Abstract
Silicone rubber is renowned for its excellent insulating properties. Enhancing its thermal conductivity can significantly improve its performance, making it more suitable for the demanding conditions of operational transformers. Adding liquid silicone rubber (ALSR) with high-performance fillers modified by coupling agents effectively enhances its thermal conductivity. This study used molecular dynamics simulations to construct Al2O3/ALSR models with unmodified and 3-aminopropyltriethoxysilane (KH550) silane coupling agent-modified alumina at grafting rates of 5% and 10%. The effects of different grafting rates of surface-modified Al2O3 on the thermodynamic properties and microstructure of silicone rubber composites were analyzed. The study found that the modification with the KH550 silane coupling agent significantly enhanced the thermodynamic properties of Al2O3/ALSR composites. Compared to the model without a grafting coupling agent, the thermal conductivity of the composite materials with grafting rates of 5% and 10%, respectively, increased by 97.91% and 127.75%. Simultaneously, excessive content of silane coupling agents can lead to instability in the molecular interface structure, resulting in a decline in the composite materials’ thermal stability and mechanical properties. By examining the free volume and mean square displacement of the composite materials, it is observed that the surface modification of Al2O3 with the silane coupling agent KH-550 restricts the mobility of silicone rubber molecular chains. However, as the grafting rate increases, this hindering effect diminishes, consequently reducing the interfacial adhesion of Al2O3/ALSR.
1 Introduction
Dry-type distribution transformers constitute pivotal assets within power distribution networks, boasting a substantial installed base (1,2,3). Nevertheless, the magnitude of units rendered obsolete annually is considerable. Effectively handling decommissioned dry-type distribution transformers to achieve their green recyclability is crucial for power grids to attain their “dual-carbon” goals (4,5). Traditional dry-type transformers employ epoxy resin as the principal insulation material, which is prone to moisture absorption and lacks recyclability or reusability upon decommissioning. This characteristic poses a significant risk of energy and resource wastage (6,7,8,9,10). Utilizing recyclable elastomeric materials for insulation in dry-type transformers significantly reduces carbon emissions and enhances the safety of grid operations and the economic viability of transformer units by effectively mitigating frequent expansion and contraction stresses.
Silicone rubber possesses characteristics such as high elasticity, resistance to extreme temperatures, excellent hydrophobicity, superior insulation properties, and safety, making it a viable alternative material (11,12,13). However, their intrinsic thermal conductivity is approximately around 0.17 W·m−1·K−1 for silicone rubber matrices, which is relatively low. This low thermal conductivity limits their application in dry-type transformers (14,15). As real-world operating conditions become increasingly complex and demanding, there is a growing demand for improved thermal conductivity and heat resistance of silicone rubber. Therefore, the thermodynamic properties of silicone rubber materials can be enhanced by incorporating high-performance fillers into the silicone rubber matrix (16,17).
The choice of fillers significantly impacts the thermal conductivity of filled polymers. Currently, various types of fillers are employed to enhance thermal conductivity, including metallic fillers (such as copper, silver, and aluminum), carbon-based fillers (like graphite, carbon nanotubes, and graphene), and inorganic fillers (such as aluminum oxide, aluminum nitride, and boron nitride) (18,19,20,21). Sun et al. found that by incorporating 30 wt% h-BN into epoxy resin, the thermal conductivity of h-BN/EP composite materials can reach 0.86 W·m−1·K−1 (22). Li et al. conducted a study and found that under a fixed filler loading of 120 phr, spherical aluminum oxide, irregular aluminum nitride, and two-dimensional boron nitride flakes exhibit varying effects on the thermal conductivity of liquid silicone rubber, with respective increases in thermal conductivity coefficients of 88%, 124%, and 316% (23). Although BN exhibits a higher enhancement effect on the thermal conductivity of silicone rubber compared to Al2O3, the mechanical performance improvement of Al2O3 far exceeds that of BN and is also more cost-effective. Therefore, aluminum oxide is widely used in industrial applications (24). However, Al2O3 tends to aggregate, making it difficult to disperse evenly within the silicone rubber matrix when directly incorporated. This aggregation can lead to poor interfacial bonding and defects at the molecular interface, thereby hindering the enhancement of adding liquid silicone rubber (ALSR) performance (25). Preventing filler aggregation and ensuring uniform dispersion within the matrix material is crucial for enhancing composite material performance. Chemical modification or functionalization of filler surfaces can effectively avoid aggregation phenomena (26,27). Kim et al. employed the silane coupling agent KH550 for surface modification of aluminum powder. Experimental results demonstrated that the surface-modified fillers significantly enhanced the composite materials’ fracture toughness, wear resistance, and adhesive strength (28).
To enhance the adhesion at the Al2O3/ALSR molecular interface and facilitate better dispersion of Al2O3 within the ALSR matrix, this study constructed models of unmodified Al2O3/ALSR as well as Al2O3/ALSR modified with KH550 silane coupling agent at grafting rates of 5% and 10%. The analysis and comparison of the effects of different grafting rates of surface-modified Al2O3 on the thermodynamic properties and microstructure of silicone rubber composite materials were conducted. Furthermore, the study investigated the influence of coupling agent content on the performance of silicone rubber composite materials, aiming to lay the groundwork for the subsequent development of new-generation environmentally friendly, dry-type recyclable transformers insulation materials.
2 Model establishment
2.1 Initial model construction
Using Material Studio software, monomer models of ALSR and Al2O3 molecules were separately constructed. VPDMS was selected as the base polymer, with PMHS as the crosslinking agent. Each VPDMS single chain consisted of 20 repeating units, while each PMHS single chain comprised 15 repeating units. Surface modification of Al2O3 molecules was performed using KH550 with grafting rates of 5% and 10%. Given the high molecular weight and initial polymerization degree of ALSR, simulating a large number of repeating unit molecules is impractical. This study examines the influence of different grafting rates of silane coupling agent-modified Al2O3 molecules on ALSR performance. Considering computational efficiency and ensuring sufficient crosslinking, 12 VPDMS chains and 6 PMHS chains were used to construct the pure silicone rubber model. The monomer models are depicted in Figure 1, while the KH550 silane coupling agent modification process is illustrated in Figure 2.
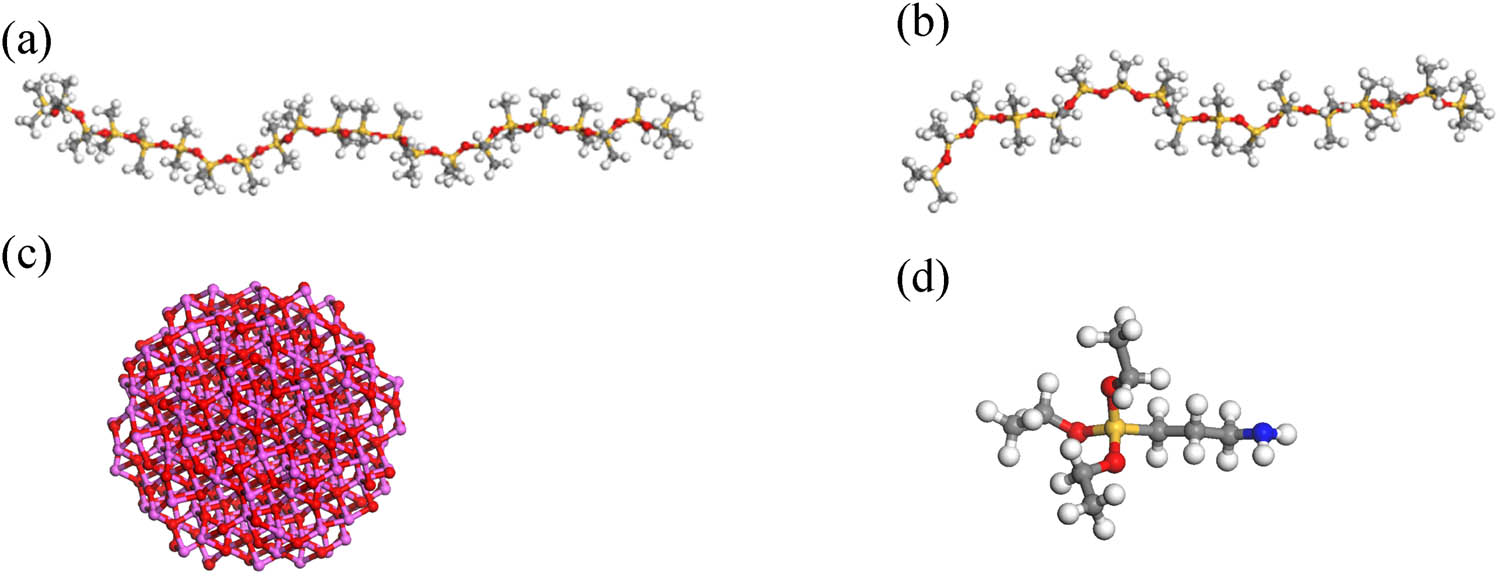
The individual monomer models: (a) VPDMS, (b) PMHS, (c) Al2O3, (d) KH550.
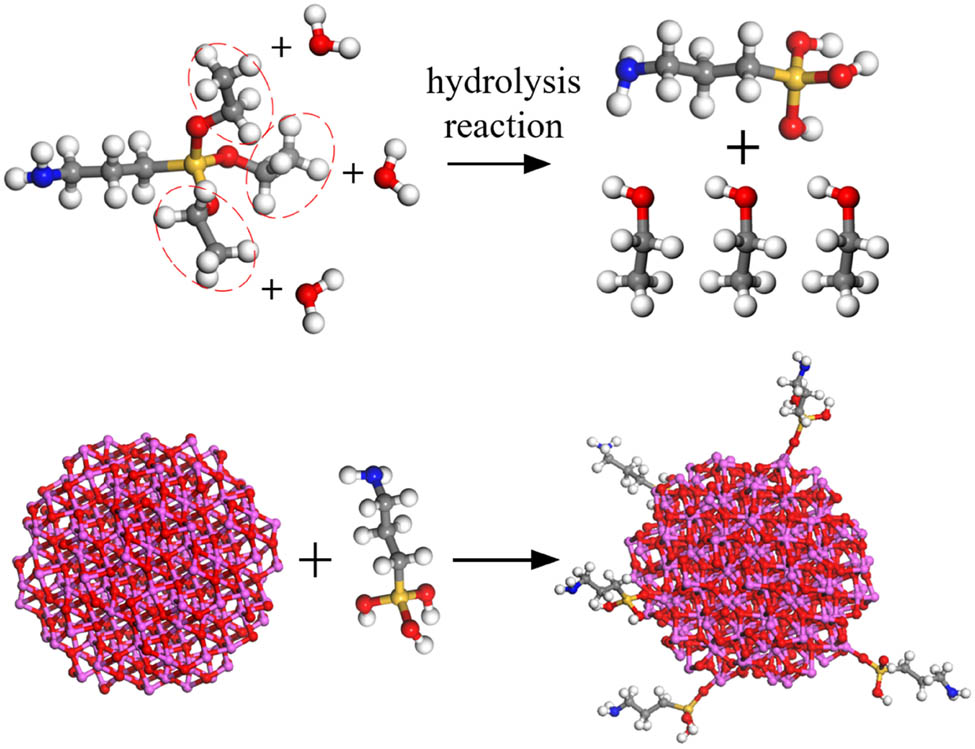
Silane coupling agent modification process.
2.2 Model construction steps
The models of pure silicone rubber, silicone rubber doped with Al2O3, and Al2O3/ALSR with different grafting rates were constructed separately. The models of pure silicone rubber and silicone rubber doped with Al2O3 and Al2O3/ALSR with different grafting rates were independently built. The initial models were established using the amorphous cell module, with an initial density of 0.6 g·cm−3 and a doping ratio of 30% for Al2O3. The model of pure silicone rubber is denoted as ALSR, silicone rubber doped with Al2O3 is ALSR-1, silicone rubber doped with 5% KH-550 grafted Al2O3 is ALSR-2, and silicone rubber doped with 10% KH-550 grafted Al2O3 is ALSR-3.
First, the initial model of pure silicone rubber undergoes geometric optimization (10,000 steps) to minimize the model energy. After geometric optimization, the model undergoes a 200 ps dynamic optimization under the NVT ensemble at a temperature of 600 K. Subsequently, a 100 ps dynamic optimization under the NPT ensemble is conducted at the same temperature. Since the remaining three models all contain Al2O3, to prevent deformation during the optimization process, it is necessary to fix the structures of Al2O3 and its grafted molecules before the optimization. Following this, geometric optimization and a 200 ps dynamic optimization under the NVT ensemble at 600 K are conducted separately for each model. The final optimized models obtained are depicted in Figure 3, which will be used for subsequent cross-linking.
The cross-linking reaction proceeds as follows: under the catalysis of a catalyst, ethylene reacts with polyhydrosiloxane through a hydrosilylation addition reaction, forming new Si–C bonds, which leads to the cross-linking of polysiloxane into a network structure. Typically, platinum-based catalysts are chosen for this purpose. The cross-linking reaction equation is shown in Figure 4. The simulation of the cross-linking reaction can be achieved through a Perl script. The initial reaction radius is 3.5 Å, the maximum reaction radius is 8 Å, and the step size is 0.5 Å. The cross-linking temperature is 438 K, and the maximum cross-linking degree is 90%.
2.3 Cross-linking model optimization
The cross-linked models obtained cannot be further investigated for performance parameters due to excessive internal stress in the system. Therefore, structural optimization and dynamic optimization are required for these models. First, geometric optimization is performed on the models, followed by five cycles of annealing under the NVT ensemble within the temperature range of 300–600 K, with a total simulation time of 140 ps. After annealing cycles, the model with the lowest potential energy is selected for a 100 ps NPT dynamic simulation at 600 K. Subsequently, dynamic simulations are conducted at 300 K, starting with a 200 ps NVT simulation followed by a 100 ps NPT simulation. The resulting model is then used for subsequent molecular simulation calculations. The final optimized cross-linked model is depicted in Figure 5, while the cross-linking bonds are illustrated in Figure 6.
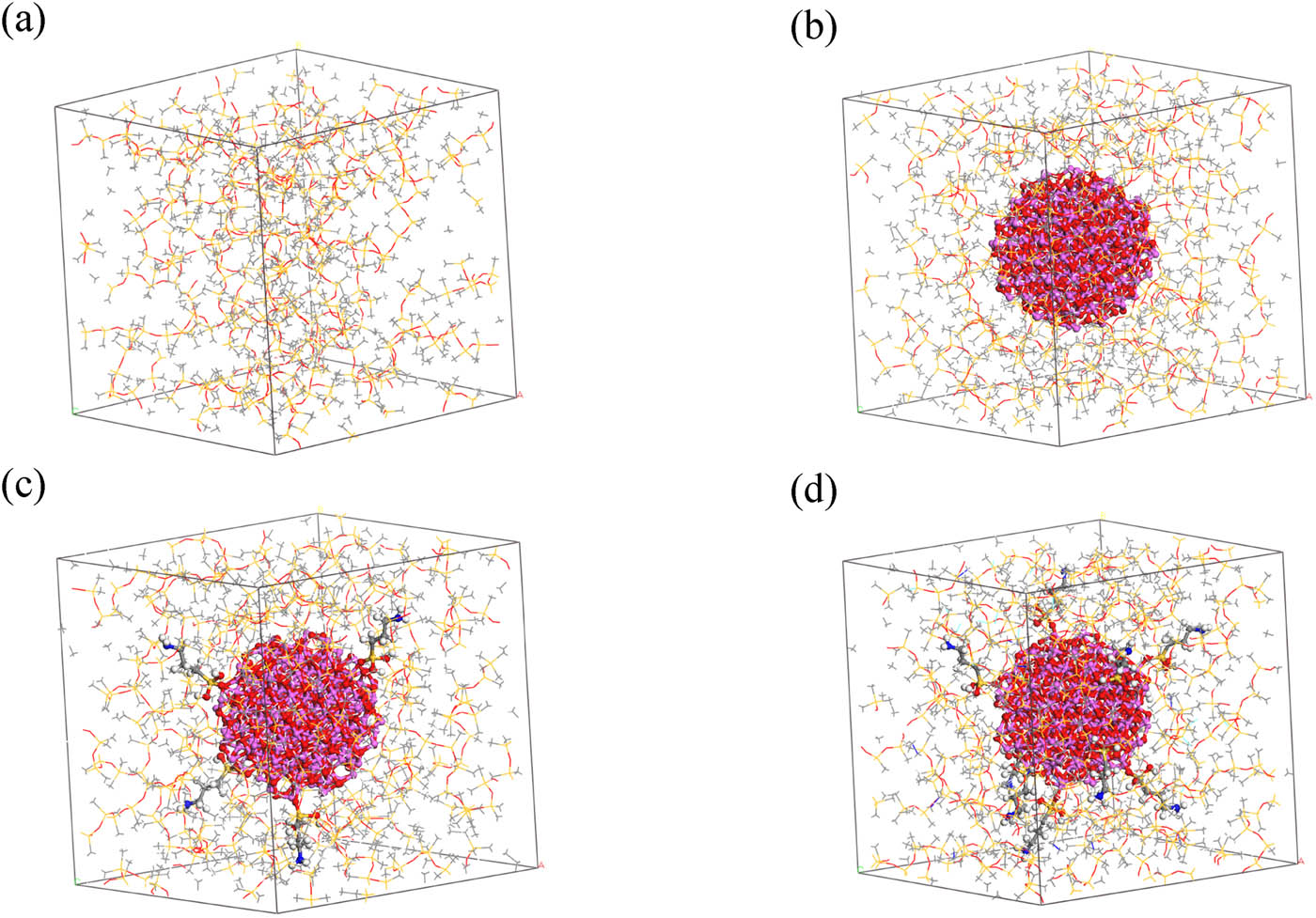
Optimized models before cross-linking: (a) ALSR, (b) ALSR-1, (c) ALSR-2, and (d) ALSR-3.
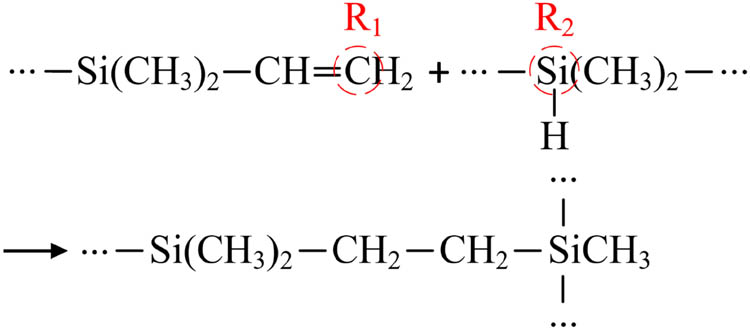
Cross-linking reaction.
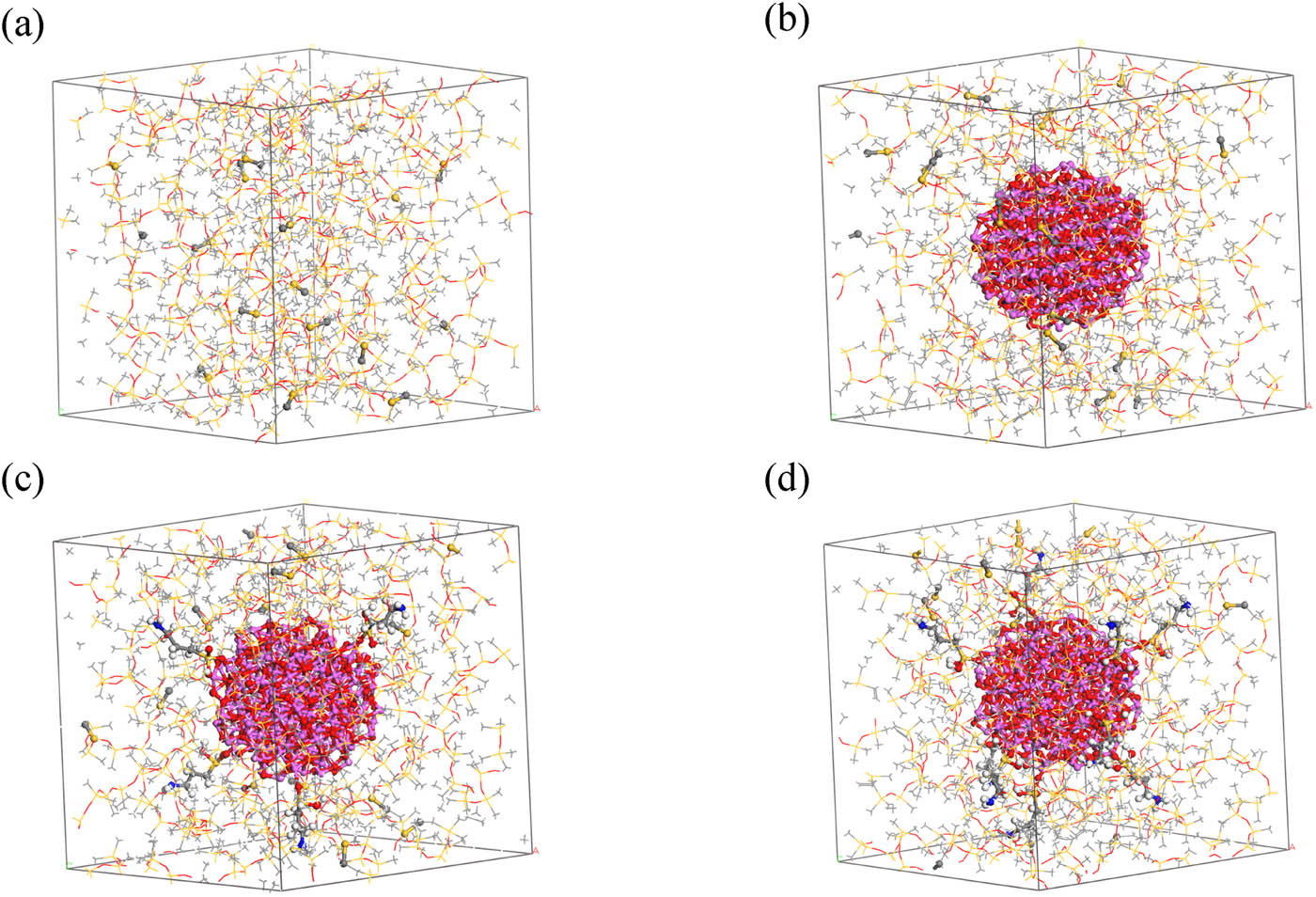
Optimized models after cross-linking: (a) ALSR, (b) ALSR-1, (c) ALSR-2, and (d) ALSR-3.
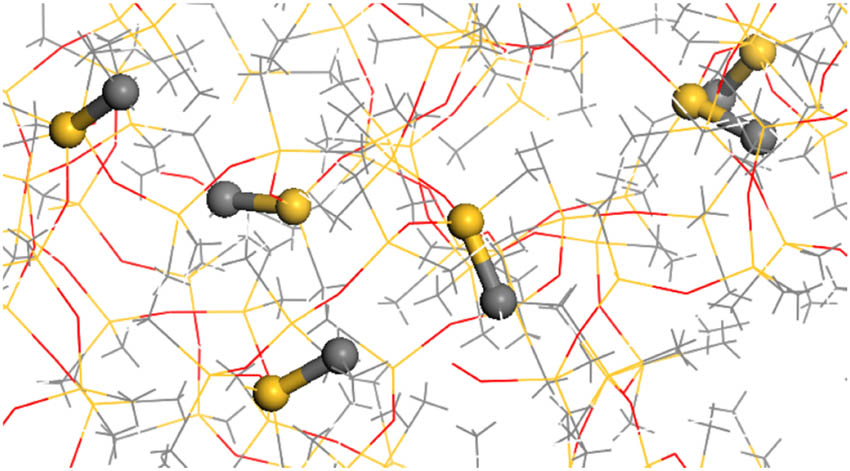
Cross-linking bonds.
In the simulation process, temperature and pressure were controlled using the Andersen and Berendsen methods. The pure silicone rubber model employed the COMPASSII force field, while the remaining models utilized the Universal force field. Electrostatic and van der Waals forces were calculated using the Ewald and Atom-based methods.
3 Thermodynamic performance calculation
3.1 Thermal conductivity
Various methods based on molecular dynamics have been extensively researched for calculating material thermal conductivity, such as Green-Kubo, non-equilibrium molecular dynamics, and reverse non-equilibrium molecular dynamics (RNEMD). RNEMD is widely adopted among these due to its high accuracy and fast convergence rate.
This article utilizes the RNEMD method to compute thermal conductivity. The model is evenly divided along a specific direction (in this study, the z-direction) into several layers (a total of 40 layers in this paper), with the outermost layers at both ends of the model termed the “hot layers” and the middle two layers referred to as the “cold layers,” as illustrated in Figure 7.
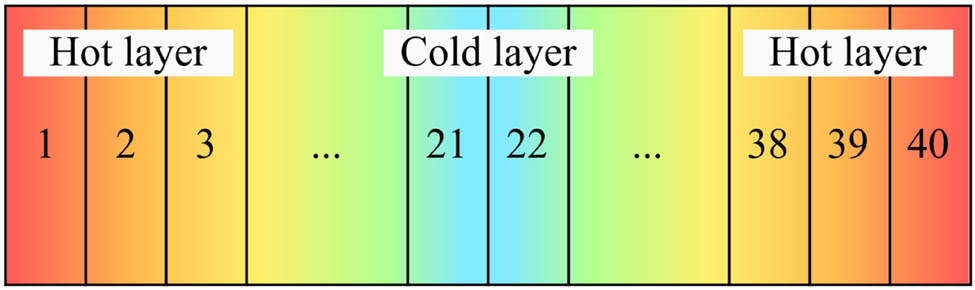
Schematic diagram of the RNEMD method model.
Then, energy exchange occurs by exchanging kinetic energy between the coldest particles in the hot layers and the hottest particles in the cold layers. After multiple energy exchanges and averaging, a stable temperature gradient is established within the system. The thermal conductivity λ is calculated based on Fourier’s heat conduction law.
where J represents the energy flux in the z-direction and dT/dz represents the temperature gradient. The negative sign indicates that the direction of energy flux is always opposite to the temperature gradient.
The energy flux J can be represented by the energy exchange ΔE between two fixed layers during each time interval Δt.
where A represents the area perpendicular to the model’s energy flux direction.
When calculating the thermal conductivity of materials, it is necessary to reconstruct the thermal conductivity calculation model while keeping the number and types of molecular chains within the unit cell unchanged. The model is enlarged by a factor of three along the z-direction to facilitate the formation of a stable temperature gradient within the system, thus enabling the calculation of thermal conductivity more effectively. When a stable temperature gradient is established within the system, the temperature variation along the z-direction of the model is depicted in Figure 8. The calculated thermal conductivity values for different types of silicone rubber are illustrated in Figure 9.
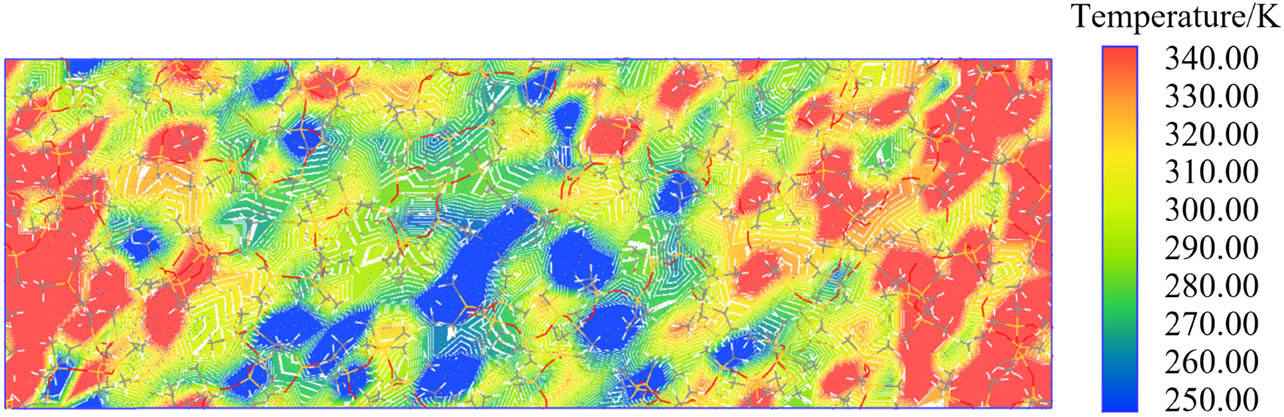
Temperature distribution of the calculated model.
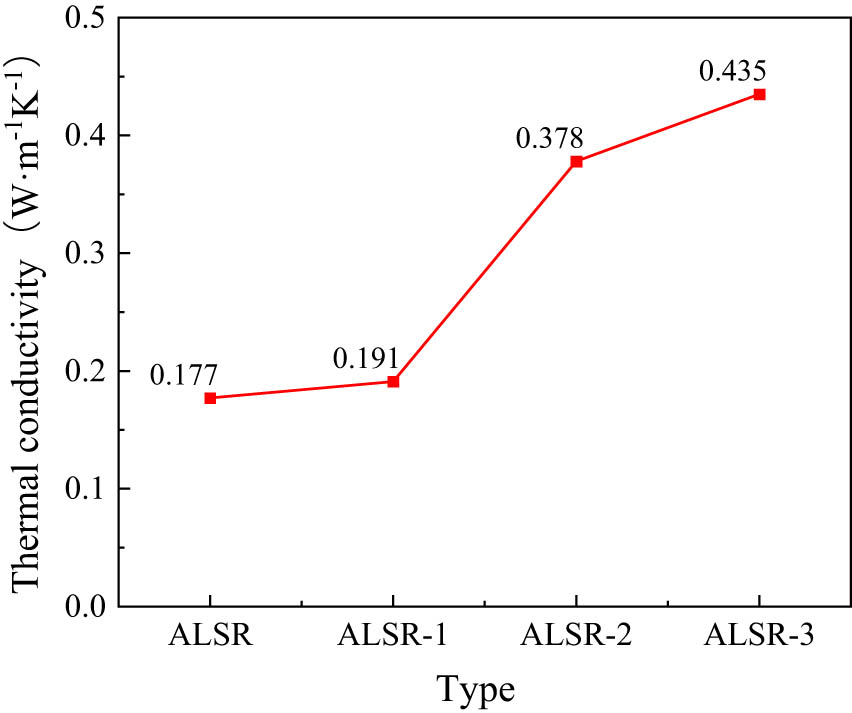
Thermal conductivity of different types of silicone rubber.
The results reveal that after doping with 30% Al2O3, the thermal conductivity of silicone rubber increased from 0.177 to 0.191 W·m−1·K−1, with the improvement not being significant. However, the thermal conductivity is greatly enhanced after modifying alumina with a KH-550 silane coupling agent. When the grafting rate of KH-550 is 5%, the thermal conductivity reaches 0.378; when the grafting rate is 10%, the thermal conductivity increases to 0.435. The thermal conductivity of the Al2O3 surface modified with silane coupling agent exhibits a significant increase compared to its unmodified state. The main reason is the addition of the coupling agent, which enhances the connection between Al2O3 molecules and silicone rubber chains, resulting in a tighter bond between them and a more pronounced modification effect. Similarly, the higher the grafting rate of the coupling agent, the more significant the enhancement effect on thermal conductivity.
3.2 Glass transition temperature
The glass transition temperature (T g) is the critical temperature at which amorphous materials (such as glass) transition from liquid to solid as the temperature decreases. This temperature marks a significant transition in the material’s structure and properties, shifting from a disordered liquid state to an ordered solid state. At the microscopic level, T g represents the temperature at which polymer chain segment motion can be activated, making it one of the critical characteristic indicators of polymer properties.
The relationship between material density and temperature can be obtained through molecular dynamics simulations of cooling experiments. The cooling range is set from 100 to 350 K with a cooling rate of 10 K per 250 ps, and molecular dynamics simulations are conducted under the NVT and NPT ensembles for 100 ps at each temperature, resulting in 25 sets of density–temperature data. By analyzing the density–temperature curve, the material’s T g can be determined. Specifically, when a distinct inflection point appears on the curve, linear regression is applied to the data on both sides of the inflection point to obtain the intersection point T g of the two linear fits, as illustrated in Figure 10.
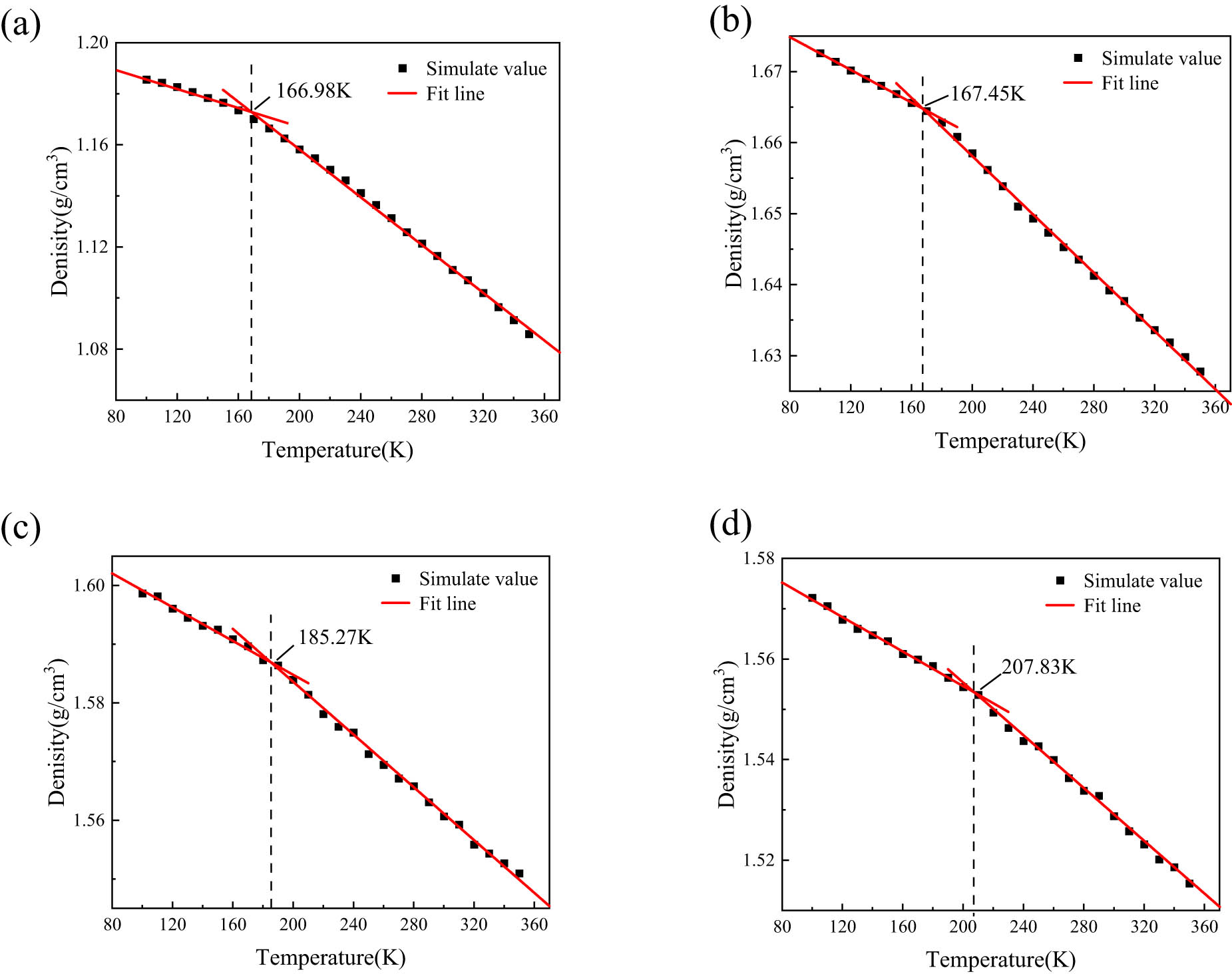
Schematic of linear fitting of density-temperature curves: (a) ALSR, (b) ALSR-1, (c) ALSR-2, (d) ALSR-3.
The graph shows that when no coupling agent is used, there is no significant change in the T g of Al2O3/ALSR compared to pure silicone rubber. However, the model’s T g is significantly increased after using the coupling agent. Specifically, with a grafting rate of 5%, T g is 185.27 K, and with a grafting rate of 10%, T g is 207.83 K. Compared to the model with Al2O3 doping but without coupling agent grafting, there is an increase of 10.65% and 24.11%, respectively. This indicates that the surface modification of Al2O3 with KH-550 silane coupling agent impedes the motion of silicone rubber molecular chains, thus requiring higher temperatures to activate the motion of silicone rubber chain segments. For both models before and after coupling agent grafting, the sharp increase in T g is mainly attributed to adding the coupling agent, which improves the aggregation phenomenon of alumina. This improves bonding between Al2O3 and the silicone rubber matrix, enhancing the molecular interface adhesion between Al2O3 and silicone rubber. It effectively assists in the modification of Al2O3-reinforced silicone rubber, improving the overall performance of silicone rubber.
3.3 Coefficient of thermal expansion (CTE)
The CTE represents the percentage change in the volume of a unit of material when the temperature changes. Understanding the CTE of a material allows for predicting dimensional changes of an object when subjected to temperature variations, thereby avoiding structural deformation, cracks, or damage caused by thermal expansion. It is an important indicator for measuring the thermal stability of materials.
The volume and temperature of the system before and after the glass transition temperature are fitted to obtain the corresponding temperature–volume curves, as shown in Figure 11. From Figure 11, the slope of the fitting curve before and after the material’s T g can be obtained. Then, the thermal expansion coefficients of the glassy state (below T g) and the rubbery state (above T g) of each system are calculated using Eq. 3, and the results are presented in Table 1.
where β CTE represents the CTE, V 0 denotes the volume of the model at the initial temperature, and P is 0.0001 GPa.
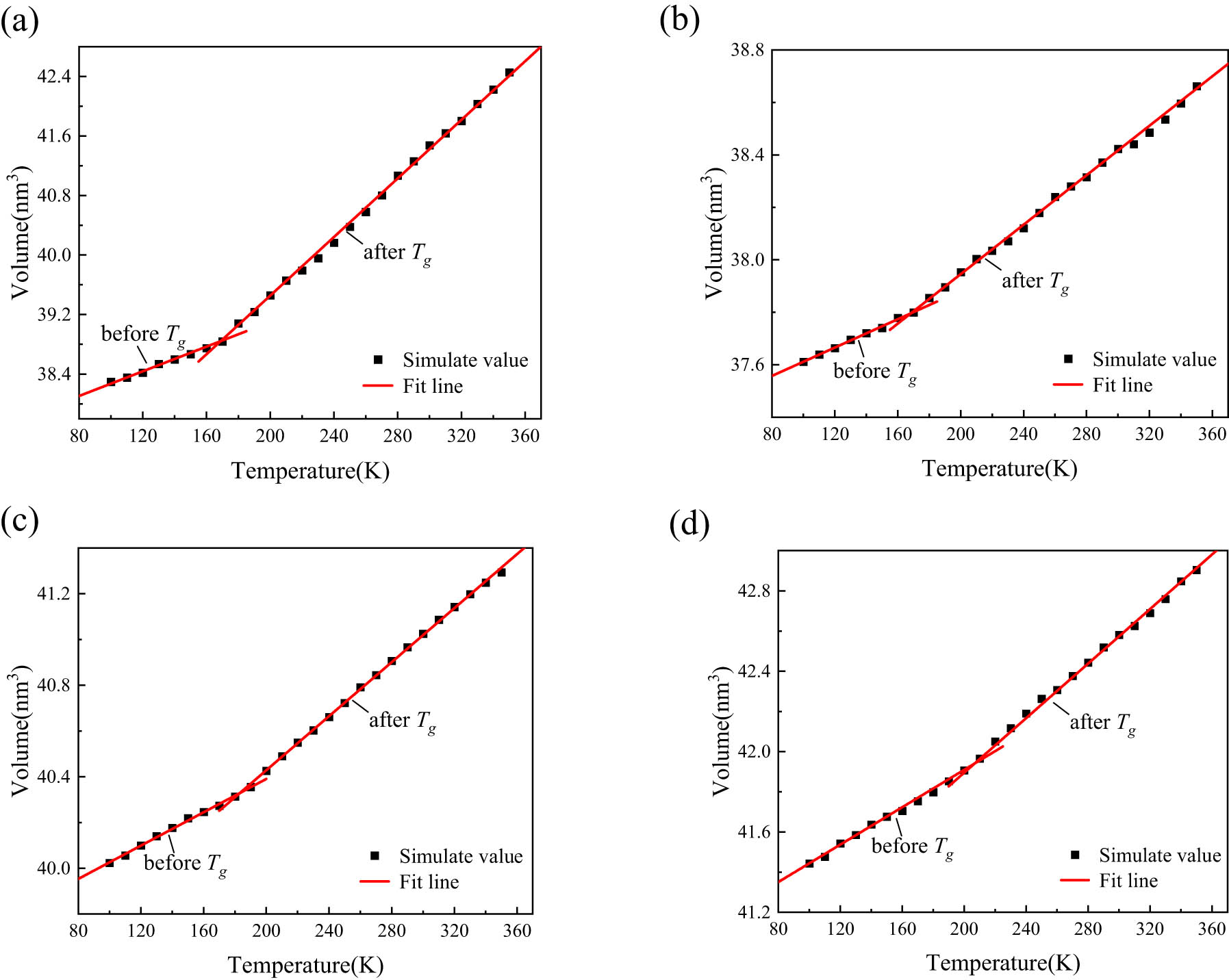
Schematic of linear fitting of volume–temperature curves: (a) ALSR, (b) ALSR-1, (c) ALSR-2, and (d) ALSR-3.
CTE for different types of silicone rubber
| Type | Glassy state β CTE (K−1) | Rubbery state β CTE (K−1) |
|---|---|---|
| ALSR | 1.94 × 10−4 | 4.66 × 10−4 |
| ALSR-1 | 0.71 × 10−4 | 1.23 × 10−4 |
| ALSR-2 | 0.89 × 10−4 | 1.44 × 10−4 |
| ALSR-3 | 1.09 × 10−4 | 1.59 × 10−4 |
Table 1 shows that Al2O3 can alter silicone rubber’s thermal expansion coefficient, resulting in a significant reduction in CTE for both its glassy and rubbery states. However, after grafting with the silane coupling agent KH-550, the CTE of the thermal expansion system compared to the nongrafting model exhibits a slight increase, which further increases with an increase in the grafting rate. Compared to pure silicone rubber, the postgrafting model exhibits considerably lower coefficients of thermal expansion, indicating that the incorporation of Al2O3 enhances the thermal stability of silicone rubber. The increase in the CTE after grafting with the coupling agent is likely due to the poor thermal stability of the coupling agent itself, which leads to changes in the thermal expansion coefficient of the composite material.
3.4 Mechanical properties
Molecular dynamics simulations can be employed to analyze stress and strain in systems undergoing minor deformation, enabling the extraction of parameters relevant to mechanical properties. Due to the isotropic behavior exhibited by silicone rubber on a macroscopic scale, it can be approximately treated as an isotropic material. Its bulk modulus K, Young’s modulus E, and shear modulus G can be obtained from the following formulas:
where λ and μ are constants that can be obtained through molecular dynamics calculations.
For models of silicone rubber and its modified materials, a geometric optimization is performed at 300 K to minimize the stress within the system as much as possible. The geometrically optimized models are then subjected to 100 ps of NPT dynamic calculations, with a model output generated every one ps, resulting in a total of 100 models. The five models with the lowest potential energy are selected, and mechanical performance calculations are conducted using the constant strain method, with a maximum strain set to 0.003. Finally, the values of λ and μ for each model at 300 K are obtained, and according to Eqs. 4–6, the corresponding models’ bulk modulus K, Young’s modulus E, and shear modulus G are calculated, resulting in five sets for each parameter. The average values are then taken to obtain the final results, as shown in Table 2. Figure 12 displays the mechanical properties of each system.
Mechanical properties of different types of silicone rubber models
| Type | K | E | G |
|---|---|---|---|
| ALSR | 1.92 | 5.02 | 2.36 |
| ALSR-1 | 4.39 | 10.05 | 5.54 |
| ALSR-2 | 18.85 | 27.68 | 11.15 |
| ALSR-3 | 16.42 | 18.86 | 7.41 |
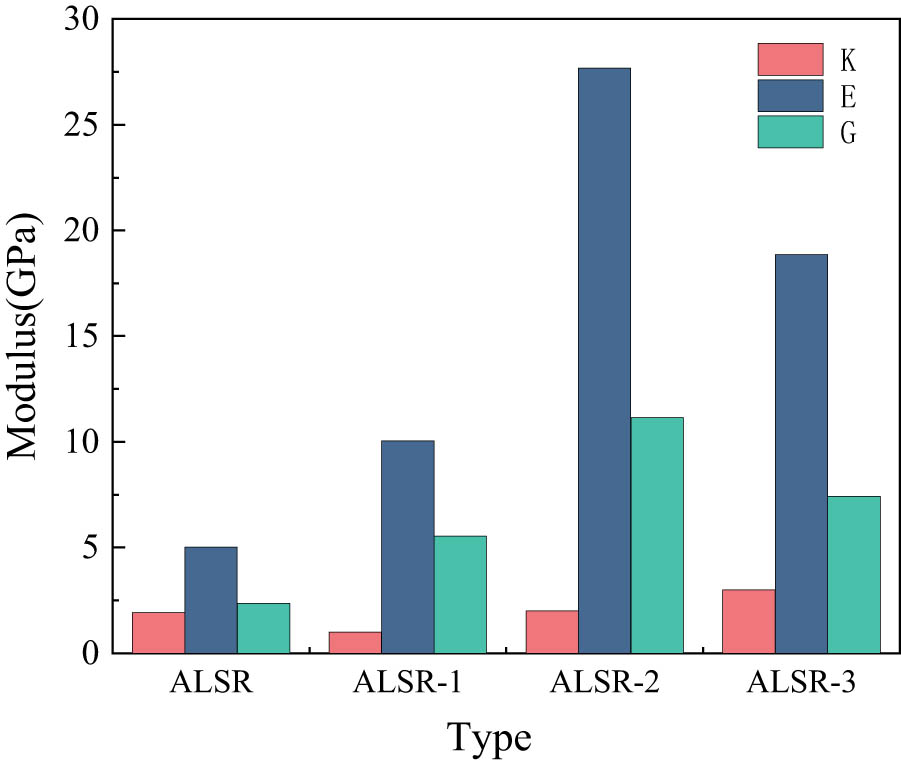
Mechanical properties of different types of silicone rubber models.
From the graph, it can be observed that the addition of Al2O3 increases the bulk modulus, Young’s modulus, and shear modulus of silicone rubber. This indicates that doping with Al2O3 enhances the rigidity of silicone rubber, making it less prone to deformation. After modification with the KH-550 silane coupling agent, the bulk modulus, Young’s modulus, and shear modulus of silicone rubber are significantly enhanced. Specifically, when the grafting rate is 5%, the silicone rubber shows a much more significant increase in these properties than pure silicone rubber and silicone rubber without the coupling agent. The bulk modulus increases by 881.8% and 329.4%, Young’s modulus increases by 451.4% and 175.4%, and the shear modulus increases by 372.5% and 101.3%, respectively. This indicates that after grafting with the silane coupling agent, the adhesion between Al2O3 and the silicone rubber matrix surface increases, making the silicone rubber composite system more stable and less prone to deformation under external forces. However, with the rise in grafting rate to 10%, a decline in mechanical performance relative to the 5% grafting rate is observed. This phenomenon may stem from the heightened concentration of the silane coupling agent at higher grafting rates, leading to potential instability in the molecular interface structure. Such instability could manifest as excessive aggregation or crystallization within the molecular interface layer, resulting in deformation and subsequent reduction in adhesion. Consequently, this adverse effect on adhesion could impact the mechanical properties, decreasing overall mechanical performance.
4 Structural parameter calculation
4.1 Radial distribution function (RDF)
The RDF is a parameter reflecting the microstructural characteristics of materials, capable of revealing the nature of interactions between nonbonded atoms. It is defined as follows:
where n B is the number of B atoms at a distance r around the A atom, N B is the total number of B atoms, and V is the volume of the entire system.
Figure 13 shows the whole atomic RDF for all model systems. The RDF of the Al2O3/ALSR model exhibits an additional peak compared to the pure silicone rubber model, mainly due to the introduction of Al2O3. In the silicone rubber model doped with Al2O3, there is no significant difference in the overall trend of the whole atomic RDF before and after coupling agent modification. Differences are only observed at various peak positions.
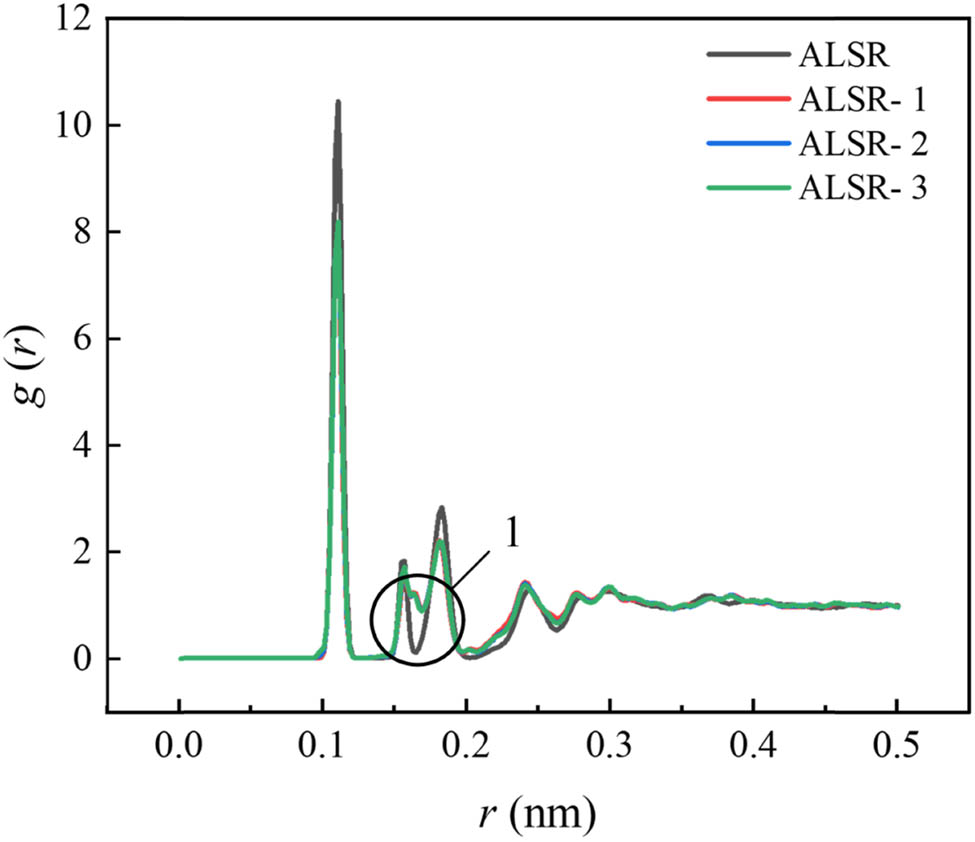
Full atomic RDF for the four system models.
The first peak of the RDF for all four types of silicone rubber occurs at 0.111 nm. The peak of pure silicone rubber is the highest, indicating the presence of a large number of C–H covalent bonds in all silicone rubber models. However, in the Al2O3/ALSR model, other covalent bonds in the system affect the proportion of C–H covalent bonds. In addition, the Al2O3/ALSR model exhibits a third peak not present in the pure silicone rubber model, as indicated by circle 1 in Figure 13. This peak corresponds to the Al–O covalent bonds in the system, effectively demonstrating the doping of Al2O3 in the model.
4.2 Free volume
A unit cell is not entirely filled with polymer molecules in molecular simulations. The space occupied by polymer chains is called the occupied volume, while the space available for polymer chain movement is termed the free volume. The magnitude of polymer-free volume indicates intermolecular cohesion in materials; a smaller free volume suggests a denser molecular packing. Temperature also affects the free volume, thereby influencing the performance of polymer materials, such as the glass transition temperature. The formula for calculating the fractional free volume (FFV) is as follows:
where V f represents the free volume and V 0 represents the occupied volume.
Figure 14 displays the free volume (depicted in blue) of the pure silicone rubber model and the Al2O3/ALSR model at a temperature of 300 K. Table 3 presents the occupied volume, free volume, and the FFV calculated using Eq. 8 for the simulated silicone rubber model.
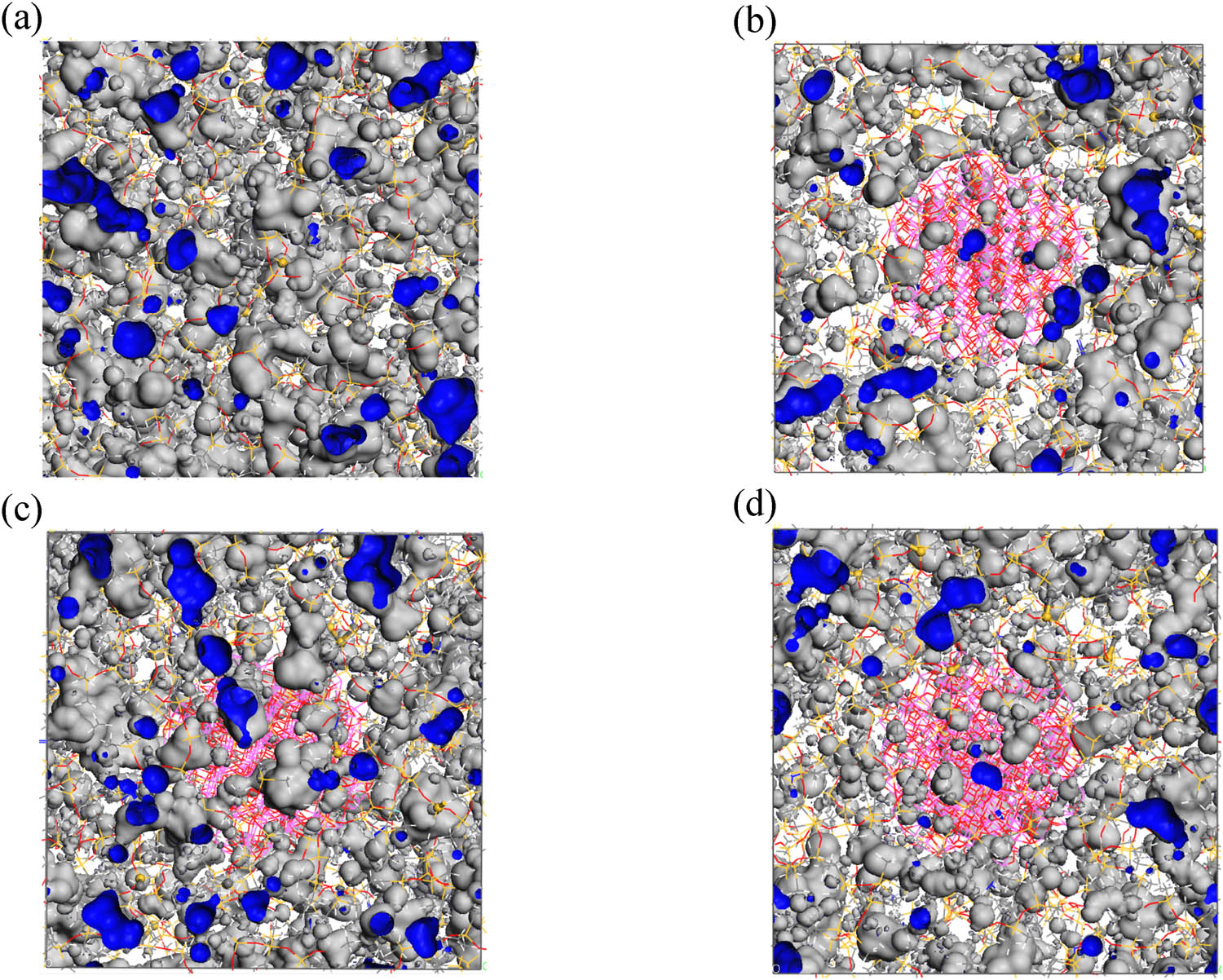
The distribution of free volume in different types of silicone rubber models: (a) ALSR, (b) ALSR-1, (c) ALSR-2, and (d) ALSR-3.
FFV of different types of silicone rubber
| Type | V f (nm3) | V o (nm3) | FFV (%) |
|---|---|---|---|
| ALSR | 5.315 | 34.951 | 13.20 |
| ALSR-1 | 2.785 | 36.656 | 9.27 |
| ALSR-2 | 3.956 | 38.721 | 7.21 |
| ALSR-3 | 3.081 | 39.624 | 7.06 |
The results indicate that the FFV of the pure silicone rubber model is the highest, at 13.20%, while the FFV of the Al2O3/ALSR model decreases to 9.27%. Furthermore, the FFV decreases again after grafting with the silane coupling agent and decreases further with increasing grafting rate. This indicates that the surface modification of Al2O3 by the silane coupling agent KH-550 hinders the movement of silicone rubber molecular chains, with the hindrance effect becoming more pronounced as the grafting rate increases.
4.3 Mean square displacement (MSD)
The atoms within the system exhibit continuous motion, and the indicator used to describe the mobility of polymer chains is called MSD, which specifically refers to the average of the squared displacements of particles in the system, defined as follows:
where R i (t) and R i (0) represent the displacement vectors of any atom i within the system at time t and the initial time, respectively.
At 300 K, the models underwent MSD analysis, resulting in MSD curves for the pure silicone rubber system and the Al2O3/ALSR system, as depicted in Figure 15. The graph shows that the MSD values of the silicone rubber composite materials doped with Al2O3 and grafted with coupling agents both exhibit a decreasing trend. However, when the coupling agent content increases from 5% to 10%, a slight upward trend in MSD is observed. This indicates that adding Al2O3 effectively impedes the movement of silicone rubber molecular chains, and the modification by coupling agents further enhances this inhibitory effect, rendering the system more stable. At the same time, the increase in MSD with the increase in coupling agent content may be attributed to the higher grafting rate of the silane coupling agent, leading to an unstable molecular interface due to excessive coupling agent content.
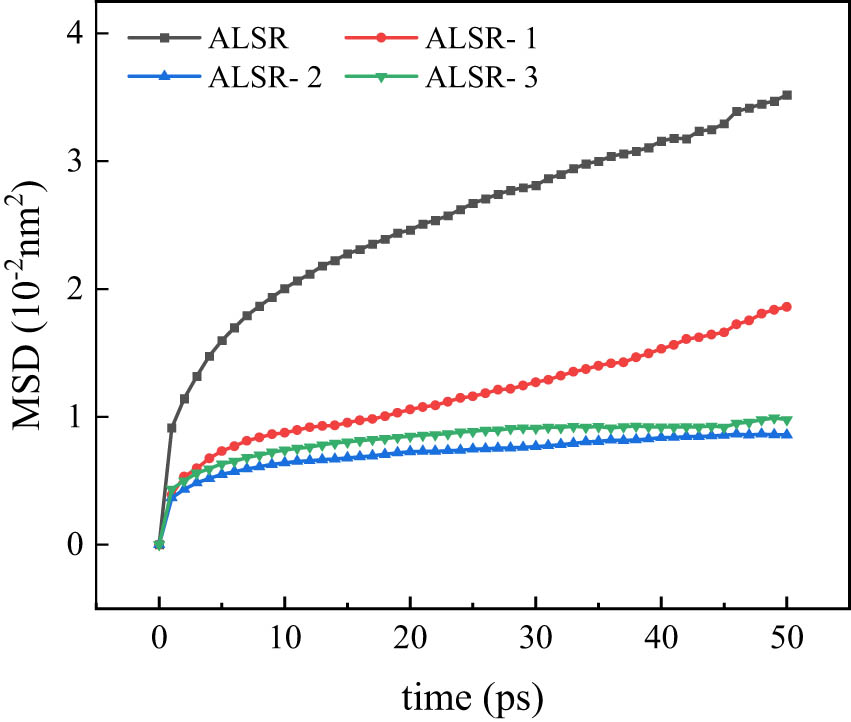
MSD curves in 50 ps for different systems.
4.4 Binding energy
The interface interaction in Al2O3/ALSR composite materials refers to the interaction between Al2O3 and silicone rubber. The magnitude of the interface interaction significantly affects the mechanical properties of silicone rubber composite materials. Therefore, the strength of the interface interaction is commonly characterized by the intermolecular interaction energy. The calculation formula for the interaction energy ΔE of silicone rubber composite materials is as follows:
where E
total represents the total potential energy of the Al2O3/ALSR composite material model;
The binding energy E bind is defined as the negative of the interaction energy ΔE, E bind = −ΔE. The calculated binding energies for different types of silicone rubber are presented in Table 4.
Binding energy of different types of silicone rubber
| Type | E total (kcal·mol−1) |
|
E ALSR (kcal·mol−1) | E bind (kcal·mol−1) |
|---|---|---|---|---|
| ALSR-1 | −149,656.51 | −95,433.23 | −49,606.72 | 4,616.56 |
| ALSR-2 | −137,791.73 | −82,556.97 | −50,443.04 | 4,791.72 |
| ALSR-3 | −130,224.33 | −74,857.43 | −50,359.44 | 5,007.46 |
Table 4 shows the binding energies of the Al2O3/ALSR interface in the unmodified model and the model modified with silane coupling agents. The binding energy for the unmodified Al2O3/ALSR model is 4,616.56 kcal·mol−1, while for the model with a grafting rate of 5%, the binding energy is 4,791.72 kcal·mol−1, and for the model with a grafting rate of 10%, the binding energy is 5,007.46 kcal·mol−1. With an increase in the grafting ratio of the coupling agent, the binding energy of the Al2O3/ALSR composites gradually rises. This indicates that the surface modification of Al2O3 by KH550 enhances the interfacial interaction between Al2O3 and the silicone rubber matrix, leading to a stronger bond between the ALSR matrix and the Al2O3 filler. Consequently, the effectiveness of the filler within the matrix is further improved.
5 Conclusions
To enhance the thermal conductivity of silicone rubber insulation materials and facilitate the dispersion of Al2O3 within the silicone rubber matrix while concurrently improving the bonding at the Al2O3/ALSR molecular interface, KH550 silane coupling agents with grafting rates of 5% and 10% were selected for surface modification of Al2O3. Based on the analysis of molecular dynamics principles, the following conclusions were drawn:
Incorporating Al2O3 into the silicone rubber matrix can alter its thermodynamic properties. Compared to pure silicone rubber, silicone rubber composite materials doped with Al2O3 exhibit improvements in thermal conductivity, glass transition temperature, CTE, and mechanical properties.
Surface modification of Al2O3-doped silicone rubber with KH550 silane coupling agent effectively enhances the thermodynamic properties of silicone rubber composite materials. However, the degree of improvement varies with different grafting rates. At a coupling agent grafting rate of 10%, the thermal conductivity and glass transition temperature have increased compared to 5%. Compared to the model without coupling agent grafting, the thermal conductivity of composite materials with grafting rates of 5% and 10% increased by 97.91% and 127.75%, respectively. However, at this point, the high content of silicone coupling agents has led to instability in the molecular interface structure, resulting in a decrease in the composite material’s thermal stability and mechanical performance.
Adding a silicone coupling agent also alters the microstructure of the composite material. Compared to the composite material without coupling agent modification, the composite material modified with coupling agent surface exhibits a decrease in free volume fraction, MSD, and an increase in binding energy. This indicates that the Al2O3 surface modified by the KH-550 silane coupling agent impedes the movement of silicone rubber molecular chains. However, as the grafting rate increases, the hindering effect decreases, leading to a decrease in the bonding at the Al2O3/ALSR molecular interface.
-
Funding information: This project was supported by National Natural Science Foundation of China (U1834203).
-
Author contributions: Lijun Zhou: writing – review and editing; Yingyi Xia: writing – original draft, conceptualization; formal analysis; Woyang Li: methodology, resources, data curation; Qian Lei: methodology, resources, data curation; Yunyun Qian: methodology, resources, data curation; Xiaohu Cai: methodology, resources, data curation; Lei Guo: visualization; Dongyang Wang: validation, writing – review and editing.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: The data presented in this study are available on request from the corresponding author.
References
(1) Awadallah SK, Milanović JV, Jarman PN. The influence of modeling transformer age related failures on system reliability. IEEE Trans Power Syst. 2014;30(2):970–9.10.1109/TPWRS.2014.2331103Suche in Google Scholar
(2) Cremasco A, Wu W, Blaszczyk A, Cranganu-Cretu B. Network modelling on dry-type transformer cooling systems. COMPEL Int J Comput Math Electr Electron Eng. 2018;37(3):1039–53.10.1108/COMPEL-12-2016-0534Suche in Google Scholar
(3) Wen M, Song J, Song Y, Liu Y, Li C, Wang P. Reliability assessment of insulation system for dry type transformers. IEEE Trans Dielectr Electr Insulation. 2013;20(6):1998–2008.10.1109/TDEI.2013.6678847Suche in Google Scholar
(4) Yang Z, Shang W, Zhang H, Garg H, Han C. Assessing the green distribution transformer manufacturing process using a cloud-based q-rung orthopair fuzzy multi-criteria framework. Appl Energy. 2022;311:118687.10.1016/j.apenergy.2022.118687Suche in Google Scholar
(5) Ekinci F. An experimental determination of the optimum cooling model for dry-type transformers for different cooling configurations. Energy Sources Part A: Recovery Util, Environ Eff. 2020;42(17):2181–97.10.1080/15567036.2020.1753858Suche in Google Scholar
(6) Pierce LW. An investigation of the temperature distribution in cast-resin transformer windings. IEEE Trans Power Deliv. 1992;7(2):920–6.10.1109/61.127099Suche in Google Scholar
(7) Ohki Y. Development of epoxy resin composites with high thermal conductivity. IEEE Electr Insul Mag. 2010;26(1):48–9.10.1109/MEI.2010.5383936Suche in Google Scholar
(8) Yuan S, Zhou L, Chen T, Wang D, Wang L. Thermally conductive h-BN/EHTPB/epoxy composites with enhanced toughness for on-board traction transformers. E-Polymers. 2022;22(1):821–33.10.1515/epoly-2022-0076Suche in Google Scholar
(9) Tian K, Yang S, Niu J, Wang H. Enhanced thermal conductivity and mechanical toughness of the epoxy resin by incorporation of mesogens without nanofillers. IEEE Access. 2021;9:31575–80.10.1109/ACCESS.2021.3058612Suche in Google Scholar
(10) Liu X, Tian F, Zhao X, Du R, Xu S, Wang YZ. Multiple functional materials from crushing waste thermosetting resins. Mater Horiz. 2021;8(1):234–43.10.1039/D0MH01053GSuche in Google Scholar PubMed
(11) Koné D, Ghunem RA, Cissé L, Hadjadj Y, El-Hag A. Effect of residue formed during the AC and DC dry-band arcing on silicone rubber filled with natural silica. IEEE Trans Dielectr Electr Insul. 2019;26(5):1620–6.10.1109/TDEI.2019.008203Suche in Google Scholar
(12) Nazir MT, Khalid A, Kabir I, Wang C, Baena JC, Akram S, et al. Flame retardancy and excellent electrical insulation performance of RTV silicone rubber. Polymers. 2021;13(17):2854.10.3390/polym13172854Suche in Google Scholar PubMed PubMed Central
(13) Hamdan MA, Pilgrim JA, Lewin PL. Thermo-mechanical analysis of solid interfaces in HVAC cable joints. IEEE Trans Dielectr Electr Insul. 2019;26(6):1779–87.10.1109/TDEI.2019.008152Suche in Google Scholar
(14) Guo L, Ding S, Yuan S, Gou X, Cai F, Wang D, et al. Study on the thermal properties and insulation resistance of epoxy resin modified by hexagonal boron nitride. E-Polymers. 2021;21(1):681–90.10.1515/epoly-2021-0069Suche in Google Scholar
(15) Guo L, Xu H, Wu N, Yuan S, Zhou L, Wang D, et al. Molecular dynamics simulation of the effect of the thermal and mechanical properties of addition liquid silicone rubber modified by carbon nanotubes with different radii. E-Polymers. 2023;23(1):20228105.10.1515/epoly-2022-8105Suche in Google Scholar
(16) Zou Z, Wu W, Wang Y, Wang L. Enhancement of thermal conductivity and tensile strength of liquid silicone rubber by three-dimensional alumina network. Soft Mater. 2019;17(3):297–307.10.1080/1539445X.2019.1601110Suche in Google Scholar
(17) Li J, Zhao X, Ji X, Lu Y, Zhang L. Graphene/Alumina micro-nano hybrid network and thermal conductive and electrical insulating silicone rubber composites. Insul Mater. 2021;52(2):49–55.Suche in Google Scholar
(18) Dang Z, Xia Y, Zha J, Yuan J, Bai J. Preparation and dielectric properties of surface modified TiO2/silicone rubber nanocomposites. Mater Lett. 2011;65(23–24):3430–2.10.1016/j.matlet.2011.07.056Suche in Google Scholar
(19) Zhou W, Yang Z, Feng Y, Lin L. Insights into the thermophysical properties and heat conduction enhancement of NaCl-Al2O3 composite phase change material by molecular dynamics simulation. Int J Heat Mass Transf. 2022;198:123422.10.1016/j.ijheatmasstransfer.2022.123422Suche in Google Scholar
(20) Shi R, Wang X, Song X, Zhan B, Xu X, He J, et al. Tensile performance and viscoelastic properties of rubber nanocomposites filled with silica nanoparticles: A molecular dynamics simulation study. Chem Eng Sci. 2023;267:118318.10.1016/j.ces.2022.118318Suche in Google Scholar
(21) Ou Z, Gao F, Zhao H, Dang S, Zhu L. Research on the thermal conductivity and dielectric properties of AlN and BN co-filled addition-cure liquid silicone rubber composites. RSC Adv. 2019;9(49):28851–6.10.1039/C9RA04771ASuche in Google Scholar PubMed PubMed Central
(22) Sun J, Wang D, Yao Y, Zeng X, Pan G, Huang Y, et al. Boron nitride microsphere/epoxy composites with enhanced thermal conductivity. High Volt. 2017;2(3):147–53.10.1049/hve.2017.0040Suche in Google Scholar
(23) Li Y, Liu W, Shen F, Zhang G, Gong L, Zhao L, et al. Processing, thermal conductivity and flame retardant properties of silicone rubber filled with different geometries of thermally conductive fillers: A comparative study. Compos Part B-Eng. 2022;238:109907.10.1016/j.compositesb.2022.109907Suche in Google Scholar
(24) Gao Z, Zhao L. Effect of nano-fillers on the thermal conductivity of epoxy composites with micro-Al2O3 particles. Mater Des (1980-2015). 2015;66:176–82.10.1016/j.matdes.2014.10.052Suche in Google Scholar
(25) Guo G, Zhang J, Chen X, Zhao X, Deng J, Zhang G. Molecular-dynamics study on the thermodynamic properties of nano-SiO2 particle-doped silicone rubber composites. Comput Mater Sci. 2022;212:11571.10.1016/j.commatsci.2022.111571Suche in Google Scholar
(26) Yang G, Qi X, Gao Q, Wang D. Electrical properties of nanosilica/epoxy resin modified by cooperation of plasma fluorination and coupling agent. High Volt Eng. 2022;48(2):689–97.Suche in Google Scholar
(27) Ding C, Liu L, Feng L. Effect of doped nano-SiO2, Al2O3, and polyhedral oligomeric sesquisiloxane on the thermodynamic properties of cellulose. AIP Adv. 2023;13:015104.10.1063/5.0132902Suche in Google Scholar
(28) Kim H, Jung D, Jung I, Cifuentes J, Rhee K, Hui D. Enhancement of mechanical properties of aluminium/epoxy composites with silane functionalization of aluminium powder. Compos Part B: Eng. 2012;43(4):1743–8.10.1016/j.compositesb.2011.12.010Suche in Google Scholar
© 2025 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- Flow-induced fiber orientation in gas-powered projectile-assisted injection molded parts
- Research on thermal aging characteristics of silicone rubber composite materials for dry-type distribution transformers
- Kinetics of acryloyloxyethyl trimethyl ammonium chloride polymerization in aqueous solutions
- Influence of siloxane content on the material performance and functional properties of polydimethylsiloxane copolymers containing naphthalene moieties
- Enhancement effect of electron beam irradiation on acrylonitrile–butadiene–styrene (ABS) copolymers from waste electrical and electronic equipment by adding 1,3-PBO: A potential way for waste ABS reuse
- Model construction and property study of poly(ether-ether-ketone) by molecular dynamics simulation with meta-modeling methods
- Zinc–gallic acid–polylysine nanocomplexes with enhanced bactericidal activity for the treatment of bacterial keratitis
- Effect of pyrogallol compounds dosage on mechanical properties of epoxy coating
- Preparation of in situ polymerized polypyrrole-modified braided cord and its electrical conductivity investigation under varied mechanical conditions
- Hydrophobicity, UV resistance, and antioxidant properties of carnauba wax-reinforced CG bio-polymer film
- Janus nanofiber membrane films loading with bioactive calcium silicate for the promotion of burn wound healing
- Synthesis of migration-resistant antioxidant and its application in natural rubber composites
- Influence of the flow rate on the die swell for polymer micro coextrusion process
- Fatty acid filled polyaniline nanofibres with dual electrical conductivity and thermo-regulatory characteristics: Futuristic material for thermal energy storage
- Hydrolytic depolymerization of major fibrous wastes
- Performance of epoxy hexagonal boron nitrate underfill materials: Single and mixed systems
- Blend electrospinning of citronella or thyme oil-loaded polyurethane nanofibers and evaluating their release behaviors
- Efficiency of flexible shielding materials against gamma rays: Silicon rubber with different sizes of Bi2O3 and SnO
- A comprehensive approach for the production of carbon fibre-reinforced polylactic acid filaments with enhanced wear and mechanical behaviour
- Electret melt-blown nonwovens with charge stability for high-performance PM0.3 purification under extreme environmental conditions
- Study on the failure mechanism of suture CFRP T-joints under/after the low-velocity impact loading
- Experimental testing and finite element analysis of polyurethane adhesive joints under Mode I loading and degradation conditions
- Optimizing recycled PET 3D printing using Taguchi method for improved mechanical properties and dimensional precision
- Effect of stacking sequence of the hybrid composite armor on ballistic performance and damage mechanism
- Bending crack propagation and delamination damage behavior of orthogonal ply laminates under positive and negative loads
- Molecular dynamics simulation of thermodynamic properties of Al2O3-modified silicone rubber under silane coupling agent modification
- Precision injection molding method based on V/P switchover point optimization and pressure field balancing
- Rapid Communication
- RAFT-mediated polymerization-induced self-assembly of poly(ionic liquid) block copolymers in a green solvent
- Corrigendum
- Corrigendum to “High-strength polyvinyl alcohol-based hydrogel by vermiculite and lignocellulosic nanofibrils for electronic sensing”
Artikel in diesem Heft
- Research Articles
- Flow-induced fiber orientation in gas-powered projectile-assisted injection molded parts
- Research on thermal aging characteristics of silicone rubber composite materials for dry-type distribution transformers
- Kinetics of acryloyloxyethyl trimethyl ammonium chloride polymerization in aqueous solutions
- Influence of siloxane content on the material performance and functional properties of polydimethylsiloxane copolymers containing naphthalene moieties
- Enhancement effect of electron beam irradiation on acrylonitrile–butadiene–styrene (ABS) copolymers from waste electrical and electronic equipment by adding 1,3-PBO: A potential way for waste ABS reuse
- Model construction and property study of poly(ether-ether-ketone) by molecular dynamics simulation with meta-modeling methods
- Zinc–gallic acid–polylysine nanocomplexes with enhanced bactericidal activity for the treatment of bacterial keratitis
- Effect of pyrogallol compounds dosage on mechanical properties of epoxy coating
- Preparation of in situ polymerized polypyrrole-modified braided cord and its electrical conductivity investigation under varied mechanical conditions
- Hydrophobicity, UV resistance, and antioxidant properties of carnauba wax-reinforced CG bio-polymer film
- Janus nanofiber membrane films loading with bioactive calcium silicate for the promotion of burn wound healing
- Synthesis of migration-resistant antioxidant and its application in natural rubber composites
- Influence of the flow rate on the die swell for polymer micro coextrusion process
- Fatty acid filled polyaniline nanofibres with dual electrical conductivity and thermo-regulatory characteristics: Futuristic material for thermal energy storage
- Hydrolytic depolymerization of major fibrous wastes
- Performance of epoxy hexagonal boron nitrate underfill materials: Single and mixed systems
- Blend electrospinning of citronella or thyme oil-loaded polyurethane nanofibers and evaluating their release behaviors
- Efficiency of flexible shielding materials against gamma rays: Silicon rubber with different sizes of Bi2O3 and SnO
- A comprehensive approach for the production of carbon fibre-reinforced polylactic acid filaments with enhanced wear and mechanical behaviour
- Electret melt-blown nonwovens with charge stability for high-performance PM0.3 purification under extreme environmental conditions
- Study on the failure mechanism of suture CFRP T-joints under/after the low-velocity impact loading
- Experimental testing and finite element analysis of polyurethane adhesive joints under Mode I loading and degradation conditions
- Optimizing recycled PET 3D printing using Taguchi method for improved mechanical properties and dimensional precision
- Effect of stacking sequence of the hybrid composite armor on ballistic performance and damage mechanism
- Bending crack propagation and delamination damage behavior of orthogonal ply laminates under positive and negative loads
- Molecular dynamics simulation of thermodynamic properties of Al2O3-modified silicone rubber under silane coupling agent modification
- Precision injection molding method based on V/P switchover point optimization and pressure field balancing
- Rapid Communication
- RAFT-mediated polymerization-induced self-assembly of poly(ionic liquid) block copolymers in a green solvent
- Corrigendum
- Corrigendum to “High-strength polyvinyl alcohol-based hydrogel by vermiculite and lignocellulosic nanofibrils for electronic sensing”

