Abstract
Knowledge of the kinetics of polymerization plays a crucial role in the optimization of a synthesis process with appropriate polymerization rate and product quality. The polymerization of acryloyloxyethyl trimethyl ammonium chloride (DAC) was investigated using a dilatometer method in an aqueous solution. The impacts of temperature, monomer concentration, and initiator concentration on the polymerization rate were examined. The results showed that the polymerization rate increased with an increase in the temperature, monomer concentration, and initiator concentration. The activation energy of polymerization under the given conditions of 2.07 mol·L−1 DAC, 1.72 × 10−3 mol·L−1 ammonium persulfate, and 1.05 × 10−4 mol·L−1 Na4 EDTA was E a = 85.25 kJ·mol−1. The overall polymerization rate equation was R p = K[M]1.69[I]0.53. Based on the experimental results, the mechanism of polymerization was discussed in detail. The studies supplied the experimental basis for the industrial implementation of this reaction.
Graphical abstract
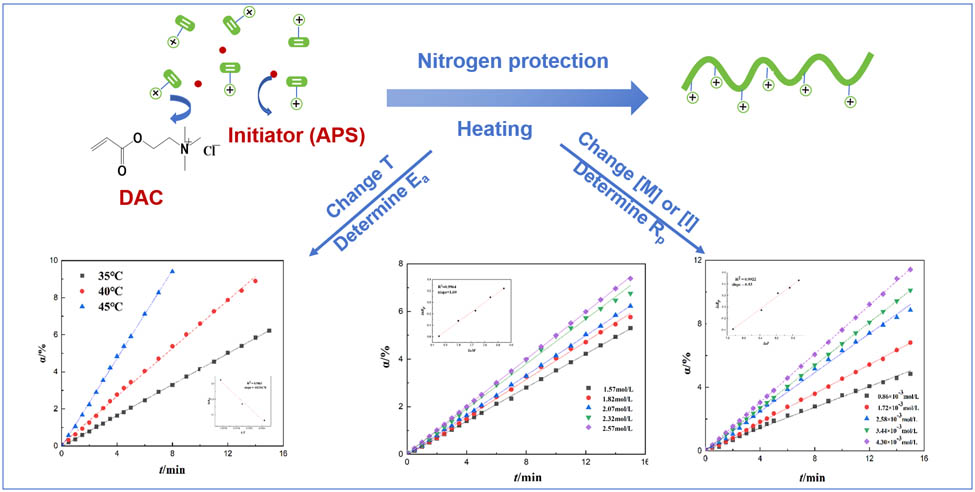
1 Introduction
Acryloyloxyethyl trimethyl ammonium chloride (DAC) is a quaternary ammonium monomer that contains a double bond, ester group, and quaternary ammonium group. It can undergo homopolymerization or copolymerization with other monomers (1). The polymers offer several advantages, including good water solubility, low toxicity, and controllable molecular weight. As a result, these polymers have found wide applications in various fields, such as oil extraction, papermaking, textile manufacturing, water treatment, biomedicine, and electronic chemicals (2,3,4). However, a specific polymer can also exhibit different properties owing to the various molecular structures, e.g., molar mass distribution, tacticity, and chain topology. These structures largely depend on the process of polymer production in a system, including reactor configurations, operation strategies, and polymerization kinetics (5). Therefore, a thorough understanding of the kinetics is crucial for the industrial implementation and effective application of these polymers. Achieving a high conversion rate in the production of polymers also necessitates a comprehensive understanding of the polymerization kinetics (6,7).
Currently, research on the synthesis methods of DAC polymers is diverse, with limited focus on the polymerization kinetics. Typically, polymerization kinetics are influenced by various factors, including the initiator concentration, monomer concentration, and temperature (6,8,9). However, there are studies available on the polymerization kinetics of similar cationic polymers. Kattner and Buback, for instance, used a single pulse–pulsed laser polymerization–electron paramagnetic resonance method to measure the rate coefficients of the termination, intramolecular transfer, and propagation for the radical polymerization of 20 wt% DAC in the temperature range 0–90°C (10). Wang and Zhang measured the polymerization rate equation for the poly-methyl acrylamide propyl trimethyl ammonium chloride (PMAPTAC) R p = K[M]1.64[I]0.71 and the activation energies E a = 153.09 kJ·mol−1 using the dilatometer method (11). Chen and Shan derived the polymerization rate equation for the inverse emulsion polymerization of acrylamide (AM) and methyl acryloyl oxygen ethyl trimethyl ammonium chloride (DMC) as R p = K[M]2.12[I]0.55[E]0.65, with an apparent activation energy of 80.65 kJ·mol−1 (12). Ayatzhan et al. obtained the polymerization rate equation for the poly-N,N-dimethyl-N,N-diallylammonium chloride (DMDAAC) and N,N-dimethyl acrylamide (DMAA) R p = K[M]2.63[I]0.40[M DMDAAC:M DMAA]−0.86 and E a = 39.56 kJ·mol−1 (6). It is evident that studies on DAC polymers were predominantly focused both domestically and internationally on the synthesis process and properties (13,14,15). However, there has been only a limited amount of research on its polymerization kinetics, an aspect that has not been as extensively explored compared to other cationic polymers. The polymerization kinetics can be investigated using different methods, such as differential scanning calorimetry (16), polarographic study (17), electrochemical methods (10), gravimetry (18), and dilatometry (6). The dilatometer method is the most common method, because of its simple operation and low cost.
The dilatometer method was utilized in this study to investigate the polymerization kinetics of poly-acryloyloxyethyl trimethyl ammonium chloride (PDAC). The polymerization rate equation and apparent activation energy were determined, and the influence of the monomer concentration and initiator concentration on the aqueous solution polymerization was determined. The studies supplied the experimental basis for the industrial implementation.
2 Experiments
2.1 Materials
DAC used in this study was of technical grade, whereas the other reagents were all of analytical reagent grade. The details of the reagents used in this study were as follows: DAC (Jiangsu Fumiao Technology Co., Ltd., Suzhou, China), ammonium persulfate (APS) (Tianjin Degussa Initiator Co., Ltd., Tianjin, China), and ethylenediamine tetraacetic acid tetrasodium salt (Na4 EDTA; Sinopharm Group Co., Ltd., Shanghai, China).
2.2 Preparation of the reaction solution
An exact amount of 12.89 g of DAC solution in water with 81.48% mass concentration, 16.69 mL of distilled water, and 0.21 mL of Na4EDTA with 1% mass concentration were placed into a four-necked flask equipped with a thermometer, a stirrer, and a nitrogen inlet and outlet tube. Then, the reaction mixture was stirred at room temperature under a nitrogen atmosphere for 20 min. About 0.21 mL of the initiator (APS) solution with 0.05% mass concentration was added to the flask. After 10 min of stirring, the reaction solution was obtained, which contained 2.07 mol·L−1 DAC, 1.72 × 10−3 mol·L−1 APS, and 1.05 × 10−4 mol·L−1 Na4EDTA. The polymerization reaction of PDAC is shown in Figure 1.

The polymerization reaction of PDAC.
2.3 Characterization
The FTIR spectra of the monomer and homo-polymer were recorded using an infrared spectrometer (FTLA2000, ABB Bomen Inc., Canada). The 1H NMR spectra of the monomer and homo-polymer were recorded using a 1H NMR spectrometer (Avance Ⅲ-500Hz, Bruker Corporation, Germany) in deuterium oxide.
2.4 Determination of polymerization kinetics
2.4.1 Determination of the activation energy
The polymerization rate was determined using the dilatometer method, which measured the volume change of the reaction mixture during polymerization (19). A specified amount of the reaction solution was placed in the dilatometer and immersed in a temperature-controlled water bath. The initial volume (V 0) was recorded. Upon initiation of polymerization, the volume started to decrease. The volume (V t) was continuously monitored over time, and the reaction solution was promptly removed from the water bath. Subsequently, the monomer conversion rate (α) was determined with Eq. 1 using the bromate–bromide titration method (Chinese National Standard GB/T 22312-2008). The polymerization rates at 35–45°C were measured. According to the Arrhenius equation (Eq. 2), by plotting ln (R p) against 1/T (where T is the temperature) as shown in Eq. 3, the slope of the graph was used to calculate E a (20,21,22):
where k is the reaction rate constant, A is the frequency factor, R is the gas constant, R p is the total rate of polymerization, [M] is the concentration of the monomer, and [I] is the concentration of the initiator.
The polymerization rate, R p, quantifies the conversion of monomer to polymer per unit time and practically illustrates the evolution of the monomer conversion rate over time. During the initial phase of the polymerization reaction, with a conversion rate below 10%, the conversion rate α was directly proportional to the reaction time (11). Consequently, the slope of the line was computed to ascertain the average polymerization reaction rate (R p), as described in Eq. 4.
where [M 0] is the initial monomer concentration.
2.4.2 Determination of the polymerization rate equation
The rate equation of the polymerization reaction is directly linked to the concentrations of the monomer and initiator and exhibits an exponential relationship (22,23). The slope (dα/dt) of the time (t)–the monomer conversion rate (α) curve in this study was obtained using the linear regression method and the polymerization rate equation using the following equation:
where k
p, k
t, and k
d are the chain growth rate constant, initiator decomposition rate constant, and chain termination rate constant, respectively, and
3 Results and discussion
3.1 Characterization
The FTIR spectra of the monomer DAC and homo-polymer PDAC were investigated and the results are shown in Figure 2(a). In the DAC spectrum, the characteristic absorption peaks of N+(CH3)3 and –COO– are found at 946 and 1,190, and 1,730 cm−1, respectively, which are displayed at 951 and 1,160, and 1,720 cm−1 in the PDAC spectrum; the characteristic absorption peaks of CH3═CH2 and C═O are found at 3,010 and 2,980, and 161 cm−1, respectively, which disappear in the PDAC spectrum. The 1H NMR spectra are shown in Figure 2(b). In the DAC spectrum, δ at 6.01–6.44 ppm are the multiple absorption peaks on the CH2═CH– group, δ at 4.63 ppm is the absorption peak on the –CH2– group connected to the carbonyl group, and δ at 3.78 ppm is the absorption peak on the –CH2– group connected to the N group. The absorption peak of δ at 3.22 ppm is the absorption peak on the –N(CH3)3 group. In the PDAC spectrum, the multiple absorption peaks on the CH2═CH– group disappear. These results are shown by the polymerization reaction of DAC.
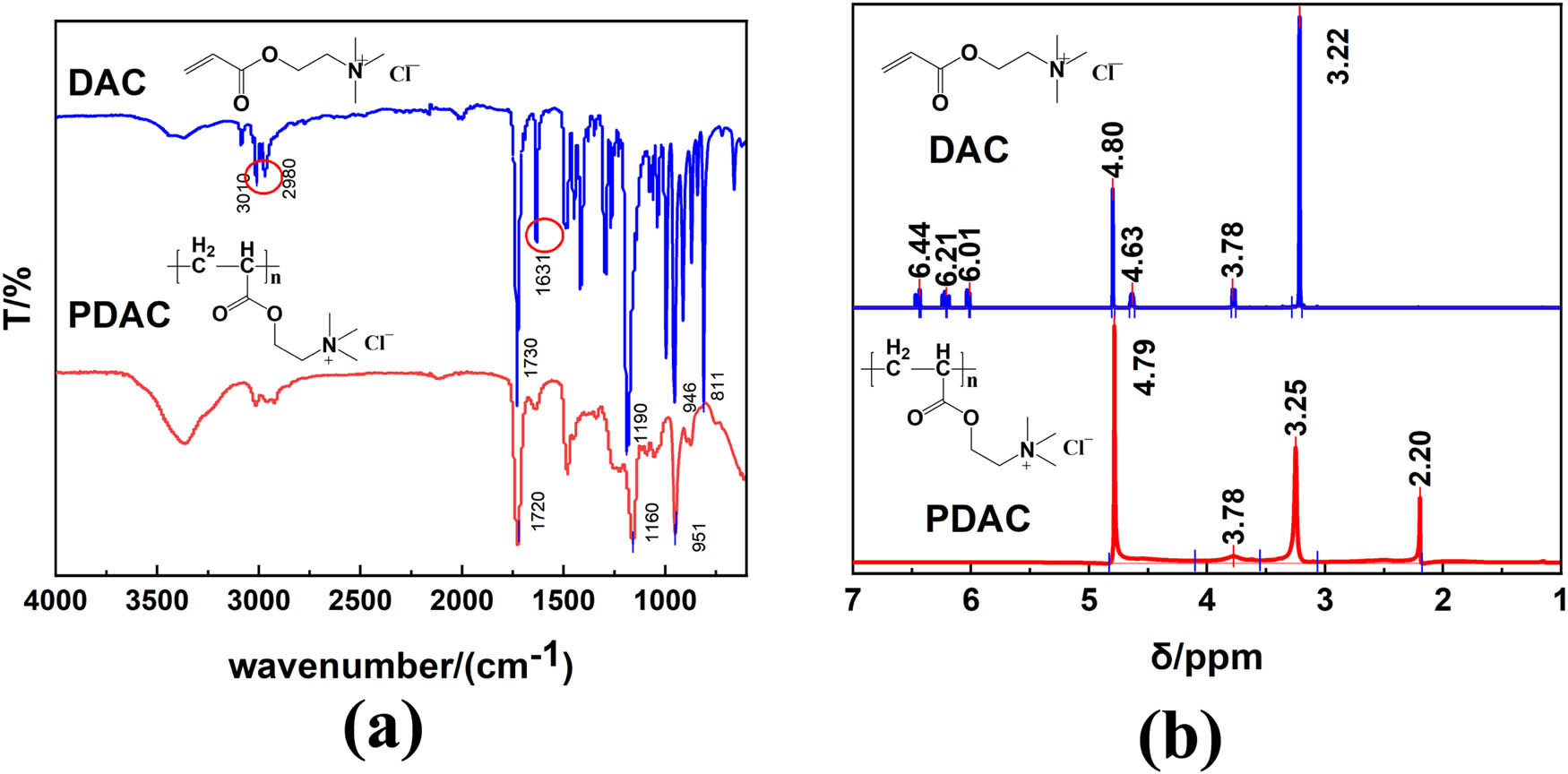
FTIR spectra (a) and 1H NMR spectra (b) of DAC and PDAC.
3.2 Determination of E a
The impact of reaction temperatures on R p was examined by adjusting the reaction temperature within the range of 35–45°C. The findings are presented in Figure 3 and Table 1. From Figure 3, it is evident that the conversion rate corresponding to a given volume shrinkage increased with increasing temperature. Furthermore, the conversion rate increased with greater volume shrinkage at constant temperatures. When temperatures varied, the conversion rate (α) increased more rapidly at higher temperatures in tandem with the volume shrinkage rate, indicating a steeper slope. This phenomenon aligns with the polymerization reaction formula, where heightened reaction temperatures accelerate the generation rate of free radicals during the initial reaction phase. As the concentration of free radicals increased, the reaction velocity increased, consistent with the impact of temperature on the polymerization reaction rate (24,25). Essentially, as the polymerization temperature increased, both the polymerization rate (R p) and the conversion rate (α) increased.
![Figure 3
Dependence of α on ΔV at different temperatures; conditions: [M] = 2.07 mol·L−1, and [I] = 1.72 × 10−3 mol·L−1.](/document/doi/10.1515/epoly-2024-0049/asset/graphic/j_epoly-2024-0049_fig_003.jpg)
Dependence of α on ΔV at different temperatures; conditions: [M] = 2.07 mol·L−1, and [I] = 1.72 × 10−3 mol·L−1.
Linear fitting results of ΔV and α
| Temperature (°C) | Linear fitting equation | R 2 |
|---|---|---|
| 35 | y = 90.75x + 0.062 | 0.9986 |
| 40 | y = 102.05x + 0.170 | 0.9975 |
| 45 | y = 147.20x + 0.169 | 0.9978 |
The fitted curve demonstrated outstanding linearity, with R 2 values surpassing 0.9975. Moreover, utilizing the fitting equation from Table 1, the relationship between the polymerization reaction time and conversion rate could be established by examining the relationship between time and ΔV throughout the polymerization process, as depicted in Figure 4.
![Figure 4
Dependence of α on t at different temperatures; conditions: [M] = 2.07 mol·L−1 and [I] = 1.72 × 10−3 mol·L−1.](/document/doi/10.1515/epoly-2024-0049/asset/graphic/j_epoly-2024-0049_fig_004.jpg)
Dependence of α on t at different temperatures; conditions: [M] = 2.07 mol·L−1 and [I] = 1.72 × 10−3 mol·L−1.
Eq. 3 demonstrates a direct linear relationship between ln R p and 1/T. The slope of the line, derived from fitting the data, is shown in Figure 5 and corresponds to −E a/R. Consequently, the activation energy, E a, for the DAC polymerization reaction was determined to be 85.25 kJ·mol−1. This value was lower than that of the similar cationic polymer PMAPTAC (E a = 153.09 kJ·mol−1) (10) and higher than the copolymer P(DMC-AM) (80.65 kJ·mol−1) (11). This difference is attributed to the significant influence of groups on the double bond on the monomer’s polymerization activity. The methyl group on the double bond of the MAPTAC monomer diminishes the monomer’s activity, while the amide group on the double bond of the AM monomer significantly enhances the monomer’s polymerization activity, thus affecting the activation energy of the polymerization reaction (26). The total activation energy E a was positive, indicating that, with an increase in the temperature, the rate constant k increased, and the total polymerization rate also increased. A higher total activation energy E a resulted in a greater effect of temperature on the polymerization rate. Polymerization temperature must be controlled in industrial production.
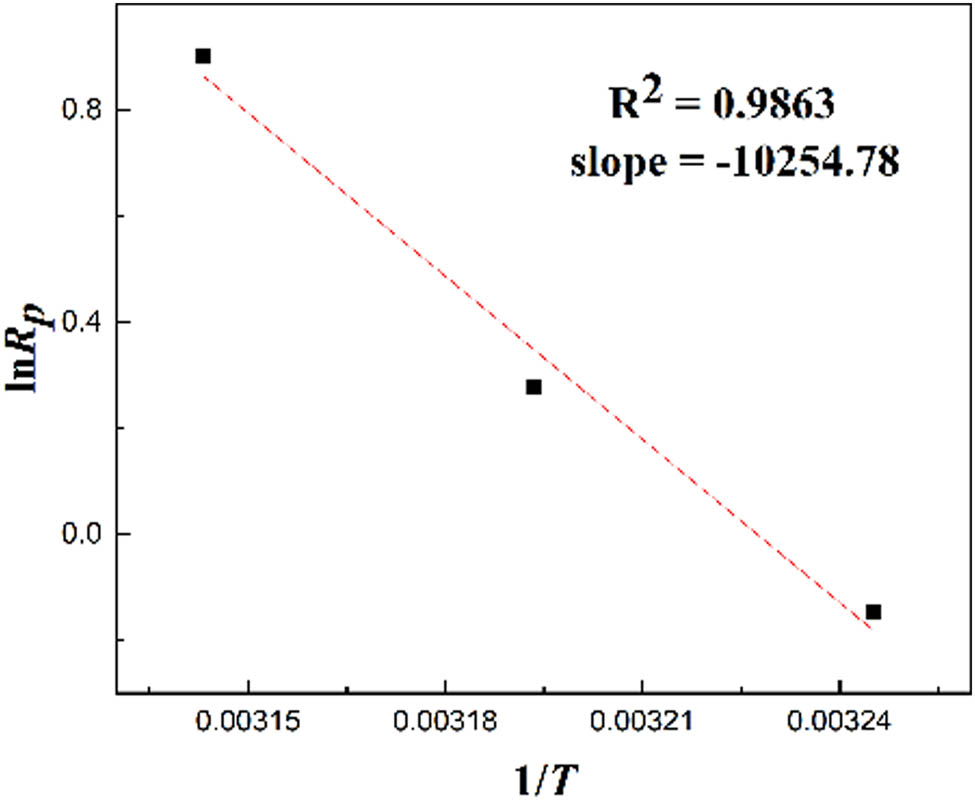
The relationship between ln R p and 1/T.
3.3 Determination of R p
3.3.1 Effect of the monomer concentration on R p
Following the method of determining the polymerization reaction rate equation (Eq. 4), the monomer concentration was sequentially altered, as shown in Figure 6, and then the DAC polymerization reaction rate equation could be obtained. Figure 6 illustrates the dependence of polymerization conversion and rate on the total monomer concentration. It was shown that, simultaneously, a higher monomer concentration led to a higher conversion rate. We propose that this phenomenon may be attributed to the formation of relatively active centers, resulting in strong interactions between the radicals (6).
![Figure 6
The relationship between α and t at different monomer concentrations; conditions: [I] = 1.72 × 10−3 mol·L−1 and T = 40°C.](/document/doi/10.1515/epoly-2024-0049/asset/graphic/j_epoly-2024-0049_fig_006.jpg)
The relationship between α and t at different monomer concentrations; conditions: [I] = 1.72 × 10−3 mol·L−1 and T = 40°C.
By fitting ln R p and ln M linearly based on the slopes of the straight lines for each monomer concentration in Figure 6 and Formula 5, as shown in Figure 7, the influence of the monomer concentration on the polymerization rate was determined to be R p ∝ [M]1.69. The monomer concentration index was notably higher than the theoretical value of 1 in the first-order reaction kinetics equation for free radical polymerization. This deviation may stem from the intricate interactions among the monomers, such as ion pairs and polarity effects, which could influence alterations in the free radical polymerization process. The results indicate that the DMC monomer had a great impact due to the steric hindrance (27).
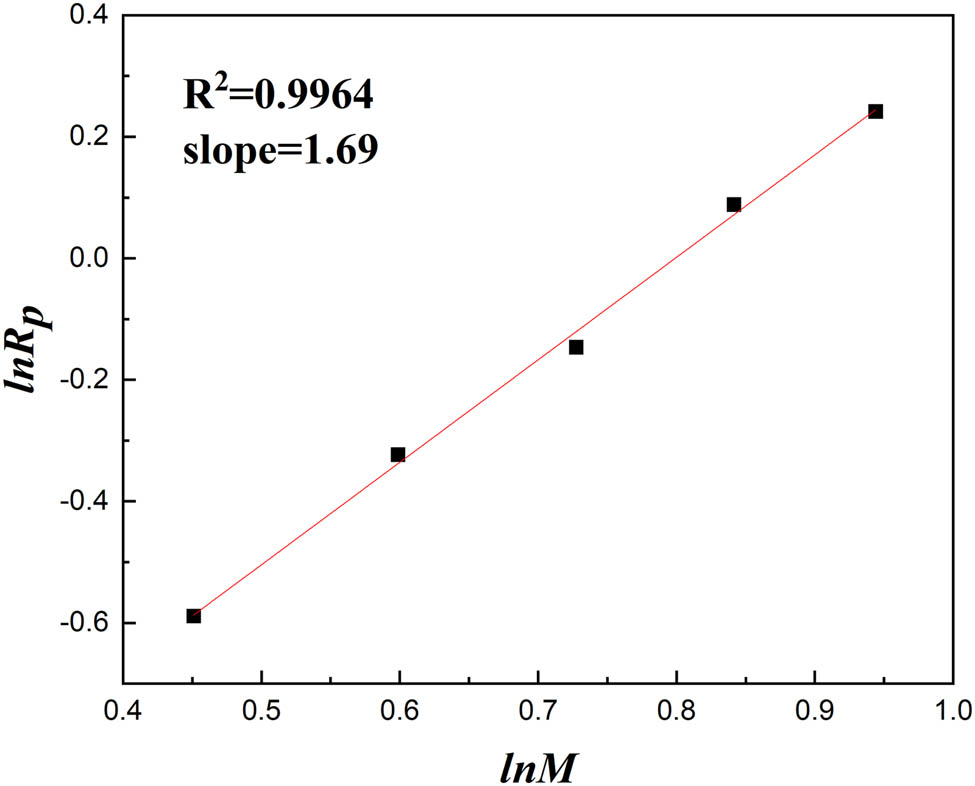
The relationship between ln M and ln R p.
3.3.2 Effect of initiator concentration on R p
With other basic process conditions being identical, the conversion rates (α) over time were determined at initiator concentrations of 0.86 × 10−3 mol·L−1, 1.72 × 10−3 mol·L−1, 2.58 × 10−3 mol·L−1, 3.44 × 10−3 mol·L−1, and 4.30 × 10−3 mol·L−1, as shown in Figure 8. This indicates that as the initiator concentrations increased, the conversion rate also increased simultaneously. Furthermore, at all times, a higher initiator concentration led to a more rapid increase in the conversion rate, resulting in a steeper slope. These phenomena could be attributed to the high concentration of the initiator, which offered an increased number of primary free radicals for interacting with the monomer molecules (28). The relationship between the polymerization rate with respect to the initiator concentration was R p ∝ [I]0.53, as shown in Figure 9. The initiator concentration index was slightly higher than the theoretical value of 0.5 in the first-order reaction kinetics equation for free radical polymerization. This implies that the primary chain termination mechanism in the reaction was predominantly double-base termination, and there was also a small number of single-base termination (6,11).
![Figure 8
The relationship between α and t at different initiator concentrations; [M] = 2.07 mol·L−1 and T = 40°C.](/document/doi/10.1515/epoly-2024-0049/asset/graphic/j_epoly-2024-0049_fig_008.jpg)
The relationship between α and t at different initiator concentrations; [M] = 2.07 mol·L−1 and T = 40°C.
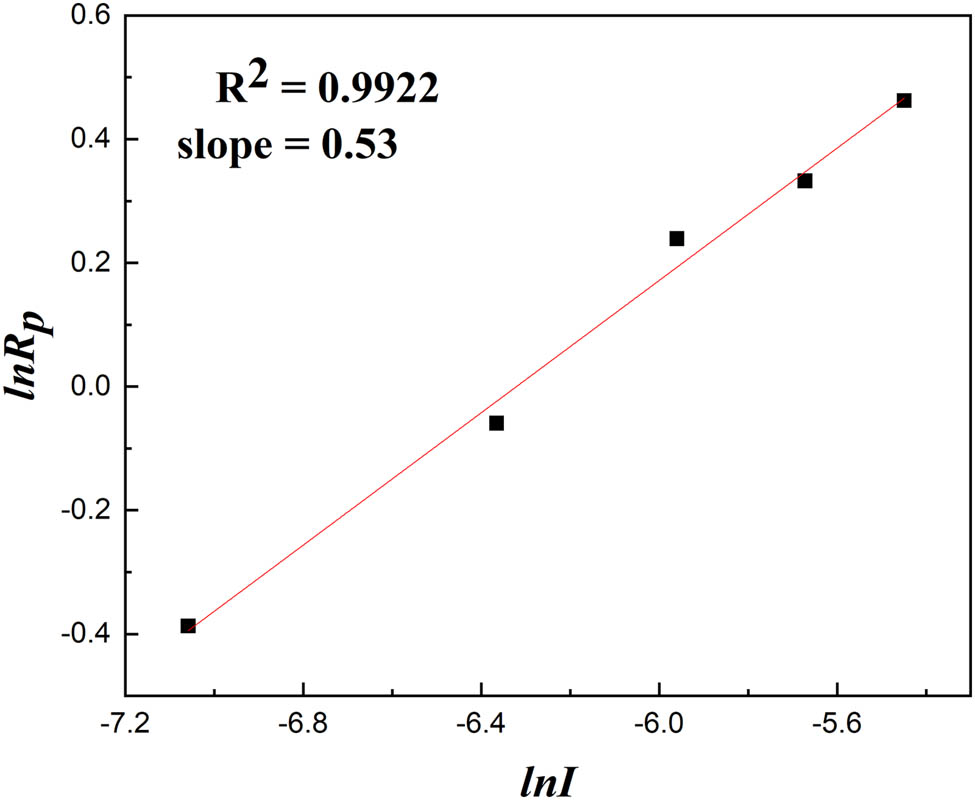
The relationship between ln I and ln R p.
4 Conclusions
In this study, the polymerization of PDAC through free radical polymerization in the presence of an ammonium persulfate initiator was investigated using a dilatometer method, and its kinetic parameters were examined. It was found that the polymerization rate increased with t increases in temperature and concentrations of the monomer and initiator. The activation energy of polymerization under the given conditions was E a = 85.25 kJ·mol−1. The overall polymerization rate equation was R p = K[M]1.69[I]0.53. This suggests that the complex interactions between monomers influenced alterations in the free radical polymerization process, and the primary chain termination mechanism in the reaction was predominantly double-base termination.
-
Funding information: This work was supported by the Scientific Research Foundation for High Level Talent of West Anhui University (WGKQ2022092, WGKQ2022003), Scientific Research Project of West Anhui University(WXZR202302), the Excellent Research and Innovation Team Project of Anhui Province (2023AH010077), and Anhui Scientific Research and Innovation Team of Quality Evaluation and Improvement of Traditional Chinese Medicine (2022AH010090).
-
Author contributions: Xingqin Fu: methodology, validation, writing – original draft, and writing – review & editing. Yifan Wang: methodology, data curation, and investigation. Huiqing Hu: validation, investigation, and resources. Wei Fu: formal analysis, visualization, and validation. Liangying Wang: data curation and investigation. Juncheng Jin: Project administration, conceptualization, resources, supervision, and writing – review & editing.
-
Conflict of interest: Authors state no conflict of interest.
References
(1) Khan S, Zheng H, Sun Q, Liu Y, Li H, Ding W, et al. Synthesis and characterization of a novel cationic polyacrylamide-based flocculants to remove Congo red efficiently in acid aqueous environment. J Mater Sci: Mater Electron. 2020;31:18832–43. 10.1007/s10854-020-04422-3.Suche in Google Scholar
(2) Foroughirad S, Haddadi-Asl V, Khosravi A, Salami-Kalajahi M. Synthesis of magnetic nanoparticles-decorated halloysite nanotubes/poly([2-(acryloyloxy)ethyl]trimethylammonium chloride) hybrid nanoparticles for removal of sunset yellow from water. J Polym Res. 2020;27(10):320–32. 10.1007/s10965-020-02293-0.Suche in Google Scholar
(3) Men J, Guo J, Zhou W, Dong N, Pang X, Gao B. Preparation of cationic functional polymer poly(acryloxyethyltrimethyl ammonium chloride)/SiO2 and its adsorption characteristics for heparin. Korean J Chem Eng. 2017;34(7):1889–95. 10.1007/s11814-017-0131-0.Suche in Google Scholar
(4) Kostova B, Georgieva D, Dundarova M, Ivanova S, Ivanova-Mileva K, Tzankova V, et al. Design and study of the potential of crosslinked cationic polymers as drug delivery systems for dermal application. J Appl Polym Sci. 2018;135(26):46420–9. 10.1002/app.46420.Suche in Google Scholar
(5) Zhou Y-N, Li J-J, Wu Y-Y, Luo Z-H. Role of external field in polymerization: mechanism and kinetics. Chem Rev. 2020;120(5):2950–3048. 10.1021/acs.chemrev.9b00744.Suche in Google Scholar PubMed
(6) Ayatzhan A, Tashenov A, Nurgeldi A, Zhanar O, Zhexenbek T, Kaldibek A, et al. P(DADMAAC-co-DMAA): synthesis, thermal stability, and kinetics. Polym Adv Technol. 2023;32(7):2669–75. 10.1002/pat.4999.Suche in Google Scholar
(7) Wehner M, Würthner F. Supramolecular polymerization through kinetic pathway control and living chain growth. Nat Rev Chem. 2020;4(1):38–53. 10.1038/s41570-019-0153-8.Suche in Google Scholar
(8) Menager C, Guigo N, Vincent L, Sbirrazzuoli N. Polymerization kinetic pathways of epoxidized linseed oil with aliphatic bio‐based dicarboxylic acids. J Polym Sci. 2020;58(12):1717–27. 10.1002/pol.20200118.Suche in Google Scholar
(9) Issa A, Luyt A. Kinetics of alkoxysilanes and organoalkoxysilanes polymerization: a review. Polymers. 2019;11(3):537–77. 10.3390/polym11030537.Suche in Google Scholar PubMed PubMed Central
(10) Kattner H, Buback M. Termination, transfer, and propagation kinetics of trimethylaminoethyl acrylate chloride radical polymerization in aqueous solution. Macromolecules. 2017;50(11):4160–8. 10.1021/acs.macromol.7b00328.Suche in Google Scholar
(11) Wang Y, Zhang Y. Kinetics of poly(3‐methacryloylamido propyl trimethyl ammonium chloride) initiated by different initiators. Polym Adv Technol. 2021;32(6):2409–15. 10.1002/pat.5269.Suche in Google Scholar
(12) Chen Y, Shan G. Kinetics of acrylamide and 2-methylacryloylxyethyltrimethyl ammonium chloride in inverse emulsion polymerization. CIESC J. 2018;69(2):563–9. 10.11949/j.issn.0438-1157.20170870.Suche in Google Scholar
(13) Sun W, Zhou S, Sun Y, Xu Y. Synthesis and evaluation of cationic flocculant P(DAC-PAPTAC-AM) for flocculation of coal chemical wastewater. J Environ Sci. 2021;99:239–48. 10.1016/j.jes.2020.07.001.Suche in Google Scholar PubMed
(14) Chen L, Sun Y, Sun W, Shah KJ, Xu Y, Zheng H. Efficient cationic flocculant MHCS-g-P(AM-DAC) synthesized by UV-induced polymerization for algae removal. Sep Purif Technol. 2019;210:10–9. 10.1016/j.seppur.2018.07.090.Suche in Google Scholar
(15) Zhou Y, Zheng H, Wang Y, Zhao R, Liu H, Ding W, et al. Enhanced municipal sludge dewaterability using an amphiphilic microblocked cationic polyacrylamide synthesized through ultrasonic-initiation: Copolymerization and flocculation mechanisms. Colloids Surf, A. 2020;594:124645–55. 10.1016/j.colsurfa.2020.124645.Suche in Google Scholar
(16) Achilias DS. Investigation of the radical polymerization kinetics using DSC and mechanistic or isoconversional methods. J Therm Anal Calorim. 2014;116:1379–86. 10.1007/s10973-013-3633-y.Suche in Google Scholar
(17) Ergozhin E, Mukhitdinova B, Shoinbekova S, Nikitina A, Nuranbaeva B. Kinetics of radical polymerization of a monomer derived from monoethanolamine vinyl ether and 1, 4-benzoquinone: A polarographic study. Russ J Appl Chem. 2003;76(3):460–3. 10.1023/A:1025621305767.Suche in Google Scholar
(18) Lin HR. Solution polymerization of acrylamide using potassium persulfate as an initiator: kinetic studies, temperature and pH dependence. Eur Polym J. 2001;37(7):1507–10. 10.1016/S0014-3057(00)00261-5.Suche in Google Scholar
(19) Batalov VS. The measurement procedure in the dilatometer method of determining the thermal diffusivity of materials. J Eng Phys. 1974;27(1):835–8. 10.1007/BF00827623.Suche in Google Scholar
(20) Nifant’ev I, Shlyakhtin A, Bagrov V, Lozhkin B, Zakirova G, Ivchenko P, et al. Theoretical and experimental studies of 1,5,7-triazabicyclo[4.4.0]dec-5-ene-catalyzed ring opening/ring closure reaction mechanism for 5-, 6- and 7-membered cyclic esters and carbonates. React Kinet Mech Catal. 2015;117(2):447–76. 10.1007/s11144-015-0952-y.Suche in Google Scholar
(21) Moad G. A critical assessment of the kinetics and mechanism of initiation of radical polymerization with commercially available dialkyldiazene initiators. Prog Polym Sci. 2019;88:130–88. 10.1016/j.progpolymsci.2018.08.003.Suche in Google Scholar
(22) Feng L, Liu J, Xu C, Lu W, Li D, Zhao C, et al. Better understanding the polymerization kinetics of ultrasonic-template method and new insight on sludge floc characteristics research. Sci Total Environ. 2019;689:546–56. 10.1016/j.scitotenv.2019.06.475.Suche in Google Scholar PubMed
(23) Ballard N, Asua JM. Radical polymerization of acrylic monomers: an overview. Prog Polym Sci. 2018;79:40–60. 10.1016/j.progpolymsci.2017.11.002.Suche in Google Scholar
(24) Ajino K, Torii A, Ogawa H, Mori H. Synthesis of ion-conductive polymers by radical polymerization of deep eutectic monomers bearing quaternary ammonium groups with urea. Polymer. 2020;204:122803–21. 10.1016/j.polymer.2020.122803.Suche in Google Scholar
(25) Li X, Zheng H, Gao B, Sun Y, Tang X, Xu B. Optimized preparation of micro-block CPAM by response surface methodology and evaluation of dewatering performance. RSC Adv. 2017;7(1):208–17. 10.1039/C6RA25245A.Suche in Google Scholar
(26) Fu X, Zhang Y, Jia X, Wang Y, Chen T. Research progress on typical quaternary ammonium salt polymers. Molecules. 2022;27(4):1267–95. 10.3390/molecules27041267.Suche in Google Scholar PubMed PubMed Central
(27) Dauletov Y, Abdiyev K, Toktarbay Z, Nuraje N, Zhursumbaeva M, Kenzhaliyev B. Radical polymerization and kinetics of N,N-diallyl-N,N-dimethylammonium chloride and vinyl ether of monoethanolamine. Fibers Polym. 2018;19(10):2023–9. 10.1007/s12221-018-6947-3.Suche in Google Scholar
(28) Jia X, Wang M, Yang K, Zhang Y, Wang G, Tao X. Effect of substitution groups on the homopolymerization activity of methyl aryl diallyl ammonium chloride. Polym Adv Technol. 2023;34(8):2684–93. 10.1002/pat.6082.Suche in Google Scholar
© 2025 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- Flow-induced fiber orientation in gas-powered projectile-assisted injection molded parts
- Research on thermal aging characteristics of silicone rubber composite materials for dry-type distribution transformers
- Kinetics of acryloyloxyethyl trimethyl ammonium chloride polymerization in aqueous solutions
- Influence of siloxane content on the material performance and functional properties of polydimethylsiloxane copolymers containing naphthalene moieties
- Enhancement effect of electron beam irradiation on acrylonitrile–butadiene–styrene (ABS) copolymers from waste electrical and electronic equipment by adding 1,3-PBO: A potential way for waste ABS reuse
- Model construction and property study of poly(ether-ether-ketone) by molecular dynamics simulation with meta-modeling methods
- Zinc–gallic acid–polylysine nanocomplexes with enhanced bactericidal activity for the treatment of bacterial keratitis
- Effect of pyrogallol compounds dosage on mechanical properties of epoxy coating
- Preparation of in situ polymerized polypyrrole-modified braided cord and its electrical conductivity investigation under varied mechanical conditions
- Hydrophobicity, UV resistance, and antioxidant properties of carnauba wax-reinforced CG bio-polymer film
- Janus nanofiber membrane films loading with bioactive calcium silicate for the promotion of burn wound healing
- Synthesis of migration-resistant antioxidant and its application in natural rubber composites
- Influence of the flow rate on the die swell for polymer micro coextrusion process
- Fatty acid filled polyaniline nanofibres with dual electrical conductivity and thermo-regulatory characteristics: Futuristic material for thermal energy storage
- Hydrolytic depolymerization of major fibrous wastes
- Performance of epoxy hexagonal boron nitrate underfill materials: Single and mixed systems
- Blend electrospinning of citronella or thyme oil-loaded polyurethane nanofibers and evaluating their release behaviors
- Efficiency of flexible shielding materials against gamma rays: Silicon rubber with different sizes of Bi2O3 and SnO
- A comprehensive approach for the production of carbon fibre-reinforced polylactic acid filaments with enhanced wear and mechanical behaviour
- Electret melt-blown nonwovens with charge stability for high-performance PM0.3 purification under extreme environmental conditions
- Study on the failure mechanism of suture CFRP T-joints under/after the low-velocity impact loading
- Experimental testing and finite element analysis of polyurethane adhesive joints under Mode I loading and degradation conditions
- Optimizing recycled PET 3D printing using Taguchi method for improved mechanical properties and dimensional precision
- Effect of stacking sequence of the hybrid composite armor on ballistic performance and damage mechanism
- Bending crack propagation and delamination damage behavior of orthogonal ply laminates under positive and negative loads
- Molecular dynamics simulation of thermodynamic properties of Al2O3-modified silicone rubber under silane coupling agent modification
- Precision injection molding method based on V/P switchover point optimization and pressure field balancing
- Heparin and zwitterion functionalized small-diameter vascular grafts for thrombogenesis prevention
- Metal-free N, S-co-doped carbon materials derived from calcined aromatic co-poly(urea-thiourea)s as efficient alkaline oxygen reduction catalysts
- Influence of stitching parameters on the tensile performance and failure mechanisms of CFRP T-joints
- Synthesis of PEGylated polypeptides bearing thioether pendants for injectable ROS-responsive hydrogels
- Rapid Communication
- RAFT-mediated polymerization-induced self-assembly of poly(ionic liquid) block copolymers in a green solvent
- Corrigendum
- Corrigendum to “High-strength polyvinyl alcohol-based hydrogel by vermiculite and lignocellulosic nanofibrils for electronic sensing”
Artikel in diesem Heft
- Research Articles
- Flow-induced fiber orientation in gas-powered projectile-assisted injection molded parts
- Research on thermal aging characteristics of silicone rubber composite materials for dry-type distribution transformers
- Kinetics of acryloyloxyethyl trimethyl ammonium chloride polymerization in aqueous solutions
- Influence of siloxane content on the material performance and functional properties of polydimethylsiloxane copolymers containing naphthalene moieties
- Enhancement effect of electron beam irradiation on acrylonitrile–butadiene–styrene (ABS) copolymers from waste electrical and electronic equipment by adding 1,3-PBO: A potential way for waste ABS reuse
- Model construction and property study of poly(ether-ether-ketone) by molecular dynamics simulation with meta-modeling methods
- Zinc–gallic acid–polylysine nanocomplexes with enhanced bactericidal activity for the treatment of bacterial keratitis
- Effect of pyrogallol compounds dosage on mechanical properties of epoxy coating
- Preparation of in situ polymerized polypyrrole-modified braided cord and its electrical conductivity investigation under varied mechanical conditions
- Hydrophobicity, UV resistance, and antioxidant properties of carnauba wax-reinforced CG bio-polymer film
- Janus nanofiber membrane films loading with bioactive calcium silicate for the promotion of burn wound healing
- Synthesis of migration-resistant antioxidant and its application in natural rubber composites
- Influence of the flow rate on the die swell for polymer micro coextrusion process
- Fatty acid filled polyaniline nanofibres with dual electrical conductivity and thermo-regulatory characteristics: Futuristic material for thermal energy storage
- Hydrolytic depolymerization of major fibrous wastes
- Performance of epoxy hexagonal boron nitrate underfill materials: Single and mixed systems
- Blend electrospinning of citronella or thyme oil-loaded polyurethane nanofibers and evaluating their release behaviors
- Efficiency of flexible shielding materials against gamma rays: Silicon rubber with different sizes of Bi2O3 and SnO
- A comprehensive approach for the production of carbon fibre-reinforced polylactic acid filaments with enhanced wear and mechanical behaviour
- Electret melt-blown nonwovens with charge stability for high-performance PM0.3 purification under extreme environmental conditions
- Study on the failure mechanism of suture CFRP T-joints under/after the low-velocity impact loading
- Experimental testing and finite element analysis of polyurethane adhesive joints under Mode I loading and degradation conditions
- Optimizing recycled PET 3D printing using Taguchi method for improved mechanical properties and dimensional precision
- Effect of stacking sequence of the hybrid composite armor on ballistic performance and damage mechanism
- Bending crack propagation and delamination damage behavior of orthogonal ply laminates under positive and negative loads
- Molecular dynamics simulation of thermodynamic properties of Al2O3-modified silicone rubber under silane coupling agent modification
- Precision injection molding method based on V/P switchover point optimization and pressure field balancing
- Heparin and zwitterion functionalized small-diameter vascular grafts for thrombogenesis prevention
- Metal-free N, S-co-doped carbon materials derived from calcined aromatic co-poly(urea-thiourea)s as efficient alkaline oxygen reduction catalysts
- Influence of stitching parameters on the tensile performance and failure mechanisms of CFRP T-joints
- Synthesis of PEGylated polypeptides bearing thioether pendants for injectable ROS-responsive hydrogels
- Rapid Communication
- RAFT-mediated polymerization-induced self-assembly of poly(ionic liquid) block copolymers in a green solvent
- Corrigendum
- Corrigendum to “High-strength polyvinyl alcohol-based hydrogel by vermiculite and lignocellulosic nanofibrils for electronic sensing”

