Abstract
C21H22FN3O, monoclinic, P21/c (no. 14), a = 17.2795(3) Å, b = 6.1659(1) Å, c = 16.7198(3) Å, β = 90.596(1)°, V = 1781.29(5) Å3, Z = 4, Rgt(F) = 0.0408, wRref(F2) = 0.1122, T = 293 K.
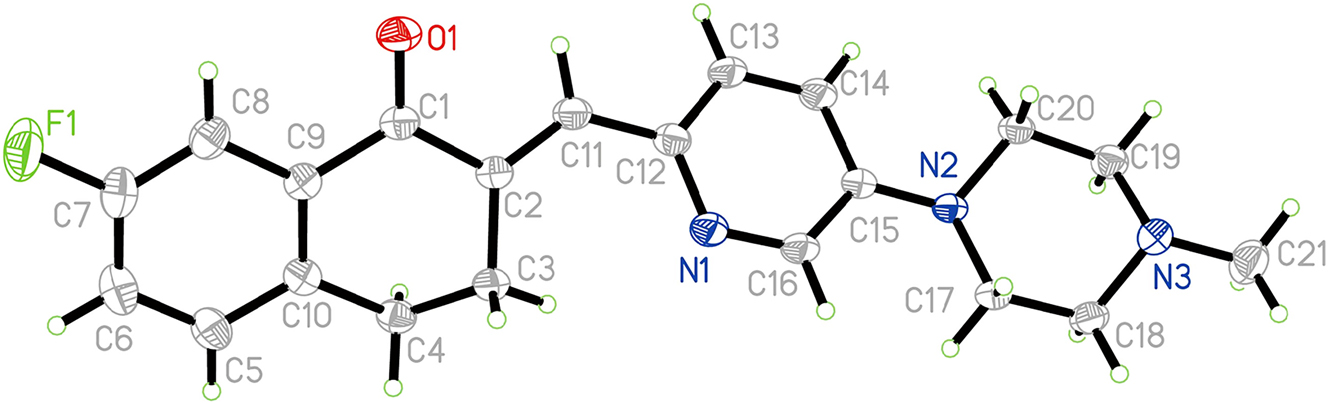
The crystal structure is shown in the figure. Displacement ellipsoids are drawn at the 30 % probability level.
Table 1 contains the crystallographic data and the list of the atoms including atomic coordinates and displacement parameters can be found in the cif-file attached to this article.
Data collection and handling.
| Crystal: | Clear light yellow block |
| Size: | 0.14 × 0.13 × 0.10 mm |
| Wavelength: | CuKα radiation (1.54178 Å) |
| μ: | 0.72 mm−1 |
| Diffractometer, scan mode: | Rigaku Synergy, ω scan |
| θmax, completeness: | 74.9°, 99 % |
| N(hkl)measured, N(hkl)unique, Rint: | 11071, 3489, 0.016 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2σ(Iobs), 3,297 |
| N(param)refined: | 236 |
| Programs: | Rigaku 1 , SHELX 2 , 3 |
1 Source of material
According to the synthesis method described in references, 4 , 5 , 6 N-methylpiperazine (16.05 g, 0.16 mol) and potassium carbonate (27.64 g, 0.20 mol) were weighed and added to a 250 mL round bottom reaction flask. N,N-dimethylformamide (15.0 mL) was used as the reaction solvent. The mixture was stirred at 353 K for about 4 h. 5-Fluorolinaldehyde (2.54 g, 0.02 mol) was added after 4 h and refluxed at 373 K to continue the reaction for about 5 h. The reaction progress is detected by Thin-Layer Chromatography (TLC, dichloromethane: methyl alcohol = 20:1, v:v). After the reaction system droped to room temperature, the precipitate was filtered and washed with dichloromethane to give a light yellow solid (22.0 mL). Spin evaporate the solvent and using dichloromethane: methyl alcohol (50:1, v:v) as the eluent to obtain the intermediate. 7-Fluoro-3,4-dihydronaphthalen-1(2H)-one (1.64 g, 0.01 mol) and intermediate (2.1 g, 0.01 mol) were reacted at 293 K for 7 h using methanol (25.0 mL) as the solvent and 25 % sodium hydroxide (20.0 mL) as the catalyst. After completion of the reaction, the precipitate was filtered and washed with 50 % methanol. 7 Finally, recrystallization gave yellow crystals of the title compound from dichloromethane and methanol solutions (1:1, v:v).
2 Experimental details
The H atoms were placed in idealized positions and treated as riding on their parent atoms, with d(C–H) = 0.96 Å (methyl), Uiso(H) = 1.5Ueq(C), and d(C–H) = 0.97 Å (methylene), Uiso(H) = 1.2Ueq(C), and d(C–H) = 0.93 Å (aromatic), Uiso(H) = 1.2Ueq(C).
3 Comment
3,4-Dihydronaphthalene-1(2H)-one derivatives are of interest because of their wide range of biological activities. 8 There is a large structural modification of space on its right side, and through skeleton assembly strategy, 3,4-dihydronaphthalene-1(2H)-one can be assembled with multiple active functional groups, which can endow it with various effects, such as anti rheumatic, anti skin disease, anti-tumor, and anti Alzheimer’s disease. 9 Pyridine or dihydropyridine modification is one of the most widely used heterocycles in the field of drug design. Their excellent therapeutic effects have expanded the scope of finding treatments for other diseases. 10 N-methylpiperazine, as another nitrogen-containing heterocyclic ring containing two N atoms, has been found to have excellent anti-inflammatory, antibacterial, and anti-tumor activities. 11 , 12 Therefore, we first spliced N-methylpiperazine and 5-fluoropicolinaldehyde, two nitrogen-containing heterocycles, into 5-(4-methylpiperazin-1-yl)picolinaldehyde through substitution reaction. 13 Secondly, the fluorine atom has a strong electronegativity, which can improve its lipophilicity and enhance its bioavailability. 14 So we used 7-fluoro-3,4-dihydronaphthalen-1(2H)-one and 5-(4-methylpiperazin-1-yl)picolinaldehyde for the Claisen–Schmidt reaction to obtain the title compound.
The single crystal structure analysis revealed that there is only one drug molecule in the asymmetric unit (cf. the figure), and the bond lengths and bond angles of the compound are within the normal range. 3,4-Dihydronaphthalene-1(2H)-one is the main active site of the title compound. 15 , 16 In this parent nucleus, the fluorine atom is connected to C(7) position, the bond length of C(7)–F(1) is 1.3585(16) Å. Introducing 5-(4-methylpiperazin-1-yl)picolinaldehyde at C(2) position can form an α,β-unsaturated ketone structure. The bond length of C(1)=O(1) is 1.2236(15) Å, and the bond length of C(2)=C(11) is 1.3439(17) Å. In addition, the twist angle of O(1)=C(1)–C(2)=C(11) is −17.37(17)°. Through the C(2)=C(11) double bond, the molecule derives a pyridine substituent, with a twist angle of −22.7(2)° for C(2)=C(11)–C(12)=N(1). The bond lengths of C(12)=N(1) is 1.3440(16) Å. The parent nucleus is not coplanar with the pyridine ring, and the dihedral angle is approximately 45.05(4)°. N-methylpiperazine is attached to C(15) position, and displays “chair” conformation. The twist angle of N(2)–C(17)–C(18)–N(3) and N(2)–C(20)–C(19)–N(3) are about 54.39(14)° and −53.52(13)°, respectively.
Funding source: Shandong Laboratory Program
Award Identifier / Grant number: SYS202205
Funding source: Shandong Provincial Natural Science Foundation
Award Identifier / Grant number: ZR2022MH159
Award Identifier / Grant number: ZR2023MH190
Funding source: Shandong Province Science and Technology-based Small and Medium-sized Enterprises Innovation Capacity Enhancement Project
Award Identifier / Grant number: 2023TSGC0870
-
Research funding: This work was supported by Shandong Laboratory Program (No. SYS202205), Shandong Provincial Natural Science Foundation (Nos. ZR2022MH159 and ZR2023MH190) and Shandong Province Science and Technology-based Small and Medium-sized Enterprises Innovation Capacity Enhancement Project (No. 2023TSGC0870).
References
1. Rigaku OD. CrysAlisPRO; Rigaku Oxford Diffraction Ltd: Yarnton, Oxfordshire, England, 2017.Search in Google Scholar
2. Sheldrick, G. M. A Short History of Shelx. Acta Crystallogr. 2008, A64, 112–122. https://doi.org/10.1107/s0108767307043930.Search in Google Scholar PubMed
3. Sheldrick, G. M. Crystal Structure Refinement with Shelxl. Acta Crystallogr. 2015, C71, 3–8. https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Luo, H. L.; Li, W. X.; Bai, X. Y.; Meng, Q. G.; Hou, Y. Crystal Structure of (E)-7-Fluoro-2-(4-Morpholinobenzylidene)-3,4-Dihydronaphthalen-1(2H)-One, C21H20FNO2. Z. Kristallogr. N. Cryst. Struct. 2023, 238 (3), 495–497. https://doi.org/10.1515/ncrs-2023-0053.Search in Google Scholar
5. Chen, Y.; Wang, J. P.; Wang, M. D.; Yu, W. X.; Cui, Y. T.; Gao, H. X.; Hou, G. G.; Ren, Y. Crystal Structure of (E)-2-(4-(1H-Imidazol-1-yl)Benzylidene)-7-Fluoro-3,4-Dihydronaphthalen-1(2H)-One, C20H15FN2O. Z. Kristallogr. N. Cryst. Struct. 2025, 240 (1), 19–21. https://doi.org/10.1515/ncrs-2024-0294.Search in Google Scholar
6. Yuan, X. Q.; Zhao, L. H.; Zhang, J. J.; Hou, G. G. Crystal Structure of (E)-7-Methoxy-2-(4-Morpholinobenzylidene)-3,4-Dihydronaphthalen-1(2H)-One, C22H23NO3. Z. Kristallogr. N. Cryst. Struct. 2023, 238, 363–365. https://doi.org/10.1515/ncrs-2022-0578.Search in Google Scholar
7. Xia, D. L.; Wang, J. P.; Yu, W. X.; Wang, M. D.; Gao, H. X.; Cui, Y. T.; Hou, G. G. Crystal Structure of (E)-6,8-Dimethoxy-4-(4-Morpholinobenzylidene)-3,4-Dihydro-1-Benzoxepin-5(2H)-One, C23H25NO5. Z. Kristallogr. N. Cryst. Struct. 2024, 239 (6), 1133–1136. https://doi.org/10.1515/ncrs-2024-0329.Search in Google Scholar
8. Kirby, A. J.; Le, L. R.; Maharlouie, F.; Mason, P.; Nicholls, P. J.; Smith, H. J.; Simons, C. Inhibition of Retinoic Acid Metabolising Enzymes by 2-(4-Aminophenylmethyl)-6-Hydroxy-3,4-Dihydronaphthalen-1(2H)-One and Related Compounds. J. Enzyme Inhib. Med. Chem. 2003, 18 (1), 27–33. https://doi.org/10.1080/1475636021000049221.Search in Google Scholar PubMed
9. Li, W. X.; Yu, L.; Chi, J. B.; Wang, J. P.; Liu, Y. J.; Wang, C. H.; Zhang, M.; Hou, G. G. Discovery of Anti-inflammatory Agents from 3,4-Dihydronaphthalene-1(2H)-One Derivatives by Inhibiting NLRP3 Inflammasome Activation. Eur. J. Med. Chem. 2024, 268, 116284. https://doi.org/10.1016/j.ejmech.2024.116284.Search in Google Scholar PubMed
10. Ling, Y.; Hao, Z. Y.; Liang, D.; Zhang, C. L.; Liu, Y. F.; Wang, Y. The Expanding Role of Pyridine and Dihydropyridine Scaffolds in Drug Design. Drug Des. Devel. Ther. 2021, 15, 4289–4338. https://doi.org/10.2147/dddt.s329547.Search in Google Scholar
11. Girase, P. S.; Dhawan, S.; Kumar, V.; Shinde, S. R.; Palkar, M. B.; Karpoormath, R. An Appraisal of Anti-mycobacterial Activity with Structure-Activity Relationship of Piperazine and its Analogues: A Review. Eur. J. Med. Chem. 2021, 210, 112967. https://doi.org/10.1016/j.ejmech.2020.112967.Search in Google Scholar PubMed
12. Qi, Q. B.; Li, W. X.; Hou, G. G.; Li, C. B. Crystal Structure of (E)-7-Bromo-2-(4-(4-Methylpiperazin-1-yl)Benzylidene)-3,4-Dihydronaphthalen-1(2H)-One, C22H23BrN2O. Z. Kristallogr. N. Cryst. Struct. 2023, 238, 235–237. https://doi.org/10.1515/ncrs-2022-0590.Search in Google Scholar
13. Yu, L.; Wang, J. P.; Wang, M. D.; Yu, W. X.; Cui, Y. T.; Gao, H. X.; Liu, Y. J.; Hou, G. G. Crystal Structure of (E)-6-(4-Ethylpiperazin-1-yl)-2-(3-Fluorobenzylidene)-3,4-Dihydronaphthalen-1(E)-One, C23H25FN2O. Z. Kristallogr. N. Cryst. Struct. 2024, 239, 515–517. https://doi.org/10.1515/ncrs-2024-0066.Search in Google Scholar
14. Li, X. W.; Miao, Y. H.; Ding, Y. X.; Hou, G. G. Crystal Structure of 9-Fluoro-4-(6-Methoxypyridin-2-yl)-5,6-Dihydrobenzo [(h)]quinazolin-2-Amine, C18H15FN4O. Z. Kristallogr. N. Cryst. Struct. 2025, 240 (1), 37–39. https://doi.org/10.1515/ncrs-2024-0330.Search in Google Scholar
15. Zhang, X. F.; Luan, M. Z.; Yan, W. B.; Zhao, F. L.; Hou, Y.; Hou, G. G.; Meng, Q. G. Anti-neuroinflammatory Effects of Novel 5,6-Dihydrobenzo [h]quinazolin-2-Amine Derivatives in Lipopolysaccharide-Stimulated BV2 Microglial Cells. Eur. J. Med. Chem. 2022, 235, 114322. https://doi.org/10.1016/j.ejmech.2022.114322.Search in Google Scholar PubMed
16. Sun, Y.; Zhou, Y. Q.; Liu, Y. K.; Zhang, H. Q.; Hou, G. G.; Meng, Q. G.; Hou, Y. Potential Anti-neuroinflammatory NF-κB Inhibitors Based on 3,4-Dihydronaphthalen-1(2H)-One Derivatives. J. Enzym. Inhib. Med. Chem. 2020, 35, 1631–1640. https://doi.org/10.1080/14756366.2020.1804899.Search in Google Scholar PubMed PubMed Central
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of (S)-N-(10-((2,2-dimethoxyethyl)amino)-1,2,3-trimethoxy-9-oxo-5,6,7,9-tetrahydrobenzo[a]heptalen-7-yl)acetamide, C25H32N2O7
- The crystal structure of 6,6′-difluoro-3,3′-dimethyl-5,5′-di(10H-phenoxazin-10-yl)- [1,1′-biphenyl]-2,2′-dicarbonitrile, C40H24F2N4O2
- Crystal structure of poly[(di-ethylenediamine-κ2N,N′)cadmium(II) tetradedocyloxidohexavanadate] (V4+/V5+ = 2/1), C4H16CdN4O14V6
- The crystal structure of poly[bis(dimethylformamide-κ1N)-(μ4-2′,3,3″,5′-tetrakis(trifluoromethyl)-[1,1′:4′,1″-terphenyl]-4,4″-dicarboxylato-κ4 O,O′: O″,O‴)dicadmium(II)], C27H15CdF12NO5
- Crystal structure of bis(μ2-ferrocenylcarboxylato-O,O′)-(μ3-oxido-κ3O:O:O)-bis(μ2-salicyladoximato-κ2N,O,O′)-(μ2-isopropoxo)-tris(isopropoxy-κ1O trititanium(IV)), C48H55N2O13Fe2Ti3
- Crystal structure of 3-(diethylamino)-7,9,11-trimethyl-8-phenyl-6H,13H-12λ4,13λ4-chromeno[3′,4′:4,5]pyrrolo[1,2-c]pyrrolo[2,1-f][1,3,2]diazaborinin-6-one, C28H26BF2N3O2
- The crystal structure of catena-poly[aqua-μ2-2-nitro-benzene-1,3-dicarboxylato-κ2O,O′)-(1,10-phenanthroline-κ2N,N′)-zinc(II)], C20H13N3O7Zn
- Crystal structure of poly[diaqua-{μ3-1-(3-carboxylatophenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylato-κ4O,O′:O′′:O′′′′}manganese(II)] hydrate
- Crystal structure of N′-((1-hydroxycyclohexyl)(phenyl)methyl)-2-methoxybenzohydrazide methanol solvate, C22H28N2O4
- The cocrystal of caffeic acid — progesterone — water (1/2/1), C51H70O9
- Crystal structure of (((oxido(quinolin-6-yl)methoxy)triphenyl-λ5-stibanyl)oxy)(quinolin-7-yl)methanolate
- Crystal structure of [(E)-6′-(diethylamino)-2-(2-(((E)-pyren-1-ylmethylene)amino)ethyl)-4′-(2-((E)-1,3,3-trimethylindolin-2-ylidene)ethylidene)-1′,2′,3′,4′-tetrahydrospiro[isoindoline-1,9′-xanthen]-3-one]-methanol, solvate C57H56N4O3
- The crystal structure of 1-(acridin-9-yl)pyrrolidine-2,5-dione, C17H22N2O2
- Crystal structure of N-(4-acetylphenyl)-2-(6-methoxynaphthalen-2-yl)propanamide, C22H21NO3
- The crystal structure of 5,10,15,20-tetrakis(4-(1H-1,2,4-triazol-1-yl)phenyl)porphyrin, C52H34N16
- Crystal structure of hexacarbonyl-μ2-[phenylmethanedithiolato-κ4S:S,S′:S′]diiron (Fe–Fe) C13H6Fe2O6S2
- Crystal structure of diiodo-bis(1-((2-propyl-1H-benzo[d]imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κ1N)cadmium(II), C34H34CdI2N10
- Crystal structure of (E)-(3-(3-bromophenyl)acryloyl)ferrocene, C19H15BrFeO
- Crystal structure of catena-poly(μ2-6-chloropyridine-2-carboxylato-κ3N,O:O′)(6-chloropyridine-2-carboxylato-κ2O,N)copper(II), C12H6Cl2N2O4Cu
- Crystal structure of poly[diaqua-μ 3-(5-(3,5-dicarboxy-2,4,6-trimethylbenzyl)-2,4,6-trimethylisophthalato)-κ 6O,O′:O″,O‴:O‴′,O‴″) terbium(III)-monohydrate], C23H28TbO12
- Crystal structure of (E)-2-(((5-chloro-3-methyl-1-phenyl-1H-pyrazol-4-yl)methylene)amino)-3′,6′-dihydroxyspiro[isoindoline-1,9′-xanthen]-3-one – ethanol (1/2), C35H33ClN4O6
- The crystal structure of 3-(5-amino-3-phenylisoxazol-4-yl)-4-chloro-3-hydroxyindolin-2-one, C17H12ClN3O3
- The crystal structure of dimethylammonium 4-[2-(4-fluorophenyl)-4, 5-diphenyl-1H-imidazol-1-yl]benzenesulfonate, C29H26FN3O3S
- Crystal structure of (R)-2-ammonio-3-((5-carboxypentyl)thio)propanoate
- Crystal structure of 4-cyclohexyl-5-(thiophen-2-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione, C12H15N3S2
- The crystal structure of 4,6-bis(dimethylamino)-2-fluoroisophthalonitrile, C12H13FN4
- Hydrogen bonding in the crystal structure of nicotin-1,1′-dium tetrabromidomanganate(II)
- The crystal structure of bis(2-bromobenzyl)(2-((2-oxybenzylidene)amino)-4-methylpentanoato-κ3N, O,O′)tin(IV), C27H27Br2NO3Sn
- Crystal structure of (E)-(3-(p-tolyl)acryloyl)ferrocene, C20H18FeO
- Crystal structure of (E)-7-fluoro-2-((5-(4-methylpiperazin-1-yl)pyridin-2-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C21H22FN3O
- Crystal structure of (E)-7-methoxy-2-((5-(4-methylpiperazin-1-yl)pyridin-2-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C22H25N3O2
- The crystal structure of poly(bis(μ2-1,3,5-tri(1H-imidazol-1-yl)benzene-κ2N:N′)-(μ2-2,3,5,6-tetrafluoroterephthalato-κ2O:O′)-manganese(II), C38H24F4N12O4Mn
- Crystal structure of (3,4-dimethoxybenzyl)triphenylphosphonium bromide ethanol solvate, C29H32BrO3P
- Crystal structure of tetraethylammonium hydrogencarbonate – (diaminomethylene)thiourea – water (2/1/3)
- Crystal structure of N, N-Dimethyl-N′-tosylformimidamide, C10H14N2O2S
- The crystal structure of ethyl 2-methyl-5-oxo-4-(2-methoxyphenyl)-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C20H23N2O4
- Crystal structure of bis(μ2-1,5-bis[(E)-1-(2-hydroxyphenyl)ethylidene] thiocarbonohydrazide)-bis(dimethylformamide)-dizinc(II) dimethylformamide solvate, C40H46N10O6S2Zn2⋅C3H7NO
- Crystal structure of azido-κ1N{hydridotris(3-tert-butyl-5-methylpyrazol-1-yl)borato-κ3N,N′,N″}copper(II), C24H40BCuN9
- The crystal structure of fac-tricarbonyl(1,10-phenanthroline-κ2N,N′)-(azido-κ1N)rhenium(I), C15H8N5O3Re
- Crystal structure of 4-((triphenylphosphonio)methyl)pyridin-1-ium tetrachloridozincate(II), C24H22Cl4NPZn
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of (S)-N-(10-((2,2-dimethoxyethyl)amino)-1,2,3-trimethoxy-9-oxo-5,6,7,9-tetrahydrobenzo[a]heptalen-7-yl)acetamide, C25H32N2O7
- The crystal structure of 6,6′-difluoro-3,3′-dimethyl-5,5′-di(10H-phenoxazin-10-yl)- [1,1′-biphenyl]-2,2′-dicarbonitrile, C40H24F2N4O2
- Crystal structure of poly[(di-ethylenediamine-κ2N,N′)cadmium(II) tetradedocyloxidohexavanadate] (V4+/V5+ = 2/1), C4H16CdN4O14V6
- The crystal structure of poly[bis(dimethylformamide-κ1N)-(μ4-2′,3,3″,5′-tetrakis(trifluoromethyl)-[1,1′:4′,1″-terphenyl]-4,4″-dicarboxylato-κ4 O,O′: O″,O‴)dicadmium(II)], C27H15CdF12NO5
- Crystal structure of bis(μ2-ferrocenylcarboxylato-O,O′)-(μ3-oxido-κ3O:O:O)-bis(μ2-salicyladoximato-κ2N,O,O′)-(μ2-isopropoxo)-tris(isopropoxy-κ1O trititanium(IV)), C48H55N2O13Fe2Ti3
- Crystal structure of 3-(diethylamino)-7,9,11-trimethyl-8-phenyl-6H,13H-12λ4,13λ4-chromeno[3′,4′:4,5]pyrrolo[1,2-c]pyrrolo[2,1-f][1,3,2]diazaborinin-6-one, C28H26BF2N3O2
- The crystal structure of catena-poly[aqua-μ2-2-nitro-benzene-1,3-dicarboxylato-κ2O,O′)-(1,10-phenanthroline-κ2N,N′)-zinc(II)], C20H13N3O7Zn
- Crystal structure of poly[diaqua-{μ3-1-(3-carboxylatophenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylato-κ4O,O′:O′′:O′′′′}manganese(II)] hydrate
- Crystal structure of N′-((1-hydroxycyclohexyl)(phenyl)methyl)-2-methoxybenzohydrazide methanol solvate, C22H28N2O4
- The cocrystal of caffeic acid — progesterone — water (1/2/1), C51H70O9
- Crystal structure of (((oxido(quinolin-6-yl)methoxy)triphenyl-λ5-stibanyl)oxy)(quinolin-7-yl)methanolate
- Crystal structure of [(E)-6′-(diethylamino)-2-(2-(((E)-pyren-1-ylmethylene)amino)ethyl)-4′-(2-((E)-1,3,3-trimethylindolin-2-ylidene)ethylidene)-1′,2′,3′,4′-tetrahydrospiro[isoindoline-1,9′-xanthen]-3-one]-methanol, solvate C57H56N4O3
- The crystal structure of 1-(acridin-9-yl)pyrrolidine-2,5-dione, C17H22N2O2
- Crystal structure of N-(4-acetylphenyl)-2-(6-methoxynaphthalen-2-yl)propanamide, C22H21NO3
- The crystal structure of 5,10,15,20-tetrakis(4-(1H-1,2,4-triazol-1-yl)phenyl)porphyrin, C52H34N16
- Crystal structure of hexacarbonyl-μ2-[phenylmethanedithiolato-κ4S:S,S′:S′]diiron (Fe–Fe) C13H6Fe2O6S2
- Crystal structure of diiodo-bis(1-((2-propyl-1H-benzo[d]imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κ1N)cadmium(II), C34H34CdI2N10
- Crystal structure of (E)-(3-(3-bromophenyl)acryloyl)ferrocene, C19H15BrFeO
- Crystal structure of catena-poly(μ2-6-chloropyridine-2-carboxylato-κ3N,O:O′)(6-chloropyridine-2-carboxylato-κ2O,N)copper(II), C12H6Cl2N2O4Cu
- Crystal structure of poly[diaqua-μ 3-(5-(3,5-dicarboxy-2,4,6-trimethylbenzyl)-2,4,6-trimethylisophthalato)-κ 6O,O′:O″,O‴:O‴′,O‴″) terbium(III)-monohydrate], C23H28TbO12
- Crystal structure of (E)-2-(((5-chloro-3-methyl-1-phenyl-1H-pyrazol-4-yl)methylene)amino)-3′,6′-dihydroxyspiro[isoindoline-1,9′-xanthen]-3-one – ethanol (1/2), C35H33ClN4O6
- The crystal structure of 3-(5-amino-3-phenylisoxazol-4-yl)-4-chloro-3-hydroxyindolin-2-one, C17H12ClN3O3
- The crystal structure of dimethylammonium 4-[2-(4-fluorophenyl)-4, 5-diphenyl-1H-imidazol-1-yl]benzenesulfonate, C29H26FN3O3S
- Crystal structure of (R)-2-ammonio-3-((5-carboxypentyl)thio)propanoate
- Crystal structure of 4-cyclohexyl-5-(thiophen-2-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione, C12H15N3S2
- The crystal structure of 4,6-bis(dimethylamino)-2-fluoroisophthalonitrile, C12H13FN4
- Hydrogen bonding in the crystal structure of nicotin-1,1′-dium tetrabromidomanganate(II)
- The crystal structure of bis(2-bromobenzyl)(2-((2-oxybenzylidene)amino)-4-methylpentanoato-κ3N, O,O′)tin(IV), C27H27Br2NO3Sn
- Crystal structure of (E)-(3-(p-tolyl)acryloyl)ferrocene, C20H18FeO
- Crystal structure of (E)-7-fluoro-2-((5-(4-methylpiperazin-1-yl)pyridin-2-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C21H22FN3O
- Crystal structure of (E)-7-methoxy-2-((5-(4-methylpiperazin-1-yl)pyridin-2-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C22H25N3O2
- The crystal structure of poly(bis(μ2-1,3,5-tri(1H-imidazol-1-yl)benzene-κ2N:N′)-(μ2-2,3,5,6-tetrafluoroterephthalato-κ2O:O′)-manganese(II), C38H24F4N12O4Mn
- Crystal structure of (3,4-dimethoxybenzyl)triphenylphosphonium bromide ethanol solvate, C29H32BrO3P
- Crystal structure of tetraethylammonium hydrogencarbonate – (diaminomethylene)thiourea – water (2/1/3)
- Crystal structure of N, N-Dimethyl-N′-tosylformimidamide, C10H14N2O2S
- The crystal structure of ethyl 2-methyl-5-oxo-4-(2-methoxyphenyl)-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C20H23N2O4
- Crystal structure of bis(μ2-1,5-bis[(E)-1-(2-hydroxyphenyl)ethylidene] thiocarbonohydrazide)-bis(dimethylformamide)-dizinc(II) dimethylformamide solvate, C40H46N10O6S2Zn2⋅C3H7NO
- Crystal structure of azido-κ1N{hydridotris(3-tert-butyl-5-methylpyrazol-1-yl)borato-κ3N,N′,N″}copper(II), C24H40BCuN9
- The crystal structure of fac-tricarbonyl(1,10-phenanthroline-κ2N,N′)-(azido-κ1N)rhenium(I), C15H8N5O3Re
- Crystal structure of 4-((triphenylphosphonio)methyl)pyridin-1-ium tetrachloridozincate(II), C24H22Cl4NPZn

