1 mg ml−1) was prepared following standard protocols with minor modifications. Briefly, DAB was dissolved in distilled water, the pH was adjusted to 3.0 using HCl, and the solution was protected from light. The working solution was obtained by adding Na2HPO3 and Tween-20, which stabilized the pH and enhanced staining efficiency. The freshly prepared solution was used immediately for assays [21].
Three replications of 100 seedlings (n = 3) were used for each treatment. Each treatment’s arithmetic mean standard errors (SEs) were determined. ANOVA and pairwise comparisons between treatments were used to analyse the data. Tukey’s test was used to calculate the means at p = 0.05 using SPSS 16.0, Chicago, IL, USA.
3 Results
3.1 Seed quality parameters
Under control condition, seed priming, involving different concentrations of S.W.E and C.A, yielded significantly higher germination percentages and all other assessed seed quality parameters. Similarly, the treatment such as S.W.E 4 % and C.A 50PPM exhibited significantly better results compared to other priming treatments as well as control for all the seed quality and physio biochemical parameters. Below are the results of the study on how priming treatments affect different lentil seed quality criteria. In this study, a range of citric acid (50 ppm, 100 ppm, 200 ppm, and 300 ppm and seaweed extract (1 %, 2 %, 3 %, and 4 % %) concentrations was applied to evaluate their effects on seed germination, seedling vigor, and key physio-biochemical parameters in lentil. The selected concentrations were based on findings from previous studies in legumes, where similar doses were effective in enhancing germination and stress tolerance. Testing multiple concentrations allowed for the assessment of dose-dependent responses and identification of the most effective levels for practical seed priming applications. Although preliminary optimization experiments were not conducted in lentil, the chosen range reflects concentrations that are both biologically relevant and feasible for field or laboratory use, providing insights into their potential as sustainable seed-priming agents.
3.1.1 Germination percentage, root and shoot length
Different concentration of seaweed and treatments with citric acid had a substantial impact on seed germination. Seaweed application, particularly at 4 %, markedly enhanced significantly germination up to 93.33 % compared to 60 % in the control as follows by 2 %, 3 % and 4 % (Figure 1A). Similarly, citric acid at 50 ppm improved germination to around 73.33 %, indicating its effectiveness. However, higher concentrations (300 PPM) showed a slight decline (Figure 1A). These findings suggest both seaweed and citric acid as promising bio stimulants for improving seed vigor and germination. In the final priming after standardisation germination percentage of seeds improved significantly over five days under different treatments shown in (Figure 2A–D), Among them, SWE-4% exhibited the significantly highest germination percentage (93.33 %) as compared to germination% of CA50 ppm (63.33 %) as shown in (Figure 2D), demonstrating its potential as an effective bio-stimulant. The data suggests SWE-4% enhances seed vigor, outperforming other treatments, including control, hydropriming and CA-50 ppm.
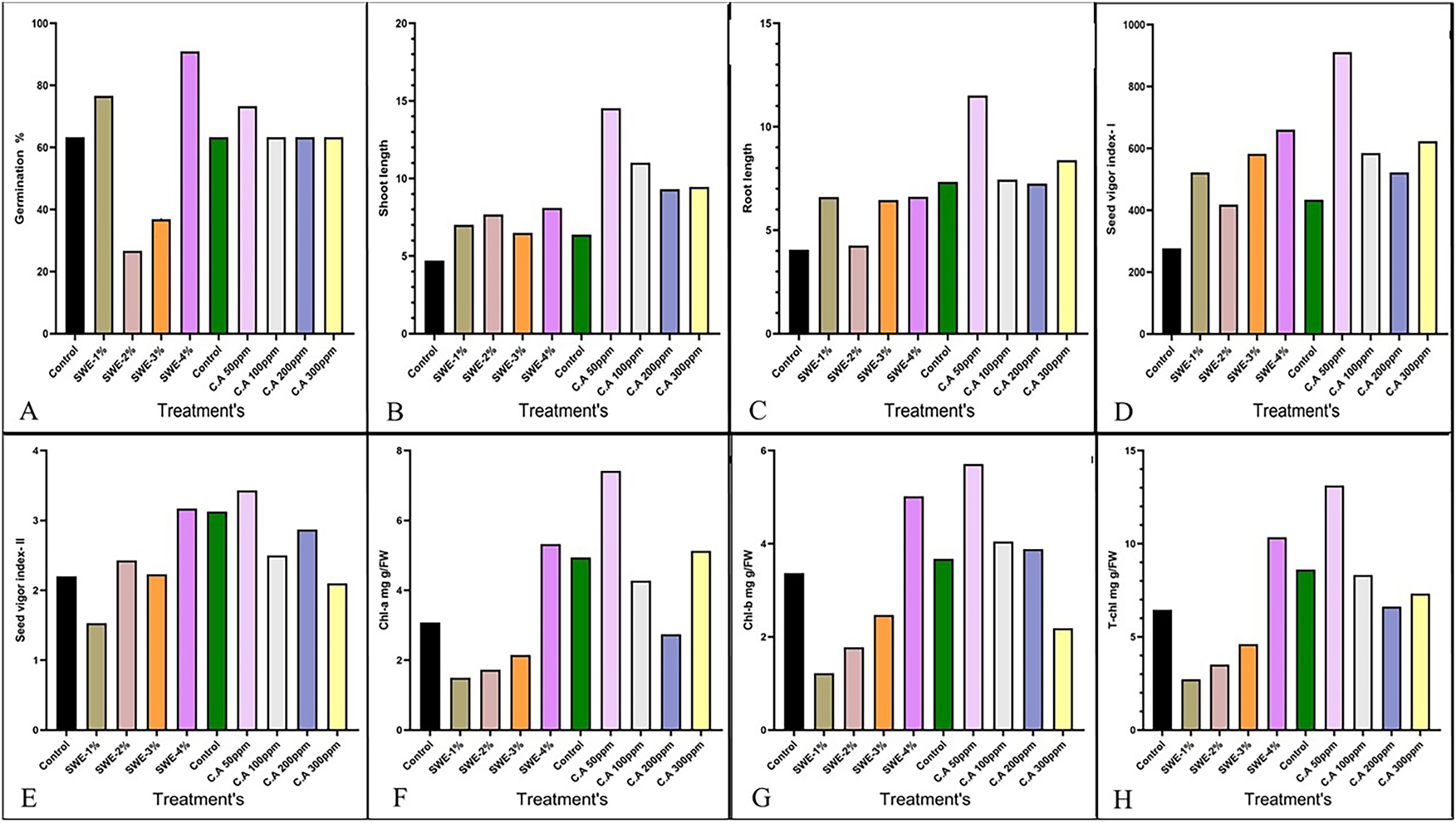
Effect of seed priming agents on the different seed quality parameters on primed seeds whereas; (A) germination percentage;(B) shoot length; (C) root length; (D) seed vigor Index-I; (E) seed vigor Index-II; (F) chlorophyll a; (G) chlorophyll b; (H) total chlorophyll content.
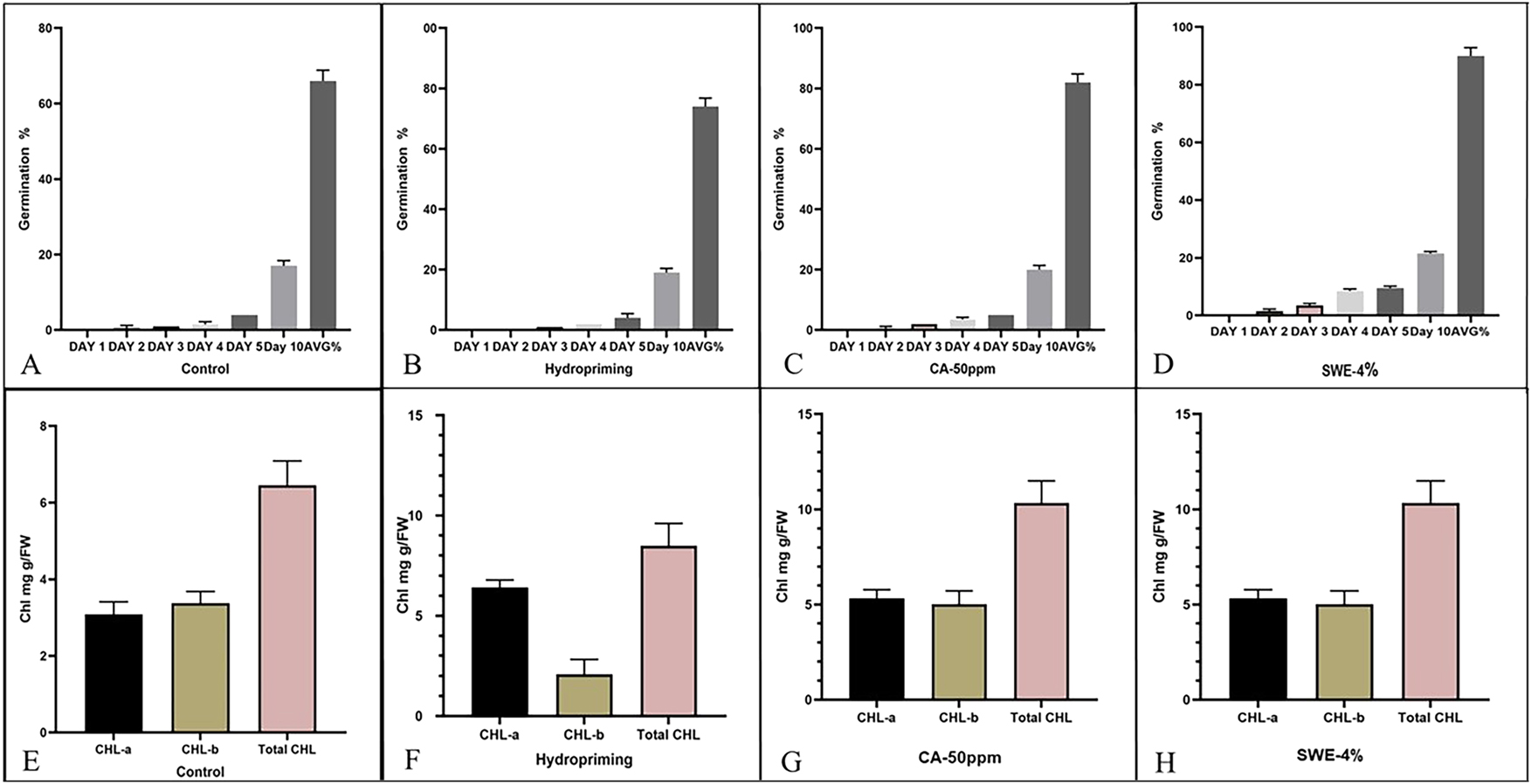
Effect of different priming agents after standardization on seed quality and physio-biochemical parameters in primed seeds whereas; (A) germination percentage in control;(B) germination percentage after hydropriming;(C) germination percentage after CA 50 ppm; (D) germination percentage after SWE 4 % treatment; (E) chlorophyll content in control;(F) chlorophyll content in hydropriming; (G) chlorophyll content in CA 50 ppm;(H) chlorophyl content in SWE 4%.
Shoot growth was positively influenced by both seaweed extract (S.W.E) and citric acid (C.A.) applications, with distinct concentration-dependent trends. Control seedlings exhibited baseline SL values of 4.76 and 4.64 cm. S.W.E at 1 % modestly enhanced SL (7.12 and 6.9 cm), while 2 % yielded higher values (7.8 and 7.56 cm) (Figure 1B). SL remained above control at 3 % (6.4 and 6.58 cm), and peaked at 4 % (7.16 and 9.04 cm), indicating optimal enhancement. Similarly, C.A. treatment showed best performance at 50 PPM, where SL surged to 14.62 and 14.44 cm from the control (6.54 and 6.24 cm), with diminishing results at higher doses. Thus, 4 % S.W.E and 50 PPM C.A. significantly improved shoot elongation in seedlings.
Root development responded distinctly to varying concentrations of seaweed extract (S.W.E) and citric acid (C.A.). In the control group, RL ranged from 4.18 to 4.34 cm. S.W.E at 1 % showed negligible change (3.94–4.18 cm) (Figure 1C)., but higher concentrations significantly improved RL 2 % (6.74, 6.56 cm), 3 % (6.48, 6.36 cm), and peaking at 4 % (6.54, 6.7 cm), indicating enhanced root elongation. Similarly, C.A. treatments showed optimal results at 50 PPM, where RL rose markedly to 11.62 and 11.4 cm from the control (7.42, 7.26 cm). Other doses showed reduced or inconsistent effects. Thus, both 4 % S.W.E and 50 PPM C.A. effectively promoted root growth in seedlings.
3.1.2 Seed vigour index-I&II
Seedling vigour, as assessed by Seedling Vigour Index-I (SVI–I) and Seedling Vigour Index-II (SVI-II), was significantly influenced by seaweed and citric acid treatments. Seaweed extract showed a marked improvement in SVI-I at 2 %, reaching 554.4, though SVI-II declined slightly, suggesting enhanced seedling length but reduced dry weight as shown in (Figure 1D). While SVI-I decreased slightly at 3 % and 4 %, higher concentrations later yielded peak values of 639.33 and 734.53 for SVI-I and 3.73 for SVI-II, indicating that higher doses improved overall vigour as shown in (Figure 1E). Citric acid treatments followed a similar trend, with optimal results at 50 PPM, where SVI-I peaked at 962.13 and SVI-II at 3.67, reflecting enhanced growth performance. Although 200 ppm maintained high values, further increase to 300 PPM led to a decline in SVI-I despite relatively stable SVI-II. Overall, the findings suggest that both seaweed and citric acid, when applied at optimal concentrations, can significantly boost seedling performance by enhancing growth and biomass accumulation.
After standardization of the treatments the results clearly demonstrate that seed priming with 4 % seaweed extract (SWE-4%) significantly enhanced seed vigor indices in lentils. In SVI-I, SWE-4% achieved the highest value of approximately 680, compared to 550 for CA-50 ppm, 420 for hydropriming, and only 250 for the control as shown in (Figure 3E). Similarly, in SVI-II, SWE-4% recorded the peak index of 2.3, outperforming CA-50 ppm (2.0), hydropriming (1.5), and the control (1.0) as shown in (Figure 3F).
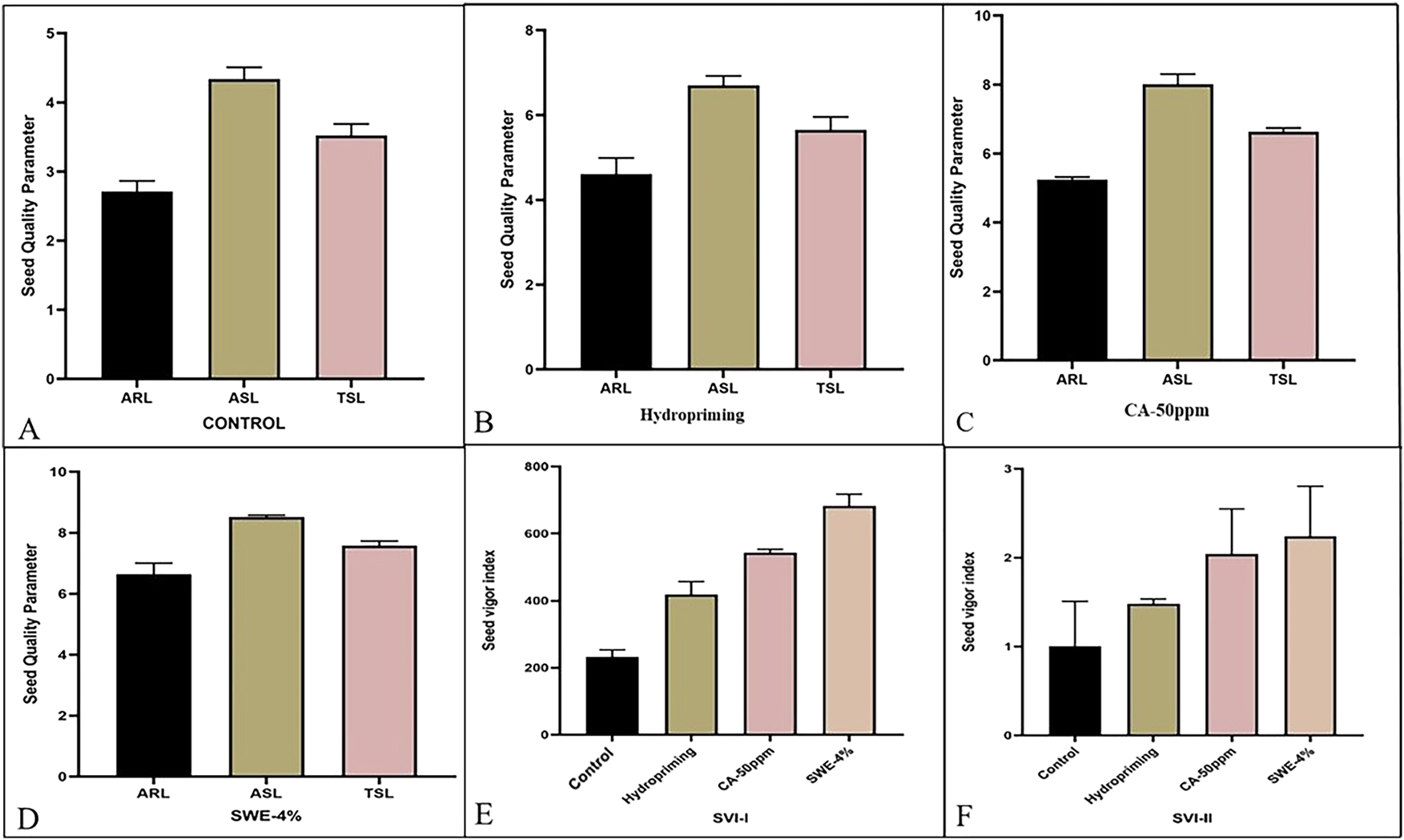
Effect of different priming agents after standardization on seed quality parameters in primed seeds whereas; (A) control (ARL, ASL and TSL); (B) hydropriming (ARL, ASL and TSL); (C) CA 50 ppm (ARL, ASL and TSL); (D) SWE 4 % (ARL, ASL and TSL); (E) seed vigor Index-I); (F) seed vigor Index-II.
3.1.3 Chlorophyll content (chl-a and chl-b)
Chlorophyll b (chl-b) levels in lentil leaves were significantly affected by (S.W.E) treatments; the control group had the greatest concentration, at 3.59 as shown in (Figure 1G). Significantly Chl-a levels gradually decreased reaching 3.15 at 1 % SW and then falling at 2 % (1.46), 3 % (0.98), and most noticeably at 4 % (1.92), indicating that stress was causing chlorophyll to degrade (Figure 1F). Interestingly, a recovery tendency was noted at greater doses, especially at 4 % SW, where levels peaked at 5.51. Significantly, citric acid (CA) treatments positively influenced chlorophyll a and total chlorophyll levels at CA-50 ppm shown in (Figure 2H), but higher concentrations led to a decline. These results potential of 4 % S.W.E and moderate C.A doses in enhancing chlorophyll stability and lentil resilience under stress.
3.1.4 Total chlorophyll
The comparative analysis of total chlorophyll content across treatments Control, Hydropriming, Citric Acid (CA-50 ppm), and Seaweed Extract (SWE-4%) demonstrates a significant enhancement under SWE-4% treatment. As shown in the (Figure 2E), the Control group recorded the lowest total CHL value at approximately 6.5 mg/g FW. Significantly, Hydropriming elevated the total CHL to nearly 9 mg/g FW as shown in (Figure 2F), indicating a moderate improvement. The CA-50 ppm treatment further increased total CHL to around 10.5 mg/g FW shown in (Figure 2G), reflecting a beneficial effect of citric acid on pigment accumulation. However, the most pronounced increase was observed under SWE-4%, with total CHL reaching approximately 11.5 mg/g FW (Figure 2H), clearly surpassing all other treatments. This suggests that SWE-4% priming not only boosts chlorophyll biosynthesis but may also enhance overall photosynthetic efficiency. These findings highlight SWE-4% as a superior biostimulant treatment for improving chlorophyll content, supporting its potential in sustainable crop enhancement strategies under stress conditions.
3.1.5 Total shoot length
Total Shoot Length (TSL) under different seed priming treatments like Control, Hydropriming, CA-50 ppm, and SWE-4% clearly shows the superior efficacy of SWE-4%. In the Control group, TSL was the lowest, approximately 3.5 cm, indicating limited seedling growth in the absence of priming agents as shown in (Figure 3A). Hydropriming showed notable improvement, raising TSL to around 5.5 cm as shown in (Figure 3B), while CA-50 ppm further enhanced it to approximately 6.5 cm, showcasing the benefit of citric acid in promoting shoot development as shown in (Figure 3C). However, the SWE-4% treatment led to the most significant increase, with TSL reaching close to 7.5 cm as shown in (Figure 3D), outperforming all other treatments. This dominance of SWE-4% in promoting shoot elongation suggests that the bioactive compounds in seaweed extract greatly stimulate cell division and elongation, improving early seedling vigor. Hence, SWE-4% emerges as a promising natural priming agent for enhancing seedling quality and shoot development in sustainable agricultural practices.
3.1.6 Histochemical analysis by DAB of hydrogen peroxide molecules
Oxidative stress in lentil seeds under different treatments was visualized using the DAB staining technique, revealing significant variation in reactive oxygen species (ROS) accumulation. Control seeds exhibited intense brown staining, indicating a high level of oxidative damage, likely due to the absence of protective treatment. Hydrogen peroxide (HP)-treated seeds showed the most severe browning, affirming its role as a positive stress inducer. In contrast, seeds treated with Seaweed Extract (SWE) at 4 % and Citric Acid (CA) at 50 ppm showed very mild or negligible brown staining, suggesting a strong antioxidative defence triggered by these priming agents as shown in (Figure 4). Notably, the SWE-4% treatment resulted in the least browning, implying superior efficiency in mitigating ROS accumulation. Quantitative analysis of staining intensity further supported these observations, positioning SWE-4% as the most effective treatment. These findings highlight the protective role of SWE and CA in enhancing seed resilience against oxidative stress, especially under external abiotic stressors.
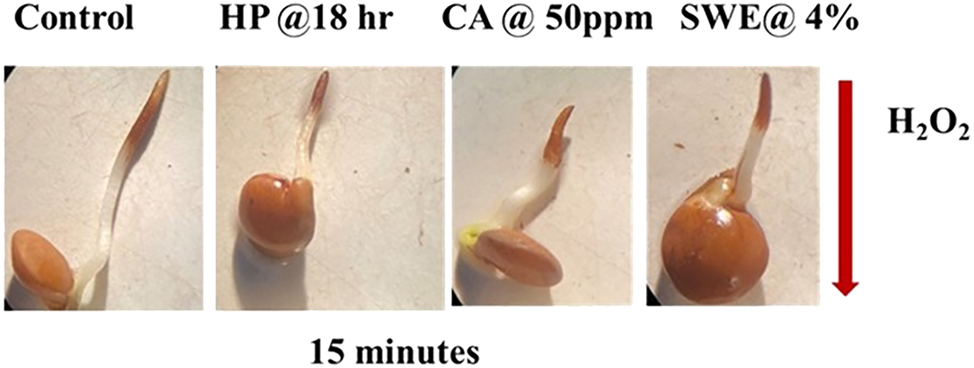
Histochemical analysis of all the treatments by using DAB solutions.
This suggests that these treatments may help mitigate the oxidative damage caused by external stressors. The quantification of staining intensity further validated these findings, demonstrating how the treatments modulated ROS accumulation. Overall, the visual depiction of oxidative damage, supported by image analysis, highlights the physiological responses of lentil seed cells to different stressors and antioxidant treatments, providing valuable insight into the seed’s cellular reactions and the potential protective role of SWE and CA in reducing oxidative damage.
3.1.7 Standardization of priming agent (S.W.E and C.A)
The experimental analysis of lentil growth revealed how different concentrations of seaweed extract (SWE) affect seedling development and germination over 10 days Fig (5). While the control group showed typical growth, seeds treated with 1 %, 2 %, and 3 % SWE inconsistent results, suggesting potential negative effects. Significantly, the 4 % SWE treatment showed germination% of 53.22 % in day 5 with longer roots and stronger shoots compared to other concentrations. However, by Day 10, lentil seedlings treated with 4 % SWE demonstrated the most vigorous growth and germination% of 93.33 %, emphasizing its potential in improving lentil production. Significantly optimal concentration of citric acid (CA) for promoting lentil seed germination and growth was found to be 50 ppm. Seedlings treated with 50 ppm CA consistently exhibited superior germination% of 41.22 % in day 5 with longer roots and stronger shoots compared to other concentrations (Figure 6). Higher concentrations of CA (100 ppm, 200 ppm, and 300 ppm) resulted in stunted growth, with shorter roots and smaller shoots, while the control group showed moderate growth. These findings highlight the potential of 50 ppm with immense germination % of 63.33 % for enhancing lentil development and improving agricultural practices for higher crop yields.
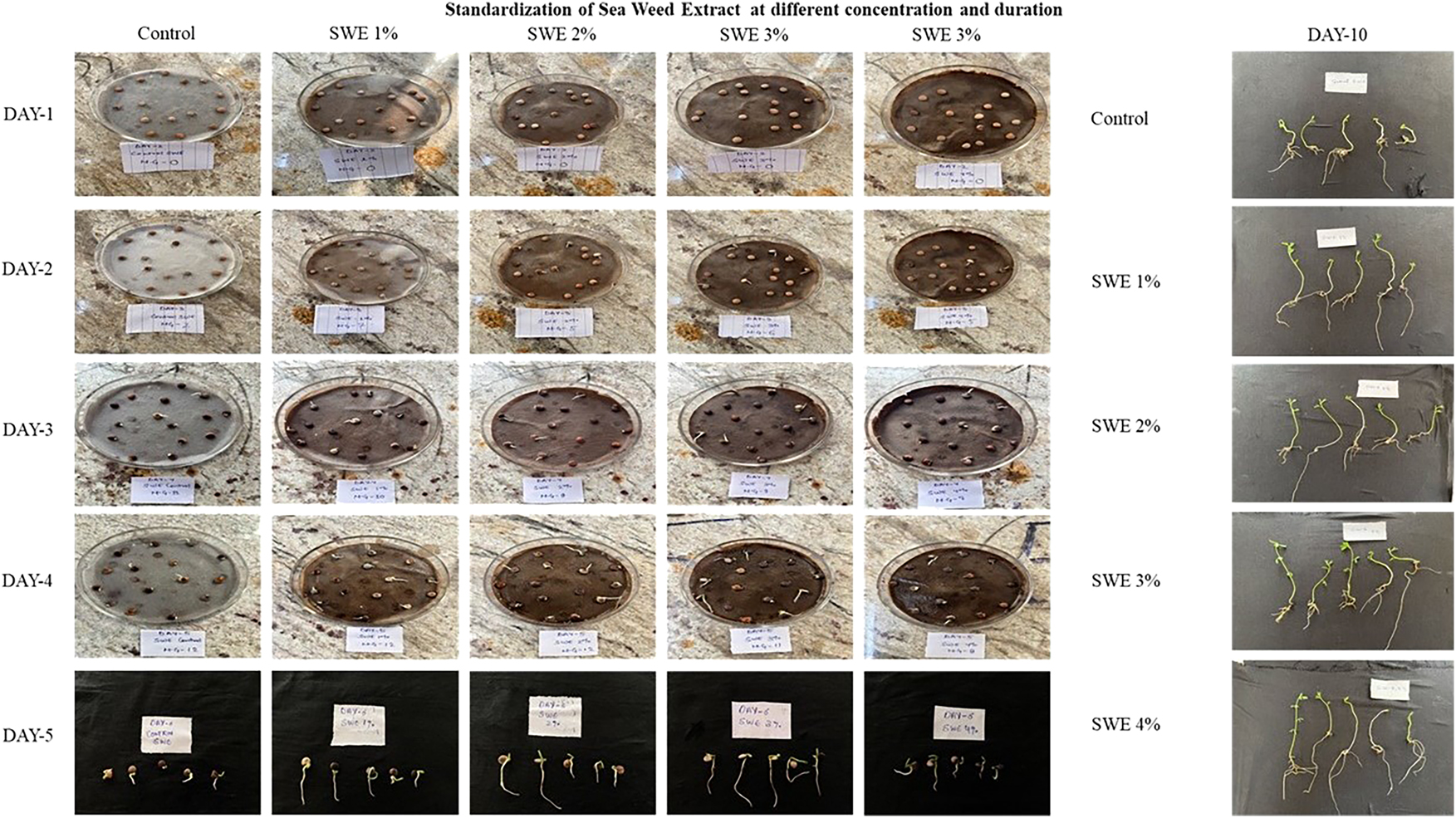
Visualization of seedlings at first and final counts after treatment with different concentrations of SWE.
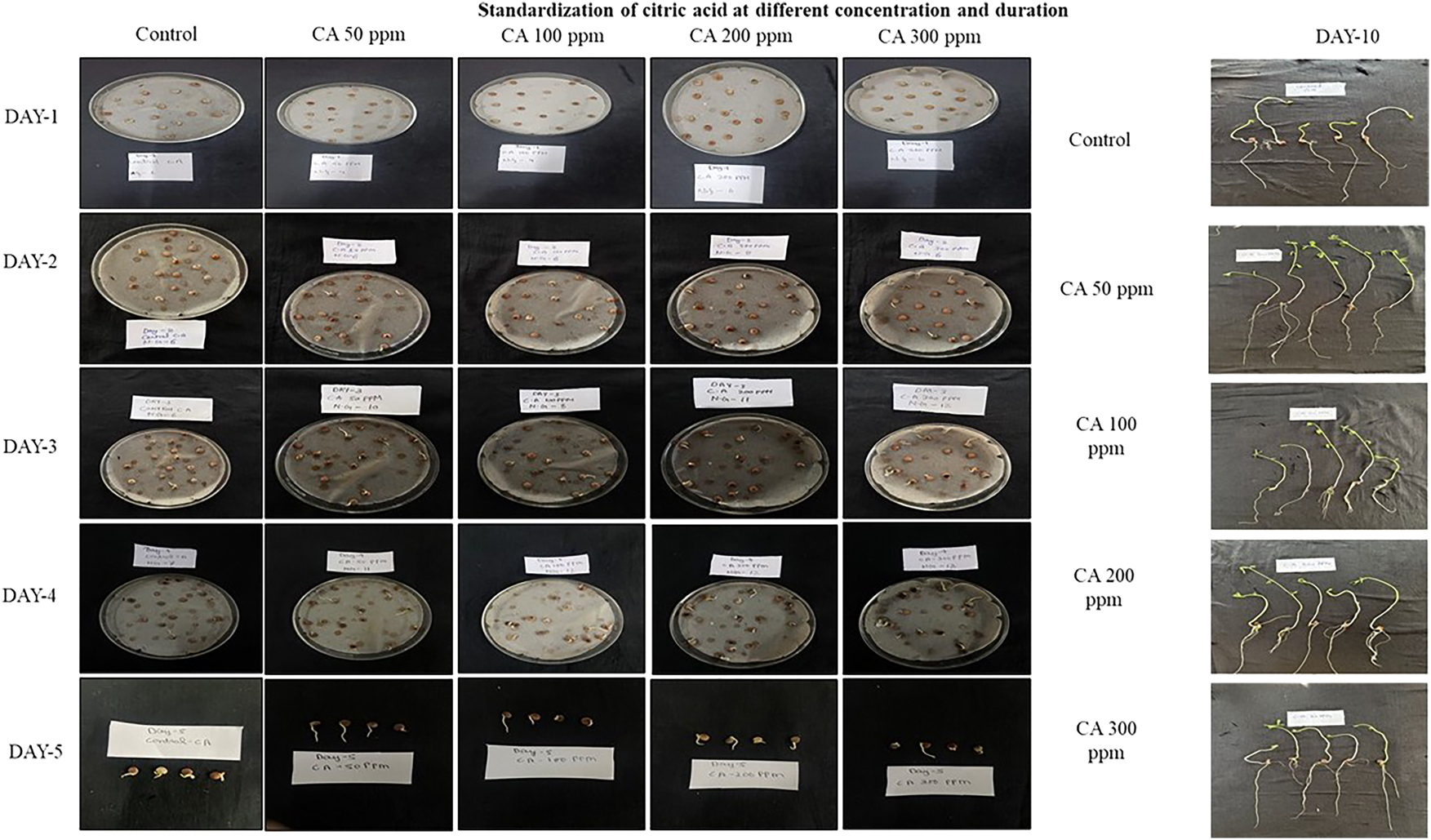
Visualization of seedlings at first and final counts after treatment with different concentrations of CA.
3.1.8 Final standardization
When comparing different seed treatments such as (Control, Hydropriming, SWE and CA) 4 % seaweed extract (SWE 4 %) emerged as the most effective for enhancing lentil seedling growth as shown in (Figure 7). By Day 10, lentil seeds treated with SWE 4 % displayed significantly better root and shoot development with immense germination percentage of 67 %, surpassing all other treatments in both size and Vigor. In contrast, control and hydropriming treatments showed minimal improvement with germination percentage of 38 % and 42 %, while 50 ppm citric acid (CA) enhanced growth moderately with germination percentage of 54 %. These results highlight the strong potential of SW 4 % for promoting seed germination and early 6growth, positioning it as an optimal treatment for boosting lentil crop performance in future agronomic practices.
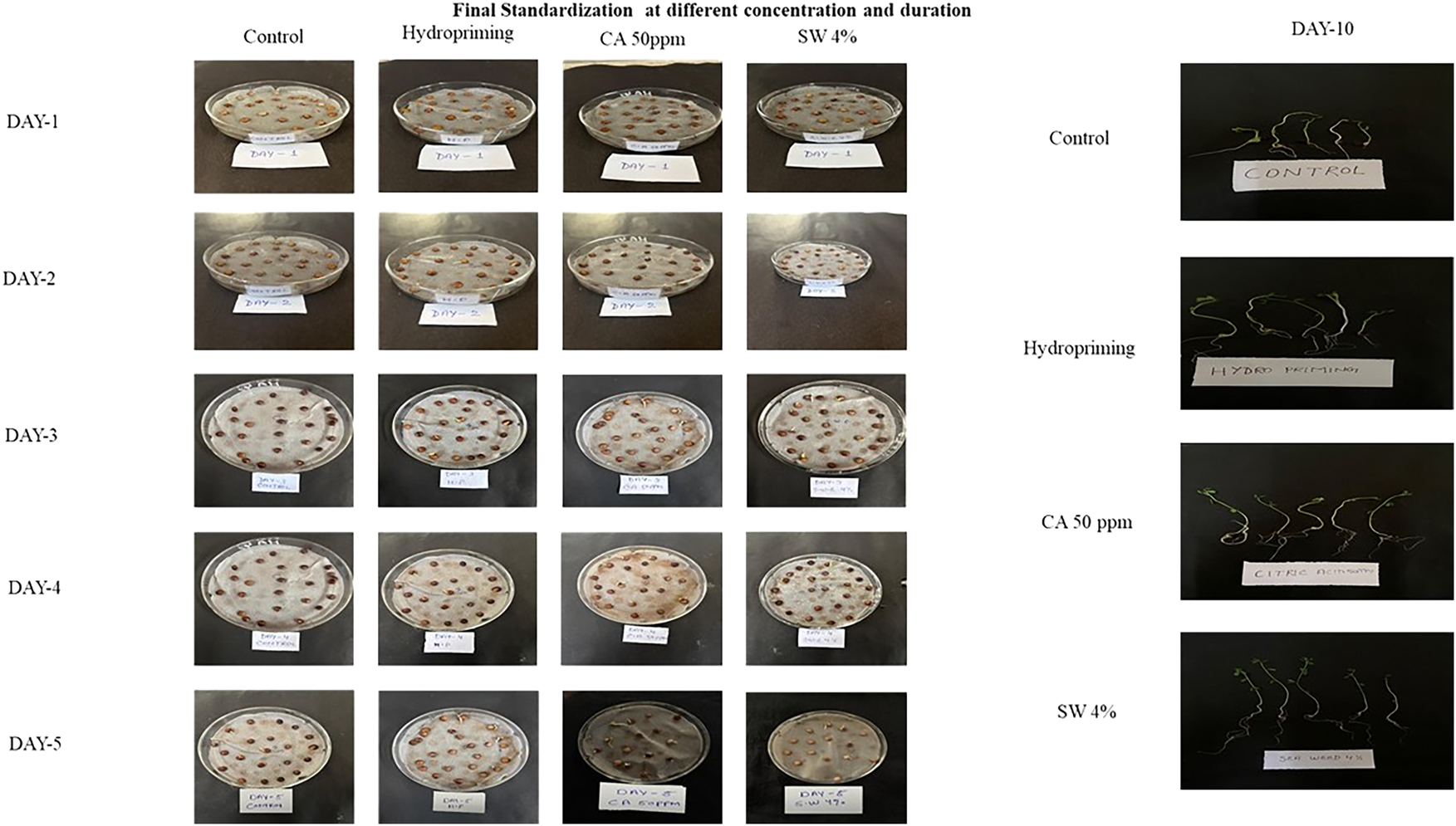
Visualization of seedlings at first and final counts after standardisation with different treatments.
4 Discussion
Seed priming represents a powerful, cost-effective tool to improve seedling establishment, particularly in pulse crops like lentil which are sensitive to early-stage environmental stress. The present study reveals that both citric acid and seaweed extract, when used as seed priming agents, markedly enhance germination metrics, physiological Vigor, and biochemical resilience, ultimately translating into improved yield components under field conditions. These outcomes strongly align with growing global interest in eco-friendly bio stimulants that serve as catalysts for sustainable crop productivity. Citric acid, a tricarboxylic acid cycle intermediate, has garnered attention for its multifaceted role in plant metabolism, especially in enhancing early germinative processes.
In this study, citric acid priming significantly boosted germination percentage (93.33 % and 63.33 % and seedling vigor index (34.53, 3.73 and 962.13, 3.67). This is in agreement with findings by [5], who showed that organic acid priming activates enzymes such as α-amylase and proteases, accelerating the mobilization of seed reserves into usable energy forms These enzymes break down stored starches and proteins into simpler sugars and amino acids, which serve as readily available energy and building blocks for cell division and elongation. This accelerated mobilization of seed reserves allows the seedling to grow faster and more uniformly, improving the seedling vigor index. These metabolic triggers are vital for the early establishment of lentils, particularly under suboptimal soil conditions where natural enzymatic activity may lag. Furthermore, [9] highlighted that citric acid acts as a signalling molecule, promoting increased mitochondrial activity during early germination. The antioxidant landscape of the treated seedlings also indicated strong activation of enzymatic defences, particularly catalase (CAT), superoxide dismutase (SOD), and peroxidase (POD). These enzymes play a central role in neutralizing reactive oxygen species (ROS), which accumulate rapidly during early germination and stress exposure. The elevated activities observed in our study reflect a protective physiological mechanism against oxidative stress, as also reported by [22] in organic acid-treated chickpeas and lentils This antioxidant upregulation further contributed to the reduction in membrane damage, as evidenced by lower malondialdehyde (MDA) and electrolyte leakage values, which are widely used indicators of lipid peroxidation,cellular integrity and priming agents citric acid and sea weed extract enhances seed germination and seedling vigor by acting as a signalling molecule that boosts mitochondrial activity and energy production. It also activates antioxidant enzymes CAT, SOD, and POD reducing ROS accumulation, protecting membranes, and maintaining cellular integrity, thereby promoting robust early seedling establishment, especially under stress conditions [23].
Seaweed extract, derived from brown macroalgae such as Ascophyllum nodosum, emerged as another potent bio stimulant. Its diverse composition including micronutrients, amino acids, phytohormones, vitamins, and polysaccharides makes it especially suitable for priming applications [6]. Our findings revealed significant increases in seed germination rate, seedling biomass, and chlorophyll content in seaweed-primed lentil plants. These results mirror those of [24], who demonstrated that seaweed extract enhances early plant vigor through phytohormonal effects, particularly via auxins, gibberellins, and cytokinin’s, which regulate cell division, elongation, and chloroplast development. Biochemically, seaweed extract-treated plants exhibited elevated levels of total soluble proteins and sugars, indicating enhanced metabolic activity and improved photosynthetic function. Such improvements are linked to the availability of essential trace elements like zinc, iron, and manganese present in seaweed formulations, which are known to activate several enzymes involved in photosynthesis and stress mitigation [25]. The observed increase in chlorophyll content further suggests better light absorption and carbon assimilation, ultimately supporting biomass accumulation, plant growth and seed priming with agents like citric acid or seaweed extract enhances chlorophyll content because it stimulates enzymatic activities involved in chlorophyll biosynthesis, such as those regulating the formation of chlorophyll precursors. Primed seeds also experience improved nutrient uptake, especially of magnesium and nitrogen, which are critical for chlorophyll formation. Additionally, priming reduces oxidative stress during germination and early growth, protecting chloroplast membranes from damage. As a result, chlorophyll accumulation is higher, which enhances photosynthetic capacity and supports more vigorous seedling growth [25].
Physiological parameters, particularly those related to water dynamics, were also favourably influenced by both citric acid and seaweed treatments. Treated plants showed higher relative water content (RWC), improved stomatal conductance, and greater water retention, suggesting better-developed root systems and improved osmotic adjustment. These traits are especially valuable under fluctuating water availability. Seed priming with citric acid and seaweed extract promotes the accumulation of osmoprotectants like proline, which help maintain cellular water balance under stress conditions. These compounds act as compatible solutes, stabilizing proteins and membranes, scavenging reactive oxygen species, and protecting cellular structures from dehydration. By enhancing osmotic adjustment and membrane integrity, priming improves seedling tolerance to drought and other abiotic stresses, supporting early growth and vigor, consistent with previous observations in legumes [26].
Agronomically, the benefits of these priming treatments extended into yield-determining stages. According to [4] citric acid-mediated seed priming significantly enhances seed metabolic activity by facilitating better enzymatic function and nutrient mobilization. Lentil plants from treated seeds were taller, more branched, and bore significantly more pods per plant. Citric acid likely contributed to this outcome by acting as a chelating agent, enhancing the bioavailability of soil nutrients such as iron and zinc [4], 27] emphasized that citric acid-based seed priming induces key biochemical changes that improve germination efficiency and seedling vigor. Enhanced nutrient uptake during vegetative and reproductive stages is critical for maximizing productivity. In contrast, seaweed extract supported root proliferation, shoot elongation, and pod filling, possibly due to its hormonal and nutrient content acting synergistically to sustain plant vigor and reproductive success. A particularly noteworthy observation is the relationship between early seedling vigor and final yield. The improvements in early-stage growth due to priming interventions effectively set the stage for more robust phenological development. This supports the notion by [28] that seed priming not only triggers metabolic readiness but may also induce molecular and epigenetic modifications that persist across the plant’s life cycle, affecting traits like flowering, pod setting, and seed filling. In this context, the initial physiological ‘boost’ offered by biostimulant priming creates a cascading effect of benefits throughout the growing season.
In terms of sustainability, both citric acid and seaweed extract stand out as environmentally benign solutions for enhancing seed quality. Citric acid, being a naturally occurring and biodegradable compound, poses minimal ecological risk, making it suitable for integrated nutrient and organic farming systems. Similarly, seaweed extract is derived from renewable marine biomass and is non-toxic to both plants and soil microbiota. These properties make them ideal components for climate-smart and resource-efficient farming strategies [29]. The application of these biostimulants also offers promise in building resilience against climate change-induced stresses. With increasing unpredictability in rainfall, rising temperatures, and soil nutrient depletion, priming strategies that improve root architecture, water uptake, and antioxidant capacity will be invaluable. As [30] noted, seed treatments that enhance early vigor are strongly correlated with yield stability in legumes under erratic environmental conditions. The findings from the present study align with this perspective, offering a pragmatic approach to future-proofing lentil cultivation.
5 Conclusions
Hence, in comparison to other priming treatments and control, seed priming with 4 % seaweed extract and 50 ppm citric acid for 18 h can greatly enhance germination parameters and physio-biochemical traits, and overall seed output. The strategy improves lentil output and plant stand development in an environmentally responsible way. Significantly Seed Weed 4 % emerged as the most effective in achieving the research objectives. (S.W.E) 4 % consistently outperformed other treatments, making it a promising option for enhancing lentil seed performance through chemical priming. Thus, this is the first report and novel study performed on seaweed extract and citric acid seed priming shows promise as a way to improve lentil crop germination, stress tolerance, nutrient uptake, and yield. The scalability and applicability of this strategy across many lentil types and environmental situations may require more investigation and field testing.
Acknowledgments
The authors wish to thank the AIOA (AMITY Institute of Organic AGRICULTRE) Amity University Uttar Pradesh, Noida (India), Crop Nanobiology and Molecular Stress Physiology Lab for providing the required necessary facilities to conduct the studies and I express my heartfelt gratitude to my esteemed faculty guide, Dr. Deepak Rao, for his unwavering support, valuable guidance, and constant encouragement throughout my research work. This study was supported by Princess Nourah bint Abdulrahman University Research Supporting Project number (PNURSP2025R579), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
-
Funding: This study was supported by Princess Nourah bint Abdulrahman Univeristy Research Supporting Project number (PNURSP2025R579), Prince Nourah bint Abdulrahmna University, Riyadh, Saudi Arabia.
-
Author Contributions: Deepak Rao: Conceptualization, Methodology, Software, Validation, Formal Analysis, Investigation, Data Curation, Visualization, Supervision; Kartik Bhardwaj: Formal Analysis, Investigation, Data Curation, writing – original draft, Writing – review & editing; Sumit Chauhan: Data Curation. All authors have read and agreed to the published version of the manuscript.
-
Data Availability Statement: All data generated or analysed during this study are included in this published article.
-
Conflicts of Interest: The authors state no conflict of interest.
References
1. Mekonnen, M, Gari, G, Tesfaye, M. Role of lentil (lens culinaris) in human nutrition and food security. Agric Sci J 2021;15:1–12.Search in Google Scholar
2. Rai, S, Verma, M, Kumar, R. Global lentil production and consumption: trends and challenges. Agron Res J 2022;16:55–64.Search in Google Scholar
3. Sarker, M, Sharma, M, Sultana, R. Agronomic practices and technological interventions for increasing lentil yield. Field Crops Res 2021;138:120–34.Search in Google Scholar
4. Rao, D, Yadav, S, Choudhary, R, Singh, D, Bhardwaj, R, Barthakur, S, et al.. Silicic and humic acid priming improves Micro- and macronutrient uptake, salinity stress tolerance, seed quality, and physio-biochemical parameters in lentil (lens culinaris spp. culinaris). Plants 2023;12:3539. https://doi.org/10.3390/plants12203539.Search in Google Scholar PubMed PubMed Central
5. Meena, S, Yadav, A, Kumar, P. The role of citric acid and seaweed extract in seed priming: mechanisms and benefits. J Agric Biotechnol. 2022;24:45–59.Search in Google Scholar
6. El Boukhari, S, Idrissi, M, Belkoura, I. The efficacy of seaweed extract as a biostimulant in enhancing plant growth and stress resistance. Plant Growth Regul 2021;93:523–32.Search in Google Scholar
7. Rahman, M, Uddin, M, Islam, M. Seed treatments for enhanced early vigor and yield stability under erratic environmental conditions. J Crop Stress Manag. 2022;6:33–42.Search in Google Scholar
8. Rao, D, Yadav, S, Choudhary, R, Singh, D, Bhardwaj, R, Barthakur, S, et al.. Unveiling the potential of silicic and humic acid priming in alleviating salinity stress on lentil (lens culinaris) seed germination in a hydroponic system. J Food Legumes 2024;37:291–6.10.59797/jfl.v37.i3.209Search in Google Scholar
9. Sharma, S, Ali, A, Jain, D. Citric acid as a signaling molecule in early germination processes in legumes. Legume Res 2021;44:889–96.Search in Google Scholar
10. Patel, R, Reddy, S, Sharma, A. Effects of citric acid priming on seed germination and stress tolerance in lentils. J Seed Sci. 2022;30:168–74.Search in Google Scholar
11. Verma, M, Gupta, V, Tripathi, R. Chelation effects of citric acid on micronutrient uptake in lentils. Agric Nutr Manag J. 2021;16:125–33.Search in Google Scholar
12. Dutta, M, Mohan, L, Kundu, S. The role of seed water extract in improving seedling vigor and stress resilience in crops. Agron J 2021;42:56–62.Search in Google Scholar
13. Singh, J, Yadav, P, Singh, S. Seed priming with seaweed extract and citric acid for improved seed performance and plant growth. J Plant Physiol 2022;182:99–107.Search in Google Scholar
14. Rani, A, Yadav, M. Impact of climate change on lentil productivity and the role of sustainable agricultural practices. Environ Sci Policy 2023;18:55–68.Search in Google Scholar
15. Rao, D, Yadav, S, Choudhary, R, Sushkova, S, Sachan, CP, Yadav, SK. Enhancing of early seedling vigour (ESV) parameters in lentils through integrated priming with silicic and humic acid. Eurasian J Soil Sci 2025;14:157–67. https://doi.org/10.18393/ejss.1646812.Search in Google Scholar
16. Patel, R, Verma, A, Reddy, P. Advances in lentil cultivation for enhanced nutritional quality and resilience. Agron Sustain Dev 2021;41:92–103.Search in Google Scholar
17. Sarkar, P, Meena, R, Ghosh, P. Citric acid as a bio stimulant in lentil seed priming: a sustainable approach for improving crop productivity. J Crop Improv 2023;17:134–45.Search in Google Scholar
18. International Rules for Seed Testing, ISTA. Introduction i–I-6 (14).:2023.10.15258/istarules.2023.1Search in Google Scholar
19. Arnon, DI. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in beta vulgaris. Plant Physiol 1949;24:1–15. https://doi.org/10.1104/pp.24.1.1.Search in Google Scholar PubMed PubMed Central
20. Singh, R, Verma, K, Das, S. Histochemical detection of hydrogen peroxide in plant tissues using DAB staining. J Plant Microsc 2014;5:12–16.Search in Google Scholar
21. Rompella, M, Malhotra, N, Arora, R. Lipid peroxidation in plant tissues: a comparative study. J Plant Biochem. 1987;2:155–60.Search in Google Scholar
22. Singh, P, Kumar, R, Mehta, N. Organic acids as seed priming agents in lentils and chickpeas for enhanced antioxidant responses. Pulse Crop J 2023;29:119–26.Search in Google Scholar
23. Roy, S, Das, T, Banerjee, A. Evaluation of seedling vigor, antioxidant defense, and cell membrane integrity in primed lentil seeds. Plant Physiol Biochem 2021;160:194–203.Search in Google Scholar
24. Kumar, V, Sharma, R, Meena, R. Seaweed extract application and its role in improving seedling vigor through phytohormonal regulation. J Plant Biochem. 2020;15:63–71.Search in Google Scholar
25. Hassan, M, Alam, F, Hossain, M. Seaweed-based biostimulants: effects on nutrient uptake and stress mitigation in pulse crops. Agric Res J 2022;59:322–30.Search in Google Scholar
26. Akter, S, Hossain, F, Rahman, M. Impact of seed priming with organic acids and seaweed on drought tolerance and water balance in legumes. J Legume Sci. 2023;15:89–98.Search in Google Scholar
27. Yadav, A, Singh, D, Sharma, M. Role of citric acid in enhancing nutrient bioavailability and promoting seedling growth. J Soil Plant Nutr. 2021;14:102–10.Search in Google Scholar
28. Khan, A, Singh, P, Verma, M. Seed priming and its molecular and epigenetic effects on plant development and yield potential. J Seed Technol 2021;40:88–97.Search in Google Scholar
29. Kalita, S, Baruah, R, Sharma, A. Sustainability of biostimulants like seaweed and citric acid for climate-smart agriculture. Sustainability Agric 2020;12:277–85.Search in Google Scholar
30. Rahman, M, Sultana, R, Bhuiyan, M. Impact of seed priming on plant stress tolerance and growth under environmental stress. Agric Sustain 2022;9:78–89.Search in Google Scholar
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Synthesis of N,S co-doped carbon quantum dots – metal complex for the detection of fluoride (F−) ion in adults and Children’s toothpastes
- Research Articles
- Optimized green synthesis of silver nanoparticles from guarana seed skin extract with antibacterial potential
- Green adsorbents for water remediation: Removal of Cr(vi) and Ni(ii) using Prosopis glandulosa sawdust and biochar
- Green approach for the synthesis of zinc oxide nanoparticles from methanolic stem extract of Andrographis paniculata and evaluation of antidiabetic activity: In silico GSK-3β analysis
- Development of a green and rapid ethanol-based HPLC assay for aspirin tablets and feasibility evaluation of domestically produced bioethanol in Thailand as a sustainable mobile phase
- A facile biodegradation of polystyrene microplastic by Bacillus subtilis
- Enhanced synthesis of fly ash-derived hydrated sodium silicate adsorbents via low-temperature alkaline hydrothermal treatment for advanced environmental applications
- Impact of metal nanoparticles biosynthesized using camel milk on bacterial growth and copper removal from wastewater
- Preparation of Co/Cr-MOFs for efficient removal of fleroxacin and Rhodamine B
- Applying nanocarbon prepared from coal as an anode in lithium-ion batteries
- Improved electrochemical synthesis of Cu–Fe/brass foil alloy followed by combustion for high-efficiency photoelectrodes and hydrogen production in alkaline solutions
- Precipitation of terephthalic acid from post-consumer polyethylene terephthalate waste fractions
- Biosynthesized zinc oxide nanoparticles: Multifunctional potential applications in anticancer, antibacterial, and B. subtilis DNA gyrase docking
- Anticancer and antimicrobial effects of green-synthesized silver nanoparticles using Teucrium polium leaves extract
- Green synthesis of eco-friendly bioplastics from Chlorella and Lithothamnion algae for safe and sustainable solutions for food packaging
- Optimizing coal water slurry concentration via synergistic coal blending and particle size distribution
- Green synthesis of Ag@Cu and silver nanowire using Pterospermum heterophyllum extracts for surface-enhanced Raman scattering
- Green synthesis of copper oxide nanoparticles from Algerian propolis: Exploring biochemical, structural, antimicrobial, and anti-diabetic properties
- Simultaneous quantification of mefenamic acid and paracetamol in fixed-dose combination tablet dosage forms using the green HPTLC method
- Green synthesis of titanium dioxide nanoparticles using green tea (Camellia sinensis) extract: Characteristics and applications
- Pharmaceutical properties for green fabricated ZnO and Ag nanoparticle-mediated Borago officinalis: In silico predications study
- Synthesis and optimization of gemcitabine-loaded nanoparticles by using Box–Behnken design for treating prostate cancer: In vitro characterization and in vivo pharmacokinetic study
- A comparative analysis of single-step and multi-step methods for producing magnetic activated carbon from palm kernel shells: Adsorption of methyl orange dye
- Sustainable green synthesis of silver nanoparticles using walnut septum waste: Characterization and antibacterial properties
- Efficient electrocatalytic reduction of CO2 to CO over Ni/Y diatomic catalysts
- Greener and magnetic Fe3O4 nanoparticles as a recyclable catalyst for Knoevenagel condensation and degradation of industrial Congo red dye
- Recycling of HDPE-giant reed composites: Processability and performance
- Fabrication of antibacterial chitosan/PVA nanofibers co-loaded with curcumin and cefadroxil for wound healing
- Cost-effective one-pot fabrication of iron(iii) oxychloride–iron(iii) oxide nanomaterials for supercapacitor charge storage
- Novel trimetallic (TiO2–MgO–Au) nanoparticles: Biosynthesis, characterization, antimicrobial, and anticancer activities
- Green-synthesized chromium oxide nanoparticles using pomegranate husk extract: Multifunctional bioactivity in antioxidant potential, lipase and amylase inhibition, and cytotoxicity
- Therapeutic potential of sustainable zinc oxide nanoparticles biosynthesized using Tradescantia spathacea aqueous leaf extract
- Chitosan-coated superparamagnetic iron oxide nanoparticles synthesized using Carica papaya bark extract: Evaluation of antioxidant, antibacterial, and anticancer activity of HeLa cervical cancer cells
- Antioxidant potential of peptide fractions from tuna dark muscle protein isolate: A green enzymatic approach
- Clerodendron phlomoides leaf extract-mediated synthesis of selenium nanoparticles for multi-applications
- Optimization of cellulose yield from oil palm trunks with deep eutectic solvents using response surface methodology
- Nitrogen-doped carbon dots from Brahmi (Bacopa monnieri): Metal-free probe for efficient detection of metal pollutants and methylene blue dye degradation
- High energy density pseudocapacitor based on a nanoporous tungsten(VI) oxide iodide/poly(2-amino-1-mercaptobenzene) composite
- Green synthesized Ag–Cu nanocomposites as an improved strategy to fight multidrug-resistant bacteria by inhibition of biofilm formation: In vitro and in silico assessment study
- In vitro evaluation of antibacterial activity and associated cytotoxicity of biogenic silver nanoparticles using various extracts of Tabernaemontana ventricosa
- Fabrication of novel composite materials by impregnating ZnO particles into bacterial cellulose nanofibers for antimicrobial applications
- Solidification floating organic drop for dispersive liquid–liquid microextraction estimation of copper in different water samples
- Kinetics and synthesis of formation of phosphate composites from low-grade phosphorites in the presence of phosphate–siliceous shales and oil sludge
- Removal of minocycline and terramycin by graphene oxide and Cr/Mn base metal–organic framework composites
- Microfluidic preparation of ceramide E liposomes and properties
- Therapeutic potential of Anamirta cocculus (L.) Wight & Arn. leaf aqueous extract-mediated biogenic gold nanoparticles
- Antioxidant-rich Micromeria imbricata leaf extract as a medium for the eco-friendly preparation of silver-doped zinc oxide nanoparticles with antibacterial properties
- Influence of different colors with light regime on Chlorella sp., biomass, pigments, and lipids quantity and quality
- Experimental vibrational analysis of natural fiber composite reinforced with waste materials for energy absorbing applications
- Green synthesis of sea buckthorn-mediated ZnO nanoparticles: Biological applications and acute nanotoxicity studies
- Production of liquid smoke by consecutive electroporation and microwave-assisted pyrolysis of empty fruit bunches
- Synthesis of MPAA based on polyacrylamide and gossypol resin and applications in the encapsulation of ammophos
- Application of iron-based catalysts in the microwave treatment of environmental pollutants
- Enhanced adsorption of Cu(ii) from wastewater using potassium humate-modified coconut husk biochar
- Adsorption of heavy metal ions from water by Fe3O4 nano-particles
- Green synthesis of parsley-derived silver nanoparticles and their enhanced antimicrobial and antioxidant effects against foodborne resistant bacteria
- Unwrapping the phytofabrication of bimetallic silver–selenium nanoparticles: Antibacterial, Anti-virulence (Targeting magA and toxA genes), anti-diabetic, antioxidant, anti-ovarian, and anti-prostate cancer activities
- Optimizing ultrasound-assisted extraction process of anti-inflammatory ingredients from Launaea sarmentosa: A novel approach
- Eggshell membranes as green carriers for Burkholderia cepacia lipase: A biocatalytic strategy for sustainable wastewater bioremediation
- Research progress of deep eutectic solvents in fuel desulfurization
- Enhanced electrochemical synthesis of Ni–Fe/brass foil alloy with subsequent combustion for high-performance photoelectrode and hydrogen production applications
- Valorization of baobab fruit shell as a filler fiber for enhanced polyethylene degradation and soil fertility
- Valorization of Agave durangensis bagasse for cardboard-type paper production circular economy approach
- Green priming strategies using seaweed extract and citric acid to improve early growth and antioxidant activity in lentil
- Gold extraction from oxide ore using iron ion-thiourea-additive
- Development of loess-derived P-type molecular sieve as a sustainable antibacterial agent
- Review Article
- Sustainable innovations in garlic extraction: A comprehensive review and bibliometric analysis of green extraction methods
- Natural sustainable coatings for marine applications: advances, challenges, and future perspectives
- Integration of traditional medicinal plants with polymeric nanofibers for wound healing
- Rapid Communication
- In situ supported rhodium catalyst on mesoporous silica for chemoselective hydrogenation of nitriles to primary amines
- Special Issue: Valorisation of Biowaste to Nanomaterials for Environmental Applications
- Valorization of coconut husk into biochar for lead (Pb2+) adsorption
- Corrigendum
- Corrigendum to “An updated review on carbon nanomaterials: Types, synthesis, functionalization and applications, degradation and toxicity”
Articles in the same Issue
- Synthesis of N,S co-doped carbon quantum dots – metal complex for the detection of fluoride (F−) ion in adults and Children’s toothpastes
- Research Articles
- Optimized green synthesis of silver nanoparticles from guarana seed skin extract with antibacterial potential
- Green adsorbents for water remediation: Removal of Cr(vi) and Ni(ii) using Prosopis glandulosa sawdust and biochar
- Green approach for the synthesis of zinc oxide nanoparticles from methanolic stem extract of Andrographis paniculata and evaluation of antidiabetic activity: In silico GSK-3β analysis
- Development of a green and rapid ethanol-based HPLC assay for aspirin tablets and feasibility evaluation of domestically produced bioethanol in Thailand as a sustainable mobile phase
- A facile biodegradation of polystyrene microplastic by Bacillus subtilis
- Enhanced synthesis of fly ash-derived hydrated sodium silicate adsorbents via low-temperature alkaline hydrothermal treatment for advanced environmental applications
- Impact of metal nanoparticles biosynthesized using camel milk on bacterial growth and copper removal from wastewater
- Preparation of Co/Cr-MOFs for efficient removal of fleroxacin and Rhodamine B
- Applying nanocarbon prepared from coal as an anode in lithium-ion batteries
- Improved electrochemical synthesis of Cu–Fe/brass foil alloy followed by combustion for high-efficiency photoelectrodes and hydrogen production in alkaline solutions
- Precipitation of terephthalic acid from post-consumer polyethylene terephthalate waste fractions
- Biosynthesized zinc oxide nanoparticles: Multifunctional potential applications in anticancer, antibacterial, and B. subtilis DNA gyrase docking
- Anticancer and antimicrobial effects of green-synthesized silver nanoparticles using Teucrium polium leaves extract
- Green synthesis of eco-friendly bioplastics from Chlorella and Lithothamnion algae for safe and sustainable solutions for food packaging
- Optimizing coal water slurry concentration via synergistic coal blending and particle size distribution
- Green synthesis of Ag@Cu and silver nanowire using Pterospermum heterophyllum extracts for surface-enhanced Raman scattering
- Green synthesis of copper oxide nanoparticles from Algerian propolis: Exploring biochemical, structural, antimicrobial, and anti-diabetic properties
- Simultaneous quantification of mefenamic acid and paracetamol in fixed-dose combination tablet dosage forms using the green HPTLC method
- Green synthesis of titanium dioxide nanoparticles using green tea (Camellia sinensis) extract: Characteristics and applications
- Pharmaceutical properties for green fabricated ZnO and Ag nanoparticle-mediated Borago officinalis: In silico predications study
- Synthesis and optimization of gemcitabine-loaded nanoparticles by using Box–Behnken design for treating prostate cancer: In vitro characterization and in vivo pharmacokinetic study
- A comparative analysis of single-step and multi-step methods for producing magnetic activated carbon from palm kernel shells: Adsorption of methyl orange dye
- Sustainable green synthesis of silver nanoparticles using walnut septum waste: Characterization and antibacterial properties
- Efficient electrocatalytic reduction of CO2 to CO over Ni/Y diatomic catalysts
- Greener and magnetic Fe3O4 nanoparticles as a recyclable catalyst for Knoevenagel condensation and degradation of industrial Congo red dye
- Recycling of HDPE-giant reed composites: Processability and performance
- Fabrication of antibacterial chitosan/PVA nanofibers co-loaded with curcumin and cefadroxil for wound healing
- Cost-effective one-pot fabrication of iron(iii) oxychloride–iron(iii) oxide nanomaterials for supercapacitor charge storage
- Novel trimetallic (TiO2–MgO–Au) nanoparticles: Biosynthesis, characterization, antimicrobial, and anticancer activities
- Green-synthesized chromium oxide nanoparticles using pomegranate husk extract: Multifunctional bioactivity in antioxidant potential, lipase and amylase inhibition, and cytotoxicity
- Therapeutic potential of sustainable zinc oxide nanoparticles biosynthesized using Tradescantia spathacea aqueous leaf extract
- Chitosan-coated superparamagnetic iron oxide nanoparticles synthesized using Carica papaya bark extract: Evaluation of antioxidant, antibacterial, and anticancer activity of HeLa cervical cancer cells
- Antioxidant potential of peptide fractions from tuna dark muscle protein isolate: A green enzymatic approach
- Clerodendron phlomoides leaf extract-mediated synthesis of selenium nanoparticles for multi-applications
- Optimization of cellulose yield from oil palm trunks with deep eutectic solvents using response surface methodology
- Nitrogen-doped carbon dots from Brahmi (Bacopa monnieri): Metal-free probe for efficient detection of metal pollutants and methylene blue dye degradation
- High energy density pseudocapacitor based on a nanoporous tungsten(VI) oxide iodide/poly(2-amino-1-mercaptobenzene) composite
- Green synthesized Ag–Cu nanocomposites as an improved strategy to fight multidrug-resistant bacteria by inhibition of biofilm formation: In vitro and in silico assessment study
- In vitro evaluation of antibacterial activity and associated cytotoxicity of biogenic silver nanoparticles using various extracts of Tabernaemontana ventricosa
- Fabrication of novel composite materials by impregnating ZnO particles into bacterial cellulose nanofibers for antimicrobial applications
- Solidification floating organic drop for dispersive liquid–liquid microextraction estimation of copper in different water samples
- Kinetics and synthesis of formation of phosphate composites from low-grade phosphorites in the presence of phosphate–siliceous shales and oil sludge
- Removal of minocycline and terramycin by graphene oxide and Cr/Mn base metal–organic framework composites
- Microfluidic preparation of ceramide E liposomes and properties
- Therapeutic potential of Anamirta cocculus (L.) Wight & Arn. leaf aqueous extract-mediated biogenic gold nanoparticles
- Antioxidant-rich Micromeria imbricata leaf extract as a medium for the eco-friendly preparation of silver-doped zinc oxide nanoparticles with antibacterial properties
- Influence of different colors with light regime on Chlorella sp., biomass, pigments, and lipids quantity and quality
- Experimental vibrational analysis of natural fiber composite reinforced with waste materials for energy absorbing applications
- Green synthesis of sea buckthorn-mediated ZnO nanoparticles: Biological applications and acute nanotoxicity studies
- Production of liquid smoke by consecutive electroporation and microwave-assisted pyrolysis of empty fruit bunches
- Synthesis of MPAA based on polyacrylamide and gossypol resin and applications in the encapsulation of ammophos
- Application of iron-based catalysts in the microwave treatment of environmental pollutants
- Enhanced adsorption of Cu(ii) from wastewater using potassium humate-modified coconut husk biochar
- Adsorption of heavy metal ions from water by Fe3O4 nano-particles
- Green synthesis of parsley-derived silver nanoparticles and their enhanced antimicrobial and antioxidant effects against foodborne resistant bacteria
- Unwrapping the phytofabrication of bimetallic silver–selenium nanoparticles: Antibacterial, Anti-virulence (Targeting magA and toxA genes), anti-diabetic, antioxidant, anti-ovarian, and anti-prostate cancer activities
- Optimizing ultrasound-assisted extraction process of anti-inflammatory ingredients from Launaea sarmentosa: A novel approach
- Eggshell membranes as green carriers for Burkholderia cepacia lipase: A biocatalytic strategy for sustainable wastewater bioremediation
- Research progress of deep eutectic solvents in fuel desulfurization
- Enhanced electrochemical synthesis of Ni–Fe/brass foil alloy with subsequent combustion for high-performance photoelectrode and hydrogen production applications
- Valorization of baobab fruit shell as a filler fiber for enhanced polyethylene degradation and soil fertility
- Valorization of Agave durangensis bagasse for cardboard-type paper production circular economy approach
- Green priming strategies using seaweed extract and citric acid to improve early growth and antioxidant activity in lentil
- Gold extraction from oxide ore using iron ion-thiourea-additive
- Development of loess-derived P-type molecular sieve as a sustainable antibacterial agent
- Review Article
- Sustainable innovations in garlic extraction: A comprehensive review and bibliometric analysis of green extraction methods
- Natural sustainable coatings for marine applications: advances, challenges, and future perspectives
- Integration of traditional medicinal plants with polymeric nanofibers for wound healing
- Rapid Communication
- In situ supported rhodium catalyst on mesoporous silica for chemoselective hydrogenation of nitriles to primary amines
- Special Issue: Valorisation of Biowaste to Nanomaterials for Environmental Applications
- Valorization of coconut husk into biochar for lead (Pb2+) adsorption
- Corrigendum
- Corrigendum to “An updated review on carbon nanomaterials: Types, synthesis, functionalization and applications, degradation and toxicity”

