Abstract
Here, we report a novel method for regulating the morphology of block copolymer assemblies through the use of reversible addition–fragmentation chain transfer dispersion polymerization in ethanol. In this method, poly(N,N-dimethylacrylamide) is used as a macromolecular chain transfer agent, and the side chain length of the ionic liquid monomer is modified. A notable change in the morphology of the alkyl-imidazole monomer (1-alkyl-3-(4-vinylbenzyl) imidazolium tetrafluoroborate) was observed when the side chain length was modified from methyl to butyl. This phenomenon can be attributed to a structural change in the alkyl imidazole monomers, which resulted in enhanced solvent-phobic properties. The polymerization kinetics and glass transition temperatures of the block polymers were investigated. These results demonstrated that the polymerization rate increased with increasing number of side chains, whereas the glass transition temperature of the block polymers decreased. It provides a novel approach for the synthesis and utilization of poly(ionic liquid) block polymers with higher-order morphologies.
Graphical abstract
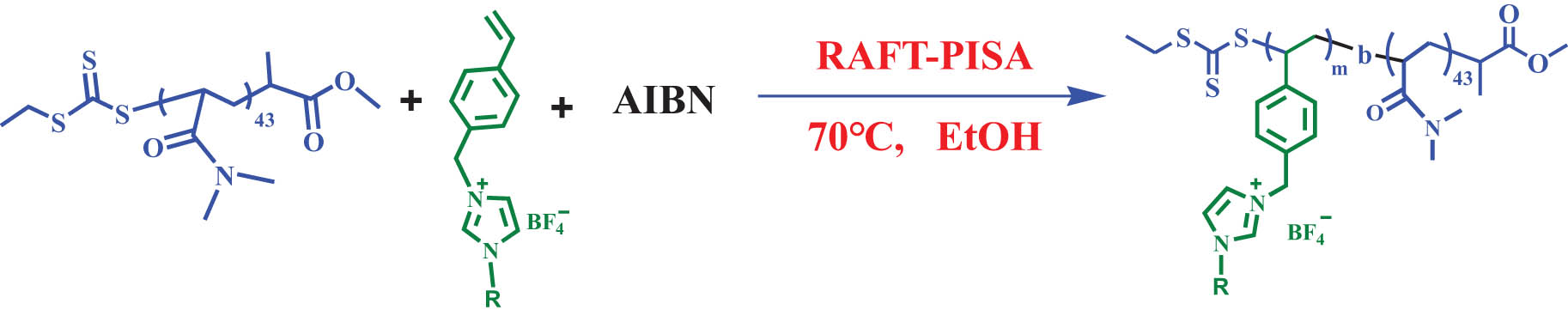
Synthesis of the poly(N,N-dimethylacrylamide)-b-poly(1-alkyl-3-(4-vinylbenzyl)imidazolium tetrafluoroborate) (PDMA43-b-PIL x ) block copolymers prepared by RAFT ethanol dispersion polymerization at 70°C.

1 Introduction
Ionic liquids are unique compounds with high ionic conductivity, good thermal stability, and low vapor pressure (1). They are typically composed of an organic cation (e.g., imidazole, pyridine, ammonium, or phosphonium) and an inorganic anion (e.g., AlCl3, non-AlCl3, or other unique ionic liquids) (2). Due to their unique molecular designability, ionic liquids have important applications in extractions and separations, organic synthesis reactions, nanomaterial preparation, electrochemical reactions, batteries, and capacitors (3,4). Poly(ionic liquids) (PILs) are a subclass of polyelectrolytes consisting of ionic liquid fragments on the side or main chains. In PILs, the advantages of both ionic liquids and polymers are combined and include a wide electrochemical window, high ionic conductivity, and highly tunable structure and polarity (5,6). Thus, PILs have important applications in electrochemical energy storage, smart responsive materials, PIL-based carbon materials, PIL-modified materials, catalysis, adsorption and separation, and other related areas (7,8).
In recent years, the efficient synthesis of PIL nanoparticles with precisely designed and tuned molecular structures has gained increasing attention (9). Among them, block copolymer (BCP) self-assembly is a well-known strategy for the preparation of various nano-objects, allowing the preparation of a wide range of BCP assemblies with specific morphologies, such as spheres, rods, worms, lamellae, vesicles, multilamellar vesicles, spongosomes, and cubosomes (10,11,12,13). Conventional radical polymerization methods generally involve polymerization to obtain the polymer, followed by modification of the solution type and polarity in a dilute solution to obtain the target features, usually at a low solid content (<10% w/v) with the need for tedious steps; thus, an advanced complex morphology is difficult to attain (14). This inefficient preparation method with a complicated procedure and many steps does not meet the demand of industrialized production and was gradually replaced by a more advanced preparation method (15). Controlled/living polymerization processes provide new possibilities for the controlled production of block polymers (16). In addition to controlled/living polymerization methods, reversible addition–fragmentation chain transfer (RAFT)-mediated polymerization-induced self-assembly is an efficient process for preparing PIL block polymers (17,18). Overcoming many of the limitations of traditional solution self-assembly processes, polymerization-induced self-assembly (PISA) is an efficient strategy for synthesizing a wide variety of high solid content (up to 50% w/w)-blocked PIL copolymer nanoparticles suitable for industrial scale-up (19,20).
Despite the significant progress made by PISA in the preparation of various functional block polymers, minimal research has been done on the synthesis of PILs using the PISA technique (21). Here, PILs have been used mainly as stabilizers and core-forming blocks. PIL-based BCP nanoparticles in water were obtained via the RAFT-mediated PISA technique using 1-[2-acryloylethyl]-3-methyl (or benzyl) imidazolium bromide as the ionic liquid monomer, and morphologies ranging from spherical to vesicular were observed (22). The synthesis of PIL block polymers of various morphologies using imidazolium ionic liquids as monomers using the RAFT-PISA method is the earliest example reported to date. Subsequently, thermosensitive PIL block polymer materials were prepared by RAFT dispersion polymerization using poly[1-(4-vinylbenzyl)-3-methylimidazolium tetrafluoroborate] trithiocarbonate in a mixed solvent (methanol/water) (23). Similarly, a rigid straight-rod block-polymerized nanoparticle, PEG-b-P (3-n-dodecyl-1-vinylimidazolium bromide), was obtained via RAFT aqueous dispersion polymerization (24). Cationic sterically stabilized diblock copolymer nanoparticles were synthesized by Armes and co-workers using quaternized poly(2-(dimethylamino)ethyl methacrylate) as a macromolecular chain transfer agent and poly(2-hydroxypropyl methacrylate) as a hydrophobic core-forming block (25). Spherical, vermicular, or vesicular nanoparticles with tunable cationic surface charge could be produced depending on the specific reaction conditions. In analogous reports, the UV sensitivity of oligochitosan-modified macromolecular chain transfer agents was exploited by their utilization as photo-interfering agents to initiate the polymerization of 2-hydroxypropyl methacrylate in acidic buffers, thereby obtaining self-assembled amphiphilic graft copolymers (26). In subsequent reports, An et al. and Yang et al. proposed changing the type of counterion by the RAFT-PISA method to obtain PIL block polymers with an advanced morphology from a spherical morphology to a vesicular morphology (27,28). However, studies on whether a change in the side chain length of the imidazole cation affects the morphological transformation of PIL block polymer assemblies have not been reported.
The aim of this study was to elucidate the comprehensive morphological transformation of BCPs comprising PIL cores formulated with ionic liquid monomers, specifically 1-methyl-3-(4-vinylbenzyl) imidazolium tetrafluoroborate ([MVBIm][BF4]) and 1-butyl-3-(4-vinylbenzyl) imidazolium tetrafluoroborate ([BVBIm][BF4]), across a range of varying chain lengths (see Scheme 1 for details). This was achieved through RAFT-mediated dispersion polymerization in ethanol at 70°C, and a systematic alteration of the average degree of polymerization (DP) of the PIL nucleated block was attained.
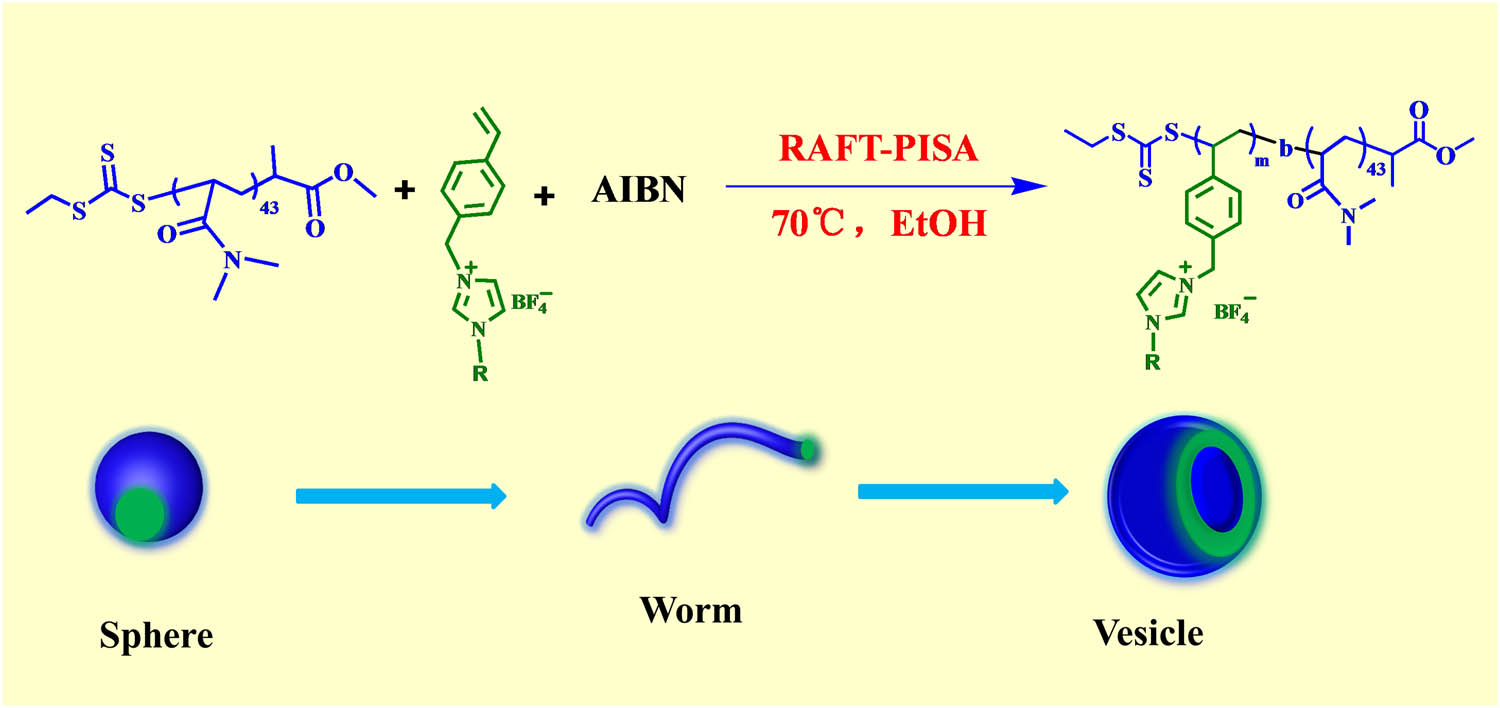
Schematic representation of the synthesis of the poly(N,N-dimethylacrylamide)-b-poly(1-alkyl-3-(4-vinylbenzyl)imidazolium tetrafluoroborate) (PDMA43-b-PIL x ) BCPs prepared by RAFT ethanol dispersion polymerization at 70°C.
2 Materials and methods
2.1 Materials
1-Methylimidazole (Adamas-beta, 98%+), 1-butylimidazole (Adamas-beta, 98%+), ethanol (EtOH, Adamas-beta, 99.5%), 4-vinylbenzyl chloride (Adamas-beta, 98%), and N,N-dimethylacrylamide (DMA, Adamas-beta, 98%+) were purchased from Shanghai Titan Co., Ltd. N,N-dimethylformamide (DMF, 99.9%), 2,2′-azobis(2-methylpropionitrile) (AIBN, 99%, recrystallized from methanol), tri-potassium phosphate trihydrate, and sodium fluoroborate were provided by Aladdin Chemical Reagent Co., Ltd. Diethyl ether anhydrous (99.5%) was obtained by Yantai Yuandong Fine Chemicals Co., Ltd. Before being used, the monomers were passed through an Al2O3 column for inhibitor removal.
2.2 Synthesis of macromolecular chain transfer agent poly(N,N-dimethylacrylamide) (PDMA43)
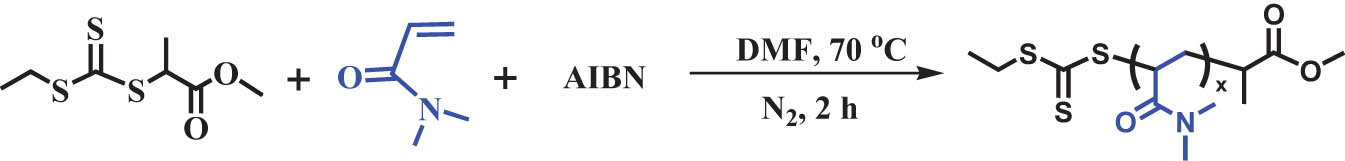
DMA (48.1198 g, 485.4 mmol), 2-ethylsulfanylthiocarbonylsulfanylpropionic acid methyl ester (1.9757 g, 8.79 mmol), AIBN (0.029 g, 0.1764 mmol), and 100 mL DMF were added to a 250 mL glass vial. After stirring for 10 min, 120 μL of the solution was removed and used as the reference sample (0 h) for proton nuclear magnetic resonance (1H NMR) spectroscopy. After flushing with nitrogen, the solution was immersed in a preheated oil bath at 70°C for 30 min. After a polymerization time of 2 h under nitrogen, the conversion of the monomer was determined to be 77% using the 1H NMR results. A yellow polymeric solid was obtained after three precipitations in excess cold diethyl ether and drying at 50°C under vacuum.
2.3 Synthesis of PDMA43-PIL x BCP nano-objects via RAFT dispersion polymerization
A systematic study of PDMA43-b-P([MVBIm][BF4]) x and PDMA43-b-P([BVBIm][BF4]) x nano-objects was performed via RAFT dispersion polymerization in ethanol at 20% w/v solids to modify the target DP of the PIL blocks ranging from 60 to 200 (Tables S1 and S2). As a representative procedure, the synthesis of PDMA43-b-P([MVBIm][BF4])100 is provided. PDMA43 (0.0407 g, 9.07 μmol) and 1-methyl-3-(4-vinylbenzyl) imidazolium tetrafluoroborate ([MVBIm][BF4], 0.2597 g, 907 μmol) were added to a 10 mL glass vial. Two milliliters of ethanol was added to dissolve all reagents. A homogeneous solution was obtained. After the reaction mixture was flushed with N2 in an ice/water bath for 0.5 h, the vial was placed in an oil bath at 70°C with stirring at 600 rpm. Next, 0.738 mg of AIBN (4.535 μmol) was dissolved in 100 μL of ethanol and added via a syringe. The composition of the BCPs and the monomer conversion were determined using 1H NMR spectroscopic techniques in DMSO-d6 after a polymerization time of 24 h under N2. Dynamic light scattering (DLS) and transmission electron microscopy (TEM) techniques were used to analyze the size and morphology of the samples.
3 Results and discussion
3.1 Synthesis of PDMA43-b-PIL x
The RAFT agent methyl-2-[ethylthio(thiocarbonyl)thio] acrylate (METCA) was prepared by the substitution reaction of ethanethiol, carbon disulfide, and methyl 2-bromopropionate in acetone solvent (for detailed preparation, refer to the Supplementary materials). The 1H NMR and 13C NMR spectra of the purified product are shown in Figure 1(a) and (b). The purity was greater than 97%; thus, the experimental requirements were met. The low dispersibility (Đ = 1.02) molecular chain transfer agent (macro-CTA) poly(N,N-dimethylacrylamide) (PDMA43) was subsequently prepared at 50% w/v solids using METCA as the chain transfer agent and DMF as the solvent for the RAFT solution polymerization. Figure 1(c) and (d) shows the 1H NMR spectrum and gel permeation chromatography (GPC) assay curves of the purified macro-CTA. The molar mass of PDMA43 was determined using triple-detection GPC, with a 2414 refractive index detector (Waters Alliance) and a Viscotek TDA 305-020 LALS/RALS detector (Malvern Instruments), and was similar to the molar mass calculated from the 1H NMR integral area, demonstrating the well-defined control of the polymer. The ionic monomers [MVBIm][BF4] and [BVBIm][BF4] were prepared from commercial 1-methyl (or butyl) imidazole and 4-vinylbenzyl chloride; this resulted in 1-alkyl-3-(4-vinylbenzyl) imidazolium chloride, which is an anion that was exchanged with NaBF4 in water. Monomer purity was monitored by 1H, 13C, and 19F NMR spectroscopy (Figures S1 and S2). After all pure macro-CTA and ionic monomers were obtained, a study was conducted on the preparation of the BCPs by RAFT-mediated polymerization self-assembly (RAFT-PISA) of 1-alkyl-3-(4-vinylbenzyl) imidazolium tetrafluoroborate in ethanol.
![Figure 1
1H and 13C NMR spectra of methyl-2-[ethylthio(thiocarbonyl)thio] acrylate (a and b) and 1H NMR spectrum and GPC trace of PDMA43 macro-CTA (c and d).](/document/doi/10.1515/epoly-2024-0077/asset/graphic/j_epoly-2024-0077_fig_001.jpg)
1H and 13C NMR spectra of methyl-2-[ethylthio(thiocarbonyl)thio] acrylate (a and b) and 1H NMR spectrum and GPC trace of PDMA43 macro-CTA (c and d).
3.2 Kinetic study for the RAFT dispersion polymerization of PDMA43-b-PIL x
The kinetic studies of PDMA43-b-PIL x were performed as follows: DMF was added to ethanol as an internal reference solvent. Aliquots (100 μL) were collected at fixed intervals (0.5, 1, and 2 h) and dissolved in 500 μL of deuterium-substituted DMSO. The solution was then analyzed using 1H NMR spectroscopy. The peak area of the vinyl monomer at the time of sampling was compared with the peak area of the vinyl monomer at 0 h to calculate the monomer conversion rate. The curve of the kinetic change is shown in Figure 2.
![Figure 2
Plots of the monomer conversion (red squares) of PDMA43-b-PILs and pseudo-first-order kinetics (black diamonds) vs polymerization time. (a) and (b) represent PDMA43-b-P([MVBIm][BF4])
x
and PDMA43-b-P([BVBIm][BF4])
x
, respectively.](/document/doi/10.1515/epoly-2024-0077/asset/graphic/j_epoly-2024-0077_fig_002.jpg)
Plots of the monomer conversion (red squares) of PDMA43-b-PILs and pseudo-first-order kinetics (black diamonds) vs polymerization time. (a) and (b) represent PDMA43-b-P([MVBIm][BF4]) x and PDMA43-b-P([BVBIm][BF4]) x , respectively.
Linear pseudo-first-order kinetics were observed for PDMA43-b-P([BVBIm][BF4]) x ; these results were similar to the kinetic curve of PDMA43-b-P([MVBIm][BF4]) x . However, the difference was the following: its monomer conversion in RAFT dispersion polymerization was significantly accelerated when the side chain was changed from methyl to n-butyl. PDMA43-b-P([BVBIm][BF4]) x showed a monomer conversion of more than 86% (Figure 4(a)) after 10 h, whereas PDMA43-b-P([MVBIm][BF4]) x showed a monomer conversion of only 83% (Figure 4(b)) after 11 h. These results were similar to the trend reported by Penfold et al. (23), in which a longer chain length of the solvent-phobic blocks correlated with a faster reaction rate of the RAFT dispersion polymerization. These results also confirmed the susceptibility to high-order morphology and were in agreement with the phenomenon that was reported in the literature (28). Interestingly, the PIL self-assembly kinetics linearly varied; these results contradicted those reported in the literature (29,30). This potentially occurred because the monomer is an ionic liquid, and in the RAFT-PISA dispersion polymerization process, the monomer has a high plasticity to the polymer; thus, the reaction rate is significantly accelerated, and similar phenomena have been reported in the literature (27).
3.3 TEM micrograph of PDMA43-b-PIL x
This dispersion polymerization formulation was thoroughly studied by systematically varying the DP of the PDMA43-b-P([MVBIm][BF4]) x blocks containing PDMA43 as macro-CTA and stabilizing blocks, with [AIBN]/[PDMA] = 0.5 and 20% w/v solids. Within 24 h, based on the 1H NMR spectroscopy results, in the majority of cases, a high level of monomer conversion (>95%) was observed (Table S1). A representative TEM micrograph is shown in Figure 3. As shown in Figure 3(a), uniform 37 nm BCP spheres were produced at a DP of 57, and the spheres coalesced into wormlike assemblies with similar diameters and a polydispersity of up to 100 nm in length. The worms then fused to form multiple “jellyfish” intermediates called lamellae (Figure 3(c)). These lamellae were subsequently encapsulated, eventually forming polydisperse small to large vesicles (Figure 3(d)–(f)) with an average vesicle wall thickness of 10 nm and an average hydrodynamic diameter of 245 nm (for the statistical particle size data, refer to Supplementary materials, Figures S3–S10). The morphological transition sequence and morphologies of the intermediates of the PIL BCPs observed in the present work were in agreement with the results reported by Yang et al. (27).
![Figure 3
TEM micrographs of the PDMA43-b-P([MVBIm][BF4])
x
BCPs at 20% w/v solids in ethanol. (a) x = 57, (b) x = 78, (c) x = 98, (d) x = 114, (e) x = 145, and (f) x = 192.](/document/doi/10.1515/epoly-2024-0077/asset/graphic/j_epoly-2024-0077_fig_003.jpg)
TEM micrographs of the PDMA43-b-P([MVBIm][BF4]) x BCPs at 20% w/v solids in ethanol. (a) x = 57, (b) x = 78, (c) x = 98, (d) x = 114, (e) x = 145, and (f) x = 192.
Next, the morphological transition features from another RAFT dispersion polymerization formulation were examined in detail by varying the monomer side chain length to systematically change the DP of the PDMA43-b-P([BVBIm][BF4]) x block at 20% w/v solid content under the same conditions (Figure 4(a)–(f) and Table S2). Notably, PDMA43-b-P([BVBIm][BF4]) x and PDMA43-b-P([MVBIm][BF4]) x underwent similar transformations, and their morphologies were pure wormlike and lamellar transition state-jellyfish-like as the side chain length of the monomers increased (Figure 4(b) and (c)). The average particle size of the spherical aggregates was 32 nm (Figure S11), the average worm length was 180 nm (Figures S12 and S13), and a distinct transition state morphology of vesicles and lamellae was also observed at an average particle size of 622 nm (Figure 4(c) and (d) and Figures S14–S16). However, this was not observed for the PDMA43-b-P([MVBIm][BF4]) x block polymer assemblies. Moreover, the PDMA43-b-P([BVBIm][BF4]) x vesicles were slightly thicker walled (∼77 nm) (Figures S17–S19), and a composite vesicle morphology was observed (Figure 4(f)). A comparison of the DPs of PDMA43-b-P([BVBIm][BF4]) x and PDMA43-b-P([MVBIm][BF4]) x at different morphologies revealed that as the side chain length of the ionic liquid monomer increased, high-order morphologies were obtained at lower DPs. These results were likely attributed to the enhanced ability of the PIL-based block solvent-sparing capacity, which led to an increase in the assembly stacking parameter, thus facilitating high-order topographic transition (31).
![Figure 4
TEM micrographs of the PDMA43-b-P([BVBIm][BF4])
x
BCPs at 20% w/v solids in ethanol at 70°C. (a) x = 48, (b) x = 69, (c) x = 78, (d) x = 88, (e) x = 115, and (f) x = 132.](/document/doi/10.1515/epoly-2024-0077/asset/graphic/j_epoly-2024-0077_fig_004.jpg)
TEM micrographs of the PDMA43-b-P([BVBIm][BF4]) x BCPs at 20% w/v solids in ethanol at 70°C. (a) x = 48, (b) x = 69, (c) x = 78, (d) x = 88, (e) x = 115, and (f) x = 132.
3.4 Differential scanning calorimetry (DSC) test of PDMA43-b-PIL x
DSC was performed to characterize the glass transition temperature (T g) of the above two PIL block polymers (PDMA43-b-P([MVBIm][BF4])114 and PDMA43-b-P([BVBIm][BF4])115) to determine the effect of side chain growth on the PIL morphological transformation (Figure 5). Initially, ethanol dispersions of the BCP nanoparticles were selected for testing using DSC; however, no meaningful data were obtained due to the evaporation of ethanol during the measurement process. Therefore, after dialysis purification, drying, and accurate weighing, the solid powder was finally selected for DSC testing. Based on the morphology transformation tendency of the PIL-based BCPs with ethanol and combined with the literature (27), the PIL BCPs underwent a certain degree of solvation, with a T g lower than the polymerization temperature (70°C). As shown in Figure 5, the glass transition temperature of PDMA43-b-P([MVBIm][BF4])114 (T g = 120°C) was much higher than that of PDMA43-b-P([BVBIm][BF4])115 (T g = 95°C). This result strongly indicates that the glass transition temperature of PIL block polymers could be significantly lowered by side chain growth; this lower T g could significantly promote the formation of higher-order morphologies (32).
![Figure 5
Second heating cycle DSC traces of PDMA43-b-P([MVBIm][BF4])114 and PDMA43-b-P([BVBIm][BF4])115.](/document/doi/10.1515/epoly-2024-0077/asset/graphic/j_epoly-2024-0077_fig_005.jpg)
Second heating cycle DSC traces of PDMA43-b-P([MVBIm][BF4])114 and PDMA43-b-P([BVBIm][BF4])115.
4 Conclusions
In summary, a variety of morphologies, including spheres, worms, jellyfish lamellae, lamellae, vesicles, and composite vesicles, were prepared using two monomers with different chain lengths, [MVBIm][BF4] and [BVBIm][BF4], for the RAFT dispersion polymerization reaction in ethanol at 70°C and 20% w/v solids. DLS and TEM were employed for morphological analysis of the two PIL BCPs with different chain lengths. Kinetics were applied to compare the monomer conversion rates of the two PILs. The T g values of the two types of PIL were determined from the DSC results; here, with increasing chain length, the solvent-phobicity and stacking parameters of the PILs increased. Thus, higher-order morphology formation was favored. This study provides useful aid in the preparation of high-order morphologies for functional materials in the PIL phase.
Acknowledgement
The financial support of the National Natural Science Foundation of China (grant number 52301100), Natural Science Foundation of Shandong Province (ZR2020QB084), and Foundation of Weifang University of Science and Technology (KJRC2019009) was greatly appreciated. We thank Professor Zesheng An of Jilin University for his guidance and advice on experimental design. All individuals included in this section have consented to the acknowledgment.
-
Funding information: This research was funded by the financial support of the National Natural Science Foundation of China (grant number 52301100), Natural Science Foundation of Shandong Province (grant number ZR2020QB084), and Foundation of Weifang University of Science and Technology (grant number KJRC2019009).
-
Author contributions: Yongqi Yang, Zekai Ren, and Xiawei Li: designed – this research; Youjun Yan, Enhao Zhao, and Hongyan Gao: conducted – this research; Yongqi Yang, Lijuan Feng, and Xin Luo: analyzed – draft data; Yongqi Yang and Hongyan Gao: wrote – original draft; Xin Luo: modified – the paper; Yongqi Yang, Xiawei Li, and Lijuan Feng: edited – the whole manuscript.
-
Conflict of interest: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.
-
Data availability statement: Data are contained within the article.
References
(1) Kondrat S, Feng G, Bresme F, Urbakh M, Kornyshev AA. Theory and simulations of ionic liquids in nanoconfinement. Chem Rev. 2023;123(10):6668–715. 10.1021/acs.chemrev.2c00728.Search in Google Scholar PubMed PubMed Central
(2) Li X, Chen K, Guo R. Ionic liquids functionalized MOFs for adsorption. Chem Rev. 2023;123(16):10432–67. 10.1021/acs.chemrev.3c00248.Search in Google Scholar PubMed
(3) Wei L, Wang L, Cui Z, Liu Y, Du A. Multifunctional applications of ionic liquids in polymer materials: A brief review. Molecules. 2023;28(9):3836. 10.3390/molecules28093836.Search in Google Scholar PubMed PubMed Central
(4) Lebedeva O, Kultin D, Kustov L. Advanced research and prospects on polymer ionic liquids: trends, potential and application. Green Chem. 2023;25(22):9001–19. 10.1039/D3GC02131A.Search in Google Scholar
(5) Al-Sodies S, Asiri AM, Khan A, Alamry KA, Hussein MA. Recent exploiting of poly (ionic liquid) s in sensing applications. Eur Polym J. 2023;205(7):112719. 10.1016/j.eurpolymj.2023.112719.Search in Google Scholar
(6) Tang Y, Zhang Y, Chen X, Xie X, Zhou N, Dai Z, et al. Up/down tuning of poly (ionic liquid) s in aqueous two‐phase systems. Angew Chem Int Ed. 2023;135(4):e202215722. 10.1002/ange.202215722.Search in Google Scholar
(7) Eddine AM, Nosov DR, Lepre LF, Serghei A, Schmidt DF, Montarnal D, et al. Dynamic ion gels from the complex coacervation of oppositely charged poly (ionic liquid) s. ACS Macro Lett. 2024;13(8):921–7. 10.1021/acsmacrolett.4c00253.Search in Google Scholar PubMed PubMed Central
(8) Ma X, Yu J, Hu Y, Texter J, Yan F. Ionic liquid/poly (ionic liquid)-based electrolytes for lithium batteries. Ind Chem Mater. 2023;1(1):39–59. 10.1039/D2IM00051B.Search in Google Scholar
(9) Hamadani CM, Dasanayake GS, Gorniak ME, Pride MC, Monroe W, Chism CM, et al. Development of ionic liquid-coated PLGA nanoparticles for applications in intravenous drug delivery. Nat Protoc. 2023;18(8):2509–57. 10.6084/m9.figshare.c.6279060.v2.Search in Google Scholar
(10) Derry MJ, Fielding LA, Armes SP. Polymerization-induced self-assembly of block copolymer nanoparticles via RAFT non-aqueous dispersion polymerization. Prog Polym Sci. 2016;52:1–18. 10.1016/j.progpolymsci.2015.10.002.Search in Google Scholar
(11) He H, Rahimi K, Zhong M, Mourran A, Luebke DR, Nulwala HB, et al. Cubosomes from hierarchical self-assembly of poly (ionic liquid) block copolymers. Nat Commun. 2017;8(1):14057. 10.1038/ncomms14057.Search in Google Scholar PubMed PubMed Central
(12) Demarteau J, de Añastro AF, Shaplov AS, Mecerreyes D. Poly (diallyldimethylammonium) based poly (ionic liquid) di-and triblock copolymers by PISA as matrices for ionogel membranes. Polym Chem. 2020;11(8):1481–8. 10.1039/C9PY01552C.Search in Google Scholar
(13) Tsoutsoura A, He Z, Alexandridis P. Effects of ionic liquids on the cylindrical self-assemblies formed by poly (ethylene oxide)-poly (propylene oxide)-poly (ethylene oxide) block copolymers in water. Polymers. 2024;16(3):349. 10.3390/polym16030349.Search in Google Scholar PubMed PubMed Central
(14) Corrigan N, Jung K, Moad G, Hawker CJ, Matyjaszewski K, Boyer C. Reversible-deactivation radical polymerization (Controlled/living radical polymerization): From discovery to materials design and applications. Prog Polym Sci. 2020;111:101311. 10.1016/j.progpolymsci.2020.101311.Search in Google Scholar
(15) Aydogan C, Yilmaz G, Shegiwal A, Haddleton DM, Yagci Y. Photoinduced controlled/living polymerizations. Angew Chem Int Ed. 2022;134(23):e202117377. 10.1002/ange.202117377.Search in Google Scholar
(16) Li X, Tang SY, Zhang Y, Zhu J, Forgham H, Zhao C, et al. Tailored fluorosurfactants through controlled/living radical polymerization for highly stable microfluidic droplet generation. Angew Chem Int Ed. 2024;63(3):e202315552. 10.1002/anie.202315552.Search in Google Scholar PubMed PubMed Central
(17) Hou W, Wu J, Li Z, Zhang Z, Shi Y, Chen Y. Efficient synthesis and PISA behavior of molecular bottlebrush block copolymers via a grafting-from strategy through RAFT dispersion polymerization. Macromolecules. 2023;56(3):824–32. 10.1021/acs.macromol.2c02233.Search in Google Scholar
(18) Zhang S, Li R, An Z. Degradable block copolymer nanoparticles synthesized by polymerization‐induced self‐assembly. Angew Chem Int Ed. 2024;136(12):e202315849. 10.1002/ange.202315849.Search in Google Scholar
(19) Galanopoulo P, Gil N, Gigmes D, Lefay C, Guillaneuf Y, Lages M, et al. RAFT‐mediated emulsion polymerization‐induced self‐assembly for the synthesis of core‐degradable waterborne particles. Angew Chem Int Ed. 2023;62(16):e202302093. 10.1002/anie.202302093.Search in Google Scholar PubMed
(20) Liu C, Hong CY, Pan CY. Polymerization techniques in polymerization-induced self-assembly (PISA). Polym Chem. 2020;11(22):3673–89. 10.1039/D0PY00455C.Search in Google Scholar
(21) Depoorter J, Yan X, Zhang B, Sudre G, Charlot A, Fleury E, et al. All poly (ionic liquid) block copolymer nanoparticles from antagonistic isomeric macromolecular blocks via aqueous RAFT polymerization-induced self-assembly. Polym Chem. 2021;12(1):82–91. 10.1039/D0PY00698J.Search in Google Scholar
(22) Luo G, Guo Y, Liu C, Han G, Ma X, Zhang W. What will happen when thermoresponsive poly (N-isopropylacrylamide) is tethered on poly (ionic liquid)s? RSC Adv. 2019;9(23):12936–43. 10.1039/C9RA01849B.Search in Google Scholar
(23) Penfold NJW, Yeow J, Boyer C, Armes SP. Emerging trends in polymerization-induced self-assembly. ACS Macro Lett. 2019;8(8):1029–54. 10.1021/acsmacrolett.9b00464.Search in Google Scholar PubMed
(24) Zhang Q, Fu M, Wang C, Wang J, Zhu S. Preparation of poly (ionic liquid) nanoparticles through RAFT/MADIX polymerization-induced self-assembly. Polym Chem. 2017;8(36):5469–73. 10.1039/C7PY01273J.Search in Google Scholar
(25) Semsarilar M, Ladmiral V, Blanazs A, Armes SP. Cationic polyelectrolyte-stabilized nanoparticles via RAFT aqueous dispersion polymerization. Langmuir. 2013;29(24):7416–24. 10.1021/la304279y.Search in Google Scholar PubMed
(26) Oumerri J, Qayouh H, Arteni AA, Six J-L, Lahcini M, Ferji K. One‐pot formulation of cationic oligochitosan coated nanoparticles via photo‐polymerization induced self‐assembly. ChemPhysChem. 2024;25(14):e202400291. 10.1002/cphc.202400291.Search in Google Scholar PubMed
(27) Yang Y, Zheng J, Man S, Sun X, An Z. Synthesis of poly (ionic liquid)-based nano-objects with morphological transitions via RAFT polymerization-induced self-assembly in ethanol. Polym Chem. 2018;9(7):824–7. 10.1039/C8PY00040A.Search in Google Scholar
(28) Yang Y, Li X, Yan Y, Pan R, Liu J, Lian M, et al. RAFT polymerization-induced self-assembly of poly (ionic liquids) in ethanol. e-Polymers. 2022;22(1):803–8. 10.1515/epoly-2022-0069.Search in Google Scholar
(29) Ikkene D, Six JL, Ferji K. Progress in aqueous dispersion RAFT PISA. Eur Polym J. 2023;188:111848. 10.1016/j.eurpolymj.2023.111848.Search in Google Scholar
(30) Ikkene D, Arteni AA, Boulogne C, Six JL, Ferji K. Multicompartment vesicles: A key intermediate structure in polymerization-induced self-assembly of graft copolymers. Macromolecules. 2022;55(11):4268–75. 10.1021/acs.macromol.2c00561.Search in Google Scholar
(31) Warren NJ, Armes SP. Polymerization-induced self-assembly of block copolymer nano-objects via RAFT aqueous dispersion polymerization. J Am Chem Soc. 2014;136(29):10174–85. 10.1021/ja502843f.Search in Google Scholar PubMed PubMed Central
(32) György C, Armes SP. Recent advances in polymerization‐induced self‐assembly (PISA) syntheses in non‐polar medi. Angew Chem Int Ed. 2023;62(42):e202308372. 10.1002/anie.202308372.Search in Google Scholar PubMed PubMed Central
© 2025 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Flow-induced fiber orientation in gas-powered projectile-assisted injection molded parts
- Research on thermal aging characteristics of silicone rubber composite materials for dry-type distribution transformers
- Kinetics of acryloyloxyethyl trimethyl ammonium chloride polymerization in aqueous solutions
- Influence of siloxane content on the material performance and functional properties of polydimethylsiloxane copolymers containing naphthalene moieties
- Enhancement effect of electron beam irradiation on acrylonitrile–butadiene–styrene (ABS) copolymers from waste electrical and electronic equipment by adding 1,3-PBO: A potential way for waste ABS reuse
- Model construction and property study of poly(ether-ether-ketone) by molecular dynamics simulation with meta-modeling methods
- Zinc–gallic acid–polylysine nanocomplexes with enhanced bactericidal activity for the treatment of bacterial keratitis
- Effect of pyrogallol compounds dosage on mechanical properties of epoxy coating
- Preparation of in situ polymerized polypyrrole-modified braided cord and its electrical conductivity investigation under varied mechanical conditions
- Hydrophobicity, UV resistance, and antioxidant properties of carnauba wax-reinforced CG bio-polymer film
- Janus nanofiber membrane films loading with bioactive calcium silicate for the promotion of burn wound healing
- Synthesis of migration-resistant antioxidant and its application in natural rubber composites
- Influence of the flow rate on the die swell for polymer micro coextrusion process
- Fatty acid filled polyaniline nanofibres with dual electrical conductivity and thermo-regulatory characteristics: Futuristic material for thermal energy storage
- Hydrolytic depolymerization of major fibrous wastes
- Performance of epoxy hexagonal boron nitrate underfill materials: Single and mixed systems
- Blend electrospinning of citronella or thyme oil-loaded polyurethane nanofibers and evaluating their release behaviors
- Efficiency of flexible shielding materials against gamma rays: Silicon rubber with different sizes of Bi2O3 and SnO
- A comprehensive approach for the production of carbon fibre-reinforced polylactic acid filaments with enhanced wear and mechanical behaviour
- Electret melt-blown nonwovens with charge stability for high-performance PM0.3 purification under extreme environmental conditions
- Study on the failure mechanism of suture CFRP T-joints under/after the low-velocity impact loading
- Experimental testing and finite element analysis of polyurethane adhesive joints under Mode I loading and degradation conditions
- Optimizing recycled PET 3D printing using Taguchi method for improved mechanical properties and dimensional precision
- Effect of stacking sequence of the hybrid composite armor on ballistic performance and damage mechanism
- Bending crack propagation and delamination damage behavior of orthogonal ply laminates under positive and negative loads
- Molecular dynamics simulation of thermodynamic properties of Al2O3-modified silicone rubber under silane coupling agent modification
- Precision injection molding method based on V/P switchover point optimization and pressure field balancing
- Heparin and zwitterion functionalized small-diameter vascular grafts for thrombogenesis prevention
- Metal-free N, S-co-doped carbon materials derived from calcined aromatic co-poly(urea-thiourea)s as efficient alkaline oxygen reduction catalysts
- Influence of stitching parameters on the tensile performance and failure mechanisms of CFRP T-joints
- Synthesis of PEGylated polypeptides bearing thioether pendants for injectable ROS-responsive hydrogels
- Rapid Communication
- RAFT-mediated polymerization-induced self-assembly of poly(ionic liquid) block copolymers in a green solvent
- Corrigendum
- Corrigendum to “High-strength polyvinyl alcohol-based hydrogel by vermiculite and lignocellulosic nanofibrils for electronic sensing”
Articles in the same Issue
- Research Articles
- Flow-induced fiber orientation in gas-powered projectile-assisted injection molded parts
- Research on thermal aging characteristics of silicone rubber composite materials for dry-type distribution transformers
- Kinetics of acryloyloxyethyl trimethyl ammonium chloride polymerization in aqueous solutions
- Influence of siloxane content on the material performance and functional properties of polydimethylsiloxane copolymers containing naphthalene moieties
- Enhancement effect of electron beam irradiation on acrylonitrile–butadiene–styrene (ABS) copolymers from waste electrical and electronic equipment by adding 1,3-PBO: A potential way for waste ABS reuse
- Model construction and property study of poly(ether-ether-ketone) by molecular dynamics simulation with meta-modeling methods
- Zinc–gallic acid–polylysine nanocomplexes with enhanced bactericidal activity for the treatment of bacterial keratitis
- Effect of pyrogallol compounds dosage on mechanical properties of epoxy coating
- Preparation of in situ polymerized polypyrrole-modified braided cord and its electrical conductivity investigation under varied mechanical conditions
- Hydrophobicity, UV resistance, and antioxidant properties of carnauba wax-reinforced CG bio-polymer film
- Janus nanofiber membrane films loading with bioactive calcium silicate for the promotion of burn wound healing
- Synthesis of migration-resistant antioxidant and its application in natural rubber composites
- Influence of the flow rate on the die swell for polymer micro coextrusion process
- Fatty acid filled polyaniline nanofibres with dual electrical conductivity and thermo-regulatory characteristics: Futuristic material for thermal energy storage
- Hydrolytic depolymerization of major fibrous wastes
- Performance of epoxy hexagonal boron nitrate underfill materials: Single and mixed systems
- Blend electrospinning of citronella or thyme oil-loaded polyurethane nanofibers and evaluating their release behaviors
- Efficiency of flexible shielding materials against gamma rays: Silicon rubber with different sizes of Bi2O3 and SnO
- A comprehensive approach for the production of carbon fibre-reinforced polylactic acid filaments with enhanced wear and mechanical behaviour
- Electret melt-blown nonwovens with charge stability for high-performance PM0.3 purification under extreme environmental conditions
- Study on the failure mechanism of suture CFRP T-joints under/after the low-velocity impact loading
- Experimental testing and finite element analysis of polyurethane adhesive joints under Mode I loading and degradation conditions
- Optimizing recycled PET 3D printing using Taguchi method for improved mechanical properties and dimensional precision
- Effect of stacking sequence of the hybrid composite armor on ballistic performance and damage mechanism
- Bending crack propagation and delamination damage behavior of orthogonal ply laminates under positive and negative loads
- Molecular dynamics simulation of thermodynamic properties of Al2O3-modified silicone rubber under silane coupling agent modification
- Precision injection molding method based on V/P switchover point optimization and pressure field balancing
- Heparin and zwitterion functionalized small-diameter vascular grafts for thrombogenesis prevention
- Metal-free N, S-co-doped carbon materials derived from calcined aromatic co-poly(urea-thiourea)s as efficient alkaline oxygen reduction catalysts
- Influence of stitching parameters on the tensile performance and failure mechanisms of CFRP T-joints
- Synthesis of PEGylated polypeptides bearing thioether pendants for injectable ROS-responsive hydrogels
- Rapid Communication
- RAFT-mediated polymerization-induced self-assembly of poly(ionic liquid) block copolymers in a green solvent
- Corrigendum
- Corrigendum to “High-strength polyvinyl alcohol-based hydrogel by vermiculite and lignocellulosic nanofibrils for electronic sensing”

