Abstract
Injectable hydrogels have gained massive attention in the past decade due to the simple operation, the ability to fill irregularly shaped wounds, and high safety. Herein, we design a PEGylated polypeptide with reactive oxygen species (ROS) responsiveness for injectable hydrogel. In brief, poly(ethylene glycol)-block-poly(2-methylthioethyl L-glutamate) was synthesized by ring opening polymerization of N-carboxylic anhydride with thioether group. The obtained amphiphilic copolymer could self-assemble into micelles, which underwent a sol–gel phase transition at 35°C. Carbon-13 nuclear magnetic resonance, dynamic light scattering, and circular dichroism measurements indicated that the partial dehydration of poly(ethylene glycol), the aggregation of micelles, and the change of β-sheet packing of PMLG15 as temperature increasing were regarded as the main driving forces of gelation. The formed hydrogel was disrupted by 10 mM H2O2 due to the oxidation of hydrophobic thioether groups into hydrophilic sulfoxides. Furthermore, in vitro experiment showed that the hydrogels consisting of thioether groups exhibited cytoprotective ability against the damage of H2O2-induced oxidative stress to L929 cells. Finally, the aqueous solution of this copolymer rapidly transformed into gel after being injected into the subcutaneous layer of mice.
Graphical abstract
Amphiphilic block copolymers only with specific degree of polymerization of both PEG segment and polypeptide chain could form injectable ROS-responsive hydrogels, which protect normal cells from oxidative damage.
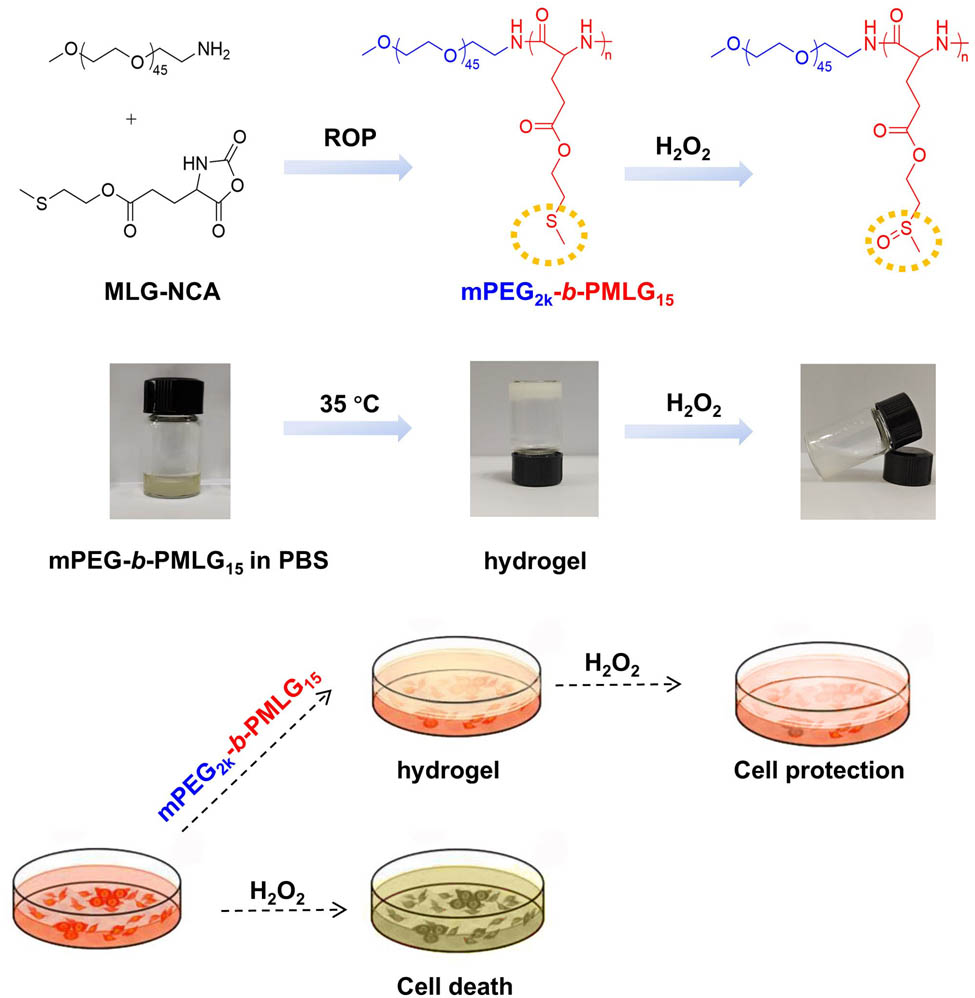
1 Introduction
Polymeric hydrogels with an obvious three-dimensional network structure and abundant water content have been widely used in biomedical fields, due to their similarity in both composition and mechanical properties to the soft tissue in the body (1,2). Among them, injectable physically cross-linked hydrogels have attracted increasing attention because of the unique sol–gel phase transition characteristic, the ability to fill irregularly shaped wounds, and adhesion to wound tissue (3,4). In addition, injectable hydrogels offer great advantages for localized drug delivery (5,6). At room temperature, the aqueous solution of the polymer has good fluidity and can evenly mix the active substances such as anticancer drugs, proteins, peptides, and nucleic acids. After injection, the polymer solution rapidly transits to a gel state at the temperature around 37°C, and the loaded drugs are released in a controllable way to achieve long-term therapeutic effect. This type of treatment maximizes the biological activity of the active substance (7,8).
Due to the aforementioned features, various biopolymer-based injectable hydrogels have been developed for biomedical applications. Wei’s group designed an injectable, thermosensitive chitosan hydrogel as a sustained-release platform for two-dimensional peptide nanosheet–doxorubicin (DOX) conjugates. This novel delivery system not only increased the solubility and bioavailability of DOX, but also improved the internalization of the hydrophobic DOX into tumor cells, achieving an enhanced cancer cell-killing efficacy (9). A temperature-sensitive injectable hydrogel has been developed based on the poly(d,l-lactide)-poly(ethylene glycol)–poly(d,l-lactide) micelles. The controlled drug release was achieved by compounding the hollow mesoporous silica containing erlotinib into the gel system (10). Ding’s group designed and synthesized a series of diblock or triblock copolymers of poly(ethylene glycol) (PEG) and poly(d,l-lactide-co-glycolide), and used them to prepare injectable hydrogels (11). They found that thermogelation is a reversible physical process primarily driven by the self-assembly of amphiphilic block copolymers in aqueous solutions into micelles. As the temperature increases, the hydrophilic blocks (such as PEG) thermally contract, leading to the exposure of the hydrophobic core, forming semi-bald micelles. With further temperature rise, hydrophobic interactions are enhanced, promoting the aggregation of micelles into a percolated network. The hydrophobic channels within this network act as physical cross-linking points, eventually resulting in the formation of a gel network (12). Hydrogels have great potential in biomedicine and can be used as carriers of NO (13), paclitaxel (14), and other drugs as well as liquid embolization agents (15). However, those polyester/polyether materials are hard to be functionalized and show obvious acid microenvironment after degradation.
Reactive oxygen species (ROS)-responsive injectable hydrogels are of particular interest owing to its relevance to anti-inflammatory and anticancer treatments. Wang et al. synthesized a ROS-sensitive cross-linked polyvinyl alcohol (PVA) hydrogel to provide basic fibroblast growth factor for myocardial repair (16). Qiu et al. developed ROS-responsive hydrogel by mixing phenylboronic acid-grafted hyaluronic acid and PVA, and the classical iron chelator desferoxamine was loaded into the hydrogel to treat traumatic brain injury by eliminating iron deposition and scavenging ROS (17). In another study, phenylboronic acid-modified gelatin methacryloyl and epigallocatechin-3-gallate (EGCG) rapidly formed hydrogel and release anti-inflammatory agent EGCG in ROS and acidic conditions for ameliorating intervertebral disk degeneration (18). Although phenylboronate bond or boronic ester bond is sensitive to ROS, a second component with cis-dihydroxy groups is always needed in these hydrogels forming via this dynamic covalent bonding, leading to a more complicated gel preparation process (19). It is urgent to develop biomaterials that can form ROS-responsive and injectable hydrogels with a single component.
Polypeptide or polyamino acids are excellent biomedical materials due to the biodegradability, biocompatibility, regular secondary structures, and the easy functionality (20,21). Stimuli-responsive peptide assemblies and the biomedical applications have been summarized elsewhere (22,23). Our group has designed ROS-responsive PEGylated polypeptide bearing thioether pendants for anticancer drug delivery (24), and synthesized tetra-poly(ethylene glycol)-b-oligo (l-methionine) copolymers that can form an injectable and oxidation-sensitive hydrogel via the hydrophobic interactions between the side chains of l-methionine (25). In this study, a ROS-responsive polypeptide block copolymer bearing thioether pendants, poly(ethylene glycol)-block-poly (2-methylthioethyl l-glutamate) (mPEG2k-b-PMLG), was synthesized for the formation of injectable hydrogels. The chemical structures of the copolymers were characterized by hydrogen-1 nuclear magnetic resonance (1H NMR) spectroscopy. The mechanism of thermal-induced gelation was studied by employing carbon-13 nuclear magnetic resonance (13C NMR), dynamic light scattering (DLS), and circular dichroism (CD) test. The oxidation of the pendant thioether groups was checked by infrared spectroscopy. Methyl thiazolyl tetrazolium (MTT) assay was conducted to prove that the mPEG2k-b-PMLG hydrogels could protect normal cells from oxidative damage. Finally, the in vivo gelation was verified by subcutaneous injection of polymer solution to rats. The experimental results indicated that the degree of polymerization (DP) of the block copolymers was one of the most critical factors for the hydrogel forming. Copolymer mPEG-b-PMLG with too long hydrophilic PEG chains or too short hydrophobic PMLG segment showed quite different self-assemble behaviors and might not form hydrogels in water. Specifically, the obtained mPEG2k-b-PMLG15 hydrogels are temperature and oxidation dual-responsive porous materials with good biocompatibility and hold great potential for drug delivery. This work exhibited the accuracy and the beauty of polymer science.
2 Materials and methods
2.1 Materials and characterization
The starting material, 1-tert-butyl N-(tert-butoxycarbonyl)-l-glutamate (Boc-Glu-OTBu), was supplied by Aladdin Bio-Chem Technology Co., Ltd (Shanghai, China). 2-(Methylthio)ethanol was acquired from Energy Chemical (Shanghai, China). Methoxy (polyethylene glycol) (mPEG2k, M n = 2,000) was purchased from Ponsure Biotechnology Co., Ltd (Shanghai, China), and mPEG2k-NH2 was synthesized following our previous procedure. Sodium hydroxide and tetrahydrofuran were purchased from Sinopharm Co., Ltd., and N,N-dimethylformamide (DMF) was obtained from Shanghai Qiangshun Chemical Reagent Co., Ltd. Dulbecco's modified eagle medium (DMEM) cell culture medium was purchased from Biotechnology Co., Ltd. Trypsin was purchased from Wuhan Seville Biotechnology Co., Ltd., and penicillin–streptomycin solution was purchased from Beijing Sevin Innovation Biology Co., Ltd. MTT was purchased from Shanghai Biyuntian Biotechnology Co., Ltd. Dichloromethane, sodium chloride, trimethylamine, ethyl acetate, anhydrous magnesium sulfate, sodium bicarbonate, and other chemicals were all bought from Xilong Scientific Co., Ltd. (Guangzhou, China).
2.2 Synthesis of mPEG2k-b-PMLG
The monomer, N-carboxyanhydride from methylthioethyl l-glutamate (MLG-NCA), was synthesized following our previous protocols (24). The di-block copolymer was synthesized through the ring-opening polymerization of MLG-NCA with mPEG2k-NH2 serving as a macroinitiator (Figure S1). Briefly, MLG-NCA and mPEG2k-NH2 macroinitiator (molar ratio 17:1) were added in a round-bottom flask under nitrogen atmosphere and dissolved by dry DMF. Following a 72 h reaction period at 25°C, the solution was subsequently subjected to dialysis and lyophilization. The product mPEG2k-b-PMLG was obtained as white solid.
2.3 Preparation of mPEG2k-b-PMLG hydrogels
The mPEG2k-b-PMLG was dissolved in a 0.01 M PBS with a pH of 7.4 at 4°C. Different concentrations of polymer solutions were prepared. Subsequently, 0.5 mL of each polymer solution was transferred to a small glass vial and placed in a water bath with a programmed warming process of 1°C per minute. And each temperature was maintained for 5 min. At a specific temperature, the glass vial was inverted. If the polymer solution did not flow within 30 s, it was considered the hydrogels have formed. Each sample was subjected to this test in triplicate for accuracy.
2.4 Dynamic rheological analysis
Dynamic rheological analysis was conducted using a US 302 (Anton Paar rheometer) with 25 mm diameter plates, operated in oscillation mode, and a gap distance of 0.5 mm. For testing, a 300 μL polymer solution was placed on the sample table, and the edges of the sample were sealed with silicone oil to prevent moisture evaporation. The changes of energy storage modulus (G′) with temperature were recorded with testing parameters of a strain (λ) of 1% and a frequency (ω) of 1 Hz. The heating rate was 0.5°C·min−1, and the temperature range of the analysis was from 20°C to 60°C.
2.5 Temperature sensitivity of the polymers
The polymer was dissolved in deionized water at a concentration of 0.05 mg·mL−1. The size and size distribution of the self-assembled particles were measured by DLS at 20°C and 60°C, respectively. CD measurement was performed over a temperature range of 10°C–60°C to monitor the secondary structure of the polypeptide mPEG2k-b-PMLG. Prior to testing, the samples were equilibrated at each temperature for 10 min. 13C NMR was performed in the temperature range of 20°C–50°C to assess the polymer structure.
2.6 Oxidative sensitivity of the polymers
The mPEG2k-b-PMLG was dissolved in 0.01 M PBS with 12.0 wt% concentration, and hydrogel was formed at 35°C. Then, the hydrogel was incubated with PBS solution containing 10 mM or 100 mM H2O2. After 6 h or 12 h, the degradation medium was removed by dialysis, and the buck products after degradation were collected, freeze–dried, and submitted for 1H NMR and Fourier transform infrared spectroscopy test.
2.7 Cytotoxicity evaluation
The in vitro cytotoxicity was assessed by using MTT assay. First, L929 cells were cultivated in 96-well plates with a seeding density of 8,000 cells per well with 200 μL DMEM medium and incubated at 37°C with 5% CO2 for 24 h. Afterward, the culture medium was removed, and 200 μL of oxidized polymer solution by H2O2 or raw polymer solution in DMEM was added, with concentrations ranging from 0.063 to 2 mg·mL−1. It should be noted that the raw mPEG2k-b-PMLG solution formed hydrogel at 37°C. The control group was cultured with DMEM without polymer. Following an additional 24 h incubation, 20 μL MTT solution was added and incubated for 4 h. Then, the culture medium was discarded, and 150 μL of DMSO was added. The absorbance or optical density (OD) at 490 nm for each well was measured using a microplate reader. The cell viability rate (cell viability) was calculated using the following formula:
2.8 Cellular protective effect of gels in oxidative environment
The L929 cells were cultured (8,000 cells/well, 200 μL DMEM/well) at 37°C for 24 h. The culture medium was removed, and 50 μL polymer solution (12 wt%) was added to each well to form a stable hydrogel on the cell surface. Subsequently, 180 μL of DMEM medium containing H2O2 (0.125, 0.25, 0.5 or 1 mM) was added. After 24 h, the medium and gel were removed. Cells were washed with PBS for 3 times to remove the remaining small amount of gel. Then, 150 μL DMEM culture solution was added. The cells that did not contain the gel were treated as the control group in the same protocols. MTT assay was used to detect the cell viability.
2.9 In vivo test
Female Sprague-Dawley (SD) rats (∼200 g) were obtained from Beijing Vital River Laboratory Animal Technology Co., Ltd. All animal experimental protocols were approved by the Animal Care and Use Committee of Changchun Institute of Applied Chemistry (No. 2022-0082). The mPEG2k-b-PMLG solution (12.0 wt% in PBS) was subcutaneously injected into the back of SD rats through a syringe. The rats were killed 15 min later. The gel formation was checked, and photographs were taken.
3 Results and discussion
3.1 Characterization of mPEG2k-b-PMLG15
The synthesis pathway for mPEG2k-b-PMLG, which is sensitive to both oxidation and temperature, is depicted in Figure S1 and detailed in the Experimental section. Initially, the thioether group is attached to glutamate through the esterification of Boc-MLG-OTBu with 2-(methylthio)ethanol. The MLG-NCA was then synthesized according to protocols in our previous report (24). The desired product, mPEG2k-b-PMLG, is ultimately obtained through the ring-opening polymerization of MLG-NCA utilizing mPEG2k-NH2 as a macromolecular initiator in anhydrous DMF. By controlling the molar ratio of MLG-NCA and mPEG2k-NH2 macromolecular initiator (17:1), the DP of PMLG is finally determined to be 15 by integrating the area of proton peaks f and i in Figure 1. GPC profiles showed that the average molecular weight of mPEG2k-b-PMLG15 is 6.1 kDa.
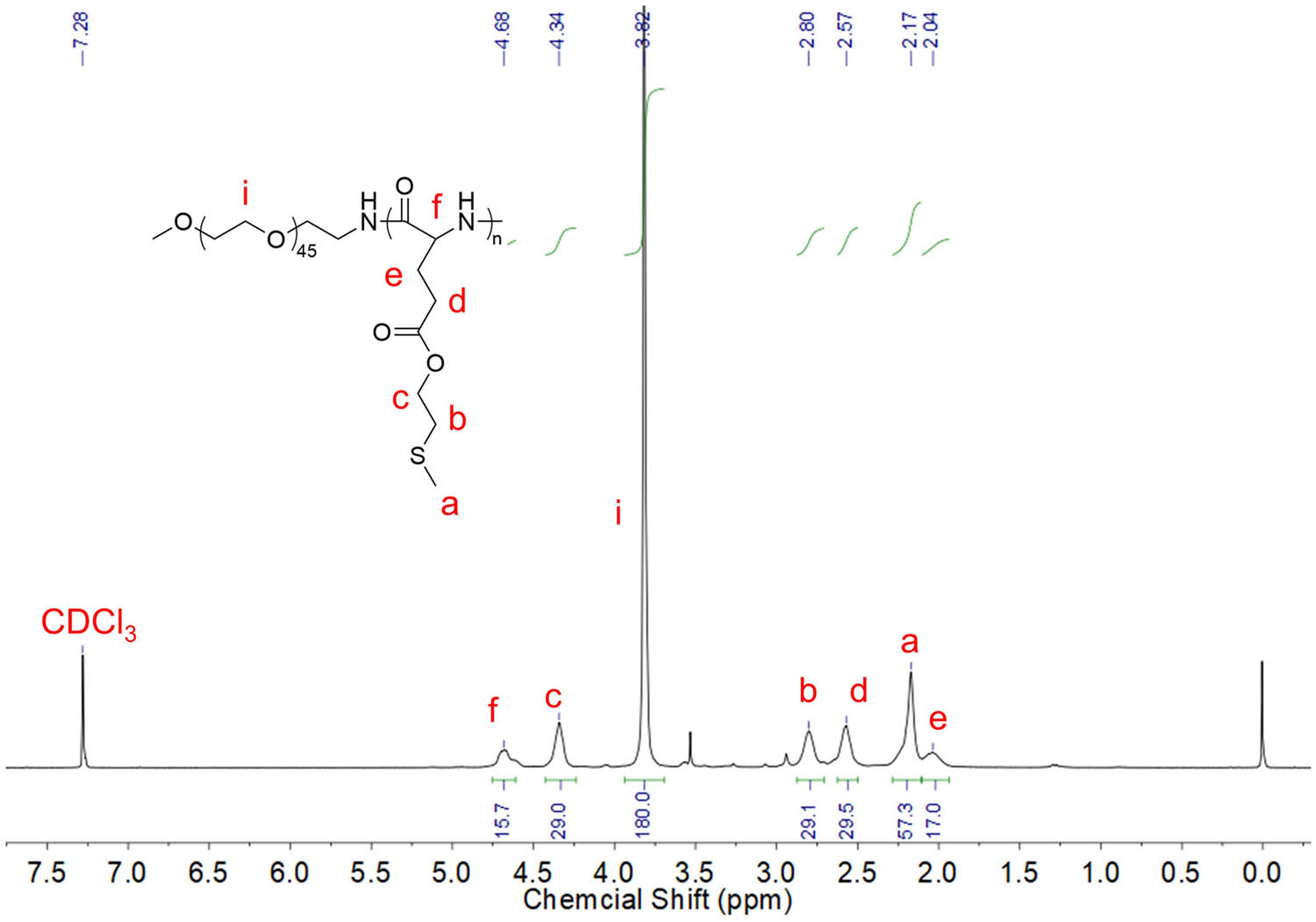
1H NMR spectrum of mPEG2k-b-PMLG15 in mixture solvent of trifluoroacetic acid-d and CDCl3.
3.2 Self-assembly of mPEG2k-b-PMLG15
Some mPEG2k-based block copolymers exhibit an interesting behavior. As the temperature increases, the enhanced interaction between the hydrophobic fragments and the partial dehydration of the mPEG2k contributes to micelle accumulation. Eventually, this leads to a phase transition in the polymer solution, resulting in the formation of a gel (26,27). Our amphiphilic mPEG2k-b-PMLG15 could self-assemble into micelles or nanoparticles (NPs) in water. The critical micelle concentration (CMC) was determined to be 0.00083 mg·mL−1 by using pyrene as fluorescence probe (Figure 2a). DLS tests have confirmed that these micelles maintain a stable 272 nm size at room temperature (Figure 2b).
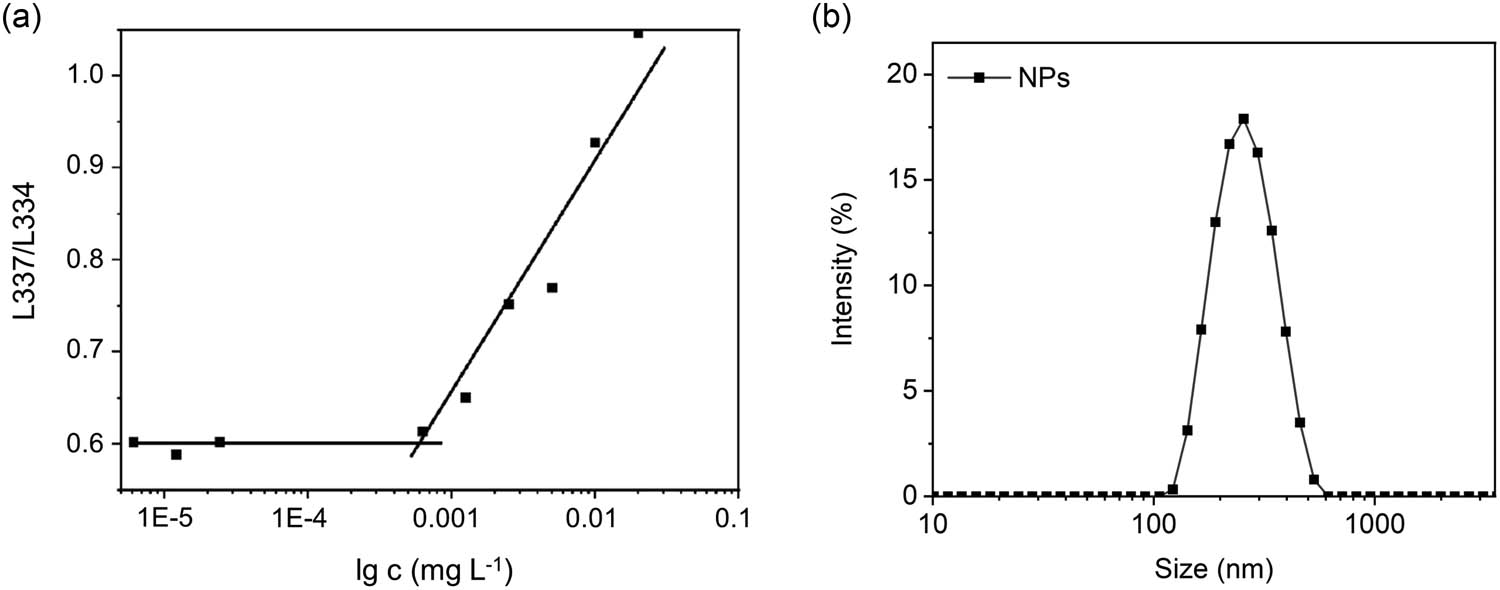
(a) CMC measurement determined by fluorescence assay of pyrene probe incubation with different concentrations of mPEG-b-PMLG15 solution and (b) DLS profiles of the self-assembled NPs at 1 mg·mL−1.
3.3 Thermal-induced gelling and the mechanism
Dynamic rheological analysis of the aqueous solution of mPEG2k-b-PMLG15 was performed at the concentration ranging from 10 to 14 wt%. As shown in Figure 3a, when the temperature is lower than the phase transition temperature, the storage modulus G′ of the polymer solution remains basically unchanged; At the vicinity of the phase transition temperature, G′ suddenly increases, indicating that there is a solution transition to gel state, and with the increase of temperature, G′ continues to increase. Specifically, the phase transition temperature was determined to be 33°C for 12 wt% polymer solution.
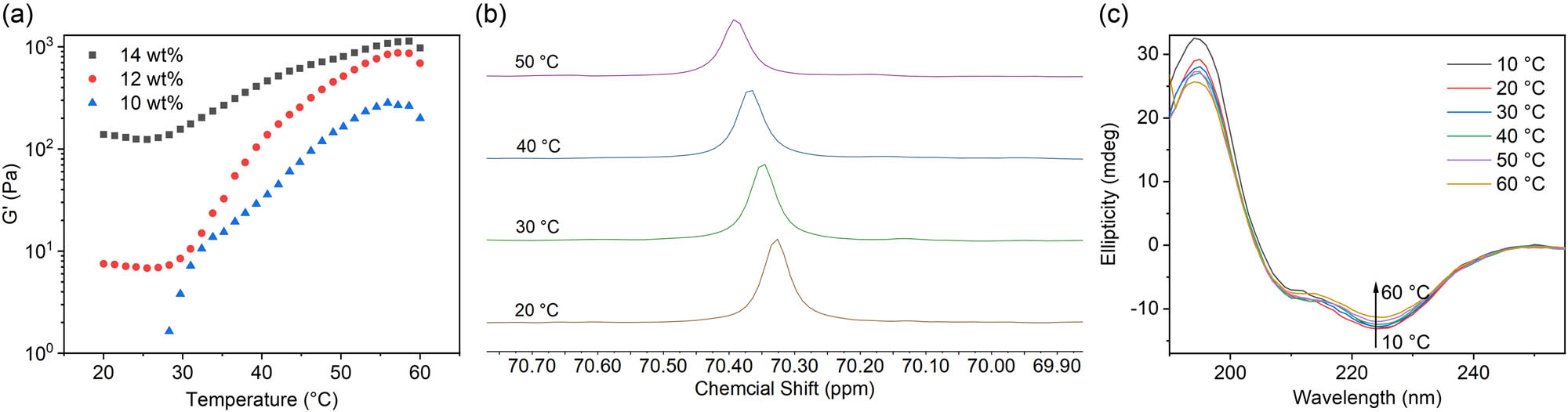
(a) The storage modulus (G′) profiles of the mPEG2k-b-PMLG15 solution (10, 12, and 14 wt%) from 10°C to 60°C, (b) 13C NMR spectra of polymer solution at temperature of 20°C–50°C, and (c) CD spectra of the mPEG2k-b-PMLG15 solution (0.05 mg·mL−1, 10°C–60°C).
As depicted in Figure 3b, the resonance absorption peak of carbon in the mPEG methylene was observed at 70.3 ppm at 20°C. As the temperature gradually increased, this peak shifted toward the lower field and tends to broaden. This shift suggests that the mPEG segment experienced gradual dehydration as the temperature increased, accompanied by partial molecular immobilization. The positive absorption peak at 195 nm and negative absorption peak at 225 nm in the CD spectra indicated a β-sheet structure of the PMLG15 polypeptide (Figure 3c). The β-sheet structure became less at higher temperature as the intensity of peak at 225 nm gradually decreased when the temperature increased from 10°C to 60°C (Figure S2). Based on the aforementioned results, it can be inferred that the changes occurring in the mPEG segment and the secondary structure of the polyamino acid chain segments played a significant role in promoting the transition from a solution to a gel state as the temperature increased (25,26,27,28).
3.4 Oxidation responsiveness of mPEG2k-b-PMLG15
First, the invert test tube method was applied to confirm the phase transition temperature of mPEG2k-b-PMLG15, i.e., by observing whether the sample flows within 30 s through the inverted tube, if it does not flow, it is considered to form a gel. As shown in Figure 4a, when the sample was inverted at 35°C, no flow occurred and hydrogel was formed. Scanning electron microscopy image exhibited the irregular pores of the hydrogel formed in vitro (Figure 4b).
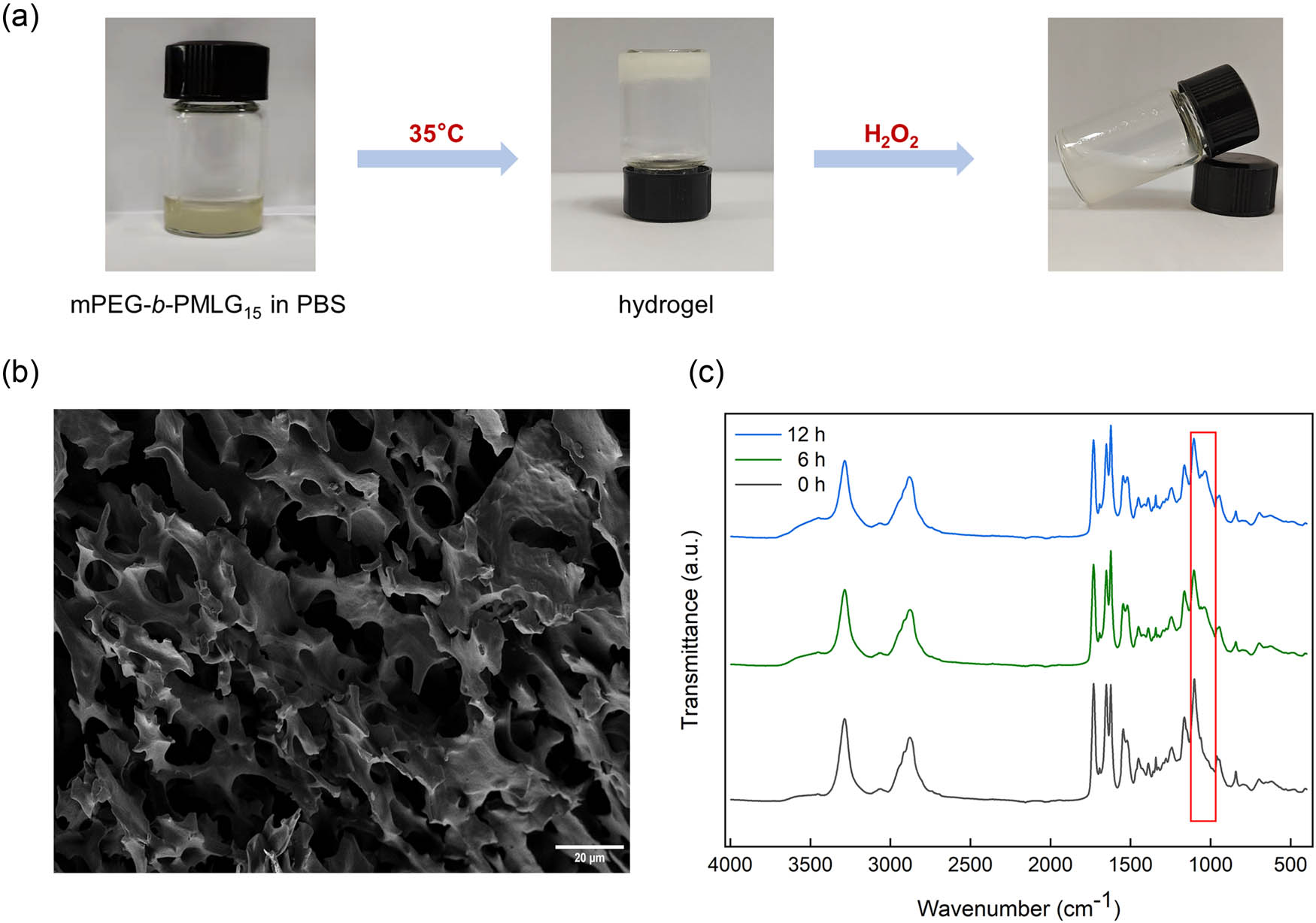
(a) Hydrogel formation at high temperature and disassociation in the presence of H2O2, (b) scanning electron microscope (SEM) image of the hydrogel formed by mPEG2k-b-PMLG15 solution at 35°C (scale bar: 20 μm), and (c) infrared spectra of hydrogel incubated with 100 mM H2O2 for 0, 6, and 12 h, respectively.
H2O2 is one of the key components of ROS generated during organism metabolism and exhibits relatively high stability compared to other ROS species. Therefore, we selected H2O2 to create a high-concentration ROS environment to assess the gel’s responsiveness to ROS. In the presence of H2O2, the hydrogel was destroyed and turned into liquid again (Figure 4a, right). As illustrated in the 1H NMR spectra in Figure S3, the three peaks at 2.11, 2.70, and 4.21 ppm corresponding to the methyl and methylene near the thioether groups (labeled as a, b, and c) gradually disappeared after incubation with H2O2 for 12 h. Concurrently, new peaks appeared at 2.68 (a′), 3.03 (b′), and 4.41 (c′) ppm, indicating the oxidation of the sulfur atom. Infrared spectra revealed a new absorption peak at 1,035 cm−1 after oxidation, corresponding to the characteristic peak of the sulfoxide group, and the intensity of this characteristic peak significantly increased with prolonged oxidation time (Figure 4c). Therefore, the oxidative response mechanism is that the thioether group in the hydrophobic portion of mPEG2k-b-PMLG15 side chains is oxidized into hydrophilic sulfoxide groups. As the hydrophilic properties of the polymer increase, its solubility also increases, which, in turn, accelerates the degradation of the hydrogel. This finding established a theoretical foundation for the elimination of ROS at inflammatory sites by mPEG2k-b-PMLG15 hydrogel and the protection of cells against oxidative damage.
3.5 Cell protection of gel in oxidized environment
The cytotoxicity of mPEG2k-b-PMLG15 and the hydrogel under oxidative conditions (10 mM H2O2) was evaluated using the MTT assay. As illustrated in Figure 5a, after a 24 h co-culture with the polymeric hydrogel and the oxidized products at concentrations ranging from 0.0156 to 1 mg·mL−1, the survival rate of L929 cells in the experimental group remained above 90%. This suggests that mPEG2k-b-PMLG15 block copolymers and their various oxidation products exhibit excellent cytocompatibility. In the presence of 0.25 and 0.125 mM H2O2, the cell survival rates of L929 were more than 80% with gel protection, significantly higher than that without gel protection (Figure 5b).
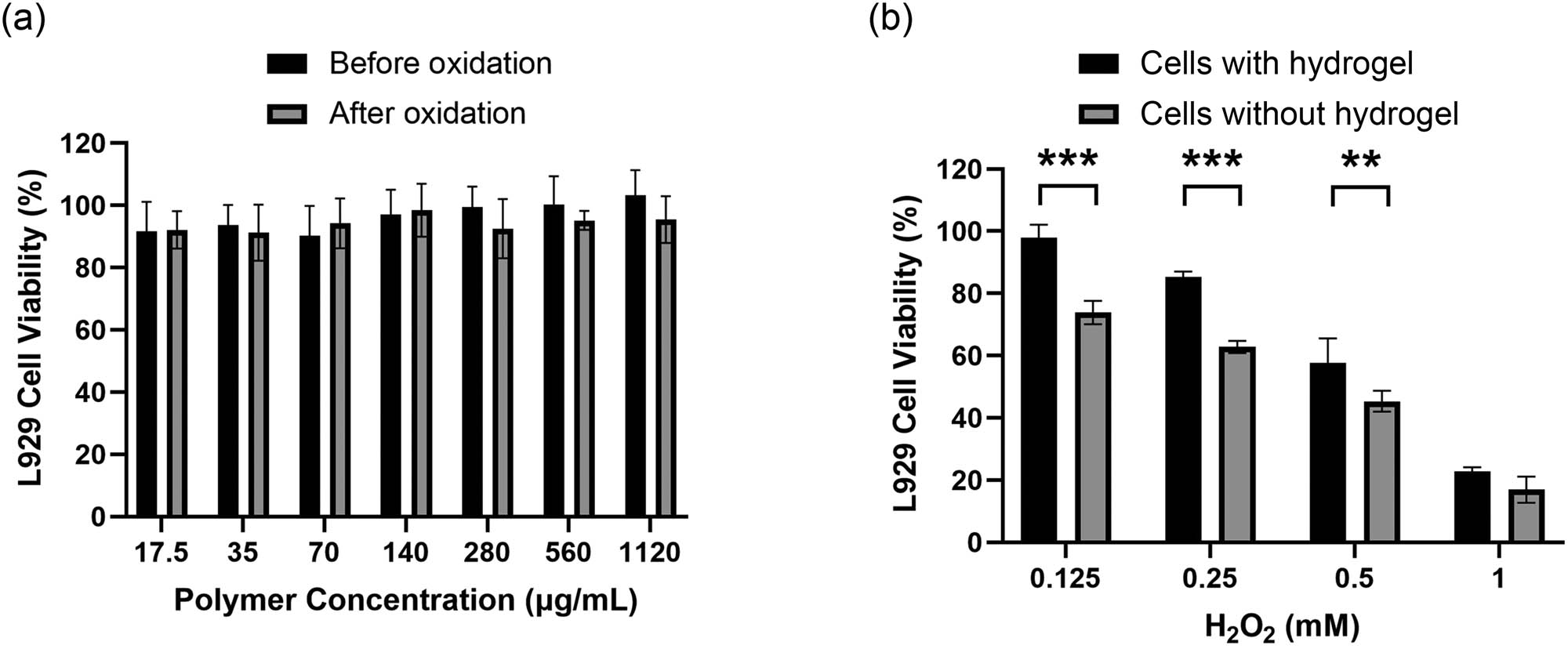
(a) Cell survival rates of mouse fibroblasts cells (L929) after 24 h incubation with mPEG2k-b-PMLG15 solution or their oxidation products at different concentrations. It should be noted that the raw mPEG2k-b-PMLG solution formed hydrogel at 37°C, and (b) cell viability of L929 cells cocultured with 12 wt% hydrogel for 24 h in the presence of 0.125,0.25,0.5, and 1 mM H2O2 in DMEM. Data are shown as mean ± SD (n = 3), **P < 0.01, ***P < 0.001.
ROS has high oxidation activity and can cause obvious damage and destruction to cells and tissues. Overexpression of ROS is the root cause of inflammation, including infectious inflammation (bacterial and viral infections) and non-infectious inflammation (such as rheumatoid arthritis), as well as a variety of senile degenerative diseases. Hydrogel formed by mPEG2k-b-PMLG15 solution could partly eliminate ROS and is potential for the treatment of oxidative stress-related diseases.
3.6 In vivo gelling of mPEG2k-b-PMLG15
A typical feature of temperature-sensitive hydrogels is that the aqueous solution of the polymer is rapidly converted into a hydrogel upon injection into the body. Therefore, SD rats were employed as an animal model to assess the subcutaneous gel process of the mPEG2k-b-PMLG15 hydrogel. The polymer solution was subcutaneously injected into the rats’ backs, and after 15 min, the rats were euthanized. The back skin was then dissected, revealing the formation of the polymer gel within the subcutaneous tissue (Figure S4).
4 Discussion
The DP is one of the most important concepts in the field of polymer science. It has been reported that the self-assembled nano-morphology of di-block amphiphilic copolymers might be spheres, cylinders, bicontinuous structures, lamellae, and enclosed membrane structures (vesicles or polymersomes), depending on the different length (or DP) ratio of the two blocks and solvent system (29,30,31). These different aggregates provide different potential applications in many fields.
Due to the different DPs of both PEG and polypeptides, our previously reported mPEG5k-b-PMLG40 and the polymer in this study exhibited quite different properties (Table S1). The previous copolymers self-assembled into micelles of 68.3 nm, which were capable of encapsulating a high content of 9.4% DOX and achieving an enhanced anticancer efficacy in 4T1-tumor bearing mice (24). The long hydrophilic PEG5k segment contributed to the stability in blood circulation, while preventing the aggregation of micelles at elevated temperatures. Thus, mPEG5k-b-PMLG40 solution could not be transformed to hydrogels. In contrast, lower-molecular mPEG2k-b-PMLG15 formed larger NPs with additional thermal sensitivity. With the increase of temperature, the PEG2k segment gradually dehydrated (Figure 3b), and the β-folding structure of PMLG15 decreased (Figure 3c), which might promote the aggregation between these large micelles, limiting the mobility of aqueous solvent, and finally forming hydrogels (Figures 3a and 4a). Actually, curiosity caused us to synthesize shorter PEGylated polypeptides, mPEG2k-b-PMLG13 and mPEG2k-b-PMLG11. The former can form hydrogels, whereas the latter cannot (data not shown).
By simply adjusting the DP, the mPEG-b-PMLG copolymers exhibited distinct properties, self-assembly behaviors, and thus had different potential biomedical applications. This is the magic and charm of polymer science. Our bi-stimulus-responsive PEG2k-b-PMLG15 hydrogels have potential applications in hydrophilic drug delivery and in wound care dressings in the future.
5 Conclusion
In summary, we successfully synthesized mPEG2k-b-PMLG15 block copolymers. These polymers can self-assemble into nanosized micelles in an aqueous solution, subsequently forming a hydrogel when the temperature raised. The gelation mechanism was attributed to the partial dehydration of PEG segment and the secondary structure change of polypeptide block as temperature increasing. In vitro experiments demonstrated that both of the polymeric hydrogel and the post-oxidized product exhibited negligible cytotoxicity. The survival rate of L929 cells incubated with the polymeric hydrogel exceeded 90% at H2O2 concentrations of 0.125 mM, effectively mitigating cellular damage in the presence of elevated H2O2 levels. In addition, a regular-shaped and solid gel was formed within 15 min injection of mPEG2k-b-PMLG15 solution in rat. This injectable hydrogel, based on essential human amino acids, holds promise for applications in the localized, on-demand release of drugs, and cellular protection in areas enriched with ROS, such as tumor site and inflammatory region.
-
Funding information: This work was supported by the Department of Science and Technology of Jilin Province (20240101113JC) and the National Natural Science Foundation of China (51803014, 52273310).
-
Author contributions: Yingying Ji: writing – original draft, experimenting, data curation; Yitao Sun: polymer synthesis and cellular experiment; Yu Zhang: funding acquisition, project administration; Hongyu Zhang: methodology; Pan He: writing – review and editing, supervision, funding acquisition.
-
Conflict of interest: The authors declare that they have no conflict of interest.
-
Ethical approval: Female SD rats were obtained from Beijing Vital River Laboratory Animal Technology Co., Ltd. The animal experimental protocols were approved by Ethics Committee of Changchun Institute of Applied Chemistry (No. 2022-0082).
-
Data availability statement: Original data can be requested from the corresponding authors.
References
(1) Liu Z, Wei J, Faraj Y, Ju XJ, Xie R, Wang W, et al. Smart hydrogels: Network design and emerging applications. Can J Chem Eng. 2018;96(10):2100–14. 10.1002/cjce.23328.Search in Google Scholar
(2) Liu JY, Zhang XN, Xiao CS, Chen XS. A drug-mineralized hydrogel orchestrated by spontaneous dynamic mineralization. Adv Funct Mater. 2024;34(12):2311844. 10.1002/adfm.202311844.Search in Google Scholar
(3) Yu L, Zhang H, Ding JD. A subtle end-group effect on macroscopic physical gelation of triblock copolymer aqueous solutions. Angew Chem Int Ed. 2006;45(14):2232–5. 10.1002/anie.200503575.Search in Google Scholar PubMed
(4) Wang S, Zhang J, Zhou W, Liu W, Ou Y, Zheng X, et al. Injectable carrier hydrogel for diabetic foot ulcer wound repair. J Mater Sci. 2023;58(28):11441–68. 10.1007/s10853-023-08730-x.Search in Google Scholar
(5) Mantooth SM, Hancock AM, Thompson PM, Varghese PJG, Meritet DM, Vrabel MR, et al. Characterization of an injectable chitosan hydrogel for the tunable, localized delivery of immunotherapeutics. ACS Biomater Sci Eng. 2024;10(2):905–20. 10.1021/acsbiomaterials.3c01580.Search in Google Scholar PubMed
(6) Hlavac N, Bousalis D, Pallack E, Li Y, Manousiouthakis E, Ahmad RN, et al. Injectable neural hydrogel as in vivo therapeutic delivery vehicle. Regen Eng Transl Med. 2023;9(3):424–30. 10.1007/s40883-022-00292-9.Search in Google Scholar PubMed PubMed Central
(7) Lee SC, Kwon IK, Park K. Hydrogels for delivery of bioactive agents: A historical perspective. Adv Drug Deliv Rev. 2013;65(1):17–20. 10.1016/j.addr.2012.07.015.Search in Google Scholar PubMed PubMed Central
(8) Ruel-Gariépy E, Leroux JC. In situ-forming hydrogels-review of temperature-sensitive systems. Eur J Pharm Biopharm. 2004;58(2):409–26. 10.1016/j.ejpb.2004.03.019.Search in Google Scholar PubMed
(9) Luan X, Hu H, Zhu D, He P, Sun Z, Xi Y, et al. Injectable chitosan hydrogels doped with 2D peptide nanosheet-drug conjugates for glutathione-responsive sustained drug delivery. Chem Eur J. 2024;30(28):e202400021. 10.1002/chem.202400021.Search in Google Scholar PubMed
(10) Zhou X, He X, Shi K, Yuan L, Yang Y, Liu Q, et al. Injectable thermosensitive hydrogel containing erlotinib-loaded hollow mesoporous silica nanoparticles as a localized drug delivery system for NSCLC therapy. Adv Sci. 2020;7(23):2001442. 10.1002/advs.202001442.Search in Google Scholar PubMed PubMed Central
(11) Cui SQ, Yu L, Ding JD. Injectable thermogels based on block copolymers of appropriate amphiphilicity. Acta Polym Sin. 2018;8:997–1015. 10.11777/j.issn1000-3304.2018.18084.Search in Google Scholar
(12) Cui SQ, Yu L, Ding JD. Semi-bald micelles and corresponding percolated micelle networks of thermogels. Macromolecules. 2018;51(16):6405–20. 10.1021/acs.macromol.8b01014.Search in Google Scholar
(13) Wang Y, Yang X, Chen X, Wang X, Wang Y, Wang H, et al. Sustained release of nitric oxide and cascade generation of reactive nitrogen/oxygen species via an injectable hydrogel for tumor synergistic therapy. Adv Funct Mater. 2022;32(36):2206554. 10.1002/adfm.202206554.Search in Google Scholar
(14) Luan J, Zhang Z, Shen W, Chen Y, Yang X, Chen X, et al. Thermogel loaded with low-dose paclitaxel as a facile coating to alleviate periprosthetic fibrous capsule formation. ACS Appl Mater Inter. 2018;10(36):30235–46. 10.1021/acsami.8b13548.Search in Google Scholar PubMed
(15) Yang H, Lei K, Zhou F, Yang X, An Q, Zhu W, et al. Injectable PEG/polyester thermogel: A new liquid embolization agent for temporary vascular interventional therapy. Mater Sci Eng C. 2019;102:606–15. 10.1016/j.msec.2019.04.075.Search in Google Scholar PubMed
(16) Li Z, Zhu D, Hui Q, Bi J, Yu B, Huang Z, et al. Injection of ROS-responsive hydrogel loaded with basic fibroblast growth factor into the pericardial cavity for heart repair. Adv Funct Mater. 2021;31(15):2004377. 10.1002/adfm.202004377.Search in Google Scholar
(17) Qiu Y, Zeng Y, Zhang C, Lv X, Ling Y, Si Y, et al. A ROS-responsive loaded desferoxamine (DFO) hydrogel system for traumatic brain injury therapy. Biomed Mater. 2024;19(2):025016. 10.1088/1748-605X/ad1dfd.Search in Google Scholar PubMed
(18) Liu L, Wang W, Huang L, Xian Y, Ma W, Fan J, et al. Injectable pathological microenvironment-responsive anti-inflammatory hydrogels for ameliorating intervertebral disc degeneration. Biomaterials. 2024;306:122509. 10.1016/j.biomaterials.2024.122509.Search in Google Scholar PubMed
(19) Ding XY, Wang Y, Li G, Xiao CS, Chen XS. Iminoboronate ester cross-linked hydrogels with injectable, self-healing and multi-responsive properties. Acta Polym Sin. 2019;50(5):505–15. 10.11777/j.issn1000-3304.2019.19015.Search in Google Scholar
(20) Zhang Y, He P, Zhang P, Yi X, Xiao CS, Chen XS. Polypeptides-drug conjugates for anticancer therapy. Adv Healthc Mater. 2021;10(11):2001974. 10.1002/adhm.202001974.Search in Google Scholar PubMed
(21) Criado-Gonzalez M, Mecerreyes D. Thioether-based ROS responsive polymers for biomedical applications. J Mater Chem B. 2022;10(37):7206–21. 10.1039/d2tb00615d.Search in Google Scholar PubMed
(22) Mu R, Zhu D, Abdulmalik S, Wijekoon S, Wei G, Kumbar SG. Stimuli-responsive peptide assemblies: Design, self-assembly, modulation, and biomedical applications. Bioact Mater. 2024;35:181–207. 10.1016/j.bioactmat.2024.01.023.Search in Google Scholar PubMed PubMed Central
(23) Luan X, Hu H, Sun Z, He P, Zhu D, Xu Y, et al. Assembling Ag2S quantum dots onto peptide nanosheet as a biomimetic two-dimensional nanoplatform for synergistic near infrared-II fluorescent imaging and photothermal therapy of tumor. J Colloid Interface Sci. 2024;663:111–22. 10.1016/j.jcis.2024.02.163.Search in Google Scholar PubMed
(24) Sun YT, Zhang Y, Guo X, Wang YP, He P, Xiao CS. Oxidation responsive PEGylated polyamino acid bearing thioether pendants for enhanced anticancer drug delivery. Macromol Biosci. 2023;23(3):2200498. 10.1002/mabi.202200498.Search in Google Scholar PubMed
(25) Xu QH, He CL, Ren KX, Xiao CS, Chen XS. Thermosensitive polypeptide hydrogels as a platform for ROS-triggered cargo release with innate cytoprotective ability under oxidative stress. Adv Healthc Mater. 2016;5(15):1979–90. 10.1002/adhm.201600292.Search in Google Scholar PubMed
(26) Kim SY, Kim HJ, Lee KE, Han SS, Sohn YS, Jeong B. Reverse thermal gelling PEG-PTMC diblock copolymer aqueous solution. Macromolecules. 2007;40(15):5519–25. 10.1021/ma070190z.Search in Google Scholar
(27) Zhu HY, Liu Y, Gu DX, Rao ZK, Li Y, Hao JY. Dual thermoresponsive mPEG-b-poly(O-benzyl-L-threonine acid) hydrogel based on β-sheet nano-structural disassembly and PEG dehydration. Polymer. 2021;226:123841. 10.1016/j.polymer.2021.123841.Search in Google Scholar
(28) Fu X, Ma Y, Shen Y, Fu W, Li Z. Oxidation-responsive OEGylated poly-l-cysteine and solution properties studies. Biomacromolecules. 2014;15(3):1055–61. 10.1021/bm5000554.Search in Google Scholar PubMed
(29) Zhang L, Eisenberg A. Multiple morphologies of “crew-cut” aggregates of polystyrene-b-poly (acrylic acid) block copolymers. Science. 1995;268(5218):1728–31. 10.1126/science.268.5218.1728.Search in Google Scholar PubMed
(30) Mai Y, Eisenberg A. Self-assembly of block copolymers. Chem Soc Rev. 2012;41(18):5969–85. 10.1039/C2CS35115C.Search in Google Scholar PubMed
(31) Blanazs A, Armes SP, Ryan AJ. Self-assembled block copolymer aggregates: from micelles to vesicles and their biological applications. Macromol Rapid Comm. 2009;30(4–5):267–77. 10.1002/marc.200800713.Search in Google Scholar PubMed
© 2025 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Flow-induced fiber orientation in gas-powered projectile-assisted injection molded parts
- Research on thermal aging characteristics of silicone rubber composite materials for dry-type distribution transformers
- Kinetics of acryloyloxyethyl trimethyl ammonium chloride polymerization in aqueous solutions
- Influence of siloxane content on the material performance and functional properties of polydimethylsiloxane copolymers containing naphthalene moieties
- Enhancement effect of electron beam irradiation on acrylonitrile–butadiene–styrene (ABS) copolymers from waste electrical and electronic equipment by adding 1,3-PBO: A potential way for waste ABS reuse
- Model construction and property study of poly(ether-ether-ketone) by molecular dynamics simulation with meta-modeling methods
- Zinc–gallic acid–polylysine nanocomplexes with enhanced bactericidal activity for the treatment of bacterial keratitis
- Effect of pyrogallol compounds dosage on mechanical properties of epoxy coating
- Preparation of in situ polymerized polypyrrole-modified braided cord and its electrical conductivity investigation under varied mechanical conditions
- Hydrophobicity, UV resistance, and antioxidant properties of carnauba wax-reinforced CG bio-polymer film
- Janus nanofiber membrane films loading with bioactive calcium silicate for the promotion of burn wound healing
- Synthesis of migration-resistant antioxidant and its application in natural rubber composites
- Influence of the flow rate on the die swell for polymer micro coextrusion process
- Fatty acid filled polyaniline nanofibres with dual electrical conductivity and thermo-regulatory characteristics: Futuristic material for thermal energy storage
- Hydrolytic depolymerization of major fibrous wastes
- Performance of epoxy hexagonal boron nitrate underfill materials: Single and mixed systems
- Blend electrospinning of citronella or thyme oil-loaded polyurethane nanofibers and evaluating their release behaviors
- Efficiency of flexible shielding materials against gamma rays: Silicon rubber with different sizes of Bi2O3 and SnO
- A comprehensive approach for the production of carbon fibre-reinforced polylactic acid filaments with enhanced wear and mechanical behaviour
- Electret melt-blown nonwovens with charge stability for high-performance PM0.3 purification under extreme environmental conditions
- Study on the failure mechanism of suture CFRP T-joints under/after the low-velocity impact loading
- Experimental testing and finite element analysis of polyurethane adhesive joints under Mode I loading and degradation conditions
- Optimizing recycled PET 3D printing using Taguchi method for improved mechanical properties and dimensional precision
- Effect of stacking sequence of the hybrid composite armor on ballistic performance and damage mechanism
- Bending crack propagation and delamination damage behavior of orthogonal ply laminates under positive and negative loads
- Molecular dynamics simulation of thermodynamic properties of Al2O3-modified silicone rubber under silane coupling agent modification
- Precision injection molding method based on V/P switchover point optimization and pressure field balancing
- Heparin and zwitterion functionalized small-diameter vascular grafts for thrombogenesis prevention
- Metal-free N, S-co-doped carbon materials derived from calcined aromatic co-poly(urea-thiourea)s as efficient alkaline oxygen reduction catalysts
- Influence of stitching parameters on the tensile performance and failure mechanisms of CFRP T-joints
- Synthesis of PEGylated polypeptides bearing thioether pendants for injectable ROS-responsive hydrogels
- Rapid Communication
- RAFT-mediated polymerization-induced self-assembly of poly(ionic liquid) block copolymers in a green solvent
- Corrigendum
- Corrigendum to “High-strength polyvinyl alcohol-based hydrogel by vermiculite and lignocellulosic nanofibrils for electronic sensing”
Articles in the same Issue
- Research Articles
- Flow-induced fiber orientation in gas-powered projectile-assisted injection molded parts
- Research on thermal aging characteristics of silicone rubber composite materials for dry-type distribution transformers
- Kinetics of acryloyloxyethyl trimethyl ammonium chloride polymerization in aqueous solutions
- Influence of siloxane content on the material performance and functional properties of polydimethylsiloxane copolymers containing naphthalene moieties
- Enhancement effect of electron beam irradiation on acrylonitrile–butadiene–styrene (ABS) copolymers from waste electrical and electronic equipment by adding 1,3-PBO: A potential way for waste ABS reuse
- Model construction and property study of poly(ether-ether-ketone) by molecular dynamics simulation with meta-modeling methods
- Zinc–gallic acid–polylysine nanocomplexes with enhanced bactericidal activity for the treatment of bacterial keratitis
- Effect of pyrogallol compounds dosage on mechanical properties of epoxy coating
- Preparation of in situ polymerized polypyrrole-modified braided cord and its electrical conductivity investigation under varied mechanical conditions
- Hydrophobicity, UV resistance, and antioxidant properties of carnauba wax-reinforced CG bio-polymer film
- Janus nanofiber membrane films loading with bioactive calcium silicate for the promotion of burn wound healing
- Synthesis of migration-resistant antioxidant and its application in natural rubber composites
- Influence of the flow rate on the die swell for polymer micro coextrusion process
- Fatty acid filled polyaniline nanofibres with dual electrical conductivity and thermo-regulatory characteristics: Futuristic material for thermal energy storage
- Hydrolytic depolymerization of major fibrous wastes
- Performance of epoxy hexagonal boron nitrate underfill materials: Single and mixed systems
- Blend electrospinning of citronella or thyme oil-loaded polyurethane nanofibers and evaluating their release behaviors
- Efficiency of flexible shielding materials against gamma rays: Silicon rubber with different sizes of Bi2O3 and SnO
- A comprehensive approach for the production of carbon fibre-reinforced polylactic acid filaments with enhanced wear and mechanical behaviour
- Electret melt-blown nonwovens with charge stability for high-performance PM0.3 purification under extreme environmental conditions
- Study on the failure mechanism of suture CFRP T-joints under/after the low-velocity impact loading
- Experimental testing and finite element analysis of polyurethane adhesive joints under Mode I loading and degradation conditions
- Optimizing recycled PET 3D printing using Taguchi method for improved mechanical properties and dimensional precision
- Effect of stacking sequence of the hybrid composite armor on ballistic performance and damage mechanism
- Bending crack propagation and delamination damage behavior of orthogonal ply laminates under positive and negative loads
- Molecular dynamics simulation of thermodynamic properties of Al2O3-modified silicone rubber under silane coupling agent modification
- Precision injection molding method based on V/P switchover point optimization and pressure field balancing
- Heparin and zwitterion functionalized small-diameter vascular grafts for thrombogenesis prevention
- Metal-free N, S-co-doped carbon materials derived from calcined aromatic co-poly(urea-thiourea)s as efficient alkaline oxygen reduction catalysts
- Influence of stitching parameters on the tensile performance and failure mechanisms of CFRP T-joints
- Synthesis of PEGylated polypeptides bearing thioether pendants for injectable ROS-responsive hydrogels
- Rapid Communication
- RAFT-mediated polymerization-induced self-assembly of poly(ionic liquid) block copolymers in a green solvent
- Corrigendum
- Corrigendum to “High-strength polyvinyl alcohol-based hydrogel by vermiculite and lignocellulosic nanofibrils for electronic sensing”

