Abstract
C32H49N3O6S, orthorhombic, P212121 (no. 19), a = 9.2618(2) Å, b = 12.4066(2) Å, c = 27.2256(5) Å, V = 3128.42(12) Å3, Z = 4, R gt (F) = 0.0517, wR ref (F 2) = 0.1355, T = 173.20(2) K.
The molecular structure is shown in the figure (the carbon bound hydrogen atoms are omitted for clarity). Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
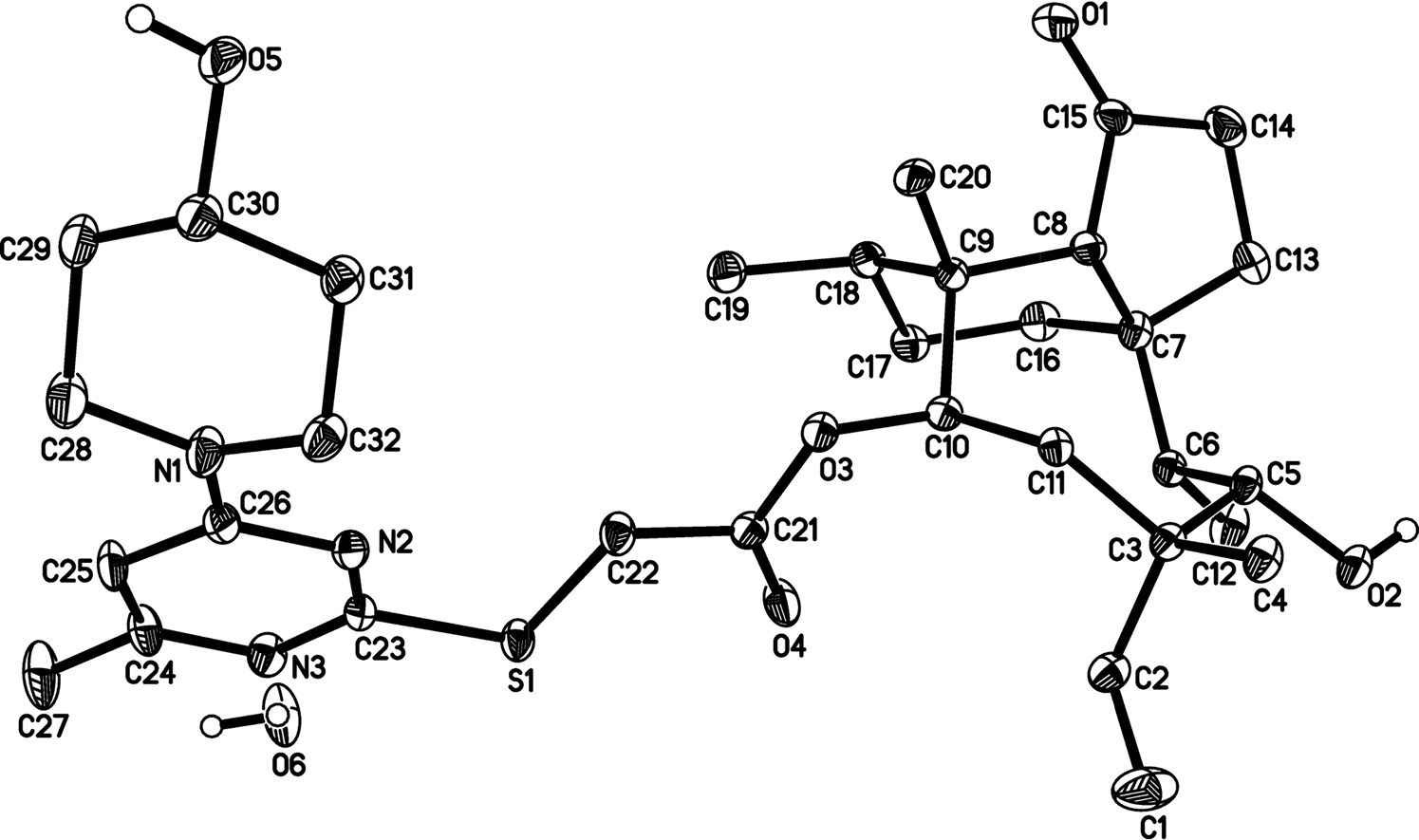
Data collection and handling.
| Crystal: | Colorless block |
| Size: | 0.18 × 0.15 × 0.12 mm |
| Wavelength: | Cu Kα radiation (1.54184 Å) |
| μ: | 1.31 mm−1 |
| Diffractometer, scan mode: | SuperNova, ω |
| θ max, completeness: | 66.6°, 98% |
| N(hkl)measured, N(hkl)unique, R int: | 9661, 5241, 0.041 |
| Criterion for I obs, N(hkl)gt: | I obs > 2 σ(I obs), 4775 |
| N(param)refined: | 397 |
| Programs: | Olex2 [1], SHELX [2], CrysAlisPRO [3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| S1 | −0.12855 (13) | −0.43111 (8) | −0.13950 (3) | 0.0269 (2) |
| O1 | −0.9226 (5) | −0.0675 (3) | −0.19578 (13) | 0.0473 (10) |
| O2 | −0.6185 (4) | −0.2359 (3) | −0.40201 (10) | 0.0302 (7) |
| H2 | −0.700767 | −0.230071 | −0.412812 | 0.045* |
| O3 | −0.3779 (4) | −0.2201 (2) | −0.20234 (9) | 0.0258 (6) |
| O4 | −0.3271 (4) | −0.3949 (3) | −0.21774 (12) | 0.0403 (9) |
| O5 | 0.0149 (5) | 0.1497 (3) | 0.00810 (11) | 0.0388 (8) |
| H5 | 0.043678 | 0.169810 | 0.035059 | 0.058* |
| N1 | 0.1182 (5) | −0.1675 (3) | −0.03142 (13) | 0.0329 (9) |
| N2 | 0.0100 (4) | −0.2957 (3) | −0.08025 (12) | 0.0255 (8) |
| N3 | 0.0126 (5) | −0.4842 (3) | −0.06024 (13) | 0.0300 (8) |
| C1 | −0.2764 (9) | −0.3181 (5) | −0.3737 (3) | 0.065 (2) |
| H1A | −0.256 (9) | −0.285 (7) | −0.401 (3) | 0.08 (3)* |
| H1B | −0.225 (7) | −0.385 (6) | −0.373 (2) | 0.052 (18)* |
| C2 | −0.3687 (5) | −0.2942 (3) | −0.34063 (15) | 0.0286 (9) |
| H2A | −0.382369 | −0.345965 | −0.316370 | 0.034* |
| C3 | −0.4593 (5) | −0.1926 (3) | −0.33547 (14) | 0.0231 (8) |
| C4 | −0.3986 (5) | −0.1031 (3) | −0.36821 (14) | 0.0281 (9) |
| H4A | −0.396006 | −0.127420 | −0.401665 | 0.042* |
| H4B | −0.302605 | −0.085233 | −0.357660 | 0.042* |
| H4C | −0.459054 | −0.040452 | −0.365819 | 0.042* |
| C5 | −0.6189 (5) | −0.2146 (3) | −0.35041 (13) | 0.0224 (8) |
| H5A | −0.673578 | −0.148010 | −0.345063 | 0.027* |
| C6 | −0.6979 (5) | −0.3077 (3) | −0.32141 (14) | 0.0242 (9) |
| H6 | −0.622497 | −0.345356 | −0.302918 | 0.029* |
| C7 | −0.8043 (5) | −0.2602 (3) | −0.28204 (15) | 0.0245 (9) |
| C8 | −0.7534 (5) | −0.1614 (3) | −0.25153 (14) | 0.0242 (9) |
| H8 | −0.713034 | −0.109552 | −0.274977 | 0.029* |
| C9 | −0.6355 (5) | −0.1810 (3) | −0.21214 (13) | 0.0226 (8) |
| C10 | −0.4917 (5) | −0.2186 (3) | −0.23766 (13) | 0.0223 (8) |
| H10 | −0.506039 | −0.292530 | −0.249300 | 0.027* |
| C11 | −0.4465 (5) | −0.1492 (3) | −0.28211 (14) | 0.0213 (8) |
| H11A | −0.346340 | −0.129169 | −0.277127 | 0.026* |
| H11B | −0.502398 | −0.083137 | −0.280575 | 0.026* |
| C12 | −0.7659 (6) | −0.3903 (4) | −0.35360 (16) | 0.0364 (11) |
| H12A | −0.812351 | −0.443843 | −0.333651 | 0.055* |
| H12B | −0.693088 | −0.423825 | −0.373479 | 0.055* |
| H12C | −0.836217 | −0.356405 | −0.374419 | 0.055* |
| C13 | −0.9411 (5) | −0.2168 (4) | −0.30832 (17) | 0.0326 (10) |
| H13A | −0.915097 | −0.180107 | −0.338515 | 0.039* |
| H13B | −1.006605 | −0.275318 | −0.316103 | 0.039* |
| C14 | −1.0110 (5) | −0.1385 (4) | −0.27232 (18) | 0.0389 (11) |
| H14A | −1.040277 | −0.073063 | −0.289058 | 0.047* |
| H14B | −1.095403 | −0.170935 | −0.257265 | 0.047* |
| C15 | −0.8973 (6) | −0.1139 (4) | −0.23374 (16) | 0.0325 (10) |
| C16 | −0.8516 (5) | −0.3471 (4) | −0.24431 (15) | 0.0288 (9) |
| H16A | −0.941663 | −0.324349 | −0.229337 | 0.035* |
| H16B | −0.870175 | −0.413855 | −0.261740 | 0.035* |
| C17 | −0.7419 (5) | −0.3692 (3) | −0.20363 (16) | 0.0272 (9) |
| H17A | −0.656507 | −0.401842 | −0.217878 | 0.033* |
| H17B | −0.782938 | −0.420177 | −0.180497 | 0.033* |
| C18 | −0.6982 (5) | −0.2665 (3) | −0.17607 (15) | 0.0271 (9) |
| H18 | −0.787980 | −0.235676 | −0.163184 | 0.033* |
| C19 | −0.6053 (6) | −0.2946 (4) | −0.13087 (15) | 0.0332 (10) |
| H19A | −0.524769 | −0.338013 | −0.140908 | 0.050* |
| H19B | −0.662635 | −0.333798 | −0.107558 | 0.050* |
| H19C | −0.570645 | −0.229360 | −0.115998 | 0.050* |
| C20 | −0.6014 (5) | −0.0749 (3) | −0.18531 (15) | 0.0288 (9) |
| H20A | −0.515813 | −0.083831 | −0.165835 | 0.043* |
| H20B | −0.680883 | −0.056064 | −0.164375 | 0.043* |
| H20C | −0.586233 | −0.018702 | −0.208972 | 0.043* |
| C21 | −0.3053 (5) | −0.3114 (3) | −0.19607 (14) | 0.0252 (9) |
| C22 | −0.1931 (5) | −0.2979 (3) | −0.15656 (15) | 0.0279 (9) |
| H22A | −0.234786 | −0.262103 | −0.128238 | 0.034* |
| H22B | −0.113654 | −0.254318 | −0.168622 | 0.034* |
| C23 | −0.0217 (5) | −0.3981 (3) | −0.08732 (14) | 0.0232 (8) |
| C24 | 0.0880 (6) | −0.4604 (4) | −0.01980 (17) | 0.0333 (11) |
| C25 | 0.1277 (6) | −0.3573 (4) | −0.00738 (15) | 0.0324 (10) |
| H25 | 0.179876 | −0.343606 | 0.021132 | 0.039* |
| C26 | 0.0866 (5) | −0.2722 (4) | −0.03939 (15) | 0.0271 (9) |
| C27 | 0.1285 (8) | −0.5551 (4) | 0.0121 (2) | 0.0506 (15) |
| H27A | 0.042728 | −0.593002 | 0.021774 | 0.076* |
| H27B | 0.178093 | −0.529761 | 0.040867 | 0.076* |
| H27C | 0.190520 | −0.602776 | −0.005938 | 0.076* |
| C28 | 0.1883 (7) | −0.1263 (4) | 0.01269 (18) | 0.0419 (13) |
| H28A | 0.287636 | −0.107604 | 0.005257 | 0.050* |
| H28B | 0.188982 | −0.181752 | 0.037810 | 0.050* |
| C29 | 0.1094 (6) | −0.0276 (4) | 0.03170 (16) | 0.0364 (11) |
| H29A | 0.160352 | 0.001030 | 0.059957 | 0.044* |
| H29B | 0.013152 | −0.048068 | 0.042186 | 0.044* |
| C30 | 0.0987 (6) | 0.0592 (4) | −0.00782 (15) | 0.0304 (10) |
| H30 | 0.195971 | 0.083615 | −0.016566 | 0.036* |
| C31 | 0.0264 (6) | 0.0123 (4) | −0.05274 (15) | 0.0303 (10) |
| H31A | 0.024181 | 0.066028 | −0.078617 | 0.036* |
| H31B | −0.072394 | −0.006902 | −0.044833 | 0.036* |
| C32 | 0.1065 (6) | −0.0869 (4) | −0.07072 (14) | 0.0315 (10) |
| H32A | 0.055511 | −0.117761 | −0.098472 | 0.038* |
| H32B | 0.202348 | −0.066417 | −0.081627 | 0.038* |
| O6 | −0.1157 (5) | −0.7014 (3) | −0.05776 (15) | 0.0516 (10) |
| H6A | −0.064702 | −0.645259 | −0.061959 | 0.077* |
| H6B | −0.082198 | −0.737619 | −0.033828 | 0.077* |
Source of material
Triethylamine (0.73 g, 7.2 mmol), 4-dimethylaminopyridine (0.01 g, 0.1 mmol) and mesitylene-2-sulfonyl chloride (0.50 g, 2.3 mmol) were added to a solution of 14-O-[(4-ol-6-methylpyrimidine-2-yl)thioacetyl] mutilin (0.75 g, 1.5 mmol) in 20 ml dry dichloromethane. After the mixture was stirred at room temperature for 3 h, piperidin-4-ol (0.76 g, 7.5 mmol) and 1-methylpyrrolidine (1.27 g, 15 mmol) were then added in one portion for stirring for 1.5 h at 273.15 K. The mixture was washed with 5% citric acid, followed by drying with Na2SO4 overnight and rotary evaporation to dryness. The crude residue was purified by silica gel column chromatography (petroleum ether: ethyl acetate 1:1–1:10 v/v) to afford the pure product with a yield of 63%. The clear light colorless crystals were obtained by slow evaporation from a solution of dichloromethane and n-hexane (1:1) at room temperature.
Experimental details
All hydrogen atoms were placed in geometrically idealized positions. The U iso values were set to 1.5U eq of the carrier atom for methyl and oxygen H atoms and 1.2 for the remaining H atoms.
Comment
The title compound may possess antibacterial activities against some Gram-positive bacteria, including Staphylococcus aureus, methicillin-resistant Staphylococcus epidermidis (MRSE), methicillin-resistant S. aureus (MRSA), Streptococcus dysgalactiae and Streptococcus agalactiae and merited further drug development as a potential agent [4]. The starting material was pleuromutilin, which was first isolated in a crystalline form from cultures of two species of basidiomycetes, Pleurotus mutilus and Pleurotus passeckerianus in 1951 [5]. The pleuromutilin class showed high antibacterial activities with no target-specific cross-resistance to other antibiotics with an unique mode of action, which involve inhibtion of protein synthesis by primarily inhibiting ribosomal peptidyl transferase center (PTC) [6, 7]. The molecular modifications of pleuromutilin led to four drugs: tiamulin, valnemulin, retapamulin and lefamulin [8], [9], [10].
The asymmetric unit is composed of a molecule of 14-O-[(4-(4-Hydroxypiperidine-1-yl)-6-methylpyrimidine-2-yl) thioacetyl] mutilin and a molecule of water. The title molecule is comprised of a 5-6-8 tricyclic carbon skeleton and a methyl pyrimidine group, in which all bond lengths are in normal ranges [11, 12]. The six-membered ring (C9, C8, C7, C16–C18) exhibits a chair conformation and the eight-membered ring (C3, C5–C11) exhibits an irregular boat conformation, which were similar as the previous reported pleuromutilin derivative [11]. The structure is stabilized by some intramolecular hydrogen bonds (for example: C10—H10⃛O4, C18–H18⃛O1, C20–H20A⃛O3, C32–H32A⃛N2). Notably, the hydrogen bond of O2–H2⃛O6, O6–H6A⃛N3 and O6–H6B⃛O5 between the target and water molecules are present.
Funding source: Quancheng Scholar Construction Project of Jinan
Award Identifier / Grant number: 00252019025
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This work was financial supported by Quancheng Scholar Construction Project of Jinan (No. 00252019025).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Dolomanov, O.-V., Bourhis, L.-J., Gildea, R.-J., Howard, J.-A.-K., Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
2. Sheldrick, G.-M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Search in Google Scholar
3. Agilent Technologies. CrysAlisPRO; Agilent Technologies: Santa Clara, CA, USA, 2017.Search in Google Scholar
4. Fan, Y., Liu, Y., Wang, H., Shi, T., Cheng, F., Hao, B.-C., Yi, Y.-P., Shang, R.-F. Novel pleuromutilin derivatives with substituted 6-methylpyrimidine: design, synthesis and antibacterial evaluation. Eur. J. Med. Chem. 2020, 207, 112735; https://doi.org/10.1016/j.ejmech.2020.112735.Search in Google Scholar
5. Kavanagh, F., Hervey, A., Robbins, W.-J. Antibiotic substances from basidiomycetes. VIII. Pleurotus multilus (Fr.) Sacc. and Pleuotus passeckerianus Pilat. Proc. Natl. Acad. Sci. U.S.A. 1951, 37, 570–574; https://doi.org/10.1073/pnas.37.9.570.Search in Google Scholar
6. Schlunzen, F., Pyetan, E., Fucini, P., Yonath, A., Harms, J.-M. Inhibition of peptide bond formation by pleuromutilins: the structure of the 50S ribosomal subunit from Deinococcus radiodurans in complex with tiamulin. Mol. Microbiol. 2004, 54, 1287–1294; https://doi.org/10.1111/j.1365-2958.2004.04346.x.Search in Google Scholar
7. Shang, R.-F., Wang, J.-T., Guo, W.-Z., Liang, J.-P. Efficient antibacterial agents: a review of the synthesis, biological evaluation and mechanism of pleuromutilin derivatives. Curr. Top. Med. Chem. 2013, 13, 3013–3025; https://doi.org/10.2174/15680266113136660217.Search in Google Scholar
8. Tang, Y.-Z., Liu, Y.-H., Chen, J.-X. Pleuromutilin and its derivatives. The lead compounds for novel antibiotics. Mini Rev. Med. Chem. 2012, 12, 53–61; https://doi.org/10.2174/138955712798868968.Search in Google Scholar
9. Moody, M.-N., Morrison, L.-K., Tyring, S.-K. Retapamulin: what is the role of this topical antimicrobial in the treatment of bacterial infections in atopic dermatitis? Skin Ther. Lett. 2010, 15, 1–4.Search in Google Scholar
10. Zhang, Y.-Y., Xie, C., Liu, Y., Shang, F., Shao, R.-S., Yu, J., Wu, C.-X., Yao, X.-H., Liu, D.-F., Wang, Z.-Y. Synthesis, biological activities and docking studies of pleuromutilin derivatives with piperazinyl urea linkage. J. Enzym. Inhib. Med. Chem. 2021, 36, 764–775; https://doi.org/10.1080/14756366.2021.1900163.Search in Google Scholar
11. Shang, R.-F., Yi, Y.-P., Liang, J.-P. Crystal structure of 14-((1-(benzyloxycarbonylamino)-2-methylpropan-2-yl) sulfanyl) acetate Mutilin, C34H49NO6S. Z. Kristallogr. N. Cryst. Struct. 2016, 231, 465–467; https://doi.org/10.1515/ncrs-2015-0142.Search in Google Scholar
12. Liang, Y., Fu, Y.-X., Liang, J.-P. Crystal structure of (3aR,4R,5R,7R,8S,9R,9aS, 12R)-7-ethyl-5-(1-hydroxy-2-((R)-3-hydroxypyrrolidin-1-yl)ethoxy)-4,7,9,12-tetramethyldecahydro-4,9a-propanocyclopenta[8] annulene-3,8-diol – a pleuromutilin derivative, C26H41NO5. Z. Kristallogr. N. Cryst. Struct. 2018, 233, 923–925; https://doi.org/10.1515/ncrs-2018-0107.Search in Google Scholar
© 2021 Si-Jie Liu et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of (E)-7-hydroxy-2-((6-methoxypyridin-3-yl)methylene)-3, 4-dihydronaphthalen-1(2H)-one, C17H15NO3
- Crystal structure of (E)-7-methoxy-2-((2-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1 (2H)-one, C18H17NO3
- The crystal structure of N 6,N 6′-di(pyridin-2-yl)-[2,2′-bipyridine]-6,6′-diamine, C20H16N6
- The crystal structure of {N 1,N 2-bis[2,4-dimethyl-6-(4-(tert-butyl)phenyl)(phenyl)methyl]acenaphthylene-1,2-diimino-κ2 N, N′}-dibromido-nickel(II) – dichloromethane(1/2), C64H64Br2Cl4N2Ni
- Synthesis and crystal structure of nonacarbonyltris[(2-thia-1,3,5-triaza-7-phosphatricylco[3.3.1.1]decane-κ1 P)-2,2-dioxide]triruthenium(0) – acetonitrile (7/6), C25.71H32.57N9.86O15P3S3Ru3
- A new polymorph of 1-(4-nitrophenyl)-1H-benzimidazole (C13H9N3O2)
- The crystal structure of 2,2′-((1E,1′E)-(naphthalene-2,3 diylbis(azanylylidene)) bis(methanylylidene))bis(4-methylphenol), C26H22N2O2
- The crystal structure of bis(μ2-iodido)-bis(η6-benzene)-bis(iodido)-diosmium(II), C12H12I4Os2
- Redetermination of the crystal structure of bis{hydridotris(3,5-dimethylpyrazol-1-yl-κN 3)borato}copper(II), C30H44B2CuN12
- Crystal structure of (E)-3-((4-(tert-butyl)phenyl)thio)-4-hydroxypent-3-en-2-one, C15H20O2S
- Crystal structure of 2,2′-(p-tolylazanediyl)bis(1-phenylethan-1-one), C23H21NO2
- Redetermination of the crystal structure of the crystal sponge the poly[tetrakis(μ3-2,4,6-tris(pyridin-4-yl)-1,3,5-triazine)-dodecaiodidohexazinc(II) nitrobenzene solvate], C72H48I12N24Zn6⋅10(C6H5NO2)
- Crystal structure of (4′E)-6′-(diethylamino)-2-[(E)-[(6-methylpyridin-2-yl)methylidene]amino]-4′-{2-[(2E)-1,3,3-trimethyl-2,3-dihydro-1H-indol-2-ylidene]ethylidene}-1′,2,2′,3,3′,4′-hexahydrospiro[isoindole-1,9′-xanthene]-3-one, C44H45N5O2
- Crystal structure of (E)-7-fluoro-2-(3-fluorobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C17H12F2O1
- Crystal structure of tetrabutylammonium sulfanilate – 1-(diaminomethylene)thiourea (1/2)
- Crystal structure of [2,2′-{azanediyl)bis[(propane-3,1-diyl)(azanylylidene)methylylidene]} bis(3,5-dichlorophenolato)-κ2O,O′]-isothiocyanato-κN-iron(III), C21H19Cl4FeN4O2S
- Crystal structure of (4-chlorophenyl)(4-hydroxyphenyl)methanone, C13H9ClO2
- Crystal structure of 6,6′-((pentane-1,3-diylbis(azaneylylidene))bis(methaneylylidene))bis(2,4-dibromolphenolato-κ4 N,N′,O,O′)copper(II),) C19H16Br4CuN2O2
- Chlorido-(2,2′-(ethane-bis(5-methoxyphenolato))-κ4 N,N′,O,O′)manganese(III) monohydrate, C19H18Cl2CuN2O2
- Crystal structure of 2,6-di-tert-butyl-4-(4-methoxybenzylidene)cyclohexa-2,5-dien-1-one, C22H28O2
- Crystal structure of [6,6′-(((2,2-dimethylpropane-1,3-diyl)bis(azanylylidene))bis(methanylylidene))bis(2-chlorophenolato)-κ4N,N′,O,O′]copper(II)
- Crystal structure of 2-chloro-3-((thiophen-2-ylmethyl)amino)naphthalene-1,4-dione, C30H20O4N2Cl2S2
- Crystal structure of bis{hydridotris(3-trifluoromethyl-5-methylpyrazolyl-1-yl)borato-κN 3}manganese(II), C30H26B2F18MnN12
- Crystal structure of 1-(2-methylphenyl)-2-(2-methylbenzo[b]thienyl)-3,3,4,4,5,5-hexafluorocyclopent-ene, C21H14F6S
- Crystal structure of 2-(3-((carbamimidoylthio)methyl)benzyl)isothiouronium hexafluorophosphate monohydrate, C10H17F6N4OPS2
- Crystal structure of 4,5-diiodo-1,3-dimesityl-1H-1,2,3-triazol-3-ium chloride – chloroform (1/1), C21H23Cl4I2N3
- Crystal structure of azido-k1 N-{6,6′-((((methylazanediyl)bis(propane-3,1-diyl))bis(azanylylidene))bis(methanylylidene))bis(2,4-dibromophenolato)k5 N,N′,N″,O,O′}cobalt(III)-methanol (1/1)), C21H23Br4CoN6O3
- The crystal structure of 2-(4-((carbamimidoylthio)methyl)benzyl)isothiouronium hexafluorophosphate monohydrate, C10H17F6N4OPS2
- Crystal structure of 1,1′-(methane-1,1-diyl)bis(3-methyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C9H14F12N4P2
- Crystal structure of (4′E)-6′-(diethylamino)-2-[(E)-[(pyren-1-yl)methylidene]amino]-4′-{2-[(2E)-1,3,3-trimethyl-2,3-dihydro-1H-indol-2-ylidene]ethylidene}-1′,2,2′,3,3′,4′-hexahydrospiro[isoindole-1,9′-xanthene]-3-one, C54H48N4O2
- Crystal structure of poly[bis(μ2-2,6-bis(1-imidazoly)pyridine-κ2 N,N′)-bis(thiocyanato-κ1 N)copper(II)] dithiocyanate, C24H18CuN12S2
- Cones with a three-fold symmetry constructed from three hydrogen bonded theophyllinium cations that coat [FeCl4]− anions in the crystal structure of tris(theophyllinium) bis(tetrachloridoferrate(III)) chloride trihydrate, C21H33Cl9Fe2N12O9
- Crystal structure of 14-O-[(4-(4-hydroxypiperidine-1-yl)-6-methylpyrimidine-2-yl)thioacetyl]-mutilin monohydrate, C32H49N3O6S
- The crystal structure of (E)-3-chloro-2-(2-(4-methylbenzylidene)hydrazinyl)pyridine, C13H12ClN3
- The crystal structure of 4-phenyl-4-[2-(pyridine-4-carbonyl)hydrazinylidene]butanoic acid, C16H15N3O3
- The crystal structure of 6-amino-5-carboxypyridin-1-ium pentaiodide monohydrate C6H9I5N2O3
- Crystal structure of bis(μ3-oxido)-bis(μ2-2-formylbenzoato-k2O:O′)-bis(2-(dimethoxymethyl)-benzoato-κO)-oktakismethyl-tetratin(IV)
- Crystal structure of 2-((E)-(((E)-2-hydroxy-4-methylbenzylidene) hydrazineylidene)methyl)-4-methylphenol, C16H16N2O2
- Crystal structure of (E)-amino(2-((5-methylfuran-2-yl)methylene)hydrazinyl) methaniminium nitrate monohydrate, C14H26N10O10
- The crystal structure of N′-(2-chloro-6-hydroxybenzylidene)thiophene-2-carbohydrazide monohydrate, C12H11ClN2O3S
- Crystal structure of catena-poly[(μ2-1,1′-(biphenyl-4,4′-diyl)bis(1H-imidazol)-κ2N:N′)-bis(4-bromobenzoate-κ1O)zinc(II)], C64H44Br4N8O8Zn2
- The crystal structure of catena-poly[(1-(4-carboxybenzyl)pyridin-1-ium-4-carboxylato-κ1O)-(μ2-oxalato-κ4 O:O′:O″:O‴)dioxidouranium(VI)], C16H11NO10U
- Crystal structure of 3-allyl-4-(2-bromoethyl)-5-(4-methoxyphenyl)-2-phenylfuran, C22H21BrO2
- Halogen bonds in the crystal structure of 4,3′:5′,4″-terpyridine — 1,3-diiodotetrafluorobenzene (1/1), C21H11F4I2N3
- Crystal structure of 2-(1H-indol-3-yl)ethan-1-aminium 2-(4-acetylphenoxy)acetate, C20H22N2O4
- Chalcogen bonds in the crystal structure of 4,7-dibromo-2,1,3-benzoselenadiazole, C6H2Br2N2Se
- The crystal structure of 1,4-bis((1H-benzimidazol-2-yl)methyl)-piperazine-2,5-dione dihydrate, C20H22N6O4
- The crystal structure of C19H20O8
- The crystal structure of KNa3Te8O18·5H2O exhibiting a ∞2[Te4O9]2− layer
- Erratum
- Erratum to: Crystal structure of (Z)-3-(6-bromo-1H-indol-3-yl)-1,3-diphenylprop-2-en-1-one, C23H16BrNO
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of (E)-7-hydroxy-2-((6-methoxypyridin-3-yl)methylene)-3, 4-dihydronaphthalen-1(2H)-one, C17H15NO3
- Crystal structure of (E)-7-methoxy-2-((2-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1 (2H)-one, C18H17NO3
- The crystal structure of N 6,N 6′-di(pyridin-2-yl)-[2,2′-bipyridine]-6,6′-diamine, C20H16N6
- The crystal structure of {N 1,N 2-bis[2,4-dimethyl-6-(4-(tert-butyl)phenyl)(phenyl)methyl]acenaphthylene-1,2-diimino-κ2 N, N′}-dibromido-nickel(II) – dichloromethane(1/2), C64H64Br2Cl4N2Ni
- Synthesis and crystal structure of nonacarbonyltris[(2-thia-1,3,5-triaza-7-phosphatricylco[3.3.1.1]decane-κ1 P)-2,2-dioxide]triruthenium(0) – acetonitrile (7/6), C25.71H32.57N9.86O15P3S3Ru3
- A new polymorph of 1-(4-nitrophenyl)-1H-benzimidazole (C13H9N3O2)
- The crystal structure of 2,2′-((1E,1′E)-(naphthalene-2,3 diylbis(azanylylidene)) bis(methanylylidene))bis(4-methylphenol), C26H22N2O2
- The crystal structure of bis(μ2-iodido)-bis(η6-benzene)-bis(iodido)-diosmium(II), C12H12I4Os2
- Redetermination of the crystal structure of bis{hydridotris(3,5-dimethylpyrazol-1-yl-κN 3)borato}copper(II), C30H44B2CuN12
- Crystal structure of (E)-3-((4-(tert-butyl)phenyl)thio)-4-hydroxypent-3-en-2-one, C15H20O2S
- Crystal structure of 2,2′-(p-tolylazanediyl)bis(1-phenylethan-1-one), C23H21NO2
- Redetermination of the crystal structure of the crystal sponge the poly[tetrakis(μ3-2,4,6-tris(pyridin-4-yl)-1,3,5-triazine)-dodecaiodidohexazinc(II) nitrobenzene solvate], C72H48I12N24Zn6⋅10(C6H5NO2)
- Crystal structure of (4′E)-6′-(diethylamino)-2-[(E)-[(6-methylpyridin-2-yl)methylidene]amino]-4′-{2-[(2E)-1,3,3-trimethyl-2,3-dihydro-1H-indol-2-ylidene]ethylidene}-1′,2,2′,3,3′,4′-hexahydrospiro[isoindole-1,9′-xanthene]-3-one, C44H45N5O2
- Crystal structure of (E)-7-fluoro-2-(3-fluorobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C17H12F2O1
- Crystal structure of tetrabutylammonium sulfanilate – 1-(diaminomethylene)thiourea (1/2)
- Crystal structure of [2,2′-{azanediyl)bis[(propane-3,1-diyl)(azanylylidene)methylylidene]} bis(3,5-dichlorophenolato)-κ2O,O′]-isothiocyanato-κN-iron(III), C21H19Cl4FeN4O2S
- Crystal structure of (4-chlorophenyl)(4-hydroxyphenyl)methanone, C13H9ClO2
- Crystal structure of 6,6′-((pentane-1,3-diylbis(azaneylylidene))bis(methaneylylidene))bis(2,4-dibromolphenolato-κ4 N,N′,O,O′)copper(II),) C19H16Br4CuN2O2
- Chlorido-(2,2′-(ethane-bis(5-methoxyphenolato))-κ4 N,N′,O,O′)manganese(III) monohydrate, C19H18Cl2CuN2O2
- Crystal structure of 2,6-di-tert-butyl-4-(4-methoxybenzylidene)cyclohexa-2,5-dien-1-one, C22H28O2
- Crystal structure of [6,6′-(((2,2-dimethylpropane-1,3-diyl)bis(azanylylidene))bis(methanylylidene))bis(2-chlorophenolato)-κ4N,N′,O,O′]copper(II)
- Crystal structure of 2-chloro-3-((thiophen-2-ylmethyl)amino)naphthalene-1,4-dione, C30H20O4N2Cl2S2
- Crystal structure of bis{hydridotris(3-trifluoromethyl-5-methylpyrazolyl-1-yl)borato-κN 3}manganese(II), C30H26B2F18MnN12
- Crystal structure of 1-(2-methylphenyl)-2-(2-methylbenzo[b]thienyl)-3,3,4,4,5,5-hexafluorocyclopent-ene, C21H14F6S
- Crystal structure of 2-(3-((carbamimidoylthio)methyl)benzyl)isothiouronium hexafluorophosphate monohydrate, C10H17F6N4OPS2
- Crystal structure of 4,5-diiodo-1,3-dimesityl-1H-1,2,3-triazol-3-ium chloride – chloroform (1/1), C21H23Cl4I2N3
- Crystal structure of azido-k1 N-{6,6′-((((methylazanediyl)bis(propane-3,1-diyl))bis(azanylylidene))bis(methanylylidene))bis(2,4-dibromophenolato)k5 N,N′,N″,O,O′}cobalt(III)-methanol (1/1)), C21H23Br4CoN6O3
- The crystal structure of 2-(4-((carbamimidoylthio)methyl)benzyl)isothiouronium hexafluorophosphate monohydrate, C10H17F6N4OPS2
- Crystal structure of 1,1′-(methane-1,1-diyl)bis(3-methyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C9H14F12N4P2
- Crystal structure of (4′E)-6′-(diethylamino)-2-[(E)-[(pyren-1-yl)methylidene]amino]-4′-{2-[(2E)-1,3,3-trimethyl-2,3-dihydro-1H-indol-2-ylidene]ethylidene}-1′,2,2′,3,3′,4′-hexahydrospiro[isoindole-1,9′-xanthene]-3-one, C54H48N4O2
- Crystal structure of poly[bis(μ2-2,6-bis(1-imidazoly)pyridine-κ2 N,N′)-bis(thiocyanato-κ1 N)copper(II)] dithiocyanate, C24H18CuN12S2
- Cones with a three-fold symmetry constructed from three hydrogen bonded theophyllinium cations that coat [FeCl4]− anions in the crystal structure of tris(theophyllinium) bis(tetrachloridoferrate(III)) chloride trihydrate, C21H33Cl9Fe2N12O9
- Crystal structure of 14-O-[(4-(4-hydroxypiperidine-1-yl)-6-methylpyrimidine-2-yl)thioacetyl]-mutilin monohydrate, C32H49N3O6S
- The crystal structure of (E)-3-chloro-2-(2-(4-methylbenzylidene)hydrazinyl)pyridine, C13H12ClN3
- The crystal structure of 4-phenyl-4-[2-(pyridine-4-carbonyl)hydrazinylidene]butanoic acid, C16H15N3O3
- The crystal structure of 6-amino-5-carboxypyridin-1-ium pentaiodide monohydrate C6H9I5N2O3
- Crystal structure of bis(μ3-oxido)-bis(μ2-2-formylbenzoato-k2O:O′)-bis(2-(dimethoxymethyl)-benzoato-κO)-oktakismethyl-tetratin(IV)
- Crystal structure of 2-((E)-(((E)-2-hydroxy-4-methylbenzylidene) hydrazineylidene)methyl)-4-methylphenol, C16H16N2O2
- Crystal structure of (E)-amino(2-((5-methylfuran-2-yl)methylene)hydrazinyl) methaniminium nitrate monohydrate, C14H26N10O10
- The crystal structure of N′-(2-chloro-6-hydroxybenzylidene)thiophene-2-carbohydrazide monohydrate, C12H11ClN2O3S
- Crystal structure of catena-poly[(μ2-1,1′-(biphenyl-4,4′-diyl)bis(1H-imidazol)-κ2N:N′)-bis(4-bromobenzoate-κ1O)zinc(II)], C64H44Br4N8O8Zn2
- The crystal structure of catena-poly[(1-(4-carboxybenzyl)pyridin-1-ium-4-carboxylato-κ1O)-(μ2-oxalato-κ4 O:O′:O″:O‴)dioxidouranium(VI)], C16H11NO10U
- Crystal structure of 3-allyl-4-(2-bromoethyl)-5-(4-methoxyphenyl)-2-phenylfuran, C22H21BrO2
- Halogen bonds in the crystal structure of 4,3′:5′,4″-terpyridine — 1,3-diiodotetrafluorobenzene (1/1), C21H11F4I2N3
- Crystal structure of 2-(1H-indol-3-yl)ethan-1-aminium 2-(4-acetylphenoxy)acetate, C20H22N2O4
- Chalcogen bonds in the crystal structure of 4,7-dibromo-2,1,3-benzoselenadiazole, C6H2Br2N2Se
- The crystal structure of 1,4-bis((1H-benzimidazol-2-yl)methyl)-piperazine-2,5-dione dihydrate, C20H22N6O4
- The crystal structure of C19H20O8
- The crystal structure of KNa3Te8O18·5H2O exhibiting a ∞2[Te4O9]2− layer
- Erratum
- Erratum to: Crystal structure of (Z)-3-(6-bromo-1H-indol-3-yl)-1,3-diphenylprop-2-en-1-one, C23H16BrNO

