Abstract
C17H20ClNO3S, orthorhombic, P212121 (no. 19), a = 6.1984(2) Å, b = 14.3940(5) Å, c = 18.9651(6) Å, V = 1692.06(10) Å3, Z = 4, Rgt(F) = 0.0263, wRref(F2) = 0.0683, T = 293(2) K.
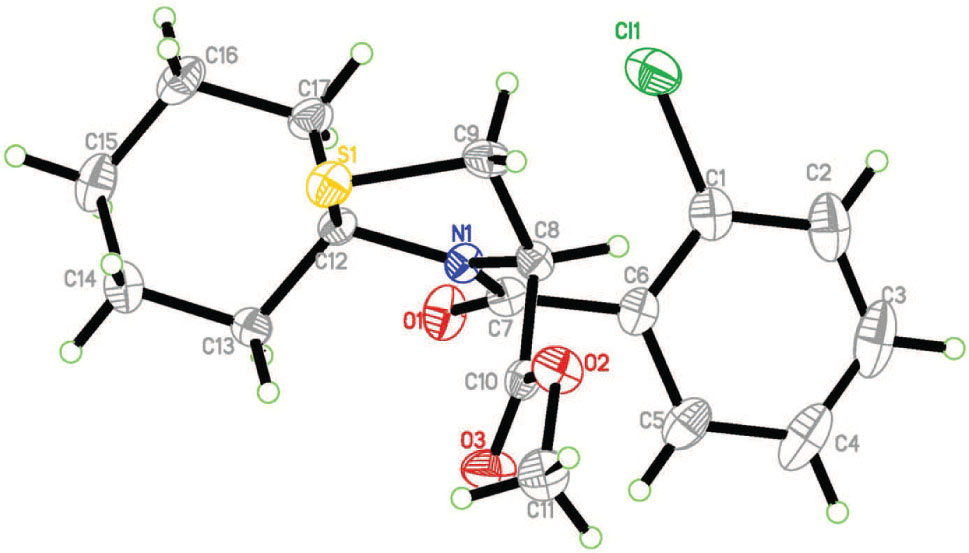
The asymmetric unit of the title crystal structure is shown in the figure. Tables 1 and 2 contain details of the measurement method and a list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.13 × 0.12 × 0.10 mm |
| Wavelength: | Cu Kα radiation (1.54178 Å) |
| μ: | 32.7 cm−1 |
| Diffractometer, scan mode: | D8 Venture Photon II, φ and ω |
| 2θmax, completeness: | 136.4°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 8944, 3042, 0.035 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 2976 |
| N(param)refined: | 210 |
| Programs: | CrysAlisPRO [1], SHELX [2], DIAMOND [3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| C1 | 0.6229(4) | 0.37442(17) | 0.41523(11) | 0.0454(6) |
| N1 | 0.6930(3) | 0.57346(11) | 0.33722(8) | 0.0301(3) |
| O1 | 0.9396(3) | 0.55611(11) | 0.42518(8) | 0.0457(4) |
| S1 | 0.48528(9) | 0.70955(4) | 0.27335(3) | 0.04108(17) |
| C2 | 0.6169(6) | 0.27880(19) | 0.42294(14) | 0.0635(8) |
| H2 | 0.5021 | 0.2505 | 0.4461 | 0.076* |
| Cl1 | 0.41335(12) | 0.44100(5) | 0.44965(4) | 0.0627(2) |
| O2 | 0.5836(3) | 0.52343(12) | 0.15470(8) | 0.0460(4) |
| C3 | 0.7821(8) | 0.22632(19) | 0.39603(16) | 0.0723(10) |
| H3 | 0.7798 | 0.1621 | 0.4015 | 0.087* |
| O3 | 0.8978(3) | 0.54158(13) | 0.21032(9) | 0.0509(4) |
| C4 | 0.9509(7) | 0.2675(2) | 0.36108(15) | 0.0689(9) |
| H4 | 1.0610 | 0.2312 | 0.3425 | 0.083* |
| C5 | 0.9573(5) | 0.36366(17) | 0.35344(12) | 0.0510(6) |
| H5 | 1.0723 | 0.3914 | 0.3300 | 0.061* |
| C6 | 0.7927(4) | 0.41821(14) | 0.38075(10) | 0.0371(5) |
| C7 | 0.8155(3) | 0.52242(14) | 0.38206(10) | 0.0323(4) |
| C8 | 0.5693(3) | 0.53352(13) | 0.27917(10) | 0.0318(4) |
| H8 | 0.5267 | 0.4698 | 0.2908 | 0.038* |
| C9 | 0.3708(3) | 0.59423(16) | 0.27211(13) | 0.0409(5) |
| H9A | 0.2723 | 0.5850 | 0.3112 | 0.049* |
| H9B | 0.2955 | 0.5822 | 0.2282 | 0.049* |
| C10 | 0.7059(3) | 0.53394(13) | 0.21172(10) | 0.0324(4) |
| C11 | 0.6937(5) | 0.5281(2) | 0.08768(12) | 0.0553(6) |
| H11A | 0.7656 | 0.5869 | 0.0835 | 0.083* |
| H11B | 0.5907 | 0.5217 | 0.0502 | 0.083* |
| H11C | 0.7978 | 0.4789 | 0.0848 | 0.083* |
| C12 | 0.6773(3) | 0.67701(13) | 0.34371(10) | 0.0310(4) |
| C13 | 0.8920(3) | 0.72515(14) | 0.33014(11) | 0.0355(4) |
| H13A | 0.9421 | 0.7098 | 0.2831 | 0.043* |
| H13B | 0.9982 | 0.7028 | 0.3636 | 0.043* |
| C14 | 0.8705(5) | 0.83019(16) | 0.33698(13) | 0.0479(6) |
| H14A | 1.0101 | 0.8590 | 0.3296 | 0.057* |
| H14B | 0.7727 | 0.8532 | 0.3011 | 0.057* |
| C15 | 0.7856(5) | 0.85626(17) | 0.40944(14) | 0.0551(7) |
| H15A | 0.7670 | 0.9231 | 0.4120 | 0.066* |
| H15B | 0.8901 | 0.8383 | 0.4450 | 0.066* |
| C16 | 0.5715(5) | 0.80891(17) | 0.42468(14) | 0.0541(6) |
| H16A | 0.4625 | 0.8332 | 0.3929 | 0.065* |
| H16B | 0.5273 | 0.8233 | 0.4725 | 0.065* |
| C17 | 0.5851(4) | 0.70318(16) | 0.41594(11) | 0.0416(5) |
| H17A | 0.6761 | 0.6775 | 0.4527 | 0.050* |
| H17B | 0.4422 | 0.6765 | 0.4210 | 0.050* |
Source of materials
L-cysteine methyl ester hydrochloride (4.29 g, 25 mmol), cyclohexanone (2.45 g, 25 mmol) and Et3N (5.05 g, 50 mmol) were stirred for 2 h in toluene (20 mL) at 65 °C under a nitrogen atmosphere. o-Chlorobenzoyl chloride (4.38 g, 25 mmol) was dropwise added to the reaction mixture at 0 °C and then reacted for 1 h. The mixture was washed with saturated NaCl solution (3 times 20 mL) and dried using anhydrous sodium sulfate. The solvent was removed under reduced pressure to yield crude title compound. The title compound was purified by column chromatography to yield 6.7 g (76%) of a white solid. Single crystals were obtained from ethyl acetate and n-hexane by slowly evaporating the solvent at room temperature.
Experimental details
The C—H atoms were constrained to an ideal geometry, with C—H distances of 0.93–0.98 Å. The Uiso values of the hydrogen atoms of methyl groups were set to 1.5 Ueq(Cmethyl) and the Uiso values of all other hydrogen atoms were set to 1.2 Ueq(C). A Flack-Parsons parameter is 0.050(6) based on 1191 quotients.
Comment
Thiazolidine derivatives are important intermediates for the synthesis of many pharmaceutical compounds, which exhibited biological importance in many fields, such as medicine [4, 5] , plant protection [6]. Recently, it was shown that some thiazolidine derivatives had superior safener activities [7]. Therefore, thiazolidine derivatives are considered to be new generation herbicide safeners and are promising alternatives to some commercial products. Based on active subunit combinations and bioisosterism [8], [9], [10], methyl (R)-4-(o-chlorobenzoyl)-1-thia-4-azaspiro[4.5]decane-3-carboxylate was designed and synthesized to protect maize from herbicide injury.
Crystal structure analysis showed that the three rings, thiazolidane ring (C8/C9/S1/C12/N1), chlorophenyl ring (C1/C2/C3/C4/C5/C6) and cyclohexane ring (C12/C13/C14/C15/C16/C17), are not parallel to each other. The cyclohexane ring is in the stable chair conformation and the dihedral angle between the best planes of the cyclohexane and thiazolidine moiety is 81.28°. The dihedral angle between the best planes of the thiazolidane ring and benzene ring is 68.82°. It has been indicated that the target compound contained a chiral carbon C8, with the R configuration. In the crystal structure, molecules are connected by van der Waals forces and non-classical intermolecular hydrogen bonds.
Acknowledgements
The authors gratefully acknowledge support by the National Nature Science Foundation of China (31572042), the China Postdoctoral Science Foundation (2015M571384), the Natural Science Foundation of Heilongjiang Province of China (C2015014, ZD2017002), the Research Science Foundation in Technology Innovation of Harbin (2015RAYXJ010) and the “Academic Backbone” Project of Northeast Agricultural University (16XG24).
References
Oxford Diffraction Ltd., CrysAlisPRO, Abingdon, Oxfordshire, England (2012).Search in Google Scholar
Sheldrick, G. M.: A short history of SHELX. Acta Crystallogr. A64 (2008) 112–122.10.1107/S0108767307043930Search in Google Scholar PubMed
Brandenburg, K.: DIAMOND. Visual Crystal Structure Information System. Version 3.2i., Crystal Impact, Bonn, Germany (2012).Search in Google Scholar
Wang, G. C.; Peng, Y. P.; Xie, Z. Z.; Wang, J.; Chen. M.: Synthesis, α-glucosidase inhibition and molecular docking studies of novel thiazolidine-2,4-dione or rhodanine derivatives. Med. Chem. Commun. 8 (2017) 1477–1484.10.1039/C7MD00173HSearch in Google Scholar
Asati, V.; Bharti, S. K.; Rathore, A.; Mahapatra, D. K.: SWFB and GA trategies for variable selection in QSAR studies for the validation of thiazolidine-2,4-dione derivatives as promising antitumor candidates. Indian J. Pharm. Educ. Res. 51 (2017) 436–451.10.5530/ijper.51.3.72Search in Google Scholar
Chen, N.; Du, H. G.; Liu, W. D.; Wang, S. S.; Li, X. Y.; Xu, J. X.: Synthesis and fungicidal activity of simple structural 1,3-thiazolidine-2-thione derivative. Phosphorus Sulfur Silicon Relat. Elem. 190 (2015) 112–122.10.1080/10426507.2014.931399Search in Google Scholar
Fu, Y.; Wang, J. Y.; Zhang, D.; Chen, Y. F.; Gao, S.; Zhao, L. X.;Ye, F.: Solvent-free synthesis and safener activity of sulfonylurea benzothiazolines. Molecules 22 (2017) 1601.10.3390/molecules22101601Search in Google Scholar PubMed PubMed Central
Fu, Y.; Wang, J.; Zhao, Q. S.; Wang, X. M.; Xing, Z. Y.; Ye, F.: Synthesis, crystal structure and biological activity of N-dichloroacetyl-3,4-dihydro-3-methyl-2H-1,4-benzoxazines. J. Heterocycl. Chem. 24 (2014) 41–46.Search in Google Scholar
Zheng, Y.; Liu, B.; Gou, Z. P.; Li, Y.; Zhang, X.; Wang Y. Q.; Shujing Yu, S. J.; Li, Y. H.; Sun, D. Q.: Design of novel CSA analogues as potential safeners and fungicides. Bioorg. Med. Chem. Lett. 25 (2015) 791–794.10.1016/j.bmcl.2014.12.085Search in Google Scholar PubMed
Fu, Y.; Chen, W. G.; Hou, Y. W.; Wang, B.; Zhao, L. X.; Ye, F.: One-pot Synthesis, Crystal structure, and bioactivity of N-phenoxyacetyl-2,4,5-trisubstituted-1,3-oxazolidines. J. Heterocycl. Chem. 54 (2017) 1660–1664.10.1002/jhet.2706Search in Google Scholar
©2018 Li-Xia Zhao et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 License.
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of (E)-1-(4-(((E)-2-hydroxy-5-methylbenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C17H18N2O2
- Crystal structure of 1,3,5,7-tetraazaadamantane-1,3-diium 2,5-dicarboxyterephthalate, C16H18N4O8
- Crystal structure of guanidinium tetrabutyl-ammonium 5-hydroxyisophthalate dihydrate, C25H50N4O7
- Crystal structure of poly[aqua-(μ2-5-methoxyisophthalate-κ3O,O′:O′′)-(μ2-1,4-bis((1H-1,2,4-triazol-1-yl)methyl)benzene-κ2N:N′)nickel(II), NiC21H20N6O6
- Crystal structure of aqua-bis(3,4-dimethoxybenzoato-κ1O)-(2,2′-bipyridine-κ2N,N′)copper(II), C28H26CuN2O9
- Crystal structure of catena-poly[aqua-(μ2-(3,5-di(1H-imidazol-1-yl)-pyridine-κ2N:N′)-(μ2-2-(carboxylatomethyl)benzoato-κ2O:O′)] cadmium(II), C20H17CdN5O5
- The crystal structure of catena-poly[chlorido-(μ2-5-methyl-1,3,4-thiadiazole-2-thiolato-κ2S:N)mercury(II)], C3H3ClHgN2S2
- Crystal structure of (E)-2,4-dichloro-6-(((4-methyl-2-nitrophenyl)imino)methyl)phenol, C14H10Cl2N2O3
- Crystal structure of a new polymorph of bis[μ-1,3-bis(diphenylphosphino)propane-κ2P:P′-disilver(I)] bis(tetrafluoroborate), [Ag(dppp)]2(BF4)2, C54H52Ag2B2F8P4
- The crystal structure of 2-phenyl-4,6-bis(R-tert-butylsulfonamido)-1,3,5-triazine – ethyl acetate (2/1), C38H58N10O6S4
- Crystal structure of 6-amino-8-(2-methoxy-phenyl)-2-methyl-2,3,8,8a-tetrahydro-1H-iso-quinoline-5,7,7-tricarbonitrile monohydrate, C20H21N5O2
- Crystal structure of methyl (1-phenylethyl)carbamate, C10H13NO2
- Crystal structure of dimethanol-(μ2-squarato-κ2O:O′)-tetrakis(tri-p-tolylphosphane-κP)disilver(I) – methanol (1/2), C92H98Ag2O8P4
- Crystal structure of catena-poly[bis(μ2-1,4-bis(triazol-1-ylmethyl)benzene-κ2N:N′)-bis(5-tert-butyl-isophthalate-κO)copper(II)]tetrahydrate, C36H46CuN6O12
- Crystal structure of 4-aminopyridinium 4-acetyl-(pyridin-4-yl)-1H-1,2,3-triazol-5-olate monohydrate, C14H16N6O3
- Crystal structure of 2-(8-bromo-2-phenylimidazo[1,2-α]pyridin-3-yl)-6,7-dimethyl-3-phenylquinoxaline, C29H21BrN4
- Crystal structure of aqua(1-(2-pyridyl)ethanone oxime-κ2N,N′)(1-(2-pyridyl)ethanone oximato-κ2N,N′) nitrate monohydrate, C14H19N5O7Cu
- Crystal structure of poly[tetraaqua-(μ4-oxalato-κ4O,O′:O′′,O′′′)-(μ8-benzene-1,2,4,5-tetracarboxylato-κ8O1:O2:O3:O4:O5:O6:O7:O8)yttrium(III)], C6H5O8Y
- Crystal structure of bis{catena-poly[(μ2-1,2-bis(4-pyridyl)ethane-κ2N:N′)silver(I)]} diaqua-bis(5-(4-carboxyphenyl)pyridine-2-carboxylato-κ2N,O)-(μ2-1,2-bis(4-pyridyl)ethane-κ2N:N′)disilver(I) octahydrate, C31H35Ag2N4O9
- Crystal structure of (E)-N-(2-(benzylamino)-2-oxo-1-(4-oxo-4H-chromen-3-yl)ethyl)-N-(4-bromophenyl)-3-chloroacrylamide hydrate, C27H22BrClN2O5
- Crystal structure of catena-poly[octaaqua-bis(μ2-4,6-dicarboxyisophthalate-κ2O:O′)cadmium(II)disodium(I)] dihydrate, C20H28CdNa2O26
- Crystal structure of acetonitrile{bis(2-benzimidazolylmethyl)amine-κ3N,N′,N′′}-{maleato-κO}zinc(II) perchlorate - acetonitrile (1/1), C24H24ClN7O8Zn
- Crystal structure of 2-amino-4-(3,5-dibromo-4-hydroxyphenyl)-7-methyl-5-oxo-2H,5H-pyrano[4,3-b]pyran-3-carbonitrile, C16H10Br2N2O4
- Crystal structure of catena-poly[diaqua-(μ2-3,5-bis(pyridin-4-ylmethoxy)benzoate-κ2N:O) manganese(II)] tetrahydrate [(3,5-bis-(pyridin-4-ylmethoxy)-benzoic-κ1Oκ1N) manganese(II)] trihydrate, C38H42MnN4O14
- The crystal structure of 2-carboxybenzaldehyde-2-phenylacetohydrazone, C16H14N2O3
- The crystal structure of poly[μ2-aqua-(μ2-2-naphthylamine-1-sulfonato-κ3O,O′:O′′)sodium(I)], C10H10N1O4S1Na
- The crystal structure of phthalazin-1(2H)-one, C8H6N2O1
- Crystal structure of 3,5-bis(trifluoromethyl)benzyl(Z)-N-(adamantan-1-yl)morpholine-4-carbothioimidate, C24H28F6N2OS
- Crystal structure of diazido-bis(μ2-pyridin-2-ylmethanolato-κ2N:O)-bis(pyridin-2-ylmethanolato-κ2N,O)dicobalt(III) – methanol (1/3), C27H35Co2N10O7
- Crystal structure of N-[[(4,6-dimethoxy-2-pyrimidinyl)amino]carbonyl]-3-(2,2,2-trifluoroethoxy)-2-pyridinesulfonamide, C14H14F3N5O6S
- Crystal structure of 1-phenyl-N′-(1-phenyl-5-(thiophen-2-yl)-1H-pyrazole-3-carbonyl)-5-(thiophen-2-yl)-1H-pyrazole-3-carbohydrazide, C28H20N6O2S2
- The crystal structure of poly[bis(4-hydroxybenzoato-κO)-(μ2-4,4′-bipyridine-κ2N:N′)copper(II)] hydrate, C24H20N2O7Cu
- Crystal structure of poly[μ3-5-(4-(2,6-di(pyridine-2-yl)pyridine-4-yl)phenoxy)isophthalato-κ5O:O′,O′′:N,N′,N′′cobalt(II)], C29H17CoN3O5
- Crystal structure of poly[μ3-5-(4-(2,6-di(pyridine-2-yl)pyridine-4-yl)phenoxy)isophthalato-κ6O:O′,O′′:N,N′,N′′)cobalt(II)] C29H17CoN3O5
- Crystal structure of diaqua-(acetato-κ3O,O′:O′′)-(μ3-4,6-di(1H-imidazol-1-yl)isophthalato-κ4O:O′:O′′,O′′′)lanthanum(III), C16H15LaN4O8
- Synthesis and crystal structure of 6-carboxy-1-(3,5-dicarboxyphenyl)-1H-benzo[d]imidazol-3-ium-5-carboxylate dihydrate, C18H12N2O8
- Crystal structure of (E)-2-hydroxybenzaldehyde O-(2-(((E)-(4-(dimethylamino)benzylidene)amino)oxy)ethyl)oxime, C18H21N3O3
- Crystal structure of bis{2-((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}zinc(II), C32H30N4O4Zn
- Crystal structure of bis(9-aminoacridin-10-ium) tetrachloridocuprate(II) monohydrate, C26H24Cl4CuN4O
- The crystal structure of 4-tert-butyl-N′-[(E)-(4-fluoro-3-methoxyphenyl)methylidene]benzohydrazide, C19H21F1N2O2
- Crystal structure of (E)-3-(3-(5-methyl-1-4-tolyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazol-4-yl)-1-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)prop-2-en-1-one, C31H26N8O
- Crystal structure of (E)-N′-(4-methoxybenzylidene)-5-methyl-1-(4-tolyl)-1H-1,2,3-triazole-4-carbohydrazide, C19H19N5O2
- Crystal structure and molecular packing of O-ethyl (2-chlorophenyl)carbamothioate, C9H10ClNOS
- Crystal structure of pyrene-2-carbaldehyde, C17H10O
- Crystal structure of (E)-2,4-diiodo-6-(4-methyl-2-nitrostyryl)phenol, C14H10I2N2O3
- Crystal structure of (E)-2,4-dichloro-6-(((4-methoxy-2-nitrophenyl)imino)methyl)phenol, C14H10Cl2N2O4
- Crystal structure of (E)-2-bromo-4-chloro-6-(4-methoxy-2-nitrostyryl)phenol, C14H10BrClN2O4
- Crystal structure of (E)-4,6-diiodo-2-(((4-methoxy-2-nitrophenyl)imino)methyl)-3-methylphenol, C14H10I2N2O4
- The crystal structure of 7-bromo-1-cyclopropyl-8-methyl-4-oxo-1,4-dihydroquinoline-3-carboxylic acid an intermediate of the ozenoxacin synthesis, C14H12BrNO3
- Crystal structure of bis(N-(1-(pyrazin-2-yl)ethylidene)nicotinohydrazonato-κ3N,N′,O)copper(II) C24H20N10O2Cu
- Crystal structure of diaqua-dinitrato-k2O,O′((Z)-N-((E)-1-(pyrazin-2-yl)ethylidene)nicotinohydrazonato-k3N,N′,O)europium(II), C12H14N7O9Eu
- Crystal structure of ethyl 4-amino-5-(5-methyl-1-(4-tolyl)-1H-1,2,3-triazole-4-carbonyl)-2-(phenylamino)thiophene-3-carboxylate, C24H23N5O3S
- The crystal structure of acridin-10-ium2-carboxybenzoate, C21H15NO4
- The crystal structure of 3-((phenylamino)methylene)-1,5-dioxaspiro[5.5]undecane-2,4-dione, C16H17N1O4
- Crystal structure of 12-chloro-5,6,7,12-tetrahydrodibenzo[c,f][1,5]oxastibocine, C14H12ClOSb
- Crystal structure of 4-((1,3-dioxoisoindolin-2-yl)methyl)phenethyl 4-methylbenzenesulfonate, C24H21NO5S
- Crystal structure of 3-methyl-2,3-dihydro-2-thioxoquinazolin-4(1H)-one, C9H8N2OS
- Crystal structure of tert-butyl (2-(4-oxo-2-thioxo-1,4-dihydroquinazolin-3(2H)-yl)ethyl)carbamate, C15H19N3O3S
- Crystal structure of ethyl 5-formyl-3,4-dimethylpyrrole-2-carboxylate–1-(propan-2-ylidene)thiosemicarbazide (1/1), C14H22N4O3S
- Crystal structure of bis-(N′-(5-ethoxycarbonyl-3,4-dimethyl-pyrrol-2-yl-methylidene)-3-hydroxybenzohydrazide-κ2O,N)copper(II) – dimethylformamide (1/2), C40H50N8O10Cu
- Crystal structure of bis(acetato-κO)bis{2-((1H-tetrazol-1-yl)methyl)-1H-benzo[d]imidazole-κN}zinc(II), C22H22N12O4Zn
- Crystal structure of 4-phenyl-3-((4-phenyl-1H-1,2,3-triazol-1-yl)methyl)-1H-1,2,4-triazole-5(4H)-thione, C17H14N6S
- Crystal structure of (Z)-N-(4-nitrophenyl)-3-phenyl-3-(phenylamino)acrylamide, C21H17N3O3
- Crystal structure of 1,1′-(pentane-1,5-diyl)bis(3-methyl-1H-imidazol-3-ium)bis(hexafluorophosphate), C13H22F12N4P2
- Synthesis and crystal structure of bis(furan-2-ylmethanaminium)-catena-[bis(μ2-phthalato-κ2O:O′)cobalt(II)], C26H24CoN2O10
- Crystal structure of methyl (R)-4-(o-chlorobenzoyl)-1-thia-4-azaspiro[4.5]decane-3-carboxylate, C17H20ClNO3S
- Crystal structure of 2-[[4-[2-[4-(4-methoxyphenyl)-1-piperazinyl]ethyl]phenyl] methyl]-1H-isoindole-1,3(2H)-dione, C28H29N3O3
- The crystal structure of benzenaminium 5,7-dihydroxy-4-oxo-2-phenyl-4H-chromene-8-sulfonate hydrate, C21H19NO8S
- Crystal structure of semiconducting potassium poly[(μ2-tetraselenido-κ2Se1:Se4)(μ2-pentaselenido-κ1Se1:Se1)argentate(I)], K3AgSe9
- Crystal structure of 2-isopropyl-8-methyl-phenanthrene-3,4-dione, C18H16O2
- Crystal structure of 2-isopropyl-8,8-dimethyl-5,6,7,8-tetrahydrophenanthrene-3,4-dione, C19H22O2
- Crystal structure of (E)-2-(1-((2-aminophenyl)imino)ethyl)-4-bromophenol, C14H13BrN2O
- Crystal structure of 1,1-di(4-cyanophenyl)-2,2-diphenylethene, C28H18N2
- Crystal structure of bis(hydroxylamido-κ2O,N)-oxido(1H-pyrazole-3-carboxylato-κ2O,N)vanadium(V), C4H7N4O5V
- The crystal structure of In1.2B3O5.6(OH)1.4
- The crystal structure of chlorido(2-(1H-pyrazol-3-yl)phenolato-κ2N,O)(2-(1H-pyrazol-3-yl)phenol-κN)copper(II), C18H15ClCuN4O2
- Crystal structure of 1-heptylpyridazin-1-ium iodide, C11H19N2I
- The crystal structure of N-butylpyridinium bis(μ2-dichlorido)-tetrachloridodicopper(II), C18H28N2Cu2Cl6
- Crystal structure of 6-hydroxy-5-((2-hydroxy-6-oxocyclohex-1-en-1-yl)(4-methoxyphenyl)methyl)-1,3-dimethylpyrimidine-2,4(1H,3H)-dione, C20H22N2O6
- Crystal structure of bis(acetonitrile)-diaqua-dichloridoiron(II), C4H10Cl2N2O2Fe
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of (E)-1-(4-(((E)-2-hydroxy-5-methylbenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C17H18N2O2
- Crystal structure of 1,3,5,7-tetraazaadamantane-1,3-diium 2,5-dicarboxyterephthalate, C16H18N4O8
- Crystal structure of guanidinium tetrabutyl-ammonium 5-hydroxyisophthalate dihydrate, C25H50N4O7
- Crystal structure of poly[aqua-(μ2-5-methoxyisophthalate-κ3O,O′:O′′)-(μ2-1,4-bis((1H-1,2,4-triazol-1-yl)methyl)benzene-κ2N:N′)nickel(II), NiC21H20N6O6
- Crystal structure of aqua-bis(3,4-dimethoxybenzoato-κ1O)-(2,2′-bipyridine-κ2N,N′)copper(II), C28H26CuN2O9
- Crystal structure of catena-poly[aqua-(μ2-(3,5-di(1H-imidazol-1-yl)-pyridine-κ2N:N′)-(μ2-2-(carboxylatomethyl)benzoato-κ2O:O′)] cadmium(II), C20H17CdN5O5
- The crystal structure of catena-poly[chlorido-(μ2-5-methyl-1,3,4-thiadiazole-2-thiolato-κ2S:N)mercury(II)], C3H3ClHgN2S2
- Crystal structure of (E)-2,4-dichloro-6-(((4-methyl-2-nitrophenyl)imino)methyl)phenol, C14H10Cl2N2O3
- Crystal structure of a new polymorph of bis[μ-1,3-bis(diphenylphosphino)propane-κ2P:P′-disilver(I)] bis(tetrafluoroborate), [Ag(dppp)]2(BF4)2, C54H52Ag2B2F8P4
- The crystal structure of 2-phenyl-4,6-bis(R-tert-butylsulfonamido)-1,3,5-triazine – ethyl acetate (2/1), C38H58N10O6S4
- Crystal structure of 6-amino-8-(2-methoxy-phenyl)-2-methyl-2,3,8,8a-tetrahydro-1H-iso-quinoline-5,7,7-tricarbonitrile monohydrate, C20H21N5O2
- Crystal structure of methyl (1-phenylethyl)carbamate, C10H13NO2
- Crystal structure of dimethanol-(μ2-squarato-κ2O:O′)-tetrakis(tri-p-tolylphosphane-κP)disilver(I) – methanol (1/2), C92H98Ag2O8P4
- Crystal structure of catena-poly[bis(μ2-1,4-bis(triazol-1-ylmethyl)benzene-κ2N:N′)-bis(5-tert-butyl-isophthalate-κO)copper(II)]tetrahydrate, C36H46CuN6O12
- Crystal structure of 4-aminopyridinium 4-acetyl-(pyridin-4-yl)-1H-1,2,3-triazol-5-olate monohydrate, C14H16N6O3
- Crystal structure of 2-(8-bromo-2-phenylimidazo[1,2-α]pyridin-3-yl)-6,7-dimethyl-3-phenylquinoxaline, C29H21BrN4
- Crystal structure of aqua(1-(2-pyridyl)ethanone oxime-κ2N,N′)(1-(2-pyridyl)ethanone oximato-κ2N,N′) nitrate monohydrate, C14H19N5O7Cu
- Crystal structure of poly[tetraaqua-(μ4-oxalato-κ4O,O′:O′′,O′′′)-(μ8-benzene-1,2,4,5-tetracarboxylato-κ8O1:O2:O3:O4:O5:O6:O7:O8)yttrium(III)], C6H5O8Y
- Crystal structure of bis{catena-poly[(μ2-1,2-bis(4-pyridyl)ethane-κ2N:N′)silver(I)]} diaqua-bis(5-(4-carboxyphenyl)pyridine-2-carboxylato-κ2N,O)-(μ2-1,2-bis(4-pyridyl)ethane-κ2N:N′)disilver(I) octahydrate, C31H35Ag2N4O9
- Crystal structure of (E)-N-(2-(benzylamino)-2-oxo-1-(4-oxo-4H-chromen-3-yl)ethyl)-N-(4-bromophenyl)-3-chloroacrylamide hydrate, C27H22BrClN2O5
- Crystal structure of catena-poly[octaaqua-bis(μ2-4,6-dicarboxyisophthalate-κ2O:O′)cadmium(II)disodium(I)] dihydrate, C20H28CdNa2O26
- Crystal structure of acetonitrile{bis(2-benzimidazolylmethyl)amine-κ3N,N′,N′′}-{maleato-κO}zinc(II) perchlorate - acetonitrile (1/1), C24H24ClN7O8Zn
- Crystal structure of 2-amino-4-(3,5-dibromo-4-hydroxyphenyl)-7-methyl-5-oxo-2H,5H-pyrano[4,3-b]pyran-3-carbonitrile, C16H10Br2N2O4
- Crystal structure of catena-poly[diaqua-(μ2-3,5-bis(pyridin-4-ylmethoxy)benzoate-κ2N:O) manganese(II)] tetrahydrate [(3,5-bis-(pyridin-4-ylmethoxy)-benzoic-κ1Oκ1N) manganese(II)] trihydrate, C38H42MnN4O14
- The crystal structure of 2-carboxybenzaldehyde-2-phenylacetohydrazone, C16H14N2O3
- The crystal structure of poly[μ2-aqua-(μ2-2-naphthylamine-1-sulfonato-κ3O,O′:O′′)sodium(I)], C10H10N1O4S1Na
- The crystal structure of phthalazin-1(2H)-one, C8H6N2O1
- Crystal structure of 3,5-bis(trifluoromethyl)benzyl(Z)-N-(adamantan-1-yl)morpholine-4-carbothioimidate, C24H28F6N2OS
- Crystal structure of diazido-bis(μ2-pyridin-2-ylmethanolato-κ2N:O)-bis(pyridin-2-ylmethanolato-κ2N,O)dicobalt(III) – methanol (1/3), C27H35Co2N10O7
- Crystal structure of N-[[(4,6-dimethoxy-2-pyrimidinyl)amino]carbonyl]-3-(2,2,2-trifluoroethoxy)-2-pyridinesulfonamide, C14H14F3N5O6S
- Crystal structure of 1-phenyl-N′-(1-phenyl-5-(thiophen-2-yl)-1H-pyrazole-3-carbonyl)-5-(thiophen-2-yl)-1H-pyrazole-3-carbohydrazide, C28H20N6O2S2
- The crystal structure of poly[bis(4-hydroxybenzoato-κO)-(μ2-4,4′-bipyridine-κ2N:N′)copper(II)] hydrate, C24H20N2O7Cu
- Crystal structure of poly[μ3-5-(4-(2,6-di(pyridine-2-yl)pyridine-4-yl)phenoxy)isophthalato-κ5O:O′,O′′:N,N′,N′′cobalt(II)], C29H17CoN3O5
- Crystal structure of poly[μ3-5-(4-(2,6-di(pyridine-2-yl)pyridine-4-yl)phenoxy)isophthalato-κ6O:O′,O′′:N,N′,N′′)cobalt(II)] C29H17CoN3O5
- Crystal structure of diaqua-(acetato-κ3O,O′:O′′)-(μ3-4,6-di(1H-imidazol-1-yl)isophthalato-κ4O:O′:O′′,O′′′)lanthanum(III), C16H15LaN4O8
- Synthesis and crystal structure of 6-carboxy-1-(3,5-dicarboxyphenyl)-1H-benzo[d]imidazol-3-ium-5-carboxylate dihydrate, C18H12N2O8
- Crystal structure of (E)-2-hydroxybenzaldehyde O-(2-(((E)-(4-(dimethylamino)benzylidene)amino)oxy)ethyl)oxime, C18H21N3O3
- Crystal structure of bis{2-((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}zinc(II), C32H30N4O4Zn
- Crystal structure of bis(9-aminoacridin-10-ium) tetrachloridocuprate(II) monohydrate, C26H24Cl4CuN4O
- The crystal structure of 4-tert-butyl-N′-[(E)-(4-fluoro-3-methoxyphenyl)methylidene]benzohydrazide, C19H21F1N2O2
- Crystal structure of (E)-3-(3-(5-methyl-1-4-tolyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazol-4-yl)-1-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)prop-2-en-1-one, C31H26N8O
- Crystal structure of (E)-N′-(4-methoxybenzylidene)-5-methyl-1-(4-tolyl)-1H-1,2,3-triazole-4-carbohydrazide, C19H19N5O2
- Crystal structure and molecular packing of O-ethyl (2-chlorophenyl)carbamothioate, C9H10ClNOS
- Crystal structure of pyrene-2-carbaldehyde, C17H10O
- Crystal structure of (E)-2,4-diiodo-6-(4-methyl-2-nitrostyryl)phenol, C14H10I2N2O3
- Crystal structure of (E)-2,4-dichloro-6-(((4-methoxy-2-nitrophenyl)imino)methyl)phenol, C14H10Cl2N2O4
- Crystal structure of (E)-2-bromo-4-chloro-6-(4-methoxy-2-nitrostyryl)phenol, C14H10BrClN2O4
- Crystal structure of (E)-4,6-diiodo-2-(((4-methoxy-2-nitrophenyl)imino)methyl)-3-methylphenol, C14H10I2N2O4
- The crystal structure of 7-bromo-1-cyclopropyl-8-methyl-4-oxo-1,4-dihydroquinoline-3-carboxylic acid an intermediate of the ozenoxacin synthesis, C14H12BrNO3
- Crystal structure of bis(N-(1-(pyrazin-2-yl)ethylidene)nicotinohydrazonato-κ3N,N′,O)copper(II) C24H20N10O2Cu
- Crystal structure of diaqua-dinitrato-k2O,O′((Z)-N-((E)-1-(pyrazin-2-yl)ethylidene)nicotinohydrazonato-k3N,N′,O)europium(II), C12H14N7O9Eu
- Crystal structure of ethyl 4-amino-5-(5-methyl-1-(4-tolyl)-1H-1,2,3-triazole-4-carbonyl)-2-(phenylamino)thiophene-3-carboxylate, C24H23N5O3S
- The crystal structure of acridin-10-ium2-carboxybenzoate, C21H15NO4
- The crystal structure of 3-((phenylamino)methylene)-1,5-dioxaspiro[5.5]undecane-2,4-dione, C16H17N1O4
- Crystal structure of 12-chloro-5,6,7,12-tetrahydrodibenzo[c,f][1,5]oxastibocine, C14H12ClOSb
- Crystal structure of 4-((1,3-dioxoisoindolin-2-yl)methyl)phenethyl 4-methylbenzenesulfonate, C24H21NO5S
- Crystal structure of 3-methyl-2,3-dihydro-2-thioxoquinazolin-4(1H)-one, C9H8N2OS
- Crystal structure of tert-butyl (2-(4-oxo-2-thioxo-1,4-dihydroquinazolin-3(2H)-yl)ethyl)carbamate, C15H19N3O3S
- Crystal structure of ethyl 5-formyl-3,4-dimethylpyrrole-2-carboxylate–1-(propan-2-ylidene)thiosemicarbazide (1/1), C14H22N4O3S
- Crystal structure of bis-(N′-(5-ethoxycarbonyl-3,4-dimethyl-pyrrol-2-yl-methylidene)-3-hydroxybenzohydrazide-κ2O,N)copper(II) – dimethylformamide (1/2), C40H50N8O10Cu
- Crystal structure of bis(acetato-κO)bis{2-((1H-tetrazol-1-yl)methyl)-1H-benzo[d]imidazole-κN}zinc(II), C22H22N12O4Zn
- Crystal structure of 4-phenyl-3-((4-phenyl-1H-1,2,3-triazol-1-yl)methyl)-1H-1,2,4-triazole-5(4H)-thione, C17H14N6S
- Crystal structure of (Z)-N-(4-nitrophenyl)-3-phenyl-3-(phenylamino)acrylamide, C21H17N3O3
- Crystal structure of 1,1′-(pentane-1,5-diyl)bis(3-methyl-1H-imidazol-3-ium)bis(hexafluorophosphate), C13H22F12N4P2
- Synthesis and crystal structure of bis(furan-2-ylmethanaminium)-catena-[bis(μ2-phthalato-κ2O:O′)cobalt(II)], C26H24CoN2O10
- Crystal structure of methyl (R)-4-(o-chlorobenzoyl)-1-thia-4-azaspiro[4.5]decane-3-carboxylate, C17H20ClNO3S
- Crystal structure of 2-[[4-[2-[4-(4-methoxyphenyl)-1-piperazinyl]ethyl]phenyl] methyl]-1H-isoindole-1,3(2H)-dione, C28H29N3O3
- The crystal structure of benzenaminium 5,7-dihydroxy-4-oxo-2-phenyl-4H-chromene-8-sulfonate hydrate, C21H19NO8S
- Crystal structure of semiconducting potassium poly[(μ2-tetraselenido-κ2Se1:Se4)(μ2-pentaselenido-κ1Se1:Se1)argentate(I)], K3AgSe9
- Crystal structure of 2-isopropyl-8-methyl-phenanthrene-3,4-dione, C18H16O2
- Crystal structure of 2-isopropyl-8,8-dimethyl-5,6,7,8-tetrahydrophenanthrene-3,4-dione, C19H22O2
- Crystal structure of (E)-2-(1-((2-aminophenyl)imino)ethyl)-4-bromophenol, C14H13BrN2O
- Crystal structure of 1,1-di(4-cyanophenyl)-2,2-diphenylethene, C28H18N2
- Crystal structure of bis(hydroxylamido-κ2O,N)-oxido(1H-pyrazole-3-carboxylato-κ2O,N)vanadium(V), C4H7N4O5V
- The crystal structure of In1.2B3O5.6(OH)1.4
- The crystal structure of chlorido(2-(1H-pyrazol-3-yl)phenolato-κ2N,O)(2-(1H-pyrazol-3-yl)phenol-κN)copper(II), C18H15ClCuN4O2
- Crystal structure of 1-heptylpyridazin-1-ium iodide, C11H19N2I
- The crystal structure of N-butylpyridinium bis(μ2-dichlorido)-tetrachloridodicopper(II), C18H28N2Cu2Cl6
- Crystal structure of 6-hydroxy-5-((2-hydroxy-6-oxocyclohex-1-en-1-yl)(4-methoxyphenyl)methyl)-1,3-dimethylpyrimidine-2,4(1H,3H)-dione, C20H22N2O6
- Crystal structure of bis(acetonitrile)-diaqua-dichloridoiron(II), C4H10Cl2N2O2Fe


