Abstract
Anticorrosive coatings are considered to be one of the most economical and effective means of protection. The adhesion of the coating is defined as the strength of the interfacial bond between the coating and the substrate, which directly determines the protective life of the coating. In this work, the epoxy–(5-{5-[2-(3,4,5-trihydroxyphenyl)-1H-1,3-benzodiazol-6-yl]-1H-1,3-benzodiazol-2-yl}benz-1,2,3-triol} (EP-BIB) composite coating was obtained by adding different mass fractions of BIB to the EP coating. The effect of mass fraction of BIB on the mechanical properties of composite coating were studied by Elongation at break, tensile shear strength, as well as Peel strength. The results showed that the mechanical properties of composite coating could be improved by adding an appropriate amount of BIB. The elongation at break, tensile shear strength, and peel strength of composite coating became better initially and then worse with the increase in BIB content in the composite coating. The composite coating with 0.9% (mass fraction) BIB had the elongation at break of 10.37 ± 0.53%, an optimal shear strength of 12.51 ± 0.48 MPa, and a maximum peel strength of 4.78 ± 0.54 N·mm−1.
1 Introduction
Marine corrosion is a problem that has been extensively researched in recent years, and the use of coating protection has become a widely used and effective means of dealing with it (1,2,3,4,5). The coating creates a physical barrier on the metal surface to isolate the direct contact between seawater and the metal matrix, thereby slowing the rate of corrosion (6,7,8,9,10). However, the protective effect of a coating is not only determined by its own chemical composition and structure, but also by the adhesion between the coating and the substrate (11). Insufficient adhesion can lead to blistering and peeling when exposed to the effects of seawater, temperature changes, and immersion, resulting in the exposure of the underlying metal and accelerated corrosion (12). Epoxy (EP) resin has gained prominence in the shipbuilding and offshore wind power industries as an anti-corrosion primer due to its proven corrosion resistance, superior mechanical properties, and notable adhesive strength (13). In order to enhance adhesion to metal surfaces, surface treatments are commonly applied, including mechanical and chemical treatments (14). However, these treatments require immediate construction, which can be inconvenient on site.
Several theories have been proposed to explain the adhesion mechanisms, including the adsorption theory (15), mechanical interlocking theory (16), diffusion theory (17), electrostatic theory, and suction theory (18). Among these theories, the key factors influencing adhesion strength are interfacial chemical composition, wetting degree, surface contamination, and surface morphology (19). For instance, Baby et al. (20) introduced catechol into EP resin to synthesize a linear polymer that, upon cross-linking, forms a primer. This primer effectively replaces the need for surface treatment, as the adhesion strength between the topcoat and the primer was found to be 2.5 times greater than the strength of direct adhesion between the topcoat and the substrate. This suggests that catechol interacts with the substrate to enhance the adhesion strength of the primer. In other studies, it was observed that introducing pyrogallol into coating resulted in even higher adhesion strength compared to catechol. However, there are limited reports on the effect of incorporating pyrogallol into EP resin when only sandblasting is used for surface treatment and its subsequent impact on coating strength.
The compound known as (5-{5-[2-(3,4,5-trihydroxyphenyl)-1H-1,3-benzodiazol-6-yl]-1H-1,3-benzodiazol-2-yl}benz-1,2,3-triol} (BIB) is a pyrogallol compound that is frequently incorporated into EP coating systems. Once the pyrogallol compound is added to the EP coating system, the pyrogallol can form non-covalent bonds with the metal surface, which can improve the corrosion protection of the corrosion protection of the EP coating (21,22,23). It has not been reported whether the adhesion strength of the system is enhanced or weakened when they are added to the EP system. In this work, EP E-44 and curing agent NX-2015 (phenolic amine curing agent, with lower toughness than Ancamine 2519) are selected to form an EP adhesive system. Different contents of BIB are added to the system, respectively. At different curing temperatures, the adhesion strength of the EP system to low carbon steel is studied.
2 Experimental
2.1 Instruments and reagents
2.1.1 Main experimental instruments
The main instruments used in the experiment are listed in Table 1.
Experimental instruments
| Name | Model | Factory |
|---|---|---|
| Electronic balance | BSA323S-CW | China, Beijng, Sartorius Scientific Instruments (Beijing) Co., Ltd. |
| Ultrasonic cleaning machine | KQ-500E | China, Jiangsu, Kunshan Ultrasonic Instrument Co., Ltd. |
| Blast drying oven | PHG-9146A | China, Shanghai, Shanghai Jinghong Experimental Equipment Co., Ltd. |
| Universal tensile testing machine | Instron 3367 | USA, Boston, Instron Instruments Co., Ltd. |
| Scanning electron microscopy | SIGMA500 | Germany, Carl Zeiss |
| Tensile test column | 20 mm × 30 mm | China, Henan, Qianfeng Machinery Co., Ltd. |
| Shearing metal sheets | 25 mm × 120 mm | China, Henan, Qianfeng Machinery Co., Ltd. |
| Vacuum air pump | 2XZ-2 | China, Shanghai Yetuo Technology Co., Ltd. |
2.1.2 Main experimental reagents
The main reagents used in the experiments are listed in Table 2.
Experimental reagents
| Name | Model | Factory |
|---|---|---|
| EP resin (E-44) | Industrial grade | China, Baling Petrochemical Co., Ltd. |
| NX2015 curing agent | Industrial grade | Germany, Evonik Industries |
| Xylene | 98% | USA, Aladdin Chemical Reagent Co., Ltd. |
| n-Butanol | 98% | USA, Aladdin Chemical Reagent Co., Ltd. |
| BYK330 | Industrial grade | Germany, BYK Chemicals |
2.2 Characterization
2.2.1 Preparation of adhesive samples
Four additive groups were set up, corresponding to 0.3%, 0.6%, 0.9%, and 1.2% of the EP resin mass, with the amount of NX-2015 curing agent set at 66% of the EP resin mass. The mixture was poured into the molds and cured at room temperature for 5 days. In addition, adhesive strips were obtained by curing for 3 days at 40°C, and then for 2 days at room temperature. Depending on the additive, the samples were named EP-BIB-0.3%, EP-BIB-0.6%, EP-BIB-0.9%, EP-BIB-1.2%.
2.2.2 Elongation at break testing
The tensile fracture strength and elongation at break were determined by conducting a series of experiments. The former was tested using a universal tensile testing machine, while the latter was analyzed using a SIGMA500 scanning electron microscope (SEM). The microscope employed an electron gun acceleration voltage of 3 kV, a magnification of 500×, and the In Lens signal mode. The fracture surface morphology was then examined using the aforementioned microscope. The tensile fracture strength and fracture elongation were subsequently calculated using the following formula:
where
where
2.2.3 Tensile strength testing
The tensile strength test was conducted in accordance with the Chinese National Standard GB/T 5210-2006. Four different types of substrates were prepared for the test, namely, low carbon steel, stainless steel, aluminum, and copper. The substrates were identical in shape, cylindrical (30 mm in height and 20 mm in diameter), with a hole in the center, and underwent sandblasting treatment.
The paint film adhered to the substrate was polished with sandpaper to ensure adequate wetting by the adhesive samples. After polishing, the different adhesive samples were applied to the film surface, and another substrate was bonded on top, ensuring that the holes in both substrates were aligned in the same plane. The specimens were left to cure for 5 days to ensure complete bonding of the adhesive. The samples cured at 40°C were first placed in a 40°C oven for 3 days and at room temperature for 4 days. The tensile strength was calculated using the built-in formula of the testing software, as shown below:
where P is the tensile strength, MPa; F is the maximum load when the test column separates, N; r is the test column radius, mm.
2.2.4 Tensile shear strength testing
The preparation for shear strength testing was carried out according to the Chinese National Standard GB/T 7124-2008 (24). The carbon steel metal plates had dimensions of 120 mm in length, 25 mm in width, and 2 mm in thickness, and were subjected to sandblasting treatment prior to testing. The bonding area was set at 312.5 mm2. The samples were cured at room temperature for 7 days. For samples cured at 40°C, the specimens were first cured in an oven at 40°C for 3 days, followed by room temperature curing for an additional 4 days. The tensile shear strength is calculated using the following equation:
where
2.2.5 Peel strength testing
The peel strength test was conducted in accordance with the Chinese National Standard GB/T 2790-81 (25), and the length of the adhesive samples is 70 mm. The tests were performed using a universal tensile testing machine to ensure accuracy and consistency in the evaluation of adhesive performance.
3 Results and discussion
3.1 Elongation at break analysis
As demonstrated in Table 3, the elongation at break of the pure EP coating was found to be 6.91 ± 0.19% and 6.82 ± 0.58% at room temperature and 40°C, respectively. This observation suggests that the pure EP coating possesses a limited elongation capacity and demonstrates high brittleness and low toughness. However, the addition of a small amount of BIB can significantly increase the elongation at break of an EP coating. It is evident that the incorporation of a small amount of BIB leads to an enhancement in the elongation at break of the EP coating, reaching a maximum of 0.9% with further addition. However, this increase is followed by a subsequent decrease as the amount of BIB increases. The maximum recorded values at 0.9% were 9.4 ± 0.81% and 10.37 ± 0.53%, respectively.
Elongation at break of EP-BIB-x% samples at different curing temperatures
| Pure EP | EP-BIB-0.3% | EP-BIB-0.6% | EP-BIB-0.9% | EP-BIB-1.2% | |
|---|---|---|---|---|---|
| Room temperature | 6.91 ± 0.19% | 7.42 ± 0.46% | 8.03 ± 0.27% | 9.4 ± 0.81% | 9.25 ± 0.33% |
| 40°C | 6.82 ± 0.58% | 7.64 ± 0.71% | 8.40 ± 0.14% | 10.37 ± 0.53% | 9.79 ± 0.9% |
As demonstrated in Figure 1, the tensile strength curve at break exhibits a similar trend to the elongation at break curve, with both parameters increasing with the amount of addition. The maximum value is observed at an addition amount of 0.9%, and subsequently, as the addition amount increases, a decrease in tensile strength is evident.
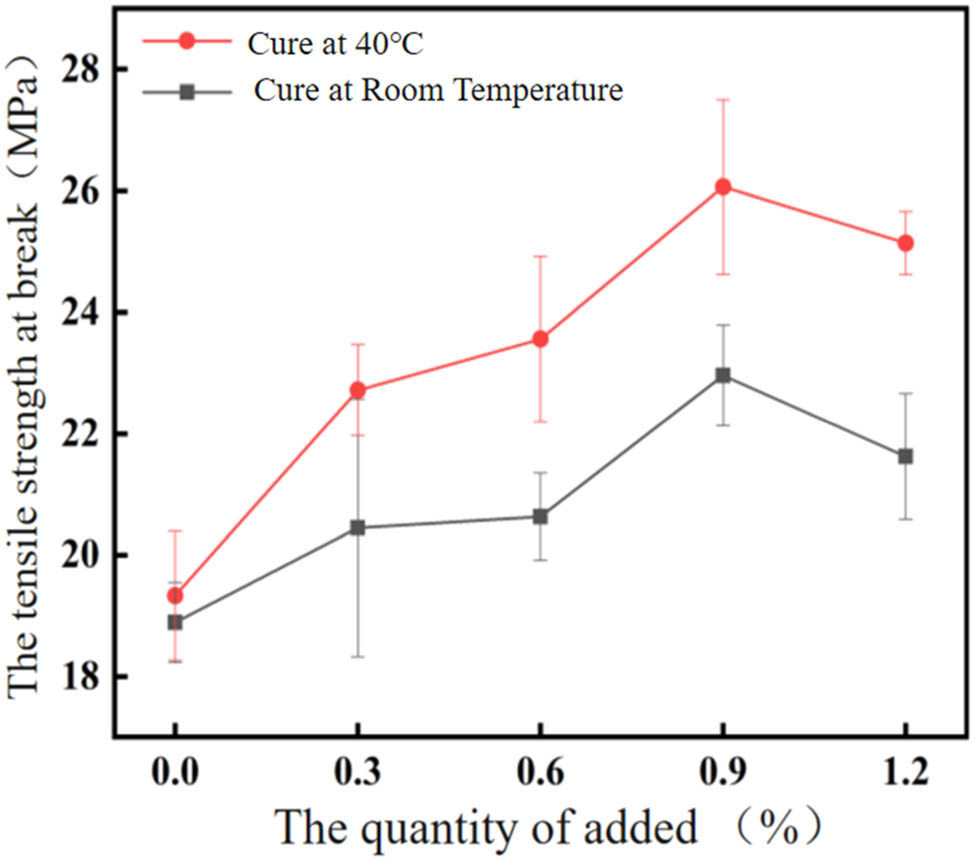
Tensile strength at break of EP-BIB-x% samples at different curing temperatures.
The fracture morphologies of EP-BIB-x% cured at room temperature are shown in Figure 2. In Figure 2(a), the fracture surface of the pure EP coating is smooth and flat, with minimal plastic deformation, predominantly displaying brittle fracture. After the addition of BIB, plastic deformation increased. As demonstrated in Figure 2(b) and (c), the fracture surfaces become less smooth, appearing rough, with layered delamination features. In Figure 2(d) and (e), the fracture surfaces are even rougher, with more pronounced layered delamination, and crack growth shows noticeable deflection. In comparison to the fracture surfaces of pure EP coating, the fracture surfaces of BIB-modified EP coating exhibit roughness and irregularity. This is attributable to the rupture of hydrogen bonds, which consequently leads to a more pronounced manifestation of ductile fracture behavior.
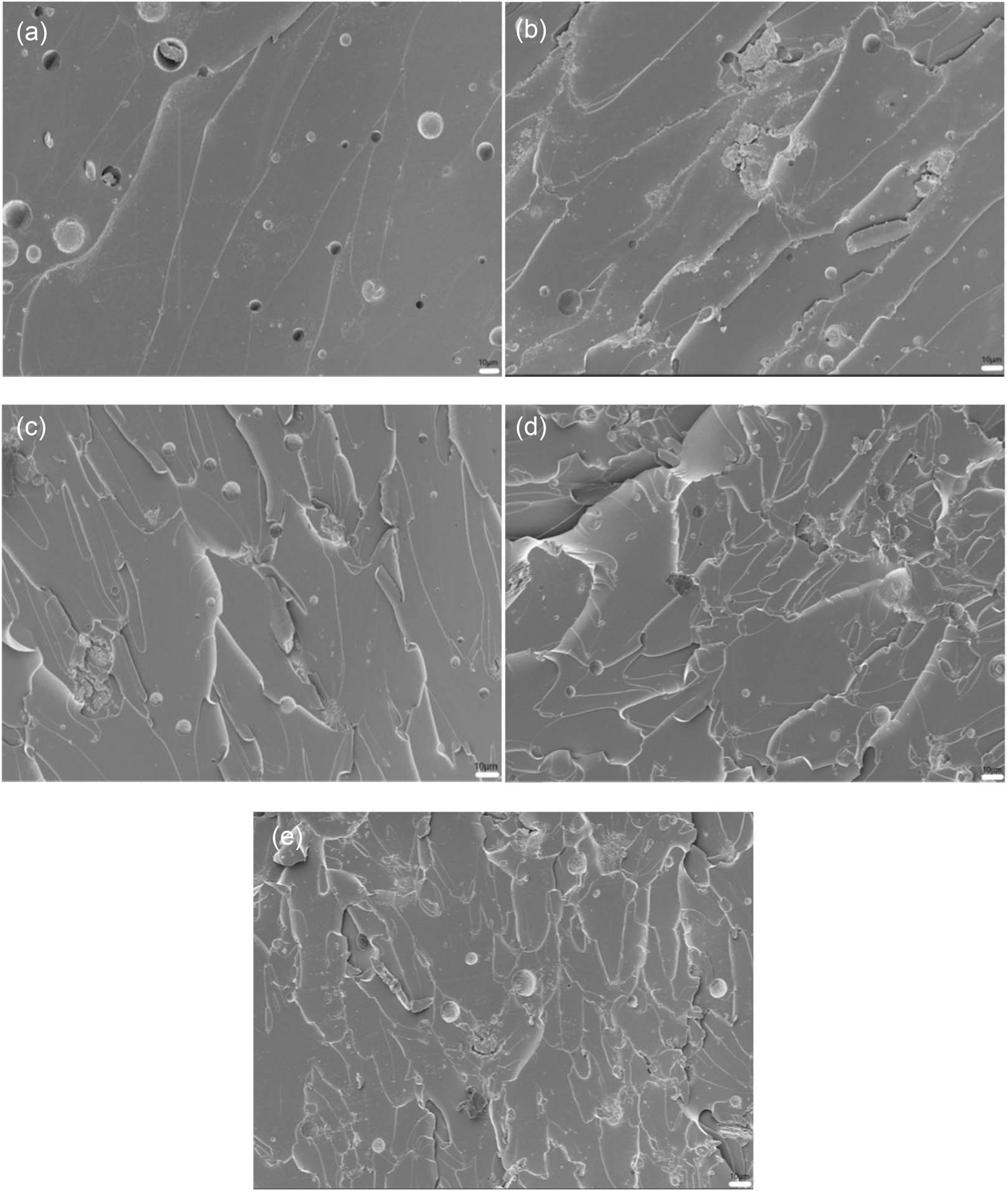
SEM images of fracture surfaces of pure EP (a), EP-BIB-0.3% (b), EP-BIB-0.6% (c), EP-BIB-0.9% (d), and EP-BIB-1.2% (e) samples cured at room temperature.
The fracture morphologies of EP-BIB-x% cured at 40°C are presented in Figure 3. The fracture morphology of the EP coating did not change, and brittle fracture was still dominant (Figure 3a). In Figure 3(b), the fracture surface becomes rougher, exhibiting a distinct layered structure and changes in crack propagation direction, which is indicative of ductile fracture. From Figure 3(c)–(e), it is evident that the roughness of the fracture surface increases further, with more pronounced layered structures and more complex crack paths. Compared to the fracture morphology observed under room temperature curing conditions, these results show that the ductile fracture of the samples are more obvious at 40°C.
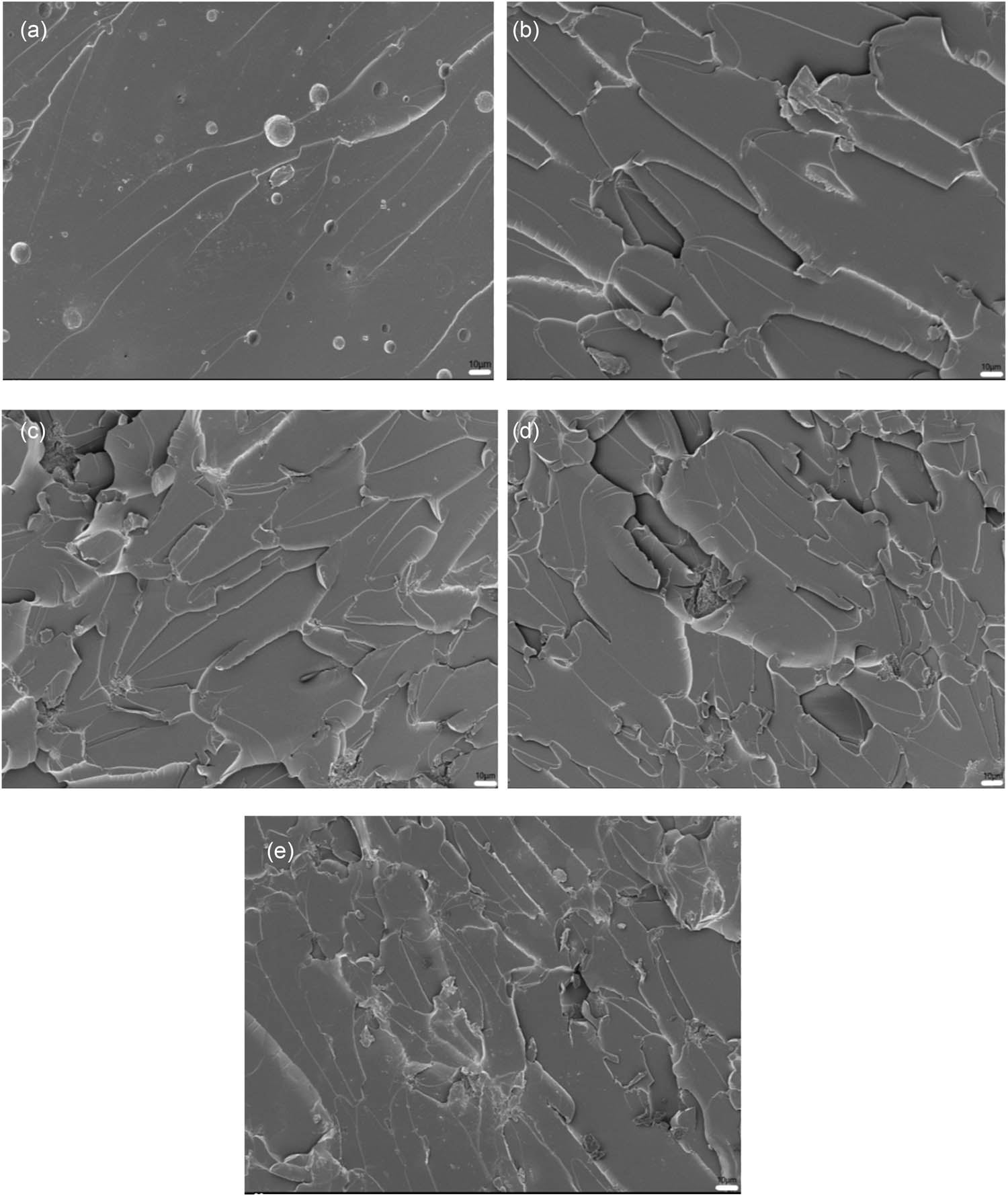
SEM images of fracture surfaces of pure EP (a), EP-BIB-0.3% (b), EP-BIB-0.6% (c), EP-BIB-0.9% (d), and EP-BIB-1.2% (e) samples cured at 40°C.
Under both room temperature and 40°C curing conditions, the addition of BIB improves the toughness of EP coating and increases the tensile fracture strength. BIB, when free in the system, acts as a plasticizer, intercalating between the EP resin molecular chains, increasing the distance between chains and enhancing their mobility (25,26). From the structure of BIB, it is evident that the presence of hydroxyl groups can form intermolecular and inter-resin hydrogen bonds. When external force is applied to the EP coating, additional force is required to break the extra hydrogen bonds (27,28).
3.2 Tensile strength analysis
Figure 4 displays the tensile strength between EP-BIB-x% and low-carbon steel. Under room temperature curing, the tensile strength of pure EP was 18.88 ± 0.81 MPa. BIB mass fractions of 0.3%, 0.6%, 0.9%, and 1.2% resulted in tensile strengths of 20.49 ± 0.59 MPa, 21.32 ± 0.67 MPa, 19.54 ± 0.34 MPa, and 19.71 ± 0.38 MPa, respectively. The tensile strength increased most significantly at a 0.6% BIB content, with a 12.9% increase, after which it began to decrease, though remaining higher than that of pure EP. Therefore, at room temperature, a 0.6% BIB mass fraction is optimal. Under 40°C curing, the tensile strength of pure EP was 17.85 ± 0.72 MPa. BIB mass fractions of 0.3%, 0.6%, 0.9%, and 1.2% resulted in tensile strengths of 19.84 ± 0.35 MPa, 19.33 ± 0.75 MPa, 18.76 ± 0.12 MPa, and 18.52 ± 0.51 MPa, respectively. The 0.3% BIB fraction produced the most significant improvement, with an 11% increase in tensile strength. Further increases resulted in decreased tensile strength, but values remained higher than those of pure EP. Therefore, at 40°C curing, a 0.3% BIB mass fraction is optimal.
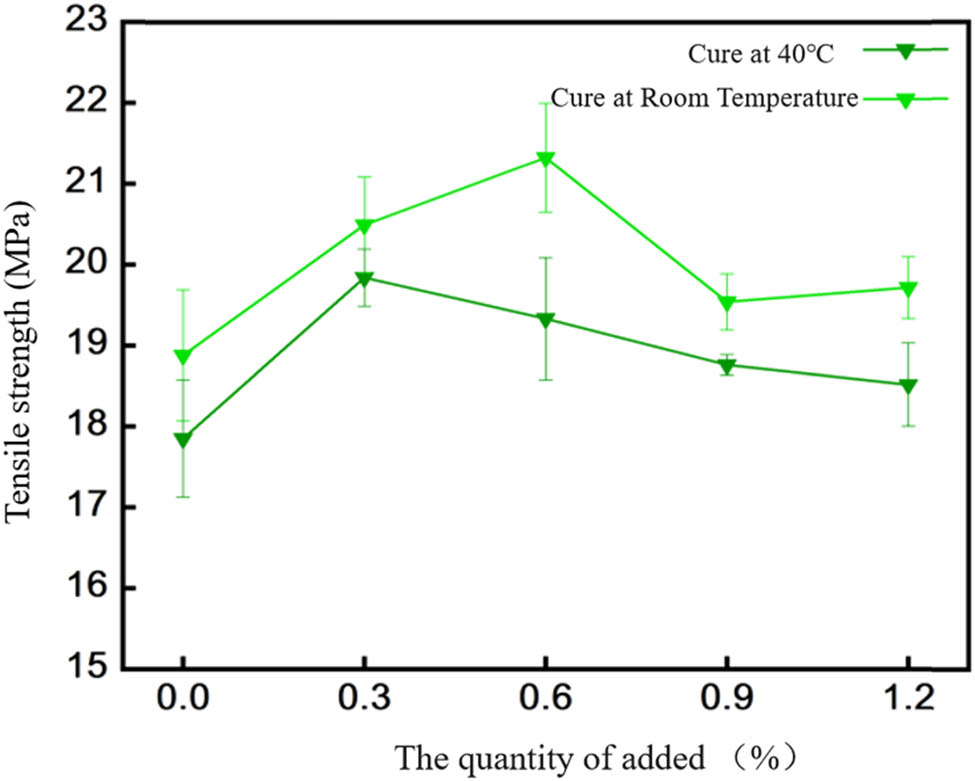
Tensile strength between EP-BIB-x% and low carbon steel.
Table 4 summarizes the failure modes of EP coating with the addition of BIB under two different curing temperatures on various substrates. Figure 4 illustrates the tensile strength between EP-BIB-x% and substrates. At room temperature curing, the pure EP coating exhibited a tensile strength of 16.91 ± 0.79 MPa. With BIB mass fractions of 0.3%, 0.6%, 0.9%, and 1.2%, the tensile strength increased to 20.73 ± 0.51 MPa, 20.89 ± 1.12 MPa, 20.85 ± 1.14 MPa, and 18.92 ± 0.51 MPa, respectively. These results indicate a significant variation in tensile strength after BIB addition, with an overall enhancement compared to pure EP adhesive. Notably, the 0.6% mass fraction yielded the highest tensile strength, achieving a 23.54% increase over pure EP. However, further increases in the BIB content led to a reduction in tensile strength, suggesting that 0.6% is the optimal mass fraction for room-temperature curing. When the curing temperature was elevated to 40°C, the tensile strength of pure EP decreased to 15.80 ± 1.16 MPa. For BIB mass fractions of 0.3%, 0.6%, 0.9%, and 1.2%, the tensile strength was measured as 17.11 ± 0.85 MPa, 16.69 ± 0.90 MPa, 17.01 ± 0.7 MPa, and 16.39 ± 1.05 MPa, respectively. While tensile strength increased with BIB addition, the 0.3% fraction showed the most significant improvement, with an 8.2% increase. Further additions led to a decrease in tensile strength, though the values remained higher than those of the pure EP adhesive.
Failure modes of EP adhesives containing BIB at different curing temperatures
| Carbon steel | Curing at room temperature | Curing at 40°C |
|---|---|---|
| Carbon steel-EP | 90% cohesion, 10% interface | 90% cohesion, 10% interface |
| EP-0.3% | 90% cohesion, 100% interface | 90% cohesion, 100% interface |
| EP-0.6% | 80% cohesion, 20% interface | 80% cohesion, 20% interface |
| EP-0.9% | 80% cohesion, 20% interface | 70% cohesion, 30% interface |
| EP-1.2% | 90% cohesion, 10% interface | 70% cohesion, 30% interface |
Under room temperature and 40°C curing conditions, the tensile strength increase with BIB addition is more significant at room temperature. According to adsorption theory, temperature influences the intermolecular distance (29). Higher temperatures increase molecular motion, leading to greater average molecular distances, which affect the formation of both chemical and non-covalent bonds. Non-covalent bond interactions occur over shorter distances, and as the molecular distance increases, the strength of non-covalent bonds diminishes or even disappears. At 40°C, the higher temperature increases BIB molecular motion, hindering the formation of non-covalent bonds, thus leading to lower tensile strength compared to room temperature curing. The increased interfacial failure at 40°C suggests a reduction in interfacial adhesion strength.
3.3 Tensile shear strength analysis
The tensile shear strength of lap joints is primarily influenced by the inherent strength of the adhesive and the interfacial adhesion between the adhesive and substrate. Toughening the EP adhesive can enhance its tensile shear strength, and improving the adhesive-substrate interfacial adhesion can also contribute to increased shear strength.
Figure 5 depicts the shear strength between EP-BIB-x% and low-carbon steel. Under room temperature curing, the pure EP shear strength is 7.76 ± 0.23 MPa, and the values at 0.3%, 0.6%, 0.9%, and 1.2% BIB are 9.39 ± 0.55 MPa, 10.33 ± 0.51 MPa, 9.57 ± 0.31 MPa, and 9.42 ± 0.66 MPa, respectively. The shear strength is highest at 0.6% BIB, with a 33% improvement over pure EP. The shear strength increases with BIB content and then decreases. Under 40°C curing, the pure EP shear strength is 7.94 ± 0.28 MPa, and the values at 0.3%, 0.6%, 0.9%, and 1.2% BIB are 10.99 ± 0.36 MPa, 11.29 ± 0.37 MPa, 12.51 ± 0.48 MPa, and 12.19 ± 0.51 MPa, respectively. The shear strength peaks at 0.9% BIB, with a 57.5% improvement over pure EP, following a similar trend to room temperature curing.
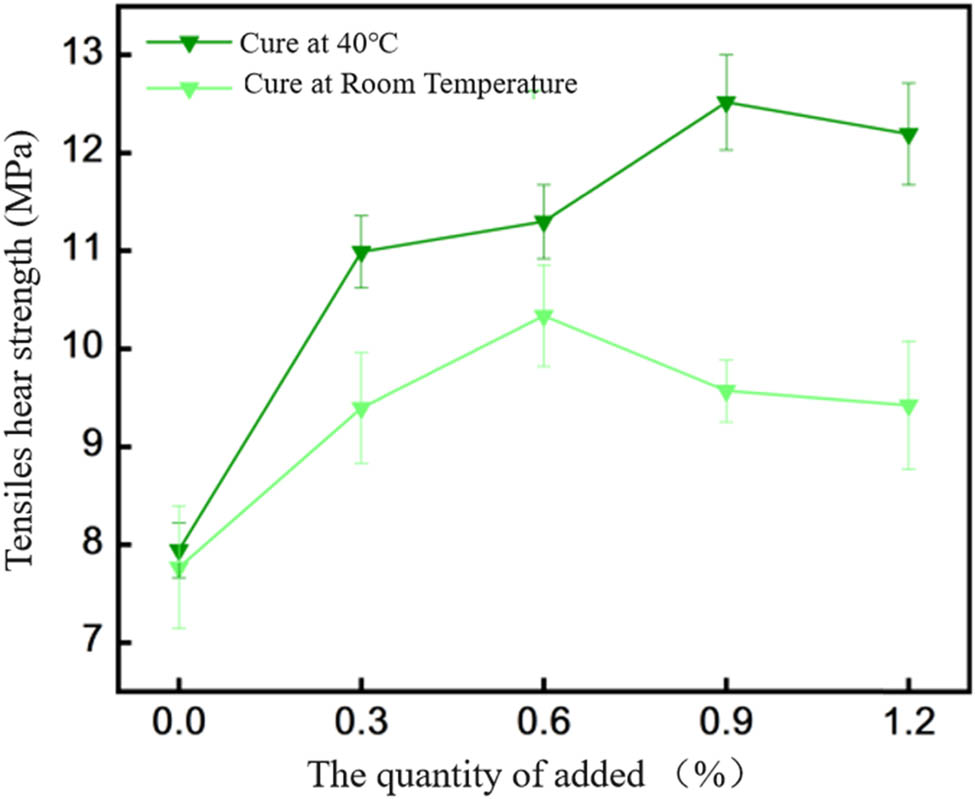
Shear strength between EP-BIB-x% and low carbon steel.
In summary, adding BIB enhances the shear strength of EP adhesives across all four metal substrates (aluminum, stainless steel, copper, and low-carbon steel) under both room temperature and 40°C curing conditions, with the degree of enhancement varying. In all cases, the shear strength initially increases with BIB content and then decreases beyond a certain point. The six hydroxyl groups in the BIB structure graft onto the EP matrix, forming hydrogen bonds between molecules and improving the toughness of the adhesive. Additionally, the hydroxyl groups can form hydrogen and coordination bonds with the metal interface, strengthening adhesion. When external forces are applied to the lap joint, the hydrogen bonds between molecules break, requiring force to rupture the adhesive. Furthermore, the presence of additional hydrogen and coordination bonds at the metal interface strengthens the bond, requiring greater force to separate the adhesive from the metal substrate. It is observed that shear strength is higher under 40°C curing compared to room temperature curing. This is attributed to the higher conversion rate of EP groups at elevated temperatures, leading to more chemical bonds forming between BIB and the EP resin. With the same curing duration, more chemical bonds form at 40°C, thus requiring more force to break the adhesive under shear stress.
When BIB is added to EP resin, the tensile strength of room temperature curing is higher than that of 40°C curing, while the shear strength of 40°C curing is higher than that of room temperature curing. This is because the decisive factors in these two tests are different. For tensile strength, the adhesion between EP adhesive and metal substrate plays a dominant role, while for the latter, the mechanical properties of EP adhesive itself play a dominant role. According to the adsorption and interlocking theory in the adhesion mechanism, EP resin infiltrates the rough surface of the metal substrate and penetrates into the rough area like a nail, while covalent bonds and non-covalent bonds are formed between the interfaces. For tensile testing, destroying the adhesion between the glue and the metal is equivalent to pulling out the nails and destroying the covalent bonds and non-covalent bonds. The direction of the force is parallel to the direction of nail pulling out. The interfacial adhesion strength determines the tensile strength. The tensile strength of the sample cured at 40°C is lower than that cured at room temperature. For shear testing, the direction of nail pulling out is perpendicular to the direction of the force, and it is not easy to pull it out. The mechanical properties of the glue itself determine the shear strength. The shear strength of 40°C cured is stronger than that of room temperature cured.
3.4 Peel strength analysis
Peel strength, similar to tensile and shear strength, is one of the critical mechanical properties of adhesives. Figure 6 illustrates the peel strength of EP adhesives with varying BIB content on different substrates.
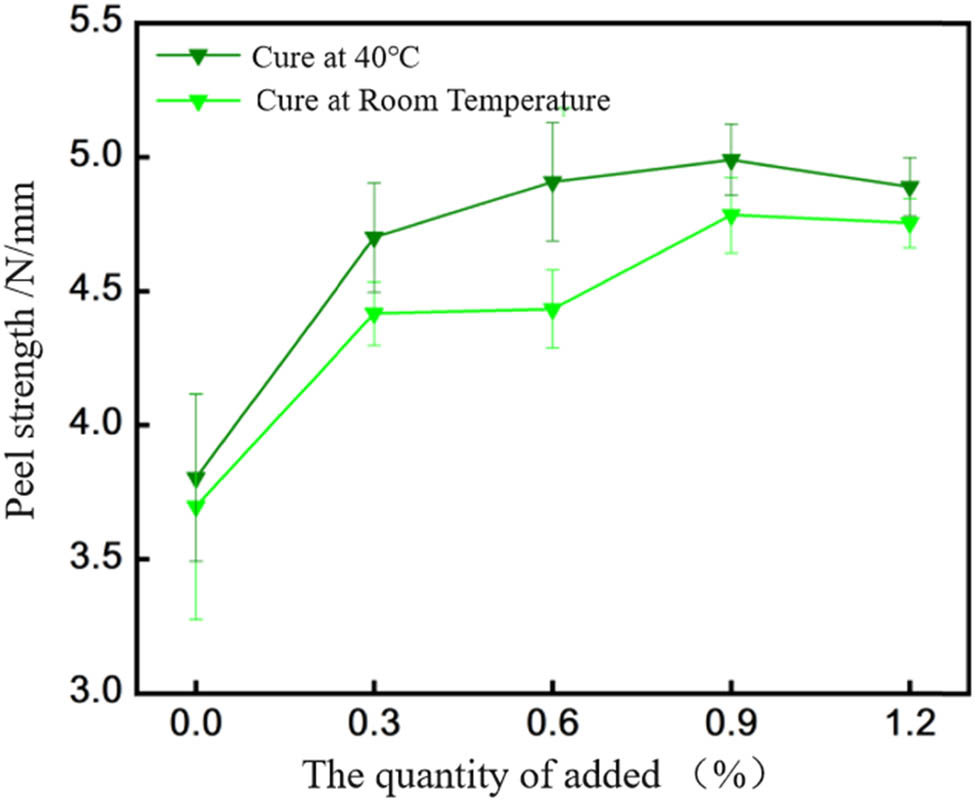
Peel strength between EP-BIB-x% and low carbon steel.
Figure 6 depicts the peel strength between EP-BIB-x% and low-carbon steel. For room temperature curing, the peel strength of pure EP is 3.69 ± 0.62 N·mm−1, with the maximum strength of 4.78 ± 0.54 N·mm−1 at 0.9% BIB, an increase of 29.5%. Under 40°C curing, the peel strength of pure EP is 3.80 ± 0.31 N·mm−1, while the maximum strength at 0.9% BIB is 4.99 ± 0.62 N·mm−1, an increase of 31.3%. Thus, a 0.9% BIB content is optimal for low-carbon steel substrates.
In summary, the peel strength of the 40°C cured EP-BIB-x% samples are slightly higher than that of the room temperature cured EP-BIB-x% samples on different substrates. The relatively lower peel strength of EP-BIB-x% on copper and aluminum substrates may be attributed to differences in surface roughness and wetting properties, which result in weaker adhesion compared to stainless steel and low-carbon steel substrates.
4 Conclusion
Adding an appropriate amount of BIB to EP resin can improve the mechanical properties of the composite coating. With the increase in BIB addition, the elongation at break, tensile strength, tensile shear strength and peel strength of the obtained composite coating first increased and then decreased.
The mechanical properties of the EP resin composite coating prepared when the mass fraction of BIB was 0.9% were the best.
Acknowledgements
This work was Supported by Guangdong S&T Program (Grant No. 2022B0101100001).
-
Funding information: Authors state no funding involved.
-
Author contributions: Weitao Huang: writing – original draft; Yong Yao: methodology; Jiayi Deng: validation; Yuqi Liu: formal analysis; Minlong Guo: visualization; Congshu Huang: data curation; Yu Liang: supervision; Yeqiang Mo: conceptualization.
-
Conflict of interest: Authors Weitao Huang, Yong Yao, Yuqi Liu, and Jiayi Deng were employed by the company Guangdong Energy Group Science and Technology Research Institute Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
-
Data availability statement: Data available on request from the authors.
References
(1) Brijder R, Hagen CHM, Cortés A, Irizar A, Thibbotuwa UC, Helsen S, et al. Review of corrosion monitoring and prognostics in offshore wind turbine structures: Current status and feasible approaches. Front Energy Res. 2022;10:991343.10.3389/fenrg.2022.991343Search in Google Scholar
(2) Montemor MF. Functional and smart coatings for corrosion protection: A review of recent advances. Surf Coat Technol. 2014;258:17–37.10.1016/j.surfcoat.2014.06.031Search in Google Scholar
(3) Eom S-H, Kim S-S, Lee J-B. Assessment of anti-corrosion performances of coating systems for corrosion prevention of offshore wind power steel structures. Coatings. 2020;10:970. 10.3390/coatings10100970.Search in Google Scholar
(4) Zhong Y, Zhang YJ, Jingwei D, Qingsong Y, Yawei S, Yanqiu Wu, et al. Effect of phytic acid on corrosion performance of epoxy coating on rust Q235 carbon steel. Corros Prot Technol. 2015;30(3):251–8.Search in Google Scholar
(5) Fan T, Wu Y, Yang M, Xu P, Li Y, Wang L, et al. Attenuation law of performance of concrete anti-corrosion coating under long-term salt corrosion. Coatings. 2024;14:1249. 10.3390/coatings14101249.Search in Google Scholar
(6) Narongdej P, Gomez R, Tseng D, Barjasteh E, Moghtadernejad S. Characterization of mechanical properties and surface wettability of epoxy resin/benzoxazine composites in a simulated acid rain environment. Coatings. 2024;14:1279. 10.3390/coatings14101279.Search in Google Scholar
(7) Končan Volmajer N, Steinbücher M, Berce P, Venturini P, Gaberšček M. Electrochemical impedance spectroscopy study of waterborne epoxy coating film formation. Coatings. 2019;9:254. 10.3390/coatings14101314.Search in Google Scholar
(8) Jin FL, Li X, Park SJ. Synthesis and application of epoxy resins: A review. J Ind Eng Chem. 2015;29:1–11.10.1016/j.jiec.2015.03.026Search in Google Scholar
(9) Bai YQ, Xue LM, Liu YF. Research progress on the modification of epoxy resin. Chem Adhes. 2007;29(4):289–92 + 304.Search in Google Scholar
(10) Anwar S, Li X. A review of high-quality epoxy resins for corrosion-resistant applications. J Coat Technol Res. 2024;21:461–80. 10.1007/s11998-023-00865-5.Search in Google Scholar
(11) van Dam JPB, Abrahami ST, Yilmaz A, Gonzalez-Garcia Y, Terryn H, Mol J. Effect of surface roughness and chemistry on the adhesion and durability of a steel-epoxy adhesive interface. Int J Adhes Adhes. 2020;96:102450. 10.1016/j.ijadhadh.2019.102450.Search in Google Scholar
(12) Graystone J, Kennedy R. Non-destructive measurement of coating adhesion. Surf Coat Int. 2000;83:389–98. 10.1007/BF02692753.Search in Google Scholar
(13) Momber AW, Plagemann P, Stenzel V. Performance and integrity of protective coating systems for offshore wind power structures after three years under offshore site conditions. Renew Energy. 2015;74:606–17. 10.1016/j.renene.2014.08.047.Search in Google Scholar
(14) Ratna D. Modification of epoxy resins for improvement of adhesion: a critical review. J Adhes Sci Technol. 2003;17(12):1655–68. 10.1163/156856103322396721.Search in Google Scholar
(15) Torry SA, Campbell A, Cunliffe AV, Tod DA. Kinetic analysis of organosilane hydrolysis and condensation. Int J Adhes Adhes. 2006;26:40–9.10.1016/j.ijadhadh.2005.03.008Search in Google Scholar
(16) Turusov RA. Adhesion interaction and adhesion mechanics. Theory and its application. Polym Sci, Ser D. 2021;14(4):522–31.10.1134/S1995421221040250Search in Google Scholar
(17) Turusov RA. Adhesion and adhesion mechanics. Features of the theory and its possibilities. Mater Sci Forum. 2020;974:638–45.10.4028/www.scientific.net/MSF.974.638Search in Google Scholar
(18) Israelachvili JN. Intermolecular and surface forces. New York: Academic Press; 2011.Search in Google Scholar
(19) Jin ZL, Wang L, Xu HX, Wang ZH, Tang YH. Discussion on the mechanism of weakening of electronic adhesive interface and analysis of influence factors. China Adhes. 2022;31(8):6–11.Search in Google Scholar
(20) Baby M, Periya VK, Sankaranarayanan SK, Maniyeri SC. Bioinspired surface activators for wet/dry environments through greener epoxy-catechol amine chemistry. Appl Surf Sci. 2020;505:144414.10.1016/j.apsusc.2019.144414Search in Google Scholar
(21) Sijbesma RP, Beijer FH, Brunsveld L, Folmer BJ, Hirschberg JH, Lange RF, et al. Reversible polymers from self-complementary monomers using quadruple hydrogen bonding. Science. 1997;278:1601–4.10.1126/science.278.5343.1601Search in Google Scholar PubMed
(22) Zhang P, Kan L, Zhang X, Li R, Qiu C, Ma N, Wei H. Supramolecularly toughened and elastic epoxy resins by grafting 2-ureido-4[1H]-pyrimidone moieties on the side chain. Eur Polym J. 2019;116:126–33.10.1016/j.eurpolymj.2019.04.001Search in Google Scholar
(23) Xiao L, Huang J, Wang Y, Chen J, Liu Z, Nie X. Tung oil-based modifier toughening epoxy resin by sacrificial bonds. ACS Sustain Chem Eng. 2019;7:17344–53.10.1021/acssuschemeng.9b04284Search in Google Scholar
(24) Chinese Standard. Adhesives-determination of tensilelapshear strength of rigid-to-rigid bonded assemblies. GB/T 7124-2008. Beijing: Standards Press of China; 2008.Search in Google Scholar
(25) Chinese Standard. Adhesives, 180° peel strength test method specimen assembly. GB/T 2790-1995. Beijing: Standards Press of China; 1995.Search in Google Scholar
(26) Li C, Strachan A. Molecular dynamics predictions of plasticization in epoxy resins. Polymer. 2015;56:209–17.Search in Google Scholar
(27) Huang XY, Wang YC, Ding C, Zhang SM, Duan XH, Ji HZ. High-performance epoxy vitrimers with the joint action of dual dynamic covalent bonds. ACS Appl Polym Mater. 2024;6(1);126–40. 10.1021/acsapm.3c01738.Search in Google Scholar
(28) Zhang H, et al. Self-healing epoxy coatings based on hydrogen-bond interactions for corrosion protection. Prog Org Coat. 2021;151:106072.Search in Google Scholar
(29) Coasne B, Gubbins KE, Pellenq RJM. Temperature effect on adsorption/desorption isotherms for a simple fluid confined within various nanopores. Adsorption. 2005;11(Suppl 1):289–94. 10.1007/s10450-005-5939-y.Search in Google Scholar
© 2025 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Flow-induced fiber orientation in gas-powered projectile-assisted injection molded parts
- Research on thermal aging characteristics of silicone rubber composite materials for dry-type distribution transformers
- Kinetics of acryloyloxyethyl trimethyl ammonium chloride polymerization in aqueous solutions
- Influence of siloxane content on the material performance and functional properties of polydimethylsiloxane copolymers containing naphthalene moieties
- Enhancement effect of electron beam irradiation on acrylonitrile–butadiene–styrene (ABS) copolymers from waste electrical and electronic equipment by adding 1,3-PBO: A potential way for waste ABS reuse
- Model construction and property study of poly(ether-ether-ketone) by molecular dynamics simulation with meta-modeling methods
- Zinc–gallic acid–polylysine nanocomplexes with enhanced bactericidal activity for the treatment of bacterial keratitis
- Effect of pyrogallol compounds dosage on mechanical properties of epoxy coating
- Preparation of in situ polymerized polypyrrole-modified braided cord and its electrical conductivity investigation under varied mechanical conditions
- Hydrophobicity, UV resistance, and antioxidant properties of carnauba wax-reinforced CG bio-polymer film
- Janus nanofiber membrane films loading with bioactive calcium silicate for the promotion of burn wound healing
- Synthesis of migration-resistant antioxidant and its application in natural rubber composites
- Influence of the flow rate on the die swell for polymer micro coextrusion process
- Fatty acid filled polyaniline nanofibres with dual electrical conductivity and thermo-regulatory characteristics: Futuristic material for thermal energy storage
- Hydrolytic depolymerization of major fibrous wastes
- Performance of epoxy hexagonal boron nitrate underfill materials: Single and mixed systems
- Blend electrospinning of citronella or thyme oil-loaded polyurethane nanofibers and evaluating their release behaviors
- Efficiency of flexible shielding materials against gamma rays: Silicon rubber with different sizes of Bi2O3 and SnO
- A comprehensive approach for the production of carbon fibre-reinforced polylactic acid filaments with enhanced wear and mechanical behaviour
- Electret melt-blown nonwovens with charge stability for high-performance PM0.3 purification under extreme environmental conditions
- Study on the failure mechanism of suture CFRP T-joints under/after the low-velocity impact loading
- Experimental testing and finite element analysis of polyurethane adhesive joints under Mode I loading and degradation conditions
- Optimizing recycled PET 3D printing using Taguchi method for improved mechanical properties and dimensional precision
- Effect of stacking sequence of the hybrid composite armor on ballistic performance and damage mechanism
- Bending crack propagation and delamination damage behavior of orthogonal ply laminates under positive and negative loads
- Molecular dynamics simulation of thermodynamic properties of Al2O3-modified silicone rubber under silane coupling agent modification
- Precision injection molding method based on V/P switchover point optimization and pressure field balancing
- Heparin and zwitterion functionalized small-diameter vascular grafts for thrombogenesis prevention
- Metal-free N, S-co-doped carbon materials derived from calcined aromatic co-poly(urea-thiourea)s as efficient alkaline oxygen reduction catalysts
- Influence of stitching parameters on the tensile performance and failure mechanisms of CFRP T-joints
- Synthesis of PEGylated polypeptides bearing thioether pendants for injectable ROS-responsive hydrogels
- Rapid Communication
- RAFT-mediated polymerization-induced self-assembly of poly(ionic liquid) block copolymers in a green solvent
- Corrigendum
- Corrigendum to “High-strength polyvinyl alcohol-based hydrogel by vermiculite and lignocellulosic nanofibrils for electronic sensing”
Articles in the same Issue
- Research Articles
- Flow-induced fiber orientation in gas-powered projectile-assisted injection molded parts
- Research on thermal aging characteristics of silicone rubber composite materials for dry-type distribution transformers
- Kinetics of acryloyloxyethyl trimethyl ammonium chloride polymerization in aqueous solutions
- Influence of siloxane content on the material performance and functional properties of polydimethylsiloxane copolymers containing naphthalene moieties
- Enhancement effect of electron beam irradiation on acrylonitrile–butadiene–styrene (ABS) copolymers from waste electrical and electronic equipment by adding 1,3-PBO: A potential way for waste ABS reuse
- Model construction and property study of poly(ether-ether-ketone) by molecular dynamics simulation with meta-modeling methods
- Zinc–gallic acid–polylysine nanocomplexes with enhanced bactericidal activity for the treatment of bacterial keratitis
- Effect of pyrogallol compounds dosage on mechanical properties of epoxy coating
- Preparation of in situ polymerized polypyrrole-modified braided cord and its electrical conductivity investigation under varied mechanical conditions
- Hydrophobicity, UV resistance, and antioxidant properties of carnauba wax-reinforced CG bio-polymer film
- Janus nanofiber membrane films loading with bioactive calcium silicate for the promotion of burn wound healing
- Synthesis of migration-resistant antioxidant and its application in natural rubber composites
- Influence of the flow rate on the die swell for polymer micro coextrusion process
- Fatty acid filled polyaniline nanofibres with dual electrical conductivity and thermo-regulatory characteristics: Futuristic material for thermal energy storage
- Hydrolytic depolymerization of major fibrous wastes
- Performance of epoxy hexagonal boron nitrate underfill materials: Single and mixed systems
- Blend electrospinning of citronella or thyme oil-loaded polyurethane nanofibers and evaluating their release behaviors
- Efficiency of flexible shielding materials against gamma rays: Silicon rubber with different sizes of Bi2O3 and SnO
- A comprehensive approach for the production of carbon fibre-reinforced polylactic acid filaments with enhanced wear and mechanical behaviour
- Electret melt-blown nonwovens with charge stability for high-performance PM0.3 purification under extreme environmental conditions
- Study on the failure mechanism of suture CFRP T-joints under/after the low-velocity impact loading
- Experimental testing and finite element analysis of polyurethane adhesive joints under Mode I loading and degradation conditions
- Optimizing recycled PET 3D printing using Taguchi method for improved mechanical properties and dimensional precision
- Effect of stacking sequence of the hybrid composite armor on ballistic performance and damage mechanism
- Bending crack propagation and delamination damage behavior of orthogonal ply laminates under positive and negative loads
- Molecular dynamics simulation of thermodynamic properties of Al2O3-modified silicone rubber under silane coupling agent modification
- Precision injection molding method based on V/P switchover point optimization and pressure field balancing
- Heparin and zwitterion functionalized small-diameter vascular grafts for thrombogenesis prevention
- Metal-free N, S-co-doped carbon materials derived from calcined aromatic co-poly(urea-thiourea)s as efficient alkaline oxygen reduction catalysts
- Influence of stitching parameters on the tensile performance and failure mechanisms of CFRP T-joints
- Synthesis of PEGylated polypeptides bearing thioether pendants for injectable ROS-responsive hydrogels
- Rapid Communication
- RAFT-mediated polymerization-induced self-assembly of poly(ionic liquid) block copolymers in a green solvent
- Corrigendum
- Corrigendum to “High-strength polyvinyl alcohol-based hydrogel by vermiculite and lignocellulosic nanofibrils for electronic sensing”

