Abstract
C10H14F6N2P, orthorhombic, Pbca (no. 61), a = 9.5929(10) Å, b = 15.9201(16) Å, c = 17.8640(19) Å, V = 2728.2(5) Å3, Z = 8, R gt (F) = 0.0587, wR ref (F2) = 0.1735, T = 296(2) K.
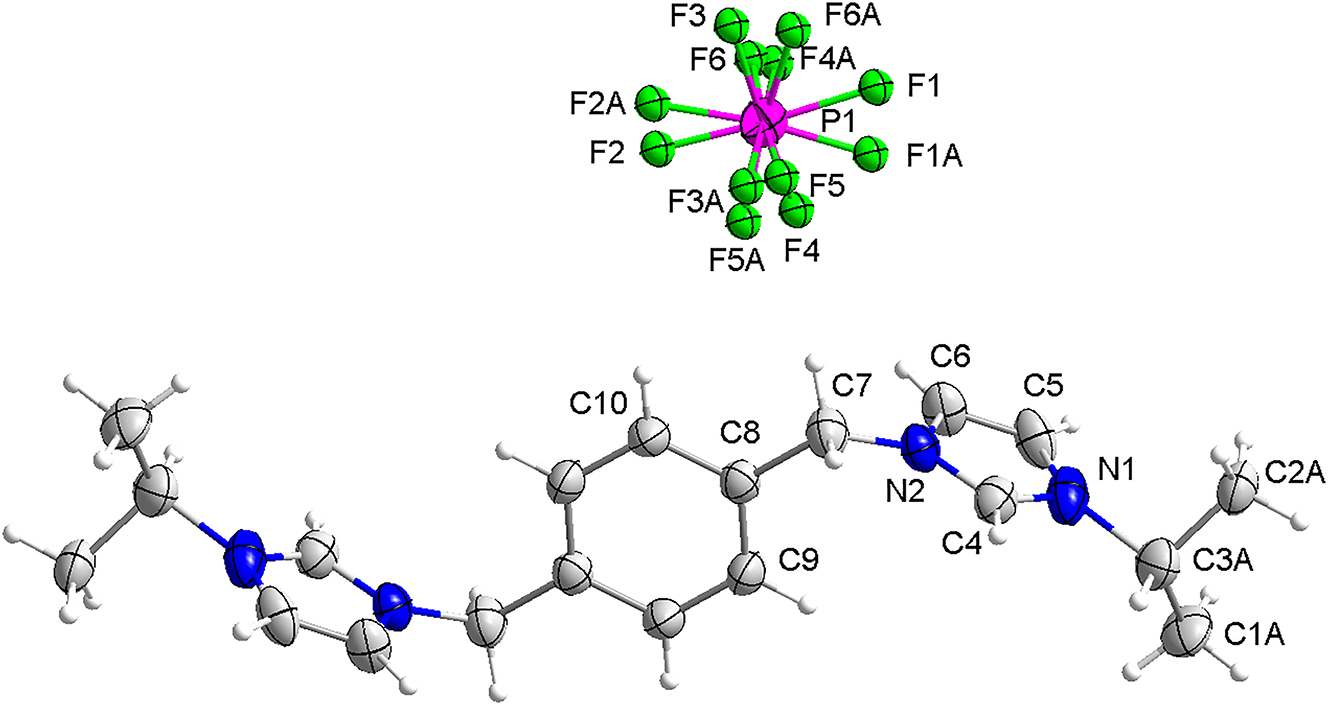
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.30 × 0.20 × 0.10 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.26 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| θmax, completeness: | 25.5°, >99 % |
| N(hkl)measured, N(hkl)unique, Rint: | 19,825, 2534, 0.036 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2σ(Iobs), 1681 |
| N(param)refined: | 240 |
| Programs: | Bruker [1], SHELX [2, 3], Diamond [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| C1Ba | 0.795 (3) | 0.382 (2) | 0.6181 (14) | 0.128 (5) |
| H1BAa | 0.846217 | 0.330437 | 0.616431 | 0.192* |
| H1BBa | 0.856549 | 0.427309 | 0.631172 | 0.192* |
| H1BCa | 0.754228 | 0.392947 | 0.569982 | 0.192* |
| C1Ab | 0.824 (3) | 0.384 (2) | 0.6436 (13) | 0.128 (5) |
| H1AAb | 0.855962 | 0.347135 | 0.605070 | 0.192* |
| H1ABb | 0.902210 | 0.406003 | 0.670888 | 0.192* |
| H1ACb | 0.773320 | 0.430089 | 0.621392 | 0.192* |
| C2Ba | 0.7279 (13) | 0.3557 (7) | 0.7572 (7) | 0.110 (3) |
| H2BAa | 0.648396 | 0.340182 | 0.786809 | 0.165* |
| H2BBa | 0.771247 | 0.404460 | 0.778589 | 0.165* |
| H2BCa | 0.793439 | 0.310086 | 0.756555 | 0.165* |
| C2Ab | 0.6658 (13) | 0.3969 (7) | 0.7521 (7) | 0.110 (3) |
| H2AAb | 0.594824 | 0.430349 | 0.728655 | 0.165* |
| H2ABb | 0.734697 | 0.433095 | 0.773970 | 0.165* |
| H2ACb | 0.624550 | 0.362734 | 0.790424 | 0.165* |
| C3Ba | 0.6806 (9) | 0.3754 (5) | 0.6762 (5) | 0.0830 (16) |
| H3Ba | 0.619437 | 0.424721 | 0.674884 | 0.100* |
| C3Ab | 0.7352 (9) | 0.3401 (5) | 0.6931 (5) | 0.0830 (16) |
| H3Ab | 0.788911 | 0.296196 | 0.718475 | 0.100* |
| C4 | 0.5966 (3) | 0.2185 (2) | 0.66835 (15) | 0.0684 (8) |
| H4 | 0.654622 | 0.189675 | 0.701257 | 0.082* |
| C5 | 0.5032 (5) | 0.3165 (2) | 0.6013 (2) | 0.1079 (14) |
| H5 | 0.485713 | 0.368605 | 0.579695 | 0.129* |
| C6 | 0.4342 (5) | 0.2463 (2) | 0.5879 (2) | 0.0984 (12) |
| H6 | 0.359116 | 0.239993 | 0.555474 | 0.118* |
| C7 | 0.4487 (4) | 0.09738 (19) | 0.63449 (16) | 0.0744 (8) |
| H7A | 0.349873 | 0.095542 | 0.645849 | 0.089* |
| H7B | 0.497900 | 0.070006 | 0.675176 | 0.089* |
| C8 | 0.4750 (3) | 0.04942 (16) | 0.56303 (14) | 0.0575 (7) |
| C9 | 0.5932 (3) | 0.06091 (18) | 0.52018 (15) | 0.0663 (7) |
| H9 | 0.656931 | 0.102310 | 0.533507 | 0.080* |
| C10 | 0.3817 (3) | −0.01204 (17) | 0.54203 (16) | 0.0655 (7) |
| H10 | 0.301275 | −0.020592 | 0.570051 | 0.079* |
| F1b | −0.0096 (16) | 0.1992 (5) | 0.6824 (6) | 0.157 (4) |
| F1Aa | 0.0752 (15) | 0.1956 (8) | 0.6549 (7) | 0.213 (7) |
| F2b | −0.0085 (16) | 0.0441 (7) | 0.5794 (7) | 0.179 (6) |
| F2Aa | −0.0693 (16) | 0.0403 (7) | 0.5947 (9) | 0.189 (6) |
| F3b | −0.1367 (12) | 0.0838 (6) | 0.6725 (7) | 0.125 (4) |
| F3Aa | 0.0921 (12) | 0.0567 (8) | 0.6733 (6) | 0.141 (5) |
| F4b | 0.1155 (10) | 0.1569 (10) | 0.5854 (8) | 0.180 (6) |
| F4Aa | −0.0969 (11) | 0.1789 (6) | 0.5791 (7) | 0.118 (4) |
| F5b | 0.0925 (14) | 0.0825 (9) | 0.6881 (5) | 0.140 (5) |
| F5Aa | 0.1121 (10) | 0.1173 (7) | 0.5648 (5) | 0.124 (4) |
| F6b | −0.1109 (8) | 0.1611 (7) | 0.5735 (6) | 0.112 (4) |
| F6Aa | −0.1090 (11) | 0.1288 (10) | 0.6916 (6) | 0.152 (5) |
| N1 | 0.6052 (3) | 0.29870 (17) | 0.65260 (15) | 0.0871 (9) |
| N2 | 0.4932 (2) | 0.18509 (15) | 0.63032 (11) | 0.0617 (6) |
| P1 | −0.00459 (9) | 0.11970 (6) | 0.62776 (4) | 0.0735 (3) |
-
aOccupancy: 0.493 (6), boccupancy: 0.507 (6).
1 Source of materials
1,4-Bis(chloromethyl)benzene (2.63 g, 0.015 mol) and 1-isopropylimidazole (3.30 g, 0.03 mol) were added to a round-bottomed flask respectively, and 30 mL of acetonitrile was added as a solvent. The mixtures were stirred vigorously for 24 h at 75 °C. After the reaction has been completed, it was poured out the upper layer of acetonitrile, the intermediate was then washed three times with ethyl acetate and anhydrous ether, respectively. The intermediate product was vacuum dried at 60 °C for 0.5 h to obtain a white powder with 93.22 % yield. During anion exchange, the intermediate (1.43 g, 0.005 mol) and potassium hexafluorophosphate (1.84 g, 0.01 mol) were dissolved in deionized water (50 mL) and stirred vigorously at 85 °C for 6 h. After the reaction time was over the resulting mixture was slowly cooled to room temperature. After washing with deionized water and drying with air, the yield was 76.76 %.
2 Experimental details
All H atoms were included in calculated positions and refined as riding atoms, with C–H = 0.90–0.97 Å with Uiso(H) = 1.5 Ueq(C) for methyl H atoms and 1.2 Ueq(C) for all other H atoms.
3 Comment
Ionic liquids (ILs) are a kind of molten salts with melting points typically below 100 °C, usually consisting of bulky and unpaired organic cations with organic or inorganic anions [5]. They have been studied with wide interest because of the unique physicochemical properties such as low toxicity, high thermal stability, and low volatility [6], [7], [8], [9]. Hexafluorophosphate ionic liquids have also gained a great deal of attention and research due to their excellent characteristics such as high oxidative stability [10]. As far as we know, previous work has focused on the synthesis of diimidazole hexafluorophosphates in which two imidazoles are linked by a flexible carbon chain [11]. Among them, a series of diimidazole hexafluorophosphates with different crystal structures were synthesized by altering the length of the flexible carbon chain, and the R group of the sidechain of the imidazole ring. However, there are relatively few reports on the synthesis of diimidazole ionic liquids with two imidazole rings linked by a phenyl-substituted flexible carbon chain. The introduction of a benzene ring combined with nitrogen to form an sp2 hybridization improves thermal stability, additional electronic effects and spatial site resistance, as well as improves solubility and facilitates catalytic extraction [12]. In view of this, equally considering the excellent properties of diimidazole hexafluorophosphate, it is of great significance to seek a diimidazole hexafluorophosphate that is coupled by phenyl [13, 14].
In the cation of the title compound, bond lengths and angles are very similar to those given in the literature [14, 15]. Both hexafluorophosphate ion and isopropyl in the molecule are disordered. All atoms of imidazole ring are on the same plane, and the two imidazole rings are parallel to each other. The dihedral angle of imidazole rings and the phenyl group is 88.6(1)°. The torsion angles of C1A–C3A–N1–C4, C3A–N1–C4–N2, C4–N2–C7–C8 and N2–C7–C8–C9 are 126.8(13)°, −176.1(4)°, −112.7(3)°, and 38.6(4)°, respectively.
Acknowledgment
X-ray data were collected at Instrumental Analysis Center Nanchang Hangkong University, Nanchang, 330063, People’s Republic of China
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This work was supported by the Natural Science Foundation of Jiangxi Province of China (No. 20202BABL205003), the Research Foundation of Education Department of Jiangxi Province of China (No. GJJ210430) and National College Students Innovation and Entrepreneurship Training Program (No. S202310410048 and 202310410313).
References
1. Bruker. APEX2, SAINT and SADABS; Bruker AXS Inc.: Madison, Wisconsin, USA, 2009.Suche in Google Scholar
2. Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122. https://doi.org/10.1107/s0108767307043930.Suche in Google Scholar PubMed
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. https://doi.org/10.1107/s2053229614024218.Suche in Google Scholar
4. Brandenburg, K. DIAMOND. Visual Crystal Structure Information System. Ver. 4.0; Crystal Impact: Bonn, Germany, 2015.Suche in Google Scholar
5. Bo, B. S., Li, S., Xuan, M. Deep desulfurization of 4,6-Dimethyldienzothiophene by an ionic liquids extraction coupled with catalytic oxidation with a molybdic compound. Ind. Eng. Chem. Res. 2014, 53, 6655–6663. https://doi.org/10.1021/ie500236b.Suche in Google Scholar
6. Gao, H. S., Luo, M. F., Xing, J. M., Wu, Y., Li, Y. G., Li, W. L., Liu, Q. F., Liu, H. Z. Desulfurization of Fuel by extraction with pyridinium based ionic liquids. Ind. Eng. Chem. Res. 2008, 21, 8384–8388. https://doi.org/10.1021/ie800739w.Suche in Google Scholar
7. Reza, M., Reza, S., Ali, A. Design of a new unsymmetrical bis (imino) pyridine Schiff-base co-complex with an ionic liquid group as a recyclable green catalyst to prepare chromenes derivatives from benzylic alcohol. Transition Met. Chem. 2023, 48, 269–280. https://doi.org/10.1007/S11243–023–00541–Y.10.1007/s11243-023-00541-ySuche in Google Scholar
8. Haddad, B., Paolone, A., Drai, M., Boumediene, M., Villemin, D., Belarbi, E., Rahmouni, M., Bresson, S., Abbas, O. Para-xylyl linked bis-imidazolium ionic liquids: a study of the conformers of the cation and of the anion-cation hydrogen bonding. J. Mol. Struct. 2019, 1175, 175–184. https://doi.org/10.1016/j.molstruc.2018.07.096.Suche in Google Scholar
9. Patil, A. R., Talebi, M., Sidisky, M. L., Armstrong, D. W. Examination of selectivities of thermally stable geminal dicationic ionic liquids by structural modification. Chromatographia 2017, 80, 1563–1574. https://doi.org/10.1007/s10337–017–3372–5.10.1007/s10337-017-3372-5Suche in Google Scholar
10. Wang, D., Takiyama, M., Hwang, J., Matsumoto, K., Hagiwara, R. A hexafluorophosphate-based ionic liquid as multifunctional interfacial layer between high voltage positive electrode and solid–state electrolyte for sodium secondary batteries. Adv. Energy Mater. 2023, 13, 2301020. https://doi.org/10.1002/aenm.202301020.Suche in Google Scholar
11. Xiong, W. M., Chen, J., Zhao, W., Zhou, Y. H., Jing, C., Nie, X. L. Crystal structure of 1,1′-methylenebis(3-ethyl-1H-imidazol-3-ium)bis(hexafluorophosphate(V)), C11H18F12N4P2. Z. Kristallogr. N. Cryst. Struct. 2020, 235, 7–9. https://doi.org/10.1515/ncrs-2019–0430.10.1515/ncrs-2019-0430Suche in Google Scholar
12. Schmolke, L., Lerch, S., Bülow, M., Siebels, M., Schmitz, A., Thomas, J., Dehm, G., Held, C., Strassner, T., Janiak, C. Aggregation control of Ru and Ir nanoparticles by tunable aryl alkyl imidazolium ionic liquids. Nanoscale 2019, 11, 4073–4082. https://doi.org/10.1039/c8nr10286d.Suche in Google Scholar PubMed
13. Zhou, Y. H., Huang, T., Nie, X. L., Chen, J., Xiong, W. M. Crystal structure of 3,3′-(1,2-phenylenebis(methylene))bis(1-methyl-1H-imidazol-3-ium)bis(hexafluoridophosphate), C16H20F12N4P2. Z. Kristallogr. N. Cryst. Struct. 2020, 235, 1217–1219. https://doi.org/10.1515/ncrs-2020–0268.10.1515/ncrs-2020-0268Suche in Google Scholar
14. Zhao, W., Liu, X. T., Wu, S. Q., Xiong, W. M., Nie, X. L. Crystal structure of 3,3′-(1,2-phenylene-bis(methylene))bis(1-ethyl-1H-imidazol-3-ium)bis(hexafluorophosphate), C18H24F12N4P2. Z. Kristallogr. N. Cryst. Struct. 2021, 236, 545–547. https://doi.org/10.1515/ncrs-2020–0639.10.1515/ncrs-2020-0639Suche in Google Scholar
15. Yuan, K., Wu, S. Q., Nie, X. L., Xiong, W. M., Cheng, J. Crystal structure of 3,3′-(1,4-phenylenebis(methylene))bis(1-allyl-1H-imidazol-3-ium)bis(hexafluorophosphate)(V), C10H12F6N2P. Z. Kristallogr. N. Cryst. Struct. 2023, 238, 231–233. https://doi.org/10.1515/ncrs-2020–0555.10.1515/ncrs-2022-0558Suche in Google Scholar
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of poly[diaqua-(μ4-3,3′-di(1H-1,2,4-triazol-1-yl)-[1,1′-biphenyl]-4,4′-dicarboxylate-N:N′:O:O′)cadmium(II)], C18H14N6O6Cd
- Crystal structure of (8R,8′S,13S,13′R)-8,8′-bis(hydroxymethyl)-9,9′,10,10′-tetramethoxy-5,5′,6,6′,8,8′,13,13′-octahydro-[13,13′-bi[1,3]dioxolo[4,5-g]isoquinolino[3,2-a]isoquinoline]-7,7′-diium chloride-methanol (1/2), C46H58N2O14Cl2
- The crystal structure of 8-methoxy-2,2-diphenyl-tosyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ig]quinoline, C29H25BN2O3S
- Crystal structure of aqua-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N″,N‴)copper(II) 5-carboxyisophthalate tetrahydrate, C25H50N4CuO11
- The crystal structure of 1-(naphthalen-2-ylsulfonyl)-2,2-diphenyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ij]quinoline, C31H23BN2O2S
- Crystal structure of iodido-(η6-benzene) (1-(pyridin-2-yl)-N-(p-fluoro-methanamine)-κ2N,Nʹ)ruthenium(II) hexaflourophosphate, (C18H15F7IN2RuP)
- The crystal structure of 1-(3-oxo-1-phenyl-3-(p-tolyl) propylidene)-1,3-dihydro-2H-inden-2-one, C25H20O2
- Crystal structure of tricyclohexyl[4-(4H-1,2,4-triazol-4-yl)-benzoato-κO]tin(IV), C27H39N3O2Sn
- Crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylate-κ4N,O,O,O)cadmium(II)]monohydrate, C12H15NO9Cd
- Crystal structure of ethyl 2-((4-(3,5-dimethylisoxazol-4-yl)-2,6-difluorophenyl)amino)benzoate, C20H18F2N2O3
- The crystal structure of 2-(hydroxymethyl)-2-(4H-1,2,4-triazol-4-yl)propane-1,3-diol, C6H11N3O3
- The crystal structure of 1,2-bis(2,4-dinitrophenyl) hydrazine, C12H8N6O8
- Crystal structure of 1-(2,6-dichloro-4-(3,5-dimethylisoxazol-4-yl)phenyl)-1,2-dihydro-4H-benzo[d][1,3]oxazin-4-one, C19H14Cl2N2O3
- The crystal structure of 5-amino-5-oxo-4-(1-oxo-4-(2-oxopyrrolidin-1-yl)isoindolin-2-yl)pentanoic acid, C17H19N3O5
- Crystal structure of N2,N6-bis(2-(((Z)-5-bromo-2-hydroxybenzylidene)amino) phenyl)pyridine-2,6-dicarboxamide, C33H23Br2N5O4
- The crystal structure of (E)-2-methoxy-6-(((5-methyl-1,3,4-thiadiazol-2-yl)imino)methyl)phenol, C11H11N3O2S
- The crystal structure of 3-((tert-butyldiphenylsilyl)methyl)-5,5-diphenyl-6-(p-tolyl) tetrahydro-2H-pyran-2-one, C41H42O2Si
- Crystal structure of 9-fluoro-4-(6-methoxypyridin-3-yl)-5,6-dihydrobenzo[h]quinazolin-2-amine, C18H15FN4O
- The crystal structure of 2-bromo-5-(4-cyanophenoxy)benzyl 1-methyl-1,2,5,6-tetrahydropyridine-3-carboxylate, C21H19BrN2O3
- Crystal structure of 3,3′-(1,4-phenylenebis(methylene))bis(1-isopropyl-1H-imidazol-3-ium) bis(hexafluorophosphate(V)), C10H14F6N2P
- The crystal structure of 2,2-di(thiophen-3-yl)-1-tosyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ig]quinoline, C24H19BN2O2S3
- Crystal structure of 5-bromo-1-(2-iodobenzoyl)-1H-indole-3-carbaldehyde, C16H9BrINO2
- The crystal structure of monocarbonyl-2-carboxypyridinato-κ2N,O-triphenylphosphine-rhodium(I) acetonitrile solvate, C26H20.50N1.50O3PRh
- Crystal structure of dichlorido-tetrakis(1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ1N)manganese(II), C60H68O4N12Cl10Mn
- Crystal structure of 3-(tert-butyldiphenylsilyl)-1-(2,6-dichlorophenyl)-2,2-diphenylpropan-1-ol, C37H36Cl2OSi
- Crystal structure of langite from Mine du Pradet (France)
- The crystal structure of 5′-(furan-2-yl)-3′-((4-methylphenyl)sulfonamido)-3′,4′,5′,6′-tetrahydro-[1,1′:3′,1″-terphenyl]-4′-carboxylic acid, C30H27NO5S
- Synthesis and crystal structure of bis{2-(((4-acetophenone)imino)methyl)-4-fluorophenolato-κ2N,O}zinc(II), C30H22F2N2O4Zn
- The crystal structure of poly[(tripyridine-κ3N,N′,N″) μ3-(pyridine-3,4-dicarboxylate-κ3N:O:O′) manganese(II)], C22H22N4O8Mn
- The crystal structure of (E)-4-chloro-N′-(1-(4-hydroxyphenyl)propylidene)benzohydrazide, C16H15ClN2O2
- Synthesis and crystal structure of bis{2-(tert-butyl)-6-((E)-((4-((E)-1-(methoxyimino) ethyl)phenyl)imino)methyl)phenolato-κ2N,O}cobalt(II), C40H46CoN4O4
- Crystal structure of tetraaqua-[(1-(carboxymethyl)-1H-pyrazole-3-carboxylato-κ2N,O)cobalt(II)], C6H12CoN2O8
- (6R,7S)-2,3,13-trimethoxy-6,7-dimethyl-5,6,7,8-tetrahydrobenzo[3′,4′]cycloocta [1′,2′:4,5]benzo[1,2-d][1,3]dioxol-1-ol, C22H26O6
- Crystal structure of 2-((2,6-dichloro-4-(3,5-dimethylisoxazol-4-yl)phenyl)amino)benzoic acid, C18H14Cl2N2O3
- Crystal structure of (5aS,6aS,8aR,9R,11aS, 11bS,13R,13aS)-1,1,8a,11a-tetramethyl-9-((S)-1-((S)-5-methyl-6-oxo-3,6-dihydro-2H-pyran-2-yl)ethyl)-3-oxo-1,7,8,8a,9,10,11,11a,11b,12,13,13a-dodecahydro-3H,6H-cyclopenta[5,6]cyclopropa[1,8a]naphtho[2,1-c]oxepin-13-yl acetate, C32H44O6
- Crystal structure of catena-poly[triaqua-(μ2-1-(4-carboxylatophenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylato-O,O′:O″)cobalt(II)], C12H12N2O8Co
- Crystal structure of 3-[(furan-2-ylmethyl)-amino]-2-(2,3,4,5-tetrafluoro-benzoyl)-acrylic acid ethyl ester, C17H13F4NO4
- Crystal structure of methyl 4-(2-ethoxy-2-oxoethoxy)-3-methoxybenzoate, C13H16O6
- Crystal structure of 4-bromo-2-(4-chlorophenyl)-1-methyl-5-(trifluoromethyl)-1H-pyrrole-3-carbonitrile, C13H7BrClF3N2
- The crystal structure of triaqua-(8-carboxymethoxy-quinoline-2-carboxylate-κ3N,O,O)nickel(II) monohydrate, C12H15NO9Ni
- Crystal structure of dihydroxy(2,4,6-triisopro-pylphenyl)telluronium trifluoromethanesulfonate, C16H25F3O5STe
- The crystal structure of 1-(carboxymethyl)-1H-imidazole 3-oxide
- The crystal structure of 1,3,5-tris(dibromomethyl)benzene, C9H6Br6
- Crystal structure of (Z)-3-(4-methoxyphenyl)-4-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-N-phenylthiazol-2(3H)-imine, C25H21N5OS
- Crystal structure of (Z)-3-(3-(4-hydroxyphenyl)-2-(phenylimino)-2,3-dihydrothiazol-4-yl)-2H-chromen-2-one, C24H16N2O3S
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of poly[diaqua-(μ4-3,3′-di(1H-1,2,4-triazol-1-yl)-[1,1′-biphenyl]-4,4′-dicarboxylate-N:N′:O:O′)cadmium(II)], C18H14N6O6Cd
- Crystal structure of (8R,8′S,13S,13′R)-8,8′-bis(hydroxymethyl)-9,9′,10,10′-tetramethoxy-5,5′,6,6′,8,8′,13,13′-octahydro-[13,13′-bi[1,3]dioxolo[4,5-g]isoquinolino[3,2-a]isoquinoline]-7,7′-diium chloride-methanol (1/2), C46H58N2O14Cl2
- The crystal structure of 8-methoxy-2,2-diphenyl-tosyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ig]quinoline, C29H25BN2O3S
- Crystal structure of aqua-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N″,N‴)copper(II) 5-carboxyisophthalate tetrahydrate, C25H50N4CuO11
- The crystal structure of 1-(naphthalen-2-ylsulfonyl)-2,2-diphenyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ij]quinoline, C31H23BN2O2S
- Crystal structure of iodido-(η6-benzene) (1-(pyridin-2-yl)-N-(p-fluoro-methanamine)-κ2N,Nʹ)ruthenium(II) hexaflourophosphate, (C18H15F7IN2RuP)
- The crystal structure of 1-(3-oxo-1-phenyl-3-(p-tolyl) propylidene)-1,3-dihydro-2H-inden-2-one, C25H20O2
- Crystal structure of tricyclohexyl[4-(4H-1,2,4-triazol-4-yl)-benzoato-κO]tin(IV), C27H39N3O2Sn
- Crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylate-κ4N,O,O,O)cadmium(II)]monohydrate, C12H15NO9Cd
- Crystal structure of ethyl 2-((4-(3,5-dimethylisoxazol-4-yl)-2,6-difluorophenyl)amino)benzoate, C20H18F2N2O3
- The crystal structure of 2-(hydroxymethyl)-2-(4H-1,2,4-triazol-4-yl)propane-1,3-diol, C6H11N3O3
- The crystal structure of 1,2-bis(2,4-dinitrophenyl) hydrazine, C12H8N6O8
- Crystal structure of 1-(2,6-dichloro-4-(3,5-dimethylisoxazol-4-yl)phenyl)-1,2-dihydro-4H-benzo[d][1,3]oxazin-4-one, C19H14Cl2N2O3
- The crystal structure of 5-amino-5-oxo-4-(1-oxo-4-(2-oxopyrrolidin-1-yl)isoindolin-2-yl)pentanoic acid, C17H19N3O5
- Crystal structure of N2,N6-bis(2-(((Z)-5-bromo-2-hydroxybenzylidene)amino) phenyl)pyridine-2,6-dicarboxamide, C33H23Br2N5O4
- The crystal structure of (E)-2-methoxy-6-(((5-methyl-1,3,4-thiadiazol-2-yl)imino)methyl)phenol, C11H11N3O2S
- The crystal structure of 3-((tert-butyldiphenylsilyl)methyl)-5,5-diphenyl-6-(p-tolyl) tetrahydro-2H-pyran-2-one, C41H42O2Si
- Crystal structure of 9-fluoro-4-(6-methoxypyridin-3-yl)-5,6-dihydrobenzo[h]quinazolin-2-amine, C18H15FN4O
- The crystal structure of 2-bromo-5-(4-cyanophenoxy)benzyl 1-methyl-1,2,5,6-tetrahydropyridine-3-carboxylate, C21H19BrN2O3
- Crystal structure of 3,3′-(1,4-phenylenebis(methylene))bis(1-isopropyl-1H-imidazol-3-ium) bis(hexafluorophosphate(V)), C10H14F6N2P
- The crystal structure of 2,2-di(thiophen-3-yl)-1-tosyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ig]quinoline, C24H19BN2O2S3
- Crystal structure of 5-bromo-1-(2-iodobenzoyl)-1H-indole-3-carbaldehyde, C16H9BrINO2
- The crystal structure of monocarbonyl-2-carboxypyridinato-κ2N,O-triphenylphosphine-rhodium(I) acetonitrile solvate, C26H20.50N1.50O3PRh
- Crystal structure of dichlorido-tetrakis(1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ1N)manganese(II), C60H68O4N12Cl10Mn
- Crystal structure of 3-(tert-butyldiphenylsilyl)-1-(2,6-dichlorophenyl)-2,2-diphenylpropan-1-ol, C37H36Cl2OSi
- Crystal structure of langite from Mine du Pradet (France)
- The crystal structure of 5′-(furan-2-yl)-3′-((4-methylphenyl)sulfonamido)-3′,4′,5′,6′-tetrahydro-[1,1′:3′,1″-terphenyl]-4′-carboxylic acid, C30H27NO5S
- Synthesis and crystal structure of bis{2-(((4-acetophenone)imino)methyl)-4-fluorophenolato-κ2N,O}zinc(II), C30H22F2N2O4Zn
- The crystal structure of poly[(tripyridine-κ3N,N′,N″) μ3-(pyridine-3,4-dicarboxylate-κ3N:O:O′) manganese(II)], C22H22N4O8Mn
- The crystal structure of (E)-4-chloro-N′-(1-(4-hydroxyphenyl)propylidene)benzohydrazide, C16H15ClN2O2
- Synthesis and crystal structure of bis{2-(tert-butyl)-6-((E)-((4-((E)-1-(methoxyimino) ethyl)phenyl)imino)methyl)phenolato-κ2N,O}cobalt(II), C40H46CoN4O4
- Crystal structure of tetraaqua-[(1-(carboxymethyl)-1H-pyrazole-3-carboxylato-κ2N,O)cobalt(II)], C6H12CoN2O8
- (6R,7S)-2,3,13-trimethoxy-6,7-dimethyl-5,6,7,8-tetrahydrobenzo[3′,4′]cycloocta [1′,2′:4,5]benzo[1,2-d][1,3]dioxol-1-ol, C22H26O6
- Crystal structure of 2-((2,6-dichloro-4-(3,5-dimethylisoxazol-4-yl)phenyl)amino)benzoic acid, C18H14Cl2N2O3
- Crystal structure of (5aS,6aS,8aR,9R,11aS, 11bS,13R,13aS)-1,1,8a,11a-tetramethyl-9-((S)-1-((S)-5-methyl-6-oxo-3,6-dihydro-2H-pyran-2-yl)ethyl)-3-oxo-1,7,8,8a,9,10,11,11a,11b,12,13,13a-dodecahydro-3H,6H-cyclopenta[5,6]cyclopropa[1,8a]naphtho[2,1-c]oxepin-13-yl acetate, C32H44O6
- Crystal structure of catena-poly[triaqua-(μ2-1-(4-carboxylatophenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylato-O,O′:O″)cobalt(II)], C12H12N2O8Co
- Crystal structure of 3-[(furan-2-ylmethyl)-amino]-2-(2,3,4,5-tetrafluoro-benzoyl)-acrylic acid ethyl ester, C17H13F4NO4
- Crystal structure of methyl 4-(2-ethoxy-2-oxoethoxy)-3-methoxybenzoate, C13H16O6
- Crystal structure of 4-bromo-2-(4-chlorophenyl)-1-methyl-5-(trifluoromethyl)-1H-pyrrole-3-carbonitrile, C13H7BrClF3N2
- The crystal structure of triaqua-(8-carboxymethoxy-quinoline-2-carboxylate-κ3N,O,O)nickel(II) monohydrate, C12H15NO9Ni
- Crystal structure of dihydroxy(2,4,6-triisopro-pylphenyl)telluronium trifluoromethanesulfonate, C16H25F3O5STe
- The crystal structure of 1-(carboxymethyl)-1H-imidazole 3-oxide
- The crystal structure of 1,3,5-tris(dibromomethyl)benzene, C9H6Br6
- Crystal structure of (Z)-3-(4-methoxyphenyl)-4-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-N-phenylthiazol-2(3H)-imine, C25H21N5OS
- Crystal structure of (Z)-3-(3-(4-hydroxyphenyl)-2-(phenylimino)-2,3-dihydrothiazol-4-yl)-2H-chromen-2-one, C24H16N2O3S


