Abstract
This study emphasizes on the production of nano-encapsulated phase change materials for the application of thermal energy storage (TES). The core is undecylenic acid (UA), a renewable latent heat storage material, which is encased inside the polyaniline (PANI) nanofibres (NFs) synthesized by interfacial polymerization technique. The morphological and structural features of the prepared NFs were examined by high-resolution transmission electron microscopy and Fourier transform infrared spectroscopy. According to differential scanning calorimetry analysis, the latent heat values were observed as 56 ± 1.4 and 57 ± 1.3 J·g−1, while the melting and freezing temperatures were recorded as 18 ± 0.2°C and 14 ± 0.3°C, respectively. Thermogravimetric analysis revealed a multi-step deterioration pattern, demonstrating the developed nano-encapsulated-phase change material’s (PCMs’) exceptional thermal stability. In addition to exhibiting reliable thermal performance over numerous cycles, PANI/UA NFs showed excellent TES and release rates. This work addresses key challenges in TES applications by introducing a PCM based on renewable materials that offers enhanced thermal reliability and efficiency. The results contribute in the development of compact and environmentally friendly thermal management systems for utilizing in the applications of electronic device cooling, building energy systems, and renewable energy storage.
1 Introduction
The dwindling fossil fuel reserves and increasing energy demand have heightened the urgency for efficient and sustainable energy storage solutions (1,2). A promising option is phase change materials (PCMs) for thermal energy storage (TES), which have the capacity to both absorb and release thermal energy during phase transitions (3). PCMs are latent heat storage systems that use phase transitions, such as solid–liquid, solid–solid, liquid–gas, and solid–gas transformations, to store and release energy (4). Solid–liquid PCMs are the most extensively used due to its exceptional versatility, better efficiency and high adaptability for a variety of applications because of their benefits, which includes a high energy storage density, uniform heat transfer during phase transition at constant temperature, and a wide range of operating temperatures. The aforementioned features make PCMs suitable for energy-efficient systems like textiles, air conditioning, electronics cooling, building materials, and renewable energy storage (5,6,7,8,9). Despite their potentials, PCMs are difficult to execute in real life, especially the organic PCMs experiencing solid–liquid transitions, due to the problems like poor thermal conductivity and risk of material seepage during phase transition (10). Encapsulation techniques have been used to circumvent these limitations, resulting in the formation of encapsulated PCMs (EPCMs) (11). PCMs shielded by this technique acquire a core-shell configuration, which also inhibits leakage and regulates variations in volume during phase transitions. In particular, nano-encapsulation has attracted a lot of interest because of its exceptional thermal performance. Nano-encapsulated PCMs are interesting candidates for TES applications owing to their high surface area-to-volume ratio, which facilitates faster heat transmission and enhanced thermodynamic efficiency (12).
Zhao et al. (13) synthesized octadecane containing nano-capsules encapsulated by poly-(ethyl methacrylate) and poly-(methyl methacrylate) (PMMA) copolymers with a core/shell ratio of 80:20 via mini-emulsion method. The nano-capsules of 119 nm showed latent heats of melting and crystallization of 198.5 and 197.1 kJ/kg, respectively. Fernández et al. (14) used an emulsion copolymerization technique to obtain a micro-encapsulated PCM shell made up of poly-(styrene-co-ethyl acrylate) for encapsulation of organic PCMs such as paraffins (Capsule A) and palmitic Acid (Capsule B) as main TES materials. Mean particle sizes of both paraffin and fatty acid encapsulated in copolymer shells were found to be 166 and 265 nm, respectively. Fang et al. (15) worked upon cold TES applications such as air-conditioning and developed a latent functionally thermal fluid comprising of nano-encapsulated PCM capsules with n-tetradecane as core and polystyrene (PS) made by ultrasonic-assisted mini-emulsion polymerization. PS/n-tetradecane nano-capsules with an average diameter of 132 nm were well distributed in the prepared slurry and formed the main energy storage components. PS nano-capsules possessed the melting and freezing temperatures of 4.0°C and −3.4°C along with their corresponding latent heat enthalpy values of 98.7 and 91.3 J·g−1, respectively.
Fu et al. (16) produced nano-encapsulated PCM slurry for cold energy storage consisting of n-tetradecane core based nano-capsules with hybrid shell wall (polystyrene-silica, PS-SiO2) dispersed in a base fluid. Tetradecane/PS-SiO2 nano-capsules with an average size of 151.3 nm had a melting enthalpy of 83.4 kJ·kg−1. Guo et al. (17) obtained nano-encapsulated PCM by encapsulating stearic acid inside PMMA nano-capsules by employing an ultrasonically initiated in situ polymerization method. Morphological results indicated the formation of nano-capsules spheres having a diameter range of 80–90 nm and differential scanning calorimetry (DSC) data suggested the thermal storage ability of fabricated PMMA/stearic acid nano-capsules found to be 155.6 J·g−1. Feng et al. (18) produced a series of nano-encapsulated core-shell PCMs containing n-dodecanol as core latent heat storage component through supramolecular lock-layer method, which showed outstanding storage reliability in the temperature range of −50°C to 50°C. The obtained PCM nano-capsules ranging from 60 to 90 nm possessed maximum encapsulation ratio and latent heat of fusion equal to 90% and 180 J·g−1, respectively. Quasi-monodispersed nano-encapsulated PCMs with the average size less than 120 nm were prepared, wherein dodecanol and in situ cross-linked acrylate copolymers were used as the heat storage core and the supramolecular lock shell layer of the nano-encapsulated PCMs, respectively. The prepared PCMs showed latent heat capacity up to 116.1 J·g−1 and form-stability under an external shear force of 101.9 kN m−2 (19).
Recent studies have investigated different encapsulating techniques, with a primary focus on paraffin PCMs generated from petroleum. Though they function remarkably well, paraffin-based PCMs are expensive, non-renewable, and adversely impact the environment. On the contrary, PCMs derived from fatty acids, including undecylenic Acid (UA) (10-undecenoic acid), offer a sustainable substitute. UA, a bio-based, renewable C11 fatty acid with a melting point of 23°C, is produced by vacuum pyrolyzing castor oil. With a carboxylic acid group and a terminal double bond, its bifunctional structure provides a wide range of applications in cosmetics, and pharmaceuticals. However, nano-encapsulated PCMs using UA as a core material remains unexplored (20,21,22,23).
Polyaniline (PANI), a well-known conducting polymer, distinguished for its exceptional chemical stability, facile synthesis, tuneable electrical properties has received much attention in the recent past for its potential applications in electrical, electronics, thermoelectric, electrochemical, electromagnetic fields, etc. (24,25). It has been observed that very few research studies (26,27,28,29) have ever been reported so far, utilizing PANI for the development of PCMs for TES applications, that accounted for the synthesis of PANI via in situ polymerization or chemical oxidative polymerization method, where the reaction is allowed to occur at low temperature (0–5°C). PANI can be viewed as a viable option for encasing PCMs for TES applications, as it can offer remarkably good structural stability along with the enhanced thermal conductivity (30,31,32). However, limited investigation done on integration of PANI with PCMs based on renewable fatty acids, implies that significant amounts of potential remain untapped. The present study addresses these gaps by producing nano-encapsulated PCMs (PANI/UA NF PCMs) utilizing interfacial polymerization technique to enclose UA, the primary latent heat storage material, within PANI nanofibers (NFs). These resulted nano-sized PCMs were further investigated for their characteristics, including their chemical properties, morphology, thermal properties, and thermal stability. This work emphasizes the necessity to integrate renewable materials and pioneering encapsulation techniques in order to advance efficient and sustainable TES systems. The results open the door for wider applications in energy-efficient technologies by aiding in the development of high-performance and environmentally friendly TES systems.
2 Materials and method
2.1 Materials
Aniline 99%, as a monomer; hydrochloric acid (37%), and ammonium persulphate (APS) 98%, as an active free radical initiator, are obtained from Loba Chemie Pvt. Ltd (India). UA 99% as core material is supplied by Avra Synthesis Pvt. Ltd (India). Toluene is received from Central Drug House (P) Ltd (CDH, India). Milli-Q water has been used during all reactions. All reagents were used as received.
2.2 Synthesis of nano-encapsulated PCMs using interfacial polymerization technique
A monomer-to-PCM ratio of 1:1 was maintained by dissolving 10.6 mL of 0.3 M aniline and 10.6 mL of core PCM fatty acid in 40 mL of toluene, to form the organic phase. The aqueous phase was prepared by adding the 0.73 g of APS initiator in 40 mL of 1 M HCl aqueous solution. The initiator solution (aqueous phase) was then added in dropwise manner to the organic phase, within a specific period of time. The reaction mixture was further stirred at 1,500 rpm for 6 h. After mixing of these two solutions (phases), PANI NFs encapsulating fatty acid PCM were formed at the interface of this biphasic system, under constant agitation. The reaction mixture was kept unperturbed for next 24 h, was filtered, and then washed with acetone, followed by distilled water multiple times, to remove unreacted monomer impurities. The synthesized nano-encapsulated PCMs obtained in blackish-green colour slurry indicates the formation of conductive emeraldine salt form of the PANI, with fibrillar morphology containing UA, as latent heat storage component within the resultant nanofibrous structures. The obtained compound was dried in oven at 50°C for 24 h. A schematic representation of the adopted experimental procedure for the synthesis of nano-encapsulated PCMs is shown in Figure 1.

A schematic representation of the experimental procedure to obtain nano-encapsulated PCMs.
3 Characterization of nano-encapsulated PCMs
The parent functional groups and idea about probable chemical structure of PANI/UA PCMs NFs were investigated by Fourier transform infrared spectrometer (FTIR; Spectrum Two, Perkin Elmer, USA). The synthesized nano-encapsulated PCMs in small amount were mixed with KBr to form pellets and the spectrum was recorded at a resolution of 4 cm−1, with 128 scans in the range of 450–4,000 cm−1. The shape and morphological appearance of the nano-encapsulated PCMs was evaluated using high-resolution transmission electron microscopy (HR-TEM; Tecnai™ G2 20). The sample was dispersed in ethanol and homogenized utilizing ultra-sonication to prevent aggregation of the suspended particles and then the sample was transferred to a copper grid and imaging was carried out. For the evaluation of form stability, leakage test was conducted, using mass-loss methods, as described by different researchers (33,34). In this, a fixed weight of sample was placed on the filter papers and subjected to hot-air oven at 50°C for 2 h. After removing from the oven, it was kept undisturbed to attain the ambient temperature. Further, it was transferred to a fresh filter paper and weighed again. The leakage test was performed thrice with three different batches of the prepared PANI/UA PCMs NFs. The leakage percent was calculated using Eq. 1 as follows:
where M o is the initial mass of the sample before heating; M t is the mass of the sample after oven heating. The criteria of leakage are shown in Table 1.
Leakage criteria considered for as-prepared PCMs
| Criteria of leakage | ||
|---|---|---|
| Leakage percentage (L%) | Phenomenon | Conclusion |
| L% ≤ 10 | Negligible or trace amounts | Highly stable |
| 10 < L% ≤ 30 | Very little leakage | Remained stable |
| 30 < L% ≤ 50 | Moderate leakage | Unstable |
| L% > 50 | Severe leakage | Highly unstable |
The principal latent TES variables of raw PCM and prepared nano-encapsulated PCMs such as melting point (T m), freezing point (T c), and the enthalpies associated during both heating (ΔH m) and cooling (ΔH c) processes were determined by DSC (DSC 8000, Perkin Elmer, USA). Each sample was tested thrice to check their heat storage parameter, along with the encapsulation content, and an average value of obtained DSC results was reported. The analysis was conducted within temperature sweep of 0–30°C at a standard heating/cooling rate of 10°C·min−1 under a nitrogen blanket, at a constant flow rate of 40 mL·min−1. The thermal stability and the weight loss studies were done on the prepared PCMs using a thermal analyser (TGA 4000, Perkin Elmer, USA). About 5–6 mg of NF sample was placed in the analyser’s silica crucible and was subjected to a temperature of 30–800°C, at a standard heating rate of 10°C·min−1, under nitrogen environment, at a constant flow rate of 20 mL·min−1.
Thermal Energy Storage/Release Ability Test (T-History) was carried for the determination of charging and discharging time of the PCM and the nano-encapsulated PCMs. The test was conducted according to a similar method suggested by Qu et al. (35). The thermal reliability of PANI-based PCMs was investigated by the experimental procedure described in literature (36). Nano-encapsulated PCMs sample was exposed to 100 thermal cycles followed by DSC analysis to investigate the changes in thermal properties after thermal cycling test. The sample was tested three times, and the reported value represents the average.
The success of the encapsulation process for the synthesis of nano-encapsulated PCMs can be deduced by calculating the encapsulation ratio (E R). Encapsulation ratio measures the effective encapsulation of a PCM as a core, inside the polymer matrix. The encapsulation ratio of UA was calculated with the following Eq. 2 based on the enthalpy values measured from the DSC analysis (37):
where ΔH m,Nano PCMs, and ΔH m,PCMs are respectively the latent heats of fusion of the nano-encapsulated PCMs and bulk UA, PCM.
Four-point probe set-up (SES Instruments Pvt. Ltd, Roorkee) consisting a digital micro voltmeter (DMV-00), low current source (LCS-02) and PID controlled oven (PID-200) were used to measure the electrical conductivity of the PANI-based nano-encapsulated PCM at room temperature.
4 Results and discussion
4.1 Form-stability test
The form-stable characteristic of the PANI/UA NF PCMs has been examined based on leakage rate (%). Figure 2 depicts the leakage stability of the prepared PCMs. The results obtained experimentally indicate the leakage rates varied linearly with increasing time period. At initial time period of 2 h, developed PCMs exhibited a negligible weight loss. It has been found that the decent form-stability behaviour with optimum and maximum leakage of 9.1% post 8 h of oven heating was observed with the sample that falls under the utility criteria of form-stability behaviour and above-prepared material can be labelled as form-stable PCM.
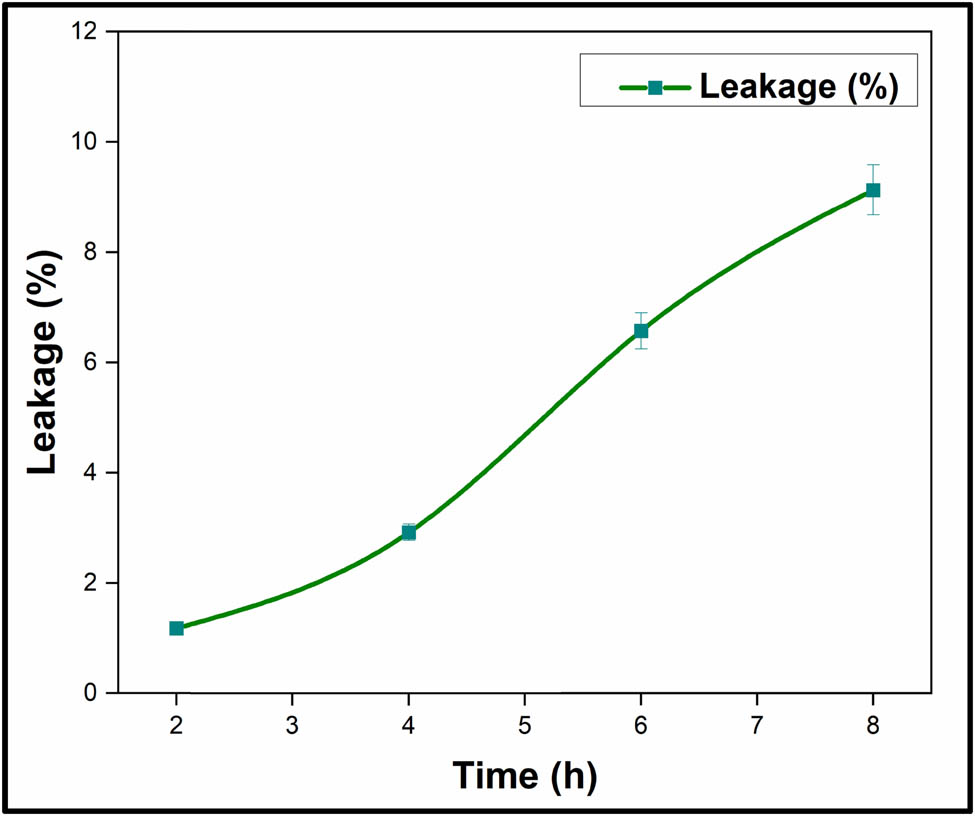
Form-stability behaviour of nano-encapsulated PCMs.
4.2 FTIR
The FTIR spectra of UA, nano-encapsulated PCMs, and PANI describing their chemical characteristics are presented in Figure 3. The raw fatty acid PCM in Figure 3(a) shows peaks at 2,856 and 1,413 cm−1, respectively, belong to C–H stretching and bending frequencies of the aliphatic chain of fatty acid. The peak at 1,710 and 1,283 cm−1 belong to the stretching vibrations of the carbonyl group of the acid. The band at 724 cm−1 corresponds to the C–H rocking modes. For raw PANI NFs as shown in Figure 3(b), the characteristics vibration bands observed at 1,599, 1,493, 1,304, 1,149, and 818 cm−1 relate to the stretching vibrations of quinoid (Q) and benzenoid (B) rings, C–N vibrational stretching in Q–B–Q and B unites, B–NH+ = Q stretching vibrations, and aromatic C–H out of-plane deformation vibrations of linear PANI backbone, respectively. The characteristic band at 1,149 cm−1 is associated with the degree of doping or delocalization of electron. In case of PANI/UA PCMs NFs, spectrum (Figure 3(c)) exhibiting similar attributes of the pure PCM as well as PANI containing distinctive vibration peaks at 2,930 and 2,853 cm−1 belongs to symmetrical and asymmetrical C–H stretch of fatty acid. Carbonyl stretching (C═O) of fatty acid was found to occur at 1,711 cm−1. Also, C═C stretching of the quinoid rings, C═C stretching of the benzenoid rings and C–N vibrational stretching in Q–B–Q and B unites have been observed at 1,568, 1,495, and 1,305 cm−1, respectively. The electronic-like absorption of N═Q═N associated with the degree of doping or delocalization of electron has been observed at 1,188 cm−1. The spectra of nano-encapsulated PCMs displayed indistinguishable characteristic peaks of both PANI and unsaturated fatty acid PCM, supporting the confirmation of the presence of UA and formation of PANI NFs. Similar findings were reported by other research groups also (38,39,40).
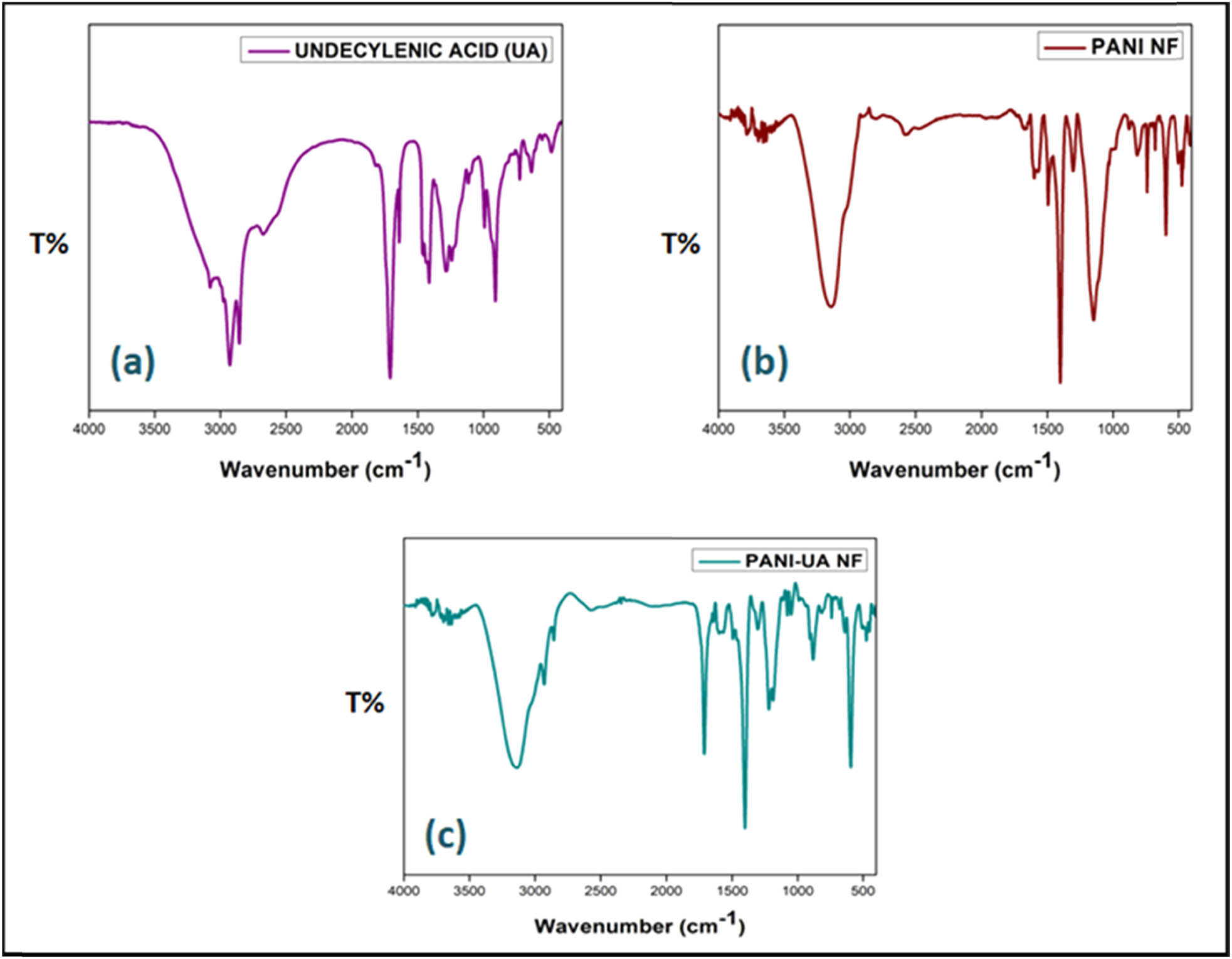
FTIR spectra of: (a) UA, (b) PANI NFs, and (c) PANI/UA NFs.
4.3 Morphology of nano-encapsulated PCMs
Molecular size and shape can be influential in affecting the physical properties of the polymers such as solubility and dispersion nature in solvents; chemical reactivity, feeding behaviour, etc. The morphology, shape profiles, and the distribution pattern of the nano-encapsulated PCMs explicated by the HR-TEM analysis and duly verified by image J software are as shown in Figure 4. Both raw PANI and fatty acid incorporated PANI PCMs produced by interfacial polymerization exhibited nanofibrillar morphologies and the NFs tend to merge and form interconnected nanofibrous network with narrow diameter distribution. Raw PANI nano-fibres as seen in Figure 4(a) and (b) show an average fibrous diameter of 53.1 nm whereas in case of fatty acid filled PANI NFs show diameter ranging from 37–78 nm with mean fibre diameter of 56.4 nm. However, few PANI molecules can also be seen in the form of spherical particles as reported in Figure 4(c) and (d).
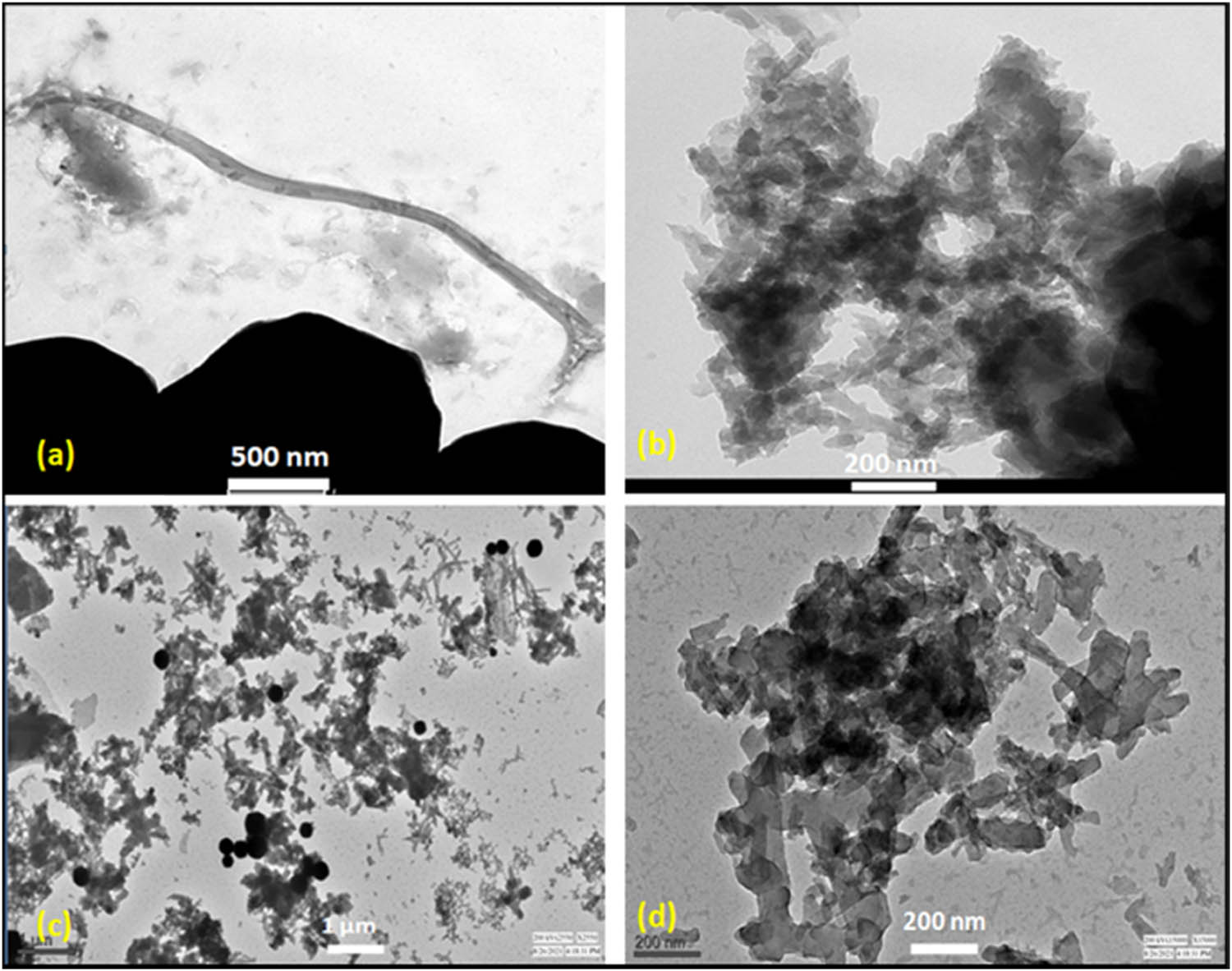
HR-TEM micrographs of the synthesized raw PANI NFs: (a) magnification-500 nm, (b) magnification-200 nm; and PANI/UA NF PCMs: (c) magnification-1 μm and (d) magnification-200 nm.
The NFs produced in the early stage of polymerization during slow-feeding reactions are subjected to secondary growth, which leads to the large agglomerates containing irregularly shaped particles and NFs. As the reaction proceeds with initiator molecules getting consumed and polymer chain grows rapidly, the doped emeraldine salt of PANI being formed is of hydrophilic nature and can quickly cast away from the interface and diffuse into the water layer. Therefore, in this way, as the NFs formed are continuously withdrawn from the reaction front, thus avoiding secondary growth and allowing high yield of new NFs to grow at this interface. It was observed that diameter of the PANI/UA-NFs seems to be increased as compared to raw PANI, which is attributed to the presence of fatty acid being encapsulated inside nanofibrous structure of PANI (41).
4.4 Thermal performance of nano-encapsulated PCMs
The phase change characteristics of the synthesized fatty acid-based nano-encapsulated PCMs and UA are analysed by DSC. The DSC curves advocating the phase-change temperatures and the latent heat enthalpies of the prepared PANI/UA NF PCMs and raw UA are shown in Figure 5 and their results are concluded in Table 2. The DSC curves of both pure PCM and the nanofibrous PCMs appear to be almost identical, thus, supporting the successful encapsulation of UA within the PANI interstices. The pure UA showed the onset of melting and crystallization temperatures at 22 ± 0.3°C and 21 ± 0.3°C, respectively. On contrary, the PANI/UA PCM NFs have showed a fair TES density, with the onset of melting at 18 ± 0.2°C and crystallization at 14 ± 0.3°C, respectively. The latent heat of fusion and the latent heat of crystallization of the pure fatty acid and nanofibrous PCMs were found to be 124 ± 2.1 J·g−1, 125 ± 1.8 J·g−1 and 56 ± 1.4 J·g−1, 57 ± 1.3 J·g−1, respectively. The observed shift in the phase transition temperatures of the synthesized nanoencapsulated PCMs is most likely due to the effects of the nanoencapsulation process. The encapsulation of the UA inside and along the domains of nano-diameter PANI fibrous entities provided higher heat transfer surface area for the PCM, thus leading to the improved heat transfer rate as a result of nano-encapsulation. The melting and crystallization peaks of the PANI/UA-NFs showed a depression compared to neat PCM (UA). The calculated encapsulation ratio (E R) of the UA obtained from the measured enthalpies values, using Eq. 1, was found to be 45%. The comparison of PCM NFs prepared in the current study with few already testified in the literature is reported in Table 3. From the attained results, it is apparent that the prepared nanofibrous PCMs exhibit good latent heat energy capacity and their storage potential can be utilized in substantial TES applications including thermo-responsive textiles, slurries, antistatic/corrosion resistant thermo-regulatory coatings, etc. (42,43,44).
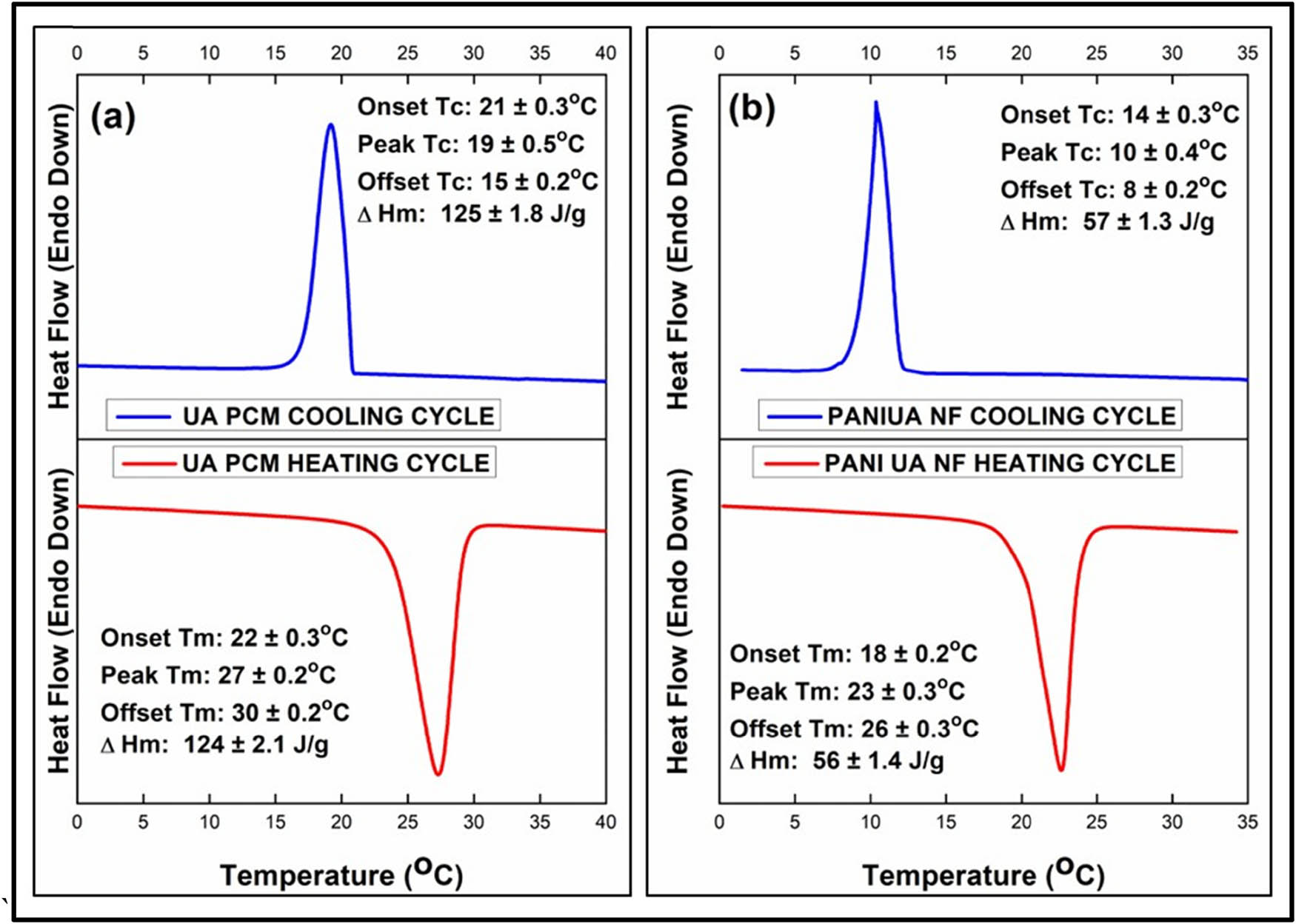
DSC curves for heating/cooling cycles for (a) Pure UA and (b) PANI/UA-NF PCMs.
DSC data of pure PCM and nano-encapsulated PCMs representing their phase change characteristics
| Sample | Undecylenic acid | PANI/UA NFs |
|---|---|---|
| Onset T m (°C) | 22 ± 0.3 | 18 ± 0.2 |
| Peak T m (°C) | 27 ± 0.2 | 23 ± 0.3 |
| Offset T m (°C) | 30 ± 0.2 | 26 ± 0.3 |
| Onset T c (°C) | 21 ± 0.3 | 14 ± 0.3 |
| Peak T c (°C) | 19 ± 0.5 | 10 ± 0.4 |
| Offset T c (°C) | 15 ± 0.2 | 8 ± 0.2 |
| ΔH m (J·g−1) | 124 ± 2.1 | 56 ± 1.4 |
| ΔH c (J·g−1) | 125 ± 1.8 | 57 ± 1.3 |
| PCM (%) | 100 | 45 |
Comparison of some encapsulated PCMs from literature and synthesized nano-encapsulated PCMs
| Core material | Shell materials | Size (nm) | ΔH m (J·g−1) | Ref. |
|---|---|---|---|---|
| Paraffin, M.P.@80°C | Poly-(styrene-co- butyl acrylate) | 52-112 | 5-25 | (45) |
| Paraffin, M.P.@26°C | PMMA-SiO2 | 120 | 71 | (46) |
| Tetradecane | Polystyrene | 132 | 98.7 | (15) |
| Tetradecane | Silica | 151.3 | 83.4 | (16) |
| UA | Polyaniline | 56.4 | 55.7 | Present study |
4.5 Thermal stability of nano-encapsulated PCMs
The thermal stability and the degradation pattern of the nano-encapsulated PCMs are studied by TGA. The TGA curves of the PANI/UA-NFs PCMs are shown in Figure 6 and, their respective degradation temperatures are given in Table 4. The pure UA degrades in a single step. The PCM shows 10% and 50% weight loss at around 158°C and 191°C, respectively. The UA undergoes complete decomposition at 199°C. Due to the compact carbon chain fatty acid chain length, UA has a low decomposition temperature and thus, experiences a progressive weight loss with the temperature rise. Similar to this, the PANI also experiences one step degradation pattern, but perceives the 50% degradation at a much higher temperature of 370°C, due to the considerably higher molecular weight of the polymer macromolecules. In contrast, the nano-encapsulated PCMs microcapsules underwent a three-stage degradation profile; the first step observed at low temperature is attributed to the vaporization of high volatile compounds such as remnant organic solvent (toluene) traces or absorbed atmospheric moisture, the second step quite similar to degradation profile corresponds to the degradation of the entrapped fatty acid where 50% thermal degradation occurring around similar temperature range as that of the PCM and followed by final degradation of conducting macromolecule around 300°C. The multi-step degradation pattern observed in the nano-encapsulated PCMs indicates successful encapsulation of the unsaturated fatty acid within the conducting polymer, which contributes to enhanced thermal stability at higher temperatures.
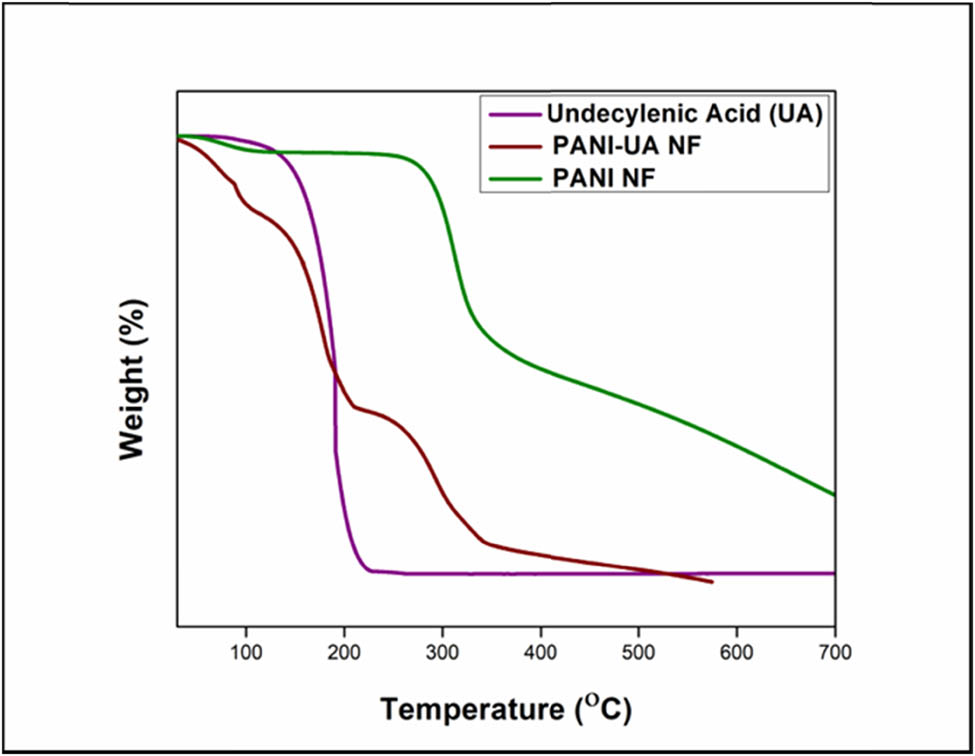
TGA cures for the UA, nano-encapsulated PCMs and PANI.
Thermal stabilities of the UA, PANI/UA-NFs PCMs and PANI
| Sample | 10% mass loss (°C) | 50% mass loss (°C) | 80% mass loss (°C) |
|---|---|---|---|
| UA | 158 | 191 | 199 |
| PANI/UA NFs | 90 | 186 | 299 |
| PANI | 288 | 370 | 677 |
4.6 Thermal energy storage/release ability of nano-encapsulated PCMs
Thermal energy storage/release ability of the nano-encapsulated PCMs was evaluated by tracking the charging-discharging rates of UA and PANI/UA-NF PCMs. The melting time was deduced from the charging cycle of UA and nano-encapsulated PCMs. Similarly, the crystallization time was observed from the discharging cycle of UA and NFs PCMs. The distinctive temperature history curves of raw PCM and nano-encapsulated PCMs are shown in Figure 7. UA attained its melting temperature (phase change) from 0°C in 117 s whereas in the case of nano-encapsulated PCMs, the time consumed was 238 s. This difference in the charging time of pure PCM and nano-encapsulated PCMs further attests the successful nano-encapsulation of UA. The discharging time for the pure PCM and nano-encapsulated PCMs to reach their onset crystallization temperatures were found to be 121 and 674 s, respectively, which confirms the prolonged latent heat energy release duration against pure PCM.
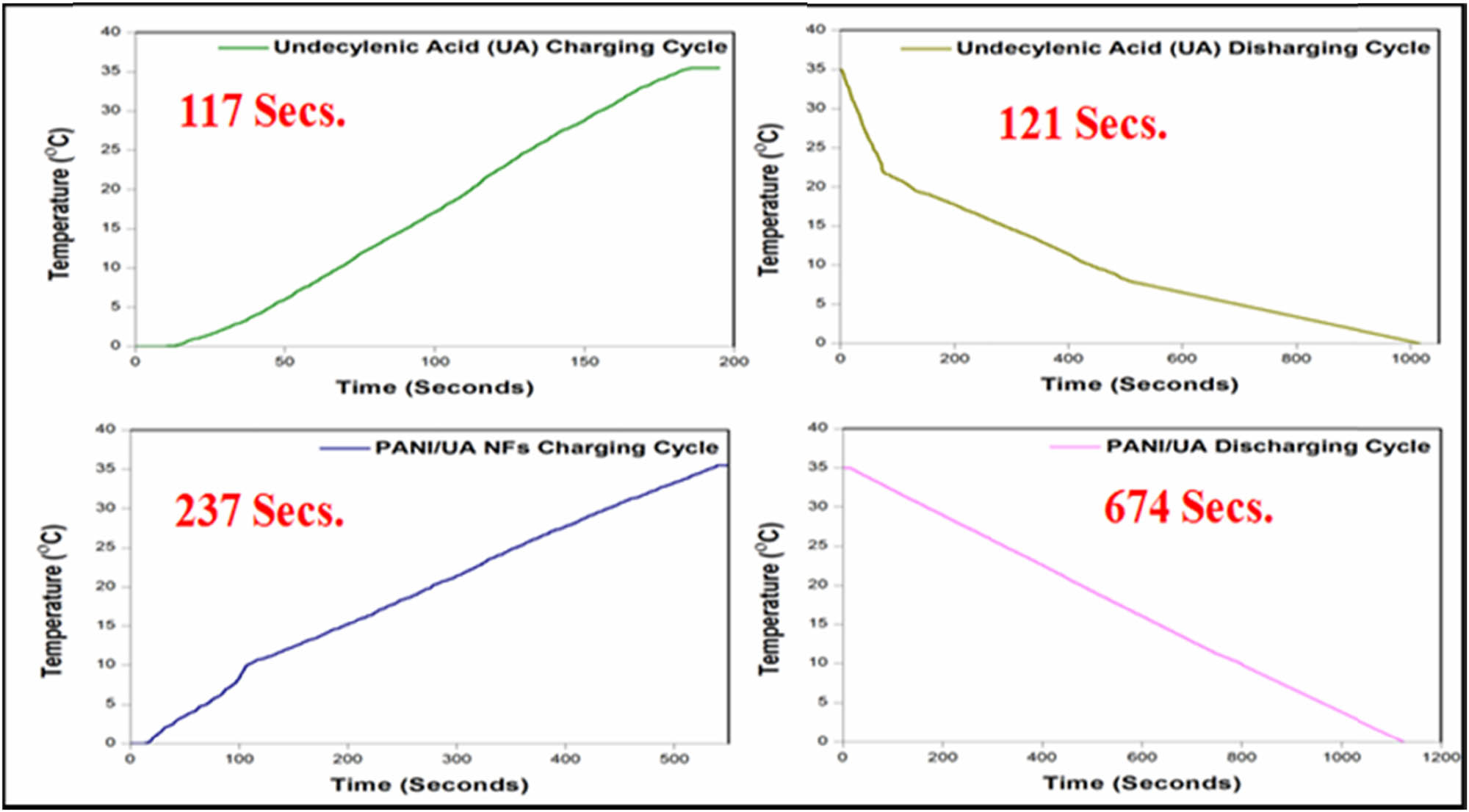
Thermal energy storage/release curves for UA and nano-encapsulated PCMs during charging and discharging cycles (T-History).
4.7 Thermal reliability of nano-encapsulated PCMs
Nano-encapsulated PCMs that are used for the purpose of TES must be enduring multiple heat-cool cycles with minor deviation in their energy storage properties. DSC plot and changes in latent heat storage characteristics of nano-encapsulated PCMs after facing 100 thermal cycles is shown in Figure 8 and Table 5, respectively. After 100 cycles, the nano-encapsulated PCMs showed latent heat enthalpies of melting and crystallization as 47 ± 1.6 and 50 ± 1.2 J·g−1, respectively. The comparison of PCM NFs synthesized in the present study with the other nano-encapsulated PCMs in the literature is reported in Table 6. It is evident from the results obtained that the minor variation in temperature or enthalpy values suggests the good thermal reliability behaviour of the synthesized PCMs suitable for utility in TES applications.
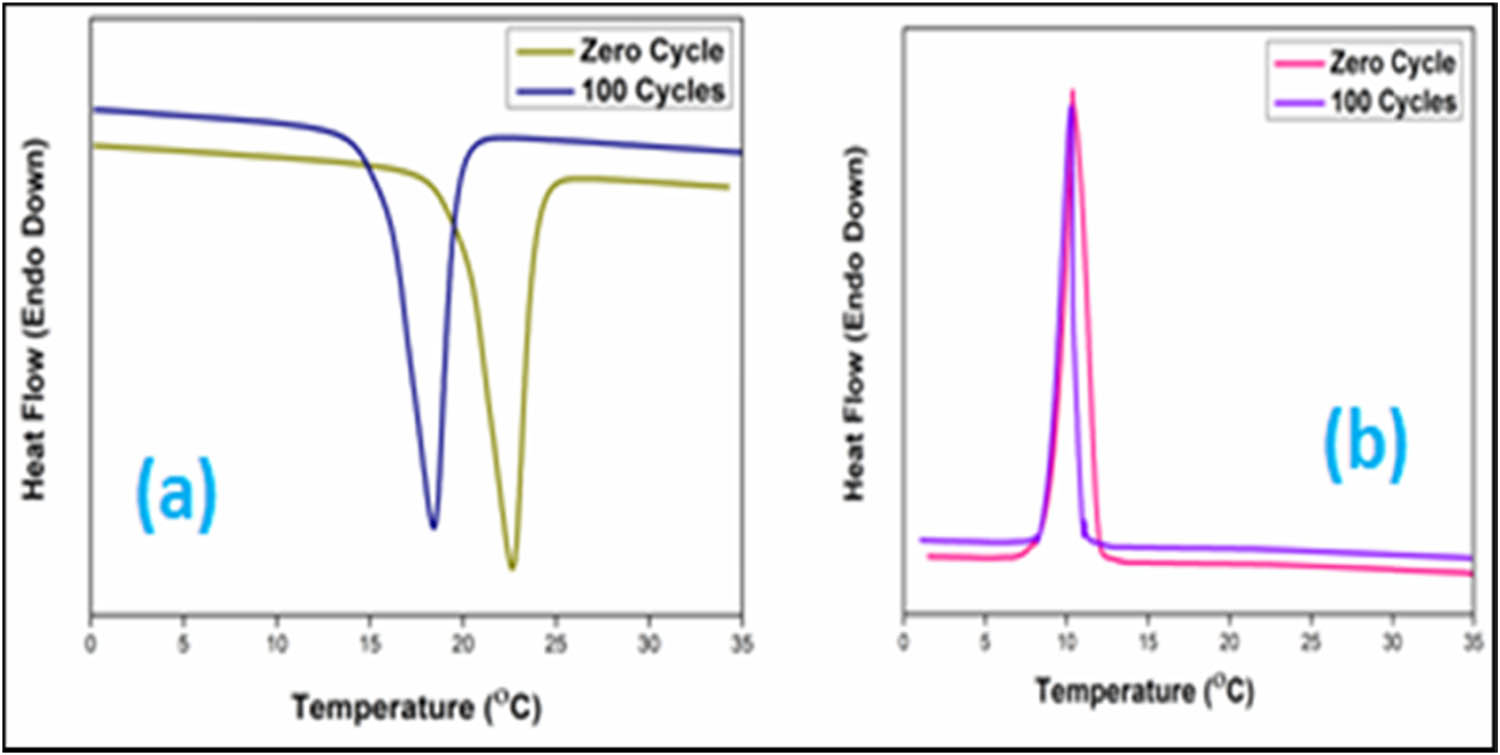
Thermal reliability curves of nano-encapsulated PCMs: (a) heating cycle and (b) cooling cycle.
Phase change characteristics of nano-encapsulated PCMs post thermal cycling exposure
| Number of cycles | Onset T m (°C) | Onset T c (°C) | ΔH m (J·g−1) | ΔH c (J·g−1) |
|---|---|---|---|---|
| 0 | 18 ± 0.2 | 14 ± 0.3 | 56 ± 1.4 | 57 ± 1.3 |
| 100 | 14 ± 0.4 | 11 ± 0.2 | 47 ± 1.6 | 50 ± 1.2 |
Comparison of some nano-encapsulated PCMs from literature and synthesized nano-encapsulated PCMs
| Core and shell | PCM (H m) | PCM (H c) | No. of cycle | Nano-encapsulated PCMs (H m) | Nano-encapsulated PCMs (H c) | Ref. |
|---|---|---|---|---|---|---|
| Octadecane PCM and organosilica shell (S3: MPS 5 mL + TEOS 10 mL, S4: MTMS 5 mL + TEOS 10 mL) MPS: γ-methacryloxypropyltrimethyl silicate, MTMS: methyltrimethoxy silane, TEOS: tetraethyl orthosilicate | 209.7 | 208.3 | 500 | S3:101.6, S4: 102.7 | S3: 94.9, S4: 100.7 | (47) |
| Octadecane PCM and polymer shell of styrene/MMA | 239.4 | 240.6 | 360 | 106.4 | 103.7 | (48) |
| D-mannitol PCM and silica–graphene oxide composite shell | 288.1 | 228 | 50 | 206.7 | 169.2 | (49) |
| Paraffin wax (RT80) PCM and polymer matrix based on styrene and butyl acrylate | 23.9 | 25 | 100 | 23.0 | 20.7 | (45) |
| Undecylenic acid PCM and polyaniline shell | 124.4 | 125.1 | 100 | 47.2 | 49.6 | Present study |
4.8 Electrical conductivity of the nano-encapsulated PCMs
Four-point probe technique was used to measure the electrical conductivity of the PANI-based nano-encapsulated PCM at room temperature. The electrical conductivity of raw PANI NFs was measured to be 0.6 S/cm. In comparison, the PANI/UA NF PCMs exhibited a higher electrical conductivity, i.e. 0.7 S/cm. This enhancement suggests that the interaction between PANI and the UA contributes positively to the material’s overall electrical performance.
5 Conclusion
The nano-encapsulated PCMs consisting of UA as core and surrounded by nano-scale PANI, as shell have been prepared by interfacial polymerization technique, for the latent heat TES applications. FTIR data have depicted the successful encapsulation of the fatty acid inside the PANI NFs. TEM results have demonstrated the nanofibrous morphologies of the synthesized nano-encapsulated PCMs with a mean fibre diameter of approximately 56.4 nm. DSC analysis showed that the prepared nano-encapsulated PCMs exhibited melting and crystallization temperatures of 18 ± 0.2°C and 14 ± 0.3°C, respectively and their corresponding latent heats equal to 56 ± 1.4 and 57 ± 1.3 J·g−1. The PCM core content of active PCM inside the PANI NFs is approximately 45%. T-History and thermal reliability test indicated that synthesized PANI based PCM NFs exhibited decent thermal cyclic stability. Based on the results obtained in the present study, it has been concluded that the synthesized nano-encapsulated PCMs can be successfully employed for TES purpose, by incorporating them in the potential areas, like PCM slurries, thermo-responsive textile fabrics, thermo-responsive functional coatings, etc.
Acknowledgments
We are thankful to Delhi Technological University for providing infrastructural facilities.
-
Funding information: Authors state no funding involved.
-
Author contributions: Surya Tanwar: conceptualization, investigation, methodology, and writing – original draft. Gunjan Varshney: writing – original draft. Raminder Kaur: conceptualization, supervision, and writing – review and editing.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: Data are contained within the article.
References
(1) Varshney G, Kaur R, Zulfequar M. Fabrication and evaluation of eicosane/poly(styrene-co-butylacrylate) microencapsulated phase change materials through ultrasonicated mini-emulsion technique. Chem Eng J. 2024;500:156994.10.1016/j.cej.2024.156994Search in Google Scholar
(2) Tanwar S, Kaur R. Fabrication and investigation on influence of metal oxide nanoparticles on thermal, flammability and UV characteristics of polyethylene glycol based phase change materials. J Energy Storage. 2022;54:105318.10.1016/j.est.2022.105318Search in Google Scholar
(3) Tanwar S, Kaur R. Development and investigation of microencapsulated caprylic acid-based phase change materials for thermal energy storage applications. Int J Energy Res. 2021 Oct;45(12):17302–14.10.1002/er.6611Search in Google Scholar
(4) Tanwar S, Kaur R. Studies on the influence of titanium dioxide nanoparticles on thermal, flammability, and UV properties of PEG-based phase change material composites. Energy Storage. 2024;6(5):1–11.10.1002/est2.678Search in Google Scholar
(5) Bastani A, Haghighat F, Kozinski J. Designing building envelope with PCM wallboards: Design tool development. Renewable Sustainble Energy Rev. 2014;31:554–62.10.1016/j.rser.2013.12.031Search in Google Scholar
(6) Ke H, Wei Q. Use of MWNTs-COOH to improve thermal energy storage and release rates of capric–palmitic–stearic acid ternary eutectic/polyacrylonitrile form-stable phase change composite fibrous membranes. Polym Eng Sci. 2019 Jan 1;59(S1):E403–11.10.1002/pen.25001Search in Google Scholar
(7) Ma Y, Sun S, Li J, Tang G. Preparation and thermal reliabilities of microencapsulated phase change materials with binary cores and acrylate-based polymer shells. Thermochim Acta. 2014;588:38–46.10.1016/j.tca.2014.04.023Search in Google Scholar
(8) Zhang L, Zhu J, Weibing Z, Wang J, Wang Y. Characterization of polymethyl methacrylate/polyethylene glycol/aluminum nitride composite as form-stable phase change material prepared by in situ polymerization method. Thermochim Acta. 2011 Sep;524:128–34.10.1016/j.tca.2011.07.003Search in Google Scholar
(9) Varshney G, Singh P, Yadav S, Kaur R. A review on unleashing the potential solution of thermal comfort: Exploring the cutting-edge progress of advanced engineering application of phase change materials integrated textiles. Sustainable Energy Technol Assess. 2024;72:104089.10.1016/j.seta.2024.104089Search in Google Scholar
(10) Tanwar S, Kaur R. Fabrication and evaluation of polyurethane supported form-stable PCMs. Energy Storage. 2024 Mar;6(2):e581.10.1002/est2.581Search in Google Scholar
(11) Chenzhen L, Rao Z, Zhao J, Yutao H, Li Y. Review on nanoencapsulated phase change materials: Preparation, characterization and heat transfer enhancement. Nano Energy. 2015;13:814–26.10.1016/j.nanoen.2015.02.016Search in Google Scholar
(12) Tanwar S, Kaur R. Role of polymers in anchoring the performance of phase change materials for thermal energy storage applications: an overview. Acad Mater Sci. 2024;1(2):1–19.10.20935/AcadMatSci6184Search in Google Scholar
(13) Zhang GH, Bon SAF, Zhao CY. Synthesis, characterization and thermal properties of novel nanoencapsulated phase change materials for thermal energy storage. Sol Energy. 2012;86(5):1149–54.10.1016/j.solener.2012.01.003Search in Google Scholar
(14) Giro-Paloma J, Konuklu Y, Fernández AI. Preparation and exhaustive characterization of paraffin or palmitic acid microcapsules as novel phase change material. Sol Energy. 2015;112:300–9.10.1016/j.solener.2014.12.008Search in Google Scholar
(15) Fang Y, Yu H, Wan W, Gao X, Zhang Z. Preparation and thermal performance of polystyrene/n-tetradecane composite nanoencapsulated cold energy storage phase change materials. Energy Convers Manag. 2013 Jul;76:430–6.10.1016/j.enconman.2013.07.060Search in Google Scholar
(16) Fu W, Liang X, Xie H, Wang S, Gao X, Zhang Z, et al. Thermophysical properties of n-tetradecane@polystyrene-silica composite nanoencapsulated phase change material slurry for cold energy storage. Energy Build. 2017;136:26–32.10.1016/j.enbuild.2016.12.001Search in Google Scholar
(17) Wang G, Xu W, Hou Q, Guo S. A simple sonochemical method for fabricating poly(methyl methacrylate)/stearic acid phase change energy storage nanocapsules. Ultrason Sonochem. 2015 Nov;27:403–7.10.1016/j.ultsonch.2015.06.007Search in Google Scholar PubMed
(18) Feng L, Dong S, Zhou H, Yang L, Yuan F, Yang Y, et al. n-Dodecanol nanocapsules with supramolecular lock shell layer for thermal energy storage. Chem Eng J. 2020;389:124483.10.1016/j.cej.2020.124483Search in Google Scholar
(19) Feng L, Zhang Y, Zhou H, Kang Y, Zhang S, Bao L, et al. Quasi-monodispersed nanocapsules with form stability at high temperature and under shear force for thermal energy storage. Chem Eng J. 2022;428:131088.10.1016/j.cej.2021.131088Search in Google Scholar
(20) Valverde C, Lligadas G, Ronda J, Galià M, Cadiz V. Hydroxyl functionalized renewable polyesters derived from 10-undecenoic acid: Polymer structure and postpolymerization modification. Eur Polym J. 2018;105:68–78.10.1016/j.eurpolymj.2018.05.026Search in Google Scholar
(21) Bigot S, Daghrir M, Mhanna A, Boni G, Pourchet S, Lecamp L, et al. Undecylenic acid: A tunable bio-based synthon for materials applications. Eur Polym J. 2016;74:26–37.10.1016/j.eurpolymj.2015.11.008Search in Google Scholar
(22) Pang C, Zhang J, Wu G, Wang Y, Gao H, Ma J. Renewable polyesters derived from 10-undecenoic acid and vanillic acid with versatile properties. Polym Chem. 2014;5(8):2843–53.10.1039/C3PY01546GSearch in Google Scholar
(23) Somisetti V, Narayan R, Kothapalli RVSN. Multifunctional polyurethane coatings derived from phosphated cardanol and undecylenic acid based polyols. Prog Org Coat. 2019;134(Complete):91–102.10.1016/j.porgcoat.2019.04.077Search in Google Scholar
(24) Duhan M, Kaur R. Nano-structured polyaniline as a potential adsorbent for methylene blue dye removal from effluent. J Compos Sci. 2021;5(1):7.10.3390/jcs5010007Search in Google Scholar
(25) Duhan M, Kaur R. Adsorptive removal of methyl orange with polyaniline nanofibers: an unconventional adsorbent for water treatment. Env Technol. 2020 Oct;41(23):2977–90.10.1080/09593330.2019.1593511Search in Google Scholar PubMed
(26) Zeng J-L, Zhu F-R, Yu S-B, Xiao Z-L, Yan W-P, Zheng S-H, et al. Myristic acid/polyaniline composites as form stable phase change materials for thermal energy storage. Sol Energy Mater Sol Cell. 2013;114:136–40.10.1016/j.solmat.2013.03.006Search in Google Scholar
(27) Ju C, Wang Y, He D, Gao Q, Gao L, Fu M. Synthesis and infrared property of polyaniline/phase-change nanocapsule composite. J Nanosci Nanotechnol. 2011 Nov;11(11):9665–70.10.1166/jnn.2011.5218Search in Google Scholar PubMed
(28) Halvaee M, Didehban K, Goodarzi V, Ghaffari M, Ehsani M, Saeb MR. Comparison of pristine and polyaniline-grafted MWCNTs as conductive sensor elements for phase change materials: Thermal conductivity trend analysis. J Appl Polym Sci. 2017 Dec;134(47):45389.10.1002/app.45389Search in Google Scholar
(29) George M, Pandey AK, Abd Rahim N, Tyagi VV, Shahabuddin S, Saidur R. A novel polyaniline (PANI)/paraffin wax nano composite phase change material: Superior transition heat storage capacity, thermal conductivity and thermal reliability. Sol Energy. 2020;204:448–58.10.1016/j.solener.2020.04.087Search in Google Scholar
(30) Jamil S, Ahmad Z, Ali M, Rauf Khan S, Ali S, Amen Hammami M, et al. Synthesis and characterization of polyaniline/nickel oxide composites for fuel additive and dyes reduction. Chem Phys Lett. 2021;776:138713.10.1016/j.cplett.2021.138713Search in Google Scholar
(31) Ali V, Kaur R, Kamal N, Singh S, Jain SC, Kang HPS, et al. Use of Cu + 1 dopant and its doping effects on polyaniline conducting system in water and tetrahydrofuran. J Phys Chem Solids. 2006;67(4):659–64.10.1016/j.jpcs.2005.10.172Search in Google Scholar
(32) Ali V, Kaur R, Lakshmi GBVS, Kumar A, Kumari K, Kumar S. Electrical conductivity and dielectric parameters of polyaniline doped with CuClO4·4BN in aqueous DMSO solvent. Adv Polym Technol. 2012;31(4):374–9.10.1002/adv.20260Search in Google Scholar
(33) Jiesheng L, Yuanyuan Y, Xiang H. Research on the preparation and properties of lauric acid/expanded perlite phase change materials. Energy Build. 2016;110:108–11.10.1016/j.enbuild.2015.10.043Search in Google Scholar
(34) Zhang L, Yang W, Jiang Z, He F, Zhang K, Fan J, et al. Graphene oxide-modified microencapsulated phase change materials with high encapsulation capacity and enhanced leakage-prevention performance. Appl Energy. 2017;197:354–63.10.1016/j.apenergy.2017.04.041Search in Google Scholar
(35) Qu Y, Wang S, Tian Y, Zhou D. Comprehensive evaluation of Paraffin-HDPE shape stabilized PCM with hybrid carbon nano-additives. Appl Therm Eng. 2019;163:114404.10.1016/j.applthermaleng.2019.114404Search in Google Scholar
(36) Liu J, Chen L, Fang X, Zhang Z. Preparation of graphite nanoparticles-modified phase change microcapsules and their dispersed slurry for direct absorption solar collectors. Sol Energy Mater Sol Cell. 2017;159:159–66.10.1016/j.solmat.2016.09.020Search in Google Scholar
(37) Peng G, Dou G, Hu Y, Sun Y, Chen Z. Phase change material (PCM) microcapsules for thermal energy storage. Adv Polym Technol. 2020;2020:9490873.10.1155/2020/9490873Search in Google Scholar
(38) Wang H, Zhao L, Chen L, Song G, Tang G. Facile and low energy consumption synthesis of microencapsulated phase change materials with hybrid shell for thermal energy storage. J Phys Chem Solids. 2017;111:207–13.10.1016/j.jpcs.2017.08.002Search in Google Scholar
(39) Patra BN, Majhi D. Removal of anionic dyes from water by potash alum doped polyaniline: investigation of kinetics and thermodynamic parameters of adsorption. J Phys Chem B. 2015 Jun;119(25):8154–64.10.1021/acs.jpcb.5b00535Search in Google Scholar PubMed
(40) Bhaumik M, McCrindle RI, Maity A, Agarwal S, Gupta VK. Polyaniline nanofibers as highly effective re-usable adsorbent for removal of reactive black 5 from aqueous solutions. J Colloid Interface Sci. 2016;466:442–51.10.1016/j.jcis.2015.12.056Search in Google Scholar PubMed
(41) Huang J, Kaner RB. Nanofiber formation in the chemical polymerization of aniline: a mechanistic study. Angew Chem Int Ed. 2004 Nov;43(43):5817–21.10.1002/anie.200460616Search in Google Scholar PubMed
(42) Carreira AS, Teixeira RFA, Beirão A, Vaz Vieira R, Figueiredo MM, Gil MH. Preparation of acrylic based microcapsules using different reaction conditions for thermo-regulating textiles production. Eur Polym J. 2017;93:33–43.10.1016/j.eurpolymj.2017.05.027Search in Google Scholar
(43) Bohdal T, Dutkowski K, Kruzel M. Experimental studies of the effect of microencapsulated PCM slurry on the efficiency of a liquid solar collector. Materials. 2022;15(13):4493.10.3390/ma15134493Search in Google Scholar PubMed PubMed Central
(44) Zhu K, Li X, Su J, Li H, Zhao Y, Yuan X. Improvement of anti-icing properties of low surface energy coatings by introducing phase-change microcapsules. Polym Eng Sci. 2018;58(6):973–9.10.1002/pen.24654Search in Google Scholar
(45) Fuensanta M, Paiphansiri U, Romero-Sanchez M, Guillem C, Lopez-Buendia A, Landfester K. Thermal properties of a novel nanoencapsulated phase change material for thermal energy storage. Thermochim Acta. 2013;565:95–101.10.1016/j.tca.2013.04.028Search in Google Scholar
(46) Shi J, Wu X, Fu X, Sun R. Synthesis and thermal properties of a novel nanoencapsulated phase change material with PMMA and SiO2 as hybrid shell materials. Thermochim Acta. 2015;617:90–4.10.1016/j.tca.2015.08.022Search in Google Scholar
(47) Zhu Y, Liang S, Chen K, Gao X, Chang P, Tian C, et al. Preparation and properties of nanoencapsulated n-octadecane phase change material with organosilica shell for thermal energy storage. Energy Convers Manag. 2015;105:908–17.10.1016/j.enconman.2015.08.048Search in Google Scholar
(48) Tumirah K, Hussein MZ, Zulkarnain Z, Rafeadah R. Nano-encapsulated organic phase change material based on copolymer nanocomposites for thermal energy storage. Energy. 2014;66:881–90.10.1016/j.energy.2014.01.033Search in Google Scholar
(49) He L, Mo S, Lin P, Jia L, Chen Y, Cheng Z. D-mannitol@silica/graphene oxide nanoencapsulated phase change material with high phase change properties and thermal reliability. Appl Energy. 2020;268:115020.10.1016/j.apenergy.2020.115020Search in Google Scholar
© 2025 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Flow-induced fiber orientation in gas-powered projectile-assisted injection molded parts
- Research on thermal aging characteristics of silicone rubber composite materials for dry-type distribution transformers
- Kinetics of acryloyloxyethyl trimethyl ammonium chloride polymerization in aqueous solutions
- Influence of siloxane content on the material performance and functional properties of polydimethylsiloxane copolymers containing naphthalene moieties
- Enhancement effect of electron beam irradiation on acrylonitrile–butadiene–styrene (ABS) copolymers from waste electrical and electronic equipment by adding 1,3-PBO: A potential way for waste ABS reuse
- Model construction and property study of poly(ether-ether-ketone) by molecular dynamics simulation with meta-modeling methods
- Zinc–gallic acid–polylysine nanocomplexes with enhanced bactericidal activity for the treatment of bacterial keratitis
- Effect of pyrogallol compounds dosage on mechanical properties of epoxy coating
- Preparation of in situ polymerized polypyrrole-modified braided cord and its electrical conductivity investigation under varied mechanical conditions
- Hydrophobicity, UV resistance, and antioxidant properties of carnauba wax-reinforced CG bio-polymer film
- Janus nanofiber membrane films loading with bioactive calcium silicate for the promotion of burn wound healing
- Synthesis of migration-resistant antioxidant and its application in natural rubber composites
- Influence of the flow rate on the die swell for polymer micro coextrusion process
- Fatty acid filled polyaniline nanofibres with dual electrical conductivity and thermo-regulatory characteristics: Futuristic material for thermal energy storage
- Hydrolytic depolymerization of major fibrous wastes
- Performance of epoxy hexagonal boron nitrate underfill materials: Single and mixed systems
- Blend electrospinning of citronella or thyme oil-loaded polyurethane nanofibers and evaluating their release behaviors
- Efficiency of flexible shielding materials against gamma rays: Silicon rubber with different sizes of Bi2O3 and SnO
- A comprehensive approach for the production of carbon fibre-reinforced polylactic acid filaments with enhanced wear and mechanical behaviour
- Electret melt-blown nonwovens with charge stability for high-performance PM0.3 purification under extreme environmental conditions
- Study on the failure mechanism of suture CFRP T-joints under/after the low-velocity impact loading
- Experimental testing and finite element analysis of polyurethane adhesive joints under Mode I loading and degradation conditions
- Optimizing recycled PET 3D printing using Taguchi method for improved mechanical properties and dimensional precision
- Effect of stacking sequence of the hybrid composite armor on ballistic performance and damage mechanism
- Bending crack propagation and delamination damage behavior of orthogonal ply laminates under positive and negative loads
- Molecular dynamics simulation of thermodynamic properties of Al2O3-modified silicone rubber under silane coupling agent modification
- Precision injection molding method based on V/P switchover point optimization and pressure field balancing
- Heparin and zwitterion functionalized small-diameter vascular grafts for thrombogenesis prevention
- Metal-free N, S-co-doped carbon materials derived from calcined aromatic co-poly(urea-thiourea)s as efficient alkaline oxygen reduction catalysts
- Influence of stitching parameters on the tensile performance and failure mechanisms of CFRP T-joints
- Synthesis of PEGylated polypeptides bearing thioether pendants for injectable ROS-responsive hydrogels
- Rapid Communication
- RAFT-mediated polymerization-induced self-assembly of poly(ionic liquid) block copolymers in a green solvent
- Corrigendum
- Corrigendum to “High-strength polyvinyl alcohol-based hydrogel by vermiculite and lignocellulosic nanofibrils for electronic sensing”
Articles in the same Issue
- Research Articles
- Flow-induced fiber orientation in gas-powered projectile-assisted injection molded parts
- Research on thermal aging characteristics of silicone rubber composite materials for dry-type distribution transformers
- Kinetics of acryloyloxyethyl trimethyl ammonium chloride polymerization in aqueous solutions
- Influence of siloxane content on the material performance and functional properties of polydimethylsiloxane copolymers containing naphthalene moieties
- Enhancement effect of electron beam irradiation on acrylonitrile–butadiene–styrene (ABS) copolymers from waste electrical and electronic equipment by adding 1,3-PBO: A potential way for waste ABS reuse
- Model construction and property study of poly(ether-ether-ketone) by molecular dynamics simulation with meta-modeling methods
- Zinc–gallic acid–polylysine nanocomplexes with enhanced bactericidal activity for the treatment of bacterial keratitis
- Effect of pyrogallol compounds dosage on mechanical properties of epoxy coating
- Preparation of in situ polymerized polypyrrole-modified braided cord and its electrical conductivity investigation under varied mechanical conditions
- Hydrophobicity, UV resistance, and antioxidant properties of carnauba wax-reinforced CG bio-polymer film
- Janus nanofiber membrane films loading with bioactive calcium silicate for the promotion of burn wound healing
- Synthesis of migration-resistant antioxidant and its application in natural rubber composites
- Influence of the flow rate on the die swell for polymer micro coextrusion process
- Fatty acid filled polyaniline nanofibres with dual electrical conductivity and thermo-regulatory characteristics: Futuristic material for thermal energy storage
- Hydrolytic depolymerization of major fibrous wastes
- Performance of epoxy hexagonal boron nitrate underfill materials: Single and mixed systems
- Blend electrospinning of citronella or thyme oil-loaded polyurethane nanofibers and evaluating their release behaviors
- Efficiency of flexible shielding materials against gamma rays: Silicon rubber with different sizes of Bi2O3 and SnO
- A comprehensive approach for the production of carbon fibre-reinforced polylactic acid filaments with enhanced wear and mechanical behaviour
- Electret melt-blown nonwovens with charge stability for high-performance PM0.3 purification under extreme environmental conditions
- Study on the failure mechanism of suture CFRP T-joints under/after the low-velocity impact loading
- Experimental testing and finite element analysis of polyurethane adhesive joints under Mode I loading and degradation conditions
- Optimizing recycled PET 3D printing using Taguchi method for improved mechanical properties and dimensional precision
- Effect of stacking sequence of the hybrid composite armor on ballistic performance and damage mechanism
- Bending crack propagation and delamination damage behavior of orthogonal ply laminates under positive and negative loads
- Molecular dynamics simulation of thermodynamic properties of Al2O3-modified silicone rubber under silane coupling agent modification
- Precision injection molding method based on V/P switchover point optimization and pressure field balancing
- Heparin and zwitterion functionalized small-diameter vascular grafts for thrombogenesis prevention
- Metal-free N, S-co-doped carbon materials derived from calcined aromatic co-poly(urea-thiourea)s as efficient alkaline oxygen reduction catalysts
- Influence of stitching parameters on the tensile performance and failure mechanisms of CFRP T-joints
- Synthesis of PEGylated polypeptides bearing thioether pendants for injectable ROS-responsive hydrogels
- Rapid Communication
- RAFT-mediated polymerization-induced self-assembly of poly(ionic liquid) block copolymers in a green solvent
- Corrigendum
- Corrigendum to “High-strength polyvinyl alcohol-based hydrogel by vermiculite and lignocellulosic nanofibrils for electronic sensing”

